Abstract
Purpose
Despite the growing body of research implying an impact of in vitro fertilization (IVF) on imprinted genes and epigenetics, few studies have examined the effects of underlying subfertility or prenatal stress on epigenetics, particularly in terms of their role in determining infant birthweights. Both subfertility and prenatal stressors have been found to impact epigenetics and may be confounding the effect of IVF on epigenetics and imprinted genes. Like IVF, both of these exposures—infertility and prenatal stressors—have been associated with lower infant birthweights. The placenta, and specifically epigenetically regulated placental imprinted genes, provides an ideal but understudied mechanism for evaluating the relationship between underlying genetics, environmental exposures, and birthweight.
Methods and results
In this review, we discuss the impacts of IVF and infertility on birthweight, epigenetic mechanisms and genomic imprinting, and the role of these mechanisms in the IVF population and discuss the role and importance of the placenta in infant development. We then highlight recent work on the relationships between infertility, IVF, and prenatal stressors in terms of placental imprinting.
Conclusions
In combination, the studies discussed, as well as two recent projects of our own on placental imprinted gene expression, suggest that lower birthweights in IVF infants are secondary to a combination of exposures including the infertility and prenatal stress that couples undergoing IVF are experiencing. The work highlighted herein emphasizes the need for appropriate control populations that take infertility into account and also for consideration of prenatal psychosocial stressors as confounders and causes of variation in IVF infant outcomes.
Keywords: Epigenetics, Imprinted genes, Prenatal depression, Prenatal anxiety, Subfertility/infertility, Placenta
Introduction
In the past 10 years, the use of in vitro fertilization (IVF) and other forms of assisted reproduction has increased by over 20% [1]. Currently, live births following IVF have risen to represent between 1 and 4% of births in many developed countries, including the USA [2], where IVF now accounts for 1.5% of live births [3]. As the number of IVF infants increases, more work has been done on the long-term outcomes of IVF offspring. However, most of the work on IVF infant outcomes has, likely by necessity of available databases, used a naturally conceived control group instead of infants whose parents experienced infertility but did not use IVF to conceive. As a result, many of these studies have not been able to control for the underlying factors that cause and are associated with infertility and this has made it difficult to differentiate between underlying genetic factors, exposures associated with infertility, and the effects of the IVF procedure.
One such exposure, maternal prenatal depression and anxiety, has been found to be more common among couples undergoing IVF than the general population [4–8] and is linked to various reproductive and developmental outcomes in the offspring and therefore represents a potentially important confounder in all studies on the outcomes of IVF. Importantly, the underlying mechanisms underlying the role of depression and stress in impacting health, through genetic and/or epigenetic effects, is only beginning to be examined, not only in the IVF population, but also in general obstetric populations are well. Recent data has pointed to a critical role of the placenta and alterations to placenta function by maternal psychosocial factors. Our group has begun to explore these mechanisms through studies of placental imprinting and epigenetics, and, in this manuscript, we synthesize that and other recent research on the effects of IVF and prenatal stress on placental epigenetics, particularly imprinted genes, and their potential role in offspring outcomes, particularly infant birthweight. We begin this review by discussing the impacts of IVF and infertility on infant birthweight, review epigenetic mechanisms and genomic imprinting, and the importance of these mechanisms to the IVF population and justify the importance of examining the placenta when considering offspring health. We then highlight a number of recent studies focusing on infertility, IVF treatments, and maternal psychosocial factors on placental imprinting, integrating their findings in an effort to provide a preliminary look at the understudied field of placental epigenetics and its role in responding to environmental factors to determine infant birthweights, in the hopes that the discussion of this work will encourage future research into the effects of IVF on the placenta and the role of prenatal stressors as both potential confounders on IVF outcomes and important exposures for infants born to infertile parents.
Birthweight outcomes in IVF and infertile populations
Infants born following IVF have been found to have a higher incidence of low birthweight (LBW) and small for gestational age (SGA) than their naturally conceived peers [9–13]. LBW has been associated with increased risk of all-cause mortality and morbidity in infancy and childhood, with an estimated 6% lower mortality risk for every kilogram increase in birthweight [14]. LBW also has been associated with adult mortality due to many diverse causes, including accidental falls in men and, in women, with musculoskeletal disease, deaths due to pneumonia, injury, and diabetes [15]. Much evidence has been found for a relationship between lower birthweights and increased risk of cardiovascular deaths [15], often with a U-shaped relationship between birthweight and poor outcomes; risks of metabolic disease and some heart disease, such as atrial fibrillation [16] and sudden cardiac death [17], as well as obesity [18], increase with both too-low (low birthweight, LBW, < 2500 g) and too-high (macrosomic, > 4000 g) birthweights, indicating an ideal range of birthweights to optimize long-term health outcomes.
Given the long-term implications of LBW and the growing use of IVF, the increased risks of LBW and SGA indicate a need for understanding and improving infant outcomes. Many of the commonly used IVF methods, including the culture media used [19], the method of stimulation for oocyte retrieval [20, 21], and the use of frozen/thawed embryos [22, 23], have been found to have an effect on infant birthweights. Whether these associations are due to an effect of the procedure, which involves the artificial manipulation of the embryo, or of the underlying genetics of those who require IVF to conceive, is still unclear. Infertility is a broad term that includes many causes, and each cause has a differing potential genetic background and environmental etiology. Among those seeking IVF in 2015, 17% had both male and female factor infertility [24], and many women and men have multiple causes of infertility. Identifying the effects of the underlying infertility on the infants later conceived is complicated by these overlapping and nonspecific diagnoses.
Most studies on IVF infant outcomes use women from the general population, rather than those who have experienced infertility but conceived naturally, as controls. These women, however, are inappropriate controls as there are likely underlying genetic and exposure factors related to infertility that the control women do not have; fertile women would not be selected as cases if they had been given the IVF procedures (as they would have no reason to receive IVF procedures) and are, therefore, not appropriate controls. Although it is easier to select a control group of fertile women since data on fertility are often not available in larger databases, the use of these women as control groups has led to a potential for confounding in many of the previous studies on IVF outcomes.
Identifying the root cause of the lower birthweights in IVF infants is essential to ensuring that IVF procedures are producing the healthiest infants possible and can also add to our understanding and treatment of LBW in normally conceived infants. Studies of outcomes of children whose parents experienced infertility but who did not receive infertility treatments, indicate that infertility, without IVF, has been associated with many of the outcomes attributed to IVF, including poor obstetric [25–27], infant and childhood outcomes [28, 29], and lower birthweight [30, 31]. However, sibling studies in which one sibling was naturally conceived and the other conceived via IVF indicate that the IVF siblings were still more likely to be born preterm (PTB, < 37 weeks) [32]. Although both infertile and IVF pregnancies have a greater risk of PTB and LBW, this risk is about 20% greater in IVF pregnancies than those in infertile mothers [2]. Therefore, the IVF procedure is likely still having some effect on infant outcomes, but the underlying infertility is also playing a role.
Studies comparing infants conceived using donor and autologous embryos supports a role for either the procedure or some underlying effect of maternal infertility, such as some component of the uterine environment. Pregnancies conceived with donor ova can be compared to autologous to evaluate the underlying role of a genetic component of infertility. Given that infants conceived using donor embryos still have increased risks of LBW and pre-term birth [33], some effect either of the IVF process or of the uterine or external environment of the women conceiving with donor ova is affecting fetal growth and parturition.
Similarly, data from women who required IVF due to tubal obstructions (TO), who make up 35% of those seeking infertility treatment [34], provide a study group of women in which most require IVF due to non-genetic causes. Women receiving IVF for ovulatory dysfunction, which can have genetic components, were more likely to have an infant with lower Apgar scores than those with TO [35]. In our study of US singleton, term (> 37 weeks) infants conceived using IVF with non-donor oocytes, we found significant relationships between birthweight and ovarian dysfunction/polycystic ovarian disorder, endometriosis, and “other causes” of infertility [22]. In contrast, the effect of tubal factor infertility on birthweight was significant only for female infants, and no effects related to birthweight were found for uterine factor, which similarly is often due to physical characteristics of the reproductive tract rather than some underlying genetic cause. These outcomes imply a role of the underlying genetics associated with infertility in addition to an effect of the procedure.
Epigenetics and imprinted genes
Epigenetics is the study of modifications to the genome that do not alter the nucleotide code but change the frequency and levels of gene transcription [36]. These mechanisms are thought to be responsive to environmental exposures, making them ideal candidate mechanisms for studying the relationship between inherited factors and the environment. One form of epigenetic modification, DNA methylation, involves enzymatic addition of a methyl group (CH3–) to cytosines, usually within regions of cytosine/guanine repetitions, called CpG islands [37] throughout the genome. These additions alter gene expression by affecting signaling and transcriptive machinery within the nucleus, usually resulting in blocking a particular gene from being expressed [36]. This mechanism is partially responsible for turning genes on and off as appropriate for cell differentiation [38]. To allow for this, global demethylation—the removal of methylation markers—of the entire genome occurs within the first few days post-fertilization, followed later by remethylation in a manner appropriate for cell differentiation and tissue specification [39, 40].
