Abstract
Purpose
Increasing intracellular energy storage by chemically activating adenosine monophosphate–activated protein kinase (AMPKα) prior to sperm cryopreservation may improve post-thawed sperm function. Using the domestic cat as a biomedical model, the objectives were to (1) confirm the expression of AMPKα and its regulatory kinases in epididymal spermatozoa and (2) assess the influence of AMPK activator, 5′-aminoimidasole-4-carboxamide-1-β-d-ribofuranoside (AICAR) on epididymal sperm function before and after cryopreservation.
Methods
In study I, sperm samples of different qualities were obtained from cauda epididymides of domestic cats and evaluated for AMPKα expression. In study II, epididymal spermatozoa were equilibrated for either 30 or 60 min in the presence of 0 (control), 0.5, 2.0, and 5.0 mM AICAR and sperm functions were assessed before and after cryopreservation. In study III, epididymal spermatozoa were treated as in study II and evaluated for AMPKα signaling protein expressions (phospho-AMPKα Thr172 and GLUT1) as well as ATP levels.
Results
AMPKα protein expression was higher in high-motility vs poor-motility samples. Thirty-minute equilibration with 0.5 mM AICAR improved motion characteristics and fertilizing ability of cryopreserved sperm to the control. Increased expressions of phospho-AMPKα Thr172 and GLUT1 as well as intracellular ATP level were confirmed in sperm samples equilibrated with 0.5 or 2.0 mM AICAR for 30 min.
Conclusions
Presence and role of AMPKα protein in cat regulating sperm function were demonstrated before and after cryopreservation. Findings could be used to potentially enhance cryopreserved sperm function in sub-fertile men.
Keywords: AMPKα protein, Energy regulation, Felids, Non-rodent model, Sperm functions
Introduction
Mitochondria play several fundamental roles in cell functions, including providing energy, regulating apoptotic pathway, and Ca2+ homeostasis [1, 2]. Mitochondria provide ATP to cells via oxidative phosphorylation (OXPHOS). During this process, high energy electrons derived from oxidation are carried by NADH+, H+, and FADH2 to the inner mitochondrial membrane and transferred through cascades of electron transport chain that convert electrons into ATP [2, 3]. In mammalian spermatozoa, mitochondria are located within the mid-piece and responsible for ATP production essential for sperm flagella movement and fertilization process [3, 4]. Dysfunction of sperm mitochondria has been linked to several pathologies, including infertility [2].
Sperm mitochondrial function has been reported to be regulated by several factors including 5′ adenosine monophosphate–activated protein kinase (AMPK). AMPK is a serine/threonine heterotrimeric protein kinase composed of three subunits (catalytic α-subunit bound with β- and γ-regulatory subunit) [5]. Activation of AMPK has been reported to enhance glucose uptake and metabolism in adipocytes leading to increased intracellular energy storage [6, 7] . Studies have shown that AMPK plays important roles in the development of male and female gonads [8]. For examples, α1AMPK-deficient metaphase II mouse oocytes contain high numbers of abnormal mitochondria and generate less ATP compared with gametes recovered from wild type individuals [8]. Alteration of αAMPK gene in mouse testes also has been shown to be associated with impairment of testicular junctional complex and high incidence of sperm with abnormal acrosome morphology [9]. In spermatozoa, AMPK is mainly located in the mid-piece and flagellum in the pig [10], chicken [11], and human [12]. Activation of AMPK has been shown to sustain mouse sperm function after long-term storage [8].
AMPK can be activated by its activators such as 5′-aminoimidasole-4-carboxamide-1-β-d-ribofuranoside (AICAR), resveratrol, or metformin [6, 9]. The activation by AICAR has been reported to increase intracellular ATP concentration while decrease reactive oxygen species (ROS) and lipid peroxidation reaction (LPO) in post-thawed chicken spermatozoa [11]. It also has been shown that AMPK activation decreases ROS levels and apoptotic-like changes as well as increases mitochondrial membrane potential but does not impact fertilizing ability of cryopreserved human spermatozoa [13].
The domestic cat is a highly relevant biomedical model to research in human fertility [14]. Furthermore, similar to inbred mice or infertile patients, epididymal and ejaculated sperm from domestic and non-domestic felids often contain high proportions of pleiomorphism and poor motility. Thus, the domestic cat is a useful model for addressing reproductive issues in human [14]. We have previously shown that sperm mitochondrial membrane potential remarkably decreases after cryopreservation that likely results in reduced fertilizing ability of cryopreserved spermatozoa [15]. Furthermore, morphologically abnormal spermatozoa, especially those with a mid-piece defect, have been shown to be extremely sensitive to cryo-induced damage [4]. Because activation of AMPK has been shown to enhance sperm metabolism after cryopreservation in human [13] and chicken [11], we hypothesize that increasing intracellular energy storage by chemically activating AMPKα prior to sperm cryopreservation improves post-thawed sperm function. Using the domestic cat as a biomedical model, the objectives were to (1) confirm the expression of this protein and its regulatory kinases in epididymal spermatozoa and (2) assess the influence of AICAR on epididymal sperm function before and after cryopreservation.
Materials and methods
All chemical substances were purchased from Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) unless otherwise indicated.
Research animals
Testes from a total of 53 adult male (1–5 years old) and ovaries from 49 adult female cats (1–5 years old) were collected opportunistically from routine castration at the Small Animal Hospital, Faculty of Veterinary Science, Chulalongkorn University, and the Veterinary Public Health Division of the Bangkok Metropolitan Administration, Thailand. Gonads were kept after excision in an isotonic normal saline solution supplemented with 0.5 IU/ml penicillin-streptomycin (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and transported to the laboratory at room temperature within 1–3 h.
Sperm cryopreservation
Sperm freezing extender and thawing medium were prepared according to the protocol described by Axnér et al. [16]. For sample collection and cryopreservation, sperm cells were recovered by cutting the cauda epididymides into small pieces in 0.5 ml of pre-warmed Tris-base buffer and allowed to swim out for 30 min. Each sample was assessed for concentration, and a portion containing 10 × 106 sperm cells processed for cryopreservation as followed: the sample was centrifuged, the supernatant removed, and the sperm pellet resuspended at 25 °C with 0.65 ml of semen extender I (containing of 3% glycerol) with or without AICAR (as described in “Experimental design”) and then cooled (for 30 min or 60 min) to 4 °C after which an equal volume of cooled semen extender II (containing 7% glycerol) was added to each sperm sample. For a given treatment, samples were then loaded into two 0.25-ml straws (approximately 0.125 ml per straw with final concentration 5% glycerol). Straws were then placed on a metal rack above liquid nitrogen vapor at 20 cm (2 min), then at 13 cm (2 min), and finally at 7 cm (1 min) [17], before being plunged into liquid nitrogen where straws were kept until evaluation. For thawing, each straw was submerged in a water bath at 37 °C for 15 s and diluted with 0.125 ml of thawing medium (1/1; v/v).
