Abstract
Purpose
It is known that sperm preparation techniques in in vitro fertilisation (IVF) are intended to select the best-quality sperm. The aim of this study is to compare sperm the density gradient method and microfluidic chip (Fertile Plus) method in infertile patients by analysing fertilisation rates, pregnancy rates, and sperm morphology and DNA fragmentation rates posed by these two methods.
Methods
Using semen samples obtained from the patients, sperms were prepared with gradient (n = 312) and microfluidic chip methods (n = 116). Fertilisation and pregnancy rates were compared in the first time and in the recurrent IVF trial patients. In addition, the morphology and DNA fragmentation comparison of sperm samples were evaluated by Toluidine blue in situ chemical staining method.
Results
There was no statistically significant difference between fertilisation and pregnancy rates when compared with study groups in first-time IVF treatment patients. However, in recurrent IVF failure patients, there was a significant difference in fertilisation rates but no statistically significant difference was found in pregnancy rates. The microfluidic chip method significantly decreased sperm DNA fragmentation index according to density gradient method.
Conclusions
Microfluidic chip method may be recommended in patients with recurrent unsuccessful in vitro trials. The sperm DNA fragmentation test prior to the treatment will be helpful in selecting the appropriate sperm-washing method.
Keywords: IVF, Microfluidic chip, Sperm, DNA fragmentation
Introduction
Infertility is a noteworthy reproductive health problem affecting 8–12% of couples in reproductive age across the world [1]. Today, infertile couples can have children with the help of assisted reproduction techniques such as in vitro fertilisation (IVF), intrauterine insemination (IUI) and intracytoplasmic sperm injection (ICSI). Regardless of these techniques, it should be noted that the success of such treatment options depends on embryo quality as well as potential of the uterus to accept pregnancy [2].
Male factor contributes 30–50% in IVF failure [3]. Sperm count along with the morphology, mobility and genomic integrity of sperms is an important parameter in the evaluation of male infertility. Several sperm preparation methods have been developed for obtaining morphologically and genetically high-quality sperms to be used in IUI and IVF procedures or experimental studies [4]. Of these, density gradient and swim-up methods are among the traditionally preferred applications. However, both methods have their own limitations and negative aspects in terms of sperm quality. In the swim-up method, motile cells get separated from immotile ones by their own motility in a modified human tubal fluid sperm–washing solution. Nonetheless, only a small number of motile sperms can be obtained in this method. Density gradient method, on the other hand, is based on the idea of separating sperms according to their density. However, as it requires centrifugation, this technique has been reported to have potential to creative a negative influence on sperm viability, increase oxygen radicals and lead to sperm DNA fragmentation [5]. At length, the deficiencies in sperm extraction processes may bring about unfavourable results in the long term in terms of fertilisation success, continuity of pregnancy and embryo health. Although it has been recently developed, preparing sperms with microfluidic technique can step in as an alternative technique in reducing DNA fragmentation at this stage. However, it should be noted that the number of clinical studies on such new methods is still very low.
The aim of this study is to compare density gradient method, which is used in sperm preparation for microinjection, and microfluidic chip (Fertile Plus) method in infertile patients who have gone through at least two unsuccessful IVF trials for unknown reasons by analysing egg fertilisation rates, pregnancy rates and sperm DNA fragmentation rates posed by these two methods. As our surveys shows, this study is the first of its kind in evaluating the results of density gradient and microfluidic chip techniques with clinical results.
Materials and methods
Patient selection
This prospective randomised study was performed in 428 infertile couples who presented at “Malatya Doğu Fertil IVF Centre” between February 2016 and June 2018 with a history of unexplained infertility and recurrent unsuccessful IVF trials. The study was submitted to and has been approved by Malatya Province Clinical Research Council of Ethics with the protocol number 2017-34.
Using semen samples obtained from the patients, sperms were prepared with gradient (n = 312) and microfluidic chip (n = 116) methods. Throughout the study, we followed and compared fertilisation and pregnancy success rates as main outcome. Patients were evaluated in two groups as first-time ICSI trial patients and recurrent unsuccessful trial patients respectively. The study covered similar patients based on several selection criteria such as female age, duration of treatment, amount of medication used, number of eggs collected, sperm count and sperm motility.
