Abstract
Lupus glomerulonephritis (GN) is an autoimmune disease characterized by with immune complex-deposition, complement activation and glomerular inflammation. In lupus-prone NZM2328 mice, the occurrence of lupus GN was accompanied by a decrease in Treg cells and an increase in proinflammatory cytokine-producing T cells. Because IL-33 in addition to IL-2 has been shown to be important for Treg cell proliferation and ST2 (IL-33 receptor) positive Treg cells are more potent in suppressor activity, a hybrid cytokine with active domains of IL-2 and IL-33 was generated to target the ST2+ Treg cells as a therapeutic agent to treat lupus GN. Three mouse models were used: spontaneous and IFN-accelerated lupus GN in NZM2328 and the lymphoproliferative autoimmune GN in MRL/lpr. Daily injections of IL233 for 5 days prevented Ad-IFNα-induced lupus GN and induced remission of spontaneous lupus GN. The remission was permanent in that no relapses were detected. The remission was accompanied by persistent elevation of Treg cells in the renal lymph nodes. IL233 is more potent than IL-2 and IL-33 either singly or in combination in the treatment of lupus GN. The results of this study support the thesis that IL233 should be considered as a novel agent for treating lupus GN.
Keywords: IL-2-IL-33 fusion protein, Treg cells, Lupus Nephritis, Autoimmunity, autoantibodies
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by inflammation and multi-organ dysfunction (1,2) with significant number (~60%) of patients developing lupus glomerulonephritis (GN) (3). Despite therapy, 15–20% of the GN patients progress to end stage renal disease (ESRD)(4). In addition, current therapies with steroid and immunosuppressive agents have significant side effects. Over the last six decades, no new therapy has been approved by US FDA for lupus GN (5,6). To improve treatment outcomes and to reduce side effects, there is an urgent need for novel therapeutic approaches for lupus GN.
IL-2 deficiency in patients with SLE was described first by Liner-Isreal et al. in 1983 (7). The significance of this deficiency has been extensively investigated in mouse models of lupus (reviewed by Lieberman and Tsokos) (8). This defect was linked with Treg cell homeostasis, activation-induced cell death (AICD) and decreased cytotoxicity. Because IL-2 deficiency has profound effect on Treg cell homeostasis, low dose IL-2 has been used to treat mouse lupus, human lupus and other immunological diseases (reviewed in (9)). In NZB/W F1, infusion of CD4+CD25+CD62LHi T-reg like cells expanded ex vivo with IL-2 plus anti-CD3/CD28 mAb-coupled beads was shown to delay the onset of proteinuria and modestly reduce the incidence of fatality (10) suggesting that increasing Treg cells may be a viable adjunct therapeutic approach with other therapeutic agents. Low dose IL-2 therapy was shown to be effective in treating SLE patients with various clinical manifestations and disease remission was accompanied by increases in Treg cells and decreases in TfH and in Th17 cell numbers (11). However none of these patients had lupus nephritis.
In addition to IL-2, IL-33 was shown to be important for the expansion of Treg cells (12). Furthermore, administration of IL-33 was shown to be effective in expanding recipient Treg cells and protecting mice against acute GVHD (13). Importantly, adoptive transfer of ST2+ (IL-33 receptor) but not ST2− Treg cells to the allogeneic bone marrow recipients were shown to be protective against GVHD. In our previous studies on the Treg cell deficient scurfy mouse, we found IL-2 to be a positive regulator of ST2 expression on Th2 cells (14,15). We hypothesized that in lupus, IL-2 deficiency may not only lead to reduced Treg cell number and function but may also cause in the reduction of the most efficient Treg cell population that expresses both IL-2 and IL-33 receptors. In this study we compared the efficiency of IL233 (an IL-2 and IL-33 fusion protein) with the efficiencies of singe therapies of IL-2, IL33 and the combination of IL-2 with IL-33 (IL-2/IL-33) in the prevention of the development of GN in a mouse model. The efficacy of IL233 in the treatment of ongoing lupus GN in mouse models was also investigated.
2. Materials and Methods
2.1. Mice and reagents
NZM2328 and MRL/MpJ-Faslpr/J (MRL/lpr) mice were used for the studies. NZM2328 mice have been bred in our facility for the past two decades (16). MRL/lpr mice originally purchased from the Jackson Laboratory was maintained in our laboratory. Only female mice were used for the experiments. Adenovirus expressing interferon alpha (Ad-IFNα) was used as described (17). Ad-IFNα lysates were amplified in early passages in 293T cells, and the titer was determined by using Adeno Rapid Titer Kit (Clontech). Recombinant IL-2, IL-33 and IL233 hybrid cytokines were produced in E.coli using methods described earlier (18). All animal procedures were approved by the University of Virginia Institutional Animal Care and Use Committee in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
2.2. Study design
Accelerated lupus was induced in female NZM2328 mice by injecting (i.v.) 5 × 107 particles of Ad-IFNα. The mice were monitored for the development of severe proteinuria (>300 mg/dl) by dipsticks (Multistix, Bayer) (16). Moribund mice were anesthetized, perfused with PBS and euthanized for tissue collection. Female (12-week old) NZM2328 mice were injected (i.p.) with 66 pmols per mouse per day for 5 days with recombinant IL-2 and/or IL-33 or IL233 in saline either before or after Ad-IFNα injection. Control mice were injected with saline only. For spontaneous lupus GN, NZM2328 mice were monitored for proteinuria as above and once mice had moderate proteinuria (>100mg/dL) for 2 consecutive weeks, they were treated with saline or IL233 as above. Female (10 weeks old) MRL/lpr mice were injected with saline or IL233 as described above. Mice were followed for renal function and general health and were euthanized once mice were moribund.
