Abstract
Background
Defective clearance of apoptotic cells (ACs) has been suggested to be involved in the pathogenesis of systemic lupus erythematosus (SLE). Mesenchymal stem cells (MSCs) exhibit promising therapeutic effects on SLE, but whether MSCs phagocytose ACs and contributes to the underlying mechanism in the treatment of SLE remain unknown.
Methods
Human umbilical cord (UC) MSCs were co-cultured with ACs, and the engulfment of ACs by MSCs was either detected by flow cytometry or observed under confocal laser scanning microscope. Peripheral blood mononuclear cells (PBMCs) from healthy controls (HCs) were cultured in MSC conditioned medium (MCM) or MSC exposed to ACs (AC-MSC) conditioned medium (ACMCM), and then CD4+ T cell proliferation was detected. Soluble factors including prostaglandin (PG)E2 in the supernatants of MSCs and AC-MSCs, as well as in the mouse peritoneal lavage fluids (PLF) were determined by enzyme-linked immunosorbent assay (ELISA). Cyclooxygenase (COX)2 inhibitors and siRNA transfection were utilized to determine the function of COX2/PGE2 in AC-MSC-mediated immunosuppression. PGE2 metabolites (PGEM) in the plasma of SLE patients were measured before and 24 h after MSC transplantation respectively.
Findings
Human UC MSCs possessed the ability to engulf ACs. AC-MSCs increased MSC-mediated suppression of CD4+ T cell proliferation compared to MSCs alone. Mechanistically, ACs stimulated MSCs to express COX2 and consequently produced PGE2 that inhibited T cell responses. NF-κB signalling pathway mediated the activation of COX2/PGE2 in AC-MSCs. Importantly, in patients with SLE, the plasma PGEM levels increased significantly in those with reduced apoptotic mononuclear cells in peripheral blood after MSC transplantation.
Interpretation
Clearance of ACs by MSCs contributes to immunosuppressive function via increasing PGE2 production. These findings reveal a previously unrecognized role of MSC-mediated phagocytosis of ACs in MSC-based immunotherapy.
Fund
This study was supported by grants from the Chinese Major International (Regional) Joint Research Project (No. 81720108020), the Jiangsu Province Major Research and Development Program (No. BE2015602) and the Jiangsu Province 333 Talent Grant (BRA2016001). WJ. Chen was supported by the Intramural Research Program of NIH, NIDCR.
Research in context.
Evidence before this study
Accumulated apoptotic cells (ACs), which were observed in patients of systemic lupus erythematosus (SLE), are prone to progress to secondary necrosis, which then expose autoantigens, leading to the breakdown of self-tolerance and tissue damage. Mesenchymal stem cells (MSCs) exhibit promising therapeutic effects on SLE. The direct interactions between MSCs and ACs are barely investigated. Previous studies showed that MSCs could directly engulf ACs, but its role in the treatment of SLE remains to be explored.
Added value of this study
In the present study, we showed that human umbilical cord (UC) MSCs engulfed ACs. MSCs exposed to ACs (AC-MSCs) increased MSC-mediated suppression of CD4+ T cell proliferation compared to MSCs alone. Mechanistically, ACs stimulated MSCs to express cyclooxygenase (COX)2 and consequently produced prostaglandin (PG)E2 that inhibited T cell responses. Further molecular studies revealed that NF-κB mediated the activation of COX2/PGE2 in AC-MSCs. Importantly, in patients with SLE, the plasma PGE2 metabolite levels increased significantly in those with reduced apoptotic mononuclear cells in peripheral blood after MSC transplantation.
Implication of all the available evidence
This study highlight the phagocytosis as a new function of MSCs to clear ACs and induce immunosuppression, and we reveal a previously unrecognized mechanism in MSC-based therapy in SLE.
Alt-text: Unlabelled Box
1. Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with multiple organs affected. The pathogenesis of SLE hinges on loss of immune tolerance, including the over-activation of T and B lymphocytes, secretion of large amounts of inflammatory cytokines and sustained autoantibody production, which is thought to result from the autoantigens released by the excessive post-apoptotic cell remnants [1,2]. Usually, apoptosis is an immunologically quiescent process dependent on normal numbers of apoptotic cells (ACs) and rapid clearance by professional and non-professional phagocytes [3]. Hence, under physiological circumstances, ACs are hardly detectable in healthy subjects. In patients with SLE, however, increased apoptosis was substantially observed and correlated to disease activity [4]. In addition, overload with dying cells in lupus-prone mice accelerated autoimmune disease [5]. The accumulated ACs, which result from imbalanced production and disposal, progress to secondary necrosis and subsequent exposure of autoantigens, which are presented by follicular dendritic cells (DCs) to autoreactive B cells, breaking self-tolerance and finally initiating systemic autoimmunity [4]. Therefore, ACs are at the apex of the cascade of pathogenetic mechanisms in SLE and investigation of approaches targeting ACs helps find novel therapies to ameliorate the disease.
Mesenchymal stem cells (MSCs) are multipotent stem cells that can be isolated from multiple organs or tissues. In addition to self-renewal and multilineage differentiation capacity, MSCs also possess an immunomodulatory function, which makes it a potential kind of cell to treat autoimmune diseases, including SLE [6,7]. Transplantation of MSCs showed safety and beneficial efficacy in both lupus-prone mice and patients with SLE [[8], [9], [10]]. Although mechanism studies revealed that immunosuppression and tolerance induction participated in MSC mediated disease remission [11,12], ACs involved in this process remain yet to be understood, especially in SLE.
In graft-versus-host disease (GvHD), Galleu et al. found that MSCs were actively induced as ACs by recipient cytotoxic cells and that this process was essential to initiate MSC-induced immunosuppression [13]. Coincidentally, a recent study showed that the infused MSCs could be phagocytosed by monocytic cells and then triggered immunomodulation [14]. These studies suggest that the infused MSCs could “be eaten” by phagocytes and thus induce immunosuppression. However, it was reported that MSCs could also directly engulf ACs, instead [15]. In addition, Jiang et al. showed that phagocytosis of ACs by adipose tissue (AT) MSCs contributed to attenuated tissue damage in the experimental vasculitis [16]. Thus, the roles of “being eaten” and “eating others” both appear to be implicated in the immunomodulation mechanisms of MSCs treating different diseases. In this study, we focus on investigating the phagocytic function of MSCs and show that umbilical cord (UC) MSCs produce prostaglandin (PG)E2 upon phagocytosis of ACs, which contributes to MSC-based immunotherapeutic effects of SLE.
