Abstract
Background
Alcohol and obesity synergise to increase the risk of liver-related mortality. We examined the influence of adiposity on clinical outcomes in alcoholic hepatitis (AH) and the underlying inflammatory crosstalk between adipose tissue (AT) and the liver.
Methods
A cohort of 233 patients with AH from the UK and USA provided data to analyse the effects of obesity in AH. Body mass index was corrected for the severity of ascites, termed cBMI. Inflammatory and metabolic profiling was undertaken by proteome analysis of human serum samples. The effect of alcohol on adipose tissue and CXCL11 expression was studied in 3 T3-derived adipocytes and in mice using the high-fat diet-plus-binge ethanol model.
Findings
Obesity was common amongst patients with AH, seen in 19% of individuals. Obesity (HR 2.22, 95%CI 1.1–4.3, p = .022) and underweight (HR 2.38, 1.00–5.6, p = .049) were independently associated with mortality at 3 months. Proteome analysis demonstrated multiple metabolic and inflammatory factors differentially expressed in obese AH verse lean AH, with CXCL11 being the most elevated factor in obese AH. In vitro analysis of cultured adipocytes and in vivo analysis of mouse models showed that alcohol induced CXCL11 expression in AT, but not in liver.
Interpretation
Obesity is common in AH and associated with a greater than two-fold increase in short-term mortality. Obese AH is associated with a different inflammatory phenotype, with the greatest elevation in CXCL11. These data confirm that adiposity is clinically important in acute alcohol-related liver disease and illustrate the adipose-liver inflammatory axis in AH.
Fund
This work was supported in part by an EASL Sheila Sherlock Physician Scientist Fellowship. The funder played no role in gathering or analysing data or writing the manuscript. This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Keywords: Alcoholic hepatitis, Adipose, Obesity
Research in context.
Evidence before this study
There is increasing recognition that the combination of alcohol and obesity is particularly dangerous with regard to liver health. Epidemiological data have demonstrated an increased risk of cirrhosis or liver–related deaths in individuals who are both obese and hazardous drinkers. However the effect of obesity in particular types of alcohol related liver disease have not been described, nor the mechanisms that cause this synergistic effect.
Added value of this study
This study examined the effect of body mass in alcoholic hepatitis, the most florid manifestation of ArLD with a poor short-term mortality. We found that obesity is associated with increased morbidity and mortality in alcoholic hepatitis. Analysis of serum showed differing inflammatory profiles in obese/AH suggesting differing pathological profiles. In vitro studie confirmed that this is due at least in part to the effect of alcohol on adipose tissue rather than the liver per se. These data add to the epidemiological data by reporting on patient-level, rather than population level, effect of obesity and start to describe mechanisms for this effect.
Implications of all the available evidence
This provides a starting point for further investigations into the combined effects of obesity and alcohol on liver function and underpins considerations for suggesting lower safe limits of alcohol when obesity is also present.
Alt-text: Unlabelled Box
1. Introduction
Alcoholic liver disease (ALD) is a common form of liver disease throughout the world and causes a significant burden on healthcare resources [1,2]. ALD is a spectrum of disease ranging from simple steatosis to cirrhosis and hepatocellular carcinoma [3]. Alcoholic hepatitis (AH) is an acute form of ALD typified by jaundice and liver failure [4]. AH has a high short-term mortality, which has not improved over time despite trials of multiple agents [5].
The presence of obesity increases the risk of clinically significant liver disease and death in hazardous drinkers [[6], [7], [8]] and increases the likelihood of AH on liver biopsy [6]. The synergy between alcohol and obesity is due to hepatic factors and extra-hepatic factors, predominantly in adipose tissue [9]. For example, flux of fatty acids from adipose tissue caused by obesity or alcohol contributes to hepatic steatosis and inflammation [10], and expression of inflammatory cytokines in adipose tissue mirrors the severity of hepatic inflammation in AH [11].
Understanding the relationship between obesity and ALD is important in the face of growing prevalence of obesity [12]. However, there are very few clinical data on the impact of obesity on mortality and morbidity in AH and mechanistic data are lacking. In this present study we examined the prevalence and clinical consequences of obesity in patients with AH and sought to phenotype the inflammatory pathways in AH and obesity.
2. Methods
2.1. Patients
This study used data regarding patients with AH identified from prospectively maintained departmental databases at University Hospitals Birmingham NHS Foundation Trust, Birmingham UK, University Hospitals Bristol NHS Foundation Trust, Bristol, UK, St Mary's Hospital, Imperial College NHS Foundation Trust, London UK, Nottingham University Hospitals NHS Trust, Nottingham, UK and Mount Sinai Medical Centre, New York, NY, USA. Standard definitions of AH were used to identify or exclude patients: alcohol use of at least 60 g/d in men and > 40 g/d in women for >5 years, hazardous drinking until at least six weeks before admission, abrupt increase in serum bilirubin, elevated transaminases >50 and < 400 IU/L, aspartate transaminase (AST) to alanine transaminase (ALT) ratio > 2:1, and exclusion of other causes of liver disease [13]. Importantly, all patients had undergone ultrasound (US) imaging of the abdomen early in their admission to hospital. Liver biopsy was performed only in cases of diagnostic uncertainty, consistent with recent NIAAA guidance for trialists [13]. AH was managed according to local protocols and specialist guidelines [3,14].
