Abstract
Purpose
TUNEL assay is the most common, direct test for sperm chromatin integrity assessment. But, lack of standardized protocols makes interlaboratory comparisons impossible. Consequently, clinical thresholds to predict the chance of a clinical pregnancy also vary with the technique adopted. This prospective study was undertaken to assess the incidence of sperm DNA fragmentation in a subfertile population and to establish threshold values of normality as compared to a fertile cohort, both before and after density gradient centrifugation in the total and vital fractions.
Method
Men presenting at a university hospital setup for infertility treatment. DNA damage via TUNEL assay was validated on fresh semen samples, as conventional semen parameters, to reduce variability of results.
Results
Total DNA fragmentation in the neat semen was significantly higher in the subfertile group, but the vital fraction was not significantly different between the two cohorts. After gradient centrifugation, DNA fragmentation increased significantly in the total fraction of the subfertile group but decreased significantly in the vital fraction. In the fertile cohort, there was a non-significant increase in total fragmentation and in the vital fraction the trend was unclear.
Conclusions
Estimating total and vital sperm DNA fragmentation, after density gradient centrifugation, increased both the sensitivity and the specificity, thereby lowering the number of false negatives and false positives encountered. These findings provide opportunities to investigate the significance of the total and the vital fractions after different assisted reproductive technologies.
Keywords: Total sperm DNA fragmentation, Vital sperm DNA fragmentation, TUNEL assay, Neat semen, Density gradient centrifugation, Threshold values
Introduction
Over the last decades significant progress has been made towards the development of reliable tests for sperm chromatin integrity as a useful and potentially independent marker of fertility [1], including sperm chromatin dispersion (SCD) [2], sperm chromatin structure (SCSA) [3], Comet [4, 5], and TUNEL assays [6, 7]. Each test evaluates a different aspect of DNA damage, and due to a lack of standardized protocols, interlaboratory comparisons become impossible.
Tests that measure DNA damage directly without a prior denaturation step, such as the terminal deoxytransferase-mediated deoxyuridine triphosphate (dUTP) nick end-labeling assay (TUNEL), are preferential especially when coupled with flow cytometry as this increases the reproducibility of the test result and the reliability of the test [8]. The conventional version of this assay underestimates DNA damage because terminal transferase cannot adequately penetrate the condensed chromatin in the sperm nucleus. Mitchell et al. [9] resolved this problem by relaxing the chromatin with a reducing agent (dithiothreitol) and, furthermore, refined the methodology by including a vital stain, thereby allowing both DNA integrity and vitality to be simultaneously detected in the same assay. However, their methodology utilized samples which had to be fixed and stored for further analysis, a step which produces a magnitude of variation when compared to fresh samples processed without fixation and storage [10, 11].
Clinical thresholds to predict the chances of sperm populations achieving a clinical pregnancy have been established but also vary with the techniques used [9]. As stated in the recent guidelines of the American Society for Reproductive Medicine, the clinical utility will only be confirmed after standardization of methodology and clinically applicable threshold values, which would be reached by the same criteria [12]. The threshold values of DNA fragmentation, measured by TUNEL, vary between 4 [13] and 36.5% [14]. These TUNEL assays incorporate both the vital and the non-vital cells, thus emphasizing the need to develop robust criteria for assessing the incidence of this damage in the sperm population that are alive, and also establishing thresholds of normality for diagnostic purposes in the same.
Chromatin integrity assays are mainly carried out on neat semen which might be beneficial for diagnostic purposes or have a predictive value on natural conception. For therapeutic purposes, semen of the infertile male should be prepared by selection procedures to obtain maximum number of highly motile and morphologically good forms of spermatozoa. Discrepancies also occur due to differences in the sperm preparation techniques resulting in controversial effects on DNA integrity [15–18]. Whether the results, influenced by sperm selection procedures will influence the predictive potential of medically assisted reproduction success is still a question of debate.
The aims of this study were to assess the incidence of total and vital sperm DNA fragmentation (sDF) in a subfertile population and to establish discriminating threshold values of normality using a standardized TUNEL methodology both before and after density gradient centrifugation.
Materials and methods
Subjects
Two different populations were included:
-
I.
Study group: subfertile population/patients: men presenting at the Centre for Reproductive Medicine, Antwerp University Hospital, Belgium, for infertility diagnosis due to prolonged time to pregnancy (≥ 1 year of subfertility) between January 2014 and December 2015.
-
II.
Control group: fertile men (who achieved pregnancy within 12 months of unprotected coitus) and sperm donors (who had self-fathered children or had achieved pregnancies within the donor program of the clinic).