2. Materials and methods
2.1. Patients and healthy controls
Twenty-two patients with SLE were recruited in this study. All patients fulfilled the American College of Rheumatology criteria for SLE. Thirteen patients underwent UC MSC transplantation as previously described. Briefly, UC MSCs of passages 2–5 were administered by intravenous infusion (1 × 106/kg of body weight), without adding steroid or other immunosuppressive drugs [9]. Peripheral blood samples of the other nine patients were collected for the induction of ACs and in vitro proliferation experiments. Blood samples from healthy controls (HCs) were obtained from the Medical Examination Centre of Nanjing Drum Tower Hospital. Informed consent was obtained from all the participants. This study was approved by the Ethics Committee at Nanjing Drum Tower Hospital and clinical study of UC MSC transplantation among lupus patients was registered at http://ClinicalTrials.gov (identifier: NCT01741857).
2.2. Mice
Female C57BL/6 (B6) mice were purchased from Model Animal Research Centre of Nanjing University. Mice were housed in a specific pathogen-free environment in the Animal Centre of Nanjing Drum Tower Hospital. To deplete the peritoneal macrophages, B6 mice were injected with 200 μl clodronate liposomes (from Vrije Universiteit Amsterdam) intraperitoneally. Flow cytometry showed that macrophages were almost eliminated completely 24 h after injection (Supplementary Fig. S1). All animal experiment protocols were approved by the Committee of Experimental Animal Administration of Nanjing Drum Tower Hospital.
2.3. Isolation and culture of UC MSCs
The study on human subjects was approved by the Ethics Committee of Nanjing Drum Tower Hospital. UC MSCs were obtained as previously described [17] and cultured with DMEM/F12 supplemented with 10% foetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin (Gibco). MSCs used in this study were within passage 3–6.
2.4. AC induction
Jurkat T cells (ATCC, Cat# TIB-152, RRID: CVCL_0367) and CD4+ T cells isolated from human peripheral blood mononuclear cells (PBMCs) were induced apoptosis by a dose of 0.5 mJ/cm2 UV irradiation and then cultured in RPMI 1640 medium for 6 h and 48 h, respectively. Apoptotic thymocytes were induced by 20 μM dexamethasone (Sigma-Aldrich, Cat#D4902) for 6 h in RPMI 1640 medium. Jurkat T cells were used as the source of ACs except in experiments that were specially indicated. Apoptosis was confirmed by Annexin V staining, as shown by supplementary Fig. S2.
2.5. Phagocytosis assay
For flow cytometry analysis of in vitro phagocytosis, ACs were labelled with pHrodo Green (Invitrogen, Cat#P35369), and added to the MSCs at a ratio of 20:1. After incubation for the indicated time, the cells were extensively washed with cold PBS, trypsinized and re-suspended in cold medium containing 1% NaN3, and then analysed by flow cytometry. APC-anti-human CD105 (eBioscience, Cat#17–1057-42, RRID: AB_1582211) staining were used to distinguish MSCs from unengulfed targets. In some experiments, phagocytosis was blocked by using cytochalasin D (1 μM, Meck Millipore, Cat#250255), pre-treating ACs with 10 μg/ml Annexin V (eBioscience, Cat#BMS306) for 30 min at 37 °C or using a transwell system, with ACs in the upper chamber (with 0.4 μm pores) and MSCs in the lower. For microscopic observation of phagocytosis, eFluor670-labelled ACs and CFSE-labelled MSCs were also co-incubated for 24 h, washed thoroughly with cold RPMI 1640 six times and visualized by Leica TCS SP8 laser scanning confocal microscopy (Leica Biosystems). For in vivo phagocytosis experiments, MSCs and ACs were labelled with eFluor670 (eBioscience, Cat#65–0840-85) and pHrodo Green, respectively. Then, 5 × 107 pHrodo labelled ACs were injected into B6 mice intraperitoneally, with 5 × 106 eFluor670 labelled MSCs infused into the peritoneal cavity subsequently. Mice were sacrificed and peritoneal exudate cells were harvested by peritoneal lavage with 5 ml PBS. The internalization of ACs by MSCs were scored as the percentage of pHrodo and eFluor670 double positive cells by flow cytometry.
2.6. MSC, AC-MSC and AC conditioned medium (MCM, ACMCM and ACM) preparation
MSCs were cultured at a concentration of 1.25 × 104 cells/cm2 in complete DMEM/F12 medium overnight to allow adherence, followed by being incubated with/without ACs at a ratio of 1:20 for 24 h. In some experiments, indomethacin and NS-398 (MCE, Cat#HY-13913) were used in the cocultures of MSCs and ACs to inhibit cyclooxygenase (COX)2 activation. Alternatively, siRNA targeting COX2 (siCOX2, 5’-GCUCCGGACUAGAUGAUAUdTdT-3′) or the negative control (siNC, 5’-UUCUCCGAACGUGUCACGUdTdT-3′) was transfected into MSCs with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Subsequently, unengulfed ACs were removed thoroughly to obtain AC-MSCs by using 0.01%EDTA-0.25%trypsin for 20–30 s to detach adherent ACs and rinsing five times with PBS. Then, the cells were cultured in serum-free RPMI 1640 medium for 24 h and the culture supernatants were collected. Afterward, the culture supernatants were obtained by centrifugation at 3000 g for 10 min and then filtered through 0.22 μm pore filters. For ACM, ACs were cultured at a concentration of 2.5 × 105 cells/cm2 in serum-free RPMI 1640 medium for 24 h and then the culture supernatants were centrifuged and filtered with the same method.
2.7. Lymphocyte proliferation assay
PBMCs were isolated from healthy subjects or SLE patients and labelled with 5 μM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, eBioscience, Cat# 65–0850-84). Labelled PBMCs were cultured with/without MSCs or AC-MSCs at a ratio of 10:1 in the complete RPMI 1640 medium or cultured in the indicated conditioned medium supplemented with 10% FBS, in the presence of 1 μg/ml anti-CD3/CD28 antibodies (RRID: AB_468854/AB_468926). After 4 days, PBMCs were harvested and stained with APC-anti-human CD4 (eBioscience, RRID: AB_1272048) to detect the CFSE dilution using flow cytometry.
