Abstract
Background
Sphingomyelin synthase 1 (SMS1) has been reported to participate in hepatitis and atherosclerosis. However, its role in autoimmune response is not clear. This study investigates the possible involvement of SMS1 in B-cell activation and lupus-like autoimmunity.
Methods
SMS1 knockout lupus-like animal model and SLE patient samples were utilized. B-cell activation and associated signal transduction were detected by flow cytometry, confocal analysis and western blotting. The SMS1 expression in B cells was measured by real-time qPCR.
Findings
SMS1 deficiency suppressed B-cell activation in culture, which was restored by exogenous SM supplementation. The BCR-mediated early signal transduction including the colocalization of BCR with F-actin or pY/pBtk, and the phosphorylation of intracellular Fyn and Syk were impaired in SMS1 knockout B cells. Furthermore, SMS1 knockout mice showed reduced production and deposition of autoantibodies, accompanied by less severe kidney pathological changes after pristane induction. SMS1 deficiency also displayed lower autoantibody titers and 24 h urine protein excretion in bm12-induced lupus, which were associated with reduced B-cell activation. Adoptively transferred wide-type B cells partially recovered B-cell activation and autoantibody production in SMS1 deficient bm12-induced lupus mice. Moreover, the SMS1 mRNA level in B cells of SLE patients was increased and positively correlated with the serum anti-dsDNA level, IgG and globulin titers.
Interpretation
These data suggest that SMS1 is involved in lupus-like autoimmunity via regulating BCR signal transduction and B cell activation. (Word count for the abstract: 230).
Keywords: Systemic lupus erythematosus, Sphingomyelin synthase 1, B cell, B cell receptor
Research in Context.
Evidence before this study
SMS1 expression has been linked to hepatitis and atherosclerosis. Our previous study showed that SMS1 is involved in TCR signal transduction and T cell activation in Con-A induced hepatitis. However, its role in lupus-like autoimmunity is not clear.
Added value of this study
In this study, we found that SMS1 is involved in BCR-mediated early signal transduction and B cell activation, and thus lupus-like autoimmunity. The SMS1 mRNA level in B-lymphocytes from SLE patients was positively correlated with the autoantibodies level.
Implications of all the available evidence
This finding suggests that SMS1 participates in lupus-like immune damage by directly regulating B cells and provides a potential intervention target for autoimmune response.
Alt-text: Unlabelled Box
1. Introduction
Systemic lupus erythematosus (SLE) is a clinically heterogeneous autoimmune disease with multiple organs involvement and characterized by immune cells activation, autoantibodies production and the deposition of circulating immune complexes [1]. These complexes are especially enriched in kidney, leading to renal failure, increased morbidity and mortality [2,3]. Abnormal immune responses with excessive production of autoantibodies against dsDNA and nuclear factor appear central to the pathogenesis of SLE [4,5].
In B cells, it is believed that B-cell antigen receptor (BCR)-mediated signaling is pivotal for the activation and differentiation of B cells [6,7]. The scattered BCRs are distributed on cell surface with low affinity for lipid rafts (LRs) in resting state. In contrast, LRs concentrate and act as platforms for BCR signal transduction cascades and trafficking upon BCR engagement with antigens [[8], [9], [10]]. The activation of B cells depends on the binding affinity of the BCR to antigen and the density of antigen. BCR clustering is required for the initiation and amplification of BCR signaling and accompanied with the morphological changes of B cells, which depend on actin cytoskeleton reorganization. Previous reports have demonstrated that membrane rafts could mediate actin cytoskeleton reorganization [11,12]. F-actin accumulated not only at the periphery but also at the center of the BCR cluster, colocalizing extensively with the BCR. In addition, F-actin is involved in regulating the signaling threshold and amplification of BCR signaling activities [13,14]. The phosphorylation of tyrosine and Btk (pY/pBtk) in the contact zone of B cells is an early event of BCR signaling [15]. Stable residency of BCRs in LRs results in accumulation of the Src-family kinase Fyn or Lyn and subsequent phosphorylation of ITAMs of BCR, recruiting Syk and multiple intracellular substrates and triggering autoantibody-producing B cells activation [16,17].
