Abstract
Purpose
To determine if levels of very long chain polyunsaturated fatty acids (VLC-PUFA; ≥ 28 carbons;4-6 double bonds) in human sperm correlate with sperm quantity and quality as determined by a complete semen analysis.
Methods
Ejaculates from 70 men underwent a complete semen analysis, which included volume, count, motility, progression, agglutination, viscosity, morphology, and pH. For lipid analysis, sperm were pelleted to remove the semen. Lipids were extracted from the cell pellet and methyl esters of total lipids analyzed by gas chromatography. The sphingolipids were enriched and sphingomyelin (SM) species measured using tandem mass spectrometry. Pair-wise Pearson correlation and linear regression analysis compared percent VLC-PUFA-SM and percent docosahexaenoic acid (DHA) to results from the semen analysis.
Results
VLC-PUFA-SM species having 28–34 carbon fatty acids were detected in sperm samples, with 28 and 30 carbon VLC-PUFA as most the abundant. The sum of all VLC-PUFA-SM species comprised 0 to 6.1% of the overall SM pool (mean 2.1%). Pair-wise Pearson analyses showed that lower levels of VLC-PUFA-SM positively correlated with lower total motile count (0.68) and lower total count (0.67). Total VLC-PUFA-SM and mole % DHA (22:6n3) were not strongly correlated (− 0.24). Linear regression analysis confirmed these findings.
Conclusion
This study revealed a positive correlation between the levels of VLC-PUFA with sperm count and total motile count and suggests that both sperm quality and quantity may depend on the presence of VLC-PUFA. The lack of correlation between VLC-PUFA and DHA suggests that low VLC-PUFA levels do not result from inadequate PUFA precursors.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01464-3) contains supplementary material, which is available to authorized users.
Keywords: Sperm lipids, Sperm, Sphingomyelin, Fatty acids, Very long chain polyunsaturated fatty acids, Motility, Sperm count, Fertility
Introduction
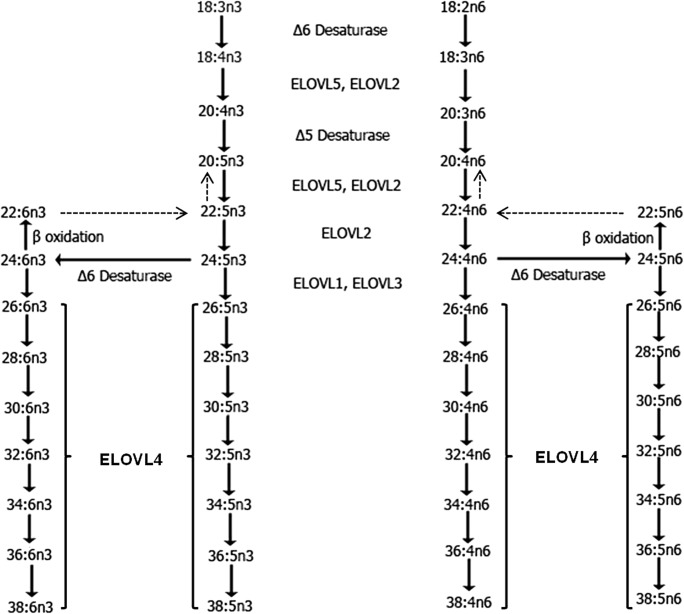
Semen analysis is the initial step for infertility evaluation of the male partner. However, the cause of abnormal semen analysis is often times unexplained even after urologic evaluation for anatomic and hormonal causes. The lipid composition of the sperm membrane plays a major role in sperm structure and function and could relate to the semen analysis abnormalities and ultimately fecundability. The lipids of sperm are relatively unique in that they contain many different sphingolipids including sphingomyelin (SM) that have long-chain (LC) polyunsaturated fatty acids (PUFA) such as docosahexaenoic acid (DHA, 22:6n3), eicosapentaenoic acid (EPA, 20:5n3), and arachidonic acid (AA, 20:4n6), as well as very long chain PUFA (VLC-PUFA; ≥ 28 carbons) [1, 2]. There is precedent for linking polyunsaturated fatty acid (PUFA) deficiencies to infertility as levels of DHA, docosapentaenoic acid (DPA), and AA were reduced when an important enzyme in PUFA biosynthesis, delta-6-desaturase (D6D), was knocked out in mice. These mice were infertile [3] and PUFA supplementation restored their fertility [4]. One type of fatty acid that is particularly unique to sperm is the VLC-PUFA (≥ 28 carbons) in the sperm membrane, which are synthesized via the enzyme elongation of very long chain fatty acids-4 (ELOVL4) from dietary essential fatty acids and incorporated into sphingolipids, primarily sphingomyelin [5]. The biosynthetic pathways for synthesis on n3 and n6 VLC-PUFA are presented in Fig. 1.