2.8. OVA specific T cell response
Ovalbumin (OVA, 4 mg/ml in PBS, Sigma) was emulsified on ice in an equal volume of Freund's Adjuvant Complete (CFA). 50 μl emulsion containing 100 μg OVA was injected subcutaneously into the footpad of the B6 mice. After one week, lymph node cells from the inguinal draining lymph node of OVA-immunized mice were cocultured with MSCs or AC-MSCs at 10:1 ratio, in the presence of OVA (100 μg/ml), for three days. The culture supernatants were collected for the assessment of IFN-γ and IL-17 levels by ELISA. Lymph node cells were incubated with 5-Bromo-2′-Deoxyuridine (BrdU, Invitrogen, Cat#B23151) for 3 h, followed by surface staining with APC-anti-CD4 and fixation and staining with FITC-anti-BrdU (eBioscience, RRID: AB_11042627). The proliferation of CD4+ T cells, as indicated by the incorporation of BrdU, were detected by flow cytometry.
2.9. ELISA and Kynurenine concentration measurement
Mouse interferon (IFN)-γ and interleukin (IL)-17 concentrations, human IL-6, monocyte chemoattractant protein (MCP)-1, transforming growth factor (TGF)-β1, PGE2, hepatocyte growth factor (HGF) and macrophage-colony stimulating factor (M-CSF) levels, as well as PGE2 and PGE2 metabolite (PGEM) levels were measured by using enzyme-linked immunosorbent assay (ELISA) Kits (eBioscience, Biolegend, Raybiotech and Cayman), according to the manufacturer's instructions. Kynurenine concentrations were analysed by high performance liquid chromatography as reported [11].
2.10. Quantitative real-time PCR and Western blot
RNA was isolated from MSCs using a total RNA isolation reagent (Vazyme Biotech), and cDNA was transcribed from RNA with HiScript® II Q RT SuperMix (Vazyme Biotech). The transcript levels of COX1 and COX2 levels were determined by StepOnePlus Real-Time PCR System (Applied Biosystems) with AceQ® qPCR SYBR® Green Master Mix (High ROX Premixed, Vazyme Biotech). Data were analysed using 2-ΔΔCT method, normalizing to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences used were as follows: COX1, forward, 5’-CGCCAGTGAATCCCTGTTGTT-3′ and reverse, 5′-AAGGTGGCATTGACAAACTCC-3′; COX2, forward, 5’-TGACCAGAGCAGGCAGATGAA-3′ and reverse, 5’-CCACAGCATCGATGTCACCATAG-3′; GAPDH, forward, 5’-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5’-TGGTGAAGACGCCAGTGGA-3′
Cell lysates of MSCs were prepared using 1 × radioimmunoprecipitation assay (RIPA) buffer containing 1% 100 × protease/phosphatase inhibitor cocktail (Cell Signaling Technology, CST). Then, the protein samples were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes (Merck Millipore). After blocking with 5% skim milk, the membranes were incubated with primary antibodies including COX2 (Proteintech, Cat#12375-1-AP, RRID: AB_2085127), IκBα and p65 (CST, Cat#4812, RRID: AB_10694416 and Cat# 3034, RRID: AB_330561) and their phosphorylated forms (Affinity, Cat#AF2006 and Cat#AF2002), followed by horseradish peroxidase-conjugated secondary antibody incubation. Anti-GAPDH antibody (Proteintech, Cat# 60004–1-Ig, RRID: AB_2107436) was used as a loading control.
2.11. Statistical analysis
Data were expressed as means ± standard deviation (SD). Analyses were performed using GraphPad Prism (GraphPad Software) using Student's t-test or one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison, as specified. p values <.05 were considered significant difference.
3. Results
3.1. UC MSCs engulf ACs in vitro and in vivo
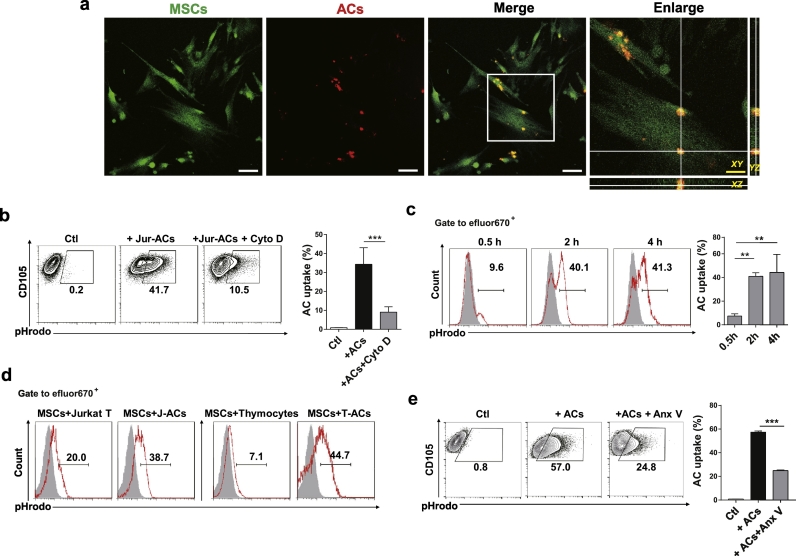
We first studied phagocytosis of ACs by UC MSCs. UC MSCs and Jurkat T cell derived ACs were stained with CFSE and eFluor670, respectively and co-cultured for 24 h. Confocal laser scanning microscopy observation confirmed that UC MSCs were able to engulf ACs (Fig. 1a). To quantify the phagocytosis, we labelled the ACs with a pH-dependent dye (pHrodo Green), whose fluorescence increased within acidic phagolysosomes. Up to 41.7% UC MSCs were detected to engulf ACs in vitro, which was significantly inhibited by cytochalasin D, a classic phagocytosis inhibitor (Fig. 1b). The in vitro phagocytosis of living cells by UC MSCs, however, was hardly detected (Supplementary Fig. S3a). We also found that UC MSCs phagocytosed ACs in both time and dose dependent manners, as longer co-culture time and increased co-culture ratio resulted in enhanced uptake of ACs (Supplementary Fig. S3b). Besides, use of CD4+ T cells from HCs and thymocytes from B6 mice as different origins of ACs resulted in similar degree of phagocytosis by UC MSCs (Supplementary Fig. S3c). To verify UC MSCs engulf ACs in vivo, eFluor670 labelled UC MSCs and pHrodo Green labelled ACs were simultaneously injected into the peritoneal cavity of B6 mice (Supplementary Fig. S3d). As shown in Fig. 1c and d, the in vivo uptake of ACs by UC MSCs achieved the highest proportion as early as 2 h after injection, which was higher than that of living cells. Importantly, AC engulfment by UC MSCs was dependent on phosphatidylserine (PtdSer) recognition, since blockade of PtdSer on ACs with Annexin V markedly inhibited phagocytosis by UC MSCs (Fig. 1e). Collectively, these data demonstrate that UC MSCs engulf ACs both in vitro and in vivo.