Sphingomyelin (SM) is the major species of sphingolipids and almost 65% of cell membrane-associated SM is enriched in LRs [18]. Sphingomyelin synthetases (SMSs), including SMS1 and SMS2, are the key synthetases for SM [19]. SMSs are ubiquitously expressed in mammal animals and SMS1 mainly contributes to the synthesis of plasma membrane SM in lymphocytes [19,20]. The addition of exogenous SM could recover the lack of SM in vitro [21]. Our previous work showed that membrane SM in LRs is crucial for TCR signal transduction and T cell activation in Con-A induced hepatitis [22,23]. Studies have reported that B cells and CD4+T cells isolated from SLE patients and lupus-prone mice displayed increased and aggregated ganglioside, an indicator of LRs [24,25]. Moreover, SMS1 deficiency attenuated Con-A induced hepatitis (7) and atherosclerosis [26]. However, it is unclear whether SMS1 participates in BCR signal transduction and lupus-like autoimmunity.
In this study, we found that SMS1 deficiency suppressed B cell activation and ameliorated lupus-like autoimmunity, as demonstrated by reduced BCR signaling, including BCR clustering by LRs moving and F-actin accumulation, and subsequent phosphorylation of tyrosine, Btk, Fyn and Syk. We also detected an increase level of SMS1 mRNA in B-lymphocytes from SLE patients, which was positively correlated with the serum anti-dsDNA titers, IgG content and globulin titers. These results suggest that SMS1 plays a pivotal role in BCR-mediated signaling and lupus-like autoimmunity.
2. Methods and materials
2.1. Characteristics of SLE patients and healthy controls
This study was approved by the ethic committee of Tongji hospital (ethical number: TJ-IRB20180102). Informed consents were obtained from all participants. The SLE patients were recruited from the Department of Rheumatology in Tongji Hospital between January 2018 and June 2018. The enrolled patients fulfilled the SLICC classification criteria of SLE [27]. No patients were treated with statins. Sex and age-matched healthy volunteers were as controls. Peripheral blood was obtained from all participants. The characteristics of patients were summarized in supplementary Table 1.
2.2. Peripheral blood samples collection and detection
Peripheral blood samples were collected from SLE patients and health controls. The serum was acquired by using a standard serum separator tube. Anti-dsDNA was quantified by autoantibodies profile assay kit (chemiluminescent microparticle immunoassay) (HOB, Jiangsu, China). Cell surface markers was stained with the following fluorochrome-labeled monoclone antibodies: FITC anti-CD19 (Clone# HIB19, Biolegend, San Diego, CA),APC anti-CD69 (Clone# FN50, Biolegend, San Diego, CA). B cells from peripheral blood were isolated with the immunomagnetic cell sorting (MACS) (Miltenyi Biotec, Bergisch Gladbach, Germany). Total RNA in B cells was extracted using total RNA purification solutions (Invitrogen, Carlsbad, CA) and then transcribed into cDNA using commercial kits (TransGen, Beijing, China). The primers were designed according to Genbank sequences and synthesized by Invitrogen Company. Primers for the following transcripts were as follows: SMS1 CAACATTGGCGTAGACAT (forward), TAGGAGGTACTCGTTCGTG-(reverse);GAPDH GCACCGTCAAGGCTGAGAAC (forward), TGGTGAAGACGCCAGTGGA (reverse). Then the expression of SMS1 was measured using Lightcycler 480II RT-PCR System (Roche, Switzerland).
2.3. Mice
C57BL/6.C-H-2bm12KhEg (bm12) female mice, strains of C57BL/6 with a spontaneous mutation in the MHC-II I-Ab molecule were from Fang Zheng as a gift. SMS1−/− mice from Hisanori Umehara and C57BL/6 mice were propagated and bred under humidity and temperature-controlled specific pathogen-free conditions in the animal facility, and female mice were used between 8 and 10 weeks of age. Generation of SMS1−/− mice was illustrated in our previous study [23]. The experimental procedures were approved by the Animal Care and Use Committee of Tongji Hospital.