Environmental exposures [41], such as maternal smoking [42]; traffic-related toxicants [43]; heavy metals such as arsenic [44] and cadmium; and childhood stressors including parental death, desertion, or maltreatment [45], have been found to alter methylation in children of prenatally exposed mothers, indicating that methylation may be a central mechanism by which genomes respond to the environment. Epigenetic changes, especially methylation, have also been identified in many cancers [46]. Abnormal methylation may affect appropriate cell growth and differentiation [47], providing a mechanism by which environmental exposures contribute to cancer development and other forms of abnormal growth.
A subset of epigenetically regulated genes, called imprinted genes, does not lose their epigenetic signals during the global demethylation that occurs soon after fertilization, and these genes thereby allow for epigenetic inheritance [48, 49]. These genes, of which about 100 have been identified in humans [50], are regulated by the silencing of one allele, either the maternal or paternal in a consistent fashion, through methylation of the gene or imprinting control regions (ICRs)—segments of DNA that contain CpG sequences that contain methylation on one of the two alleles, and which regulate gene expression [48, 51]. Some imprinted genes will have a maternally methylated allele and the paternal allele will be un-methylated, while others will exhibit the opposite pattern. This results in about 50% expression of the gene compared to what would be possible if both alleles were unmethylated [37, 52], indicating an exquisite, fine-tuning of the expression of these genes.
Since imprinted genes are highly involved in infant growth and development [53–55], if these genes have inappropriate methylation patterning leading to the gene being under- or over-expressed, a too-large or too-small infant can result, depending on whether the inappropriately expressed gene is maternally or paternally controlled [48]. For example, the imprinted genes H19—which is thought to suppress fetal growth and placental development and is involved in inhibiting trophoblastic growth and development [56]—and IGF2—which increases fetal and placental growth—are both regulated by the same ICRs: ICR1 and ICR2. Methylation at ICR1 is associated with expression of the paternal IGF2 allele and maternal H19 allele [57]. Demethylation of this ICR would, therefore, result in low birthweight, as seen in Russell–Silver syndrome, which can be due to loss of imprinting at ICR1 [58]. In support of imprinted genes’ importance in fetal development, loss of imprinted methylation patterns is associated with poor postnatal outcomes [48], including low birthweight, intrauterine growth restriction [59], placental hyperplasia [60], and altered lipid metabolism [61].
Although ICRs are central to the regulation of imprinted genes, changes to ICR methylation usually result in extreme phenotypic outcomes, as seen in the severe overgrowth, neurological deficiencies, and tumors characteristic of imprinting disorders such as Angelman and Beckwith–Wiedemann syndromes [62]. Methylation in non-ICRs, however, also can affect imprinted gene RNA expression [63] and results in more subtle differences in expression which may be a cause of normal phenotypic variations seen in the general population. Currently, most research on imprinted genes has focused on ICRs, but more work on non-ICRs is needed to begin to understand the cause of less extreme variation in the population, such as that seen in the IVF population in comparison with the naturally conceived population. Although ICRs are regulated by inherited methylation established during gamete development in utero [48, 49], the non-ICR regions are thought to undergo the global demethylation that occurs post-fertilization and therefore may be better markers of response to early environmental exposures, particularly during prenatal development.
Imprinted genes in the placenta
The placenta controls the supply of nutrients and hormones to the fetus by preventing fetal exposure to toxicants and acts as an endocrine organ [64, 65], overall resulting in control of the fetal environment. Since imprinted genes are epigenetically regulated, they have the potential to be affected by environmental exposures. Given that environmental exposures, such as maternal BMI and diet, have been found to impact the development of the placenta, determining placental size and shape, imprinted genes provide a novel approach to studying the effects of environmental exposures [66, 67]. In turn, placental size and shape are indicative of its efficiency and function and have been found to be predictive of infant size and long-term health [67, 68]. The ratio of birthweight to placental size is representative of placental efficiency, with small placentas that produce large infants considered to be highly efficient and vice versa [69]. Poor placental efficiency has been correlated with cardiovascular risk in adulthood [70], and placental phenotype has been correlated with heart disease, heart failure, asthma, and several cancers [70]. The placenta, therefore, provides a potential mechanism by which environmental exposures may affect the growth rate of the developing fetus.
In the placenta, imprinted genes appear to regulate the distribution of nutritional resources between the mother and the fetus [71], with paternally expressed genes (where the maternal allele is silenced) increasing nutrient transfer to the fetus and maternally expressed genes (where the paternal allele is silenced) conserving the mother’s resources and protecting them for her use; this concept is termed the conflict hypothesis [72]. Changes in the epigenetic regulation of imprinted genes have been identified in placentae and offspring cord blood from mothers exposed to famine [73], heavy metals such as lead, arsenic, and cadmium [74, 75], maternal and paternal obesity [76, 77], prenatal physical activity [78], and folic acid intake [79], among many other exposures. Imprinted genes, therefore, are key in understanding the mechanisms by which environmental exposures affect birthweight and may be markers of modifiable exposures that later lead to too-low or too-high birthweights.
As touched on above, though, most studies on imprinted genes focus on cord blood or buccal cells and look at a few specific genes rather than the entire panel of known imprinted genes. There are, therefore, several genes, such as IGF2 and H19, which have been extensively studied, and many others about which little is known. Additionally, although there is a great deal of work looking at many exposures in the murine, porcine, bovine, and other mammalian placentas, the human placenta is vastly different from the placentas of other mammals in terms of histological structure and this work cannot be easily extrapolated to humans [80, 81]. Further work characterizing the complete set of imprinted genes in placental tissues is needed to begin to understand how environmental exposures affect the placenta’s ability to regulate the parental environmental and encourage appropriate growth.
Imprinted genes in IVF-conceived children and placentas
Epigenetics, as a both genetically and environmentally determined marker, can be used to help parse out the effect of underlying infertility from the effect of IVF procedures. IVF infants have been found to have a higher incidence of imprinted gene disorders due to imprinting errors, rather than deletions or other genetic alterations which also can cause the same syndromes [82]; for example, over 90% of those with Beckwith–Wiedemann syndrome conceived using IVF had an imprinting disorder (a methylation defect) compared to just 40–50% in those not conceived using IVF. These findings have led to a growing body of research on the impact of IVF on epigenetics and imprinted genes. Based on this, several studies have looked at imprinted gene expression and methylation in buccal cells, cord blood, and placentae from infants conceived using IVF [83–87], with a particular focus on genes known to be associated with imprinted gene disorders. For example, Loke et al. have reported decreased H19/IGF2 methylation in buccal swabs from IVF twins, most specifically those conceived through intracytoplasmic sperm injection [83], indicating that there may be epigenetic effects of IVF on specific imprinted genes.
The identification of methylation differences in mouse and bovine models [88, 89], where infertility is not a factor, supports that at least some of the variation in imprinted gene methylation and expression identified in IVF infants is due to IVF approach. However, imprinting differences between infants born using donor and autologous eggs have also been identified [90], indicating that there is also epigenetic variation associated with some aspect of the underlying infertility. Song et al. evaluated methylation levels at CpGs associated with genes that had previously been identified as being differentially methylated in IVF children in comparison with naturally conceived controls. However, they compared naturally conceived infants, autologous IVF infants, and infants conceived with a donor oocyte. They found that 67% of the differences identified between the entire IVF cohort and the controls were present in both the donor and autologous groups. Therefore, some of those findings were due to the IVF process and not the underlying infertility, but there were some that were found only in the autologous group that could be due to the underlying infertility [90]. Additionally, some methylation changes identified as associated with IVF treatment, such as increased methylation of SNRPN—an imprinted gene associated with imprinting disorders—have been found to be of increased magnitude with increasing time to conception when adjusted for maternal age, indicating that imprinted gene methylation status may have a correlation with severity of infertility [91].
Some of the epigenetic differences identified as being associated with IVF actually may be related to the cause of infertility. In cases of male factor infertility, differences in human sperm support the role of inherited parental epigenetic differences [92–94]. In murine models, advanced maternal age also appears to increase methylation variation and, given the increased average age of IVF mothers, this could disproportionately affect IVF offspring [95]. Other causes of female infertility, such as endometriosis [96] and polycystic ovarian syndrome (PCOS) [97], and especially the hyperandrogenic state resulting from PCOS [98], have also been associated with altered methylation. Identification of the role of parental epigenetic variation in offspring and placental epigenetic differences could provide essential guidance for the treatment approaches used in IVF, particularly whether donor eggs or sperm may be warranted in some cases. However, little work has been done on imprinted genes to date and the effects of IVF on imprinted gene expression remain to be determined.
However, as discussed above, most studies on IVF and imprinted genes to date have focused on methylation in offspring cord blood or buccal cells. Little is known about the effect of IVF and infertility on imprinted genes in the placenta. First, differentiation of the placenta from the overall cell mass occurs at the blastocyst stage, 5 days after fertilization, when the trophectoderm and the inner cell mass begin to form [64, 99]. For IVF embryos, this early placental differentiation is occurring right around the time of transfer to the uterus, depending on the day of transfer, and embryos are therefore exposed to an in vitro environment or a potentially non-typical intrauterine environment during early placental development.