Fig. 1.
UC MSCs Engulf ACs in vitro and in vivo. (a) Engulfment of apoptotic cells (ACs) by UC MSCs observed under laser scanning confocal microscopy in all three axes. White scale bar: 75 μm;Yellow scale bar: 30 μm. (b) In vitro AC uptake of UC MSCs (n = 4) by flow cytometry. (c-d) pHrodo labelled living cells or ACs were injected into B6 mice intraperitoneally with eFluor670 labelled UC MSCs. Mice were sacrificed and peritoneal exudate cells were collected. (c) The internalization of ACs by UC MSCs (n = 3) were scored 0.5 h, 2 h and 4 h after injection. (d) The internalization of living cells and ACs by UC MSCs were determined 2 h after injection. (e) Annexin V (Anx V) blocked the engulfment of ACs by UC MSCs (n = 4). Bar graphs show means ± SD. **p < .01, ***p < .001 (One-way ANOVA with Tukey's multiple comparisons test). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Phagocytosis of ACs increases immunosuppressive function of MSCs
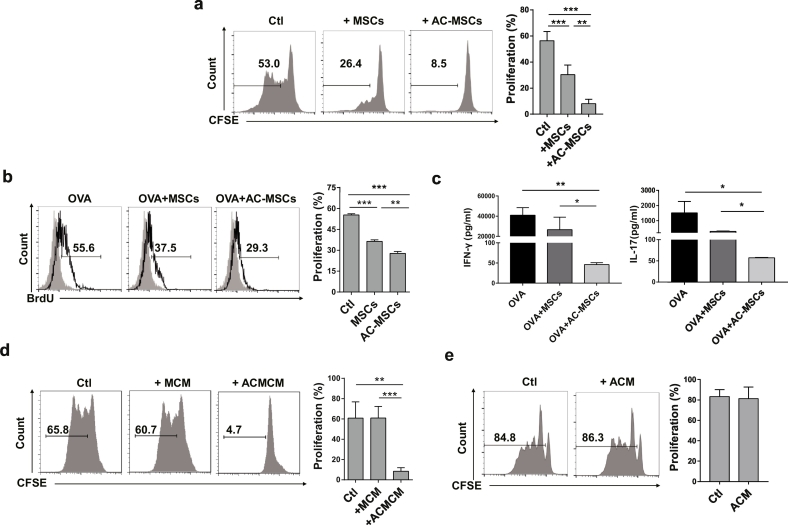
We next investigated whether phagocytosis of ACs affected the immunosuppressive function of MSCs. We determined the inhibition of MSCs and MSCs exposed to ACs (AC-MSCs) on CD4+ T cell proliferation in cultures. AC-MSCs exhibited enhanced suppressive effects on CD4+ T cell proliferation stimulated by anti-CD3 plus anti-CD28 (Fig. 2a). Additionally, the inhibition of antigen specific T cell response by AC-MSCs was stronger than by MSCs, with decreased OVA specific T cell proliferation and reduced secretion of IFN-γ and IL-17 in an immunization model in B6 mice with OVA protein and CFA (Fig. 2b and c).
Fig. 2.
Phagocytosis of ACs Increases Immunosuppressive Function of MSCs. (a) MSCs exposed to ACs (AC-MSCs) showed enhanced suppression on CD4+ T cell proliferation (n = 5). (b-c) Lymph node cells from the inguinal draining lymph node of OVA-immunized mice (n = 3) were cocultured with MSCs or AC-MSCs at 10:1 ratio, in the presence of OVA, for three days. (b) OVA-specific CD4+ T cell proliferation. (c) IFN-γ and IL-17 levels in the coculture supernatants. (d) Conditioned media of AC-MSCs (ACMCM) significantly inhibited CD4+ T cell proliferation (n = 6). (e) Conditioned media of ACs (ACM) could not suppress CD4+ T cell proliferation (n = 7, student's t-test). Bar graphs show means ± SD. *p < .05, **p < .01, ***p < .001 (One-way ANOVA with Tukey's multiple comparisons test).
Next, we determined that the immunosuppressive function of MSCs upon exposure to ACs was mediated by soluble factors secreted by MSCs. We utilized conditioned media from MSCs, AC-MSCs and ACs alone (MCM, ACMCM and ACM) to culture normal human PBMCs respectively. We showed that only ACMCM significantly inhibited T cell proliferation to anti-CD3 and anti-CD28 antibodies (Fig. 2d and e). The T cell inhibitory effects of AC-MSCs was not caused by their accelerated proliferation or altered phenotype, as AC-MSCs showed similar viability and surface markers to untreated control MSCs (Supplementary Fig. S4a and 4b). Taken together, these data suggest that phagocytosis of ACs promots the immunosuppressive function of MSCs by stimulating soluble factor (s) secretion in MSCs.