2.4. Pristane-induced lupus model
For lupus induction, 0.5 mL pristane (Sigma-Aldrich, St. Louis, MO) were administrated by intraperitoneal injection, and PBS treatment as controls. Serum and urine samples were obtained every month, and spleen and kidneys were harvested 6 months post injection. The serum titers of anti-dsDNA and ANA were quantified by commercial ELISA kits (Alpha Diagnostic International, San Antonio, TX). Total protein in urine sample was measured by BCA methods (Beyotime, Shanghai, China). Creatinine (Cr) was quantified using a Creatinine Companion kit (BioAssay Systems, Hayward, CA). Cr estimated as the 24 h urinary protein excretion is the ratios of total urine protein [28]. Kidneys were fixed in 10% neutral-buffered formalin and then embedded in paraffin. 4-μm-thick sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) according to the standard methods. PAS-stained sections were graded in a semiquantitative scoring system as previous study [28,29]. The glomerular activity score (GAS) includes glomerular proliferation, karyorrhexis, fibrinoid necrosis, inflammatory cells infiltration, cellular crescents, and hyaline deposits. The tubulointerstitial activity score (TIAS) includes interstitial inflammation, tubular cell necrosis and/or flattening, and epithelial cells or macrophages infiltration in the tubular lumen [29,30]. IgG deposition in glomeruli was detected by immunofluorescent staining with goat anti-mouse IgG (Sigma-Aldrich, St. Louis, MO).
2.5. The induction of cGVHD lupus-like model
The donor (bm12) and recipient (SMS1−/− or C57BL/6) mice were age- and gender- matched. Details of this lupus-like model were described previously [[31], [32], [33]]. Briefly, C57BL/6 or SMS1−/− recipients received i.p. injection of single-cell suspensions containing 7 × 107 cells from spleens and lymph nodes (superficial cervical, axillary and inguinal) of bm12 donors. Serum and urine specimens were collected at the day of injection (as day 0) and weekly thereafter until 4 weeks. Urine samples and ANA titers were detected as above in the pristane-induced lupus model.
2.6. Adoptive transfer of B cells
B cells from WT C57BL/6 spleen were purified using MACS negative isolation (Miltenyi Biotec, Bergisch Gladbach, Germany), the purity of sorted B cells >95%. Single B cells suspension (1 × 107 cells in 300 μLPBS) was transferred i.v. into SMS1−/− mice, and control group was injected with 300 μL PBS. Six hours after transfer, transferred mice and WT group were induced for cGVHD lupus-like model as described above.
2.7. Flow cytometry
Spleens from lupus model were separated in cold RPMI 1640, and then lapped gently and made into signal cell suspension. Cell surface markers was stained with the following fluorochrome-labeled monoclone antibodies (Biolegend, San Diego, CA): PerCP/Cyanine5.5 anti-CD19 (Clone# 6D5),APC anti-CD69 (Clone#H1.2F3), FITC anti-CD80 (Clone#16-10A1), PE anti-CD86 (Clone#GL-1), and PE anti-CD138 (Clone# 281–2). Then the cells were washed with buffer and identified by flow cytometry (FACS Aria, BD Biosciences, USA), data acquisition were analyzed with FlowJo 7.0 software.
2.8. The treatment of B-lymphocytes in vitro
B cells from spleens were purified using MACS isolation (Miltenyi Biotec, Bergisch Gladbach, Germany), and stimulated by anti-IgM F(ab’)2 fragment (anti-IgM, 10 μg/mL) (Jackson ImmunoResearch Laboratories, Philadelphia, PA), anti-IgM added SM (30 μg/mL) (Sigma-Aldrich, St. Louis, MO) or D609 (50 μg/mL) (Sigma-Aldrich, St. Louis, MO) for indicated time. Then the cells were cultured for 24 h and harvested. The isolated B cells were treated with anti-IgM (10 μg/mL) (Jackson ImmunoResearch Laboratories, Philadelphia, PA) and anti-CD40 (100 ng/mL) (R&D systems) for 48 h and then measured by flow cytometry for the differentiation assay.