Expression of AMPKα, phospho-AMPKα Thr 172, and GLUT1 proteins
Sperm protein was extracted by incubation with protein lysis buffer (CellLytic™ MT) supplemented with protease inhibitor for 30 min on a shaker at room temperature (25 °C) followed by centrifugation at 12,000g at 4 °C for 10 min. Sperm pellet was discarded, and the supernatant was measured for total protein amount using NanoDrop 100 (Thermo Fisher Scientific, Waltham, MA, USA). Final protein concentration was adjusted to 15 μg/μl by dilution in PBS and stored at − 80 °C until western blot analysis. Western blot analysis was performed as follows; the protein sample was mixed with SDS sample loading buffer (4 × Laemmli sample buffer and β-mercaptoethanol; Bio-Rad, Hercules, CA, USA) and processed on SDS-PAGE (4–15% Miniprotein® TGX Precast Gels; Bio-Rad) for 1 h. Precision Plus Protein Dual Color Standards (Bio-Rad) loaded into the first lane were used as molecular weight standard. Gels were then transferred to polyvinylidene difluride membranes (Immobilon-P, Millipore, Billerica, MA, USA.) and blocked with 2.5% skim milk (Bio-Rad) diluted in washing buffer (Tris-buffered saline plus 0.1% (v/v) Tween) for 1 h at room temperature (25 °C) and incubated on a shaker in the dark at 4 °C overnight with one of the following primary antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA; (I) alpha-Tubulin Rabbit antibody (1:1000); (II) AMPKα Rabbit Antibody (1:1000); (III) Phospho-AMPKα Thr172 (D76.5E) Rabbit Antibody (1:2000); and (IV) GLUT1 (D3J3A) Rabbit Antibody (1:1000). Samples incubated with normal rabbit IgG (1:1000) served as negative controls. After overnight incubation, the blot membrane was incubated with secondary antibody (anti-rabbit IgG antibody conjugated with HRP, 1:2000; Cell Signaling Technology) for 1 h at room temperature (25 °C). Immunoreactivity was detected by colorimetric method (Opti 4CN™ Substrate Kit, Bio-Rad). Volume intensity of each protein band and background were measured using Synegen software (Frederick, MD, USA). Relative quantification volume was normalized by dividing the volume intensity of AMPKα, phospho-AMPKα Thr172, and GLUT1 antibodies (volume intensity of each protein band minus background) by the alpha-Tubulin volume intensity.
Intracellular ATP concentration
Sperm intracellular ATP extraction was performed using the protocol previously described by Thuwanut at al. [18]. In brief, sperm samples were washed in ice-cold 0.15 M sodium chloride by centrifugation at 600g for 6 min. Sperm pellet was resuspended in ice-cold Triton X and 10% perchloric acid, centrifuged at 20,000g for 10 min, and supernatant stored at 4 °C. Evaluation of intracellular ATP concentration was performed within 1 h of extraction by high-performance liquid chromatography (HPLC) (UltiMate HPLC System, Thermo Fisher Scientific Inc.). Peaks from chromatographic band were analyzed by absorbance at 254 nm with reverse-phase HPLC on Ultrasphere 18-mm column. Intracellular ATP concentration was identified by comparison with the external standard method (r2 = 0.90). Each sample was analyzed in duplicate, and data were present as means of the duplicate.
Sperm motility, motion characteristics, and morphology
For percent motility and progressive motility score, 5 μl of sperm sample was placed on a pre-warmed glass slide and assessed under a phase contrast microscope at × 200 magnification [18]. Sperm motion patterns were evaluated by Sperm Class Analyzer® CASA system (Microptic S.L, Barcelona, Spain). Three to five randomly selected optical fields of each sperm sample were evaluated for sperm motion patterns, including straight line velocity (VSL), average path velocity (VAP), curve linear velocity (VCL), and amplitude lateral head displacement (ALH) as described by Thuwanut et al. [15]. For sperm head morphology, 5 μl of sperm sample was placed on a pre-warmed glass slide, air-dried, and stained by William’s staining method. Two hundred spermatozoa were evaluated under a light microscope at × 1000 magnification. To assess head morphology, spermatozoa were classified as having normal head, narrow at the base, pear shape, head contour, or varied in size (giant, board, round shape). For mid-piece and tail morphology, 5 μl of sperm sample was fixed in 100 μl formal saline, and 5 μl of the fixed sample was placed on a glass slide. Two hundred spermatozoa were analyzed under the phase contrast microscope at × 400 magnification and classified as having normal mid-piece and tail morphology, bent tail, coiled tail, abaxial mid-piece, proximal droplet, or distal droplet.
Integrity of sperm plasma membrane and acrosome, and mitochondrial membrane potential
Sperm plasma membrane and acrosome integrity was determined by double-fluorescent labeling techniques (sperm membrane integrity: EthD-1 and SYBR-14, [Molecular Probes Inc., OR, USA] and acrosome integrity: FITC-PNA mixed with propidium iodide [PI; Molecular Probes Inc.]) [16]. Sperm mitochondrial membrane potential was evaluated using fluorochrome 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; Molecular Probes Inc.) [19]. For all analyses, 200 spermatozoa were counted for each sample. For plasma membrane integrity, spermatozoa fluoresced green of SYBR-14 were considered “live,” cells fluoresced both green and red were considered moribund, and cells fluoresced red were classified as “dead.” To evaluate acrosome integrity status, spermatozoa were classified into three categories: intact acrosome (spermatozoa fluoresced green of FITC-PNA at acrosomal cap area), reacted acrosome (spermatozoa fluoresced green of FITC-PNA at acrosomal cap area and red from PI from DNA at equatorial segment), and missing acrosome or acrosomal loss (spermatozoa fluoresced red from PI at all sperm head region). For mitochondria potential, 200 spermatozoa were evaluated and classified into two categories: high (spermatozoa fluoresced orange from JC-1) and low mitochondrial membrane potential (spermatozoa fluoresced green from JC-1).
Homologous in vitro fertilization
Oocyte recovery and in vitro culture (IVM) media were prepared as described by Thuwanut et al. [18]. A total of 513 oocytes with more than two layers of compact cumulus cells and presence of dark homogeneous ooplasm were collected from ovaries of domestic cats after a routine ovariohysterectomy. Oocytes were gently washed in oocyte recovery medium (Medium 199 supplemented with penicillin-streptomycin [0.5 U/ml] and Hepes [0.2 mg/ml]) and then IVM medium (Medium 199 supplemented with bovine serum albumin [4 mg/ml], glutamine [0.29 mg/ml], pyruvate [0.026 mg/ml], estradiol [0.001 mg/ml], follicle stimulating hormone [0.05 U/ml], epidermal growth factor [0.25 mg/ml] and penicillin-streptomycin [0.5 U/ml]). Approximately 8–10 oocytes were cultured in a drop of 50-μl IVM medium overlaid with mineral oil at 38.5 °C under humidified 5% CO2. Cumulus cells were then partially removed at 24 h post-IVM. Oocytes were washed and cultured in a drop of IVF medium (Origio, Måløv, Denmark) and inseminated with cryopreserved spermatozoa (0.5 × 106/ml) for 18 h, and cumulus cells were then completely removed and oocytes cultured in IVC medium (Origio) for additional 6 h. Cleavage rate was recorded after 24 h post-insemination.