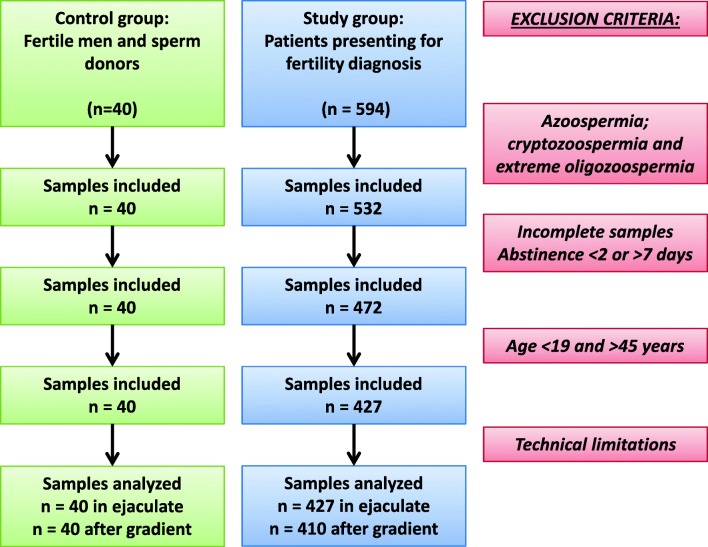
In both, the control and study group, exclusion criteria were based on initial semen analysis (Fig. 1 flow chart). Azoospermic and cryptozoospermic samples were excluded. TUNEL assay via flow cytometry required a minimum of 2 M/ejaculate per run. From the samples included, only complete samples with 2–7 days of abstinence were considered. Age being a confounding factor was held between 19 and 45 years in both groups. Lifestyle parameters and clinical pathologies affecting DNA fragmentation could not be regarded, which is a limitation for the study.
Fig. 1.
Flow diagram illustrating the inclusion and exclusion criteria
All subjects gave written informed consent for participation in this study, and the project was approved by the Institutional Ethical Committee (Ref. 17/24/285).
Semen analysis
Semen samples were collected at the laboratory, weighed to determine volume and the analysis initiated within 30 min after ejaculation conform international standards of ISO 15189 (International Standards Organization, 2012). Standard semen parameters including sperm concentration, motility, and morphology were determined using WHO 2010 [19] recommendations and complying with the checklist for acceptability reported by Björndahl et al. [20].
All staff members were trained in basic semen analysis (ESHRE-European Society for Human Reproduction and Embryology Basic Semen Analysis Courses) [21, 22] and participated regularly in internal and external quality control programs (Institute of Public Health, Belgium and ESHRE External Quality Control Schemes, Sweden) [23].
Sperm processing
A part of the same semen sample was treated with a two-step (40% and 80%) discontinuous density gradient [24] using Puresperm® (Nidacon, International AB, Gothenburg, Sweden). Briefly, 40% and 80% Puresperm® density gradient were prepared using 1.5 ml of each suspension. Semen was layered on the top of each gradient and centrifuged for 15 min at 300g. Subsequently, the upper layer seminal plasma, the 40% upper layer, and the 40–80% interface was discarded, and the remaining spermatozoa in the 80% pellet were collected from the bottom of the tube and washed once with human tubal fluid for 5 min at 300 g (HTF Hepes, Gynotec, Malden, and The Netherlands).
TUNEL assay
Assessment of sDF was performed using the TUNEL assay described by Mitchell et al. [9]. Briefly, spermatozoa were incubated for 30 min at 37 °C with LIVE/DEAD ® Fixable Dead Cell Stain (far red) (Molecular Probes, Life technologies, Oregon, USA) after which the cells were washed 2× with phosphate-buffered saline (PBS, GIBCO Life technologies, Paisley, UK) before being incubated with 2 mM dithiothreitol (DTT, Sigma-Aldrich, Belgium) for 45 min. Following which the samples were washed 2× in PBS and fixed in 3.7% formaldehyde (Sigma-Aldrich, Belgium) for 20 min at 4 °C. As we have previously shown that storage of the sample at 4 °C affects reproducibility [10], the assay was carried out directly after fixation but, without storage in 0.1 M glycine. For the assay, the spermatozoa were washed 2× and centrifuged before being resuspended in 500 μl of fresh permeabilization solution (100 mg sodium citrate, 100 μl Triton X–100 in 100 ml dH2O) and incubated for 5 min at 4 °C. The cells were washed 2× with PBS. The positive control samples were treated with 5 μl of DNase I (Qiagen, Germany) 1500 Kunitz Units for 30 min at room temperature. The assay was performed using the fluorescein In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany) using Accuri C6 flow cytometer (BD Sciences, Erembodegem, Belgium). For each sample, 5000–10,000 events were recorded at a flow rate of 35 μl/min.
For diagnostic purposes, the test was done on neat semen and for therapeutic purposes after density gradient centrifugation. DNA fragmentation was analyzed and the results are presented as
Total sDF: percentage of the entire sperm population that was positive for DNA fragmentation.
Vital sDF: percentage of the entire sperm population that was alive and positive for DNA fragmentation.