In addition, we randomly selected 30 male patients inside from 428 infertile patients and divided sperm samples into three groups as ejaculate (control), gradient and microchip group in order to compare DNA fragmentation index and normal morphology sperm ratio by Toluidine blue in situ chemical sperm staining method between groups.
Preparation of sperms with density gradient method
We collected sperm specimens from the IVF patients on the day of egg collection and placed them in sterile containers. We used Irvine scientific sperm-washing medium (catalog ID 9983) for both methods which is a modified human tubal fluid that is completely sterile, and endotoxin and mouse embryo assays are maiden and these are assisted reproductive technologies which mimics natural conditions. The samples were incubated to dissolve at 37 °C for 20 min. We calculated the sperm concentration with the help of a microscope (× 20). We placed 1 ml of 80% gradient solution in a conical tube and added 1 ml of 40% gradient solution over the first solution. Then, we added 2 ml of liquefied sperm sample on top of the solutions. After centrifugation (279×g, 20 min), we applied 2-ml sperm-washing solution to the pellet. The centrifuged material (468×g, 10 min) was then added 0.1 ml of sperm wash solution and placed in the incubator at 37 °C with a 45° angle position in order to increase top surface area. A portion of the sperms were then saved for the microinjection process while the rest was preserved on slides for sperm DNA fragmentation and sperm morphology examinations.
Preparation of sperms with microchip method
For the preparation of sperms with this technique, we used the microchip method called Fertile Plus Chip® (Koek Biotechnology, İzmir, Turkey). This is an apparatus that gives opportunity to select sperms passively (without centrifugation steps) by sperm’s own motility similar to swim-up method. In addition, there is a membrane filter with pores on it that allows to pass morphologically normal–headed sperms. First of all, sperm samples from IVF patients were first collected in sterile containers to dissolve at 37 °C for 20 min for the liquefaction and then the sperm concentration was calculated with a microscope (× 20). For sperm sorting, we placed 800 μl of sperm samples into the loading chamber of the microchip via a pipette with care and added 1 ml of sperm-washing solution to the top chamber. The microchip was incubated at 37 °C for 30 min in order to get sperms passing through the membrane filter from the ejaculate group to the washing medium [6]. At length, we collected the sperm samples from the collection chamber to be used in microinjection while the rest of the sperm samples were spread on slides for sperm DNA fragmentation and sperm morphology examination.
Sperm DNA fragmentation examination
Sperm specimens gathered from the study groups were left to dry for 1 day at room temperature. The preparations were suspended in acetone + ethyl alcohol solution at + 4 °C for 30 min and then kept in HCl solution at + 4 °C for 5 min. This was followed by a washing process three times in distilled water. Next, the preparations were stained with 0.01% Toluidine blue stain prepared in 50% mcIIvain buffer solution for 5 min. Having dried the preparations, we then examined the dry material under the microscope at × 1000 magnification with immersion oil by the help of an analysis program. We regarded the indigo DNA fragmentation positive and the light blue ones negative. We scored 300 sperms in each group and proportioned the DNA fragmented cells by dividing the number of fragmented cells by the total number.
Morphological evaluation of the sperms
We analysed Toluidine blue stained sperm samples under the microscope at × 1000 magnification with immersion oil. In the evaluation of sperm morphology, the Kruger criteria were taken into consideration in analysing the sperm head, neck and tail.
Statistical analysis
In the study groups, we used the Kolmogorov-Smirnov normality test to study the distribution of variables. Due to lack of a normal distribution, the average values of the groups were compared with the Mann-Whitney U test. Because of the independent and non-parametric conditions between the groups, we analysed the fertilisation and pregnancy rates by using the chi-square test. For the DNA fragmentation and morphology comparisons, we chose 30 patients by using power analysis. These randomly taken 30 sperm samples were divided into three groups as control, gradient and microfluidic chip groups. Due to the presence of multiple dependent groups, we used the Friedman and Wilcoxon tests for comparisons and considered p < 0.05 value statistically significant.
Results
The sperm samples of 428 patients were divided into two groups as gradient group (n = 312) and microfluidic chip group (n = 116). Throughout the study, no difference was observed between the groups in terms of age, duration of treatment, amount of medication used, egg count, sperm count or motility (Table 1).
Table 1.
