Abstract
We recently demonstrated that NLY01, a novel glucagon-like peptide receptor 1 (GLP1R) agonist, exerts neuroprotective effects in two mouse models of Parkinson disease (PD) in a glia-dependent manner. NLY01 prevented microglia from releasing inflammatory mediators known to convert astrocytes into a neurotoxic A1 reactive subtype. Importantly, we provided evidence that this neuroprotection was not mediated by a direct action of NLY01 on neurons or astrocytes (e.g. by activating neurotrophic pathways or modulating astrocyte reactivity per se). In the present article, we provide a generalist review of microglia and astrocytes in neurodegeneration and discuss the emerging paradigm of A1 astrocyte neurotoxicity in more detail. We comment on specific inferences that are naturally suggested by our work in this area and the differential level of support it offers to each. Finally, we discuss implications for the overall goal of creating disease-modifying therapies for PD, survey emerging methodologies for accelerating translational research on glia in neurodegeneration, and describe expected challenges for developing glia-directed therapies that do not impede essential physiologic functions carried out by glia in the central nervous system.
Keywords: Parkinson disease, Microglia, Astrocytes, NLY01, Neurodegeneration
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative condition worldwide.1 For nearly a century, scientific consensus has maintained that the disease preferentially ravages the dopaminergic neurons of the ventral midbrain, thereby causing the principal motor signs experienced by PD patients. Efforts to understand why these neurons are disproportionately affected in PD have historically assumed that answers would lie primarily within the neurons themselves. The resultant inquiries have generated comprehensive models of neuronal function. Additionally, the study of aberrant gene product activities in monogenic forms of PD have clarified why dopaminergic neurons in the substantia nigra may be especially vulnerable to the disease process. For example, these cells sustain calcium-dependent pacemaker firing activity and variegated axonal projections to distant targets, leading to extraordinary metabolic demands that induce damaging mitochondrial oxidant stress.2 However, cell autonomous or neuron-centric perspectives of neurodegeneration are reckoning with the increasingly accepted notion that the non-neuronal cells of the brain are not passive bystanders in either healthy or diseased states.
Neurobiological paradigms of PD—and other neurodegenerative diseases such as Alzheimer disease (AD), Huntington disease (HD) and amyotrophic lateral sclerosis (ALS)—are undergoing rapid conceptual refinement to accommodate newfound or revisited roles for glial cells and their contributions to brain homeostasis. For example, microglia are not merely phagocytic sinks or debris receptacles, but motile and dynamic surveillants that patrol the central nervous system (CNS) parenchyma. They are also exquisitely responsive to extracellular signals and undergo remarkable transformations to accommodate a variety of functions, such as selective synaptic pruning during development.3 Similarly, astrocytes appear to undergo phenotypic diversification in the presence of damage- or disease-associated signals, altering their relationship to neighboring neurons.4
In the present Scientific Perspectives article, we discuss recent discoveries regarding the relevance of glial cells to models of neurodegeneration, with a focus on PD. Our commentary centers on findings that may bear especial therapeutic promise insofar as they suggest fruitful targets of pharmacologic intervention. As an example, we highlight our own work on NLY01.5 We also temper this discussion with attention to the essential and adaptive roles of glia in the aging CNS, which is requisite to understanding the impacts of modulating their activity. We conclude the perspective by highlighting emerging methodologies that may accelerate translational research centered on glial function and answer outstanding questions for the field.
Microglia – More than Macrophages
In 1918, Pío del Río-Hortega devised an adapted staining technique that enabled him to morphologically distinguish microglia from other types of glia.6 Río-Hortega subsequently laid the descriptive foundation for this newly resolved population of cells, but one full century later, much remains to be understood about them. For example, the developmental origin of microglia was a surprisingly controversial topic until the present decade. Microglia are now seen as mesodermal in origin, arising from the embryonic yolk sac like other tissue macrophages and colonizing the developing CNS.7 During an adult human lifespan, it is estimated that the microglial population self-renews multiple times through ordered proliferation, although the cues that govern this process across different brain regions are not well understood.8 Similarly, while microglia exhibit region- and age-dependent phenotypes,9–12 it remains profoundly unclear how these differences and changes relate to health and disease.
As for other tissue macrophages, investigators often use the metaphor of “activation” to describe the process by which microglia transform from a surveilling or homeostatic state to a reactive state. This can be catalyzed by the detection of a damage- or pathogen-associated substance such as bacterial lipopolysaccharide or misfolded proteins.13 In the context of histologic analysis, these phenotypes can be discriminated based on cell markers and morphology. The appearance of homeostatic microglia elegantly reflects their patrolling activities: a relatively small rod-shaped cell body with highly ramified processes that dynamically protract and retreat to sample the extracellular milieu.14 The morphology observed to predominate near diseased or infarcted brain tissue is distinctively less intricate—these reactive microglia appear as though retracting inwardly, exhibiting larger cell bodies and few short, dense processes. In this form, microglia are specialized for signal amplification and cytokine biosynthesis rather than surveillance. Traditionally, activated microglia are further dichotomized into pro-inflammatory “M1” and alternative immunoregulatory “M2” microglia subtypes. While this particular split is now reflexively acknowledged to be an oversimplification,15, 16 it remains in use as a generally useful heuristic. Reactive microglia have been recognized for decades as a pathologic hallmark of neurodegenerative foci in PD and other neurodegenerative conditions.17 In recent years, it has become increasingly difficult to contend that these changes are primarily a secondary effect of neuronal destruction that bear little instigative relevance to neurodegenerative pathogeneses. However, it is similarly difficult to coherently link specific cellular changes to disease etiology, as they are typically not inherently adaptive or pathological.
Transcriptomic analysis of microglia in neurodegenerative models has provided some valuable starting points for how these cells respond locally at neurodegenerative foci, giving rise to the idea of disease-associated microglia (DAM)18 or the microglial neurodegenerative (MGnD) phenotype.19 Interestingly, these microglia differentially express markers that are traditionally associated with both M1 and M2 microglia—i.e., markers from both population are either upregulated or downregulated (see Shi & Holtzman, 2018).20 At a broad level, this signature is associated with increased expression of phagocytic and inflammatory genes, as well as regulators of microglial ontogeny, specifically colony stimulating factor 1 (Csf1). Mice lacking the receptor for this gene (Csf1r) do not develop microglia and its pharmacologic inhibition leads to microglia depletion in adult animals.21 Whether DAM represent an overall beneficial population in terms of resisting neurodegeneration remains largely unclear, but it appears likely that they exert both protective functions (e.g., uptake and digestion of misfolded proteins and dead neurons) and adverse effects (e.g. phagocytosis of stressed but not dying neurons). Environmental influences, genetic variation, epigenetic status, and peripheral immune changes may also bias DAM one way or the other, but much remains to be understood about them.