2.3. Renal structure and function
Urine samples were collected weekly or before euthanasia and analyzed as described before (19) for proteinuria using Multistix 10 SG (Bayer). Kidney sections fixed in 10% buffered formalin were paraffin embedded and stained with Hematoxylin and Eosin (H & E) at the University of Virginia Research Histology Core. The sections were scored in a blinded manner on a scale of 0 to 4 for abnormalities (glomerular hypertrophy, mesangial expansion, mononuclear infiltrates and tubulo-interstitial inflammation), as 0 (no abnormality), 1 (less than 20%), 2 (20 to 40%), 3 (40 to 60%), and 4 (more than 60%) of 200x magnified field with above-mentioned abnormalities. IgG, C3, IgG2a and IgG2b deposition in kidneys was analyzed by direct immunofluorescence as before (19). Briefly, frozen sections of mouse kidneys were fixed in methanol at −20°C for 10 min, blocked with 2% normal goat serum in PBS containing 3% BSA, and then stained with goat anti mouse IgG-FITC, goat anti mouse IgG2a-TXRD, goat anti mouse IgG2b-TXRD (Southern Biotech) and goat anti-mouse Complement C3-FITC (MP Biomedicals). The frozen sections stained for immune complex deposits were analyzed by immunofluorescence captured in 200x amplified images from at least 30 random glomeruli for each group and quantitated using ImageJ software and expressed as total immunofluorescence.
2.4. Flow cytometry
Single cell suspensions from peripheral blood, renal lymph nodes (LN) and spleen were prepared as before and analyzed for cell surface markers and intracellular Foxp3 expression by flow cytometry using fluorescently labeled antibodies (BioLegend and eBiosciences) (14,20). For intracellular cytokine analysis, single cell suspensions were stimulated ex vivo for 5 hours with phorbol 12-myristate 13-acetate (PMA, 20ng/ml) and ionomycin (1μg/ml) in the presence of 1μM monensin (all from Sigma, USA). Gated CD4+ T cells were analyzed with a 5-color upgraded (Cytek Development) FACScan™ (BD Biosciences) equipped with CellQuest™ and Rainbow™ software for data acquisition. The data was analyzed using FlowJo™ software (FlowJo LLC).
2.5. Treg cell activity assay
Treg cell activity for suppression of CD4+ T cell proliferation was measured using the CFSE-dilution assay as described before (18). Briefly, CD4+CD25+ cells were isolated from the pooled lymph nodes of saline or IL233 treated NZM2328 mice using EasySep™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit II (STEMCELL Technologies) according to manufacturer’s instructions. The CD4+CD25− cells from untreated NZM2328 were obtained using the same kit, labeled with 0.5μM carboxy-fluorescein-diacetate succinimidyl ester (CFDA-SE, Molecular Probes). Different ratios of Treg cells were added to the CD4+CD25− cells, which were then stimulated with anti-CD3/CD28 beads (Life Technologies) at 1:1 ratio for 72 hours. The %divided cells were analyzed by flow cytometry.
2.6. Anti-ds DNA assay
Serum samples collected at necropsy were analyzed for anti-dsDNA antibody by ELISA as described earlier (19). Briefly, pGEM-3zf plasmid DNA was cut with S1-nuclease (ThermoFisher Scientific) and biotinylated using Photoprobe Biotin (Vector Labs) following manufacturer’s protocol. Biotinylated dsDNA (1ng/ml) were incubated in Pierce Streptavidin-coated plates (ThermoFisher Scientific) 30 minutes at room temperature. Plates were blocked with 1%BSA for 1 hour. Diluted serum samples (1:100) were added to the wells for 2 hours at room temperature. After 6-times washing with PBS/Tween 20, anti-dsDNA Ab were quantified with anti-mouse IgG, IgG2a or IgG2b HRP-conjugated antibodies (1:3000 dilution). After development with OPD solution (Sigma Chemical Company) following manufacturer’s recommendations, OD values were recorded using ELISA plate reader (Dynex Opsys).
2.7. Statistics
Two way ANOVA or two-tailed unpaired Student t-tests were used to evaluate the significance of the results with assigned p-values and treatment. Mouse survival in Kaplan-Meier plots were analyzed with log-rank test for significance.
3. Results
3.1. IL-2 production and Treg cell homeostasis is defective in NZM2328 mice.
Two months old female NZM2328 mice with no renal disease were compared with 6 month old NZM2328 when about 50% of females have established GN (16) to ascertain the development of IL-2 deficiency and reduction in Treg cells. As shown in Figure 1A, the CD4+ T-cells isolated from the peripheral blood of older NZM2328 mice had a much lower proportion of IL-2 producing cells (7.7±0.7%) compared to young mice (13.7±2%). The proportion of Foxp3+ cells among CD4+ T-cells was also markedly reduced in older mice (1.4±1.2%) as compared to younger mice (5.4±1.2%) (Figure 1B). Interestingly, the CD4+ T-cells in the older NZM2328 mice also had a lower proportion (2.2±0.3%) of cells producing the immuno-regulatory cytokine IL-10 (Figure 1C) as compared to the younger mice (8.3±2.6%). We also observed higher numbers of CD4+ T cells that expressed TNFα, in the older NZM2328 mice as compared to younger mice (Figure 1D). The total number of splenic Treg cells was lower in the 6 month old mice than the 2 month old mice (Figure 1E). In addition, the total number of ST2+ Treg cells was reduced in the 6 months old NZM2328 mice as compared to the 2 months old mice (Figure 1F). These data indicate that the lupus-prone NZM2328 mice produce decreasing amounts of IL-2 with age, accompanied with a decrease in the proportion of ST2+ immuno-regulatory Foxp3+ T cells.
