Abstract
Zika virus (ZIKV) is mainly transmitted through Aedes mosquito bites, but sexual and post-transfusion transmissions have been reported. During acute infection, ZIKV is detectable in most organs and body fluids including human semen. Although it is not currently epidemic, there is a concern that the virus can still reemerge since the male genital tract might harbor persistent reservoirs that could facilitate viral transmission over extended periods, raising concerns among public health and assisted reproductive technologies (ART) experts and professionals. So far, the consensus is that ZIKV infection in the testes or epididymis might affect sperm development and, consequently, male fertility. Still, diagnostic tests have not yet been adapted to resource-restricted countries. This manuscript provides an updated overview of the cellular and molecular mechanisms of ZIKV infection and reviews data on ZIKV persistence in semen and associated risks to the male reproductive system described in human and animal models studies. We provide an updated summary of the impact of the recent ZIKV outbreak on human-ART, weighing on current recommendations and diagnostic approaches, both available and prospective, with special emphasis on mass spectrometry-based biomarker discovery. In the light of the identified gaps in our accumulated knowledge on the subject, we highlight the importance for couples seeking ART to follow the constantly revised guidelines and the need of specific ZIKV diagnosis tools for semen screening to contain ZIKV virus spread and make ART safer.
Keywords: Zika virus, Male reproductive tract, Assisted reproduction, Semen, Metabolomics
Overview
ZIKV is a positive sense single-stranded RNA virus belonging to the Flavivirus genus. Its molecular structure is very similar to other flaviviruses, like West Nile and dengue (DENV); however, cryoelectron microscopy analysis revealed that ZIKV is distinctively more stable at different temperatures than DENV [1].
ZIKV is mainly transmitted by female Aedes aegypti mosquitoes [2], but reports show that Aedes albopictus and Culex quinquefasciatus may also act as vectors [3, 4]. Besides transmission by vector and nonvector routes (blood transfusions, breastfeeding, etc.), ZIKV has also been shown to be transmitted by sexual contact, predominantly from male-to-female [5]. Female-to-male, as well as male-to-male transmission, has also been reported, and while female-to-female has not, it is considered biologically plausible [6–8].
The incidence of infection is highly variable, ranging from 10,510 cases per 100.000 in Saint Barthelemy epidemic outbreak to 65% of a major urban center population in Brazil [9, 10]. The most common symptoms are rashes, fever, arthralgia, arthritis, and conjunctivitis. Any combination of one of the first two with any of the latter three constitutes a suspected ZIKV case according to the World Health Organization (WHO) guidelines. A confirmed case is diagnosed by the presence of viral RNA or antigen on serum or other fluids, or a positive immunoglobulin M (IgM) test against ZIKV with exclusion of other flaviviruses [11].
Besides the common symptoms, other major complications have been associated with ZIKV. In adults, Guillain-Barre syndrome (GBS) [12–14], myelitis, and meningoencephalitis [15, 16] following ZIKV infection was observed. In newborns, GBS, congenital microcephaly and hydranencephaly, and stillbirth have been reported [17–19]. During the 2015 outbreak in the Northeast region of Brazil, the incidence of microcephaly was 20 times higher than in other periods, and viral RNA was found in fetal brain tissue of microcephalic babies as well as in the amniotic fluid and placentas of pregnant mothers [20]. It is estimated that 10% of babies from infected mothers may develop birth defects [21]. A recent case-report study confirmed that congenital Zika syndrome can be the result of sexual transmission [22].
In November 2016, the WHO ended its global health emergency over the spread of ZIKV and, in the following year, the number of new cases dramatically decreased [23, 24]. The change in designation and the drop in clinical cases, however, do not represent a downgrading of ZIKV importance. Specialists say that epidemics of ZIKV and other arboviruses are likely to continue to emerge [25]. Continents/countries at risk of ZIKV outbreaks include Africa, Asia, the Caribbean, Central America (including Mexico), the Pacific Islands, and South America (including Brazil) [26]. A recent study confirmed the presence of natural ZIKV infection in neotropical non-human primates in urban areas of Brazil, suggesting they might have a role in establishing a sylvatic cycle in the region [27]. Therefore, ZIKV infection continues to be a serious threat, especially to pregnant women and their fetuses, and immune-naive newborns [24].
Introduction
ZIKV infection produces a short viremia in the blood leading to systemic infection [28]; however, over 80% of infected people are asymptomatic, making them potential reservoirs [29]. Through December 2018, 36 cases of probable or confirmed sexual transmission of ZIKV were registered in over 10 different countries [30]. In fact, researchers stated that the magnitude of person-to-person ZIKV transmission is substantially underestimated, and the role of sexual transmission is potentially much larger than previously identified [31].
ZIKV replication has been detected in most organs so far, including the brain [32–35], placenta [36–38], ovaries, spleen [39], spinal cord [40], and testes [40, 41]. Although the major host cells sustaining ZIKV replication in vivo are of myeloid lineages found in the blood and tissues [42], other vulnerable cell types await further investigation. Cells of the male reproductive tract, namely interstitial Leydig cells, Sertoli cells, and germ cells in the testes have been found to be targeted by ZIKV in mice [43]. Importantly, the male genital tract might harbor persistent reservoirs of ZIKV that could facilitate viral transmission over extended periods to uninfected hosts [44].
In response, health authorities have published guidelines for women and couples planning pregnancy. Women wishing to become pregnant and who are carrying out ART treatment in ZIKV endemic areas are encouraged to protect themselves from mosquitoes and from their partner’s potentially infected semen as well. Other recommendations include counseling on the risk of infection during pregnancy, delaying pregnancy attempts until the risk is minimal, and undergoing ZIKV nucleic acid testing (NAT) and immunity testing before proceeding with ART [45].
The duration of excretion of virulent particles in semen needs further evaluation, but it is known that ZIKV RNA can persist in the semen up for 188 days (range 3–188 days) after the acute viremic phase in humans; infectious ZIKV was isolated in semen up to 69 days after symptom onset [46]. It is unknown, however, what impact this long-standing presence of the virus can have on male fertility and on the risk of sustained sexual transmission. This data and some specificities of ZIKV infection in the male genital tract represent a challenge to both public health and reproductive medicine including in vitro fertilization treatments, namely, the persistence of the virus in semen after the acute viremic phase, the teratogenicity of the virus, as well as the possible risk of infertility. Thus, an updated summary on the cellular and molecular ZIKV mechanisms and host cell-virus interactions related to the infection of the male reproductive system along with the description of identified damage observed in humans and animal models are presented. The potential impact of the virus on human ART and insights on the most recent treatment and diagnostics methods focused on the presence of the virus in semen, including an analytical approach based on lipidomics and its potential applications in prospective ZIKV diagnosis, will also be discussed in this review.