Experimental design
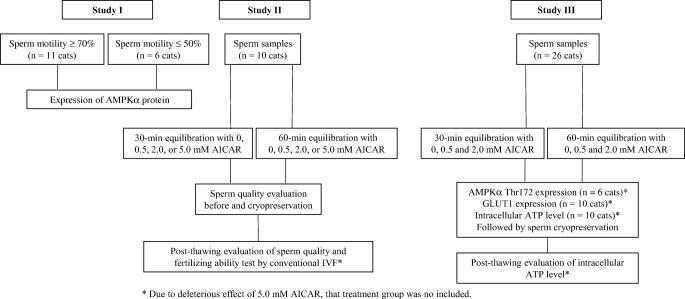
Experimental design is summarized in Fig. 1.
Fig. 1.
Summary of experimental design. *Due to deleterious effect of 5.0 mM AICAR, that treatment group was not included
Study I: expression of AMPKα protein in epididymal cat spermatozoa
Epididymal spermatozoa were obtained from 17 domestic cats. Sample from each individual cat was diluted with Tris-base buffer to reach a final volume containing 10 × 106 cells. Sperm morphology and subjective motility were evaluated to classify each sample as either high motility (≥ 70% of motile sperm cells; n = 11 cats) or poor motility (≤ 50% of motile sperm cells; n = 6 cats). Proteins from each individual sample were extracted and kept at − 80 °C until western analysis as described above.
Study II: influence of AICAR on fertilizing ability of epididymal spermatozoa
Epididymal spermatozoa were obtained from 10 domestic cats, and each individual sample was diluted with Tris-base buffer to reach a final volume containing 10 × 106 cells. Sperm motility, motion characteristics, integrity of sperm plasma membrane and acrosome, and high mitochondrial membrane potential of each individual sample were immediately assessed (fresh control). Each individual sample was then diluted with Semen extender I and divided into eight treatments: (1) presence of 0 (control), (2) 0.5, (3) 2.0, or (4) 5.0 mM AICAR and equilibrated for 30 min; (5) presence of 0 (control), (6) 0.5, (7) 2.0, or (8) 5.0 mM AICAR and equilibrated for 60 min to 4 °C. At the end of each equilibration period, sperms in each treatment were assessed for subjective motility, progressive motility, motion characteristics, integrity of plasma membrane and acrosome as well as mitochondrial membrane potential. The remaining aliquot was then diluted with Semen extender II (ratio 1:1 with Semen extender I), cryopreserved as described above, and subjected to the same assessments as the cooled samples. Fertilizing ability (percentage of cleavage after IVF) of cryopreserved sperm from each individual cat was also assessed as described above. Due to deleterious effects of 5.0 mM AICAR to sperm function, samples equilibrated in the presence of 5.0 mM AICAR were not processed for fertilizing ability outcome.
Study III: influence of AICAR on sperm characteristics before and after cryopreservation and AMPKα signaling proteins
Epididymal spermatozoa from 26 domestic cats were placed in Tris-base buffer to reach a final volume containing 10 × 106 cells. Samples from six cats were used for phospho-AMPKα Thr172 analysis, 10 cats for GLUT1, and other ten cats for ATP level measurement. For each analysis, individual sample was diluted in Semen extender I and divided into six treatments: (1) exposure to 0 (control), (2) 0.5, (3) 2.0 mM AICAR and equilibrated for 30 min; (4) exposure to 0 (control), (5) 0.5, (6) 2.0 mM AICAR and equilibrated for 60 min to 4 °C. Cooled samples were assessed for either AMPK analysis (10 cats) or GLUT1 (six cats). For ATP analysis, the cooled samples were then diluted 1:1 with Semen extender II and cryopreserved as described above. Cryopreserved samples were thawed and assessed for intracellular ATP level (n = 10 cats). Due to deleterious effects of 5.0 mM AICAR to sperm function (detected in studies I and II), samples equilibrated in the presence of 5.0 mM AICAR were not processed for AMPKα signaling proteins and ATP levels.
Statistical analysis
Data analyses were performed using ANOVA with SAS (SAS Institute Inc., Cary, NC, USA). Normal distribution of residuals from statistical models was tested using the UNIVARIATE procedure option NORMAL. Statistical models included fixed effects of AICAR concentration and equilibration duration. Dependent variables (percentage of motility, motion characteristics, percentages of intact cytoplasmic (live) and acrosomal membranes, percentage of spermatozoa with high mitochondrial potential, relative western blot band intensity and intracellular ATP level) were analyzed using ANOVA (GLM procedure). Differences between treatments in each evaluation criterion were compared using a Tukey-Kramer test. The level of progressive motility was compared using NPAR1WAY procedure and a Kruskal-Wallis test. The embryo development was analyzed by a binomial score (1 or 0). Values are presented as mean ± SD. The level of statistical significance was set at P ≤ 0.05.
Results
Study I: expression of AMPKα protein in epididymal cat sperm
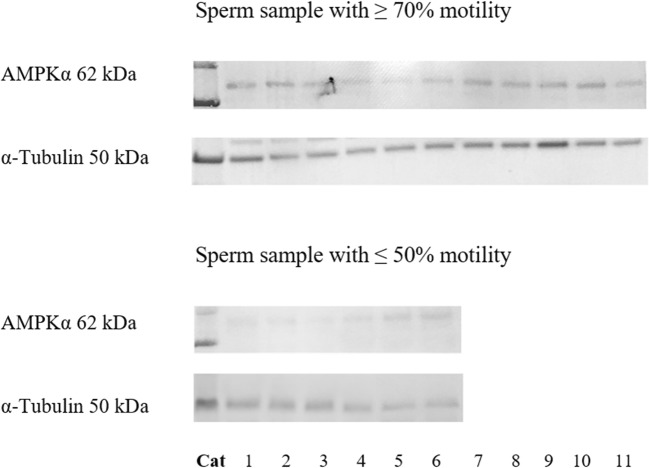
AMPKα protein expression was detected in both samples with high and poor motility (Fig. 2). However, relative AMPKα protein band intensity in samples with ≥ 70% motility (0.35 ± 0.13) was higher (P ≤ 0.05) than those with ≤ 50% motility (0.27 ± 0.09; Fig. 2).