A more granular strategy for exploring microglial function in neurodegenerative contexts is to consider how disease-linked gene variants may alter microglial function. This approach is exemplified by recent studies of triggering receptor expressed on myeloid cells 2 (TREM2), a gene with hypofunctioning alleles linked strongly to AD and to a lesser extent, PD.22–24 Human carriers of the AD-linked R47H variant of TREM2 not only have higher risk for AD, but earlier symptom onset and more rapid AD progression.22, 24 A growing body of literature suggests that microglial TREM-2 enables adaptive regulation of amyloid plaque formation.20, 25–27 Microglia trim extracellular filamentous β-amyloid in a TREM-2 dependent manner; this process compacts it into plaques, which blocks its spread and enables the formation of barriers to reduce its local neurotoxicity.28, 29 Finally, TREM-2 appears to regulate adoption of the DAM transcriptional signature, as the appearance of this population in the 5xFAD mouse model of AD is blocked in mice that lack TREM2.18 Because reduced TREM-2 function can be linked to both DAM reduction and increased risk for neurodegeneration, this may suggest an overall neuroprotective role for DAM, but direct evidence for this claim is lacking.
The successful work on microglial TREM2 in AD suggests that it may be important to reconsider the functions of classic PD-associated genes—such as LRRK2, PINK1, and PRKN (Parkin)—and how their mutation or loss could impact microglial biology. For example, a recent investigation found that mice overexpressing mutant human LRRK2 (either R1441G or G2019S) exhibit increased dopamine neuron loss and neuroinflammation in response to systemic endotoxin challenge.30 This study concluded that LRRK2 mediated the link between peripheral inflammation and neuroinflammation by augmenting peripheral production of CNS-modulating cytokines. If supported by future research, this finding could also partly explicate the association between PD and Crohn’s disease. Genetic variants in LRRK2 have been found that are linked to not only PD but various clinical characteristics of Crohn’s disease. For example, the N2081D risk allele for Crohn’s disease affects the kinase domain of the LRRK2 protein, similarly to the G2019S mutation that increases PD risk.31 Interestingly, anti–tumor necrosis factor (anti-TNF) regimens for inflammatory bowel diseases may reduce the incidence of PD.32 This could suggest a broader relationship between colonic inflammation and PD, such as facilitating the theoretical spread of α-synuclein aggregates from the enteric nervous system to the brainstem33 or modulating microbiomic control over neuroinflammation.34 Given the exquisite sensitivity of both microglia and astrocytes to inflammatory signals, either of these mechanisms would have clear relevance to glial function.
PRKN and PINK1 are essential to mitochondrial quality control (see Pickrell & Youle, 2015),35 which could be important in microglia, as mitochondria serve as convergence points for several innate immune signaling pathways.36 For example, Parkin-mediated mitophagy is involved in the abrogation of NF-κB-mediated inflammation in macrophages, a process that appears to be closely linked to the degree of mitochondrial damage that immunostimulants cause.37 Parkin deficiency could be an instructive model for PD-related microglial dysfunction because its inactivation is linked to both sporadic and heritable forms of the diease.38 The extensive nature of parkin’s ubiquitylome39 suggests that it helps to regulate diverse and conserved signaling pathways with relevance to age-dependent neuroinflammation. While there is evidence for elevated inflammatory cytokine production in immunostimulated parkin-knockout microglia,40 mechanistic insights or translation from macrophage work is lacking, suggesting fertile ground for future investigation.
Astrocytes: Claiming a Starring Role
Astrocytes are “non-excitable” cells (i.e., they do not fire action potentials like neurons) that are often associated with metaphors evoking a passive, structural, or supportive set of functions. Such connotations may be inherited from antecedent ideas that glia might form a sort of “connective substance” for the brain, possibly one largely devoid of cellular components. The term neuroglia (German: nervenkitt, “nerve glue”) was introduced in 1856 by Rudolf Virchow, an authoritative figure in 19th century pathology and cellular biology.41 In a lecture, Virchow later acknowledged that, “where neuroglia is met with, it also contains a certain number of cellular elements”.42 It seems likely that, as astrocytes became recognized as the predominant type of glial cell, they inherited much of the antecedent conceptual framework for neuroglia in general.
While not unreasonable, earlier metaphors of “glia as glue” almost certainly shaped the nature of later inquiries and parameterized which functions could be plausibly ascribed to astrocytes. However, one can also appreciate early questioning of these assumptions. The esteemed neuropathologist Santiago Ramón y Cajal wrote in 1899:
“The prejudice that the relation between neuroglial fibers and neuronal cells is similar to the relation between connective tissue and muscle or gland cells, that is, a passive weaving for merely filling and support (and in the best case, a gangue for taking nutritive juices), constitutes the main obstacle that the researcher needs to remove to get a rational concept about the activity of the neuroglia”.43
This argument by Ramón y Cajal is interesting to compare with evolving perspectives on astrocytes because “supportive” and “passive” are not fixed metaphors, but relative ones. He and his contemporaries pioneered an understanding of important functional niches fulfilled by astrocytes, such as ion buffering, neurotransmitter handling, metabolic regulation, and microvasculature control.44 Today, however, these functions seem to form the new reference point for a fundamentally supportive glial identity, which may lead to miscommunication when proposing—or revisiting—putative astrocyte behaviors.
These considerations notwithstanding, longstanding paradigms of astrocyte function have already provided important insights into PD models. For example, astrocytes and serotonergic neurons are the primary cell types that express monoamine oxidase B (MAO-B), the enzyme that metabolizes the prodrug 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) into the neurotoxin MPP+.45 Administration of this compound has been widely used to induce parkinsonism in model organisms because dopamine neurons are most drastically affected by the MPP+ neurotoxin.46 Thus, it is not a new revelation that astrocytes can actively contribute to a PD-relevant pattern of neuronal dysfunction; however, their role in this model has not typically been the focus of studies that leverage MPTP toxicity. For example, if an experimental intervention is shown to ameliorate MPTP-induced neurodegeneration, it may often be assumed that the intervention operates by buttressing neuronal resistance to MPTP toxicity (e.g. by reducing mitochondrial dysfunction) or activating neurotrophic signaling pathways. The extent to which the requisite “upstream” glial MPTP metabolism might be affected by the intervention may not be routinely considered an important confound, although most if not all studies assess whether the intervention interferes with the conversion of MPTP to MPP+. Work in the MPTP model of PD provided compelling evidence that MPTP kills in both a cell autonomous manner through neuronal production of nitric oxide (NO) and activation of parthanatos47, 48 and a non-cell autonomous mechanism, through microglia production of NO through inducible NO synthase49. Consistent with this notion, It was recently shown that MPP+ can enter microglia via cation transporters, inducing oxidative stress and inflammation that ultimately exacerbate dopaminergic neurodegeneration.50 Growing recognition that glial involvement cannot be discounted in neuropathological investigations has thus inspired a reconsideration and examination of existing literature on PD models and mechanisms.