ZIKV replication mechanisms on male reproductive tract
Viruses intricately interact with and modulate hosts cells at structural and molecular levels at several stages of their replication. ZIKV infection of skin cells showed receptor-mediated virus entry and increased virus replication upon stimulation of autophagy [47]. ZIKV seems to induce autophagosome formation to promote replication and may trigger apoptosis to foster viral dissemination. Similarly to DENV, ZIKV appears to use the C-type lectin receptor DC-SIGN and members of the TIM and TAM (two receptor families that mediate the phosphatidylserine (PtdSer)-dependent phagocytic removal of apoptotic cells) on host cell surface to gain access to the cytoplasm via receptor-mediated endocytosis [47]. Widespread remodeling of intracellular membranes upon ZIKV infection have been observed during this process, including structural rearrangements of the endoplasmic reticulum associated with RNA genome replication and budding of virions inside it, as well as viruses in transit through the Golgi apparatus for viral maturation and subsequent cellular exit [48]. These findings show a major role for the phosphatidylserine-receptor tyrosine kinase Axl as a ZIKV entry receptor and for cellular autophagy in enhancing ZIKV replication in permissive cells [47]. Further genomic analyses revealed that many antiviral response-related genes are induced by ZIKV and upregulation of genes related to immune response, and apoptosis pathways were also observed. Conversely, many genes involved in cell proliferation, differentiation, migration, and organ development were downregulated [49].
ZIKV infection and male fertility: spermatogenesis and endocrine abnormalities
Axl receptors are thought to be the main access route of ZIKV to immune-privileged sites, like the human male reproductive tract. Similarly to what has been observed in the brain, it is hypothesized that ZIKV particles persisting in the blood could enter the endothelium cells of capillaries in the testis, which are known to have several receptors for flaviviruses (including Axl) replicate inside them and be released in the interstitial space in contact with Leydig cells, macrophages, dendritic cells, and fibroblast [50].
A physical blood-testis-barrier (BTB) formed by tight junctions between adjacent Sertoli cells, associated with mechanisms that suppress immune responses, aids developing sperm to remain protected from an autoimmune attack [51]. However, in vitro studies showed that Sertoli cells are very permissive to ZIKV infection, possibly because they, unlike Leydig cells, highly express the AXL gene. A breach of the immune privilege of the testis could be a serious threat to spermatogenesis as it would compromise the function of key cells in the process (e.g., Sertoli and Leydig cells) [51]. Infection of Sertoli cells led to the dysregulation of several mRNA transcripts, notably the upregulation of fibroblast growth factor (FGF2) that could possibly lead to lower fertility [52].
Concrete information on the fertility of men who have been exposed to ZIKV is still scarce. In a prospective observational study, Joguet et al. [53] studied 15 male volunteers (mean age 35 years) with acute ZIKV infection and positive ZIKV RNA detection in blood or urine. Blood, urine, and semen were collected at days 7, 11, 20, 30, 60, 90, and 120 after symptom onset, and semen characteristics (such as total sperm count, sperm motility, vitality, and morphology) and reproductive hormone concentrations (testosterone, inhibin, follicle-stimulating hormone, and luteinizing hormone) were assessed. The authors demonstrated that total sperm count has decreased from median 119 × 106 spermatozoa at day 7 to 45·2 × 106 at day 30, and 70 × 106 at day 60, respectively, after ZIKV infection. Inhibin values increased from 93·5 pg/mL (55–162) at day 7 to 150 pg/mL (78–209) at day 120 when total sperm count recovered [53]. Taken together, these findings demonstrated harmful effects of ZIKV on human spermatozoa production. The authors concluded that semen alterations occurring early after acute ZIKV infection might affect fertility and could be explained by virus effects on the testis and epididymis, which needs further investigation. Clinical implications of ZIKV compartmentalization in whole semen are still currently not understood. This is a matter of concern as ZIKV seems to be the only arbovirus that causes such severe symptoms of infertility in humans.
Insights from ZIKV infection of the male reproductive tract from animal models
Since the characterization of a novel murine model to study ZIKV by Rossi et al. [54], many experimental infection studies reported that ZIKV severely damages the testes of mice [41, 43, 55].
A longitudinal study in wild-type C57BL/6 mice infected with ZIKV (strain H/PF/2013—French Polynesia 2013), a mouse-adapted Dakar 41,519 (Senegal 1984), or DENV serotype 2 (strain D2S20) was recently conducted [43]. The persistence of ZIKV in semen was associated with testicular damages from both Sertoli and Leydig cells infection, oligospermia, and severely impaired male fertility [43]. Three weeks after infection, both testicles had shrunk to one-tenth of their normal size and the internal structure was completely destroyed [43]. The infected cell types found in this study included spermatogonia, primary spermatocytes, Sertoli cells, and, to a lesser extent, Leydig cells [43]. The symptoms affected the mice ability to produce two important sex hormones, including testosterone and inhibin B, both of which are important in sperm production [43]. This made their sperm counts fall and greatly reduced their fertility (Fig. 1). In addition, fecundity showed a 75% decrease in female mice mated with ZIKV-infected male mice than in female mice mated with uninfected controls. In contrast, male mice infected with DENV showed no evidence of acute or persistent infection in their reproductive systems [43].
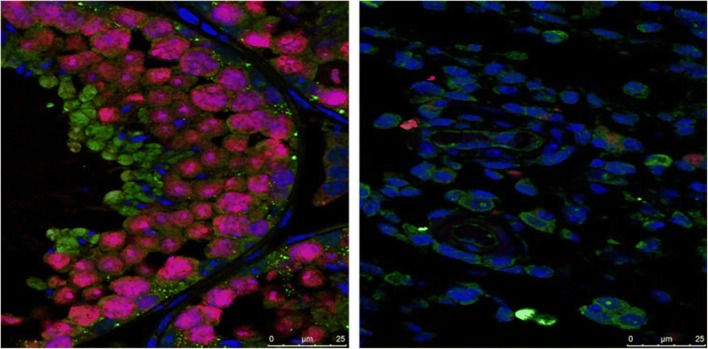
Fig. 1.