Fig. 1.
Schematic of the biosynthesis of n3 and n6 VLC-PUFA from shorter chain polyunsaturated fatty acids. Pathways of in vivo very long chain polyunsaturated fatty acid (VLC-PUFA) biosynthesis from essential fatty acids (EFA) 18:3n3 and 18:2n6 mediated by ELOVL4 and other ELOVL enzymes. Desaturation and elongation of the EFAs are performed by fatty acid desaturase-1 (FADS1 or Δ5 desaturase), fatty acid desaturase-2 (FADS2 or Δ6 desaturase), and ELOVL1-5. Apart from ELOVL4, which mediates C28-C36 VLC-PUFA biosynthesis, other ELOVL enzymes are nonspecific or multifunctional and act at several steps. Reproduced with permission from Man Yu et al., (2012). J. Lipid Res. 53:(3) 494–504
VLC-PUFA are found in the testis, sperm, and the retina [1, 6–9]. While studying several different transgenic and knock-in/knockout rodent models of ELOVL4 (juvenile-onset Stargardt-like macular dystrophy-3), we discovered that some of the affected males bred poorly or not at all, depending on the severity of the expression of the mutation. Consequently, we examined the VLC-PUFA in their testes and discovered that the poor-breeding ELOVL4-affected animals had significantly reduced levels (unpublished data). Knowing that lipids play integral roles in energy production and membrane structure, we hypothesized that a subset of human males who suffer from poor fertility would have comparatively low levels of VLC-PUFA in their sperm. The objective of our study was to determine if levels of VLC-PUFA in the sperm correlate with sperm quantity and quality as determined by a complete semen analysis.
Materials and methods
Institutional review board approval was received from the University of Oklahoma Health Sciences Center and the study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. This prospective cohort study enrolled males age 18–55 presenting for semen analysis at an academic fertility center. Subjects were excluded if they were unable to provide a sample, had a current history of sexually transmitted disease, used testosterone or anabolic steroids in the last 2 years, had a history of vasectomy or known testicular obstruction, or had a history of retrograde ejaculation.
In all cases, semen was collected by masturbation and processed after liquefaction had occurred. Sperm count and motility was conducted according to the World Health Organization (WHO) 5th edition for semen evaluation [10]. After thorough mixing, semen volume was determined using a serological pipette and pH was determined using a commercial strip of pH paper (pHydrion, Micro Essential Laboratory, Brooklyn, New York) by comparison to a color standard. Each sample was given a viscosity score of 0, 1, or 2 (normal, slightly thick, and thick, respectively) using a wide-bore pipette and assessing flow of the semen column. Seven microliters of semen was placed in a pre-warmed Microcell counting chamber (VitroLife, Göteborg, Sweden) and allowed to equilibrate for at least 1 min on a 37 °C stage warmer. Sperm concentration (million/ml) and motility (percent) were then determined. The rate of forward progression (RFP) was scored was scored as 0 to 4; (0, no motility; 1, sluggish or poor motility; 2, moderate motility; 3, good motility and 4, vigorous or rapid motility), according to the American Association of Bioanalysts (AAB)/College of Reproductive Biology (CRB) andrology proficiency testing standards. Each sample was given an agglutination score of 0, 1, or 2 for none, some/mixed, and complete, respectively. The total motile sperm count (TMSC) was calculated using the formula “volume × count × motility.” For strict morphology (Kruger) [11], approximately 10 μl of semen was placed on a clean glass slide, spread evenly, and allowed to dry completely and then stained with Stat III andrology stain (Mid-Atlantic Diagnostics, Mount Laurel, New Jersey). A minimum of 200 sperms were then counted at 100× under oil immersion and the percent normal forms was recorded. Following clinical semen evaluation, the remaining sample was stored at − 80 °C.