Neurotoxic Behaviors of Microglia and Astrocytes in Neurodegeneration
The role of glia in neurodegeneration is arguably among the most striking narratives that have emerged from the explosion of interest in glia over the past decade. Microglia are inextricably linked to astrocytes in this context, as they are proposed to exert phenotypic control via the secretion of inflammatory factors. Astrocytes have long been known to proliferate at a site of CNS injury and assist in scar formation; this is the classical clinico-pathologic significance of reactive astrocytosis.51 Certain harmful effects of reactive astrocytes have been observed, studied, and debated for decades, such as interference with axonal regeneration.52 The notion that reactive astrocytes could directly contribute to neurodegenerative disease (e.g., by secretion of neurotoxic factors) has not been the subject of as much investigation. In 2007, a pair of twin publications demonstrated that overexpression of human mutant superoxide dismutase-1 (SOD-1) in mouse astrocytes induced them to release soluble factor(s) that killed motor neurons in vitro.53, 54 This cell death was reduced by inhibition of the BAX protein, a key regulator of mitochondrial apoptosis.54 This suggested that, at least in ALS, selective neurotoxicity mediated by astrocytes constitutes an important new disease mechanism and target for therapeutic intervention.
Transcriptional analysis has demonstrated subtype heterogeneity among reactive astrocytes that depends heavily on the extracellular environment and type of injury. For example, reactive astrocytes produced in the context of either neuroinflammation induced by systemic LPS injection or middle cerebral artery occlusion (MCAO) upregulate transcription of a core set of “pan-reactive” genes. However, sub-populations of reactive astrocytes differentially regulate transcriptional modules, enabling them to be further categorized and robustly discriminated.55 Subsequent research has designated the reactive sub-populations induced by neuroinflammation (LPS) and ischemia (MACO) as the “A1” and “A2” reactive astrocyte subtypes (see Liddelow & Barres 2017).4 Notably, LPS injection does not cause A1 astrocytes to appear in mice without microglia (Csf1r knockout), suggesting that microglial activation is required for A1 astrocyte production.56 More specifically, genetic ablation or receptor neutralization of interleukin-1α (IL-1α), tumor necrosis factor α (TNF-α), and complement component subunit 1q (C1q) abrogates the induction of A1 reactive astrocytes by microglia.56
Analogous to what was seen in the SOD-1 overexpression reports, astrocyte-conditioned medium from A1 astrocytes (A1-ACM) is potently neurotoxic; this can be reduced by pre-treatment with compounds that interfere with apoptosis, but not necroptosis.56 Blockade of AMPA, NMDA, and kainate receptors all failed to reduce A1-ACM neurotoxicity, suggesting that A1 neurotoxicity cannot be explained in this model simply by a “loss of function” for glutamate handling that engenders glutamatergic excitotoxicity.56 Furthermore, A1 astrocytes—identified in vivo by upregulated expression of C3 complement component—were demonstrated not only in diseased tissue from patients with ALS, but also AD, HD, PD, and multiple sclerosis (MS), suggesting a broader relevance to neurodegenerative processes.56
What adaptive or homeostatic function could potentially be fulfilled by A1 astrocytes remains an open question. The removal of unsalvageable neurons from neural circuits is one potential evolutionary reason for their existence, i.e. eliminating those which have lost functional synaptic connections or have become virally compromised. Eliminating unsupported neurons could also have significance during development. For example, microglia are actively involved with synaptic pruning during neurodevelopment3 and A1 astrocytes could plausibly serve a complementary or parallel function. Additionally, it is increasingly recognized that microglia activated in disease or injury assume a transcriptomic profile that largely overlaps with developmental populations.12 Thus, reactivation of developmental pathways in microglia may have undesirable effects later in life or in the context of chronic disease; analogous processes could theoretically be relevant to astrocytes. Regardless of their ontological basis, A1 phenotype regulators are likely to be seen as candidate targets for therapeutic interventions if identified.
Damage Control: Reducing A1 Astrocyte Neurotoxicity with NLY01, a GLP1R Agonist
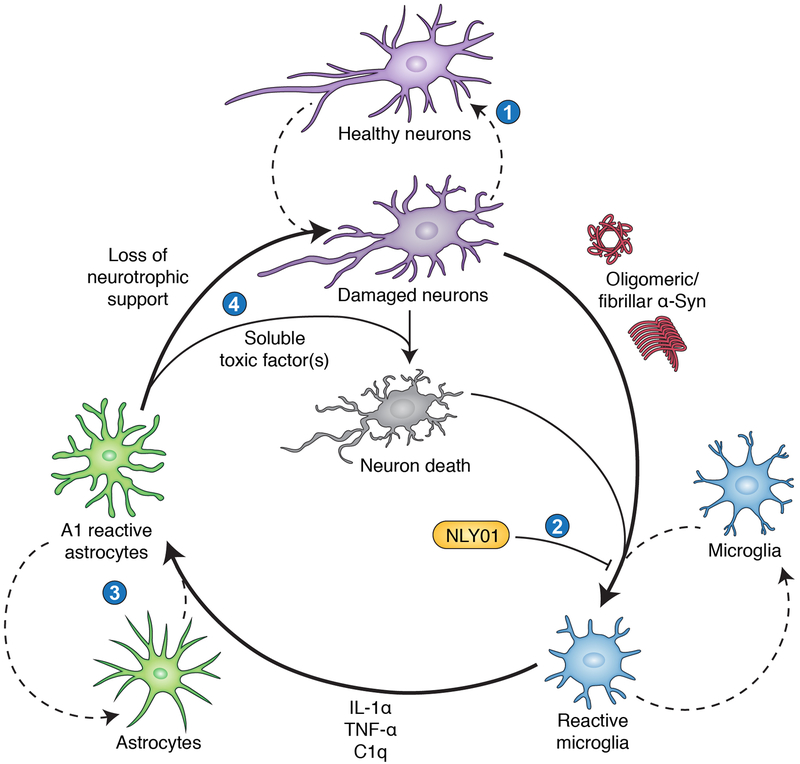
In Figure 1, we summarize the A1 astrocyte mechanism of neurodegeneration thus far reviewed. This paradigm is fundamentally cyclical and feedback-driven, which presents unique challenges. Damaged or stressed neurons can theoretically initiate and amplify an inflammatory microglial response, perpetuating the generation of neurotoxic A1 astrocytes. In PD, neuron-derived pathologic α-synuclein can bind microglial toll-like receptor 2 (TLR2), activating a canonical TLR/MyD88 inflammatory signal transduction pathway.13 However, such a cycle presents multiple points for therapeutic intervention. For example, it may eventually be possible to identify the soluble factor (or factors) produced by A1 astrocytes and selectively block generation or release. This approach could be facilitated by evolving modes of gene therapy as they are validated and acquire more mainstream acceptance. An alternative strategy is to intervene one step prior in the cycle by blocking the ability of microglia to convert bystander astrocytes into an A1 reactive phenotype. Our lab recently demonstrated the feasibility of this approach using a novel, highly brain-penetrant agonist of the glucagon-like peptide-1 receptor (GLP1R).
Figure 1: The neurotoxic reactive astrocyte paradigm of neurodegeneration.