2.9. Confocal analysis
Isolated splenic B cells from WT or SMS1 knockout mice were incubated with AF594-mB-F(ab)2 anti-mouse Ig(G + M) (Jackson ImmunoResearch) at 4 °C for 30 min, and then cells were incubated with streptavidin (SA) for 10 min at 4 °C to amplify the signal. After washing, cells were cultured at 37 °C for varying lengths of time (0 min, 5 min, 10 min, 30 min). Cells were fixed with 4% paraformaldehyde (Thermo) and permeabilized with 0.05% saponin (Sigma), and then stained for AF488-anti-F-actin, anti-phosphotyrosine proteins (pY, Merck-millipore), anti-phosphorylated Brutons tyrosine kinase (pBtk, Abcam), AF488-Cholera Toxin Subunit B (CTB, Thermo fisher) and analyzed by a Zeiss LSM 780 confocal fluorescence microscope. >30 cells were individually analyzed, and the correlation coefficient was quantified using NIS-Elements AR 3.2 software.
2.10. Western blot analysis
After stimulating with anti-IgM F(ab’)2 (10 μg/mL) for 5 min, isolated B cells were lysated and detected with western blotting and probed for Syk, Fyn, p-Syk (Y525 + Y526), p-Fyn (Y530) and β-actin (Abcam, Cambridge, UK).
2.11. Intracellular calcium measurement
Isolated B cells were loaded with a concentration of 5 μM Fluo-3-AM (Beyotime, China) for 50 min at 37 °C and changes in dye fluorescence with anti-IgM F(ab’)2 (10 μg/mL) stimulation were analyzed by using LSR II flow cytometer. Kinetic change of Fluo-3 fluorescence was plotted by FlowJo software.
2.12. Statistical analysis
Statistical analysis was analyzed with GraphPad Prism 5 (San Diego, CA, USA). Experimental data are presented as mean ± SEM. The comparisons between the data were analyzed using two-tailed unpaired Student's t-test and Pearson's correlation analysis. p value <.05 was set as statistically significance.
3. Results
3.1. SMS1 contributes to B cell activation and differentiation
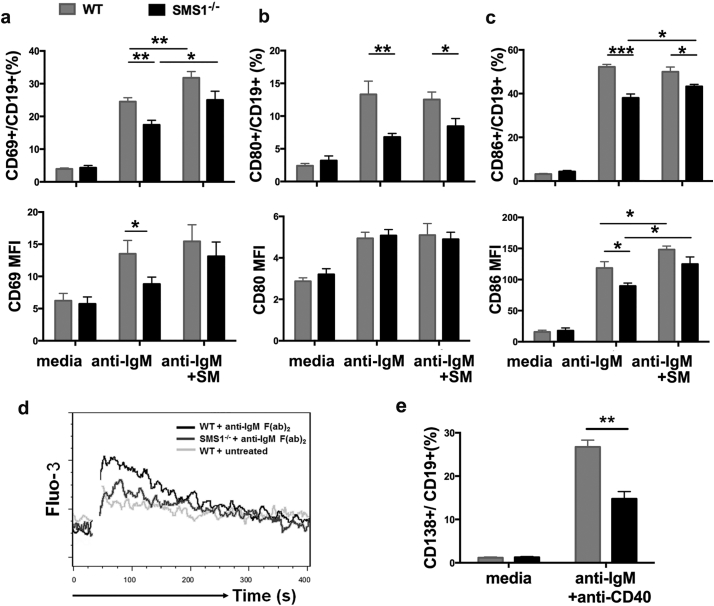
B cell activation and subsequent autoantibody production play a pivotal role in the development of SLE. To confirm the effect of SMS1 on B cell activation and differentiation, we detected the B cells in in vitro B cells culture system. The expression of CD69, CD80 and CD86 on B cells were increased after anti-IgM F(ab’)2 stimulation in WT B cells, while anti-IgM F(ab’)2-induced upregulation of CD69 and CD86 was markedly lower in SMS1 deficient group (Fig. 1a-c). Moreover, exogenous SM supplementation potentiated the expression of CD69 and CD86 on WT B cells, and this SM supplementation partially recovered the upregulation of CD69 and CD86 in SMS1 knockout group (Fig. 1a and Fig. 1c). As the major early event during B lymphocyte activation, the Ca2+ influx in SMS1−/− B cells were lower than that in WT group (Fig. 1d). Autoantibodies production depend on the differentiation of B lymphocytes into plasma cells which express CD138 molecule. As shown in Fig. 1e, SMS1 deficiency itself did not affect the expression of CD138 on plasma cells without stimulation. After anti-IgM F(ab’)2 and anti-CD40 stimulation, the expression of CD138 was elevated in WT B cells, while SMS1 knockout reduced the proportion of plasma cells compared with that in WT group.