3.3. Phagocytosis of ACs by MSCs releases PGE2
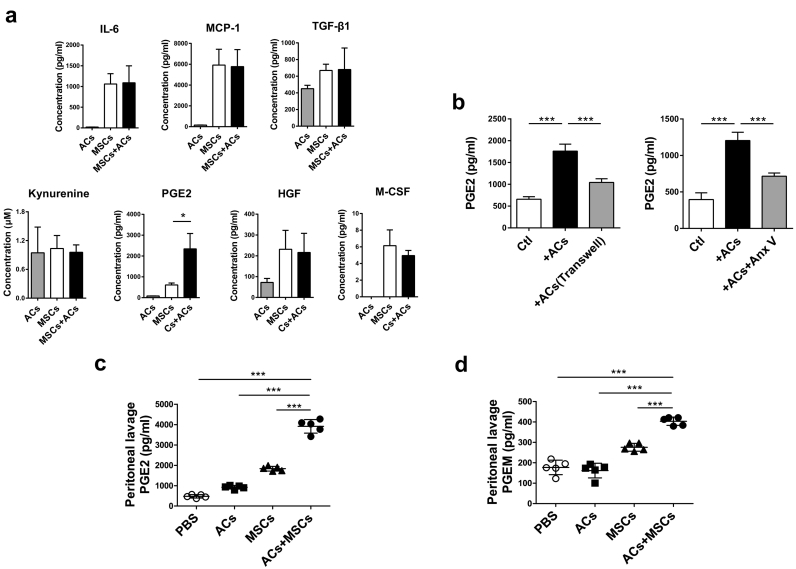
To identify which soluble factor(s) participated in the enhanced immunosuppressive function of MSCs, we examined IL-6, MCP-1, TGF-β1, kynurenine, PGE2, HGF and M-CSF in the coculture supernatants of MSCs exposed to ACs. Notably, only PGE2 was significantly increased in the supernatants of MSC-AC cocultures (Fig. 3a and Supplementary Fig. S5a). Moreover, even if ACs were removed from MSCs after 24 h-coculture, continuous elevation of PGE2 was observed in the culture supernatant of AC-MSCs (Supplementary Fig. S5b). To ascertain whether the increased PGE2 levels were caused by phagocytosis of ACs, we used a transwell system and Annexin V to block the direct contact between MSCs and ACs and phagocytosis, respectively. As shown in Fig. 3b, PGE2 levels were remarkably reduced when the direct interaction was removed and phagocytosis inhibited, indicating that ACs promoted MSCs to secrete PGE2 through direct cell contact and being engulfed. To explore whether in vivo interaction of MSCs and ACs also resulted in the production of PGE2, we administered mice with ACs, MSCs or both ACs and MSCs intraperitoneally. Increased PGE2 concentration in the peritoneal lavage fluid (PLF) was observed in all the three groups (Supplementary Fig. S6). We guessed that the increased PGE2 in mice injected with ACs might be produced by peritoneal macrophages, which could also engulf ACs and release PGE2. To exclude the interference of peritoneal macrophages, we depleted them by pre-treating mice with clodronate liposomes twenty-four hours before injection of ACs, MSCs or both ACs and MSCs. As shown in Fig. 3c, PGE2 in AC-treated group increased slightly, while highest in mice treated with ACs plus MSCs. Since PGE2 was rapidly converted into its metabolites in vivo, we also measured PGE2 metabolite (PGEM) levels in the PLF. We showed that PGEM levels increased in both MSC-treated group and MSC plus AC treated group, but much higher in the latter (Fig. 3d). These data altogether indicate that phagocytosis of ACs lead to increased PGE2 production by MSCs.
Fig. 3.
Phagocytosis of ACs by MSCs Releases PGE2. (a) ACs and MSCs were cultured alone or cocultured together at a ratio of 20:1 for 24 h (n = 4, **P < .01, student's t-test). Related soluble factor levels in the culture supernatants. (b) ACs were cultured in the upper chamber of the transwell system (with 0.4 μm pores), with MSCs in the lower (n = 4). Alternatively, ACs were pre-treated with Annexin V and cocultured with MSCs at a ratio of 20:1 for 24 h (n = 4). PGE2 levels in the culture supernatants were measured. (c-d) B6 mice (n = 5/group) were pre-treated with clodronate liposomes to deplete peritoneal macrophages one day before ACs, MSCs, or ACs plus MSCs (10:1) injection. After 4 h, mice were sacrificed and peritoneal lavage fluid (PLF) was harvested for PGE2 (c) and PGEM (d) concentration assessment. Data were presented as means ± SD. *p < .05, ***p < .001 (One-way ANOVA with Tukey's multiple comparisons test).
3.4. PGE2 contributes to immunosuppression by MSC uptake of ACs
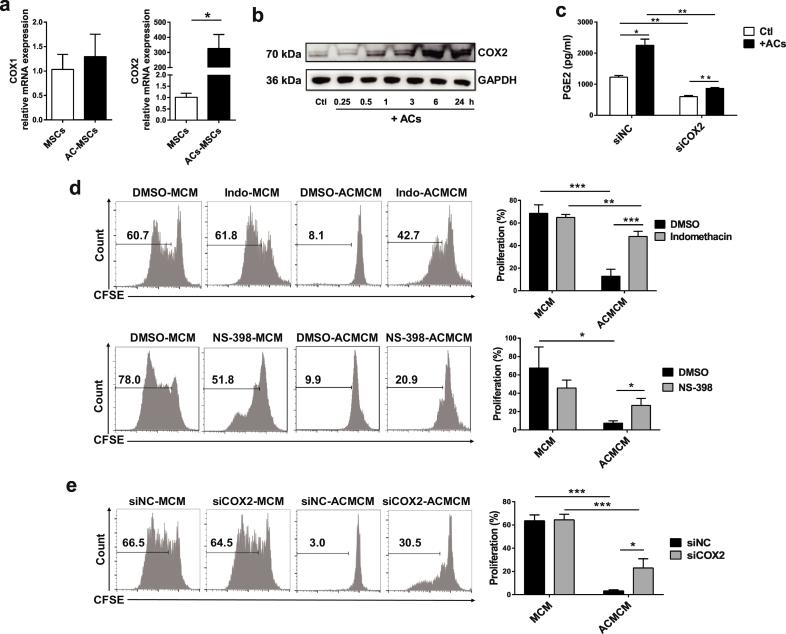
As PGE2 was synthesized by COX1 and COX2, we then determined COX1 and COX2 mRNA expression in AC-MSCs. The expression of COX2, but not COX1 increased compared to MSCs (Fig. 4a). Western blot analysis further confirmed the gradually increased protein level of COX2 in AC-MSCs as time elapsed (Fig. 4b). Moreover, knock-down of COX2 in MSCs with small interfering RNA abolished the increase in PGE2 secreted by AC-MSCs (Fig. 4c). Thus, COX2 drives PGE2 increase in AC-MSCs.