In support of this, an increased occurrence of implantation and nutrient transport disorders following IVF has been found in murine placentae conceived via IVF, including different methylation and imprinted gene profiles and downregulation of many of the known nutrient transport genes [100]. Although little is known about the effect of IVF on human placentae, IVF is associated with greater incidence of placental lakes and infarction on histology [101] and increased risk of placental disorders such as placenta previa [9]. IGF2 RNA expression has been found to be decreased and H19 RNA expression increased in human IVF placentae [85], indicating that the IVF environment is sufficiently different from the in vivo environment to change imprinting patterns and that imprinting may be a mechanism by which the IVF environment alters nutrient transport. However, there is still little work on the effects of IVF on human placentae.
The placenta as a buffer for prenatal stress exposure
In the placenta’s role as an endocrine organ, it produces, among other hormones, human placental lactogen—which has similar metabolic activity to growth hormone—and placental growth hormone—a variant of growth hormone [64]. These placental hormones contribute to fetal growth and appropriate development and provide signals to the mother’s body, altering her hormone responses and nutrient supply [102, 103]. Additionally, the placenta provides a buffer from maternal hormones, especially stress hormones. For example, placental 11β-hydroxysteroid-dehydorgenase-2 (11β-HSD2) converts maternal cortisol to the inactive corticosterone, preventing the fetus from being exposed to too-high levels of maternal cortisol [64, 102]. There is, however, a threshold at which the placenta can no longer sufficiently convert maternal cortisol, resulting in fetal exposure to inappropriately high cortisol levels, as prenatal stress results in reduced expression of 11β-HSD2 mRNA and decreased enzymatic activity [102, 104]. Such high levels of cortisol are associated with LBW and inappropriate stress response and anxiety in the infant and child [105], indicating the importance of the placenta as a buffer.
Prenatal stress and depression are also associated with changes in brain development and function, emotional problems into adolescence, lower birthweights, and increased risk of preterm birth [106, 107]. The effect on birthweight may be due to cortisol’s ability to increase placental cortisol-releasing hormone concentrations, which have in turn been found to be inversely correlated with fetal growth [107]. Depression during pregnancy also appears to affect fetal development and has been associated with poorer obstetric and developmental outcomes, increased neonatal cortisol levels, fussiness, and sleep problems [108]. Long-term, prenatal depression is associated with increased risk of attention deficit hyperactivity disorder, emotional problems, impaired cognitive development [109], and lower childhood IQ scores [110]. Prenatal depression has been associated with SGA and LBW [111–113], although there are also many studies that have found no association between prenatal depression and offspring birthweights, especially when premature birth is properly accounted for [112, 114, 115]. Depression during pregnancy also decreases the likelihood of breastfeeding initiation [116], which may affect growth. Some of these growth effects may be due to depression reducing sufficient dietary intake during pregnancy, thereby limiting infant growth [117].
The link between severe prenatal stressors, such as physical intimate partner violence [118], and lower birthweight outcomes is more well established, though the role of more mild stressors, such as psychosocial work-related stress [119], and anxiety is still debated, with some studies finding lower birthweights [120] and others no effect [121]. Despite the mixed findings, sufficient evidence exists to imply that the stress experienced by women undergoing IVF may result in lower birthweight outcomes among their offspring, especially given that stressful exposures prior to conception appear to have an effect as well; women who experienced a stressful event—which included a death of a parent, divorce or separation, or death of their partner, and, notable, fertility problems—prior to conception were found to be 38% more likely to have an LBW infant [122]. These studies all point to prenatal stressors as important determinants of infant development.
Couples struggling with infertility and undergoing IVF are under considerable stress, with greater rates of depression and anxiety than their fertile peers’ experience [4, 5]. Increased IVF failures have been found to increase depression and anxiety [6–8], and infertility has been associated with decreased life satisfaction [123]. Additionally, pregnancy-related stress—stress related to being pregnant and concerning the health of the child—has been found to be a more significant factor in infant development than other forms of anxiety [107]. Women who struggle to get pregnant may have higher levels of this form of stress. Although the placenta buffers much of the maternal cortisol from reaching the fetus, 40% of the variance in fetal cortisol is determined by maternal cortisol concentrations [124]. Inappropriately high cortisol levels can interfere with normal development of the fetus’s stress axis, resulting in inappropriate stress responses throughout life. Anxiety during pregnancy interferes with the placenta’s ability to buffer the infant from maternal cortisol by reducing placental 11-β-HSD2 mRNA levels [110], thereby setting the infant up for inappropriate development of his or her stress-regulating systems, with long-term consequences; infants who were exposed to prenatal stress have been found to have dysregulated hormonal stress systems and behavior [110].
Identifying which birthweight outcomes among IVF conceived infants are due to the genetics of underlying stress and to the stress of infertility and a difficult to conceive pregnancy is challenging. In an attempt to differentiate between the two, Rice et al. examined offspring outcomes in IVF infants who were conceived using autologous and donor oocytes [125]. If lower birthweights were due to the maternal stress, rather than underlying genetics, the expected outcomes would be present even in those conceived using donor oocytes. They found that maternal stress, even in the donor pregnancies, was significantly correlated with reduced birthweights, indicating that there are components of the prenatal environment that are being affected by maternal stress, and this effect is not just due to a genetic component. Child antisocial behavior and anxiety were also found to be associated with maternal prenatal stress, even in donor pregnancies.
As stated above, maternal prenatal stress has been found to affect the placenta’s ability to buffer infants from maternal cortisol and has been found to do so by altering the expression of 11-β-HSD2 [104] via epigenetic mechanisms. Increased 11-β-HSD2 methylation, which would result in lower gene expression, is associated with lower birthweights [126]. Perceived prenatal stress has been associated with increased 11-β-HSD2 methylation [127], while stressors such as increased socioeconomic adversity are associated with decreased methylation [128]. Prenatal anxiety and mood disorders have been found to affect methylation of the NR3C1 gene in cord blood leukocytes [129]; NR3C1 encodes for a glucocorticoid receptor and, therefore, responsivity to stress hormones [130]. Stressors such as intimate partner violence and war-related events have also been found to affect cord blood and placental methylation of NR3C1 regulatory regions, as well as other stress axis–related genes [102, 131, 132], indicating that epigenetic mechanisms are involved in responding to, and are affected by, prenatal stressors.
Studies to date, however, have mostly focused on mechanisms related to stress response and the stress axis, such as cortisol processing and receptivity. Little is known about how prenatal stress affects imprinted genes and other neurodevelopmental and growth processes. Prenatal depression has been found to be associated with differences in regions associated with three imprinted genes—MEG3, IGF2, and PLAGL1—all of which are involved in infant growth [133]. This finding indicates that imprinted genes may be involved in the birthweight outcomes observed in infants of prenatally stressed and depressed mothers. PEG3, another imprinted gene associated with growth, has also been found to have decreased placental expression in infants whose mothers were diagnosed with depression during pregnancy [134]. In addition, prenatal maternal stress has been associated with IGF2 regulatory region methylation [135] and decreased average ICR methylation [136] in infant cord blood. Additional research is needed, though, to verify this association and better understand the mechanism by which stress affects imprinted genes and birthweights.
Separating out the effects of prenatal stressors from the underlying causes of those stressors is complicated by the large number of factors which are associated with stress, low birthweight, and epigenetic changes. For example, cigarette smoking is more common in women who are anxious or depressed [137] and those of lower SES [138, 139], who are also more likely to be stressed. Both of these factors—smoking and SES—are also associated with lower birthweights and epigenetic effects [140–142]. Additionally, there is much debate about the effects of pharmacological treatments for depression and anxiety on both birthweight and epigenetics [143, 144]. Access to such treatments, however, and the reasons why women may or may not take psychiatric medications are complicated by underlying cultural and demographic factors [145, 146], making it difficult to distinguish the effects of the underlying condition and those of the treatment for it. Appropriate study populations with sufficient data to control for these factors are required to properly identify the effects of prenatal stress and depression on imprinted genes.
Placental imprinted genes, infertility, and prenatal depression and anxiety in the RICHS cohort
To differentiate between the impact of infertility and that of IVF on birthweight, and to explore the epigenetic mechanism behind these relationships, we used the Rhode Island Child Health Study (RICHS) cohort, which contains data on over 800 mothers who delivered a full-term, healthy infant at Women and Infants’ Hospital in Rhode Island between 2009 and 2014. In addition to in depth demographics and exposure data, placental RNA expression data are available for all of these mother–infant pairs for the comprehensive library of known and putative imprinted genes (a total of 108 genes). RNA data were acquired via Nanostring and a subset was verified using RNA-seq. From RICHS, we designed a nested matched cohort that included IVF pregnancies (n = 18), infants whose parents self-reported infertility, defined as difficulty conceiving for a year or more, but did not use IVF (n = 79) and controls (n = 158) [63].
Within our nested matched cohort, we compared placental imprinted gene RNA expression between infants whose mothers reported infertility, infants conceived with IVF, and fertile controls. We found that the majority of the differences in imprinted gene expression were between the controls and those with infertility (45 genes), with no differences between the controls and those conceived with IVF. Five genes were differentially expressed between those with IVF and those with infertility, but four of these genes were also found to be differentially expressed between the infertile group and the controls. These findings indicate that there are differences in imprinted gene expression that are associated with infertility but are not associated with the IVF procedure. Given the small number of known imprinted genes (108 analyzed in this study), a finding of 45, or even 5, genes with differential RNA expression between two groups represents a large percentage of the genes evaluated. Given our stringent analytical approaches, these findings would be unlikely to have been found by chance, and given the important role of imprinting, a potentially clinically important intermediary worthy of further examination.