Main characteristic properties of patients included in the study
| Gradient group, X ± SD | Microchip group, X ± SD | p value | |
|---|---|---|---|
| Female age (years) | 31.49 ± 0.5 | 31.07 ± 0.3 | 0.390 |
| Duration of IVF treatment (days) | 9.4 ± 0.05 | 9.3 ± 0.08 | 0.260 |
| Amount of medication used (units) | 1828 ± 35 | 1721 ± 60 | 0.150 |
| Egg count | 13.6 ± 0.39 | 13.06 ± 0.56 | 0.900 |
| Sperm count (million/ml) | 53.5 ± 1.5 | 49.5 ± 3 | 0.190 |
| Sperm motility (%) | 54.1 ± 0.85 | 52.3 ± 1.5 | 0.150 |
In order to determine whether IVF was successful, we compared the fertilisation and pregnancy rates between the groups by using chi-square test (Table 2). The fertilisation rates were calculated by dividing the number of successfully fertilised eggs by the number of ICSI-applied eggs. As for the pregnancy rates, we considered > 100 IU positive using beta-HCG tests conducted after 12 days and biochemical pregnancies were not included in the study. We did not find any significant difference between the patients who received IVF treatment for the first time (n = 336) in terms of fertilisation and pregnancy rates.
Table 2.
The comparison between the fertilisation and pregnancy rates according to gradient and microchip methods in first-time IVF trial patients
| Gradient group, (n = 256) | Microchip group, (n = 80) | p value | |||
|---|---|---|---|---|---|
| Rate | % | Rate | % | ||
| Fertilisation rates | 1950/2776 | 70.20 | 563/811 | 69.40 | 0.650 |
| Pregnancy rates | 130/256 | 50.70 | 43/80 | 53.75 | 0.640 |
Table 3 shows the comparison between the fertilisation and pregnancy rates according to gradient and microfluidic chip techniques in the 92 patients who had previously received at least two IVF trials before the study. Although there was no significant difference in pregnancy rates between the groups, there was a notable difference in terms of fertilisation rates with p < 0.05.
Table 3.
The comparison between the fertilisation and pregnancy rates according to gradient and microchip methods in the patients who had previously received at least two IVF trials before the study
| Gradient group, (n = 56) | Microchip group, (n = 36) | p value | |||
|---|---|---|---|---|---|
| Rate | % | Rate | % | ||
| Fertilisation rates | 272/432 | 62.9 | 271/369 | 73.4* | 0.002 |
| Pregnancy rates | 23/56 | 50 | 19/36 | 52 | 0.900 |
*Significant between the groups
Table 4 shows the morphological evaluation of sperm cells in the control, gradient and microfluidic chip groups. We observed that the gradient and microchip sperm-washing methods increased the number of sperms in good morphological condition compared with the control group in a statistically significant way. Nonetheless, there was no difference between the gradient and microchip sperm-washing techniques in terms of sperm morphology.
Table 4.
The comparison of morphological evaluation of sperm cells among groups
| Groups | The Kruger morphological evaluation (%) (n = 30) | Standard deviation (max–min) | p value |
|---|---|---|---|
| Control group | 4.5 | 2.1 (2–10) | p < 0.05 |
| Gradient group | 6.4* | 2.9 (3–12) | |
| Microchip group | 5.8* | 3.0 (2–12) |
*Significant from the control
The sperm DNA fragmentation comparison between the two study groups and the control group is shown in Table 5. Statistically speaking, there was no difference between the treatment groups and control group in terms of DNA fragmentation rates. However, the DNA fragmentation rate of the density gradient group was significantly higher by p = 0.01 compared with the microfluidic chip group.
Table 5.
The sperm DNA fragmentation comparison between the groups
| Groups | Sperm DNA fragmentation (%) | Standard deviation (max–min) | p value |
|---|---|---|---|
| Control group (n 10) | 24.1 | 11.8 (6–52) | 0,01 |
| Gradient group (n 10) | 29.5* | 21.9 (5–85) | |
| Microchip group (n 10) | 22.3 * | 13.9 (2–54) |
*Significant difference between the study groups
Figure 1 shows the microscopic views of sperm cells stained with Toluidine blue for the DNA fragmentation analysis.
Fig. 1.