A cooperative and cyclical pattern of aberrant neuron-glia interactions initiates and sustains neuronal loss in this model. We identify four key opportunities for therapeutic intervention in this cycle. (1) Aging, injury, and/or environmental or genetic influences present stresses that damage neurons via mechanisms such as dysproteostasis, mitochondrial dysfunction, oxidative/nitrative stress, lysosomal inadequacy, and DNA damage. Strategies that facilitate neuronal homeostasis or provide neurotrophic support could help reverse these processes and block progression to irreversible neuron loss. This approach may also reduce the release of immunostimulatory substances by damaged or dying neurons, including oligomeric and fibrillar α-Syn species. (2) Our work has shown that in multiple PD models, the GLP1R agonist NLY01 blocks microglial activation. This prevents subsequent transformation of astrocytes into the neurotoxic A1 reactive subtype, reducing downstream neuronal loss. (3) Future work may identify key molecular determinants of the A1 reactive astrocyte phenotype (e.g., transcriptional regulators) and targets for selectively blocking or reversing their appearance in a neuroinflammatory environment. (4) A1 astrocytes lose neurotrophic and synaptotrophic functions that normally support neuronal health, which may exacerbate disease. They also release a soluble neurotoxic factor that kills neurons. Depending on the nature of this unidentified factor, it may be possible to repress its production or release, or to render neurons resistant to its effects.
GLP1 is an endogenously produced peptide and an important member of the incretin class of hormones, which facilitate insulin release in a manner that depends on enteric glucose levels.57 This property made its receptor (GLP1R) an intriguing therapeutic for type II diabetes mellitus, as the risk of hypoglycemia is reduced by the glucose-level dependent nature of its insulin-modifying effects (see Lovshin & Drucker 2009 for a review of incretinergic therapy in the context of diabetes).58 Prior preclinical research has also suggested that GLP1R could be a promising therapeutic target in the context of neurodegeneration. Early studies focused on exenatide, a synthetic version of the GLP1 analog exendin-4 that crosses the blood-brain barrier (BBB).59 GLP1 had previously been a posited as a neuroactive hormone that regulated feeding behaviors; thus, it was easily revisited to evaluate neuroprotective properties in disease models.60 GLP1R recruits adenylyl cyclases to increase cyclic AMP (cAMP) production in pancreatic β-cells61; similarly, neuronal cAMP elevation has been proposed to explain neuroprotective effect of GLP1R agonism.62 However, these exendin-4 studies relied upon toxin-based PD models, such as intraperitoneal MPTP injection.62 Another utilized intracerebral injection of either lipopolysaccharide (LPS) or 6-hydroxydopamine (OHDA), making it difficult to determine whether efficacy of exendin-4 administration is best attributed to intraneuronal pathways or non-cell autonomous effects.63 Interestingly, the latter study did not explicitly consider the possibility GLP1R agonism could attenuate neuroinflammation, but rather speculated on neurotrophic resistance to the inflammatory milieu conferred by exendin-4.
Our study5 leveraged a different set of PD mouse models to test the neuroprotective properties of GLP1R agonism with NLY01. We first characterized its pharmacologic properties, showing that it exhibits an approximately 40-fold longer plasma half-life than exendin-4 in non-human primates, but stimulates cellular cAMP production in vitro that is essentially identical to exendin-4. Having shown this, we moved to evaluate its efficacy in the α-synuclein preformed fibrils (PFF) mouse model of PD, in which intrastriatally injected PFF progressively induces nigrostriatal synucleinopathy and dopaminergic cell loss within six months.64 One month after PFF injection—allowing enough time for fibril seeding and initial histopathologic changes65, 66—we initiated subcutaneous NLY01 administration twice weekly until the six-month time point. NLY01 treatment normalized key PD-relevant behavioral changes (e.g. amphetamine rotation, pole test), biochemical alterations (e.g., phosphorylation of α-synuclein serine 129), and histopathological hallmarks (e.g., reduced number of tyrosine hydroxylase-positive neurons in the substantia nigra). To test whether this neuroprotection could extend to other PD models, we also demonstrated that NLY01 extended lifespan and reduced synucleinopathy in human A53T α-synuclein transgenic mice.67
We considered that a non-cell autonomous mechanism could be critical for explaining the neuroprotective properties of NLY01 that we observed in these initial experiments. We established a conditioned-medium cell culture assay in order to test the hypothesis that NLY01 could prevent the generation of A1 astrocytes, thereby indirectly promoting neuronal survival. PFF has been used in vitro to study its effects on cultured neurons68 and we reasoned a similar approach could be adapted to study the responses of primary astrocytes and microglia. Treating astrocytes with PFF directly did not induce a transcriptional response consistent with A1 activation,55, 56 but microglia-conditioned medium (MCM) of PFF-treated microglia (MCM-PFF) did cause an A1 response. This property of MCM-PFF was reduced when microglia—but not astrocytes—were pretreated with NLY01, which suggested to us that NLY01 reduces the ability of activated microglia to affect the A1 astrocyte phenotype (Figure 1, section 2). Consistent with this hypothesis, PFF-treated microglia exhibited hallmarks of reactive microgliosis—such as increased Iba-1 immunoreactivity and microglial density in culture—that were blocked by NLY01. Additionally, a marked upregulation of IL-1α, TNF-α, and C1q—the three cytokines that most potently induce A1 reactivity in astrocytes56—was abrogated by NLY01. Finally, we showed that microglial NF-κB activation was similarly increased by PFF exposure and reduced by NLY01, although we did not extensively characterize this or other signal transduction pathways that could be affected.
We next sought to establish that this microglial reactivity and consequent A1 astrocyte activation is important to the biological endpoint of neurotoxicity. Adapting the protocol of a prior study,56 we investigated the neurotoxicity of astrocyte-conditioned medium (ACM) after provoking this A1 response with MCM-PFF (Figure 1, section 4). The resultant PFF-ACM exhibited potent neurotoxicity to primary cortical neurons from mouse, and to a lesser extent, human dopaminergic neurons. Both effects were rescued when microglia had been pre-treated with NLY01, enabling us to conclude that NLY01 ultimately reduces the neurotoxicity of reactive astrocytes in vitro. To corroborate this finding in vivo, we showed that NLY01 reduces astrogliosis and the density of A1 astrocytes in the ventral midbrain of intrastriatal PFF-injected mice. Interestingly, the toxicity we observed for neurons in vitro did not require any obvious cellular stressors or damage, suggesting that the toxin can kill healthy cortical neurons in vitro. However, as discussed above, we expect that in vivo, the toxin is able to exert selectivity for damaged neurons. Our lab remains interested in better understanding this ostensibly contradictory finding. It may be the case that cultured neurons in vitro are more vulnerable to the toxin than neurons that occupy supportive networks in vivo. For example, cultured neurons may externalize a toxin-binding molecule via a pathway that is repressed by neurotrophic support in vivo, as proposed elsewhere.4 Identifying such neuronal factors could in turn suggest novel targets for inhibiting astrocytic neurotoxicity.