Immunofluorescence staining showing that the testis of ZIKV-free mice (left) are full of developing sperm (pink), while the testes of ZIKV-infected mice (right) contain very few sperm. Font: Reprinted from Govero et al. [43], with permission from Macmillan Publishers Ltd.: [Nature Publishing Group], advance online publication, Oct 31, 2016 (10.1038/nature20556)
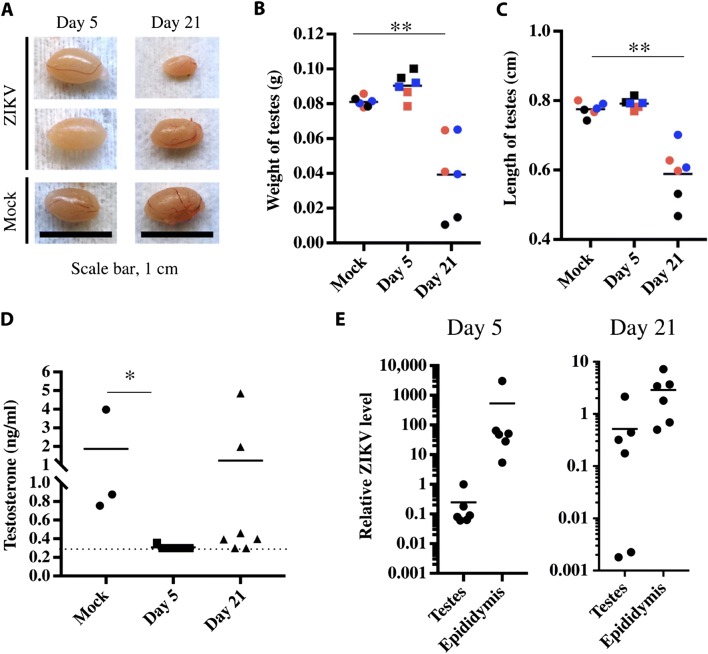
Using C57BL/6 murine infected with American-derived ZIKV (MEX2-81 strain), Uraki and colleagues [41] showed that Leydig cells were able to support ZIKV replication, possibly acting as a reservoir for ZIKV, and that persistent infection may lead to hypofertility. The weight and length of testes were also significantly affected when compared to uninfected mice (Fig. 2), indicating progressive testicular atrophy. In addition, a high amount of antigen associated with the sperm mass within the epididymal duct lumen was observed [41]. In type I interferon receptor-deficient (IFNAR−/−) mice, highest levels of ZIKV RNA (107 FFU equivalents per gram) were also observed in the testes, which could explain male-to-female sexual transmission of ZIKV observed in humans [41].
Fig. 2.
Testicular abnormalities after ZIKV infection. The testes of Ifnar1−/− mice infected with 105 plaque-forming units (PFU) of ZIKVMEX via subcutaneous route and of mock-infected mice were harvested at 5 and 21 days post-infection (dpi). a The photographs compare the testis of infected mice. Scale bar, 1 cm. b, c Weight and length of whole testis from infected animals were measured (n = 6 testes comprising three mice; **P < 0.01). Same color indicates the same mice. d The levels of testosterone in blood of infected mice investigated by enzyme-linked immunosorbent assay (*P < 0.05). The detection limit is 0.3 ng/ml. e The relative viral RNA levels in testis and epididymis at 5 and 21 dpi were examined by qRT-PCR. Data are normalized to mouse b-actin. Font: Reprinted from Uraki et al. [41], with permission from Science Advances
A few studies have also been carried in non-human primates model. A summary of the main outcomes of previously cited and other key studies on the effects of ZIKV infection on the male reproductive tract of different models, including humans, are presented in Table 1. Collectively, these data will allow further investigation of ZIKV pathogenesis and persistence in the male reproductive tract but, although alarming, these findings have to be analyzed with caution.
Table 1.
Main outcomes of key studies on the effects of ZIKV infection on the male reproductive tract of different models
| Publication (year/authors) | Model | Main outcomes |
|---|---|---|
| Mice | ||
| 2016/Ma et al. [55] | Ifnar1−/− mice (C57BL/6 background) |
• ZIKV caused orchitis-epididymitis that led to infertility; • Peritubular cell and spermatogonia could be the potential repositories for ZIKV. |
| 2016/Govero et al. [43] | Wild-type C57BL/6 mice (treated with a single dose of Ifnar1–blocking antibody) | • Observed persistence of ZIKV in the testis and epididymis associated with tissue injury that caused diminished testosterone and inhibin B levels and oligospermia. |
| 2017/Uraki et al. [41] | Ifnar1−/− mice (C57BL/6 background) |
• ZIKV replication persists within the testes even after clearance from the blood; • ZIKV infection caused a reduction in serum testosterone. |
| 2017/Sheng et al. [56] | AG6 mice | • Observed marked atrophy of seminiferous tubules and reduction in lumen size accompanied by positive staining of ZIKV antigens on Sertoli cells. |
| 2018/Clancy et al. [57] | Male Ifnar1−/− mice and female AG129 mice | • Confirmed the passage of virus from epididymal flush and seminal plasma to naïve females via artificial insemination. |
| Non-human primates | ||
| 2016/Osuna et al. [42] | Indian-origin rhesus monkeys and cynomolgus monkeys |
• Viral RNA was detectable in seminal fluids 3 weeks after the resolution of viremia in the blood; • Re-infection of animals 45 days after primary infection resulted in complete protection. |
| Humans | ||
| 2017/Joguet et al. [53] | Humans |
• Total sperm count decreased progressively after acute Zika virus infection for 30 days, at day 120 sperm count was recovered; • Zika virus RNA was found in motile spermatozoa obtained after density gradient separation. |
| 2018/Kumar et al. [52] | Primary human Sertoli and Leydig cell cultures | • Sertoli but not Leydig cells are highly susceptible to Zika virus infection, this proccess is dependent on the TAM family receptor Axl. |
Immunocompetent mice are generally resistant to ZIKV infection, so, many groups (including the ones cited above) opted for using immunocompromised strains to carry out studies about the disease [58]. In humans, ZIKV antagonizes type I interferon (IFN) response through its NS5 protein leading to degradation of a transcription factor (STAT2) that mediates signaling by the type I IFN receptor, IFNAR. However, ZIKV NS5 did not promote degradation of mouse STAT2 which may explain their immunity. Therefore, mice strains lacking the Ifnar1 gene, including A129 and Ifnar1−/− C57BL/6, were used and developed severe disease [58].
Meanwhile, it is known that ZIKV infection of rhesus and cynomolgus macaques share many key clinical findings with humans [27]. The recent finding that New World primates, like the common marmoset in Brazil, are naturally infected by ZIKV points to a possible new and easier to manage experimental model for the study of arboviral infections [27].
Detection and diagnosis, impact on ART, and protective approaches
The human immune response to ZIKV is complex and can be both protective and pathogenic. Also, there are cross-reactions among different viruses such as DENV and chikungunya (CHIKV) serotypes [59]. This fact has implications not only for the understanding of clinical manifestations but also for diagnosis strategies. So far, no diagnostic tests have been adapted to low-resource countries, and there are no rapid tests specific for semen. Currently, there are at least 14 nucleic acid detection lab-based tests (NAT), and 5 serology tests that have received authorization for emergency use from the U.S. Food and Drug Administration (FDA) and likely more from other regulatory agencies around the world [60]. In addition, there are several companies with kits at various stages of product development. [61].
Theel and Hata [60] reviewed the main studies regarding the efficiency of the serological and molecular tests available in the USA. Of the five serological tests available at the time, only three had peer-reviewed studies assessing their efficiency beyond those performed by their manufacturers. The tests based on the detection of IgM class antibodies to ZIKV use mostly serum as a specimen and, in general, have similar results to molecular assays. Comparison of serological and molecular tests showed high correlation between positive results, however, negative results agreement was inconsistent between the two assays [60]. This reinforces the recommendation of the Centers for Disease Control (CDC) to use its plaque-reduction neutralization testing (PRNT) for the confirmation of presumed positive, equivocal, or inconclusive tests. To further demonstrate active infection, either viral proteins or viable infectious viral particles would need to be recovered from a patient’s samples [62].