Fig. 4.
PGE2 Contributes to Immunosuppression by MSC Uptake of ACs. (a) Increased COX2 mRNA expression in AC-MSCs 24 h after MSCs and ACs coculture (n = 3). (b) Protein levels of COX2 in MSCs at the indicated time points after coculturing with ACs. (c) COX2 knockdown by siRNA transfection in MSCs decreased PGE2 releasing (n = 3). (d) Indomethacin and NS-398 (dissolved in DMSO) were added into the MSC and AC cocultures and then the conditioned media were prepared. Indomethacin and NS-398 partly reversed the inhibition of CD4+ T cells (n = 4) by the conditioned media of AC-MSCs (ACMCM). (e) MSCs were transfected with siRNA targeted to COX2 (siCOX2) or negative control siRNA (siNC) and then the conditioned media were prepared. COX2 knockdown partially reversed CD4+ T cell suppression (n = 4) by the ACMCM. Bar graphs show means ± SD. *p < .05, **p < .01, ***p < .001 (Student's t-test).
Next, we investigated whether the activation of COX2/PGE2 was involved in the enhanced immunosuppressive function of AC-MSCs. Indomethacin (COX1/2 inhibitor), NS-398 (COX2 specific inhibitor) and siRNA targeted COX2 were added into the MSC and AC cocultures. As shown in Fig. 4d-e, COX2 inhibition or COX2 knock-down significantly reversed the suppression of CD4+ T cell proliferation caused by AC-MSCs. Together, our data reveal that phagocytosis of ACs by MSCs mediated immunosuppression is via the activation of COX2/PGE2 pathway.
3.5. PGE2 production in AC-MSCs is dependent on NF-κB signalling
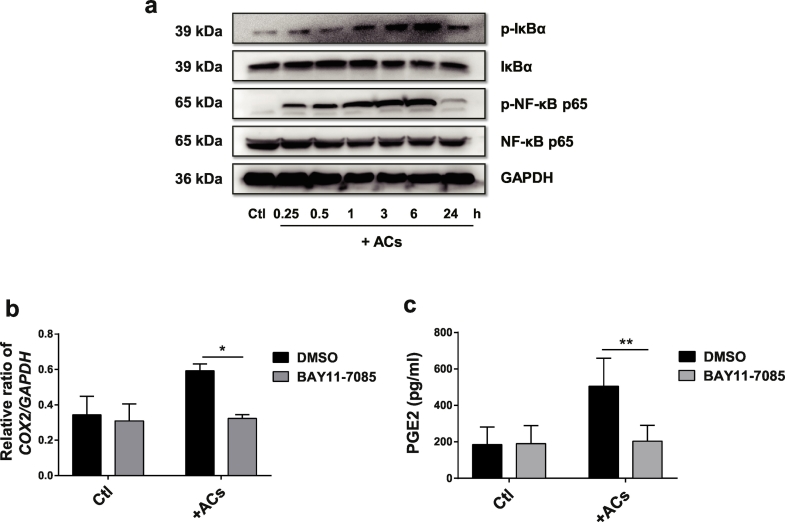
We then focused on the signalling pathways responsible for the activation of COX2/PGE2 in AC-MSCs. Since PGE2 secretion could be upregulated by activation of NF-κB pathway, we assessed whether it was required for the COX2/PGE2 upregulation in AC-MSCs. As revealed in Fig. 5a, phosphorylated (p)-IκB and p-p65 in MSCs were induced by AC treatment. The inhibitor of NF-κB pathway (BAY 11–7085) significantly diminished the expression of COX2 (Fig. 5b) and PGE2 production (Fig. 5c) in AC-MSCs. Therefore, the data indicate that NF-κB signalling pathway participates in the COX2/PGE2 production in AC-MSCs.
Fig. 5.
PGE2 Production in AC-MSCs is Dependent on NF-κB Signalling. (a) MSCs were cocultured with ACs at a ratio of 1:20 for the indicated time. The expressions of IκBα, p65 and their phosphorylated forms in MSCs were analysed by western blot. (b-c) MSCs were cocultured with ACs for 6 h in the presence or absence of BAY 11–7085. (b) The relative ratio of COX2/GAPDH expression in MSCs by western blot analysis (n = 3). (c) PGE2 levels in the culture supernatants (n = 4). Bar graphs show means ± SD. *p < .05, **p < .01 (Student's t-test).
3.6. MSC transplantation increases PGEM in patients with SLE
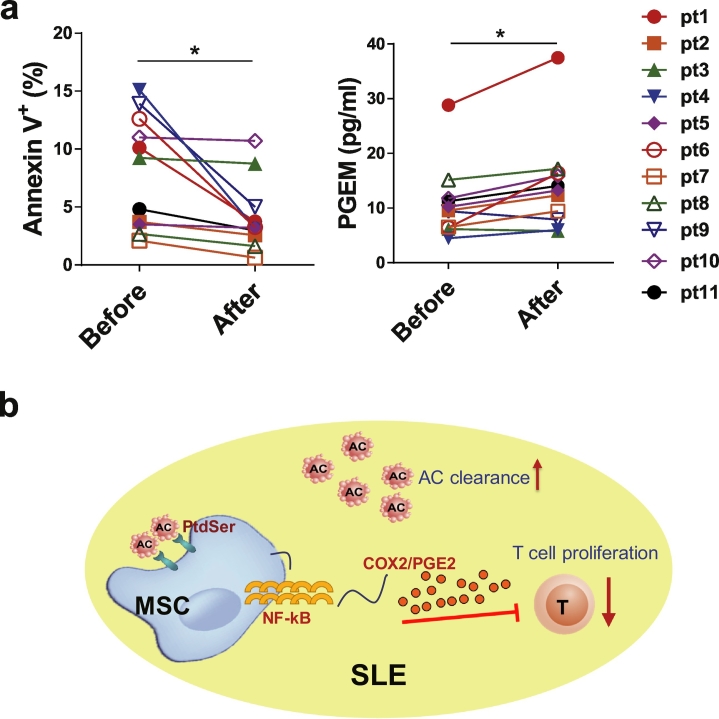
Lastly, we attempted to confirm the aforementioned findings in SLE patients. We measured PGEM levels, which provided a reliable estimate of actual PGE2 production, in the plasma of SLE patients before and 24 h after allogeneic UC MSC infusion. Intriguingly, the plasma PGEM levels increased significantly in eleven patients who were correlated with decreased ACs after MSC transplantation (Fig. 6a). In the other two patients, treatment of MSCs did not reduce the AC levels, and the PGEM level was only slightly increased in one patient and decreased in another (supplementary Fig. S7a). Importantly, MSCs exposed to ACs derived from SLE patients (SLEAC-MSCs) also displayed enhanced inhibition of T cell responses (supplementary Fig. S7b). The data suggest that the infused MSCs might release PGE2 upon clearance of ACs in SLE patients.