Lipid extraction
A 100 μl aliquot of sample was spun at 4 °C for 12 min at 9000×g to pellet the sperm cells, and the supernatant was discarded. Total lipids were extracted from the cell pellets using the method of Folch et al. [12] and stored under nitrogen at − 20 °C.
Sphingomyelin analysis
Total lipid extracts were subjected to mild base hydrolysis to hydrolyze phospholipids. The resulting mixture was then extracted with chloroform:methanol to obtain the intact sphingolipids [13]. The resulting lipid extract was dried under nitrogen and resuspended in 200 μl of 2-propanol/methanol/chloroform (4:2:1 v/v/v) containing 20 mM ammonium formate and 0.5 μM SM (d18:1/12:0) as internal standard. Samples were introduced into a TSQ Ultra triple quadrupole mass spectrometer (Thermo Scientific, Waltham, Massachusetts) using a nanomate chip–based nano-ESI source (Advion Biosciences, Ithaca, New York) operating in infusion mode and SM species were measured using precursor ion scanning of m/z 184 [14]. Abundances of SM molecular species were calculated using the lipid mass spectrum analysis (LIMSA) software (University of Helsinki) and are represented as a relative percent of the sum based upon their response values.
Fatty acid analysis
Fatty acid profiles were determined for total lipid extracts. The internal standards 15:0 and 17:0 were added and the lipid extracts were subjected to acid hydrolysis/methanolysis to generate fatty acid methyl esters (FAME) [15]. FAME were quantified using an Agilent Technologies (Santa Clara, California) 7890B gas chromatograph equipped with a flame ionization detector [16].
Statistical analyses
Descriptive statistics such as mean, median, and variance were determined for each continuous variable. Frequency counts were determined for categorical variables. Pair-wise Pearson correlation was calculated for each measured clinical and lipid parameter. Values greater than |0.5| are considered significantly correlated. The relationship between each pair of variables was examined using linear and logistic regression models with and without consideration for race and age.
Results
Seventy males were consented with an average age of 33 years old (range 23–53). The racial makeup of all participants was as follows: American Indian 2.7%, Asian 2.7%, African American 2.7%, Native Hawaiian/Pacific Islander 0.9%, White 81.3%, and unreported 9.8%. The mean semen analysis parameters are listed in Table 1. The standard deviations were large in most categories, especially in count and TMSC, reflecting the heterogeneity of the population being studied.
Table 1.
Semen analysis (n = 70)
| Sperm parameters | Mean ± Stdevd | Range |
|---|---|---|
| Volume (ml) | 3.0 ± 1.5 | 0.1–6.5 |
| pH | 7.5 ± 0.4 | 6.6–8.0 |
| Count (million/ml) | 37.2 ± 33.7 | 0.1–176 |
| % Motility | 46.9 ± 15.6 | 0–73 |
| Rate of forward progressiona | 2.0 ± 0.3 | 0–3 |
| Agglutinationb | 0.5 ± 0.5 | 0–2 |
| Viscosityc | 0.4 ± 0.6 | 0–2 |
| Total motile sperm count (million) | 52.8 ± 49.5 | 0–216.2 |
| Morphology (% normal) | 4.6 ± 2.9 | 0–11 |
aThe rate of forward progression (RFP) was scored as 0 to 4)
bEach sample was given an agglutination score of 0, 1, or 2 for none, some/mixed, and complete, respectively
cViscosity score of 0, 1, or 2 was assigned for normal, slightly thick, and thick, respectively
dStDev = standard deviation
Analysis of the SM molecular species of sperm cells from 70 human participants was performed. The relative percent of each SM species for each sample was calculated (Fig. 2; Online Resource 1) to determine the amount of VLC-PUFA-SM present. VLC-PUFA-SM species having 28–34 carbon fatty acids were detected, with 28 and 30 carbon VLC-PUFA being the most abundant. For comparison purposes, the percentages of all individual VLC-PUFA-SM were summed (∑ VLC-PUFA-SM). The sum of all VLC-PUFA-SM species made up anywhere from 0 to 6.1% of the overall SM pool, with the average being 2.1%. The most abundant SM species was SM (16:0), averaging 47.1%. Saturated fatty acid–containing SM collectively made up 82.1% of the overall SM pool, on average. The most abundant monounsaturated fatty acid–containing SM was SM (24:1), averaging 6.4% of the overall SM pool. Interestingly, DHA (22:6n3) was not a component of SM.