As for molecular diagnostics approaches for ZIKV, the tests are based mainly in virus RNA detection in serum or urine samples collected during the first 2 weeks of symptom onset. Due to DENV, CHIKV, and ZIKV comorbidity, a laboratory test to sort out the three infections in emergency situations, named Trioplex RT-PCR was the first to be approved by the FDA and showed highly accurate results, although, a false-positive has been reported, urging the CDC to release a statement about it. At least three other molecular assays had similar results [60]. The challenges for the current methods being developed include the lack of specificity for antibody-based assays [6, 63], especially in semen [64], and a very narrow time window for PCR (< 1 week) [65]. Serum and urine are the still the most used samples for practical reasons; however, the recent report of detection of ZIKV RNA in semen of asymptomatic blood donors by RT-PCR [66] highlights the need for readily-available, cost-effective methods of detection of viral load in semen.
Detection of ZIKV in semen
Recently, Joguet and colleagues [53] demonstrated that replicative ZIKV can persist in semen after clinical remission despite its clearance in other fluids, such as blood and urine. It is important, however, to note that long-term persistence of ZIKV in the male genital tract does not necessarily reflect infectivity [31].
The time between onsets of symptoms in the partner after presumed sexual transmission range from 4 to 44 days, the average reported to be between 9.5 and 13 days [31, 67]. Viral load in the semen was roughly 100,000 times that of blood or urine 2 weeks after symptom onset [68]. Recent data reported the detection of ZIKV in the spermatozoa head obtained in the semen of a 32-year-old man returning from French Guiana using immunohistochemical analysis [68]. The proportion of spermatozoa contaminated by ZIKV was estimated at 3.52%.
In motile spermatozoa obtained after density gradient separation, ZIKV RNA was found in three out of 14 patients at day 7, four of 15 at day 11, and four of 15 at day 20. Replication-competent virus was found in the tested patient, showing replication-competent virus in seminal plasma and in spermatozoa after sperm isolation by semen preparation methods [53, 69]. However, only one semen preparation protocol has been evaluated so far; therefore, more studies are needed to assess the effectiveness of different procedures.
In fact, several questions on the effect of ZIKV infection on human health remain open [70]. Further studies are needed to identify the precise locations in male genital tract targeted by ZIKV infection, to explain the longtime presence of the virus in semen in comparison to other body fluids, determine the maximal duration of ZIKV infectivity of semen, and the long-term impact of infection on male fertility [70]. It is also of great importance to investigate semen preparation protocols capable of eliminating ZIKV transmission through ART.
Current and prospective diagnosis approaches for semen samples
So far, the only mention in literature of a method of detection of ZIKV in semen being developed is a single patent by Mariani and Stern [71] that describe “a highly sensitive and robust method for Zika detection in semen, as well as related compositions that include: (a) extracting nucleic acids from a human semen sample; (b) detecting Zika virus nonstructural protein 5 (NS5) mRNA using real-time reverse-transcription polymerase chain reaction (rRT-PCR); and (c) simultaneously, in the rRT-PCR, detecting human beta-actin mRNA as positive control.”
Another prospective diagnosis approach involves lipid screening in semen samples. A recent study indicated that lipids might be useful biomarkers for identifying the infection by ZIKV in Aedes mosquitoes [72], and the same might be speculated for humans. In the past few years, mass spectrometry (MS)-based strategies in combination with bioinformatics have been successfully applied to identify and quantify global changes in cellular proteins, lipids, peptides, and metabolites in response to viral infection. Structural lipids and proteins, the main components of biological membranes, play an important role in viral infection and, notably, Flavivirus replication induces invaginations in the membranes of the endoplasmic reticulum, forming vesicles where RNA replication occurs. The redistribution of cellular lipids to replication sites that follows appears to favor virus replication and survival but is not well understood [73]. Perera et al. [74] used high-resolution MS to evaluate global changes in lipid metabolism upon DENV infection. They found drastic alterations in the global lipid profile in the cells of both human (hepatic cells, Huh-7) and mosquito (Ae. albopictus derived C6/36 cells). Furthermore, genes associated with lipid metabolism were regulated after bacterial or DENV infection in mosquito cells, suggesting that lipid droplets have a role in mounting an antiviral response [73, 75]. Electron spin resonance studies suggest that the virus membrane lipid composition produces a more ordered membrane in the virus compared with the host cell plasma membrane from which it buds [76], such changes in the lipid profile of cells infected by DENV and HIV could also be present on sperm cells infected by ZIKV and readily detected by MS.
Initial exploratory results of our group indicate that informative lipid profiles can be obtained from small amounts of sperm. It seems likely that lipid markers can be characterized for semen infected with ZIKV using widespread metabolomics approaches [77] or using novel biomarker discovery methods such as the MRM-profiling, which has been reported for Parkinson’s disease and polycystic ovarian syndrome [78, 79]. Specific panels of small molecules could become a diagnostic tool to support semen screening before the use of ART in humans and even a prognostic method if metabolic signatures related to infertility due to virus-induced damage can be found.
ZIKV and assisted reproductive technologies
In September 2016, the American Society of Reproductive Medicine (ASRM) published a set of guidelines modified from combined CDC, FDA, and WHO publications, with a focus upon caring for patients with infertility and donors of sperm, oocytes, and embryos [80]. A copy of the table present in the updated ASRM document summarizing recommendations for diverse cases can be seen in Table 2.
Table 2.
CDC recommendations for preconception counseling and prevention of sexual transmission of Zika virus among persons with possible Zika virus exposure
| Exposure scenario | Recommendations (update status) |
|---|---|
| Only the male partner travels to an area with risk for Zika virus transmission and couple planning to conceive |
The couple should use condoms or abstain from sex for at least 3 months after the male partner’s symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). (Updated recommendation) |
| Only the female partner travels to an area with risk for Zika virus transmission and couple planning to conceive |
The couple should use condoms or abstain from sex for at least 2 months (8 weeks) after the female partner’s symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). (No change in recommendation) |
| Both partners travel to an area with risk for Zika virus transmission and couple planning to conceive | The couple should use condoms or abstain from sex for at least 3 months from the male partner’s symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). (Updated recommendation) |
| One or both partners have ongoing exposure (i.e., live in or frequently travel to an area with risk for Zika virus transmission) and couple planning to conceive |
The couple should talk with their health care provider about their plans for pregnancy, their risk for Zika virus infection, the possible health effects of Zika virus infection on a baby, and ways to protect themselves from Zika. If either partner develops symptoms of Zika virus infection or tests positive for Zika virus infection, the couple should follow the suggested timeframes listed above before trying to conceive. (No change in recommendation) |
| Men with possible Zika virus exposure whose partner is pregnant | The couple should use condoms or abstain from sex for the duration of the pregnancy. (No change in recommendation) |
Source: Polen et al. [81]
As shown by those guidelines, ZIKV infection affects not only ongoing ART treatment but fertility preservation programs as well, and the handling of semen, a leukocyte-rich material and therefore highly susceptible to viral infection, is given special attention. A recent study argued that ZIKV present in spermatozoa likely survives both the freezing and thawing processing of semen cryopreservation [82], thereby resulting in frozen sperm having the ability to transmit the virus at a later point in time. Regarding oocytes, there is only one case reporting the presence of ZIKV to date [83], and it is unknown if they will be susceptible to lasting ZIKV infection. Since there is no rapid test available for ZIKV IgM and IgG for semen sample, neither a standardized method to test human oocytes or embryos derived from women who underwent oocyte retrieval at a time of a positive serum ZIKV PCR result [84], the presence of ZIKV in semen and potentially in the female genital tract [85, 86] must be taken into account when reviewing all protocols used in ART.