Statistical analysis
Statistical analyses were conducted using Medcalc® version 13.0.6.0 and SPSS version 21. Between-group comparisons were conducted using a non-parametric test (Mann-Whitney U). The difference in the parameters evaluated before and after density gradient centrifugation was tested by means of the Wilcoxon signed rank test. Comparisons of the data distributions were conducted by constructing receiver operating characteristic (ROC) curve analysis. Twenty-eight fertile and 322 patients were required to detect an area under a ROC curve of 0.66 compared to a null hypothesis value of 0.5 taking into account the required significance level (type I error, α-level = 0.05) and power of the test (type II, β-level = 0.20). Threshold criteria were determined using Youden J index. For all statistical tests, differences with a P value < 0.05 were considered significant.
Results
Semen parameters in the control and study groups
Four hundred and twenty-seven patient samples and 40 control samples were analyzed in our study. Out of 427 samples, 57.7% had normal semen values, 21.7% had one, 13.8% had two, and 6.8% showed all three semen abnormalities. All mean semen parameters (sperm concentration, motility, and morphology) were well within the normal levels in the study group but significantly lower as compared to the control group (Table 1).
Table 1.
Semen parameters and sDF in the control and study groups
| Study group (n = 427) | Control group (n = 40) | P value | |
|---|---|---|---|
| Sperm concentration (M/ml) | 51.5 ± 49.9 (0.1–313.1) | 82.0 ± 53.4 (22.8–263.8) | p < 0.0001 |
| Total sperm count (M/ejaculate) | 189.7 ± 184.7 (0.2–1372.8) | 286.9 ± 211.0 (59.2–874.9) | p = 0.0006 |
| Progressive motility (%) | 47.4 ± 16.9 (0–77) | 57.8 ± 9.2 (34–74) | p = 0.0001 |
| Total motility (%) | 57.2 ± 17.0 (0–81) | 66.6 ± 8.7 (46–82) | p = 0.0005 |
| Sperm morphology (%) | 5.3 ± 3.8 (0–16) | 7.9 ± 4.3 (1–22) | p = 0.0002 |
| Total sDF in ejaculate (%) | 14.2 ± 9.8a (0.0–56.0) | 11.3 ± 9.2 (1.0–55.0) | p = 0.0241 |
| Vital sDF in ejaculate (%) | 1.9 ± 2.6b (0.0–22.0) | 1.5 ± 1.7 (0.0–7.0) | p = 0.5684 |
| Total sDF after gradient (%) | 20.7 ± 15.6a (2.0–90.0) | 14.4 ± 15.0 (1.0–70.0) | p = 0.0013 |
| Vital sDF after gradient (%) | 1.4 ± 1.6b (0.0–10.0) | 1.6 ± 3.6 (0.0–19.0) | p = 0.0972 |
Mean ± SD (range); n = number of observations; sDF = sperm DNA fragmentation
aStatistical significance between the total sDF in ejaculate and after gradient p < 0.0001
bStatistical significance between vital sDF in ejaculate and after gradient p = 0.0001
Total sDF: percentage of the entire sperm population that was positive for DNA fragmentation
Vital sDF: percentage of the entire sperm population that was alive and positive for DNA fragmentation
sDF in the control and study groups both before and after gradient centrifugation
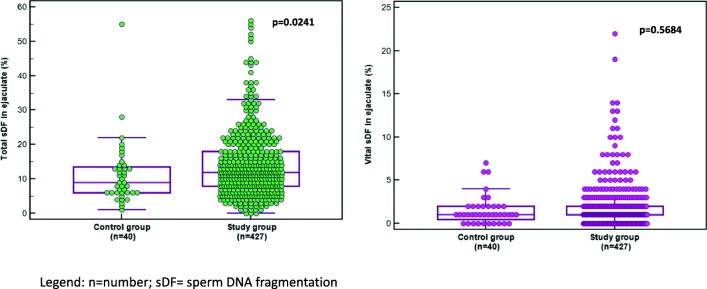
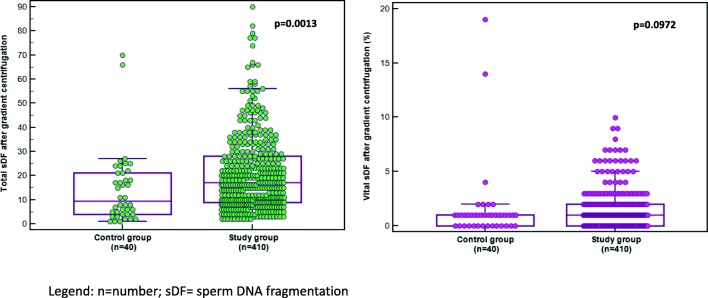
Total sDF was significantly (Table 1 and Fig. 2) higher in the study group as compared to the control cohort (14.2 ± 9.8% and 11.3 ± 9.2%, respectively). DNA fragmentation in the vital fraction (Fig. 2) in the neat semen was not significantly different between the two cohorts (1.9 ± 2.6% and 1.5 ± 1.7%, respectively). In the subfertile group, 410/427 (96.0%) samples were analyzed after density gradient centrifugation. The total sDF increased significantly (p < 0.0001) after density gradient centrifugation from 14.2 ± 9.8 to 20.7 ± 15.6%, respectively (Table 1 and Fig. 3).