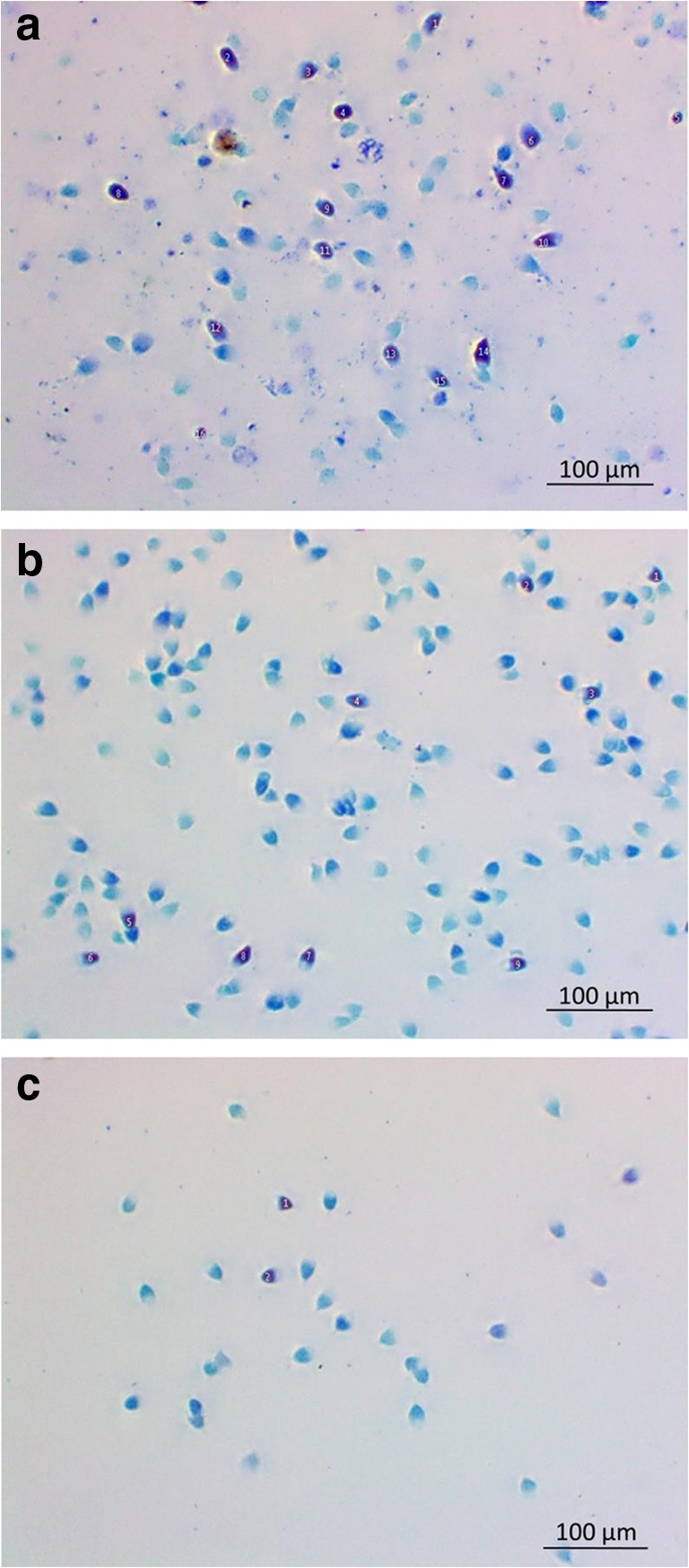
Microscopic views of sperm cells stained with Toluidine blue for the DNA fragmentation analysis (× 1000). (Numbered and dark blue sperm heads show damage to DNA.) a Control group, b gradient group and c microchip group
Discussion
Male factor is very important in the absence of pregnancy after 1 year of unprotected sex. Semen analysis is the first and basic standard for a full understanding of male infertility and the success of IVF treatment [7]. However, traditional semen analysis methods may sometimes fall short in diagnosing underlying problems and applying necessary treatments. For example, despite the existence of a correlation between sperm morphology and DNA integrity, there may be DNA fragmentation in morphologically intact normal sperms [8–10]. The purpose of microchips used in the preparation of sperms is to choose the best sperms in a fast and gentle way without damaging sperms by way of creating an impression of natural conditions. This is achieved with the help of the membrane in the microchip. As it does not require centrifugation, this method comprises all the advantages of traditional sperm preparation techniques.