Implications of NLY01 for PD research and treatment
There are multiple tiers of inference that can be supported by our work on NLY01 in PD models, each with acknowledged caveats. The most direct and clinically salient implication is that long-acting GLP1R agonists, such as NLY01, have exciting potential as disease-modifying PD therapies. In 2017, once-weekly dosing of exenatide (2 mg) was shown to have clinically significant effects on PD progression in a randomized, placebo-controlled trial.69 At 60 weeks, in the exenatide group, participants actually experienced a slight reduction in motor impairment—assessed by MDS-UPDRS III motor score in the “off” state—whereas scores worsened in the placebo group. Furthermore, a post-hoc analysis demonstrated that greater benefits were observed in individuals with shorter disease duration, suggesting that it is an effective preventive treatment for halting pathology early in disease.70 The trial has been celebrated as a validation of the preclinical research on GLP1 analogs discussed earlier in this Perspective and as proof-of-concept supporting their use in PD. Future work on exenatide is underway to determine whether the improvement observed reflects a disease-modifying property or merely a potent symptomatic treatment. Similarly, it remains to be seen whether compounds with relatively longer cerebral half-life, such as NLY01, might provide additive benefits. Perhaps an ideal test of GLP1R agonists would be a long-term trial in patients afflicted with polysomnography-confirmed idiopathic REM behavior disorder (iRBD). Because this condition represents the strongest known predictor of an incipient α-synucleinopathy,71 reduced progression from iRBD to PD could indicate that GLP1R agonism halts α-synucleinopathogenesis.
A post-hoc analysis from the exenatide trial found some improvement in metrics of depression and emotional well-being that was not correlated with motor impairment reduction,72 suggesting that GLP1 agonists could alleviate the prevalent mood changes seen in PD (see Schapira et al, 2017).73 GLP1 agonists have also been observed to have anxiogenic and antidepressant properties in rodents, suggesting that these changes could be independent of changes to PD-specific pathology or reduction of neuroinflammation.74 Regardless, this additional benefit could provide an additional indication for GLP1 agonist use in PD; thus, we expect future trials validating GLP1 agonists in the PD population to address non-motor symptom changes as an outcome of primary interest.
A second tier of inference from our work is that the most proximal effect of NLY01 was to quell reactive microgliosis. Importantly, direct treatment of neurons with NLY01 did not protect against PFF or ACM-PFF in vitro. We also showed that GLP1R was upregulated only in microglia—not neurons or astrocytes—when treated with PFF. These observations notwithstanding, we cannot exclude a role for non-microglial GLP1R in mediating the neuroprotection afforded by NLY01 in vivo. Cell type-specific effects of GLP1R could theoretically be investigated with conditional knockout mice, but from a clinical perspective, this would not constitute immediately useful information about the therapeutic benefits of GLP1R agonists in neurodegeneration. On the other hand, it could have implications for how researchers think about microglial contributions to disease. It is currently controversial whether selectively but uniformly “turning off” microglia would be expected to have a net benefit for patients with neurodegenerative conditions. There is some evidence that pharmacologic CSF-1R inhibition, which suppresses microgliosis and even depletes microglia from the brain at high doses,21 can reduce neuronal death and synaptic dysfunction in AD mouse models.75–77 Conversely, microglia clearly participate in the clearance of misfolded proteins and could have mainly adaptive roles in preclinical disease. They are also essential to CNS recovery in the aftermath of acute insults such as ischemia78 and experimental TDP-43 proteinopathy.79 Therefore, rational therapeutic design will require a consideration of how microglial behaviors shift over the course of a chronic neurodegenerative disease, or even how to harness their most adaptive functionalities. We might speculate that, analogous to the self-limiting effects of GLP1R agonists on blood glucose levels, compounds such as NLY01 could offer a self-limiting reduction of microgliosis by targeting pathways that are not intrinsically linked to microglial survival. A more comprehensive understanding of which microglial pathways are affected by NLY01 could provide some insight into how the compound modulates their activity.
The final tier of inference is that the reduced death of dopaminergic neurons observed in NLY01-treated mice can be attributed specifically to a reduction in A1 astrocyte generation or activity. Our in vivo data support this premise by showing that A1 astrocyte density is reduced in the substantia nigra by NLY01 in PD models, even after adjusting for the total astrogliosis (GFAP immunoreactivity). However, establishing a definitive causal link will require more selective interrogation of reactive astrocytosis and specific control of A1 astrogenesis. The current “A1 vs. A2” astrocyte dichotomy is likely to be replaced in the future with a more granular paradigm of reactive astrocytosis.4 TREM-2 is currently seen as a master regulator of the DAM phenotype in microglia, but whether a similar paradigm applies to astrocytes is unknown. However, A1 astrocytes are likely to be only one of many reactive subtypes; theoretically, there may be other “disease-associated astrocytes” that mirror or complement the DAM population. Selectively blocking or even reversing the A1 transcriptional program could redirect astrocytes to more supportive phenotypes, although the master regulators of A1 differentiation are not known. Once they are, selective ablation of this population could enable researchers to determine with more certainty how these astrocytes kill neurons and to identify molecular targets for intervention (Figure 1, section 3).
Bringing glia into focus in PD research
To-date, genome-wide association (GWA) studies have identified over forty risk loci for PD and the implicated regions contain an overrepresentation of genes with relevance to autophagy and lysosomal function.80 As discussed below in this section, failure of both neurons and glia to degrade misfolded α-synuclein is likely an important event in PD pathogenesis, which may form the basis for these linkages. Inflammatory pathways also feature prominently; for example, the rs34043159 polymorphism is found near the interleukin 1 receptor type 2 (IL1R2) gene.80 This highlights the significance of the IL-1 family and generalized inflammation to PD; additionally, it is worth noting that IL-1α is among the three factors driving A1 astrocyte polarization. Thus, variants in this receptor’s structure could govern astrocyte function during inflammation. Polymorphisms in regulatory regions of the class II major histocompatibility complex (MHC-II) receptors HLA-DQ and HLA-DR have also been consistently linked to PD.80, 81 MHC-II molecules have been implicated in the activation of microglia by α-synuclein and dopaminergic neuron loss in PD models,82 but understanding how this translates to human disease remains an extremely complex problem. Glial inflammation can also recruit cytotoxic T cells, which directly recognize α-synuclein peptides and kill dopamine neurons via MHC class I molecules.83
Using the results of GWA studies to inform more targeted research projects on glial behaviors relevant to PD are complicated on both temporal and spatial fronts. PD is fundamentally a disease of aging,84, 85 making it important to understand how microglia and astrocyte functionalities are expected to change across an individual’s lifespan. Aging entails a variety of cellular dysfunctions, including impaired proteostasis, increased oxidative/nitrative stress, mitochondrial dysfunction, and loss of lysosomal activity.86 While the mechanisms by which these changes lead to neuronal death in PD have been the subject of much scrutiny, it is less clear how aging glia could engender disease. Interestingly, astrocytes and microglia increase expression of classic molecular markers of cellular senescence in the PS19 mouse model of neurodegenerative tauopathy.87 This is consistent with recent transcriptomic evidence suggesting that on average, both microglia and astrocytes enter more pro-inflammatory states with aging.9, 12, 89 The total pool of A1 astrocytes also increases as a function of age in mice,90 which would be expected if the overall level of neuroinflammation is increasing. Future work may explore whether these astrocytes contribute to age-related cognitive decline, which would corroborate existing evidence that A1 astrocytes lose synaptotrophic functions (e.g., production of glypicans and thrombospondins).56, 91, 92 Whether A1 astrogenesis is an early/inciting or late/reinforcing event in the timeline of dopaminergic neurodegeneration observed in PD is also very much open to future investigation. In our work, PFF-injected mice were given NLY01 for 5 months, from 30 days after injection until pathologic evaluation at 180 days. However, it is possible that there is a therapeutic window when A1 conversion block is most consequential.