Fig. 2.
Total and vital sDF in the ejaculate in the control and study groups. n = number; sDF = sperm DNA fragmentation
Fig. 3.
Total and vital sDF after gradient centrifugation in the control and study groups. n = number; sDF = sperm DNA fragmentation
In the control group, there was a non-significant (p = 0.0907) increase in total sDF. On the other hand, in the vital fraction, the percentage damage was significantly decreased in the patient group (p = 0.0001) while in the controls, the difference between the neat semen and after processing was not significant (p = 0.0961).
Threshold values before and after gradient centrifugation
The area under the curve (AUC) gives an indication of the ability to discriminate two independent populations (Table 2). If a threshold of ≥ 14% was used as a selection criterion, then 42.9% of the patient cohort exceeded this value, as compared to the 25.0% of the fertile cohort. After gradient centrifugation, the Youden threshold value of ≥ 9% was captured by 77.8% of the patient group but also by 50.0% of the fertile group, emphasizing that both the cohorts reacted in a similar way to DNA damage after processing. In the vital fraction, the threshold of ≥ 3% was observed in 20.6% of the ejaculates of the subfertile group but also in 15.0% of the fertile cohort. After gradient centrifugation, the threshold criterion of ≥ 2% was observed in 32.2% of the patient group and in 17.5% of the fertile cohort. Estimating both the total and vital sDF after density gradient increased both the sensitivity and the specificity, thereby lowering the number of false negatives and false positives encountered.
Table 2.
Predictive properties of sDF by receiver operating curve analysis in the various fractions analyzed
| Fractions | AUC | Associated criterion | Sensitivity | Specificity | PPV | NPV | p value |
|---|---|---|---|---|---|---|---|
| Total sDF in ejaculate | 0.608 | ≤ 13 | 42.9 | 75.0 | 94.8 | 10.9 | 0.0243 |
| Vital sDF in ejaculate | 0.526 | ≤ 2 | 20.6 | 85.0 | 93.6 | 9.1 | 0.5847 |
| Total sDF after gradient | 0.653 | ≤ 8 | 77.8 | 50.0 | 94.1 | 18.0 | 0.0014 |
| Vital sDF after gradient | 0.575 | ≤ 1 | 32.2 | 82.5 | 95.0 | 10.6 | 0.1160 |
sDF sperm DNA fragmentation, AUC area under the curve, PPV positive predictive value, NPV negative predictive value
Total sDF: percentage of the entire sperm population that was positive for DNA fragmentation
Vital sDF: percentage of the entire sperm population that were alive and positive for DNA fragmentation
Discussion
Although significant data are available to suggest that higher levels of DNA damage are an indication of a potentially negative impact on both natural and assisted conception outcomes [25], there is scarce information of this damage in the vital spermatozoa. The data generated in this study provided the first insights into the size of the patient and fertile population exhibiting vital sDF both in the neat semen and after semen processing.
It has been postulated that fertile men with normal semen parameters have almost uniformly low levels of DNA breakage, whereas infertile men, especially those with compromised semen parameters, have increased proportions of nicks and breaks in the chromatin [26, 27]. In our previous study [10], no differences in the levels of total sDF were observed between WHO normal and subnormal samples suggesting that sperm DNA damage may be one of the factors related to unexplained male infertility, especially in normozoospermia [28, 29]. In the present study, although the mean semen parameters in the subfertile group were well above the lower reference limits of the WHO manual [19], they were significantly lower than the means observed in the fertile group. Semen analysis alone provides important information on male fertility [30], but is less reliable in distinguishing between fertile and subfertile men. Although clinical prediction models of natural conception have been designed, including semen parameters [31, 32], there is clear evidence that infertile men possess substantially more sperm DNA damage than fertile men [4, 27, 33, 34]. Many studies have also demonstrated its negative impact on medically assisted reproduction outcome [35–38] and on the health of offspring in animal models [39]. We confirm that total sDF was significantly higher in the study group, but the vital fraction showed no significant differences between the two cohorts. Aitken et al. [40] observed, in a small group of patients (n = 50), that ~ 5% of live cells were TUNEL positive. Our values in a much larger patient population are lower but the ranges indicate that there might be situations which might lead to detrimental effects on male infertility. Moreover, the vital fraction is significantly negatively affected by total count, immature germ cells, and peroxidase positive leucocytes [10]. Testicular insufficiency and idiopathic oligozoospermia, presumably associated with abnormal spermatogenesis, showed the highest mean levels of DNA fragmentation index [41]. While leukocytes and immature/abnormal spermatozoa are major culprits responsible for lowering the antioxidant capacity of human semen [42–44], the large range of damage in the vital fraction might suggest that sDF be considered as an independent attribute of semen quality for all infertility patients, detecting problems not seen with semen analysis alone.