The microfluidic chips that we employed in our study were essentially produced for IUI cycles. In our study, we tested whether sperm cells using the Fertile Plus chips create a difference compared with the gradient method in the selection of best sperms in terms of morphological and genomic integrity and success of pregnancy in microinjection (ICSI) cycles. A recent study comparing swim-up and microfluidic chip methods in IVF patients with unknown reasons reports that there was no difference between pregnancy and fertilisation rates [11]. In our study, we used gradient method instead of the swim-up method and we, too, failed to observe any difference between the two methods in pregnancy rates. This is in line with reports in the literature and it is safe to claim that our study is compatible with similar studies. However, our study differs from the corresponding studies in that we observed a significant difference in the fertilisation rates in infertility patients who had previously failed more than twice in fertility treatment and underwent microchip method during the course of our study. Therefore, microfluidic chips may be considered as an alternative to increase fertilisation rates in patients with recurrent IVF failure.
The initial purpose of current sperm preparation methods in assisted reproductive therapies is to obtain the best sperms with the highest insemination capacity by removing unwanted biochemical agents and non-sperm round cells in the seminal fluid. In selecting high-quality sperms, DNA fragmentation tests are applied in addition to examining morphological features, count and mobility of sperms. Throughout the study, we discovered that both the gradient and microchip washing methods significantly increased sperm concentration with good morphology. In our study, there was no difference between gradient and microfluidic chip groups. Some studies report that gradient method was found to be significantly superior in selecting sperms with well-constructed morphology as well as in terms of ongoing pregnancy rates compared with the swim-up method [12, 13].
On the other hand, a considerable number of studies comparing these methods report that gradient method was ineffective in terms of sperm DNA fragmentation. In their 2016 study, Muratori et al. report that there was a 50% increase in DNA fragmentation in the sperm samples after the application of gradient method. They also suggest that the gradient method may not be safe for all infertility patients and that it would be healthier to use different washing methods in such patients [14]. In a study of 22 semen samples in 2000, Zini et al. observe that the swim-up method is the most effective approach as far as DNA integrity is concerned [15]. Again, Zini et al. also report in another study in 2000 including a total of 53 men (44 infertile; 9 fertile) that there was no significant difference in terms of DNA fragmentation in the samples from the fertile subjects after the gradient method application while there was an increase in fragmentation rates in the infertile group. Accordingly, they suggest that density gradient method should urgently be revised to minimise DNA damage in sperms [16]. A similar study conducted by Wang et al. report that density gradient method reduces DNA fragmentation except for samples from severe oligozoospermia patients [17]. In a study led by Ghaleno et al. comparing gradient method with centrifugation and modified swim-up method without centrifugation as well as centrifugal swim-up methods in 28 men, it has been suggested that DNA fragmentation was most visible in the gradient method application and that there was no difference between the two swim-up methods as far as DNA fragmentation is concerned [18]. In our study, the DNA fragmentation rate which was 25% in the control group increased to 30% in the gradient group and decreased to 22% in the microfluidic chip group. As such, our study shows an ever-growing significant difference between the gradient and microfluidic chip methods. It is known that sperm DNA damage may increase in vitro after 3 h of incubation duration. So, we tried our best to minimise that time and we start to collect sperms 1 h before ICSI. It took almost 1 h to prepare sperms with liquefaction time for both gradient and microchip groups. Through the course of our study, we used two layers of gradient solution. Ghaleno et al. had used a three-layer gradient solution and applied a higher centrifugal g value. The difference in gradient solution amount and the difference between the physical forces caused by centrifugation in the two studies may have increased the DNA fragmentation rates in our study. However, there are studies that suggest that gradient method reduces DNA fragmentation [9, 19, 20]. The increase or decrease of DNA fragmentation levels in gradient method applications and the varying results in these studies may result from initial cellular DNA fragmentation rates or centrifugation. In a 2018 study sponsored by the microchip manufacturer, Quinn et al. compare gradient and microfluidic chip methods. The results of the study shows that DNA fragmentation rates in the microchip method were reduced to almost 0% compared with the gradient method [21]. Nonetheless, the study does not evaluate pregnancy rates, the main objective in IVF treatment.