The group of five genes identified in IVF conceived placentae—IGF2-AS, IGF2, NAPIL5, PAX8-AS1, and TUBGCP5—may be associated with more extreme infertility rather than the IVF procedure. However, due to our small sample size of IVF placentae, we cannot rule out an effect of IVF on placental imprinted genes. We can, though, conclude that there are differences in these genes related to infertility in comparison with the general population. Studies on imprinted genes and epigenetics in IVF rarely take participants’ underlying infertility into account and rarely use an infertile population as the control group. Our findings indicate that future work needs to control for the underlying reasons for IVF to ensure that findings indicating epigenetic effects of IVF are not actually identifying an underlying cause or effect of infertility.
In our prior study on infertility [63], we were able to control for many factors that affect birthweight but we did not control for the potential underlying stress that women who have difficulty conceiving and who receive IVF treatments may be experiencing. However, within the cohort studied, rates of depression and anxiety among those women experiencing infertility and IVF were higher than those in the general public, as expected in light of the studies indicating higher levels of depression and anxiety among those with infertility and undergoing IVF [4–8, 123]. Within the nested, matched cohort used in our study on IVF and infertility, 20% of women in the infertile and IVF groups had depression during pregnancy compared with 13% of the control population. Similarly, 25% of the infertile and IVF groups had diagnosed anxiety/OCD/panic during pregnancy compared with only 17% of the control population.
Because of the small sample size of women with infertility and IVF and the lack of a measure specifically for levels of psychiatric stress or depression in the RICHS cohort, it was not possible to control for these stressors when examining the role of IVF and infertility in imprinted gene expression. However, we can establish whether there is an effect of depression and anxiety on imprinted genes within a healthy cohort, and little is known about the effects of the more common levels of stress and depression that pregnant women are exposed to and the potential effects of slightly higher levels that may be generated by IVF and infertility. Therefore, to determine the effect of depression and anxiety on placental imprinted gene RNA expression, we expanded our study group to include the full RICHS cohort that had complete data to allow for a greater sample size.
Within the RICHS cohort, we compared women who had both depression and anxiety during pregnancy, as diagnosed by a physician at a prenatal visit, (n = 54) with those who had no history of either stressor prior to or during pregnancy (n = 458) [147]. We identified 21 imprinted genes for which the expression levels were different based on exposure to prenatal depression and anxiety, with an additional six genes that had significant differences in placental RNA expression in infants whose mothers were taking a psychiatric medication during pregnancy (n = 29) in comparison with controls. Most of the 21 genes identified as being associated with prenatal depression and anxiety were also significantly differentially expressed in placentae from infants exposed to prenatal psychiatric medication. None of these genes were significantly different among those with depression or anxiety who were and were not taking medication; we do not think, therefore, that these findings were due to an effect of prenatal psychiatric medication use but rather that those taking medications had a greater level of symptoms resulting in a greater effect on gene expression. This is supported by the fact that the genes with greatest difference in expression between those with depression and anxiety and controls had an even greater effect size in those taking medications in comparison with controls. Our results suggest that the levels of stress resulting from diagnosed prenatal depression and anxiety are sufficient to result in an effect on placental imprinted gene expression.
Relationships between genes with differences in placental imprinted gene RNA expression associated with infertility and with prenatal depression and anxiety
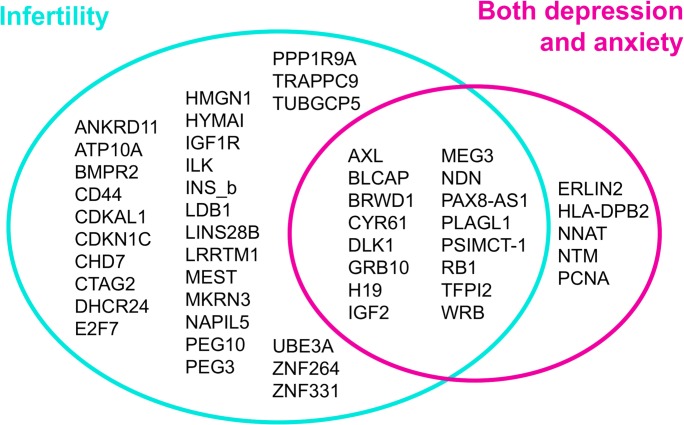
Given the prevalence of prenatal depression and anxiety in the IVF and infertile populations, as well as the similarities in infant birthweight and obstetric outcomes between the two groups, we hypothesized that the apparent effects of IVF and infertility on infant outcomes may in fact be confounded by underlying prenatal depression and anxiety. To evaluate the potential role of prenatal stressors in imprinted gene differences associated with infertility, we compared the genes identified as being differentially expressed in association with infertility and with depression/anxiety (Table 1). Of the 45 genes identified as being differentially expressed between those with infertility and the controls, 16 were differentially expressed in those with both depression and anxiety during pregnancy (Fig. 1; Table 1). An additional six genes were identified as being differentially expressed in association with both infertility and with taking psychiatric medication during pregnancy, with similar effect sizes identified with both exposures (Table 2). As we are considering use of a psychiatric medication as a more extreme phenotype of depression/anxiety, the observed differences in expression are similarly likely related to underlying stress. Because different cohorts from within the RICHS cohort were used to estimate effect sizes associated with infertility and prenatal anxiety/depression, these two studies are not directly comparable. However, effect sizes for each gene in both studies were similar, which implies that the findings related to infertility may have been due, at least in part, to underlying depression and anxiety that was not examined in that study.
Table 1.
Genes with differences in expression associated with both infertility and prenatal depression and anxiety
| Effect change associated with infertility | Effect change associated with prenatal stressors | |
|---|---|---|
| AXL | − 0.464 | − 0.411 |
| BLCAP | − 0.162 | − 0.127 |
| BRWD1 | − 0.261 | − 0.211 |
| CYR61 | − 0.447 | − 0.455 |
| DLK1 | − 0.586 | − 0.570 |
| GRB10 | − 0.282 | − 0.224 |
| H19 | − 0.331 | − 0.297 |
| IGF2 | − 0.495 | − 0.435 |
| MEG3 | − 0.620 | − 0.791 |
| NDN | − 0.343 | − 0.460 |
| PAX8-AS1 | − 0.292 | − 0.343 |
| PLAGL1 | − 0.568 | − 0.626 |
| PSIMCT-1 | − 0.309 | − 0.268 |
| RB1 | − 0.189 | − 0.218 |
| TFPI2 | 0.201 | 0.304 |
| WRB | − 0.149 | − 0.167 |
A negative value indicates decreased RNA expression in the case group in comparison with controls. Data has been previously published in [68] and [151]
Fig. 1.
Venn diagram showing similarities and differences in genes identified as being differentially expressed between those with infertility and the controls and genes differentially expressed in those with both depression and anxiety during pregnancy
Table 2.
Genes with differences in expression associated with infertility and with prenatal depression and anxiety in those who used psychiatric medication during pregnancy
| Effect change associated with infertility | Effect change associated with prenatal psychiatric medication use | |
|---|---|---|
| CD44 | − 0.317 | − 0.333 |
| CTAG2 | 0.259 | 0.473 |
| E2F7 | 0.170 | 0.311 |
| HYMAI | − 0.227 | − 0.406 |
| ILK | − 0.071 | − 0.131 |
| MEST | − 0.281 | − 0.298 |
A negative value indicates decreased RNA expression in the case group in comparison with controls. Data has been previously published in [68] and [151]
Our findings and the growing body of literature on epigenetic effects of the prenatal environment provide evidence to suggest that differential expression of imprinted genes associated with infertility may be due to the underlying anxiety and depression that has been found to be of a higher incidence in the IVF and infertile populations. Many of the genes identified as having differences in placental RNA expression associated with infertility had differences in expression between cases and controls of similar magnitudes to those associated with prenatal depression and anxiety. Therefore, the cause of the lower birthweights and other outcomes seen in IVF infants, many of which have also been found to be associated with prenatal stressors, may be due to underlying stress rather than infertility or IVF. However, there were also 23 genes which were identified as having differential expression related to infertility that were not found to have differential expression associated with prenatal stressors.
Some of these genes may be identified as being associated with prenatal stressors in a study with a larger sample size and greater power to detect differences. However, we were able to detect differences of similar effect sizes associated with infertility and depression/anxiety, so this is unlikely to be the case. These data also suggest that the lower birthweights observed in IVF infants are likely due to a combination of exposures including (1) the underlying genetics of the infertility requiring IVF; (2) the stress that mothers are experiencing prenatally, which is likely due to both genetics and the procedures and effort required to achieve pregnancy; and (3) the IVF techniques used, which may be affecting early placental development and imprinted gene expression.
Limitations and future directions
Our work can help to guide future studies on the effects of IVF and infertility on infant outcomes, as it highlights the importance of accounting for exposure to prenatal depression and anxiety in the IVF and infertile populations. Although the RICHS cohort provides a comprehensive profile of placental RNA expression for the complete library of known and putative imprinted genes, RICHS was not designed specifically to study IVF, infertility, or prenatal depression or anxiety. Therefore, although the RICHS cohort contains data on infertility, these data are self-report and no cause is provided. IVF is also self-reported in this cohort, and data on the type of procedure used are usually not available. As a result, we could not look at the effect of type of infertility on imprinted gene expression using this cohort.