As an effort to keep ZIKV out of sperm banks and clinics supply, organs and tissue donations, including reproductive tissues and gamete cells, were suspended to avoid infection of future patients. Other measures included the ineligibility of tissue and semen donors that have traveled to, or live in, an area of active ZIKV transmission, under current FDA guidance [87]. Patients must also be informed that direct viral RNA testing with RT-PCR could result in both false-negative and false-positive results. Testing outside of the time of treatment for infertility does not indicate viral infectious status at the time of treatment [80]. In addition, a negative serum RNA result cannot rule out infection in reproductive tissues [80]. More data regarding the maximal duration of ZIKV infectivity in the semen of men and new accurate diagnostic tools are needed in order to allow for different recommendations.
In some cases, however, ART procedures may be urgent, (e.g., fertility preservation before cancer treatment or women with poor ovarian reserve) [88, 89]. For cases like this, the French Biomedicine Agency based its recommendations for ART management on the ZIKV infection status of the male patient, through combining ZIKV serology (IgM and IgG) and RT-PCR testing of a semen sample [90, 91]. If both ZIKV serology and RT-PCR testing of semen are negative, ART can be performed with no specific ZIKV-related measures. In case of positive results or previously diagnosed ZIKV infection, sperm preparation through selection on a density gradient and washing procedure is recommended. Importantly, ZIKV RT-PCR should be performed on both seminal fluid and selected sperm, and only ZIKV-negative sperm should be used for fertilization [92]. Extreme caution is still advised since the risks involve possible ZIKV transmission to a noninfected partner, to the couple’s embryo, to other embryos and gametes, and to the laboratory team [89].
Protection against ZIKV in the male reproductive tract
Recent studies described promising results utilizing a DNA vaccine to reduce the damage the male reproductive tract of mice. Griffin et al. [93] demonstrated that administration of a synthetic DNA vaccine encoding ZIKV pre-membrane and envelope (prME) was able to completely protect mice against the damage described by Govero and collaborators in 2016 [43] to the reproductive tract of immunocompromised mice and was also capable of preventing viral persistence in the testes. Zou et al. [94] reported similar results as well as the protection against maternal-to-fetal transmission in pregnant subjects after the administration of a single dose of a plasmid-launched live-attenuated Zika vaccine (DNA-LAV), suggesting that its simplicity and effectiveness might lead to the development of a universal vaccine platform for ZIKV and other RNA viruses.
Summary and perspectives
While rodent models will keep helping to improve our understandings of tissue tropism and viral spread, their relevance to human infection and disease must be critically evaluated. Immunocompetent non-human primates infected with ZIKV produce clinical responses that closely resemble those of humans and, therefore, are likely more relevant.
There is an urgent need for more and better research focusing on prospective cohort study designs to determine the frequency and duration of viral shedding in the male genital tract. Equally important is determination of the origins of ZIKV in semen and the seminal components, and elucidating whether ZIKV could be present in semen as free particles or associated with leucocytes and epithelial cells in vasectomized/azoospermic men, whether ZIKV preferably adheres to the spermatozoa or could be present in both germ cells and the seminal fluid in normospermic men, and to look for specific methods to obtain ZIKV-free gametes in infected men undergoing ART. Overall, the observations reported by Joguet and colleagues have provided valuable baseline information about endocrine abnormalities and spermatogenesis commitment in ZIKV-infected men. Studies in areas with high rates of ZIKV occurrence, however, are still lacking to determine the impact of ZIKV on men’s reproductive health. Identification of fusexins, fusion proteins essential for both sexual reproduction, and exoplasmic merging of plasma membranes may contribute to our better understanding of how ZIKV infect their target hosts and could help developing diagnostic approaches. Altogether, these studies provide valuable insights into ZIKV infection and about strategies for helping to manage the virus in ART procedures as new protocols to purify ZIKV-infected semen.
In the face of the gaps in knowledge around ZIKV persistence/infectivity in semen, the successively revised guidelines are important to both limit public health risks and deliver general information to couples planning to conceive, gamete donors, and patients requesting ART. Nonetheless, novel sensitive and specific ZIKV diagnosis tools for semen screening are urgent public health instruments to contain ZIKV virus spread and make ART safer.
Funding
Financial support from the Purdue University Women’s Global Health Institute (WGHI) and Indiana Clinical and Translational Sciences Institute (CTSI) through Mildred Elizabeth Edmundson Research (MEER) award PDT 728 2017 is acknowledged.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, et al. Structure of the thermally stable Zika virus. Nature. 2016;533(7603):425–8. 10.1038/nature1799410.1038/nature17994. [DOI] [PubMed]
- 2.Sikka V, Chattu VK, Popli RK, Galwankar SC, Kelkar D, Sawicki SG, et al. The emergence of Zika virus as a global health security threat: a review and a consensus statement of the INDUSEM joint working group (JWG). J Global Infect Dis. 12016:3–15. [DOI] [PMC free article] [PubMed]
- 3.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa) – 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guedes DR, Paiva MH, Donato MM, Barbosa PP, Krokovsky L, Rocha SWDS, Saraiva K, Crespo MM, Rezende TM, Wallau GL, Barbosa RM, Oliveira CM, Melo-Santos MA, Pena L, Cordeiro MT, Franca RFO, Oliveira AL, Peixoto CA, Leal WS, Ayres CF. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6(8):e69. doi: 10.1038/emi.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus - new York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(28):716–717. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- 7.Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P. Male-to-male sexual transmission of Zika virus--Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65(14):372–374. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Transmission Methods. https://www.cdc.gov/zika/prevention/transmission-methods.html. 2019. Accessed 10 Apr 2019.
- 9.Hills SL, Fischer M, Petersen LR. Epidemiology of Zika virus infection. J Infect Dis. 2017;216(suppl_10):S868–S874. doi: 10.1093/infdis/jix434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lourenço J, Maia de Lima M, Faria NR, Walker A, Kraemer MU, Villabona-Arenas CJ, et al. Epidemiological and ecological determinants of Zika virus transmission in an urban setting. Elife. 2017;6. [DOI] [PMC free article] [PubMed]
- 11.WHO. Zika virus disease: interim case definitions. 2016. http://apps.who.int/iris/bitstream/10665/204381/1/WHO_ZIKV_SUR_16.1_eng.pdf. Accessed 10 0ct 2018.