Baseline clinical and biochemical characteristics, specific treatments for AH and presence of complications during admission (infection or AKI) were collected. The diagnosis of infection was according to criteria published by Bajaj [15]. Renal failure was diagnosed in accordance to a recent expert consensus statement [16]. Follow-up was to three months after admission. Only observational data were collected, no procedures, tests or interventions were performed outside of standard care. Blood samples from patients with AH were collected within 48 h of admission, after obtaining written confirmation of informed consent. The collection of samples was approved by research ethics committee (reference 06/Q27108/11).
2.2. Analysis of body composition and correction for ascites
Body mass index was adjusted for the presence of ascites by subtracting the weight of ascites from measured body weight, and a corrected BMI (cBMI) was calculated. Patients were categorised as underweight (cBMI) <18.5 kg/m2), normal (cBMI 18–5-24.9 kg/m2), overweight (cBMI 25–29.9 kg/m2) or obese (cBMI ≥30 kg/m2). This is described in detail in the supplementary information.
2.3. Inflammatory phenotyping
Blood samples were separated into serum by centrifuging and frozen to −80 °C within an hour of collection, and stored until analysis. Inflammatory and metabolic profiling (see supplementary table 1 for complete list) was undertaken by proteome analysis of serum samples (proteome profiler, R&D Systems, USA). This system uses multiple antibody dots on a film to semi-quantitatively detect multiple proteins in serum. The size of the resulting dot when the film is developed corresponds to the abundance of the protein in serum. Enzyme-linked immunosorbant assay (ELISA) was performed to confirm the results of the proteomic analysis using Quantikine ELISA kit (R&D Systems, USA). All assays were performed according to the manufacturer's instructions.
2.4. In vitro experiments
3 T3-derived adipocytes were used for in vitro work to examine the influence of alcohol on adipocytes. Briefly, for adipocyte differentiation, the 3 T3-derived adipocytes were plated 1 × 105 cells/well. After confluence, cells were induced differentiation with 10% FBS + 5 μg/mL insulin, 0.5 mM IBMX, 1 μM Dex, T3 and Rosiglitazone. After 2 days, cells were changed to a medium containing 10% FBS, insulin, T3 and Rosiglitazone. After 4 days, cells were changed with the same medium again. Cells were cultured to confluence and then incubated with varying concentrations of alcohol: 0, 17.4 and 50 mmol/L. The concentrations of alcohol used in experiments were chosen to reflect serum concentrations of alcohol after binge drinking [17]: a blood alcohol concentration of 0.08 g/dL corresponds to 17.4 mmol/L. Concentrations much in excess of this are lethal [18].
2.5. In vivo experiments of high-fat diet (HFD)-plus-binge ethanol feeding
To investigate the combination of obesity and alcohol on liver function, a model of HFD-plus-binge ethanol feeding was used as described previously [19,20]. Briefly, C57/B6 mice were given a high fat diet (HFD, 60% of calories from fat, catalog no. D12492; Research Diets, New Brunswick, NJ) for three months. Mice were divided into four groups of five mice. In addition to HFD, groups received either single or multiple alcohol binges of alcohol or calorie-matched maltose via gavage. For single binge, mice were administered a single gavage of 5 g/kg ethanol. For multiple binges, mice were administered 3 g/kg ethanol twice a week for a total of 8 times during the final month of HFD feeding. At the end of the experiment mice underwent terminal anaesthesia 9 h after the final gavage. Blood for analysis of serum was obtained through cardiac puncture. Liver and epididymal adipose tissue was harvested, snap frozen in liquid nitrogen and stored at −80 °C until analysis.
2.5.1. Biochemical assays
The levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG) and cholesterol (Chol) were measured with IDEXX Catalyst Dx analyzer (IDEXX Laboratories, Westbrook, Maine).
2.5.2. Real time-Polymerase chain reaction (PCR)
Total RNA was isolated from AT, liver and 3 T3-derived adipocytes by using Trizol Reagent (Invitrogen). cDNA was synthesized by using cDNA RT kit (Applied Biosystems, Forster City, CA). The expression levels of genes were measured with quantitative real-time PCR by using ABI PRISM 7500 real-time PCR detection system (Applied Biosystems, Foster City, CA).
2.5.3. Oil-red-O staining
3 T3-derived adipocytes cells were fixed with 10% formaldehyde and washed with 60% isopropanol. Oil-red-O (sigma cat. O-0625) solution was added to the completed dried well and incubated at room temperature for 10 min. The O.D values were measured at 520 nm in a spectrophotometer.
2.6. Statistical analysis
Correlation between BMI and volume of adipose and muscle tissue were examined by linear regression to calculate coefficient of determination (r2) values. cBMI groups were compared with one-way analysis of variance (ANOVA). Survival was analysed with univariate Kaplan Meier analysis and multivariate Cox proportional hazard analysis, to calculate hazard ratios (HR). SPSS v23 (IBM, New York USA) was used for statistical analyses.