Although studied intensively, sDF remains controversial and even confusing depending on the detection method [45, 46]. Uncontrolled confounding, heterogeneity and a potential selection bias are of concern in the majority of evidence and are the reasons for the disparity of results [12]. While using the TUNEL assay, fixation, and storage of samples, lack of a reducing agent to decompact the chromatin and different protocols for labeling DNA breaks produces a large heterogeneity in the values for sDF. The reported amounts for sDF in similar groups of subfertile men varied from 11.1 ± 8.00% (n = 140) [47] through 29.5 ± 18.7% (n = 194) [48] to 40.9 ± 14.3% (n = 66) [49]. Even while using DTT to relax the chromatin, Aitken et al. [50] revealed high TUNEL signals in the study population (n = 50; 34.3 ± 2.5%) due to sample storage. Consequently, our findings may be compared only partially to other studies and with caution. Fertile populations, on the other hand, are distinctly different from subfertile men in that they are enriched with good quality samples expressing low levels of damage. Irrespective of the methodological variations, our values for total sDF 11.3 ± 9.2 agree with that found in literature [48, 49] (11.9 ± 6.8% and 13.1 ± 7.3%, respectively). Although, again much lower than those reported by Aitken et al. [50] using the same method (30.9 ± 1.7%).
For therapeutic purposes, semen is processed via density gradient centrifugation to select spermatozoa with better morphology, motility [24], and chromatin maturity [51]. Whether density gradient increases or decreases sDF is currently unclear. Induction of DNA damage after semen preparation has been observed previously on independent occasions [15, 52–54] although not consistent with the findings of others [50, 55, 56]. These studies have concentrated on percentage damage of the entire sperm population. Our study is unique, in using a vital dye which covalently labels intracellular amines in non-viable cells allowing assessment of DNA damage and cell viability simultaneously.
In our study, we observed an increase in the damage in the total fraction in both cohorts, but a concomitant decrease after processing in the vital fraction only in the patient population. When focusing on viable spermatozoa, Aitken et al. [50] observed a significant increase in DNA fragmentation in their patient population, but in their cohort of semen donors, there was no significant effect of density gradient on the vital TUNEL signals. They proposed that the shearing forces generated at the sperm surface during centrifugation trigger free radical generation and oxidative DNA damage. In a later study [52], the same group of authors, using the same methodology, on a cohort of semen donor samples, found significantly elevated oxidative damage using PureSperm® for density gradients. This induction of DNA damage, according to the authors, in the total fraction was not associated with increased ROS generation, but due to elevated levels of metals present in commercial media that are known to precipitate sperm DNA damage. Surprisingly, the effect on the vital fraction after density gradient was omitted. According to Muratori et al. [57], the higher sDF observed after density gradient centrifugation is due to the induction of a de novo DNA damage during the procedure. However, only in 50% of the samples the authors noted an increase in DNA damage indicating that metal contamination of gradients is not sufficient to induce the damage. They hypothesize that the concomitant presence of intrinsic features (such as defects in sperm chromatin maturation) or of other sperm abnormalities (such as lower sperm defenses to oxidative attack or high levels of ROS in semen) render the sample more susceptible to the noxious agents. In our study, since this iatrogenically induced oxidative DNA damage was observed much more in the total fraction of both cohorts, it could be potentially attributed to the presence of transition metals in sperm preparation media. In the vital fraction, there was a significant decrease in damage, probably due to the capacity of density gradient centrifugation to blunt the amount of immature germ cells and leukocytes thereby reducing oxidative damage in the vital fraction.
In establishing the cut-off or normal threshold values, it is important to consider whether a test is to be used as a screening/diagnostic test or as a predictor of an established endpoint [48]. High sensitivity is important for a screening/diagnostic test so that it can be offered to a larger population. However, specificity becomes critical if a test is to be offered as a predictive marker of a defined endpoint. Our results of total and vital sDF in the neat semen show a high specificity, thus ruling in those patients with the defect. Parallel testing of the total and vital sDF after density gradient increased both the sensitivity and the specificity, quantifying the avoiding of both false negatives and positives, simultaneously.