- 12.do Rosario MS, de Jesus PA, Vasilakis N, Farias DS, Novaes MA, Rodrigues SG, et al. Guillain-Barre syndrome after Zika virus infection in Brazil. Am J Trop Med Hyg. 2016;95(5):1157–1160. doi: 10.4269/ajtmh.16-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet. 2016;387(10026):1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- 15.Mecharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481. doi: 10.1016/S0140-6736(16)00644-9. [DOI] [PubMed] [Google Scholar]
- 16.Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, Espinal M, Low N, Dye C. Zika virus as a cause of neurologic disorders. N Engl J Med. 2016;374(16):1506–1509. doi: 10.1056/NEJMp1602708. [DOI] [PubMed] [Google Scholar]
- 17.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 18.Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis. 2016;10(2):e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects--reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 20.Carod-Artal FJ. Neurological complications of Zika virus infection. Expert Rev Anti-Infect Ther. 2018;16(5):399–410. doi: 10.1080/14787210.2018.1466702. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, Ellington SR, Evert N, Reagan-Steiner S, Oduyebo T, Brown CM, Martin S, Ahmad N, Bhatnagar J, Macdonald J, Gould C, Fine AD, Polen KD, Lake-Burger H, Hillard CL, Hall N, Yazdy MM, Slaughter K, Sommer JN, Adamski A, Raycraft M, Fleck-Derderian S, Gupta J, Newsome K, Baez-Santiago M, Slavinski S, White JL, Moore CA, Shapiro-Mendoza CK, Petersen L, Boyle C, Jamieson DJ, Meaney-Delman D, Honein MA, U.S. Zika Pregnancy Registry Collaboration. U.S. Zika Pregnancy Registry Collaboration. Adair J, Ruberto I, Haselow DT, Im L, Jilek W, Lehmann MS, Olney R, Porse CC, Ramstrom KC, Sowunmi S, Marzec NS, Davis K, Esponda-Morrison B, Fraser MZ, O’Connor CA, Chung W, Richardson F, Sexton T, Stocks ME, Woldai S, Bundek AM, Zambri J, Goldberg C, Eisenstein L, Jackson J, Kopit R, Logue T, Mendoza R, Feldpausch A, Graham T, Mann S, Park SY, Carter KK, Potts EJ, Stevens T, Simonson S, Tonzel JL, Davis S, Robinson S, Hyun JK, Jenkins EM, Piccardi M, Reid LD, Dunn JE, Higgins CA, Lin AE, Munshi GS, Sandhu K, Scotland SJ, Soliva S, Copeland G, Signs KA, Schiffman E, Byers P, Hand S, Mulgrew CL, Hamik J, Koirala S, Ludwig LA, Fredette CR, Garafalo K, Worthington K, Ropri A, Ade JN, Alaali ZS, Blog D, Brunt SJ, Bryant P, Burns AE, Bush S, Carson K, Dean AB, Demarest V, Dufort EM, Dupuis II AP, Sullivan-Frohm A, Furuya AM, Fuschino M, Glaze VH, Griffin J, Hidalgo C, Kulas KE, Lamson DM, Lance LA, Lee WT, Limberger R, Many PS, Marchewka MJ, Naizby BE, Polfleit MJ, Popowich M, Rahman T, Rem T, Robbins AE, Rowlands JV, Seaver C, Seward KA, Smith L, Sohi I, St. George K, Souto MI, Wester RE, Wong SJ, Zeng L, Ackelsberg J, Alex B, Ballen V, Baumgartner J, Bloch D, Clark S, Conners E, Cooper H, Davidson A, Dentinger C, Deocharan B, DeVito A, Fu J, Hrusa G, Iqbal M, Iwamoto M, Jones L, Kubinson H, Lash M, Layton M, Lee CT, Liu D, McGibbon E, Moy M, Ngai S, Parton HB, Peterson E, Poy J, Rakeman J, Stoute A, Thompson C, Weiss D, Westheimer E, Winters A, Younis M, Chan RL, Cronquist LJ, Caton L, Lind L, Nalluswami K, Perella D, Brady DS, Gosciminski M, McAuley P, Drociuk D, Leedom V, Witrick B, Bollock J, Hartel MB, Lucinski LS, McDonald M, Miller AM, Ponson TA, Price L, Nance AE, Peterson D, Cook S, Martin B, Oltean H, Neary J, Baker MA, Cummons K, Bryan K, Arnold KE, Arth AC, Bollweg BC, Cragan JD, Dawson AL, Denison AM, Dziuban EJ, Estetter L, Silva-Flannery L, Free RJ, Galang RR, Gary J, Goldsmith CS, Green C, Hale GL, Hayes HM, Igbinosa I, Keating MK, Khan S, Kim SY, Lampe M, Lewis A, Mai C, Martines RB, Miers B, Moore J, Muehlenbachs A, Nahabedian J, Panella A, Parihar V, Patel MM, Rabeneck DB, Rasmussen SA, Ritter JM, Rollin DC, Sanders JH, Shieh WJ, Simeone RM, Simon EL, Sims JR, Spivey PJ, Talley-McRae H, Tshiwala AK, VanMaldeghem K, Viens L, Wainscott-Sargent A, Williams T, Zaki S. Vital signs: update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure - U.S. Zika pregnancy registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(13):366–373. doi: 10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarrington C, Hamer D, Kuohung W, Lee-Parritz A. Congenital Zika syndrome arising from sexual transmission of Zika virus, a case report. Fertil Res Pract. 2019;5(1):1. doi: 10.1186/s40738-018-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Fifth meeting of the Emergency Committee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. 2016. https://www.who.int/en/newsroom/detail/18-11-2016-fifth-meeting-of-the-emergency-committee-under-the-international-health-regulations(2005)-regarding-microcephaly-other-neurological-disorders-and-zika-virus. Accessed 10 Oct 2018.
- 24.Cohen J. Where has all the Zika gone? Science. 2017;357(6352):631–632. doi: 10.1126/science.357.6352.631. [DOI] [PubMed] [Google Scholar]
- 25.Ali S, Gugliemini O, Harber S, Harrison A, Houle L, Ivory J, Kersten S, Khan R, Kim J, LeBoa C, Nez-Whitfield E, O’Marr J, Rothenberg E, Segnitz RM, Sila S, Verwillow A, Vogt M, Yang A, Mordecai EA. Environmental and social change drive the explosive emergence of Zika virus in the Americas. PLoS Negl Trop Dis. 2017;11(2):e0005135. doi: 10.1371/journal.pntd.0005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portal da Saúde - Ministério da Saúde. Confirmação do Zika Vírus no Brasil. 2015. http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/noticias-svs/17702-confirmacaodo-zika-virus-no-brasil. Accessed 3 Dec 2018.