Fig. 2.
Western blot analysis of AMPKα protein in fresh epididymal cat spermatozoa (sperm sample with ≥ 70% motility: n = 11 cats; sperm sample with ≤ 50% motility: n = 6 cats)
The percentage of sperm head normal morphology in all sperm samples included in this study was 91.3 ± 3.7 whereas the mid-piece and tail normal morphology was 57.4 ± 8.1.
Study II: AMPKα regulatory kinase effects on epididymal sperm functions
Equilibrating sperm cells with 0.5 mM AICAR for 30 min promoted (P ≤ 0.05) post-thawed motility (Table 1) and motion characteristics (VCL and VSL) (Table 2) compared with the non-supplemented control and higher concentration treatments. Increase exposure time to 60 min did not enhance post-thawed motility and motion characteristics in all AICAR concentrations (Tables 1 and 2). Furthermore, incubating sperm with the highest AICAR concentration (5.0 mM) significantly decreased (P ≤ 0.05) progressive motility and motion characteristics of cooled and frozen-thawed sperm regardless of incubation period (Tables 1 and 2).
Table 1.
Influence of 30-min or 60-min equilibration in 0 (control), 0.5, 2.0, or 5.0 mM of AICAR on the percentage of motile spermatozoa, score of progressive motility, and percentage of membrane integrity in fresh or frozen-thawed epididymal cat spermatozoa. Values are expressed as mean ± SD (n = 10 cats)
| Metrics | Exposure time | Fresh | Control | 0.5 mM AICAR | 2.0 mM AICAR | 5.0 mM AICAR |
|---|---|---|---|---|---|---|
| Subjective motility (%) | Cooled sperm | 74.0 ± 5.4A | ||||
| 30 min | 68.0 ± 4.5A,B,a | 71.0 ± 4.2A,B,a | 68.0 ± 4.5A,B,a | 63.0 ± 5.0B,a | ||
| 60 min | 67.0 ± 9.7A,a | 65.0 ± 7.1A,B,a | 57.0 ± 3.3B,b | 46.0 ± 5.5C,b | ||
| Cryopreserved sperm | ||||||
| 59.3 ± 2.1A,a | 65.6 ± 3.2B,a | 60.2 ± 3.9A,B,a | 50.3 ± 2.4C,a | |||
| 30 min | 56.5 ± 4.2A,a | 59.7 ± 2.9A,b | 50.1 ± 4.5B,b | 40.7 ± 4.9C,b | ||
| 60 min | ||||||
| Score of progressive motility | Cooled sperm | 3.8 ± 0.4A | ||||
| 30 min | 3.9 ± 0.2A,a | 3.8 ± 0.3A,a | 3.6 ± 0.5A,a | 3.4 ± 0.5A,a | ||
| 60 min | 3.5 ± 0.4A,a | 3.5 ± 0.4A,a | 2.9 ± 0.2B,b | 3.0 ± 0.3B,b | ||
| Cryopreserved sperm | ||||||
| 3.1 ± 0.3A,B,a | 3.2 ± 0.2A,a | 3.2 ± 0.1A,a | 2.8 ± 0.3B,a | |||
| 30 min | 3.0 ± 0.2A,a | 3.1 ± 0.1A,a | 2.5 ± 0.3B,b | 2.4 ± 0.2B,b | ||
| 60 min | ||||||
| Membrane integrity (%) | Cooled sperm | 72.2 ± 3.9A | ||||
| 30 min | 66.5 ± 1.9A,a | 66.4 ± 4.7A,a | 64.5 ± 1.8A,a | 56.8 ± 9.0B,a | ||
| 60 min | 60.3 ± 2.3B,b | 60.1 ± 6.2B,b | 59.5 ± 3.7B,b | 52.9 ± 2.9C,b | ||
| Cryopreserved sperm | ||||||
| 57.7 ± 3.3A,a | 59.1 ± 5.4A,a | 56.6 ± 2.9A,a | 49.4 ± 5.6B,a | |||
| 30 min | 52.5 ± 2.8A,b | 50.2 ± 3.2A,b | 50.2 ± 1.7A,b | 44.3 ± 4.3B,a | ||
| 60 min |
Fresh sample data were only compared with cooled samples
Within the same row, values with different uppercase letters between treatment groups are statistically different (P ≤ 0.05)
Within a treatment group, values with lowercase letters between incubation times are statistically different (P ≤ 0.05)
Table 2.
Influence of 30-min or 60-min equilibration in 0 (control), 0.5, 2.0, or 5.0 mM of AICAR on sperm motion (SCA program analysis) in fresh or frozen-thawed epididymal cat spermatozoa. Values are expressed as mean ± SD (n = 10 cats)
| Metrics | Fresh | Control | 0.5 mM AICAR | 2.0 mM AICAR | 5.0 mM AICAR | |
|---|---|---|---|---|---|---|
| VCL (μm/s) | Cooled sperm | 69.3 ± 5.5 A | ||||
| 30 min | 68.1 ± 13.3A,a | 70.9 ± 10.7A,a | 66.5 ± 9.4A,a | 57.3 ± 17.1B,a | ||
| 60 min | 60.3 ± 2.9B,b | 71.5 ± 2.1A,a | 58.1 ± 0.7B,b | 49.5 ± 2.3C,b | ||
| Cryopreserved sperm | ||||||
| 58.2 ± 2.2A,a | 65.7 ± 4.4B,a | 61.3 ± 9.1A,B,a | 48.7 ± 11.2B,a | |||
| 30 min | 57.4 ± 4.9A,a | 64.3 ± 5.1B,a | 54.4 ± 8.3A,a | 44.1 ± 6.6C,a | ||
| 60 min | ||||||
| VAP (μm/s) | Cooled sperm | 36.1 ± 5.1A | ||||
| 30 min | 39.1 ± 6.5A,a | 40.2 ± 6.1A,a | 34.3 ± 3.4A,a | 32.5 ± 6.7A,a | ||
| 60 min | 35.9 ± 1.1A,a | 34.8 ± 2.8A,a | 33.9 ± 1.6A,a | 25.2 ± 4.5B,b | ||
| Cryopreserved sperm | ||||||
| 30.7 ± 7.8A,B,a | 32.9 ± 6.2A,a | 29.6 ± 4.1A,B,a | 27.5 ± 3.8B,a | |||
| 30 min | 31.1 ± 5.8A,a | 29.2 ± 4.5A,a | 28.4 ± 6.7A,a | 22.6 ± 5.9B,a | ||
| 60 min | ||||||
| VSL (μm/s) | Cooled sperm | 48.3 ± 2.6 A | ||||
| 30 min | 46.3 ± 3.7A,a | 47.3 ± 4.0A,a | 44.8 ± 2.6A,a | 41.2 ± 1.1B,a | ||
| 60 min | 44.9 ± 3.4A,a | 48.1 ± 2.2A,a | 38.8 ± 3.1B,b | 35.4 ± 3.7B,b | ||