In a meta-analysis, Zini et al. [34] observed that a clinically relevant cut-off level was not used (i.e., the authors did not establish a normal range based on the evaluation of a fertile population). In these studies, the cut-off was selected based on previously reported cut-offs [34], the median value for the study population [58], or receiver-operating characteristics curves [59]. Our results (≤ 13% total sDF) for the neat semen are comparable to the threshold values of 12% [60]. Sharma et al. [48] reported a threshold value of 19.25% for TUNEL, but these values were reduced to 16.8% [61] when the same group used a bench top flow cytometer. The associated criterion for the vital fraction was low (≤ 2%) and not significant. In the fertile cohort, 25.0% exceeded the total sDF value and 15.0% the vital SDF value proving that the breaks in the male genome may be repaired by the oocyte [62] to a certain extent. Muratori et al. [57] found that the pre-density gradient values for sDF were not different between infertile couples achieving or not achieving pregnancy. On the other hand, post-density gradient values were more evident, approaching significance after adjustment for male and female age (after IVF). From our study it is evident that density gradient affects total sDF but selects a better vital fraction with reduced sDF in the subfertile group after processing. We will not be able to prove the impact of this selection in our fertile cohort; however, in a subfertile population, the thresholds of normality obtained both before and after sperm preparation can be tested in future after different medically assisted reproductive technologies.
Acknowledgments
The authors acknowledge Christine Croes, Els Smet, Julie Biltjes, and Zehra Kara for preparing the sperm samples.
Authors’ contributions
U.P. substantial contributions to conception, acquisition of data, analysis and interpretation of data, drafting the article. D.D.N. revising it critically for important intellectual content. H.V.M. and I.G. conducted the DNA damage analyses. E.R. was involved in statistical analysis and interpretation of data. All authors revised and approved the final version of the manuscript.
Funding
This work was supported by funds from the Centre for Reproductive Medicine, Antwerp University Hospital and by the Fonds Wetenschappelijk Onderzoek (FWO), Research Foundation Flanders (grant number T007016N).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evenson DP, Darzynkiewicz Z, Melamed MR. Realation of mammalian sperm chromatin heterogeneity to fertility. Sci. 1980;210:1131–1133. doi: 10.1126/science.7444440. [DOI] [PubMed] [Google Scholar]
- 2.Muriel L, Garrido N, Fernández JL, Remohí J, Pellicer A, de los Santos MJ, Meseguer M. Value of the sperm DNA fragmentation level, measured by the sperm chromatin dispersion (SCD) test, in the IVF and ICSI outcome. Fertil Steril. 2006;85:371–383. doi: 10.1016/j.fertnstert.2005.07.1327. [DOI] [PubMed] [Google Scholar]
- 3.Evenson DP, Kasperson K, Wixon R. Analysis of sperm DNA fragmentation using flow cytometer and other techniques. Soc Reprod Fertil (Suppl) 2007;65:93–113. [PubMed] [Google Scholar]
- 4.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 5.Lewis SE, Agbaje IM. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis. 2008;23:163–170. doi: 10.1093/mutage/gem052. [DOI] [PubMed] [Google Scholar]
- 6.Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56:602–607. doi: 10.1095/biolreprod56.3.602. [DOI] [PubMed] [Google Scholar]
- 7.Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27:53–59. doi: 10.2164/jandrol.05068. [DOI] [PubMed] [Google Scholar]
- 8.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2010;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi U, Van Mulders H, Goovaerts I, Peeters K, Clasen K, Janssens P, Zemstova O, De Neubourg D. Sperm DNA fragmentation in the total and vital fractions before and after density gradient centrifugation: significance in male fertility diagnosis. Clin Biochem. 2018;62:47–54. doi: 10.1016/j.clinbiochem.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Curi SM, Chenlo PH, Billordo LA, Baz P, Sardi ML, Ariagno JI, Repetto H, Mendeluk GR, Pugliese MN. Flow cytometry TUNEL standardization for assaying Sperm DNA Fragmentation. J Androl. 2012;33:1. doi: 10.2164/jandrol.110.011734. [DOI] [PubMed] [Google Scholar]
- 12.Practice Committee of the American Society for Reproductive medicine The clinical utility of sperm DNA integrity testing a guideline. Fertil Steril. 2013;99:673–677. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Huang CC, Lin DPC, Tsao HM, Cheng TC, Liu CH, Lee MS. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril. 2005;84:130–140. doi: 10.1016/j.fertnstert.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, Gips H, Schill WB, Kruger TF. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–972. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 15.Zini A, Mak V, Phang D, Jarvi K. Potential adverse effect of semen processing on human sperm deoxyribonucleic acid integrity. Fertil Steril. 1999;72:496–499. doi: 10.1016/S0015-0282(99)00295-2. [DOI] [PubMed] [Google Scholar]