Fig. 6.
MSCs Transplantation Increases PGE2 in patients with SLE. (a) Apoptotic levels of PBMCs and plasma levels of PGE2 metabolites (PGEM) in eleven patients with SLE before and after MSC transplantation. (b) Scheme of MSCs promote AC clearance and induced immunosuppression in SLE. *p < .05 (Student's t-test).
4. Discussion
Accumulated ACs are a contributing factor for the exposure of autoantigens to the immune system that eventually leads to autoimmunity like SLE [1,18]. Herein we showed that ACs could be engulfed by UC MSCs and these MSCs exhibited enhanced immunosuppressive function through the activation release of PGE2, which involved NF-κB signalling pathway. Furthermore, increased PGEM was observed in patients with SLE with decreased ACs after MSC transplantation.
In addition to professional phagocytes such as macrophages, non-professional phagocytes, such as epithelial cells, have gained increasing attention for their ability to clear ACs. Although less efficient, nonprofessional phagocytes play a critical role under circumstances that macrophages are scarce or defective [3]. Besides, promoting AC engulfment by non-professional phagocytes has shown benefits in an experimental colitis model [19]. Here we discovered that UC MSCs were also capable of ingesting ACs both in vitro and in vivo. Moreover, PtdSer, the most studied ‘eat me’ signal during phagocytosis, mediated the interaction between UC MSCs and ACs, which further confirmed phagocytosis as a common function of MSCs. Thus, the transplantation of UC MSCs, a novel non-professional phagocytes, might provide an alternative mechanism to promote AC clearance in the treatment of SLE, in which impaired clearance of ACs was observed and contributed to the breakdown of self-tolerance.
Previous studies demonstrate that the immunoregulatory activities of MSCs do not only rely on the characteristics of themselves, but are also largely affected by various factors from the microenvironments [20,21]. In fact, MSCs are silent and cannot suppress immune responses unless they are first activated by the stimuli [22]. In the present study, we found the immunosuppressive effects of MSCs were enhanced after uptake of ACs, then we conceived that the phagocytosis of ACs might act as a stimulator as well to empower immunosuppressive function of MSCs. Interestingly, our data provide an unexpected link between phagocytosis of ACs and MSC immunosuppression. The uptake of ACs activated MSCs to secrete soluble factors to inhibit T cells, and we identified it was PGE2. A previous study showed AC-MSCs exerted an immune supportive role, with the induction of more Th17 cells and increased IL-17 levels [15]. The reasons for the discrepancy remain unknown but might be attributed to the different origins of MSCs used and different culture conditions, which influenced the immunoregulatory capacities.
Various studies have reported the immunomodulatory effects of ACs. The underlying mechanisms depend on not only the interaction of ACs with innate immune cells including dendritic cells and macrophages, but also the induction of regulatory T cells (Treg) and regulatory B cells (Breg) [13,[23], [24], [25]]. AC clearance by professional phagocytes is known to elicit anti-inflammatory mediators, such as TGF-β, PGE2 and IL-10, which dampen immune response and inflammation [4,26,27]. It was reported that bronchial epithelial cells, which were non-professional phagocytes, also produced large amounts of TGF-β and PGE2 upon the ingesting of ACs [28]. Here, we observed a significant increased secretion of PGE2 by MSCs after exposed to ACs. PGE2 has been demonstrated as an important mediator in the immunoregulatory activities of MSCs, including inhibition of T cell proliferation and alteration of T cell and DC differentiation and cytokine production [29,30]. Notably, COX2/PGE2 also mediated the immunosuppressive effects by AC-MSCs. Although indoleamine 2, 3-dioxygenase (IDO), which is always induced by inflammatory cytokines in MSCs, is a well-known and mostly reported factor mediating T cell suppression [11,22,31], we excluded its involvement in phagocytosis activated immunosuppression with the unchanged enzyme activity of IDO in AC-MSCs, indicative of levels of kynurenine, the tryptophan metabolite.
As a small-molecule derivative of arachidonic acid, PGE2 was synthesized by cyclooxygenase including COX1 and COX2. COX1 is constitutively expressed in most cell types and important to maintain homeostasis and normal physiology, while COX2 is inducible and can be activated by a variety of stimuli to promote PGE2 production [32]. In our study, a slightly increase was also observed in the transwell condition that phagocytosis was blocked. Thus, in addition to the phagocytosis, there could be some factors that ACs release acting to stimulate MSCs to produce PGE2, which required further investigation. The signalling pathways of how AC engulfment is linked to the release of anti-inflammatory mediators in professional phagocytes are still unclear. As for MSCs, we found the activation of NF-κB signalling pathway was required for the amplification of COX2/PGE2 when ingesting ACs. Coincidentally, Diaz et al reported that biomechanical forces amplified COX2/PGE2 production by MSCs via the NF-κB signalling axis as well [33]. Despite that the activation of NF-κB pathway usually leads to a pro-inflammatory response, however, in MSCs, this “pro-inflammatory” pathway resulted in anti-inflammatory factor releasing and an inhibition of immune response. Yet specific mechanisms of how AC internalization activate NF-κB pathway to promote COX2/PGE2 production need further investigations, which may involve the participant of danger-associated molecular patterns (DAMPs), such HMGB1 (data not shown).
Lastly, phagocytosis of ACs by MSCs mediated immunosuppression was verified in patients with SLE. ACs derived from SLE patients consistently enhanced the immunosuppressive activity of MSCs. Of importance, a higher PGEM level was observed in patients with reduced ACs after MSC treatment. Although the inflammatory cytokines in SLE microenvironment may contribute to the increased PGEM production [21], yet the role of phagocytosis of ACs in this process should not be ignored, with the closely association of the reduced ACs and increased PGEM levels in patients received MSC transplantation. We also compared the PGEM levels between SLE patients and healthy subjects, but no significant differences were found. The plasma levels of PGEM did not have a correlation to the disease activities among SLE patients (data not shown). Thus, further studies and clinical follow-up are required to clarify whether PGEM could be an indicator to predict the efficacy of MSC treatment in SLE.