Similarly, because we used obstetric medical records to determine prenatal depression and anxiety exposure, which resulted in a binary variable of physician diagnosis during pregnancy, we were not able to examine a possible dose–response relationship between prenatal stressors and birthweight. A depression scale, and ideally maternal cortisol levels, would be needed to determine the mechanism behind prenatal stressors such as infertility and IVF and imprinted gene expression. We also did not have sufficient sample size to explore the effects of depression and anxiety as related to infertility and IVF in the RICHS cohort. Further work in a cohort with more IVF conceptions and better data on levels of depression and anxiety is needed to explore the potential dose–response relationship between stressors and birthweight. A cohort that focuses on IVF and infertility is needed to help to fully elucidate how much of the lower birthweights seen in IVF and infertile populations is actually associated with infertility and IVF rather than prenatal stressors. Such a cohort would ideally include maternal and infant cortisol levels and measures of anxiety and depression during each trimester to allow for evaluation of potentially differing effects of stressors based on developmental stage.
Although this lack of detailed IVF and depression/anxiety data prevented us from narrowing our study to specific types of infertility or levels of depression/anxiety, our ability to find differences in gene expression, even though our infertile group contained a range of diagnoses, infertility was a self-reported variable, and we did not have specific trimesters or severity for depression/anxiety diagnosis, supports the strength of our findings and the conclusion that there is a true difference in placental gene expression in those with and without infertility and in those exposed to prenatal stressors. RICHS is one of the largest cohorts available for study of placental epigenetics and imprinted genes, and our data on imprinted gene expression are much more comprehensive than most similar studies since placental RNA expression levels for all known or putative imprinted genes are available for most of the RICHS participants; most studies on imprinted genes focus instead on a select few genes that are suspected to be involved in the pathway of interest. The detailed demographic and exposure data available within the RICHS cohort provides the ability to match on and control for many potentially confounding factors, allowing for complicated and detailed experiments. Therefore, despite the small sample size available in this cohort, we were able to design well-powered experiments that identified novel relationships between prenatal exposures and imprinted gene expression.
Conclusions
Overall, these findings advocate for more careful examination of the potential role that prenatal stressors and underlying infertility play in the outcomes, both molecular and phenotypic, experienced following IVF procedures. Specifically, future work on birthweight outcomes in the IVF and infertile populations needs to account for the underlying stress these women experience due to pregnancy anxiety, difficulty conceiving, the procedures they are undergoing, and other life stressors. Our results indicate that this underlying stress represents an important confounder to the relationship between infertility and birthweight outcomes, as studied through imprinted gene expression. Our work highlights the need to include prenatal depression and anxiety as a variable in studies on IVF and infertile couples’ infant outcomes, especially those exploring the mechanisms behind lower birthweights.
Additionally, the imprinted genes identified as associated with lower birthweight provide an opportunity for intervention that could improve birthweight outcomes, thereby improving the long-term health of IVF conceived infants. Findings regarding the etiology of lower birthweights in association with IVF, infertility, and prenatal stressors can also be applied to the general population. In particular, a greater focus on reducing prenatal stress and depression could be a powerful public health goal for improving birthweight outcomes, especially within the infertile population.
Funding information
This work is funded by grants from the National Institutes of Health (NIH-NIMH R01MH094609, NIH-NIEHS R01ES022223).
Compliance with ethical standards
Disclaimer
Funding sources had no involvement in data collection, data analysis, or generation of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention; American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. 2014 Assisted reproductive technology fertility clinic success rates report [Internet]. US Dept Heal. Hum. Serv. 2016. Available from: https://www.cdc.gov/art/pdf/2014-report/art-2014-national-summary-report.pdf. Accessed 17 Feb 2019.
- 2.Barnhart KT. How do we explain the association between assisted reproductive technologies and perinatal morbidity? Fertil Steril [Internet] American Society for Reproductive Medicine. 2015;103:896–897. doi: 10.1016/j.fertnstert.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Sunderam S, Kissin D, Crawford S, Folger S, Jamieson D, Barfield W. Assisted reproductive technology surveillance - United States. 2014. MMWR Surveill Summ. 2014;63:1–28. [PubMed] [Google Scholar]
- 4.Shani C, Yelena S, Reut BK, Adrian S, Sami H. Suicidal risk among infertile women undergoing in-vitro fertilization: incidence and risk factors. Psychiatry Res. [Internet]. Elsevier; 2016;240:53–9. Available from: 10.1016/j.psychres.2016.04.003 [DOI] [PubMed]
- 5.Vahratian A, Smith YR, Dorman M, Flynn HA. Longitudinal depressive symptoms and state anxiety among women using assisted reproductive technology. Fertil Steril [Internet]. Elsevier Ltd; 2011;95:1192–4. [DOI] [PubMed]
- 6.Pasch LA, Gregorich SE, Katz PK, Millstein SG, Nachtigall RD, Bleil ME, et al. Psychological distress and in vitro fertilization outcome. Fertil Steril [Internet]. Elsevier; 2012;98:459–64. [DOI] [PMC free article] [PubMed]
- 7.Wu G, Yin T, Yang J, Xu W, Zou Y, Wang Y, et al. Depression and coping strategies of Chinese women undergoing in-vitro fertilization. Eur J Obstet Gynecol [Internet]. Elsevier Ireland Ltd; 2014;183:155–8. Available from: 10.1016/j.ejogrb.2014.10.019. [DOI] [PubMed]
- 8.Biringer E, Howard LM, Kessler U, Stewart R, Mykletun A. Is infertility really associated with higher levels of mental distress in the female population? Results from the North-Trøndelag Health Study and the Medical Birth Registry of Norway. J Psychosom Obstet Gynecol. 2015;36:38–45. doi: 10.3109/0167482X.2014.992411. [DOI] [PubMed] [Google Scholar]
- 9.Palomba S, Homburg R, Santagni S, La Sala GB, Orvieto R. Risk of adverse pregnancy and perinatal outcomes after high technology infertility treatment: a comprehensive systematic review. Reprod Biol Endocrinol [Internet] Reprod Biol Endocrinol. 2016;14:76. doi: 10.1186/s12958-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Angelo D V., Whitehead N, Helms K, Barfield W, Ahluwalia IB. Birth outcomes of intended pregnancies among women who used assisted reproductive technology, ovulation stimulation, or no treatment. Fertil. Steril. [Internet]. Elsevier Ltd; 2011;96:314–320.e2. Available from: 10.1016/j.fertnstert.2011.05.073 [DOI] [PubMed]
- 11.Bradbury K, Sutcliffe A. The health of children born following assisted reproductive technologies. Paediatr Child Health (Oxford). [Internet]. Elsevier Ltd; 2014;24:172–6.
- 12.Halliday J. Outcomes of IVF conceptions: are they different? Best Pract Res Clin Obstet Gynaecol. 2007;21:67–81. doi: 10.1016/j.bpobgyn.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M, Bower C. The impact of assisted reproductive technologies on intra-uterine growth and birth defects in singletons. Semin. Fetal Neonatal Med. [Internet]. Elsevier Ltd; 2014;19:228–33. Available from: 10.1016/j.siny.2014.03.002 [DOI] [PubMed]
- 14.Watkins WJ, Kotecha SJ, Kotecha S. All-cause mortality of low birthweight infants in infancy, childhood, and adolescence: population study of England and Wales. PLoS Med [Internet] 2016;13:1–20. doi: 10.1371/journal.pmed.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syddall HE, Sayer AA, Simmonds SJ, Osmond C, Cox V, Dennison EM, et al. Birth weight, infant weight gain, and cause-specific mortality: the Hertfordshire Cohort Study. Am J Epidemiol. 2005;161:1074–80. [DOI] [PubMed]
- 16.Johnson LSB, Salonen M, Kajantie E, Conen D, Healey JS, Osmond C, et al. Early life risk factors for incident atrial fibrillation in the Helsinki Birth Cohort Study. J Am Heart Assoc [Internet]. 2017;6:1–8. [DOI] [PMC free article] [PubMed]
- 17.Barker DJP, Larsen G, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The placental origins of sudden cardiac death. Int J Epidemiol. 2012;41:1394–1399. doi: 10.1093/ije/dys116. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson JG, Salonen MK, Kajantie E, Osmond C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki birth cohort study, 1924-1944. Am. J. Kidney Dis. [Internet]. Elsevier Inc; 2017;71:20–6. Available from: 10.1053/j.ajkd.2017.06.030 [DOI] [PubMed]
- 19.Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27:1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 20.Kondapalli LA, Perales-Puchalt A. Low birth weight: is it related to assisted reproductive technology or underlying infertility? Fertil. Steril. [Internet]. Elsevier Inc.; 2013;99:303–10. Available from: 10.1016/j.fertnstert.2012.12.035 [DOI] [PMC free article] [PubMed]
- 21.Mak W, Kondapalli LA, Celia G, Gordon J, Dimattina M, Payson M. Natural cycle IVF reduces the risk of low birthweight infants compared with conventional stimulated IVF. Hum Reprod. 2016;31:789–794. doi: 10.1093/humrep/dew024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litzky JF, Boulet SL, Esfandiari N, Zhang Y, Kissin DM, Theiler RN, et al. Effect of frozen/thawed embryo transfer on birthweight, macrosomia, and low birthweight rates in US singleton infants. Am J Obstet Gynecol [Internet]. Elsevier Inc.; 2018;218:433.e1–433.e10. [DOI] [PMC free article] [PubMed]
- 23.Luke B, Brown MB, Wantman E, Stern JE, Toner JP, Coddington CC. Increased risk of large-for-gestational age birthweight in singleton siblings conceived with in vitro fertilization in frozen versus fresh cycles. J Assist Reprod Genet. 2017;34:191–200. doi: 10.1007/s10815-016-0850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer UE, Barfield WD, Morris K, Folger SG, Boulet SL, Chang J, et al. 2015 Assisted reproductive technology national summary report. Centers Dis Control Prev Am Soc Reprod Med Soc Assist Reprod Technol [Internet]. Atlanta: US Dept of Health and Human Services; 2017. pp. 1–80.