Given the centrality of α-synuclein to PD, there is also much interest in understanding how glia prevent or promote the pathologic accumulation of toxic α-synuclein species. Astrocytes can internalize neuron-released α-synuclein93 or PFF α-synuclein species94 for lysosomal degradation and have been shown to transfer α-synuclein aggregates throughout their own cellular network.95 This mechanism is probably important for the physiologic elimination of misfolded species, as astrocytes reportedly degrade α-synuclein aggregates more proficiently than neurons.96 However, because each astrocyte supports contacts with many neurons and synapses, astrocytic dysproteostasis could engender an alternative pathway for purely interneuronal aggregate transmission.66 In support of this concept, A1 astrocytes exhibit reduced phagocytic activity for myelin debris and may generally downregulate proteostasis pathways to support an ostensibly secretory phenotype.56 Interestingly, induced pluripotent stem-cell (iPSC)-derived astrocytes from PD patients carrying the LRRK2 G2019S mutation exhibit dysfunctional autophagy.97 Furthermore, when these astrocytes were co-cultured with healthy control-derived neurons, the astrocytes accumulated α-synuclein and the neurons progressively died, suggesting that astrocytic dysfunction can serve as a primary deficit underlying neurodegeneration in PD.97 Therefore, it seems likely that “loss-of-function” astrocyte changes are as crucial for disease pathogenesis in vivo as the acquisition of directly toxic functions, such as the release of neurotoxic proteins. Activated astrocytes may also sustain microglial activation by releasing chemoattractants and proliferation factors.98
The heuristic link between aging and neuroinflammation is a useful rule-of-thumb, but it is also important to consider that microglial9, 11, 12 and astrocytic89, 99 transcriptomes are regionally heterogeneous. There is evidence that even within the relatively small purview of the ventral midbrain, microglia of different nuclei are morphologically and transcriptionally differentiable.10 Fine spatial resolution of specialization could reflect region-specific demands on microglia for overall parenchymal health, but also opens up the possibility of localized dysfunction. Naturally, one may wonder if glia of the ventral tegmental area (VTA) offer more neurotrophic support than those in the substantia nigra, as it is mainly the dopaminergic neurons of the latter that die off in PD. Similarly, using the A1 neurotoxicity paradigm, one could hypothesize that VTA astrocytes are less likely to adopt the A1 phenotype than those in the substantia nigra.
Glia also represent a relatively novel and untapped source of biomarkers for neurodegenerative diseases. In AD patients, peripherally circulating astrocyte-derived exosomes (ADEs) extracted from blood plasma contain increased levels of complement proteins.100 Because C3 complement protein is a key marker of A1 astrocytes (which are elevated in AD tissue56), these data suggest that ADEs could encapsulate an accessible measure of astrocyte reactivity. Imaging-based biomarkers are also plausible; positron-emission tomography (PET) scans with ligands selective for reactive microglia or astrocytes could be used to approximate the severity of ongoing gliosis in a diagnosed patient. An existing example is PET imaging for gliosis with ligands for the translocator protein (TSPO), which is upregulated by reactive microglia and astrocytes in neurodegenerative disease.101 Interestingly, patients with iRBD may have increased TSPO binding in the substantia nigra,102 suggesting that gliosis may be part of prodromal PD. The study also noted a more salient reduction in striatal18F-DOPA uptake in the iRBD cases, suggesting that TSPO imaging may not afford any increased sensitivity relative to dopamine transporter (DAT) metrics for screening or diagnostic purposes. Thus, PET imaging could useful for monitoring responses to disease-modifying regimens, especially for glia-directed therapies. The development of new and improved radiotracers—for TSPO or other glial targets such as PET imaging of the CSF1R103 —may accelerate our ability to image neuroinflammation, moving from primarily “descriptive” uses to more clinically salient applications (see Jacobs et al., 2012).104
Methodologies for differentiating patient-derived induced pluripotent stem cells (iPSCs) into glial cells may also accelerate translational research. In this context, aberrant neuron-glia interactions could be modeled in vitro with (hopefully) a high degree of fidelity to the in vivo situation. Three-dimensional triculture of neurons, astrocytes, and microglia has been accomplished using cell lines in vitro, suggesting that this approach could be adopted by stem cell researchers seeking to monitor the progression of disease with patient samples.105 Currently, protocols exist to differentiate iPSCs into microglia-like106 and astrocyte-like107 cells for disease-specific investigations, but whether regional phenotypes can be recapitulated in vitro remains an open question.
Concluding remarks
It has rapidly become trite to state that the role of glia in neurodegeneration has been historically underappreciated. However, much of the work highlighted in this review is not exceptionally recent, making it difficult to discern how much of the current interest in glia is driven by new findings as opposed to a sociocultural shift among neurobiologists. This shift is expected to positively impact both neuron-focused and glia-focused researchers, not only by facilitating dialogue between specialists, but also by encouraging younger researchers to develop basic competency in both neuronal and glial biology. Increased input from immunologists is also demanded by the central role of canonical inflammatory signaling to neuron-glia and glia-glia communication. Such communication may accelerate translational insights and prevent cases of “reinventing the wheel” when developing research strategies that draw upon findings in other disciplines.
Acknowledgements:
We are grateful to I-Hsun Wu for the rendering of our illustration.
Relevant Financial Disclosures: V.L.D. and T.M.D. are co-founders of Neuraly Inc. and hold ownership equity in the company. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Full financial disclosures (preceding 12 months):
J.T.H.recognizes support through the Johns Hopkins Medical Scientist Training Program (NIH/NIGMS T32 GM73009).