3. Results
3.1. cBMI correlates with adiposity in AH
Body composition was analysed in a subgroup of 60 patients from the entire cohort who underwent CT imaging during admission. This allowed for accurate determination of the volume of ascites, subcutaneous and visceral adipose tissue and muscle volume. Analysis of body composition showed that cBMI correlated with volume of subcutaneous and to a lesser extent intra-abdominal adipose tissue (r2 = 0.62, p < .001 and r2 = 0.11 p = .024 respectively) (supplementary fig. 2). cBMI did not correlate with volume of muscle (r2 = 0.03, p = .215). Thus, cBMI is a measure of adiposity in alcoholic hepatitis.
3.2. Prevalence and consequences of obesity
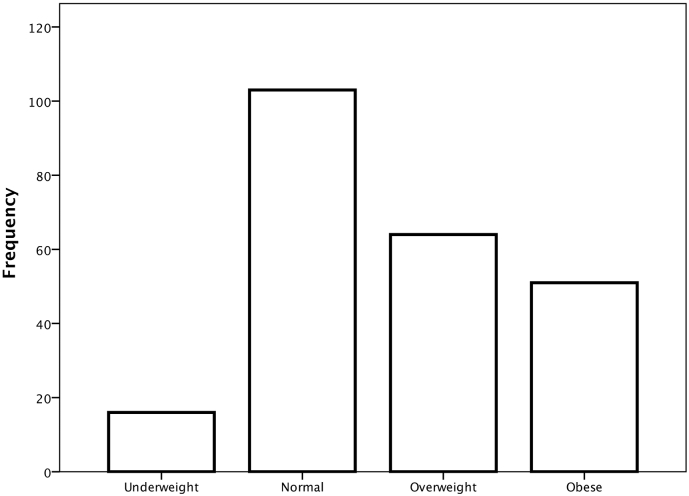
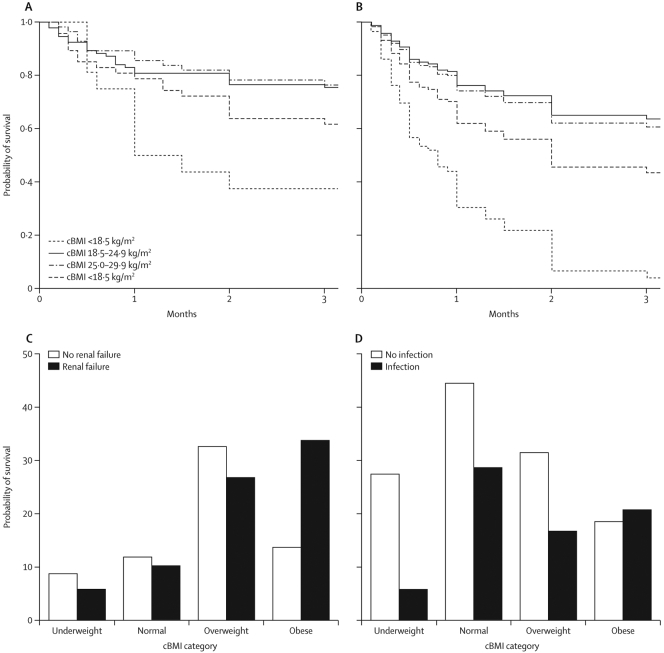
The total cohort of 233 patients were used to examine the effect of obesity in AH. Characteristics of patients are shown in Table 1. cBMI was used to classify patients as underweight, normal, overweight or obese. Overweight and obesity were common amongst patients with AH (29% and 19% respectively) (Fig. 1). Characteristics of each cBMI category are shown in Table 1. Groups differed with respect to serum sodium concentration (one-way ANOVA p < .001), severity of ascites (p = .002) and urea (p = .007). Univariate Kaplan-Meier analysis showed that cBMI category was associated with 90-day survival (log rank p = .005) (Fig. 2A).
Table 1.
Baseline characteristics of patients included in the study.
| All n = 233 |
Underweight n = 25 |
Normal n = 95 |
Overweight n = 67 |
Obese n = 45 |
p ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Variance | Mean | Variance | Mean | Variance | Mean | Variance | Mean | Variance | ||
| Age years | 49.0 | 53.0 | 47.0 | 37.0 | 50.0 | 49.0 | 49.0 | 38.0 | 50.0 | 45.0 | 0.469 |
| Ascites severity⁎ | 1.4 | 1.1 | 2.0 | 1.0 | 1.5 | 1.1 | 1.2 | 1.1 | 1.1 | 1.0 | 0.017 |
| Prothrombin time seconds | 26.0 | 8.8 | 24.7 | 4.5 | 25.6 | 8.8 | 26.2 | 9.3 | 27.2 | 9.2 | 0.700 |
| Bilirubin Mmol/L | 238 | 162 | 234 | 129 | 239 | 151 | 236 | 177 | 242 | 177 | 0.998 |
| Creatinine mmol/L | 91.3 | 70.8 | 63.0 | 41.5 | 89.8 | 68.3 | 83.4 | 49.6 | 112.6 | 96.7 | 0.059 |
| Sodium mmol/L | 130.9 | 8.2 | 123 | 7.6 | 132 | 7.9 | 131 | 8.3 | 132 | 8.1 | 0.002 |
| Urea mmol/L | 8.6 | 13.4 | 4.6 | 5.1 | 6.5 | 8.1 | 7.7 | 9.7 | 15.4 | 22.7 | 0.001 |
| Albumin g/L | 24.2 | 11.3 | 26.0 | 7.1 | 24.8 | 11.4 | 23.9 | 11.6 | 22.6 | 11.9 | 0.665 |
| White cell count x106/L | 12.5 | 13.7 | 13.0 | 7.9 | 12.4 | 7.8 | 11.1 | 5.5 | 14.1 | 26.2 | 0.716 |
Compared by Fisher's exact test.