- 25.Palomba S, Santagni S, Gibbins K, La Sala GB, Silver RM. Pregnancy complications in spontaneous and assisted conceptions of women with infertility and subfertility factors. A comprehensive review. Reprod. Biomed. Online [Internet]. Elsevier Ltd; 2016;33:612–28. Available from: 10.1016/j.rbmo.2016.08.007 [DOI] [PubMed]
- 26.Thomson F, Shanbhag S, Templeton A, Bhattacharya S. Obstetric outcome in women with subfertility. Bjog [Internet]. 2005;112:632–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15842289. [DOI] [PubMed]
- 27.Luke B, Stern JE, Hornstein MD, Kotelchuck M, Diop H, Cabral H, et al. Is the wrong question being asked in infertility research? J Assist Reprod Genet. 2016;33:3–8. [DOI] [PMC free article] [PubMed]
- 28.Zhu JL, Basso O, Obel C, Hvidtjørn D, Olsen J. Infertility, infertility treatment and psychomotor development: the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2009;23:98–106. doi: 10.1111/j.1365-3016.2008.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso O, Baird DD. Infertility and preterm delivery, birthweight, and caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18:2478–2484. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- 30.Cooney MA, Buck Louis GM, Sun W, Rice MM, Klebanoff MA. Is conception delay a risk factor for reduced gestation or birthweight? Paediatr Perinat Epidemiol. 2006;20:201–209. doi: 10.1111/j.1365-3016.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper AR, O’Neill KE, Allsworth JE, Jungheim ES, Odibo AO, Gray DL, et al. Smaller fetal size in singletons after infertility therapies: the influence of technology and the underlying infertility. Fertil. Steril. [Internet]. Elsevier Ltd; 2011;96:1100–6. Available from: 10.1016/j.fertnstert.2011.08.038, 2011. [DOI] [PMC free article] [PubMed]
- 32.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Sö derström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 33.Elenis E, Sydsjö G, Skalkidou A, Lampic C, Svanberg AS. Neonatal outcomes in pregnancies resulting from oocyte donation: a cohort study in Sweden. BMC Pediatr [Internet] BMC Pediatrics. 2016;16:1–10. doi: 10.1186/s12887-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dun EC, Nezhat CH. Tubal factor infertility. Diagnosis and Management in the era of assisted reproductive technology. Obstet. Gynecol. Clin. North Am. [Internet]. Elsevier Inc; 2012;39:551–66. Available from: 10.1016/j.ogc.2012.09.006 [DOI] [PubMed]
- 35.Grigorescu V, Zhang Y, Kissin DM, Sauber-Schatz E, Sunderam M, Kirby RS, et al. Maternal characteristics and pregnancy outcomes after assisted reproductive technology by infertility diagnosis: ovulatory dysfunction versus tubal obstruction. Fertil. Steril. [Internet]. Elsevier; 2014;101:1019–25. Available from: 10.1016/j.fertnstert.2013.12.030 [DOI] [PMC free article] [PubMed]
- 36.Holliday R. Epigenetics: a historical overview. Epigenetics. 2006;1:76–80. doi: 10.4161/epi.1.2.2762. [DOI] [PubMed] [Google Scholar]
- 37.Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review) Mol Med Rep. 2012;5:883–889. doi: 10.3892/mmr.2012.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature [Internet] 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 39.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol [Internet] Nat Publ Group. 2013;20:282–9. [DOI] [PubMed]
- 40.Smallwood SA, Tomizawa S-I, Krueger F, Ruf N, Carli N, Segonds-Pichon A, et al. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat Genet [Internet] Nat Publ Group. 2011;43:811–814. doi: 10.1038/ng.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsit CJ. Influence of environmental exposure on human epigenetic regulation. J Exp Biol [Internet]. 2015;218:71–9. [DOI] [PMC free article] [PubMed]
- 42.Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21 and miR-146a in the placenta. Epigenetics. 2010;5:583–589. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kingsley SL, Eliot MN, Whitsel EA, Huang YT, Kelsey KT, Marsit CJ, et al. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ. Int. [Internet]. Elsevier Ltd; 2016;92–93:43–9. Available from: 10.1016/j.envint.2016.03.020 [DOI] [PMC free article] [PubMed]
- 44.Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, et al. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environ Health [Internet]. 2013;12:58. [DOI] [PMC free article] [PubMed]
- 45.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7:9–17. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma M, Rogers S, Divi RL, Schully SD, Nelson S, Su J, et al. Epigenetic research in cancer epidemiology: trends, opportunities, and challenges. Cancer Epidemiol Biomark Prev. 2014;23:223–233. doi: 10.1158/1055-9965.EPI-13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammoud SS, Cairns BR, Jones DA. Epigenetic regulation of colon cancer and intestinal stem cells. Curr Opin Cell Biol. 2013;25:177–183. doi: 10.1016/j.ceb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piedrahita JA. The role of imprinted genes in fetal growth abnormalities. Birth Defects Res (Part A) Clin Mol Teratol [Internet] 2011;91:682–692. doi: 10.1002/bdra.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelissen ECM, van Montfoort APA, Dumoulin JCM, Evers JLH. Epigenetics and the placenta. Hum Reprod Update [Internet] 2011;17:397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- 50.Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet. 2005;21:457–465. doi: 10.1016/j.tig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Maupetit-Méhouas S, Montibus B, Nury D, Tayama C, Wassef M, Kota SK, et al. Imprinting control regions (ICRs) are marked by mono-allelic bivalent chromatin when transcriptionally inactive. Nucleic Acids Res. 2016;44:621–35. [DOI] [PMC free article] [PubMed]
- 52.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 53.Lambertini L, Marsit CJ, Sharma P, MacCani M, Ma Y, Hu J, et al. Imprinted gene expression in fetal growth and development. Placenta. 2012;33:480–486. doi: 10.1016/j.placenta.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kappil MA, Green BB, Armstrong DA, Sharp AJ, Lambertini L, Marsit CJ, et al. Placental expression profile of imprinted genes impacts birth weight. Epigenetics [Internet] 2015;10:842–849. doi: 10.1080/15592294.2015.1073881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green BB, Kappil M, Lambertini L, Armstrong DA, Guerin DJ, Sharp AJ, et al. Expression of imprinted genes in placenta is associated with infant neurobehavioral development. Epigenetics [Internet] 2015;10:834–841. doi: 10.1080/15592294.2015.1073880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao W-L, Liu M, Yang Y, Yang H, Liao Q, Bai Y, et al. The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets Nodal Modulator 1 (NOMO1) RNA Biol [Internet] 2012;9:1002–1010. doi: 10.4161/rna.20807. [DOI] [PubMed] [Google Scholar]
- 57.Tabano S, Colapietro P, Cetin I, Grati FR, Zanutto S, Mandò C, et al. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2010;5:313–24. [DOI] [PubMed]
- 58.Netchine I, Rossignol S, Dufourg MN, Azzi S, Rousseau A, Perin L, et al. 11p15 imprinting center region 1 loss of methylation is a common and specific cause of typical Russell-Silver syndrome: clinical scoring system and epigenetic-phenotypic correlations. J Clin Endocrinol Metab. 2007;92:3148–54. [DOI] [PubMed]
- 59.Tycko B, Morison IM. Physiological functions of imprinted genes. J Cell Physiol. 2002;192:245–258. doi: 10.1002/jcp.10129. [DOI] [PubMed] [Google Scholar]
- 60.Bressan FF, De Bem THC, Perecin F, Lopes FL, Ambrosio CE, Meirelles F V., et al. Unearthing the roles of imprinted genes in the placenta. Placenta [Internet]. Elsevier Ltd; 2009;30:823–34. Available from: 10.1016/j.placenta.2009.07.007. [DOI] [PubMed]
- 61.Himes KP, Young A, Koppes E, Stolz D, Barak Y, Sadovsky Y, et al. Loss of inherited genomic imprints in mice leads to severe disruption in placental lipid metabolism. Placenta [Internet]. Elsevier Ltd; 2015;36:389–96. Available from: 10.1016/j.placenta.2015.01.012. [DOI] [PMC free article] [PubMed]