Figure 1. NZM2328 mice develop a defect in expression of IL-2, IL-10, Foxp3 and ST2 on CD4+ T-cells.
In the blood, 6 months old NZM2328 female mice had lower expression of IL-2+ T cells as compared to 2 months old mice (n=4 per group) (A), Foxp3+ T cells (B) and IL-10+ T cells (C) as compared to 2 months old mice. The proportion of TNFα producing cells was higher (D) and absolute number of Foxp3+ cells (E) and ST2+Foxp3+ T cells (F) were lower in older NZM2328 mice as compared to young mice (n≥ 3). Treatment strategy of NZM2328 mice with IL-2 or IL233 (G) increased Foxp3+ and IL-10 producing CD4+ T cells in the blood (H). IL233, but not IL-2 inhibited the generation of TNFα+ and increased the ratio of IL-10+ T cells /TNFα+ T cells. Individual values are shown; # comparison with IL-2 only group; p value: *<0.05; **<0.01, ***<0.001.
3.2. IL233 is more efficient than low-dose IL-2 for protective immunomodulation.
We have shown that IL233 was more efficient than IL-2 in increasing Treg cells and modulating cytokine production in B6 mice (18). Hence, we investigated whether IL233 was also more efficient in regulating Treg cell generation and cytokine production in lupus-prone mice. NZM2328 mice (3 months old) were treated with low-dose IL-2 (66 pmols; equivalent to 1μg) or 66 pmols of IL233 per mouse per day or saline daily for 5 days (Figure 1G). On day 9 after the initiation of cytokine treatments, peripheral blood mononuclear cells were analyzed for Foxp3+ Treg cell and CD4+ T-cell populations for the production of TNFα and IL-10 (Figure 1H). Treatment with both IL-2 and IL233 induced an increase in circulating Treg cells as compared to saline treated control. However, mice treated with IL233 showed greater inhibition of TNFα, indicating that IL233 treatment was more efficient in suppressing the proinflammatory cytokine production and increasing the immunoregulatory Foxp3+ and IL-10+ CD4 T cells, such that the ratio of IL-10+ T-cell/TNFα-producing T cells ratios were significantly higher in mice treated with IL233 than those treated with saline or IL-2 alone. The data indicates that IL233 is more efficient than IL-2 in modulating Treg cell generation in NZM2328 and downregulating inflammatory cytokine production.
3.3. Treatment with IL233 protects NZM2328 mice from Ad-IFNα accelerated lupus GN with modulation of Treg cell population in the renal lymph nodes.
For more synchronous and predictable disease onset, we used the IFNα - accelerated lupus GN model (17) to compare the efficiency of IL-2, IL-33, IL-2 plus IL-33 (IL-2/IL-33) and IL233 in preventing the development of lupus GN. Female NZM2328 mice (12 weeks old) were injected with 66 pmols of IL-2, IL-33, IL-2/IL-33 or IL233 hybrid cytokine or saline daily for 5 days. On day 9, mice were injected with Ad-IFNα particles and were followed for the development of severe proteinuria (Figure 2A). As shown in Figure 2A, 83% of mice injected with saline developed severe proteinuria by week 9 post Ad-IFNα treatment. Mice treated with IL-2 or IL-33 alone showed a delay in the development of severe proteinuria post Ad-IFNα treatment. However, by week 10, the proportion of mice treated with IL-2 or IL-33 alone that was free of severe proteinuria was similar to that of mice injected with saline. Mice treated with IL-2/IL-33 further delayed the development of severe proteinuria. However, at 14 weeks after Ad-IFNα treatment when they were euthanized, more than 50% of the mice treated with IL-2/IL-33 had severe proteinuria. In contrast, a significantly greater proportion of mice (83%) of the mice treated with IL233 remained free from severe proteinuria. The survival data were similar to severe proteinuria development (not shown). Figure 2B shows a representative normal kidney from a mouse treated with IL233. Figure 2C shows that there was a significant increase in proportion of the CD4+ T cell populations in the kidney draining (renal) lymph nodes in mice treated with IL-2/IL-33 and IL233 in comparison with mice treated with IL-2 or IL-33 or saline. A significantly greater increase in the proportion of Treg cells was seen in the IL233 treated mice in comparison with that of the IL-2/IL-33-treated mice. Similar observations were seen in the total numbers of Treg cells in the renal lymph nodes with the exception that treatment with IL-2 appeared to moderately increase the total number of Treg cells in the renal lymph nodes in comparison with that from saline treated mice (Figure 2D). In the spleen, IL-2, IL-33, IL-2/IL-33 and IL233 increased the proportion of Treg cells in the CD4+ population (Figure 2E). The increase was more marked in the IL-2/IL-33 and IL233 treated groups. A statistically significant increase in the total numbers of Treg cells in the spleen was observed only in the IL2/IL33- and IL233- treated mice (Figure 2F). In addition, there was a decrease in numbers of TNFα+ T cells in the renal lymph nodes in the IL233 treated mice (data not shown). There was also an increase in the ratios of Treg cells/TNFα+ T cells and Treg cells/IFNγ+ T cells in the renal lymph nodes upon treatment with IL233 as compared to the other treatment regimen (data not shown).
Figure 2. IL-2/IL-33 and IL233 prevent the development of IFNα accelerated lupus GN in NZM2328.