In summary, our findings highlight the phagocytosis as a new skill of MSCs to clear ACs and induce immunosuppression, and we reveal a previously unrecognized mechanism in MSC-based therapy in SLE.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by grants from the Chinese Major International (Regional) Joint Research Project (No. 81720108020), the Jiangsu Province Major Research and Development Program (No. BE2015602) and the Jiangsu Province 333 Talent Grant (BRA2016001). WJ. Chen was supported by the Intramural Research Program of NIH, NIDCR. The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
We thank Alexander Cain, NIDCR, NIH for critically reading the manuscript. We also thank Dr. Di Yu, from Australian National University, for his kind suggestion in the revision of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.06.016.
Appendix A. Supplementary data
Supplementary material
References
- 1.Tsokos G.C., Lo M.S., Costa Reis P., Sullivan K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–730. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan A., Herrmann M., Munoz L.E. Clearance deficiency and cell death pathways: A model for the pathogenesis of SLE. Front Immunol. 2016;7:35. doi: 10.3389/fimmu.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arandjelovic S., Ravichandran K.S. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz L.E., Lauber K., Schiller M., Manfredi A.A., Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 5.Shoshan Y., Mevorach D. Accelerated autoimmune disease in MRL/MpJ-Faslpr but not in MRL/MpJ following immunization with high load of syngeneic late apoptotic cells. Autoimmunity. 2004;37:103–109. doi: 10.1080/08916930410001666622. [DOI] [PubMed] [Google Scholar]
- 6.Liang J., Sun L. Mesenchymal stem cells transplantation for systemic lupus erythematosus. Int J Rheum Dis. 2015;18:164–171. doi: 10.1111/1756-185X.12531. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 8.Sun L., Akiyama K., Zhang H., Yamaza T., Hou Y., Zhao S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Zhang H., Liang J., Wang H., Hua B., Feng X. A long-term follow-up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Rep. 2018;10(3):933–941. doi: 10.1016/j.stemcr.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Li J., Zhang Y., Zhang M., Chen J., Li X. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther. 2014;16:R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Feng X., Lu L., Konkel J.E., Zhang H., Chen Z. A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2234–2245. doi: 10.1002/art.38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Huang S., Yuan X., Liang J., Xu R., Yao G. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14:423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam7828. pii:eaam7828. [DOI] [PubMed] [Google Scholar]
- 14.de Witte S.F.H., Luk F., Sierra Parraga J.M., Gargesha M., Merino A., Korevaar S.S. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of msc by monocytic cells. Stem Cells. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 15.Tso G.H., Law H.K., Tu W., Chan G.C., Lau Y.L. Phagocytosis of apoptotic cells modulates mesenchymal stem cells osteogenic differentiation to enhance IL-17 and RANKL expression on CD4+ T cells. Stem Cells. 2010;28:939–954. doi: 10.1002/stem.406. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D., Muschhammer J., Qi Y., Kugler A., de Vries J.C., Saffarzadeh M. Suppression of neutrophil-mediated tissue damage-a novel skill of mesenchymal stem cells. Stem Cells. 2016;34:2393–2406. doi: 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Feng R., Niu L., Huang S., Deng W., Shi B. Human umbilical cord mesenchymal stem cells inhibit T follicular helper cell expansion through the activation of inos in lupus-prone B6.MRL-Fas(lpr) mice. Cell Transplant. 2017;26:1031–1042. doi: 10.3727/096368917X694660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H., Fu S., Zhao M., Lu L., Lu Q. Dysregulation of cell death and its epigenetic mechanisms in systemic lupus erythematosus. Molecules (Basel, Switzerland) 2016;22:E30. doi: 10.3390/molecules22010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C.S., Penberthy K.K., Wheeler K.M., Juncadella I.J., Vandenabeele P., Lysiak J.J. Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity. 2016;44:807–820. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattar P., Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:560. doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo E., Fajka-Boja R., Kriston-Pal E., Hornung A., Makra I., Kudlik G. Licensing by inflammatory cytokines abolishes heterogeneity of immunosuppressive function of mesenchymal stem cell population. Stem Cells Dev. 2015;24:2171–2180. doi: 10.1089/scd.2014.0581. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 23.Kasagi S., Wang D., Zhang P., Zanvit P., Chen H., Zhang D. Combination of apoptotic T cell induction and self-peptide administration for therapy of experimental autoimmune encephalomyelitis. EBioMedicine. 2019 doi: 10.1016/j.ebiom.2019.05.005. pii: S2352–3964(19)30306–8, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notley C.A., Brown M.A., McGovern J.L., Jordan C.K., Ehrenstein M.R. Engulfment of activated apoptotic cells abolishes TGF-beta-mediated immunoregulation via the induction of IL-6. J Immunol. 2015;194:1621–1627. doi: 10.4049/jimmunol.1401256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray M., Miles K., Salter D., Gray D., Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory b cells. Proc Natl Acad Sci U S A. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perruche S., Zhang P., Liu Y., Saas P., Bluestone J.A., Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 27.Kasagi S., Zhang P., Che L., Abbatiello B., Maruyama T., Nakatsukasa H. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci Transl Med. 2014;6:241ra78. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- 28.Juncadella I.J., Kadl A., Sharma A.K., Shim Y.M., Hochreiter-Hufford A., Borish L. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 30.Rozenberg A., Rezk A., Boivin M.N., Darlington P.J., Nyirenda M., Li R. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl Med. 2016;5:1506–1514. doi: 10.5966/sctm.2015-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R., Li X., Zhang Z., Zhou M., Sun Y., Su D. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci Rep. 2015;5 doi: 10.1038/srep12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz M.F., Vaidya A.B., Evans S.M., Lee H.J., Aertker B.M., Alexander A.J. Biomechanical forces promote immune regulatory function of bone marrow mesenchymal stromal cells. 2017;35:1259–1272. doi: 10.1002/stem.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material