| Cryopreserved sperm | ||||||
| 30 min | 38.2 ± 2.2A,a | 43.9 ± 2.1B,a | 39.3 ± 3.6A,B,a | 34.8 ± 5.7B,a | ||
| 60 min | 39.1 ± 3.8A,a | 42.9 ± 3.4A,a | 34.7 ± 4.4B,a | 30.8 ± 6.9B,a | ||
| ALH (μm) | Cooled sperm | 2.3 ± 0.9 A | ||||
| 30 min | 2.5 ± 0.7A,a | 2.7 ± 0.7A,a | 2.8 ± 0.6A,a | 3.1 ± 0.7B,a | ||
| 60 min | 3.3 ± 0.4B,a | 3.3 ± 0.7B,a | 2.9 ± 0.5A,a | 3.3 ± 0.5B,a | ||
| Cryopreserved sperm | ||||||
| 2.6 ± 0.3A,a | 2.7 ± 0.9A,a | 3.1 ± 0.7A,a | 3.3 ± 0.4A,a | |||
| 30 min | 3.5 ± 0.6A,b | 3.0 ± 0.8A,a | 3.0 ± 0.3A,a | 3.7 ± 0.6A,a | ||
| 60 min |
Fresh sample data were only compared with cooled samples
Within the same row, values with different uppercase letters between treatment groups are statistically different (P ≤ 0.05)
Within a treatment group, values with lowercase letters between incubation times are statistically different (P ≤ 0.05)
Although equilibrating sperm with 0.5 or 2.0 mM AICAR for either 30 or 60 min did not affect (P > 0.05) plasma membrane integrity of cooled and post-thawed samples compared with the control, percentages of plasma membrane integrity in 60-min-equilibrated samples were significantly lower than those of the fresh control (Table 1). In addition, incubating sperm to 5.0 mM AICAR concentration even for a short period (30 min) decreased plasma integrity of cooled and cryopreserved sperm.
Incubating sperm with AICAR dose-dependently decreased (P ≤ 0.05) the percentage of sperm with intact acrosome compared with the non-supplemented control. However, equilibrating sperm with the low dosage of AICAR (0.5 mM) for 30 min before freezing sustained acrosomal integrity post-thawed as the percentage of sperm with intact acrosome in this treatment tended to be higher than that in the non-supplemented control and higher AICAR dosages (Table 3).
Table 3.
Influence of 30-min or 60-min equilibration in 0 (control), 0.5, 2.0, or 5.0 mM of AICAR on percentages of acrosome integrity, acrosome reaction, and high mitochondrial membrane potential in fresh or frozen-thawed epididymal cat spermatozoa. Values are expressed as mean ± SD (n = 10 cats)
| Metrics | Fresh | Control | 0.5 mM AICAR | 2.0 mM AICAR | 5.0 mM AICAR | |
|---|---|---|---|---|---|---|
| Acrosome integrity (%) | Cooled sperm | 66.4 ± 7.2A | ||||
| 30 min | 54.0 ± 4.6B,C,a | 55.6 ± 2.6B,C,a | 50.6 ± 6.3C,a | 46.6 ± 7.2C,a | ||
| 60 min | 55.7 ± 2.2B,C,a | 50.5 ± 4.6C,a | 51.1 ± 4.1C,a | 41.0 ± 4.8D,b | ||
| Cryopreserved sperm | 46.4 ± 3.1A,B,a | 49.5 ± 4.4B,a | 43.7 ± 5.6A,C,a | 40.1 ± 3.8C,a | ||
| 30 min | 43.1 ± 5.2A,a | 43.2 ± 2.9A,b | 42.9 ± 4.9A,a | 37.8 ± 1.5B,a | ||
| 60 min | ||||||
| Acrosome reaction (%) | Cooled sperm | 24.2 ± 5.2A | ||||
| 30 min | 32.4 ± 3.8A,B,a | 38.3 ± 5.1B,a | 36.7 ± 6.6A,a | 45.4 ± 6.2C,a | ||
| 60 min | 38.2 ± 3.7B,b | 40.2 ± 3.9B,a | 37.9 ± 3.1B,a | 45.6 ± 6.2C,a | ||
| Cryopreserved sperm | ||||||
| 30 min | 37.5 ± 4.4A,a | 41.2 ± 4.9A,B,a | 39.6 ± 5.8A,a | 45.3 ± 1.5B,a | ||
| 60 min | 45.8 ± 3.7A,b | 47.3 ± 5.6A,b | 40.4 ± 2.3B,a | 49.9 ± 4.7A,a | ||
| High mitochondrial membrane potential (%) | Cooled sperm | 67.6 ± 3.9A | ||||
| 30 min | 62.8 ± 2.6A,a | 63.4 ± 4.5A,a | 55.2 ± 4.1B,a | 51.6 ± 4.4B,a | ||
| 60 min | 54.1 ± 2.7B,b | 55.8 ± 4.0B,b | 53.5 ± 4.5B,a | 47.3 ± 4.5C,b | ||
| Cryopreserved sperm | 53.7 ± 3.7A,a | 55.8 ± 5.3A,a | 50.1 ± 3.9A,a | 44.1 ± 4.2B,a | ||
| 30 min | 49.8 ± 2.2A,a | 51.1 ± 4.6A,a | 49.5 ± 4.8A,a | 41.3 ± 5.1B,a | ||
| 60 min |
Fresh sample data were only compared with cooled samples
Within the same row, values with different uppercase letters between treatment groups are statistically different (P ≤ 0.05)
Within a treatment group, values with lowercase letters between incubation times are statistically different (P ≤ 0.05)
The low AICAR concentration did not impact mitochondrial membrane potential compared with the non-supplemented control. However, incubating sperm with one of the two higher dosages decreased mitochondrial membrane potential, especially in cooled samples. Spermatozoa equilibrated for 60 min without AICAR or with 0.5 mM AICAR reduced mitochondrial membrane potential in cooled samples (Table 3).
For embryo development after homologous IVF, percentage of cleavage was significantly higher (P ≤ 0.05) after equilibration (30 or 60 min) with 0.5 mM AICAR than 0 or 2.0 mM AICAR (Table 4).
Table 4.