A. NZM2328 mice (12 week old) were injected (i.p.) with 66 pmols each of IL-2, IL-33 (n=6 for both), mixture of IL-2 and IL-33, IL233 or saline (n=11 for all) daily for 5 days and with Ad-IFNα (i.v.) on day9. IL-2/IL33(IL-2 and IL-33), especially IL233 protected NZM2328 mice from the development of Ad-IFNα-accelerated GN and severe proteinuria. B. Glomerular hypertrophy, mesangial expansion, and leukocytic infiltration were seen in the saline control mice (left panel) but not in IL233 treated mice (right panel). C. Treatment with IL-2/IL-33 and especially with IL233 led to a persistent increase in the proportion of Foxp3+ Treg cells in the CD4+ T cell populations in renal lymph nodes. D. The total numbers of Treg cells in the renal lymph nodes are depicted. E. There were increases in the proportion of Treg cells in the spleen in mice treated with IL-2, IL33, IL-2/IL-33 and IL233. F. Increase in the total numbers of Treg cells in spleens were seen only in IL-2/IL-33 and IL233 treated mice. Green scale bars in (B) = 50μM; p values: *<0.05, **<0.01, ***<0.001, ****<0.0001 and NS>0.05.
3.4. IL233-mediated protection from GN does not correlate with circulating autoantibodies and immune complex deposition.
Terminal sera were collected from mice that were treated with saline, IL-2, IL-33, IL-2 /IL-33 and IL233 and assayed for anti-dsDNA Ab titers as a surrogate maker for auto-Ab production. As shown in Figure 3A, no significant differences were detected in all 5 groups. By immunofluorescence, there were no differences in the glomerular staining for total IgG and C3 between the saline-mice treated that succumbed to GN and the IL233-treated mice that were protected from GN (Figure 3B). Quantitation of subclass Ab deposition, however, showed statistically significant differences in IgG2a deposition, but no significant difference in IgG2b glomerular deposits in the kidney (Figure 2, C–E). The corresponding isotype controls for the Ab used in immunofluorescence did not stain the glomeruli of the saline or IL233 -treated mice (not shown).
Figure 3. IL233-mediated protection from GN development was not accompanied with decreases in circulating anti-dsDNA antibodies and immune complex deposits.
Levels of circulating anti-dsDNA IgG (A) Anti-dsDNA Ab levels by ELISA in the terminal sera of a representative cohort (n=6) of NZM2328 mice treated with different cytokines. B. Representative images of IgG and C3 deposits in kidneys of saline and IL233 treated mice. C. Immunofluorescence studies of IgG2a and IgG2b immune complex deposits in mice treated with saline or with IL233. D. Significant increase in IgG2a deposits in the glomeruli of IL233-treated mice. E. No significant difference in IgG2b immune complex deposits in the glomeruli in mice treated with saline or IL233. Green scale bar in (B) and (C) is 50μM; p value: **<0.01, N.S. >0.05.
3.4. IL233 treatment induces persistent remission in mice with established IFNα accelerated GN.
To interrogate whether IL233 is effective in treating established IC mediated GN, we injected 12-week old NZM2328 mice with Ad-IFNα. When mice had moderate proteinuria (>100mg/dL) for two consecutive weeks, they were injected either with saline or 66 pmols of IL233 for 5 consecutive days and monitored for the development of severe proteinuria and early mortality. As shown in Figure 4A, all the saline treated control mice continued to have severe proteinuria and were moribund within 14 weeks of Ad-IFNα injection, whereas 9 out of 11 mice treated with IL233 showed reduced proteinuria. The survival curves of the treated and control groups were plotted in Figure 4B. The majority of IL233-treated mice exhibit normal appearance with mild proteinuria till they were euthanized at the age of 14 months. Figure 4C and Figure 4D show that the Foxp3+ Treg cells and the IL-10+ CD4+ T cells in the IL233 treated mice stayed elevated when analyzed at necropsy in response to the one-time induction therapy administered several months earlier. Blood samples were collected 90 days and 300 days post administration of Ad-IFNα and assayed for the proportion of Foxp3+ cells in the CD4+ T cell population. Treg cell levels were still elevated in mice 10 months after the initial therapy when compared to 28 days after the injection of Ad-IFNα (Figure 4E).
Figure 4. IL233 treatment after the onset of proteinuria reverses established Ad-IFNα accelerated lupus GN in NZM2328.
NZM2328 female mice (12 week old) were injected (i.v.) with Ad-IFNα and monitored for proteinuria. Once the mice had moderate proteinuria (>100mg/dL) for 2 weeks, they were injected with 66pmol/mouse of IL233 (n=11) or saline (n=5) daily for 5 days. IL233 treatment induced persistent remission from severe proteinuria (A) (also see supplemental Figure 1A) and mortality (B) in Ad-IFNα-accelerated lupus GN. The IL233 treated mice had elevated levels of Foxp3+CD4+ T cells (C) and IL-10+CD4+ T-cells (D) day 90 after Ad-IFNα injection. E. Elevation of circulating Foxp3+CD4+ T cells 300days post Ad-IFNα in IL233 treated mice as compared to saline-treated controls. Lines represent individual mice in (A) and arrows indicate IL233 cytokine treatment. The truncation of red lines in panel (A) indicate that mice either died or were euthanized due to severe proteinuria. p value: *<0.05; **<0.01, ***<0.001.