- 16.Zini A, Nam RK, Mak V, Phang D, Jarvi K. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril. 2000;74:824–827. doi: 10.1016/S0015-0282(00)01495-3. [DOI] [PubMed] [Google Scholar]
- 17.Gandini L, Lombardo F, Paoli D, Caruso F, Eleuteri P, Leter G, Ciriminna R, Culasso F, Dondero F, Lenzi A, Spanò M. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19:1409–1417. doi: 10.1093/humrep/deh233. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RE, Bormann CL, Hassun PA, Rocha AM, Motta ELA, Serafini PC, Smit GD. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril. 2010;94:2626–2630. doi: 10.1016/j.fertnstert.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Laboratory manual for the examination of human semen and semen-cervical mucus interaction. 5. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 20.Björndahl L, Barratt CL, Mortimer D, Jouannet P. ‘How to count sperm properly’: checklist for acceptability of studies based on human semen analysis. Hum Reprod. 2016;31:227–232. doi: 10.1093/humrep/dev305. [DOI] [PubMed] [Google Scholar]
- 21.Punjabi U, Spiessens C. Basic semen analysis courses: experience in Belgium. In: Ombelet W, Bosmans E, Vandeput H, Vereecken A, Renier M, Hoomans E, editors. Modern ART in the 2000’s – andrology in the nineties. London: The Parthenon Publishing Group; 1998. pp. 107–113. [Google Scholar]
- 22.Björndahl L, Barratt CL, Fraser LR, Kvist U, Mortimer D. ESHRE basic semen analysis courses 1995–1999: immediate beneficial effects of standardized training. Hum Reprod. 2002;17:1299–1305. doi: 10.1093/humrep/17.5.1299. [DOI] [PubMed] [Google Scholar]
- 23.Punjabi U, Wyns C, Mahmoud A, Vernelen K, China B, Verheyen G. Fifteen years of Belgian experience with external quality assessment of semen analysis. Andrology. 2016;13:1–10. doi: 10.1111/andr.12230. [DOI] [PubMed] [Google Scholar]
- 24.Punjabi U, Gerris J, Van Bijlen J, Delbeke L, Buytaert P. Comparison between different pre-treatment techniques for sperm recovery prior to IUI-GIFT-IVF. Hum Reprod. 1990;5:75–83. doi: 10.1093/oxfordjournals.humrep.a137046. [DOI] [PubMed] [Google Scholar]
- 25.Barratt CL, Aitken RJ, Björndahl L, Carrell DT, de Boer P, Kvist U, Lewis SE, Perreault SD, Perry MJ, Ramos L, Robaire B, Ward S, Zini A. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications – a position report. Hum Reprod. 2010;25:824–838. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, Sakkas D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 2001;16:2160–2165. doi: 10.1093/humrep/16.10.2160. [DOI] [PubMed] [Google Scholar]
- 27.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75:674–677. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 28.Oleszczuk K, Augustinsson L, Bayat N, Giwercman A, Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology. 2013;1:357–360. doi: 10.1111/j.2047-2927.2012.00041.x. [DOI] [PubMed] [Google Scholar]
- 29.Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril. 2014;101:58–63. doi: 10.1016/j.fertnstert.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, Xu D, Vogel DL, National Cooperative Reproductive Medicine Network Sperm morphology, motility and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 31.Hunault CC, Laven JS, van Rooij IA, Eijkemans MJ, te Velde ER, Habbema JD. Prospective validation of two models predicting pregnancy leading to live birth among untreated subfertile couples. Hum Reprod. 2005;20:1636–1641. doi: 10.1093/humrep/deh821. [DOI] [PubMed] [Google Scholar]
- 32.Van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Broekmans FJ, van Dessel HJ, Bossuyt PM, van der Veen F, Mol BW, CECERM study group (collaborative effort for clinical evaluation in reproductive medicine) Pregnancy is predictable: a large-scale prospective external validation of the prediction of spontaneous pregnancy in subfertile couples. Hum Reprod. 2007;22:536–542. doi: 10.1093/humrep/del378. [DOI] [PubMed] [Google Scholar]
- 33.Spanò M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Dannish first pregnancy planner study team. Fertil Steril. 2000;73:43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 34.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–2668. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 35.Tarozzi N, Bizzaro D, Flamigni C, Borini A. Clinical relevance of sperm DNA damage in assisted reproduction. Reprod BioMed Online. 2007;14:746–757. doi: 10.1016/S1472-6483(10)60678-5. [DOI] [PubMed] [Google Scholar]
- 36.Tamburrino L, Marchiani S, Montoya M, Elia Marino F, Natali I, Cambi M, Forti G, Baldi E, Muratori M. Mechanisms and clinical correlates of sperm DNA damage. Asian J Androl. 2012;14:24–31. doi: 10.1038/aja.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonuclei acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: asystematic review and meta-analysis. Reprod BioMed Online. 2015;30:120–127. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, Bilbao A, Bermejo-Alvarez P, de Dios HJ, de Fonseca FR, Gutiérrez-Adán A. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–772. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]