- 62.Butler MG. Genomic imprinting disorders in humans: a mini-review. J Assist Reprod Genet. 2009;26:477–486. doi: 10.1007/s10815-009-9353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Litzky JF, Deyssenroth MA, Everson TM, Armstrong DA, Lambertini L, Chen J, et al. Placental imprinting variation associated with assisted reproductive technologies and subfertility. Epigenetics [Internet] Taylor & Francis. 2017;12:1–9. doi: 10.1080/15592294.2017.1336589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heffner LJ, Schust DJ. The reproductive system at a glance. 4. West Sussex: John Wiley & Sons Ltd.; 2014. [Google Scholar]
- 65.Díaz P, Powell TL, Jansson T. The role of placental nutrient sensing in maternal-fetal resource allocation. Biol Reprod [Internet] 2014;91:1–10. doi: 10.1095/biolreprod.114.121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winder NR, Krishnaveni GV, Veena SR, Hill JC, Karat CLS, Thornburg KL, et al. Mother’s lifetime nutrition and the size, shape and efficiency of the placenta. Placenta. 2011;32:806–10. [DOI] [PMC free article] [PubMed]
- 67.Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29:187–196. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roland MCP, Friis CM, Voldner N, Godang K, Bollerslev J, Haugen G, et al. Fetal growth versus birthweight: the role of placenta versus other determinants. PLoS One. 2012;7:e39324. [DOI] [PMC free article] [PubMed]
- 69.Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, et al. Placental adaptation: what can we learn from birthweight:placental weight ratio? Front Physiol. 2016;7:1–13. doi: 10.3389/fphys.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. 2015;213:S14–S20. doi: 10.1016/j.ajog.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reik W, Constância M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, et al. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore T, Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 1991;7:45–49. doi: 10.1016/0168-9525(91)90040-W. [DOI] [PubMed] [Google Scholar]
- 73.Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun [Internet] Nat Publ Group. 2014;5:1–8. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smeester L, Yosim AE, Nye MD, Hoyo C, Murphy SK, Fry RC. Imprinted genes and the environment: Links to the toxic metals arsenic, cadmium and lead. Genes (Basel) 2014;5:477–496. doi: 10.3390/genes5020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vidal AC, Semenova V, Darrah T, Vengosh A, Huang Z, King K, et al. Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol Toxicol BMC Pharmacology and Toxicology. 2015;16:1–9. doi: 10.1186/2050-6511-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds RM, Jacobsen GH, Drake AJ. What is the evidence in humans that DNA methylation changes link events in utero and later life disease? Clin Endocrinol. 2013;78:814–822. doi: 10.1111/cen.12164. [DOI] [PubMed] [Google Scholar]
- 77.Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, et al. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med [Internet] 2013;11:1–10. doi: 10.1186/1741-7015-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCullough LE, Mendez MA, Miller EE, Murtha AP, Murphy SK, Hoyo C. Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics. 2015;10:597–606. doi: 10.1080/15592294.2015.1045181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoyo C, Murtha AP, Schildkraut JM, Jirtle R, Demark-Wahnefried W, Forman MR, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6:928–936. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt A, Morales-Prieto DM, Pastuschek J, Fröhlich K, Markert UR. Only humans have human placentas: molecular differences between mice and humans. J. Reprod. Immunol. [Internet]. Elsevier Ireland Ltd; 2015;108:65–71. Available from: 10.1016/j.jri.2015.03.001 [DOI] [PubMed]
- 81.Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol [Internet] 2014;27:11–18. doi: 10.1293/tox.2013-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Owen CM, Segars JH. Imprinting disorders and assisted reproductive technology. Semin Reprod Med [Internet] American Society for Reproductive Medicine. 2009;27:417–428. doi: 10.1055/s-0029-1237430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loke YJ, Galati JC, Saffery R, Craig JM. Association of in vitro fertilization with global and IGF2/H19 methylation variation in newborn twins. J Dev Orig Health Dis [Internet]. 2015;6:115–24. [DOI] [PubMed]
- 84.Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–78. [DOI] [PMC free article] [PubMed]
- 85.Sakian S, Louie K, Wong EC, Havelock J, Kashyap S, Rowe T, et al. Altered gene expression of H19 and IGF2 in placentas from ART pregnancies. Placenta [Internet]. Elsevier Ltd; 2015;36:1100–5. Available from: 10.1016/j.placenta.2015.08.008 [DOI] [PubMed]
- 86.Katagiri Y, Aoki C, Tamaki-Ishihara Y, Fukuda Y, Kitamura M, Matsue Y, et al. Effects of assisted reproduction technology on placental imprinted gene expression. Obstet Gynecol Int [Internet] 2010;2010:4–7. doi: 10.1155/2010/437528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huntriss JD, Picton HM. Epigenetic consequences of assisted reproduction and infertility on the human preimplantation embryo. Hum Fertil (Camb) [Internet] 2008;11:85–94. doi: 10.1080/14647270802116250. [DOI] [PubMed] [Google Scholar]
- 88.Chen S, Sun F-Z, Huang X, Wang X, Tang N, Zhu B, et al. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice. Sci Rep [Internet] 2015;5:10596. doi: 10.1038/srep10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan K, An L, Miao K, Ren L, Hou Z, Tao L, et al. Impaired imprinted X chromosome inactivation is responsible for the skewed sex ratio following in vitro fertilization. Proc Natl Acad Sci U S A [Internet] 2016;113:3197–3202. doi: 10.1073/pnas.1523538113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song S, Ghosh J, Mainigi M, Turan N, Weinerman R, Truongcao M, et al. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin Epigenetics [Internet] 2015;7:41. doi: 10.1186/s13148-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitelaw N, Bhattacharya S, Hoad G, Horgan GW, Hamilton M, Haggarty P. Epigenetic status in the offspring of spontaneous and assisted conception. Hum Reprod. 2014;29:1452–1458. doi: 10.1093/humrep/deu094. [DOI] [PubMed] [Google Scholar]
- 92.Kobayashi H, Hiura H, John RM, Sato A, Otsu E, Kobayashi N, et al. DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet [Internet] Nat Publ Group. 2009;17:1582–1591. doi: 10.1038/ejhg.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gunes S, Arslan MA, Hekim GNT, Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet [Internet] Journal of Assisted Reproduction and Genetics. 2016;33:553–569. doi: 10.1007/s10815-016-0682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kitamura A, Miyauchi N, Hamada H, Hiura H, Chiba H, Okae H, et al. Epigenetic alterations in sperm associated with male infertility. Congenit Anom (Kyoto) 2015;55:133–144. doi: 10.1111/cga.12113. [DOI] [PubMed] [Google Scholar]
- 95.Paczkowski M, Schoolcraft WB, Krisher RL. Dysregulation of methylation and expression of imprinted genes in oocytes and reproductive tissues in mice of advanced maternal age. J Assist Reprod Genet. 2015;32:713–723. doi: 10.1007/s10815-015-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stilley JAW, Birt JA, Sharpe-Timms KL. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012;349:849–862. doi: 10.1007/s00441-011-1309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qu F, Wang FF, Yin R, Ding GL, El-prince M, Gao Q, et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J Mol Med. 2012;90:911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- 98.Xu N, Chua AK, Jiang H, Liu N-A, Goodarzi MO. Early embryonic androgen exposure induces transgenerational epigenetic and metabolic changes. Mol Endocrinol [Internet] 2014;28:1329–1336. doi: 10.1210/me.2014-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts V, Myatt L. Placental development and physiology [Internet]. UpToDate. 2017. Available from: https://www.uptodate.com/contents/placental-development-and-physiology?search=placental development and physiology&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed 18 Feb 2019.