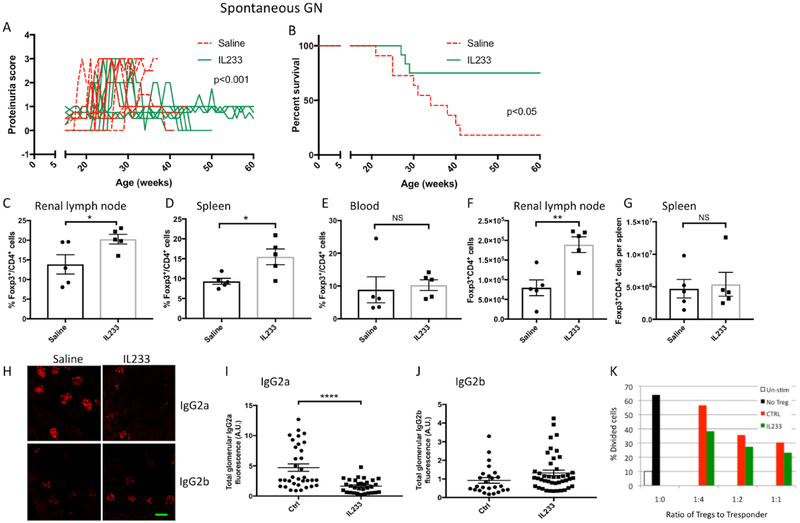
3.5. IL233 treatment induces persistent remission in mice with established spontaneous GN.
To address the effect of IL233 treatment on spontaneous lupus GN, NZM2328 mice with aGN (proteinuria > 100mg/dL for two weeks), were treated with saline or 66pmol daily of IL233 for 5 days (Figure 5A). They were monitored for the development of severe proteinuria and early mortality. The majority (9/11) of saline-treated mice developed severe proteinuria and died. The remaining 2 saline-treated mice spontaneously recovered from mild proteinuria and survived till 60 weeks of age (Figure 5B). In contrast, proteinuria was reversed in 9/12 IL233 treated mice and they survived until 14 months of age with only 3/12 of IL233 treated mice dying of severe proteinuria (Figure 5B). Flow cytometric analysis showed a significant increase in numbers of Treg cells in the CD4+ T cell populations in the renal lymph nodes (Figure 5C) and spleen (Figure 5D) but not in the blood (Figure 5E) in mice treated with IL233. There was a significant increase in the total number of Treg cells in the renal lymph nodes (Figure 5F) but not in the spleen (Figure 5G) of NZM2328 mice treated with IL233. Immunofluorescence studies showed that the kidneys from mice treated with IL233 had a highly significant attenuation of IgG2a deposits (Figure 5H and 5I). Although there were no statistically significant difference in the IgG2b deposition between the two groups (Figure 5J), there is a trend for increase in IgG2b immune complex deposits in the kidneys of IL233 treated mice.
Figure 5. IL233 treatment reverses proteinuria in NZM2328 with spontaneous lupus GN with prolonged survival.
Female NZM2328 mice were monitored for proteinuria. Once the mice had moderate proteinuria (>100mg/dL) for 2 weeks, they were injected (i.p.) with 66pmol/mouse of IL233 (n=12) or saline (n=11) daily for 5 days. IL233 treatment reversed severe spontaneous proteinuria (A) (also see supplemental Figure 1B) and reduced mortality (B) in NZM2328 mice compared to saline controls. Levels of Tregs in NZM2328 mice in renal lymph nodes (C and F), Spleen (D and G) and blood (E). H. Glomerular immune complex deposits were analyzed for IgG2a (I) and IgG2b (J) subtypes by immunofluorescence and quantified by ImageJ as described in section 2.3 in materials and methods. K. CD4+CD25+ Tregs isolated from pooled lymph nodes of IL233 treated mice (4 mice) more efficiently inhibited the proliferation of CD4+CD25− Tresponder cells compared to pooled Tregs isolated from saline controls (4 mice). Lines in (A) represent individual mice. The truncation of red lines in panel (A) indicates that mice either died or were euthanized due to severe proteinuria; Green scale bar in (H) is 50μM; p value: *<0.05; **<0.01, ****<0.0001 and NS p>0.05.
3.6. Treg cells from IL233 treated mice are more potent suppressor T cells.
CD4+CD25+ Treg cells were isolated from pooled lymph nodes of NZ2328 treated with saline or IL233. The isolated Treg cells were assayed for their suppressor activity in a T cell proliferation assay. As shown in Figure 5K, Treg cells from mice treated with IL233 were more effective in suppressing T cell proliferation suggesting that they were more potent Treg cells.
3.7. IL233 treatment protects MRL/lpr mice from GN.
MRL/lpr mice (10 weeks old) were injected either with saline or 66pmoles of IL233 for 5 consecutive days and monitored for the development of severe proteinuria. The mice were euthanized at 20 weeks of age. As shown, the saline-treated MRL/lpr mice had a higher proteinuria score and became moribund, whereas the majority of IL233 treated mice had lower proteinuria scores (Figure 6A) and decreased incidence of mortality (not shown). Histopathology showed severe inflammation, glomerular hypertrophy, mesangial expansion and glomerulosclerosis in the majority of saline control mice, whereas the IL233 mice had preserved renal structure (Figure 6B and 6C). The IL233-treated MRL/lpr mice had a significantly higher proportion of Treg cells (Figure 6D) and IL-2 producing CD4+ T-cells (Figure 6E) in their renal lymph nodes (Figure 5E and 5F). The changes in these T cell populations was accompanied with a lower proportion of activated (CD69+), effector memory (CD44hiCD62Llo) and follicular T-helper cells in the IL233-treated group as compared to the saline-treated mice (Figure 6, F–H). In addition, the IL233 treated mice had lower proportions of CD11b+ and CD11c+ myeloid cells (Figure 6I and 6L), which showed lower expression of co-stimulatory receptors CD80 and CD86 (Figure 6, J–N). There was also a trend for increase in the expression of Foxp3 and Gata3 in the kidneys of IL233 treated mice as compared to the saline controls, it did not reach statistical significance (not shown). Although, we did not observe statistically significant changes in the levels of circulating anti-dsDNA IgG or immune complex deposits (not shown), there was a trend for skewing of anti-dsDNA antibody to more IgG2b in IL233 group and higher IgG2a in the saline group (Figure 6O, 6P and 6Q).