- 40.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 41.Smit M, Romijn JC, Wildhagen MF, Weber RF, Dohle GR. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril. 2010;94:1748–1752. doi: 10.1016/j.fertnstert.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 42.Aitken RJ, West KM. Analysis of the relationship between reactive oxygen species production and leucocyte infiltration in fractions of human semen separated on Percoll gradients. Int J Androl. 1990;13:433–451. doi: 10.1111/j.1365-2605.1990.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 43.Aitken RJ, West K, Buchkingham D. Leukocytic infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J Androl. 1994;15:343–352. [PubMed] [Google Scholar]
- 44.Aitken RJ, Baker HW. Seminal leukocytes: passengers, terrorists or good Samaritans? Hum Reprod. 1995;10:1736–1739. doi: 10.1093/oxfordjournals.humrep.a136165. [DOI] [PubMed] [Google Scholar]
- 45.Voncina SM, Golob B, Ihan A, Kopitar AN, Kolbezen M, Zorn B. Sperm DNA fragmentation and mitochondrial membrane potential combined are better for predicting natural conception than standard sperm parameters. Fertil Steril. 2016;103:637–644. doi: 10.1016/j.fertnstert.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro S, Muratori M, De Geyter M, De Geyter C. TUNEL labeling with BrdUTP/anti-BrdUTP greatly underestimates the level of sperm DNA fragmentation in semen evaluation. PLoS One. 2017;12(8):1–18. doi: 10.1371/journal.pone.0181802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muratori M, Piomboni P, Baldi E, Filimberti E, Pecchioli P, Moretti E, Gambera L, Baccetti B, Biagiotti R, Forti G, Maggi M. Functional and ultrastructural features of DNA-fragmented human sperm. J Androl. 2000;21:903–912. [PubMed] [Google Scholar]
- 48.Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, Agarwal A. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76:1380–1386. doi: 10.1016/j.urology.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 49.Sergerie M, Laforest G, Bujan L, Bissonnette F, Bleau G. Sperm DNA fragmentation: threshold value in male fertility. Hum Reprod. 2005;20:3446–3451. doi: 10.1093/humrep/dei231. [DOI] [PubMed] [Google Scholar]
- 50.Aitken RJ, De Luliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25:2415–2426. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 51.Sellami A, Chakroun N, Ben Zarrouk S, Sellami H, Kebaili S, Rebai T, Keskes L. Assessment of chromatin maturity in human spermatozoa: useful aniline blue assay for routine diagnosis of male infertility. Ther Adv Urol. 2013;2013:578631. doi: 10.1155/2013/578631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fariello RM, Del Giudice PT, Spaine DM, Fraietta R, Bertolla RP, Cedenho AP. Effect of leukocytospermia and processing by discontinuous density gradient on sperm nuclear DNA fragmentation and mitochondrial activity. J Assist Reprod Genet. 2009;26(2–3):151–157. doi: 10.1007/s10815-008-9288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brahem S, Mehdi M, Elghezal H, Saad A. Semen processing by density gradient centrifugation is useful in selecting sperm with higher double-strand DNA integrity. Andrologia. 2011;43(3):196–202. doi: 10.1111/j.1439-0272.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 54.Aitken RJ, Finnie JM, Muscio L, Whiting S, Connaughton HS, Kuczera L, Rothkirch TB, De Luliis GN. Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum Reprod. 2014;29:2136–2147. doi: 10.1093/humrep/deu204. [DOI] [PubMed] [Google Scholar]
- 55.Donnelly ET, McClure N, Lewis SE. Antioxidant supplementation in vitro does not improve human sperm motility. Fertil Steril. 1999;72:484–495. doi: 10.1016/S0015-0282(99)00267-8. [DOI] [PubMed] [Google Scholar]
- 56.Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, Bianchi U. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod. 2000;15:1112–1116. doi: 10.1093/humrep/15.5.1112. [DOI] [PubMed] [Google Scholar]
- 57.Muratori M, Tarozzi N, Cambi M, Boni L, Iorio AL, Passaro C, Luppino B, Nadalini M, Marchiani S, Tamburrino L, et al. (2016) Med 95:1–9. [DOI] [PMC free article] [PubMed]
- 58.Frydman N, Prisant N, Hesters L, Frydman R, Tachdjian G, Cohen-Bacrie P, Fanchin R. Adequate ovarian follicular status does not prevent the decrease in pregnancy rates associated with high sperm DNA fragmentation. Fertil Steril. 2008;89:92–97. doi: 10.1016/j.fertnstert.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 59.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Francois’ Guerin J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 60.Agarwal A, Guta S, Sharma R. Measurement of DNA fragmentation in spermatozoa by TUNEL assay using bench top flow cytometer. In: Agarwal A, Guta S, Sharma R, editors. Andrological evaluation of male infertility a laboratory guide. New York: Springer; 2016. pp. 181–203. [Google Scholar]
- 61.Sharma RK, Ahmad G, Esteves SC, Agarwal A. Terminal deoxyribonucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values and quality control. J Assist Reprod Genet. 2016;33:291–300. doi: 10.1007/s10815-015-0635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18:357–365. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]