Fig. 1.
distribution of cBMI in patients with alcoholic hepatitis. Underweight cBMI<18.5 kg/m2, normal cBMI 18.5–24.9 kg/m2, overweight cBMI 25–29.9 kg/m2, obese cBMI ≥ 30 kg/m2.
Fig. 2.
A univariate analysis of survival after admission with alcoholic hepatitis by cBMI category log rank p < .001. B multivariate Cox proportional hazard analysis of survival taking into account baseline factors C incidence of acute renal failure was greater in obese patients with AH (Chi squared p = .032) D no difference in the incidence of infection was observed.
90-day mortality was worse in underweight patients (63%) and obese patients (40%) compared to normal or overweight patients (90-day mortality 34% and 24% respectively). These findings remained when analysis was limited to patients without ascites to remove any possibility of interference in assessment of adiposity by ascites (supplementary fig. 3). Multivariate analysis (to control for baseline differences in the severity of ascites, serum urea and sodium concentration between cBMI groups) confirmed that, compared to a normal cBMI, underweight (HR 3.37, 95%CI 1.40–8.09, p = .007) and obesity (HR 2.81, 1.21–4.25, p = .022) were independently associated with mortality at 3 months after admission (Fig. 2B). Overweight was not associated with mortality (HR 1.09, 0.52–2.29, p = .816) Obesity was also associated with greater incidence of renal failure (Chi-squared p = .032) (Fig. 2C) but not infection (Fig. 2D).
3.3. Inflammatory and metabolic phenotyping
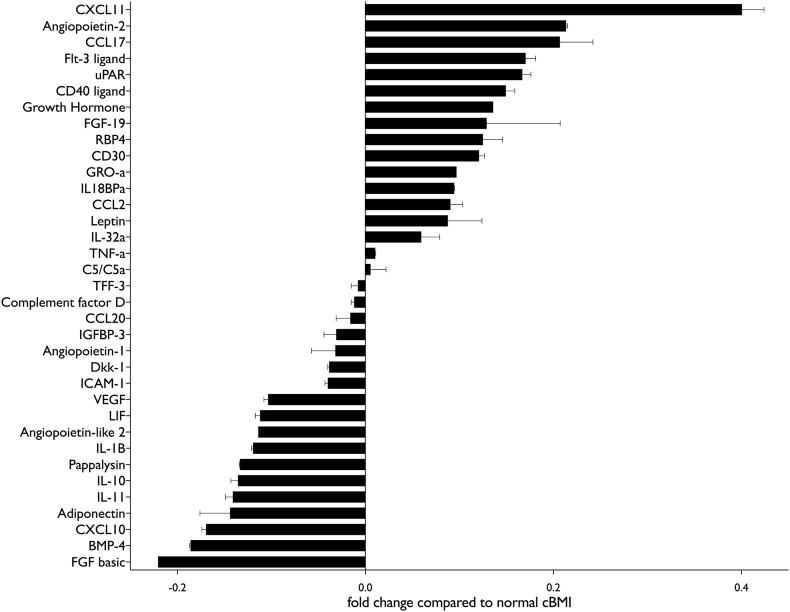
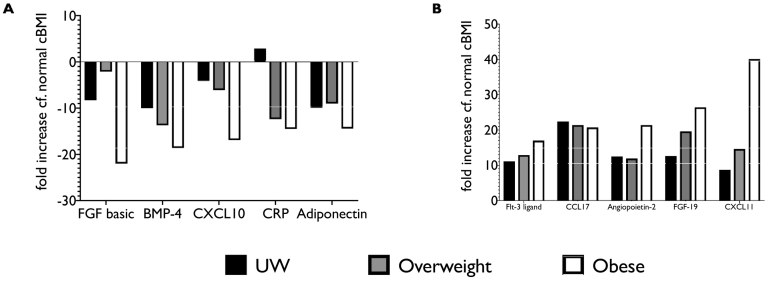
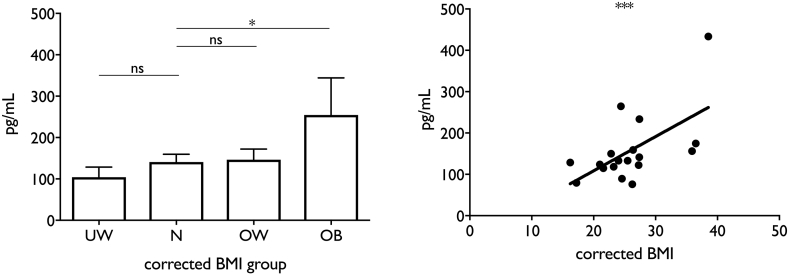
The serum inflammatory phenotype was markedly different in patients with AH and obesity compared to normal cBMI (Table 2, Fig. 3). There was a clear continuum from overweight to obese in most of the factors analysed, especially when the most up or down-regulated factors were considered (Fig. 4). Otherwise inflammatory and metabolic pathways were remarkably similar between extremes of cBMI with only a few pathways differing in proteomic analysis. Interestingly, although leptin concentration was up-regulated to a greater extent in obese patients as might be expected, adiponectin was relatively decreased in both underweight and obese patients compared to normal cBMI. The most striking difference was seen in CXCL11, which was markedly up-regulated (40% greater) in obese patients compared to the group with normal cBMI. To confirm this result, single-target ELISA was performed to which showed that serum CXCL11 concentration was correlated with cBMI in patients with AH (r2 0.35, p = .001) (Fig. 5).