Figure 6. IL233 prevents lupus-GN prone MRL/lpr mice from the development GN.
Female MRL/lpr mice (10 weeks old) were injected (i.p.) with 66 pmol/mouse of IL233 or saline daily for 5 days. A. IL233 treatment protected MRL/lpr mice from the development of severe proteinuria (individual mice are shown above and mean ± SEM is shown in the lower panel). B. Kidneys of saline-treated, but not IL233-treated mice showed enlarged glomeruli with marked cellular infiltration and sclerosis, interstitial cellular infiltration, dilated tubules and fibrosis. C. Decrease in inflammatory scores in the kidneys of IL233 treated mice. D. The pathological changes in IL233 were accompanied by the increase in Treg (fold change in %Foxp3+ cells) and IL-2 producing cells (E). IL233 treated MRL/lpr mice had reduced proportions of recently activated (CD69+) (F), memory (CD44+CD62L+) (G) and follicular T-helper cells (H). In the renal lymph nodes there were fewer CD11b+ (I) and CD11c+ (L) cells, which had lower expression of CD80 (J and M) and CD86 (K and N). In IL233-treated mice, there was a trend for lower anti-dsDNA IgG2a (O) and higher anti-ds IgG2b (P) antibodies, with a higher trend for the ratio of IgG2b to IgG2a anti-dsDNA in the IL233 group (Q). Yellow circles - glomeruli and green scale bars - 50μM in panel B. Symbols represent individual mice; n=10 for (A to B) and 7–9 for D to N; p value: *<0.05, **<0.001, and ***<0.001.
4. Discussion
In addition to IL-2, IL-33 has been shown to expand Treg cells via its engagement with ST2, the IL-33 receptor (12). The ST2+ Treg cells have been shown to be more potent suppressors of T cells (13). These observations led us to postulate that a combination of IL-2 and IL-33 may be more potent than either IL-2 or IL33 as a single agent to expand Treg cells in general and ST2+ Treg cells in particular. Because both IL-2 and IL-33 have diverse biological functions, the use of high doses of these cytokines are not desirable. Thus we have designed a fusion protein of IL-2 and IL-33, namely IL233, with the functional domains of these two cytokines linked by a polypeptide linker to target the ST2+ Treg cell population (18). In the present study, IL233 was shown to be more potent than IL-2 at a comparable dose in immunomodulation of T cells accompanied with a marked increase in Treg cells and IL-10+ CD4 T cell populations and a decrease in TNFα+ CD4+ T cells. These desirable functions of IL223 suggest that the fusion cytokine may be an appropriate agent for the treatment of lupus GN in particular and SLE in general.
In this study, IL233 was shown to induce a durable remission from established IFNα accelerated lupus GN in the NZM2328. This hybrid cytokine was superior in preventing the development of severe proteinuria in this model. More importantly IL-2 or IL-33 or in combination does not have significant effects preventing the development of lupus GN. Our results are in agreement with the conclusion that the treatment with low-dose IL-2 was ineffective in treating IC–mediated GN in the NZB/W F1 mouse model (21,22). The conclusion that IL233 is superior than IL-2, IL-33 or these two cytokine in combination in the treatment of lupus GN is congruent with our earlier observation that IL233 was more potent than equimolar concentrations of IL-2 or IL-33 or a mixture of IL-2 and IL-33 in increasing Tregs cells and protecting B6 mice from Ischemia reperfusion injury (18). In another recently published study, IL233 administration was found to robustly increase Treg cells, protecting BALB/cJ mice from nephrotoxic injury induced by doxorubicin (20). Thus IL223 has been demonstrated in both autoimmune and non-autoimmune strains to be effective in the induction of Treg cells without significant short term and long-term side effects.
With aging and the onset of lupus GN, there is a decline in the Treg cell population in NZM238 mice and an increase in T cells that are pro-inflammatory. Importantly there is a decline in the ST2+ Treg cell population with age. We expected that IL233 would be able to restore the T cell homeostasis in aging NZM2328 rendering it effective in the prevention and in the treatment of lupus GN. Indeed, our results demonstrate that IL233 is effective in treating ad-IFNα accelerated lupus GN in NZM2328 mice. More importantly, IL233 is effective in treating established lupus GN in three models i.e. the IFNα-accelerated GN in NZM2328 mice, spontaneous GN in NZM2328 and in MRL/lpr mice. The therapeutic effect is accompanied by an increase in Treg cells and a reduction in proinflammatory T cells in the renal lymph nodes. The efficacy of IL233 as a single therapeutic agent for lupus GN is beyond our expectation.
It is of interest to note that IL233 prevents and successfully treats ad-IFNα accelerated GN and spontaneous GN in the NZM2328 and in MRL/lpr mice without significantly affecting autoantibody production, IC deposits and C3 activation within the glomeruli. This observation is similar to that by Wolsy and Seamen (23) that shows anti-CD3 Ab treatment ameliorates lupus GN in the BXW F1 model without decreasing IC deposition and autoantibody production. The mechanisms by which renal damage can be dissociated from IC deposition and C activation will be considered further.