Influence of 30-min or 60-min equilibration in 0.5 or 2.0 mM AICAR on percentages of cleavage after homologous in vitro fertilization with frozen-thawed epididymal cat sperm cells. Values are expressed as mean ± SD (n = 10 replications)
| Incubation before freezing | Number of oocytes | Percentage of cleavage | ||
|---|---|---|---|---|
| Control | 0.5 mM AICAR | 2.0 mM AICAR | ||
| 30 min | 261 | 61.1 ± 3.1A | 68.6 ± 2.2B | 60.3 ± 4.4A |
| 60 min | 252 | 58.3 ± 4.2A | 67.4 ± 3.2B | 58.4 ± 3.7A |
Within rows, values with different letters are statistically different (P ≤ 0.05)
Study III: influence of AICAR on AMPKα signaling proteins
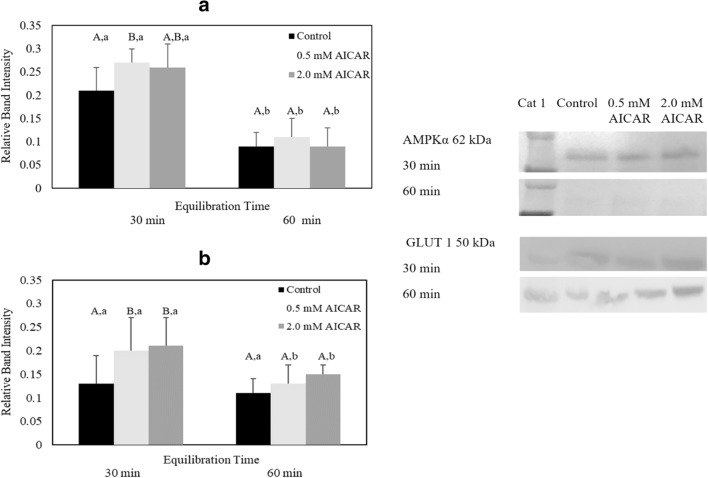
Equilibrating cat epididymal spermatozoa with 5.0 mM AICAR compromised sperm motility, motion characteristics, plasma membrane and acrosome integrity, and mitochondrial membrane potential (Tables 1, 2, 3, and 4). Thus, samples exposed to this high AICAR concentration were excluded from phospho-AMPKα Thr172, GLUT1, and intracellular ATP analyses. For the remaining treatments, western blot analysis revealed appreciable levels of phospho-AMPKα Thr 172 and GLUT1 expression (Fig. 3A and B). However, the highest level of phospho-AMPKα Thr172 protein was observed in spermatozoa equilibrated with 0.5 mM AICAR for 30 min (0.27 ± 0.11) which was significantly higher than the control group (0.21 ± 0.09) (P ≤ 0.05) (Fig. 3A). Extending equilibration period to 60 min did not enhance phospho-AMPKα Thr172 expression, as there were no differences (P > 0.05) in band intensity among treatments. Equilibrating samples at 4 °C for 60 min decreased the expression of phospho-AMPKα Thr172 compared with the level observed after 30-min equilibration in the respective treatment (Fig. 3A).
Fig. 3.
Relative band intensity of (A) phospho-AMPKα Thr172 protein (n = 6 cats) and (B) GLUT1 protein (n = 6 cats) in epididymal cat spermatozoa equilibrated with 0.5 or 2.0 mM AICAR for 30 or 60 min before cryopreservation (values expressed as mean ± SD). Within the same equilibration time, values with different uppercase letters are statistically different (P ≤ 0.05). For a given treatment, values with lowercase letters between equilibration times are statistically different (P ≤ 0.05). Western blot bands represent AMPKα signaling protein expression from one selected cat
Sperm samples equilibrated with 0.5 mM and 2.0 mM AICAR for 30 min expressed a higher relative GLUT1 level (0.5 mM AICAR, 0.20 ± 0.17, and 2.0 mM AICAR, 0.21 ± 0.19) than the control group (0.13 ± 0.15) (P ≤ 0.05) (Fig. 3B). However, increase in exposure time to 60 min in either 0.5 mM or 2.0 mM AICAR treatment decreased GLUT1 expression to the level that was similar to the control (Fig. 3B) (P > 0.05).
The equilibration time did not affect the intracellular ATP level of both cooled and cryopreserved samples (P > 0.05) (Table 5). However, AICAR significantly influenced ATP level in cooled and post-thawed samples (Table 5). Spermatozoa equilibrated with 0.5 mM (30 min and 60 min) and 2.0 mM AICAR (60 min) exhibited a higher intracellular ATP level than the control group (P ≤ 0.05) (Table 5). Similar to the cooled samples, the intracellular ATP level of post-thawed spermatozoa significantly increased in 0.5 mM treatment (for both 30-min and 60-min equilibration) compared with the control (P ≤ 0.05).
Table 5.
Influence of 30-min or 60-min equilibration in 0.5 or 2.0 mM AICAR on intracellular ATP level extracted from frozen-thawed epididymal cat sperm cells. Values are expressed as mean ± SD (n = 10 cats)
| Intracellular ATP level (μg/1 × 106 spermatozoa) | |||
|---|---|---|---|
| Control | 0.5 mM AICAR | 2.0 mM AICAR | |
| Cooled sperm | |||
| 30 min | 0.09 ± 0.02A,a | 0.18 ± 0.03B,a | 0.17 ± 0.05B,a |
| 60 min | 0.08 ± 0.03A,a | 0.15 ± 0.04B,a | 0.11 ± 0.03AB,a |
| Cryopreserved sperm | |||
| 30 min | 0.05 ± 0.009A,a | 0.11 ± 0.03B,a | 0.09 ± 0.02B,a |
| 60 min | 0.04 ± 0.01A,a | 0.07 ± 0.03A,a | 0.08 ± 0.04A,a |
Within the same row, values with different uppercase letters between treatment groups are statistically different (P ≤ 0.05)
Within a treatment group, values with lowercase letters between incubation times are statistically different (P ≤ 0.05)
Discussion
Regulation of cellular energy homeostasis by AMPK is fundamental to cell functions. The present study demonstrated, for the first time, the expression of AMPK protein in domestic cat epididymal spermatozoa. We discovered that AMPK expression level was associated with sperm motility: high sperm motility samples expressed a higher protein level than the poor-motility cohorts. We also showed that (1) equilibrating cat spermatozoa in 0.5 mM AICAR for 30 min increased motility and motion characteristics (VCL and VSL) and fertilizing ability of cryopreserved spermatozoa; (2) high dosage of AICAR (5.0 mM) negatively impacted sperm function both before and after cryopreservation; (3) AICAR dose-dependently influenced phosphorylation of Thr172 level after 30-min equilibration at 4 °C; and (4) prolonged exposure of spermatozoa to AICAR decreased expression of AMPK and GLUT1 as well as intracellular ATP.