In our study, we used Toluidine blue staining technique to evaluate sperm DNA fragmentation. Studies often employ methods such as SCSA, SCD, TUNEL, COMET, Aniline blue and Toluidine blue for this purpose. Meta-analysis of these applications shows that there is no significant difference between these tests in detecting sperm DNA fragmentation levels [22, 23]. In a study comparing TUNEL, SCSA and Toluidine blue methods, it has been suggested that Toluidine blue method has totally matched positive DNA fragmentation index and that this method can be a reliable test in routine practice for the evaluation of sperm DNA integrity and/or abnormal chromatin structures. Moreover, it has been reported that the DNA fragmentation rates do not exceed 35% in this method in fertile individuals and the application has been appointed a specific cut-off value [24]. So much so, Toluidine blue method has been successfully standardised in studies working on sperms from various species as a fast and effective method [23, 25, 26]. Therefore, we also preferred Toluidine blue staining technique in our study as it provides practical and reliable results in DNA fragmentation imaging. However, the Toluidine blue staining results need to be corroborated using other methodologies for DNA testing.
A study on DNA fragmentation in infertile patients reports that the time required to have children for individuals with a sperm DNA fragmentation index between 27 and 30% is considerably longer than the average [27]. Another study points that microinjection method is more successful in achieving pregnancy in patients with a sperm DFI of > 30% 23 [28]. Our study differs from these studies in that the post-IVF treatment clinical evaluation did not show any significant differences after the applications on these sperm cells in terms of pregnancy rates after IVF treatment.
An overall evaluation of these results imply that microchip washing method may be an alternative to density gradient method as it does not require centrifugation, one of the factors that is thought to effect sperm DNA. However, it should be noted that the microfluidic chip technique does not always give the expected clinical results in patients undergoing their first IVF trial possibly due to the absence of the physical effect created by centrifugation. It should also be added that the method led to a significant increase in fertilisation rates in patients with recurrent IVF in our study. Therefore, it would be wiser for patients with a history of recurrent IVF treatment to undergo DNA fragmentation tests prior to the treatment as their sperms are likely to have a more fragile structure. We also suggest that the washing technique should be determined in accordance with the results of these pre-treatment tests especially if the results are higher. An overview of the literature has led us believe that our study is the first of its kind providing a comparative analysis of the application of density gradient and microfluidic chip methods in patients with a history of single or multiple IVF trials with special focus on fertilisation and pregnancy success rates. The number of subjects in our study can be considered as a limiting factor. In order to determine the long-term results of the data proposed in our study, the number of subjects should be increased and the patients should be called in for follow-up visits over a longer period of time.
Funding information
This study was funded by Inonu University Research Fund (2016/147).
Compliance with ethical standards
The study was submitted to and has been approved by Malatya Province Clinical Research Council of Ethics with the protocol number 2017-34.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 4.Tanphaichitr N, Agulnick A, Seibel M, Taymor M. Comparison of the in vitro fertilization rate by human sperm capacitated by multiple-tube swim-up and Percoll gradient centrifugation. J In Vitro Fert Embryo Transf. 1988;5(3):119–122. doi: 10.1007/BF01131172. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988;9(6):367–376. doi: 10.1002/j.1939-4640.1988.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 6.Tasoglu S, Safaee H, Zhang X, Kingsley JL, Catalano PN, Gurkan UA, et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small. 2013;9(20):3374–3384. doi: 10.1002/smll.201300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belva F, Bonduelle M, Roelants M, Michielsen D, Van Steirteghem A, Verheyen G, et al. Semen quality of young adult ICSI offspring: the first results. Hum Reprod. 2016;31:2811–2820. doi: 10.1093/humrep/dew245. [DOI] [PubMed] [Google Scholar]