Fig. 2.
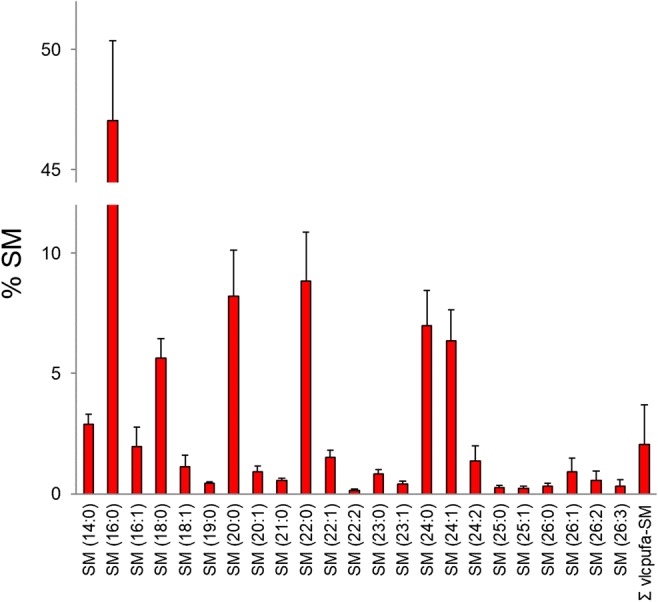
Molecular species composition of human sperm sphingomyelin (n = 70). Values are relative percent of each sphingomyelin (SM) species ± standard deviation. The value in parenthesis represents the amide-bound fatty acid in the SM molecule. The number to the left of the colon represents the number of carbons in the fatty acid and the number to the right of the colon represents the number of double bonds in the fatty acid. ∑ VLC-PUFA-SM = sum of VLC-PUFA-containing sphingomyelin (SM) species
The fatty acid profile of the sperm cell pellet total lipid extracts was likewise measured (Fig. 3; Online Resource 2). The most abundant fatty acids were the saturates 16:0 (46.6%) and 18:0 (23.4%). DHA (22:6n3) was the third most abundant fatty acid with an average value of 9.1 relative mole percent.
Fig. 3.
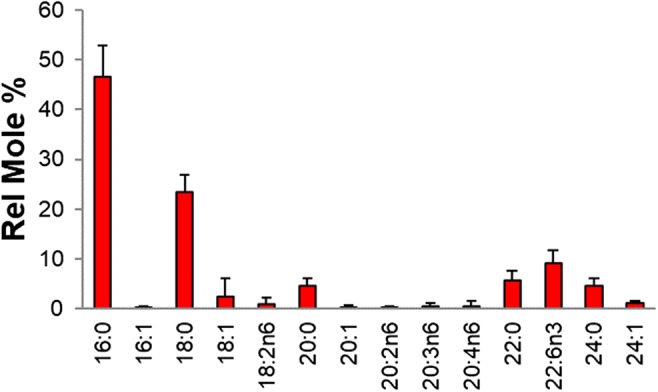
Fatty acid profile of sperm pellet total lipid extract (n = 70). Values are relative mole percent of each species ± standard deviation. The number to the left of the colon represents the number of carbons in the fatty acid and the number to the right of the colon represents the number of double bonds in the fatty acid
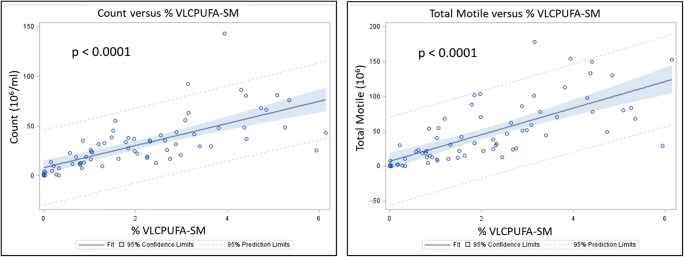
Pair-wise Pearson analysis (Online Resource 3) was used to compare the ∑ VLC-PUFA-SM to results from the semen analysis (volume, count, motility, RFP, agglutination, viscosity, TMSC, morphology, and pH) performed on the same sample to look for correlations. The pair-wise Pearson analyses showed that lower levels of VLC-PUFA-SM positively correlated with lower TMSC (0.68; p < 0.0001) and lower total count (0.67; p < 0.0001), with no differences attributed to race or age. Linear regression analysis confirmed these findings (Fig. 4), showing a positive correlation between VLC-PUFA in SM and sperm count (p < 0.0001) and sperm motility (p < 0.0001).