- 27.Terzian ACB, Zini N, Sacchetto L, Rocha RF, Parra MCP, Del Sarto JL, et al. Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci Rep. 2018;8(1):16034. doi: 10.1038/s41598-018-34423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, et al. Adaptive immune responses to Zika virus are important for controlling virus infection and preventing infection in brain and testes. J Immunol. 2017;198(9):3526–3535. doi: 10.4049/jimmunol.1601949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- 30.CDC. Zika Travel Information. 2016. https://wwwnc.cdc.gov/travel/page/zika-travel-information. Accessed 3 Nov 2018.
- 31.Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017;23(5):296–305. doi: 10.1016/j.cmi.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Brault JB, Khou C, Basset J, Coquand L, Fraisier V, Frenkiel MP, Goud B, Manuguerra JC, Pardigon N, Baffet AD. Comparative analysis between Flaviviruses reveals specific neural stem cell tropism for Zika virus in the mouse developing neocortex. EBioMedicine. 2016;10:71–76. doi: 10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanners NW, Eitson JL, Usui N, Richardson RB, Wexler EM, Konopka G, Schoggins JW. Western Zika virus in human fetal neural progenitors persists long term with partial cytopathic and limited immunogenic effects. Cell Rep. 2016;15(11):2315–2322. doi: 10.1016/j.celrep.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Hammack C, Ogden SC, Cheng Y, Lee EM, Wen Z, Qian X, Nguyen HN, Li Y, Yao B, Xu M, Xu T, Chen L, Wang Z, Feng H, Huang WK, Yoon KJ, Shan C, Huang L, Qin Z, Christian KM, Shi PY, Xu M, Xia M, Zheng W, Wu H, Song H, Tang H, Ming GL, Jin P. Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016;44(18):8610–8620. doi: 10.1093/nar/gkw765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, Ming GL. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1(13). [DOI] [PMC free article] [PubMed]
- 37.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, et al. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20(1):83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165(5):1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10(5):e0004658. doi: 10.1371/journal.pntd.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19(5):720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, Homer RJ, Iwasaki A, Fikrig E. Zika virus causes testicular atrophy. Sci Adv. 2017;3(2):e1602899. doi: 10.1126/sciadv.1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EFC, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22(12):1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice. Nature. 2016;540(7633):438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387(10037):2501. doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]
- 45.Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, Rabe IB, Kuhnert-Tallman WL, Martin SW, Walker AT, Gregory CJ, Ades EW, Carroll DS, Rivera M, Perez-Padilla J, Gould C, Nemhauser JB, Ben Beard C, Harcourt JL, Viens L, Johansson M, Ellington SR, Petersen E, Smith LA, Reichard J, Munoz-Jordan J, Beach MJ, Rose DA, Barzilay E, Noonan-Smith M, Jamieson DJ, Zaki SR, Petersen LR, Honein MA, Meaney-Delman D. Update: interim guidance for Health care providers caring for pregnant women with possible Zika virus exposure - United States (including U.S. territories), July 2017. MMWR Morb Mortal Wkly Rep. 2017;66(29):781–793. doi: 10.15585/mmwr.mm6629e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016;21(32). [DOI] [PMC free article] [PubMed]
- 47.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89(17):8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossignol ED, Peters KN, Connor JH, Bullitt E. Zika virus induced cellular remodelling. Cell Microbiol. 2017;19:e12740. doi: 10.1111/cmi.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19(1):120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 50.da Silva LRC. Zika virus trafficking and interactions in the human male reproductive tract. Pathogens. 2018;7(2):51. doi: 10.3390/pathogens7020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stassen LA-O, Armitage CW, van der Heide DJ, Beagley KW, Frentiu FA-O. Zika virus in the male reproductive tract. Viruses. 2018;10(4):E198. doi: 10.3390/v10040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar A, Jovel J, Lopez-Orozco J, Limonta D, Airo AM, Hou S, Stryapunina I, Fibke C, Moore RB, Hobman TC. Human Sertoli cells support high levels of Zika virus replication and persistence. Sci Rep. 2018;8(1):5477. doi: 10.1038/s41598-018-23899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, Guyomard S, Prisant N, Lamarre P, Dejucq-Rainsford N, Pasquier C, Bujan L. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200–1208. doi: 10.1016/S1473-3099(17)30444-9. [DOI] [PubMed] [Google Scholar]
- 54.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg. 2016;94(6):1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167(6):1511–1524. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 56.Sheng ZY, Gao N, Wang ZY, Cui XY, Zhou DS, Fan DY, Chen H, Wang PG, An J. Sertoli cells are susceptible to ZIKV infection in mouse testis. Front Cell Infect Microbiol. 2017;7:272. doi: 10.3389/fcimb.2017.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CSA-Ohoo C, Van Wettere AJ, Morrey JD, Julander JG. Coitus-free sexual transmission of Zika virus in a mouse model. Sci Rep. 2018;8(1):15379. doi: 10.1038/s41598-018-33528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison TE, Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol. 2017;91(8):e00009–e00017. doi: 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016;113(28):7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theel ES, Hata DJ. Diagnostic testing for Zika virus: a post outbreak update. J Clin Microbiol. 2018;56(4):e01972–e01917. doi: 10.1128/JCM.01972-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chua A, Prat I, Nuebling CM, Wood D, Moussy F. Update on Zika diagnostic tests and WHO's related activities. PLoS Negl Trop Dis. 2017;11(2):e0005269. doi: 10.1371/journal.pntd.0005269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lum FM, Lin C, Susova OY, Teo TH, Fong SW, Mak TM, Lee LK, Chong CY, Lye DCB, Lin RTP, Merits A, Leo YS, Ng LFP. Sensitive detection of Zika virus antigen in patients' whole blood as an alternative diagnostic approach. J Infect Dis. 2017;216(2):182–190. doi: 10.1093/infdis/jix276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fauci AS, Morens DM. Zika virus in the Americas--yet another Arbovirus threat. N Engl J Med. 2016;374(7):601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 64.Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill. 2016;21:32. doi: 10.2807/1560-7917.ES.2016.21.32.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill. 2016;21:26. doi: 10.2807/1560-7917.ES.2016.21.26.30269. [DOI] [PubMed] [Google Scholar]
- 66.Musso D, Richard V, Teissier A, Stone M, Lanteri MC, Latoni G, Alsina J, Reik R, Busch MP, Recipient Epidemiology and Donor Evaluation Study (REDS-III) ZIKV Study Group Detection of ZIKV RNA in semen of asymptomatic blood donors. Clin Microbiol Infect. 2017;23(12):1001.e1–1001.e3. doi: 10.1016/j.cmi.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yakob L, Kucharski A, Hue S, Edmunds WJ. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis. 2016;16(10):1100–1102. doi: 10.1016/S1473-3099(16)30324-3. [DOI] [PubMed] [Google Scholar]
- 68.Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, Delobel P, Gonzalez-Dunia D, Malnou CE, Izopet J, Martin-Blondel G. Zika virus in semen and spermatozoa. Lancet Infect Dis. 2016;16(10):1106–1107. doi: 10.1016/S1473-3099(16)30336-X. [DOI] [PubMed] [Google Scholar]
- 69.Cassuto NG, Marras G, Jacomo V, Bouret D. Persistence of Zika virus in gradient sperm preparation. J Gynecol Obstet Hum Reprod. 2018;47(5):211–212. doi: 10.1016/j.jogoh.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Barzon L, Lavezzo E, Palù G. Zika virus infection in semen: effect on human reproduction. Lancet Infect Dis. 2017;17(11):1107–1109. doi: 10.1016/S1473-3099(17)30495-4. [DOI] [PubMed] [Google Scholar]
- 71.Mariani BD, Stern HJ, Inventors: methods and compositions for detecting zika virus in semen patent US20180142310A1. 2017.