AMPK is expressed in male germ cells and plays important roles in regulating sperm function in several mammalian species [8]. In the rat testis, the AMPK activity is associated with one catalytic subunit (α-subunit) and two regulatory subunits (γ- and β-subunit) [20]. In spermatozoa, AMPK (α-subunit) is dominantly expressed in boar [10], human [12], and chicken [11]. AMPK expression level has been shown to link to sperm function [10, 11]. In the present study, we demonstrated that AMPKα protein expression was elevated in high sperm motility samples compared with those with low percentages of motile cells. Our finding is consistent with that reported previously in human [12]. Furthermore, it has been shown that spermatozoa obtained from AMPKα1-knockout (KO) mice are immotile and exhibit high incidences of head abnormality [9]. The same authors also demonstrated mitochondrial dysgenesis in AMPKα-KO mice [9]. Furthermore, incubating boar semen with the AMPK inhibitor, compound C, has been shown to diminish sperm motility [21]. Collectively, the findings reported in the present study and those reported previously confirm the roles of AMPKα in regulating sperm motility.
Our findings demonstrated that AMPKα activation improved post-thawed sperm motility and motion characteristics (VCL and VSL). It has been shown that ATP is produced in mammalian sperm via either glycolysis or OXPHOS pathways at the sperm mid-piece region (mitochondria) or at the flagellum [3, 22]. In stallion, OXPHOS pathway has been considered as the main energy source for sperm flagella sliding and movement [23]. The same investigators also have shown that inhibition of the glycolytic pathway by glucose analog (2-deoxy-D-glucose) negatively impacts stallion sperm motility and motion characteristics (VCL, VSL, and VAP) [23]. Similar to the stallion, a decrease in human sperm motility has been observed after glucose deprivation or exposure of spermatozoa to a glycolysis blockage, methanandamide [24]. Furthermore, mouse spermatozoa incubated in glucose-free medium for 30 min become non-motile indicating that glycolysis is critical for sperm motility [25]. For the cat, it has been shown that the ratio of glucose uptake to lactate production in normospermic individuals (mean of sperm motility index, 80) is ~ 60% higher than teratospermic (mean of sperm motility index, 66) counterparts confirming the crucial role of glucose uptake in sperm flagella movement [26].
In the present study, we reported, for the first time, that AMPKα activation enhanced fertilizing ability of cat spermatozoa. The correlation between sperm motility and fertility has been previously established in many mammalian species, including human [27]. In human, sperm motion characteristics, including VCL and VSL, are positively correlated with fertilization rate [27]. A separate study in human has shown that fertilizing potential of sperm obtained from 26 patients is positively correlated with sperm motility, motion characteristics, and mitochondrial membrane potential [28]. Increased ATP production has been shown to be vital for vigorous flagella movement, sperm capacitation, hyperactivation, and tyrosine phosphorylation leading to successful fertilization in human and mouse [29, 30]. Therefore, we hypothesized that improved cleavage rate observed in the 0.5 mM AICAR exposure group is a resultant of increased ATP production essential for supporting normal sperm function.
It has been demonstrated that the activation of AMPK is operated by the phosphorylation of Thr172 of the α-subunit (phospho-AMPKα Thr 172) that, in turn, stimulates glucose uptake and increases intracellular energy storage [6, 7, 31]. Our western blot analysis revealed that AMPKα protein in cat spermatozoa was indeed phosphorylated at Thr172. We also demonstrated that the impact of AICAR on kinase phosphorylation level depended on dosages and exposure time. A previous report in chicken has shown that the most efficient AICAR concentration for enhancing motility and viability is 2.0 mM whereas higher dosages of this compound (5.0 mM and 10 mM) significantly decrease sperm quality [11]. In addition, high AICAR dosages have been shown to diminish Atp1b1 and Aqp9 gene expression, both of which play important roles in regulating embryo development (blastocyst expansion) in mouse [32]. In the present study, exposure of cat spermatozoa to AICAR for 60 min decreased the level of phospho-AMPKα Thr 172 compared with 30-min equilibration. This finding is similar to that reported in chicken where equilibrating sperm with AICAR increases Thr172-phosphorylated AMPK after 10- and 25-min equilibration while extending equilibration time to 40 min decreases the level of the phosphorylated protein [11]. However, our finding is in contrast to the study in pig [10]. In that study, AMPK protein increases during 60-min period and remains detectable up to 24 h after 38.5 °C incubation [10]. The discrepancy between the findings from our study to those in pig may be due to species-specific differences in the sensitivity to AICAR or the variations in equilibration temperature between the two studies. The synthesis of intracellular ATP is dependent on temperature [33]. As temperature decreases, cell metabolism decreases, hence, lowering ATP production. Because AMPK regulates cell metabolism, it is likely that the function of this protein is also temperature dependent, thus explaining the low Thr172-phosphorylated AMPKα in cat spermatozoa observed after 60-min equilibration at 4 °C in AICAR. It has been shown that the glycolytic pathway and GLUT1 protein activity at the cell membrane are regulated by the AMPK activation [8]. In rat, AMPK enhances GLUT1 expression and glucose uptake in the Sertoli cells that leads to the increase of lactate production [34]. Furthermore, a study in stallion spermatozoa has revealed that the AMPK activation by antidiabetic compound (rosiglitazone) dramatically increases phospho-AMPKα signaling as well as ATP content and glucose uptake [35]. In the present study, equilibrating epididymal spermatozoa with AICAR for 30 min increased GLUT1 protein expression level. However, extending the equilibration period to 60 min decreased GLLUT1 expression. This finding was consistent with the result of phospho-AMPKα Thr172 protein expression level which decreased after 60-min equilibration, thus confirming the role of AMPK in regulating glucose transport and ATP content in cat spermatozoa. Because upregulation of GLUT1 is a downstream effect of AMPK activation, it is not surprising that 60-min equilibration decreased GLUT1 protein expression as decreased AMPKα activity was also observed in this treatment.
In conclusion, the present study confirmed that AMPKα was expressed in the domestic cat spermatozoa and played essential roles in supporting normal sperm function. However, the limitation of this study is that sperm samples were obtained from normospermic samples which might not fully address infertility associated with poor semen quality observed in human. Therefore, future research should focus on examining the impact of AMPKα activation in teratospermic individuals. Findings from such studies could be used to potentially improve fertilizing ability of sperm in sub-fertile men.
Acknowledgments
The authors are thankful for Dr. Em-Orn Olanrattamanee for statistical analysis.