Fig. 4.
Linear regression analysis of % VLC-PUFA-SM (sum of VLC-PUFA-containing sphingomyelin) versus sperm count (million/ml) and total motile sperm count (volume × count × motility)
Discussion
We found that lower levels of VLC-PUFA strongly correlated with decreased sperm count and decreased total motile sperm count. The chance to conceive increases with higher sperm concentrations [17, 18]. Total motile sperm count also positively correlates with the chance of pregnancy in infertility treatments such as intrauterine insemination (IUI), a treatment frequently recommended for idiopathic male infertility [19, 20]. The semen analysis is a superficial external view of the sperm quantity and quality. There are not reliable and reproducible tests for sperm function such as capacitation, the acrosome reaction, binding and penetration of the zona pellucida, and ultimately fertilization [21]. VLC-PUFA-containing sphingomyelin are concentrated in the head of the spermatozoa and undergo changes with capacitation, thereby playing a role in fertilization [22]. The VLC-PUFA content of the sperm plasma cell membrane is crucial for stability of the membrane, and varying levels of VLC-PUFA have resulted in changes in the cell membrane fluidity, flexibility, and/or rigidity [23, 24]. Certainly, the components of the sperm plasma membrane play an important role in sperm development as well as sperm function.
The lipids of sperm are relatively unique in that they contain many different sphingolipids that have long-chain polyunsaturated fatty acids (LC-PUFA) such DHA (22:6n3), EPA (20:5n3), and AA (20:4n6), as well as very long chain PUFA containing 28 or more carbons [1, 2]. Some studies in humans found an association between sperm quality and the sperm levels of n3 PUFA [25, 26]. In one study published in Clinical Nutrition [27] proven fertile men (n = 78) were found to have higher blood and spermatozoa levels of n3 PUFA. Infertile men (n = 82) had significantly higher AA (n6)/DHA(n3) and AA (n6)/EPA(n3) ratios that were both negatively correlated with total sperm count, sperm motility, and sperm morphology. Another study reported a positive correlation (p < 0.001) between sperm motility and DHA levels [27]. Esmaeili et al. [5] reviewed the literature on dietary fatty acid effects on sperm quality and concluded that there were differences in n3 and n6 PUFA levels in sperm of fertile and infertile men and that supplementation with DHA could improve the quality of human sperm, although this was not shown in all publications. Interestingly, there have been no reported clinical trials to determine the efficacy of DHA supplementation on male fertility using pregnancy and live births as an outcome measure. Neither has there been any detailed analysis of VLC-PUFA in human sperm from fertile and infertile males.
VLC-PUFA are essential fatty acids (EFA) and must be synthesized from shorter chain EFA that are derived from the diet (Fig. 1). Delta-6 desaturase is an important enzyme in PUFA biosynthesis and plays a key role in converting 18:3n3 to 18:4n3, and 24:5n3 to 24:6n3; the latter is then retro-converted in peroxisomes to DHA (22:6n3). We collaborated with a group at the University of Illinois Urbana and found that male mice lacking delta-6 desaturase activity were infertile and showed an arrest in spermatogenesis [4]. We showed in this same study that dietary supplementation with DHA, a precursor of VLC-PUFA, completely reversed the infertility and sperm defects, which clearly established an essential role for DHA in mouse fertility. However, at that time, we did not appreciate the potential role of VLC-PUFA, products of elongation of DHA, in fertility. Zadravec et al. [28] showed that deletion of ELOVL2, the enzyme that converts C-22 PUFA to C-24/26 PUFA (precursors of VLC-PUFA), also resulted in mouse male infertility. In these mice, providing DHA did not restore fertility, indicating that DHA deficiency in and of itself is not the problem. Likely, it is the lack its elongation products (VLC-PUFA). Rabionet et al. [29] were the first to show the importance of VLC-PUFA in sperm maturation in mice; they characterized a number of sphingolipids, mostly fucosylated, several of which were composed primarily of VLC-PUFA. Depletion of ceramide synthase-3 led to a significant decrease in VLC-PUFA-containing sphingolipids and a loss of spermatogenesis. However, they did not confirm infertility with breeding studies.