T.M.D.: Dr. Dawson acknowledges the Adrienne Helis Malvin and Diana Henry Helis Medical Research Foundations and their direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and The Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs. His work is also supported by SPARC, Inhibikase, NIH/NINDS P50NS038377, NIH/NINDS U01NS097049, NIH/NINDS U01NS102035, NIH/NINDS R37NS067525, NIH/NIA R44AG053092, NIH/NIA RF1AG059686–01, NIH/NIDA P50 DA044123, the JPB Foundation, the Michael J. Fox Foundation and the Friedreich Ataxia Research Alliance, DONG-A ST CO LTD and the Bachmann Strauss Foundation. Dr. Dawson is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. Dr. Dawson is a member of the Dystonia Prize committee of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation and the Michael J. Fox Foundation. He is a member of the Board of Directors of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation. Dr. Dawson is a member of Scientific Advisory Board of CurePSP. Dr. Dawson was a member of the Executive Scientific Advisory Board of Michael J. Fox Foundation for Parkinson’s Research. Dr. Dawson is a consultant and advisor to Sun Pharma Advanced Research Company Ltd. Dr. Dawson is an advisor to the Sergey Brin Family Foundation, in partnership with the Milken Institute Center for Strategic Philanthropy, on the Parkinson’s Disease Research Roadmap (PDRR) Initiative. Dr. Dawson serves on the Advisory Council for Aligning Science Across Parkinson’s Disease. Dr. Dawson has received personal compensation in an editorial capacity for Journal of Clinical Investigation. Dr. Dawson has received personal compensation for consulting with Sun Pharma Advanced Research Company. Dr. Dawson is a paid consultant and advisory board member to DONG-A ST. Dr. Dawson has received personal compensation for consulting and serving on a scientific advisory board with Hopstem, Inc. Dr. Dawson is a member of American Gene Technologies International Inc., advisory board and owns stock options in the company. Dr. Dawson is a consultant for Mitokinin and owns stock options in the company. Dr. Dawson is a consultant to Inhibikase Therapeutics and owns stock options in the company. Dr. Dawson is a founder of Valted, LLC and holds an ownership equity interest in the company. Dr. Dawson is an inventor of technology of Neuraly, Inc. that has optioned from Johns Hopkins University. Dr. Dawson is a founder of, and holds shares of stock options as well as equity in, Neuraly, Inc. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
V.L.D.: Dr. Dawson acknowledges the Adrienne Helis Malvin and Diana Henry Helis Medical Research Foundations and their direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and The Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs. Her work is also supported by NIH/NINDS P50NS038377, NIH/NINDS R37NS067525, NIH/NINDS R44NS103695, NIH/NINDS R03NS104503, NIH/NIA RF1AG059686–01, NIH/NIDA P50DA044123, MSCRF MSCRFD-4338, the Parkinson’s Foundation PF-CRA-1895, the Michael J. Fox Foundation. Dr. Dawson has received personal compensation for consulting and serving on a scientific advisory board with Hopstem, Inc. Dr. Dawson serves on the Advisory Boards for the Burke Medical Center, NeuroMab, and the New York Stem Cell Foundation. Dr. Dawson has received personal compensation in an editorial capacity for eNeuro. Dr. Dawson is a consultant to Inhibikase Therapeutics and owns stock options in the company. Dr. Dawson is a founder of Valted, LLC and holds an ownership equity interest in the company. Dr. Dawson is an inventor of technology of Neuraly, Inc. that has optioned from Johns Hopkins University. Dr. Dawson is a founder of, and holds shares of stock options as well as equity in, Neuraly, Inc. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nature Reviews Disease Primers 2017;3:1–21. [DOI] [PubMed] [Google Scholar]
- 2.Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nature Reviews Neuroscience 2017;18:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens B, Allen NJ, Vazquez LE, et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007;131:1164–1178. [DOI] [PubMed] [Google Scholar]
- 4.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017;46:957–967. [DOI] [PubMed] [Google Scholar]
- 5.Yun SP, Kam T-I, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nature Medicine 2018;24:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hortega PdR Noticia de un nuevo y fácil método para la coloración de la neuroglia y el tejido conjuntivo. Trab Lab Invest Biol Univ Madrid 1918;15:367–378. [Google Scholar]
- 7.Ginhoux F, Greter M, Leboeuf M, et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010;701:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askew K, Li K, Olmos-Alonso A, et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Reports 2017;18:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabert K, Michoel T, Karavolos MH, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nature Neuroscience 2016;19:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Biase LM, Schuebel KE, Fusfeld ZH, et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017;95:341–356.e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olah M, Patrick E, Villani AC, et al. A transcriptomic atlas of aged human microglia. Nature Communications 2018;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond TR, Dufort C, Dissing-Olesen L, et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019;50:253–271 e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim C, Ho D-H, Suk J-E, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nature Communications 2013;4:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005;308:1314–1319. [DOI] [PubMed] [Google Scholar]
- 15.Murray PJ, Allen JE, Biswas SK, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ransohoff RM. A polarizing question: Do M1 and M2 microglia exist. Nature Neuroscience 2016;19:987–991. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988;38:1285–1285. [DOI] [PubMed] [Google Scholar]
- 18.Keren-Shaul H, Spinrad A, Weiner A, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017;169:1276–1290.e1217. [DOI] [PubMed] [Google Scholar]
- 19.Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017;47:566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nature Reviews Immunology 2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmore MRP, Najafi AR, Koike MA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014;82:380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benitez BA, Cruchaga C. TREM2 and Neurodegenerative Disease . New England Journal of Medicine 2013;369:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerreiro R, Wojtas A, Bras J, et al. TREM2 Variants in Alzheimer’s Disease. New England Journal of Medicine 2013;368:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. New England Journal of Medicine 2013;368:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulland TK, Song WM, Huang SCC, et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017;170:649–663.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Cella M, Mallinson K, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015;160:1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh FL, Hansen DV, Sheng M. TREM2, Microglia, and Neurodegenerative Diseases. Trends in Molecular Medicine 2017;23:512–533. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Ulland TK, Ulrich JD, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. The Journal of Experimental Medicine 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan P, Condello C, Keene CD, et al. TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron 2016;90:724–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozina E, Sadasivan S, Jiao Y, et al. Mutant LRRK2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain 2018;141:1753–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui KY, Fernandez-Hernandez H, Hu J, et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter I, Dubinsky M, Bressman S, et al. Anti-Tumor Necrosis Factor Therapy and Incidence of Parkinson Disease Among Patients With Inflammatory Bowel Disease. JAMA Neurol 2018;75:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 2014;128:805–820. [DOI] [PubMed] [Google Scholar]
- 34.Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016;167:1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickrell AM, Youle RJ. The roles of PINK1, Parkin, and mitochondrial fidelity in parkinson’s disease. Neuron 2015;85:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nature Reviews Immunology 2011;11:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016;164:896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson’s disease. Movement Disorders 2010;25:S32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarraf SA, Raman M, Guarani-Pereira V, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 2013;496:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouton-Liger F, Rosazza T, Sepulveda-Diaz J, et al. Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia 2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virchow R Gesammelte Abhandlungen zur wissenschaftlischen Medizin. 1856.
- 42.Virchow R Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. 1871. [PubMed]
- 43.Cajal SRy. Textura del Sistema Nervioso del Hombre y de los Vertebrados. 1899.