Funding information
The present study was financially supported by (I) The Thailand Research Fund (Contract No. MRG 5980231) and (II) Research Unit of Reproductive Medicine and Fertility Preservation, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–280. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 2.Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15:553–572. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process?(Review) Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- 4.Peña FJ, Rodríguez Martínez H, Tapia JA, Ortega Ferrusola C, González Fernández L, Macías García B. Mitochondria in mammalian sperm physiology and pathology: a review. Reprod Domest Anim. 2009;44(2):345–349. doi: 10.1111/j.1439-0531.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 5.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem. 2006;281(36):25956–25964. doi: 10.1074/jbc.M602992200. [DOI] [PubMed] [Google Scholar]
- 7.Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, Ceddia RB. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50(4):704–715. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoldo MJ, Guibert E, Faure M, Ramé C, Foretz M, Viollet B, Dupont J, Froment P. Specific deletion of AMP-activated protein kinase (α1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS One. 2015;10(3):e0119680. doi: 10.1371/journal.pone.0119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tartarin P, Guibert E, Touré A, Ouiste C, Leclerc J, Sanz N, Brière S, Dacheux JL, Delaleu B, McNeilly JR, McNeilly AS, Brillard JP, Dupont J, Foretz M, Viollet B, Froment P. Inactivation of AMPKα1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology. 2012;153(7):3468–3481. doi: 10.1210/en.2011-1911. [DOI] [PubMed] [Google Scholar]
- 10.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PLoS One. 2012;7(6):e38840. doi: 10.1371/journal.pone.0038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TM, Seigneurin F, Froment P, Combarnous Y, Blesbois E. The 5'-AMP-activated protein kinase (AMPK) is involved in the augmentation of antioxidant defenses in cryopreserved chicken sperm. PLoS One. 2015;10(7):e0134420. doi: 10.1371/journal.pone.0134420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calle-Guisado V, de Llera AH, Martin-Hidalgo D, Mijares J, Gil MC, Alvarez IS, Bragado MJ, Garcia-Marin LJ. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl. 2017;19(6):707–714. doi: 10.4103/1008-682X.185848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shabani Nashtaei M, Nekoonam S, Naji M, Bakhshalizadeh S, Amidi F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 5′ AMP-activated protein kinase activation. Cell Tissue Bank. 2017;26:87–95. doi: 10.1007/s10561-017-9642-5. [DOI] [PubMed] [Google Scholar]
- 14.Comizzoli P, Paulson EE, McGinnis L. The mutual benefits of research in wild animal species and human-assisted reproduction. J Assist Reprod Genet. 2018;35(4):551–560. doi: 10.1007/s10815-018-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuwanut P, Chatdarong K, Johannisson A, Bergqvist AS, Söderquist L, Axnér E. Cryopreservation of epididymal cat spermatozoa: effects of in vitro antioxidative enzymes supplementation and lipid peroxidation induction. Theriogenology. 2010;73(8):1076–1087. doi: 10.1016/j.theriogenology.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Axnér E, Hermansson U, Linde-Forsberg C. The effect of Equex STM paste and sperm morphology on post-thaw survival of cat epididymal spermatozoa. Anim Reprod Sci. 2004;84:179–191. doi: 10.1016/j.anireprosci.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Rota A, Ström B, Linde-Forsberg C, Rodriguez-Martinez H. Effects of Equex STM paste on viability of frozen-thawed dog spermatozoa during in vitro incubation at 38 degrees C. Theriogenology. 1997;47:1093–1101. doi: 10.1016/S0093-691X(97)00066-6. [DOI] [PubMed] [Google Scholar]
- 18.Thuwanut P, Arya N, Comizzoli P, Chatdarong K. Effect of extracellular adenosine 5′-triphosphate on cryopreserved epididymal cat sperm intracellular ATP concentration, sperm quality, and in vitro fertilizing ability. Theriogenology. 2015;84(5):702–709. doi: 10.1016/j.theriogenology.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Thuwanut P, Chatdarong K, Bergqvist AS, Söderquist L, Thiangtum K, Tongthainan D, Axnér E. The effects of antioxidants on semen traits and in vitro fertilizing ability of sperm from the flat-headed cat (Prionailurus planiceps) Theriogenology. 2011;76(1):115–125. doi: 10.1016/j.theriogenology.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–669. doi: 10.1042/bj3460659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Hidalgo D, Hurtado de Llera A, Yeste M, Cruz Gil M, Bragado MJ, Garcia-Marin LJ. Adenosine monophosphate-activated kinase, AMPK, is involved in the maintenance of the quality of extended boar semen during long-term storage. Theriogenology. 2013;80(4):285–294. doi: 10.1016/j.theriogenology.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Amaral A, Lourenço B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction. 2013;146(5):R163–R174. doi: 10.1530/REP-13-0178. [DOI] [PubMed] [Google Scholar]
- 23.Plaza Davila M, Martin Muñoz P, Tapia JA, Ortega Ferrusola C, Balao da Silva CC, Peña FJ. Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS One. 2015;10(9):e0138777. doi: 10.1371/journal.pone.0138777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbonetti A, Vassallo MR, Fortunato D, Francavilla S, Maccarrone M, Francavilla F. Energetic metabolism and human sperm motility: impact of CB1 receptor activation. Endocrinology. 2010;151(12):5882–5892. doi: 10.1210/en.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 26.Terrell KA, Wildt DE, Anthony NM, Bavister BD, Leibo SP, Penfold LM, Marker LL, Crosier AE. Evidence for compromised metabolic function and limited glucose uptake in spermatozoa from the teratospermic domestic cat (Felis catus) and cheetah (Acinonyx jubatus) Biol Reprod. 2010;83(5):833–841. doi: 10.1095/biolreprod.110.085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, Tsunoda H, Sato I. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 2001;18(4):213–218. doi: 10.1023/A:1009420432234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, Fujie M, Suzuki K, Hirata S, Hoshi K. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J Androl. 2002;4(2):97–103. [PubMed] [Google Scholar]
- 29.Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276(10):7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- 30.Hereng TH, Elgstøen KB, Cederkvist FH, Eide L, Jahnsen T, Skålhegg BS, Rosendal KR. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum Reprod. 2011;26(12):3249–3263. doi: 10.1093/humrep/der317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirwany NA, Zou MH. AMPK: a cellular metabolic and redox sensor. A minireview. Front Biosci (Landmark Ed) 2014;19:447–474. doi: 10.2741/4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calder MD, Edwards NA, Betts DH, Watson AJ. Treatment with AICAR inhibits blastocyst development, trophectoderm differentiation and tight junction formation and function in mice. Mol Hum Reprod. 2017;23(11):771–785. doi: 10.1093/molehr/gax050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer D, Bretschneider HJ. Metabolic reduction in hypothermia: pathophysiological problems and natural examples--part 1. Thorac Cardiovasc Surg. 1990;38(4):205–211. doi: 10.1055/s-2007-1014020. [DOI] [PubMed] [Google Scholar]
- 34.Galardo MN, Riera MF, Pellizzari EH, Cigorraga SB, Meroni SB. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J Mol Endocrinol. 2007;39(4):279–288. doi: 10.1677/JME-07-0054. [DOI] [PubMed] [Google Scholar]
- 35.Swegen A, Lambourne SR, Aitken RJ, Gibb Z. Rosiglitazone improves stallion sperm motility, ATP content, and mitochondrial function. Biol Reprod. 2016;95(5):107;1–10712. doi: 10.1095/biolreprod.116.142687. [DOI] [PubMed] [Google Scholar]