To our knowledge, this is the first time that a correlation has been made between the health of human sperm and VLC-PUFA. Future studies should evaluate if fertilization rates in in vitro fertilization correlate with levels of sperm VLC-PUFA. Additionally, we need to evaluate pregnancy and live birth as a consequence of VLC-PUFA and see if this improves with supplementation. Future animal studies will focus on ELOVL4 knockout mice and determine if fertility can be restored through VLC-PUFA supplementation in their diet. If successful, we would anticipate that some forms of human male infertility could be reversed with VLC-PUFA treatment.
Electronic supplementary material
(DOCX 27 kb)
Acknowledgments
We thank Sonny Icks of the Dean McGee Eye Institute for his invaluable assistance with all matters related to IRB approvals and updates. We also thank Michelle Scruggs, RN, and Kisha Turner, RN, for their assistance in recruitment, consenting, and data management. And finally, we thank Dr. Sixia Chen for statistical assistance.
Funding
This work was supported by the Presbyterian Health Foundation Team Science Grant (L.B.C, R.EA and M-P.A), the National Institutes of Health Grants EY04149 (R.E.A.) and P30EY021725 (R.E.A); an unrestricted grant from Research to Prevent Blindness to the University of Oklahoma Health Sciences Center’s Department of Ophthalmology; OU College of Medicine Alumni Association, BrightFocus Foundation, and Oklahoma Center for Advancement of Science and Technology to M-P.A; and the Oklahoma Shared Clinical and Translational Resource Institute (NIGMS U54 GM104938).
Compliance with ethical standards
Conflict of interest
LB Craig, MT Sullivan, and MT Zavy declare that they have no conflicts of interest. RS Brush, MP Agbaga and RE Anderson have a US Patent for biological synthesis of VLC-PUFA (United States Patent 8,021,874 B2).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants
Research involved human participants. Institutional Review Board approval was obtained prior to initiating the trial.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Furland NE, Oresti GM, Antollini SS, Venturino A, Maldonado EN, Aveldaño MI. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J Biol Chem. 2007;282(25):18151–18161. doi: 10.1074/jbc.M700709200. [DOI] [PubMed] [Google Scholar]
- 2.Poulos A, Johnson DW, Beckman K, White IG, Easton C. Occurrence of unusual molecular species of sphingomyelin containing 28-34-carbon polyenoic fatty acids in ram spermatozoa. Biochem J. 1987;248(3):961–964. doi: 10.1042/bj2480961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoffel W, et al. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 2008;27(17):2281–2292. doi: 10.1038/emboj.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roqueta-Rivera M, Stroud CK, Haschek WM, Akare SJ, Segre M, Brush RS, Agbaga MP, Anderson RE, Hess RA, Nakamura MT. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J Lipid Res. 2010;51(2):360–367. doi: 10.1194/jlr.M001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmaeili V, Shahverdi AH, Moghadasian MH, Alizadeh AR. Dietary fatty acids affect semen quality: a review. Andrology. 2015;3(3):450–461. doi: 10.1111/andr.12024. [DOI] [PubMed] [Google Scholar]
- 6.Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51(7):1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett LD, Anderson RE. Current Progress in deciphering importance of VLC-PUFA in the retina. Adv Exp Med Biol. 2016;854:145–151. doi: 10.1007/978-3-319-17121-0_20. [DOI] [PubMed] [Google Scholar]
- 8.Aveldano MI. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J Biol Chem. 1987;262(3):1172–1179. [PubMed] [Google Scholar]
- 9.Aveldano MI, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem. 1987;262(3):1180–1186. [PubMed] [Google Scholar]