Table 2.
serum proteome from patients with alcoholic hepatitis. All values are relative to patients with a normal corrected BMI (18.5–24.9 kg/m2).
| cBMI category |
||||
|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | |
| FGF basic | −8.3 | 0.0 | −2.1 | −22.0 |
| BMP-4 | −10.0 | 0.0 | −13.7 | −18.6 |
| CXCL10 | −4.1 | 0.0 | −6.1 | −16.9 |
| CRP | 2.9 | 0.0 | −12.4 | −14.5 |
| Adiponectin | −9.9 | 0.0 | −9.0 | −14.4 |
| IL-11 | −10.4 | 0.0 | −14.8 | −14.1 |
| IL-10 | −8.9 | 0.0 | −15.5 | −13.6 |
| Pappalysin | −2.1 | 0.0 | −14.0 | −13.3 |
| IL-1B | −10.3 | 0.0 | −11.5 | −12.0 |
| Angiopoietin-like 2 | −0.4 | 0.0 | 1.5 | −11.4 |
| LIF | −8.2 | 0.0 | −11.8 | −11.2 |
| VEGF | −9.1 | 0.0 | −11.0 | −10.3 |
| Angiopoietin-1 | −2.4 | 0.0 | 0.5 | −7.6 |
| ICAM-1 | −2.3 | 0.0 | −12.1 | −4.0 |
| Dkk-1 | 6.7 | 0.0 | −5.5 | −3.8 |
| IGFBP-3 | 11.9 | 0.0 | −1.8 | −3.1 |
| CCL20 | 3.3 | 0.0 | −0.6 | −1.6 |
| Complement factor D | −6.5 | 0.0 | −3.1 | −1.2 |
| TFF-3 | 8.2 | 0.0 | 3.7 | −0.8 |
| FGF-19 | −15.2 | 0.0 | 1.9 | −0.6 |
| C5/C5a | 10.3 | 0.0 | −4.7 | 0.6 |
| TNF-a | −0.8 | 0.0 | −2.4 | 1.0 |
| Angiopoietin-1 | 7.8 | 0.0 | 5.9 | 1.2 |
| Leptin | −4.7 | 0.0 | 2.0 | 2.5 |
| IL-32a | 27.1 | 0.0 | 3.6 | 5.9 |
| CCL2 | 11.3 | 0.0 | 5.7 | 6.8 |
| IL18BPa | 9.0 | 0.0 | 1.1 | 9.4 |
| GRO-a | 14.8 | 0.0 | 1.5 | 9.7 |
| CCL2 | 18.0 | 0.0 | 2.9 | 11.3 |
| CD30 | 6.2 | 0.0 | −2.5 | 12.1 |
| RBP4 | 9.7 | 0.0 | 1.4 | 12.5 |
| Growth Hormone | 7.3 | 0.0 | −0.2 | 13.6 |
| CD40 ligand | 11.2 | 0.0 | 0.0 | 15.0 |
| Leptin | −2.4 | 0.0 | 6.7 | 15.1 |
| uPAR | 12.1 | 0.0 | 3.7 | 16.7 |
| Flt-3 ligand | 11.1 | 0.0 | 12.9 | 17.0 |
| CCL17 | 22.4 | 0.0 | 21.4 | 20.7 |
| Angiopoietin-2 | 12.5 | 0.0 | 11.9 | 21.4 |
| FGF-19 | 12.6 | 0.0 | 19.6 | 26.4 |
| CXCL11 | 8.7 | 0.0 | 14.6 | 40.1 |
Fig. 3.
proteome analysis of inflammatory factors in serum from patients with AH and obesity. All changes are compared to patients with AH and normal cBMI (18.5–24.9 kg/m2).
Fig. 4.
relative values of A the five most-downregulated factors and B the five most-upregulated factors by cBMI group.