- 8.Schieve LA, Peterson HB, Meikle SF, Jeng G, Danel I, Burnett NM, et al. Live-birth rates and multiple-birth risk using in vitro fertilization. JAMA. 1999;282(19):1832–1838. doi: 10.1001/jama.282.19.1832. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, Sakkas D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 2001;16(10):2160–2165. doi: 10.1093/humrep/16.10.2160. [DOI] [PubMed] [Google Scholar]
- 10.Avendaño C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94:549–555. doi: 10.1016/j.fertnstert.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Yetkinel S, Kilicdag EB, Aytac PC, Haydardedeoglu B, Simsek E, Cok T. Effects of the microfluidic chip technique in sperm selection for intracytoplasmic sperm injection for unexplained infertility: a prospective, randomized controlled trial. J Assist Reprod Genet. 2018;18:1375–1372. doi: 10.1007/s10815-018-1375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Zwalmen P, Bertin-Segal G, Geerts L, Debauche D, Schoysman R. Sperm morphology and IVF pregnancy rate: comparison between Percoll gradient centrifugation and swim-up procedures. Hum Reprod. 1991;6(4):581–588. doi: 10.1093/oxfordjournals.humrep.a137383. [DOI] [PubMed] [Google Scholar]
- 13.Prakash P, Leykin L, Chen Z, Toth T, Sayegh R, Schiff I, Isaacson K. Preparation by differential gradient centrifugation is better than swim-up in selecting sperm with normal morphology (strict criteria) Fertil Steril. 1998;69(4):722–726. doi: 10.1016/S0015-0282(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 14.Muratori M, Tarozzi N, Cambi M, Boni L, Iorio AL, Passaro C, Luppino B, Nadalini M, Marchiani S, Tamburrino L, Forti G, Maggi M, Baldi E, Borini A. Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: a possible new male predictive parameter of pregnancy? Medicine (Baltimore) 2016;95(20):e3624. doi: 10.1097/MD.0000000000003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56(6):1081–1084. doi: 10.1016/S0090-4295(00)00770-6. [DOI] [PubMed] [Google Scholar]
- 16.Zini A, Nam RK, Mak V, Phang D, Jarvi K. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril. 2000;74(4):824–827. doi: 10.1016/S0015-0282(00)01495-3. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Sun J, Wang L, Gao X, Lu X, Wu Z, Wang Y, Liu K, Tao J, Wu Y. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet. 2014;31(12):1655–1663. doi: 10.1007/s10815-014-0339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaleno LR, Valojerdi MR, Janzamin E, Chehrazi M, Sharbatoghli M, Yazdi RS. Evaluation of conventional semen parameters, intracellular reactive oxygen species, DNA fragmentation and dysfunction of mitochondrial membrane potential after semen preparation techniques: a flow cytometric study. Arch Gynecol Obstet. 2014;289:173–180. doi: 10.1007/s00404-013-2946-1. [DOI] [PubMed] [Google Scholar]
- 19.Jackson RE, Bormann CL, Hassun PA, Rocha AM, Motta EL, Serafini PC. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril. 2010;94:2626–2630. doi: 10.1016/j.fertnstert.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 20.Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29:557–563. doi: 10.1007/s10815-012-9742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33(8):1388–1393. doi: 10.1093/humrep/dey239. [DOI] [PubMed] [Google Scholar]
- 22.Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, Mol BW, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0165125. doi: 10.1371/journal.pone.0165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rui BR, Angrimani D, Bicudo LC, Losano J, Nichi M, Pereira R. A fast, low-cost and efficient method for the diagnosis of sperm DNA fragmentation in several species. Reprod Domest Anim. 2018;53:171–175. doi: 10.1111/rda.13087. [DOI] [PubMed] [Google Scholar]
- 24.Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, Spano M. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod. 2004;19:2277–2282. doi: 10.1093/humrep/deh417. [DOI] [PubMed] [Google Scholar]
- 25.Tsarev I, Bungum M, Giwercman A, Erenpreisa J, Ebessen T, Ernst E, Erenpreiss J. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum Reprod. 2009;24(7):1569–1574. doi: 10.1093/humrep/dep068. [DOI] [PubMed] [Google Scholar]
- 26.Pourmasumi S, Khoradmehr A, Rahiminia T, Sabeti P, Talebi A, Ghasemzadeh J, et al. Evaluation of sperm chromatin integrity using aniline blue and toluidine blue staining in infertile and normozoospermic men. J Reprod Infertil. 2019;20(2):95–101. [PMC free article] [PubMed] [Google Scholar]
- 27.Evenson DP, Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. J In Vitro Fert Embryo Transf. 2006;65(5):979–991. doi: 10.1016/j.theriogenology.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Zini A, Jamal W, Cowan L, Al-Hathal N. Is sperm DNA damage associated with IVF embryo quality? A systematic review. J Assist Reprod Genet. 2011;28(5):391–397. doi: 10.1007/s10815-011-9544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