- 10.Cooper TG, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 11.Kruger TF, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46(6):1118–1123. doi: 10.1016/S0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 13.Brush RS, Tran JTA, Henry KR, McClellan ME, Elliott MH, Mandal MNA. Retinal sphingolipids and their very-long-chain fatty acid-containing species. Invest Ophthalmol Vis Sci. 2010;51(9):4422–4431. doi: 10.1167/iovs.09-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busik JV, Reid GE, Lydic TA. Global analysis of retina lipids by complementary precursor ion and neutral loss mode tandem mass spectrometry. Methods Mol Biol. 2009;579:33–70. doi: 10.1007/978-1-60761-322-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford DA, Monda JK, Brush RS, Anderson RE, Richards MJ, Fliesler SJ. Lipidomic analysis of the retina in a rat model of Smith-Lemli-Opitz syndrome: alterations in docosahexaenoic acid content of phospholipid molecular species. J Neurochem. 2008;105(3):1032–1047. doi: 10.1111/j.1471-4159.2007.05203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Benham A, Logan S, Brush RS, Mandal MNA, Anderson RE, Agbaga MP. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. J Lipid Res. 2012;53(3):494–504. doi: 10.1194/jlr.M021386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, Xu D, Vogel DL, National Cooperative Reproductive Medicine Network Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345(19):1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 18.Bonde JP, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352(9135):1172–1177. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 19.Van Voorhis BJ, et al. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril. 2001;75(4):661–668. doi: 10.1016/S0015-0282(00)01783-0. [DOI] [PubMed] [Google Scholar]
- 20.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010;93(1):79–88. doi: 10.1016/j.fertnstert.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 21.Fritz MA, Speroff L, editors. Clinical Gynecologic Endocrinology and Infertility. 8th ed. Vol. Chapter 30. 2012. p. 1272–1275.
- 22.Sassa T, Kihara A. Metabolism of very long-chain fatty acids: genes and pathophysiology. Biomol Ther (Seoul) 2014;22(2):83–92. doi: 10.4062/biomolther.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahumada-Gutierrez H, Peñalva DA, Enriz RD, Antollini SS, Cascales JJL. Mechanical properties of bilayers containing sperm sphingomyelins and ceramides with very long-chain polyunsaturated fatty acids. Chem Phys Lipids. 2019;218:178–186. doi: 10.1016/j.chemphyslip.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Lenzi A, et al. Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update. 1996;2(3):246–256. doi: 10.1093/humupd/2.3.246. [DOI] [PubMed] [Google Scholar]
- 25.Gulaya NM, Margitich VM, Govseeva NM, Klimashevsky VM, Gorpynchenko II, Boyko MI. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch Androl. 2001;46(3):169–175. doi: 10.1080/01485010151096405. [DOI] [PubMed] [Google Scholar]
- 26.Tang LX, Yuan DJ, Wang QL, Jiang F, Guo J, Tang YG, Zheng LX, Kang JX. Association of decreased spermatozoa omega-3 fatty acid levels and increased oxidative DNA damage with varicocele in infertile men: a case control study. Reprod Fertil Dev. 2016;28(5):648–654. doi: 10.1071/RD14276. [DOI] [PubMed] [Google Scholar]
- 27.Safarinejad MR, Hosseini SY, Dadkhah F, Asgari MA. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: a comparison between fertile and infertile men. Clin Nutr. 2010;29(1):100–105. doi: 10.1016/j.clnu.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Zadravec D, Tvrdik P, Guillou H, Haslam R, Kobayashi T, Napier JA, Capecchi MR, Jacobsson A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J Lipid Res. 2011;52(2):245–255. doi: 10.1194/jlr.M011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabionet M, van der Spoel AC, Chuang CC, von Tümpling-Radosta B, Litjens M, Bouwmeester D, Hellbusch CC, Körner C, Wiegandt H, Gorgas K, Platt FM, Gröne HJ, Sandhoff R. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J Biol Chem. 2008;283(19):13357–13369. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 27 kb)