Fig. 5.
analysis of CXCL11 concentration in serum from patients with AH. A CXCL11 significantly overexpressed in obese AH, B serum CXCL11 correlates with cBMI.
3.4. Ethanol induces CXCL11 gene expression in vivo and in vitro models of ALD
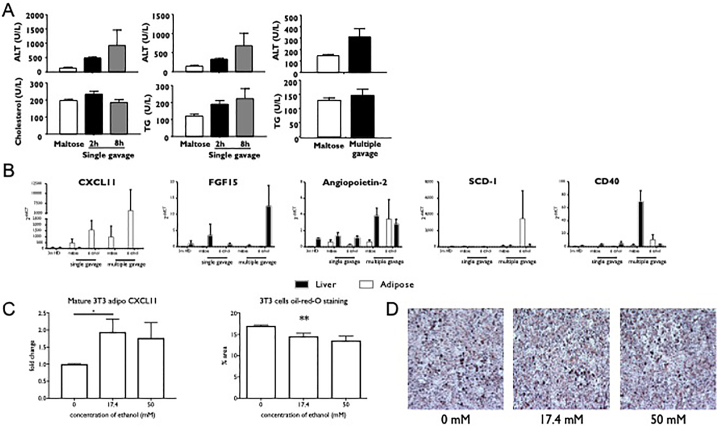
A murine model of obesity and alcoholic hepatitis induced by high-fat diet (HFD) feeding plus binge ethanol was used to investigate the relative contributions of adipose and liver tissue to the inflammatory changes observed in patients with AH. The experimental diets induced liver injury, evidenced by increased circulating ALT and AST concentration and typical changes of steatohepatitis on histology (supplementary fig. 4). Consistent with previous results, HFD and binges of alcohol caused greater liver injury compared to maltose fed mice evidenced by higher circulating transaminases, cholesterol and triglycerides (Fig. 6A). The relative gene expression of targets identified in analysis of human proteome was analysed. The combination of alcohol and obesity altered gene expression with the most obvious change being expression of CXCL11, which was markedly up-regulated in adipose tissue without any significant changes being seen in liver tissue (two way ANOVA p = .008)(Fig. 6B).
Fig. 6.
C57/B6 mice were fed with a high fat diet for three months and received binge alcohol to induce liver injury. A circulating concentration of transaminases and triglycerides were increased by HFD and alcohol. B HFD and multiple alcohol binges caused differential changes in gene expression in liver and adipose tissue. CXCL11 was predominantly increased in adipose tissue. 3 T3-derived adipocytes were incubated with ethanol. C Increased gene expression of CXCL11 was seen at physiological doses at ethanol. D triglyceride loading of adipocytes was reduced by ethanol in a dose-dependent manner illustrated with Oil-red O staining. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To confirm alcohol-mediated alterations in CXCL11 in adipose tissue, 3 T3-derived adipocytes were incubated with alcohol for 7 days. CXCL11 gene expression was up-regulated, with most marked increase seen at 17.4 mmol/L of ethanol - a physiological dose (Fig. 6C). In parallel with changes in gene expression, a reduction in the lipid content of 3 T3-derived adipocytes was observed when assessed by oil-red-O staining (Fig. 6D) consistent with increased lipotoxicity secondary to impaired adipocyte lipid storage induced by alcohol.
4. Discussion
Previous epidemiological studies have observed synergy between obesity and alcohol in terms of the risk of liver disease [6,7] and the risk of liver-related death [8]. Clinical studies have confirmed that adipose tissue is inflamed in patients with alcoholic liver disease and correlates with the severity of liver inflammation [11,21]. Our data add to this knowledge by showing that in patients with AH, the severe form of ALD, obesity is a risk factor for short-term morbidity and mortality. This is in part driven by a greater incidence of renal failure. Patients with obesity and AH have a different inflammatory phenotype where CXCL11 in particular was seen in high concentrations. This inflammatory phenotype is driven by adipose tissue, as experiments with isolated adipocytes and in animal models of AH showed that induction of CXCL11 by alcohol is specific to adipose tissue.
It is striking that whilst the inflammatory phenotype described in the serum proteome showed a progression from overweight to obese (Fig. 4, Fig. 5) this did not translate into a similar progression in clinical events, where mortality and morbidity was markedly increased in obese patients and but not in overweight patients. Our data cannot provide and explaination for this phenomenon. It could be speculated that the accumulation of multiple dysregulated pathways leads to worse clinical outcomes. Larger studies would be required to allow for robust mortality analysis based on smaller ranges of cBMI to look for increasing mortality with small increases in body mass. This is a limitation of these data.
This study is based on careful evaluation of the severity of ascites to allow accurate assessment of BMI and body composition. Other systems of correcting for the presence of ascites in the assessment of BMI are used [22] but are not based on analysis of body composition. This methodology allowed us to be confident that BMI was a surrogate marker of overall adiposity. Conversely, this analysis also underpins previous work that has shown that sarcopaenia cannot be adequately assessed by BMI in patients with liver disease [23]. In keeping with other systems, we categorised ascites into grades of severity based on US findings. This categorisation may have introduced an element of inaccuracy to our clinical findings as it relies on the judgment of clinicians, however our results remained valid when we only considered patients without any evidence of ascites where no correction to BMI was necessary.