Two studies have shown that kidney damage is prevented by shifting the autoimmune response to a Th2 dominant response with IgG2b IC deposition accompanied with little complement activation in the kidney (24,25). Since IL233 retains the functional capacity of IL-33 that was initially shown to affect Th2 polarization (26), it might be expected that IL233 would induce a Th2 immune response. However, any significant immune deviation to a Th2 response was not detected in IL233 treated mice in the IFNα accelerated lupus GN model. Similarly, in the two spontaneous lupus GN models of NZM2328 and MRL/lpr mice, there was a trend of shifting to a Th2 dominant response, which did not reach statistical significance. Thus it is not likely that shifting to a Th2 immune response is responsible for the prevention or the reversal of lupus GN by IL233 treatment.
Two additional mechanism for uncoupling IC formation and renal damage in lupus GN are worthy of further consideration. Clynes et al (27) demonstrated the dissociation of IC formation and kidney damage in a mutant mice with the deletion of Fc receptors. This observation suggests that modulating effector function is effective in treating lupus GN. Another mechanism is to confer end organ resistance to damage by the IC-mediated mechanisms. As shown in the studies by Ge et al (19), NZM2328.c1R27 mice are resistant to kidney damage despite deposition of IC and complement. This resistance is genetically determined. Recently Maeda et al (28) were able to prevent renal damage by delivering a CaMK4 specific inhibitor to the podocytes in autoimmune and non-autoimmune kidney diseases. Thus targeting podocytes with drugs may render the podocytes resistant to damage. Because of the marked immunomodulatory effect of IL233, it is likely that the hybrid cytokine modulates effector function of proinflammatory cells and promotes anti-inflammatory cellular functions. Whether IL233 may act on podocyte indirectly to render them resistant to damage should be considered.
In the IFNα accelerated BXW F1 model, the GN recurs after successfully treating with immunosuppressive agents (29). It is also observed that relapses are frequent in human lupus GN (30). Thus, the prolonged effect by IL233 to prevent recurrence of lupus GN in the NZM2328 mice is unexpected. It may be due to the persistent elevation of Treg cells in the treated mice. It is also of interest to note the persistent elevation of Treg cells in renal lymph nodes suggest a continued local generation of Treg cells that may be specific to suppress renal reactive T cells. These Treg cells may be more potent in suppressing inflammatory cells (see Figure 5). At any rate, in this study, IL233 was found to be effective as a single agent to treat lupus GN in mouse models, without the use of prednisone and without relapses. In view of the desire to treat SLE in general and lupus GN in particular without chronic steroid administration (31) and the difficulty to achieve relapse-free remission in lupus GN (30), IL233 should be an excellent candidate for future clinical trials.
5. Conclusions
The primary effect of a combination IL-2 and IL-33 therapy via IL233 (a hybrid cytokine) is to promote Treg cell homeostasis to inhibit the lupus-associated immune dysregulation. Importantly, an increase in Treg cells is sufficient to prevent end-organ damage in lupus GN in three mouse models, irrespective of an effect on circulating autoantibody levels or immune complex deposition. A single course of daily injections for 5-days of IL233 is sufficient to sustain Treg cell homeostasis months after the treatment. The induced remission of lupus GN is long-lasting without relapses. The increase in the Treg cells was more pronounced in the renal lymph nodes suggesting an antigen-specific response. Thus IL233 as a single agent without the use of steroid induces remission of mouse lupus GN without relapses, suggesting that IL233 should be considered in clinical trials for lupus GN.
Highlights:
Lupus prone NZM2328 mice have a deficit in IL-2, IL-10 and Foxp3+ST2+ Tregs
IL-2 and IL-33 combination in the form of IL-2 and IL-33 fusion cytokine (IL233) induced a sustained increase in Tregs and suppressed inflammation without affecting autoantibodies.
IL233, as a single agent not only prevented the onset, but also induced persistent remission in ongoing lupus glomerulonephritis (GN) in IFNα-accelerated and spontaneous models in NZM2328 mice and in MRL/lpr mice.
Acknowledgements
This research was mostly reported supported by R01DK105833 (multi-PI: RS and SMF) by the National Institute of Diabetes and Kidney Diseases, R01 AR047988 (PI:SMF) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a grant TIL332615 (PI: SMF) from Lupus Research Alliance. Additional supports were from NIH grants R01DK104963 (PI: RS), R21DK112105 (multi-PI: RS, M. Rosner and K. Lynch), R01AI116725 (F. Perrino, subcontract to RS) from the National Institute of Allergy and Infectious Diseases, a UVA AstraZeneca Research Alliance award (RS) and LaunchPad Diabetes Fund (RS). We thank the UVA Research Histology Core for processing tissue samples and the UVA Flow Cytometry Core Facility, which is supported through the University of Virginia Cancer Center National Cancer Institute P30-CA044579–23 Center Grant. The histology data was gathered on an “MBF Bioscience and Zeiss microscope system for stereology and tissue morphology” funded by National Institutes of Health grant 1S10RR026799–01 (MDO). The content is solely the responsibility of the authors and do not represent the official views of the National Institutes of Health or other funding agencies.