- 72.Melo CF, de Oliveira DN, Lima EO, Guerreiro TM, Esteves CZ, Beck RM, et al. A lipidomics approach in the characterization of Zika-infected mosquito cells: potential targets for breaking the transmission cycle. PLoS One. 2016;11(10):e0164377. doi: 10.1371/journal.pone.0164377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pando-Robles V, Batista CV. Aedes-borne virus-mosquito interactions: mass spectrometry strategies and findings. Vector Borne Zoonotic Dis. 2017;17(6):361–375. doi: 10.1089/vbz.2016.2040. [DOI] [PubMed] [Google Scholar]
- 74.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8(3):e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barletta AB, Alves LR, Silva MC, Sim S, Dimopoulos G, Liechocki S, et al. Emerging role of lipid droplets in Aedes aegypti immune response against bacteria and dengue virus. Sci Rep. 2016;6:19928. doi: 10.1038/srep19928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kielian M, Chatterjee PK, Gibbons DL, Lu YE. Specific roles for lipids in virus fusion and exit examples from the alphaviruses. In: Hilderson H, Fuller S, editors. Fusion of biological membranes and related problems. Boston: Springer US; 2002. pp. 409–455. [Google Scholar]
- 77.Crutchfield CA, Thomas SN, Sokoll LJ, Chan DW. Advances in mass spectrometry-based clinical biomarker discovery. Clin Proteomics. 2016;13(1):1. doi: 10.1186/s12014-015-9102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferreira CR, Yannell KE, Mollenhauer B, Espy RD, Cordeiro FB, Ouyang Z, Cooks RG. Chemical profiling of cerebrospinal fluid by multiple reaction monitoring mass spectrometry. Analyst. 2016;141(18):5252–5255. doi: 10.1039/c6an01618a. [DOI] [PubMed] [Google Scholar]
- 79.Cordeiro FB, Ferreira CR, Sobreira TJP, Yannell KE, Jarmusch AK, Cedenho AP, Lo Turco EG, Cooks RG. Multiple reaction monitoring (MRM)-profiling for biomarker discovery applied to human polycystic ovarian syndrome. Rapid Commun Mass Spectrom. 2017;31(17):1462–1470. doi: 10.1002/rcm.7927. [DOI] [PubMed] [Google Scholar]
- 80.Ball D, Bracero N, Bustillo M, Davis O, Gitlin S, Hershlag A et al. Guidance for providers caring for women and men of reproductive age with possible Zika virus exposure. Guidelines from the American Society for Reproductive Medicine regarding Zika virus, incorporates guidance from the CDC, FDA and WHO. 2016.
- 81.Polen KD, Gilboa SM, Hills S, Oduyebo T, Kohl KS, Brooks JT, Adamski A, Simeone RM, Walker AT, Kissin DM, Petersen LR, Honein MA, Meaney-Delman D. Update: interim guidance for preconception counseling and prevention of sexual Transmission of Zika virus for men with possible Zika virus exposure – United States. MMWR Morb Mortal Wkly Rep. 2018;67:868–871. doi: 10.15585/mmwr.mm6731e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.BFS. British Fertility Society. Zika virus, fertility treatment and gamete donation. Updated Advice. 2016. https://britishfertilitysociety.org.uk/2016/02/01/zika-virus-fertility-treatment-and-gamete-donation/. Accessed 3 Nov 2018.
- 83.Araújo Filho E, Fácio C, Machado-Paula L, Martinhago C, Previato L. Case report of Zika virus during the controlled ovarian hyperstimulation: results from oocytes. JBRA Assist Reprod. 2017;21(3):276–292. doi: 10.5935/1518-0557.20180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cordeiro CN, Bano R, Washington Cross CI, Segars JH. Zika virus and assisted reproduction. Curr Opin Obstet Gynecol. 2017;29(3):175–179. doi: 10.1097/GCO.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 85.Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, Herrmann C, Janky E, Joguet G. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16(9):1000–1001. doi: 10.1016/S1473-3099(16)30193-1. [DOI] [PubMed] [Google Scholar]
- 86.Visseaux B, Mortier E, Houhou-Fidouh N, Brichler S, Collin G, Larrouy L, Charpentier C, Descamps D. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16(11):1220. doi: 10.1016/S1473-3099(16)30387-5. [DOI] [PubMed] [Google Scholar]
- 87.CDC. CDC identifies potential risk of Zika virus transmission since June 15, 2016, in Miami-Dade, Broward, and Palm Beach counties. 2017. https://www.cdc.gov/media/releases/2017/s0313-risk-of-zikatransmission-florida.html. Accessed 3 Nov 2018.
- 88.WHO. World Health Organization. Prevention of sexual transmission of Zika virus. 2016. http://www.who.int/csr/resources/publications/zika/sexual-transmission-prevention/en. Acessed on 10 Oct 2018.
- 89.Bujan L, Joguet G, Pavili L, Talarmin A, Pasquier C. Zika virus and assisted reproductive technologies. BMJ. 2016;352:i1062. [Google Scholar]
- 90.Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro surveill. 2016;21:16. doi: 10.2807/1560-7917.ES.2016.21.16.30203. [DOI] [PubMed] [Google Scholar]
- 91.Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill. 2016;21(50):30426. doi: 10.2807/1560-7917.ES.2016.21.50.30426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Epelboin S, Dulioust E, Epelboin L, Benachi A, Merlet F, Patrat C. Zika virus and reproduction: facts, questions and current management. Hum Reprod Update. 2017;23(6):629–645. doi: 10.1093/humupd/dmx024. [DOI] [PubMed] [Google Scholar]
- 93.Griffin BD, Muthumani K, Warner BM, Majer A, Hagan M, JA-O A, et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat Commun. 2017;8:15743. doi: 10.1038/ncomms15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou J, Xie X, Luo H, Shan C, Muruato AE, Weaver SC, Wang T, Shi PY. A single-dose plasmid-launched live attenuated Zika vaccine induces protective immunity. EBioMedicine. 2018;36:92–102. doi: 10.1016/j.ebiom.2018.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]