- 100.Li B, Chen S, Tang N, Xiao X, Huang J, Jiang F, et al. Assisted reproduction causes reduced fetal growth associated with downregulation of paternally expressed imprinted genes that enhance fetal growth in mice. Biol Reprod [Internet] 2016;94:1–11. doi: 10.1095/biolreprod.115.136051. [DOI] [PubMed] [Google Scholar]
- 101.Cooley S, Donnelly JC, Walsh T, Geary M, Gillan J. 507: subfertility and the placenta: is infertility a risk factor for placental disease? Am J Obstet Gynecol [Internet] 2008;199:S149. doi: 10.1016/j.ajog.2008.09.536. [DOI] [Google Scholar]
- 102.Janssen AB, Kertes DA, McNamara GI, Braithwaite EC, Creeth HDJ, Glover VI, et al. A role for the placenta in programming maternal mood and childhood behavioural disorders. J Neuroendocrinol [Internet] 2016;28:1–6. doi: 10.1111/jne.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keverne EB. Significance of epigenetics for understanding brain development, brain evolution and behaviour. Neuroscience [Internet] IBRO. 2014;264:207–217. doi: 10.1016/j.neuroscience.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 104.O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology [Internet]. Elsevier Ltd; 2012;37:818–26. Available from: 10.1016/j.psyneuen.2011.09.014 [DOI] [PubMed]
- 105.Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci. Biobehav. Rev. [Internet]. Elsevier Ltd; 2014;43:137–62. Available from: 10.1016/j.neubiorev.2014.03.022. [DOI] [PubMed]
- 106.Lewis AJ, Austin E, Knapp R, Vaiano T, Galbally M. Perinatal maternal mental health, fetal programming and child development. Healthcare [Internet]. 2015;3:1212–27. [DOI] [PMC free article] [PubMed]
- 107.Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry [Internet] 2014;23:943–956. doi: 10.1007/s00787-014-0566-3. [DOI] [PubMed] [Google Scholar]
- 108.Field T. Prenatal depression effects on early development: a review. Infant Behav. Dev. [Internet]. Elsevier Inc.; 2011;34:1–14. Available from: 10.1016/j.infbeh.2010.09.008. [DOI] [PubMed]
- 109.Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract. Res. Clin. Obstet. Gynaecol. [Internet]. Elsevier Ltd; 2014;28:25–35. Available from: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed]
- 110.Waters CS, Hay DF, Simmonds JR, van Goozen SHM. Antenatal depression and children’s developmental outcomes: potential mechanisms and treatment options. Eur Child Adolesc Psychiatry [Internet] 2014;23:957–971. doi: 10.1007/s00787-014-0582-3. [DOI] [PubMed] [Google Scholar]
- 111.Szegda K, Markenson G, Bertone-Johnson ER, Chasan-Taber L. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960–967. doi: 10.3109/14767058.2013.845157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Accortt EE, Cheadle ACD, Schetter CD. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015;19:1306–1337. doi: 10.1007/s10995-014-1637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet [Internet]. Elsevier Ltd; 2014;384:1800–19. Available from: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed]
- 114.Chang HY, Keyes KM, Lee K, Choi IA, Kim JS, Kim KW, et al. Prenatal maternal depression is associated with low birth weight through shorter gestational age in term infants in Korea. Early Hum Dev. 2014;90:15–20. doi: 10.1016/j.earlhumdev.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ecklund-flores L, Myers MM, Monk C, Perez A, Odendaal HJ, Fifer WP. Maternal depression during pregnancy is associated with increased birth weight in term infants. Dev Psychobiol. 2016;59:314–323. doi: 10.1002/dev.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis C, Koren G, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74:e321–e341. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- 117.Saeed A, Raana T, Saeed AM, Humayun A. Effect of antenatal depression on maternal dietary intake and neonatal outcome: a prospective cohort. Nutr J [Internet] Nutrition Journal. 2016;15:1–9. doi: 10.1186/s12937-016-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koen N, Wyatt GE, Williams JK, Zhang M, Myer L, Zar HJ, et al. Intimate partner violence: associations with low infant birthweight in a South African birth cohort. Metab Brain Dis. 2014;29:281–99. [DOI] [PMC free article] [PubMed]
- 119.Lee B-E, Ha M, Park H, Hong Y-C, Kim Y, Kim YJ, et al. Psychosocial work stress during pregnancy and birthweight. Paediatr Perinat Epidemiol [Internet]. 2011;25:246–54. [DOI] [PubMed]
- 120.Pinto TM, Caldas F, Nogueira-silva C, Figueiredo B. Maternal depression and anxiety and fetal-neonatal growth. J Pediatr (Rio J) [Internet] Sociedade Brasileira de Pediatria. 2017;93:452–459. doi: 10.1016/j.jped.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 121.Abeysena C, Jayawardana P, de Seveviratne R A. Effect of psychosocial stress and physical activity on low birthweight: a cohort study. J Obstet Gynaecol Res. 2010;36:296–303. doi: 10.1111/j.1447-0756.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- 122.Witt WP, Cheng ER, Wisk LE, Litzelman K, Chatterjee D, Mandell K, et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. Am J Public Health. 2014;104:S81–9. [DOI] [PMC free article] [PubMed]
- 123.Rostad B, Schmidt L, Sundby J, Schei B. Infertility experience and health differentials - a population-based comparative study on infertile and non-infertile women (the HUNT Study) Acta Obstet Gynecol Scand. 2014;93:757–764. doi: 10.1111/aogs.12404. [DOI] [PubMed] [Google Scholar]
- 124.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet [Internet]. 1998;352:707–8 Available from: https://www.sciencedirect.com/science/article/pii/S0140673605608240. [DOI] [PubMed]
- 125.Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med [Internet] 2010;40:335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am J Psychiatry. 2016;173:705–713. doi: 10.1176/appi.ajp.2015.15091171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Appleton AA, Armstrong DA, Lesseur C, Lee J, Padbury JF, Lester BM, et al. Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS One. 2013;8:e74691. [DOI] [PMC free article] [PubMed]
- 129.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 130.Cao-Lei L, de Rooij SR, King S, Matthews SG, Metz GAS, Roseboom TJ, et al. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. [Internet]. Elsevier; 2017;1–13. Available from: 10.1016/j.neubiorev.2017.05.016. [DOI] [PubMed]
- 131.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry [Internet] Nat Publ Group. 2011;1:e21 Available from: https://www.ncbi.nlm.nih.gov/pubmed/228325235. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3309516/pdf/tp201121a.pdf [DOI] [PMC free article] [PubMed]
- 132.Mulligan CJ, D’Errico NC, Stees J, Hughes DA. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 2012;7:853–857. doi: 10.4161/epi.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics [Internet] 2012;7:735–746. doi: 10.4161/epi.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Janssen AB, Capron LE, O’Donnell K, Tunster SJ, Ramchandani PG, Heazell AEP, et al. Maternal prenatal depression is associated with decreased placental expression of the imprinted gene PEG3. Psychol Med. 2016;46:1–13. doi: 10.1017/S0033291716001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vangeel EB, Izzi B, Hompes T, Vansteelandt K, Lambrechts D, Freson K, et al. Abstract: DNA methylation in imprinted genes IGF2 and GNASXL is associated with prenatal maternal stress. Genes, Brain Behav. [Internet]. Elsevier Ltd; 2015;14:573–82. Available from: 10.1016/j.psyneuen.2015.07.430. [DOI] [PubMed]
- 136.Mansell T, Novakovic B, Meyer B, Rzehak P, Vuillermin P, Ponsonby A-L, et al. The effects of maternal anxiety during pregnancy on IGF2/H19 methylation in cord blood. Transl Psychiatry [Internet] Nature Publishing Group. 2016;6:e765. doi: 10.1038/tp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gallo LC, Roesch SC, Fortmann AL, Carnethon MR, Penedo FJ, Perreira K, et al. Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Psychosom Med [Internet]. 2015;76:468–75 Available from: https://www.ncbi.nlm.nih.gov/pubmed/24979579%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4349387. [DOI] [PMC free article] [PubMed]
- 138.Bonevski B, Regan T, Paul C, Baker AL, Bisquera A. Associations between alcohol, smoking, socioeconomic status and comorbidities: evidence from the 45 and Up Study. Drug Alcohol Rev. 2014;33:169–176. doi: 10.1111/dar.12104. [DOI] [PubMed] [Google Scholar]
- 139.Clare P, Bradford D, Courtney RJ, Martire K, Mattick RP. The relationship between socioeconomic status and ‘hardcore’ smoking over time - greater accumulation of hardened smokers in low-ses than high-ses smokers. Tob Control. 2014;23:e133–e138. doi: 10.1136/tobaccocontrol-2013-051436. [DOI] [PubMed] [Google Scholar]
- 140.King K, Murphy S, Hoyo CC. Epigenetic regulation of newborns’ imprinted genes related to gestational growth: patterning by parental race/ethnicity and maternal socioeconomic status. J Epidemiol Community Health [Internet]. 2015;69:639–47 Available from: https://www.ncbi.nlm.nih.gov/pubmed/256787125Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4466032. [DOI] [PMC free article] [PubMed]
- 141.Juárez SP, Merlo J. Revisiting the effect of maternal smoking during pregnancy on offspring birthweight: a quasi-experimental sibling analysis in Sweden. PLoS One. 2013;8:e61734. doi: 10.1371/journal.pone.0061734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Joubert BR, Håberg SE, Bell DA, Nilsen RM, Vollset SE, Midttun Ø, et al. Maternal smoking and DNA methylation in newborns: in utero effect or epigenetic inheritance? Cancer Epidemiol Biomark Prev. 2014;23:1007–1017. doi: 10.1158/1055-9965.EPI-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Viuff A-CF, Pedersen LH, Kyng K, Staunstrup NH, Børglum A, Henriksen TB. Antidepressant medication during pregnancy and epigenetic changes in umbilical cord blood: a systematic review. Clin Epigenetics [Internet] Clin Epigenetics. 2016;8:1–12. doi: 10.1186/s13148-016-0262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nezvalova-Henriksen K, Spigset O, Brandlistuen RE, Ystrom E, Koren G, Nordeng H. Effect of prenatal selective serotonin reuptake inhibitor (SSRI) exposure on birthweight and gestational age: a sibling-controlled cohort study. Int J Epidemiol. 2016;45:2018–2029. doi: 10.1093/ije/dyw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kopelman RC, Moel J, Mertens C, Stuart S, Arndt S, O’Hara MW. Barriers to care for antenatal depression. Psychiatr Serv. 2008;59:429–432. doi: 10.1176/ps.2008.59.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stevenson F, Hamilton S, Pinfold V, Walker C, Dare CRJ, Kaur H, et al. Decisions about the use of psychotropic medication during pregnancy: a qualitative study. BMJ Open. 2016;6:e010130. [DOI] [PMC free article] [PubMed]
- 147.Litzky JF, Deyssenroth MA, Everson TM, Lester BM, Lambertini L, Chen J, et al. Prenatal exposure to maternal depression and anxiety on imprinted gene expression in placenta and infant neurodevelopment and growth. Pediatr Res. 2018;83:1075–83. [DOI] [PMC free article] [PubMed]