Disclosures
A US patent was granted in December 2017 for the IL-2 and IL-33 fusion protein (IL233; US Patent No. 9,840,545).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsokos GC (2011) Systemic lupus erythematosus. The New England journal of medicine 365, 2110–2121 [DOI] [PubMed] [Google Scholar]
- 2.Crow MK (2009) Developments in the clinical understanding of lupus. Arthritis research & therapy 11, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydintug AO, Chwalinska-Sadowska H, de Ramon E, Fernandez-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, and Hughes GR (2003) Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 82, 299–308 [DOI] [PubMed] [Google Scholar]
- 4.Ward MM (2000) Changes in the incidence of end-stage renal disease due to lupus nephritis, 1982–1995. Archives of internal medicine 160, 3136–3140 [DOI] [PubMed] [Google Scholar]
- 5.Paz Z, and Tsokos GC (2013) New therapeutics in systemic lupus erythematosus. Current opinion in rheumatology 25, 297–303 [DOI] [PubMed] [Google Scholar]
- 6.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, and Winkelmayer WC (2011) Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis and rheumatism 63, 1681–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, and Horwitz DA (1983) Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol 130, 2651–2655 [PubMed] [Google Scholar]
- 8.Lieberman LA, and Tsokos GC (2010) The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. Journal of biomedicine & biotechnology 2010, 740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizui M, and Tsokos GC (2016) Low-Dose IL-2 in the Treatment of Lupus. Current rheumatology reports 18, 68. [DOI] [PubMed] [Google Scholar]
- 10.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, and Daikh DI (2006) Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol 177, 1451–1459 [DOI] [PubMed] [Google Scholar]
- 11.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, and Li Z (2016) Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nature medicine 22, 991–993 [DOI] [PubMed] [Google Scholar]
- 12.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, Demetris AJ, Liew FY, Wood KJ, and Thomson AW (2011) IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol 187, 4598–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, Lott JM, Uhl FM, Pfeifer D, Feser CJ, Smith MJ, Liu Q, Zeiser R, Blazar BR, and Turnquist HR (2016) Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood 128, 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R, Sharma PR, Kim YC, Leitinger N, Lee JK, Fu SM, and Ju ST (2011) IL-2-controlled expression of multiple T cell trafficking genes and Th2 cytokines in the regulatory T cell-deficient scurfy mice: implication to multiorgan inflammation and control of skin and lung inflammation. J Immunol 186, 1268–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma R, Sung SS, Abaya CE, Ju AC, Fu SM, and Ju ST (2009) IL-2 regulates CD103 expression on CD4+ T cells in Scurfy mice that display both CD103-dependent and independent inflammation. J Immunol 183, 1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, and McDuffie M (2001) NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol 100, 372–383 [DOI] [PubMed] [Google Scholar]
- 17.Dai C, Wang H, Sung SS, Sharma R, Kannapell C, Han W, Wang Q, Davidson A, Gaskin F, and Fu SM (2014) Interferon alpha on NZM2328.Lc1R27: enhancing autoimmunity and immune complex-mediated glomerulonephritis without end stage renal failure. Clin Immunol 154, 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stremska ME, Jose S, Sabapathy V, Huang L, Bajwa A, Kinsey GR, Sharma PR, Mohammad S, Rosin DL, Okusa MD, and Sharma R (2017) IL233, A Novel IL-2 and IL-33 Hybrid Cytokine, Ameliorates Renal Injury. Journal of the American Society of Nephrology : JASN 28, 2681–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge Y, Jiang C, Sung SS, Bagavant H, Dai C, Wang H, Kannapell CC, Cathro HP, Gaskin F, and Fu SM (2013) Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. The Journal of experimental medicine 210, 2387–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabapathy V, Cheru NT, Corey R, Mohammad S, and Sharma R (2019) A Novel Hybrid Cytokine IL233 Mediates regeneration following Doxorubicin-Induced Nephrotoxic Injury. Sci Rep 9, 3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen KL, Shibata T, Izui S, and Walker SE (1989) Recombinant interleukin-2 therapy of systemic lupus erythematosus in the New Zealand black/New Zealand white mouse. Journal of biological response modifiers 8, 366–374 [PubMed] [Google Scholar]
- 22.Taylor EB, Sasser JM, Maeda KJ, and Ryan MJ (2019) Expansion of regulatory T cells using low-dose interleukin-2 attenuates hypertension in an experimental model of systemic lupus erythematos. American journal of physiology. Renal physiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wofsy D, and Seaman WE (1985) Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. The Journal of experimental medicine 161, 378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, Hamano S, Nakashima H, and Yoshida H (2005) Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1). J Immunol 175, 7185–7192 [DOI] [PubMed] [Google Scholar]
- 25.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, Bixler SA, Ambrose CM, Scott ML, and Stohl W (2006) Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol 177, 2671–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liew FY, Girard JP, and Turnquist HR (2016) Interleukin-33 in health and disease. Nature reviews. Immunology 16, 676–689 [DOI] [PubMed] [Google Scholar]
- 27.Clynes R, Dumitru C, and Ravetch JV (1998) Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052–1054 [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, Otomo K, Yoshida N, Abu-Asab MS, Ichinose K, Nishino T, Kono M, Ferretti A, Bhargava R, Maruyama S, Bickerton S, Fahmy TM, Tsokos MG, and Tsokos GC (2018) CaMK4 compromises podocyte function in autoimmune and nonautoimmune kidney disease. The Journal of clinical investigation 128, 3445–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Bethunaickan R, Huang W, Ramanujam M, Madaio MP, and Davidson A (2011) IFN-alpha confers resistance of systemic lupus erythematosus nephritis to therapy in NZB/W F1 mice. J Immunol 187, 1506–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana LF, and Jayne D (2016) Sustained remission in lupus nephritis: still a hard road ahead. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 31, 2011–2018 [DOI] [PubMed] [Google Scholar]
- 31.Lightstone L, Doria A, Wilson H, Ward FL, Larosa M, and Bargman JM (2018) Can we manage lupus nephritis without chronic corticosteroids administration? Autoimmunity reviews 17, 4–10 [DOI] [PubMed] [Google Scholar]