The patients included in this study were diagnosed with AH on clinical grounds; biopsy was not required for inclusion. Published data suggest that clinical diagnoses of AH are accurate [24], especially when patients have a marked hyperbilirubinaemia [25], which was clearly the case in our cohort. This is reflected in the recent NIAAA statement regarding the need for biopsy in AH [13], where biopsy is only suggested for cases of clinical uncertainty.
Obesity is increasingly common on a population level [26], where hazardous drinking and early alcoholic liver disease are often associated with obesity [6,27]. Although obesity may often fade or resolve in advanced cirrhosis as sarcopaenia develops, it is common to manage patients where both alcohol and the metabolic syndrome can both contribute to the development of liver disease. Our data relate only to adiposity and we are unable to describe the role of other aspects of metabolic syndrome such as insulin resistance, hypertension or dyslipidaemia but data in animals and other human liver disease, principally NAFLD that suggest they are deleterious [28].
We observed a different inflammatory phenotype in obese AH patients verse non-obese AH patients. When we explored this further in the laboratory we found that this was due in part to a contribution from adipose tissue. In particular alcohol caused the expression of CXCL11 expression to increase in adipose tissue but not in the liver. CXCL11 is classically a T-lymphocyte chemoattractant via interaction with CXCR3. Interestingly CXCL11 may be downregulated by adiponectin. Adiponectin, an adipokine that reduces adipose inflammation and systemic insulin resistance, [29] is well recognised to be increased by alcohol consumption [30,31] and is increased in ArLD [32] [33]. This is in contrast to obesity where decreased concentrations of adiponectin are observed [34]. The finding of increased CXCL11 in obese AH suggests that there may be a failure of down-regulation of CXCL11 by adiponectin, or that alcohol use in obesity may still be associated with decreased adiponectin and consequent increased CXCL11 production. This was indeed the case in our cohort where the combination of AH and obesity was associated with a marked decrease in adiponectin, compared to normal BMI (supplementary table 1).
The expression of CXCL11 in our murine model of alcohol and obesity was investigated with analysis of gene expression in response to high fat diet feeding, with or without administration of ethanol; however, the function of CXCL11 was unable to explored in C57BL/6 mice due to a frame shift germline mutation of the Cxcl11 gene in C57BL/6 mice [35]. This mutation results in expression of a variant form of the CXCL11 protein that is not functional [36]. Thus whilst the expression of mRNA can be measured, seeking to antagonize the effect of CXCL11 cannot be performed in this standard model of obesity and alcohol abuse.
Obesity exerts a deleterious effect in AH. Important differences are seen in the clinical and inflammatory changes that occur, between non-obese and obese patients. Our data highlight CXCL11 as one of the pathways that differ. The interplay between alcohol, metabolic factors and hepatic inflammation is complex and is far from fully explored. Careful further studies are required to understand this in more detail. Given the prevalence of both alcohol abuse and the metabolic syndrome these studies are urgently needed.
Acknowledgments
Acknowledgements
This work was supported in part by an EASL Sheila Sherlock Physician Scientist Fellowship. The funder played no role in gathering or analysing data or writing the manuscript.
Author contributions
RP: conceived study, collected data, performed experiments, analysed data, drafted manuscript.
SJK: performed experiments, analysed data, reviewed manuscript.
GYI: collected data, reviewed manuscript.
JN: collected data.
BD: analysed radiological images.
NV: collected data, reviewed manuscript.
AG: collected data, reviewed manuscript.
MR: performed experiments.
AM: reviewed manuscript.
GPA: reviewed manuscript.
PN: reviewed manuscript.
CJW: supervised experiments, reviewed manuscript.
AH: reviewed manuscript.
BG: supervised experiments, reviewed manuscripts.
Conflicts of interest
No authors have any conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.046.