- 44.Navarrete M The Cajal school and the physiological role of astrocytes: a way of thinking. Frontiers in Neuroanatomy 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proceedings of the National Academy of Sciences of the United States of America 1982;79:6385–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron 2003;39:889–909. [DOI] [PubMed] [Google Scholar]
- 47.Mandir AS, Przedborski S, Jackson-Lewis V, et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A 1999;96:5774–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A 1996;93:4565–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liberatore GT, Jackson-Lewis V, Vukosavic S, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 1999;5:1403–1409. [DOI] [PubMed] [Google Scholar]
- 50.He Q, Wang Q, Yuan C, Wang Y. Downregulation of miR-7116–5p in microglia by MPP+sensitizes TNF-α production to induce dopaminergic neuron damage. Glia 2017;65:1251–1263. [DOI] [PubMed] [Google Scholar]
- 51.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 2005;50:427–434. [DOI] [PubMed] [Google Scholar]
- 52.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of Neurite Outgrowth in a Model of Glial Scarring Following CNS Injury Is Correlated With the Expression of Inhibitory Molecules on Reactive Astrocytes. Journal of Neuroscience 1991;11:3398–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nature Neuroscience 2007;10:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature Neuroscience 2007;10:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamanian JL, Xu L, Foo LC, et al. Genomic Analysis of Reactive Astrogliosis. Journal of Neuroscience 2012;32:6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017;541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreymann B, Ghatei MA, Williams G, Bloom SR. Glucagon-like peptide-17–36: a physiological incretin in man. The Lancet 1987;2:1300–1304. [DOI] [PubMed] [Google Scholar]
- 58.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nature Reviews Endocrinology 2009;5:262–269. [DOI] [PubMed] [Google Scholar]
- 59.Banks W, During M, Niehoff M. Brain Uptake of the Glucagon-Like Peptide-1 Antagonist Exendin(9–39) after Intranasal Administration. Journal of Pharmacology and Experimental Therapeutics 2004;309:469–475. [DOI] [PubMed] [Google Scholar]
- 60.Turton M A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72. [DOI] [PubMed] [Google Scholar]
- 61.Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proceedings of the National Academy of Sciences 2009;106:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. Journal of Neuroinflammation 2008;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dawson TM, Golde TE, Lagier-Tourenne C. Animal models of neurodegenerative diseases. Nature Neuroscience 2018;21:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luk K, Kehm V, Lee V. Pathological a-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012;338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mao X, Ou MT, Karuppagounder SS, et al. Pathological a-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016;353:aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee MK, Stirling W, Xu Y, et al. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A 2002;99:8968–8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volpicelli-Daley LA, Luk KC, Lee VMY. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nature Protocols 2014;9:2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. The Lancet 2017;390:1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Athauda D, Maclagan K, Budnik N, et al. Post hoc analysis of the Exenatide-PD trial-Factors that predict response. Eur J Neurosci 2019;49:410–421. [DOI] [PubMed] [Google Scholar]
- 71.Postuma RB, Gagnon JF, Bertrand JA, Génier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: Preparing for neuroprotective trials. Neurology 2015;84:1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Athauda D, Maclagan K, Budnik N, et al. What Effects Might Exenatide have on Non-Motor Symptoms in Parkinson’s Disease: A Post Hoc Analysis. J Parkinsons Dis 2018;8:247–258. [DOI] [PubMed] [Google Scholar]
- 73.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017;18:435–450. [DOI] [PubMed] [Google Scholar]
- 74.Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 2016;65:54–66. [DOI] [PubMed] [Google Scholar]
- 75.Olmos-Alonso A, Schetters STT, Sri S, et al. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 2016;139:891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sosna J, Philipp S, Albay RI, et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Molecular Neurodegeneration 2018;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spangenberg EE, Lee RJ, Najafi AR, et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 2016;139:1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szalay G, Martinecz B, Lénárt N, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nature Communications 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spiller KJ, Restrepo CR, Khan T, et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nature Neuroscience 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang D, Nalls MA, Hallgrimsdottir IB, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 2017;49:1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wissemann WT, Hill-Burns EM, Zabetian CP, et al. Association of Parkinson disease with structural and regulatory variants in the HLA region. Am J Hum Genet 2013;93:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harms AS, Cao S, Rowse AL, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 2013;33:9592–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 2017;546:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collier TJ, Kanaan NM, Kordower JH. Aging and Parkinson’s disease: Different sides of the same coin? Movement Disorders 2017;32:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pringsheim T, Jette N, Frolkis A, Steeves TDL. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders 2014;29:1583–1590. [DOI] [PubMed] [Google Scholar]
- 86.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Almolda B, Gonzalez B, Castellano B. Microglia and brain macrophages in health and disease. Frontiers in Biosciences 2011;16:1157–1171. [DOI] [PubMed] [Google Scholar]
- 89.Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Reports 2018;22:269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proceedings of the National Academy of Sciences of the United States of America 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allen NJ, Bennett ML, Foo LC, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012;486:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005;120:421–433. [DOI] [PubMed] [Google Scholar]
- 93.Lee H-J, Suk J-E, Patrick C, et al. Direct Transfer of α-Synuclein from Neuron to Astroglia Causes Inflammatory Responses in Synucleinopathies. Journal of Biological Chemistry 2010;285:9262–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindström V, Gustafsson G, Sanders LH, et al. Extensive uptake of a-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Molecular and Cellular Neuroscience 2017;82:143–156. [DOI] [PubMed] [Google Scholar]
- 95.Rostami J, Holmqvist S, Lindstrom V, et al. Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes. J Neurosci 2017;37:11835–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loria F, Vargas JY, Bousset L, et al. alpha-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol 2017;134:789–808. [DOI] [PubMed] [Google Scholar]
- 97.di Domenico A, Carola G, Calatayud C, et al. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease . Stem Cell Reports 2019;12:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kano S-i Choi EY, Dohi E, et al. Glutathione S-transferases promote proinflammatory astrocyte-microglia communication during brain inflammation. Science Signaling 2019;12:eaar2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itoh N, Itoh Y, Tassoni A, et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proceedings of the National Academy of Sciences 2017:201716032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Annals of Neurology 2018;83:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cagnin A, Brooks DJ, Kennedy AM, et al. In-vivo measurement of activated microglia in dementia. Lancet 2001;358:461–467. [DOI] [PubMed] [Google Scholar]
- 102.Stokholm MG, Iranzo A, Østergaard K, et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. The Lancet Neurology 2017;16:789–796. [DOI] [PubMed] [Google Scholar]
- 103.Horti A, Naik R, Foss C, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobs AH, Tavitian B. Noninvasive molecular imaging of neuroinflammation. Journal of Cerebral Blood Flow and Metabolism 2012;32:1393–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park J, Wetzel I, Marriott I, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nature Neuroscience 2018;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muffat J, Li Y, Yuan B, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nature Medicine 2016;22:1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krencik R, Hokanson KC, Narayan AR, et al. Dysregulation of astrocyte extracellular signaling in Costello syndrome. Science Translational Medicine 2015;7:286ra266. [DOI] [PMC free article] [PubMed] [Google Scholar]