Appendix A. Supplementary data
Supplementary material
References
- 1.Rehm J., Samokhvalov A.V., Shield K.D. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Blachier M., Leleu H., Peck-Radosavljevic M., Valla D.-C., Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Easl clinical practical guidelines: management of alcoholic liver diseaseJ Hepatol. 2012;57(2):399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Lucey M.R., Mathurin P., Morgan T.R. Alcoholic hepatitis. New Engl J Med. 2009;360(26):2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 5.Hughes E., Hopkins L. R P. survival from alcoholic hepatitis has not improved over time. PLoS One. 2018;13(2):1–10. doi: 10.1371/journal.pone.0192393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naveau S., Giraud V., Borotto E., Aubert A., Capron F., Chaput J.-C. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25(1):108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 7.Liu B., Balkwill A., Reeves G., Beral V. Body mass index and risk of liver cirrhosis in middle aged Uk women: prospective study. BMJ. 2010;340:c912. doi: 10.1136/bmj.c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart C.L., Morrison D.S., Batty G.D., Mitchell R.J., Smith G.D. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ: Br Med J. 2010:340. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle M., Masson S., Anstee Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J Hepatol. 2017;68:251–267. doi: 10.1016/j.jhep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Parker R., Kim S.J., Gao B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol. 2018;15:50–59. doi: 10.1038/nrgastro.2017.116. [DOI] [PubMed] [Google Scholar]
- 11.Naveau S., Cassard-Doulcier A.M., Njike-Nakseu M. Harmful effect of adipose tissue on liver lesions in patients with alcoholic liver disease. J Hepatol. 2010;52(6):895–902. doi: 10.1016/j.jhep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Ogden C.L., Carroll M.D., Lawman H.G., Fryar C.D., Kruszon-Moran D., Kit B.K. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crabb D.W., Bataller R., Chalasani N.P. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the Niaaa alcoholic hepatitis consortia. Gastroenterology. 2016;150(4):785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'shea R.S., Dasarathy S., Mccullough A.J. Alcoholic liver disease. Hepatology. 2010;51(1):307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj J.S., O'leary J.G., Reddy K.R. Second infections independently increase mortality in hospitalized patients with cirrhosis: the north American consortium for the study of end stage liver disease (Nacseld) experience. Hepatology. 2012;56(6):2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeli P., Gines P., Wong F. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the international club of ascites. J Hepatol. 2015;62:968–974. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Niaaa/nih Drinking Levels Defined. 2017. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- 18.Poikolainen K. Estimated lethal ethanol concentrations in relation to age, aspiration, and drugs. Alcohol Clin Exp Res. 1984;8(2):223–225. doi: 10.1111/j.1530-0277.1984.tb05843.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang W., Xu M.J., Cai Y. Inflammation is independent of Steatosis in a murine model of Steatohepatitis. Hepatology. 2017;66:108–123. doi: 10.1002/hep.29129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z., Xu M., Cai Y. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5(3):399–413. doi: 10.1016/j.jcmgh.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voican C.S., Njike-Nakseu M., Boujedidi H. Alcohol withdrawal alleviates adipose tissue inflammation in patients with alcoholic liver disease. Liver Int. 2015;35(3):967–978. doi: 10.1111/liv.12575. [DOI] [PubMed] [Google Scholar]
- 22.Newsome P.N., Mckiernan P.J., Millson C. Guidelines for liver transplantation for patients with non-alcoholic Steatohepatitis. Gut. 2012;61:484–500. doi: 10.1136/gutjnl-2011-300886. [DOI] [PubMed] [Google Scholar]
- 23.Vugt J.L.A., Levolger S., Bruin R.W.F., Rosmalen J., Metselaar H.J., Ijzermans J.N.M. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 24.Roth N.C., Saberi B., Macklin J. Prediction of histologic alcoholic hepatitis based on clinical presentation limits the need for liver biopsy. Hepatol Commun. 2017;1(10):1070–1084. doi: 10.1002/hep4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamid R., Forrest E. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut. 2011;60:A233. [Google Scholar]
- 26.cdc CFDCAP . MMWR morbidity and mortality weekly report. 2004. Trends in intake of energy and macronutrients—United States, 1971-2000; p. 80. 53(4) [PubMed] [Google Scholar]
- 27.Sayon-Orea C., Martinez-Gonzalez M.A., Bes-Rastrollo M. Alcohol consumption and body weight: a systematic review. Nutr Rev. 2011;69(8):419–431. doi: 10.1111/j.1753-4887.2011.00403.x. [DOI] [PubMed] [Google Scholar]
- 28.Chitturi S., Abeygunasekera S., Farrell G.C. Nash and insulin resistance: insulin Hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35(2):373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 29.Turer A.T., Scherer P.E. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 30.Sierksma A., Patel H., Ouchi N. Effect of moderate alcohol consumption on Adiponectin, tumor necrosis factor-α, and insulin sensitivity. Diabetes Care. 2004;27(1):184–189. doi: 10.2337/diacare.27.1.184. [DOI] [PubMed] [Google Scholar]
- 31.Hillemacher T., Weinland C., Heberlein A. Increased levels of Adiponectin and Resistin in alcohol dependence - a possible link to craving. Drug Alcohol Depend. 2009;99(1):333–337. doi: 10.1016/j.drugalcdep.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Kasztelan-Szczerbinska B., Surdacka A., Slomka M., Rolinski J., Celinski K., Smolen A. Association of Serum Adiponectin, Leptin, and Resistin concentrations with the severity of liver dysfunction and the disease complications in alcoholic liver disease. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/148526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaser S., Moschen A., Kaser A. Circulating Adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis. J Intern Med. 2005;258(3):274–280. doi: 10.1111/j.1365-2796.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- 34.Kern P.A., Di Gregorio G.B., Lu T., Rassouli N., Ranganathan G. Adiponectin expression from human adipose tissue relation to obesity, insulin resistance, and tumor necrosis factor-a expression. Diabetes. 2003;52(7):1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 35.Groom J.R., Luster A.D. Cxcr3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawford M.A., Zhu Y., Green C.S. Antimicrobial effects of interferon-inducible cxc chemokines against bacillus Anthracis spores and bacilli. Infect Immun. 2009;77(4):1664–1678. doi: 10.1128/IAI.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material