Fig. 1.
SMS1 contributes to B cell activation and differentiation.
(a-c) The isolated splenic B cells from WT or SMS1 knockout mice were incubated with 30 μg/mL exogenous sphingomyelin for 6 h, and then stimulated with anti-IgM F(ab’)2 (10 μg/mL) for 24 h in vitro. The percentage and mean fluorescence intensity (MFI) of CD69, CD80 and CD86 were measured by flow cytometry, respectively, n = 8–12 for each group. (d) Calcium mobilization in anti-IgM-stimulated B cells. (e) The percentage of CD138+ plasma cells analyzed by flow cytometry, n = 7 for each group. Anti-IgM, anti-IgM F(ab’)2; data are represented as mean ± SEM and p-values are calculated using unpaired student's t-tests, two-tailed, ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001.
D609 is an inhibitor of SMSs. After D609 pretreatment, anti-IgM F(ab’)2-induced upregulations of CD69, CD80 and CD86 on B cells were suppressed and this suppressive effect could last up to 72 h after treatment (Supplemental Fig. 2a-c). The suppression on CD40 expression was also observed post D609 treatment (Supplemental Fig. 2d). These data support that SMS1 is essential for B cells activation and differentiation.
3.2. SMS1 regulates BCR signaling via modulating lipid raft moving and F-actin accumulation
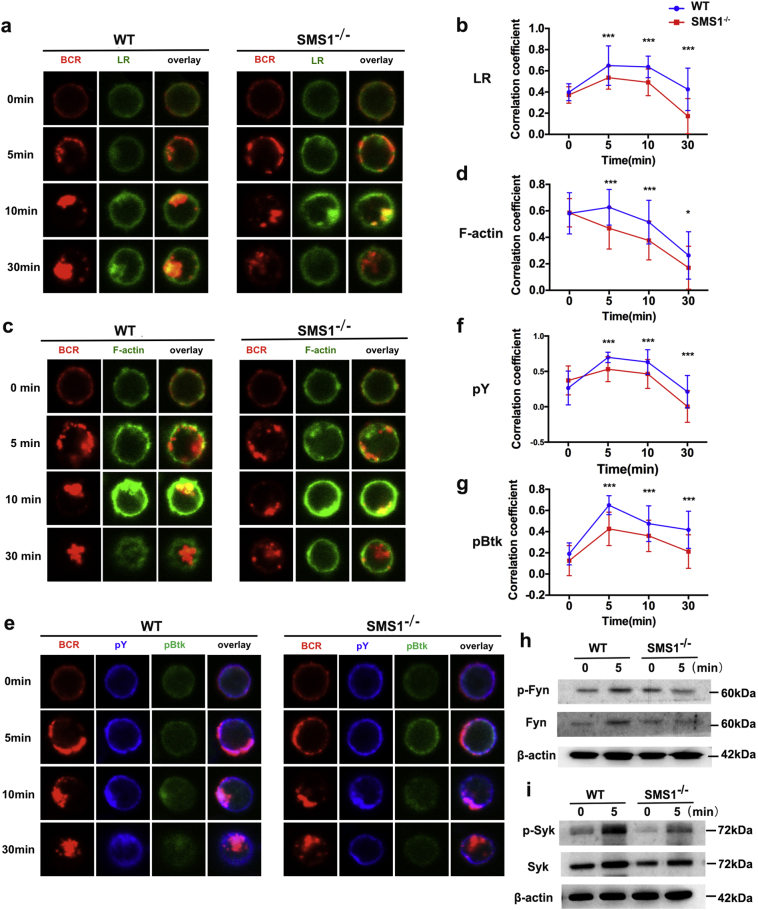
BCR-mediated signaling in response to BCR cross-linking is important for B cell activation and differentiation. BCR clustering, which depends on LR and F-actin accumulation, is required for BCR signaling initiation and amplification. We examined whether SMS1 is involved in BCR signaling using confocal microscopy. As shown in Fig. 2a and Supplemental Fig. 4f, we found that the percentage of BCRs remaining on cell surface was higher in SMS1 knockout group compared with that in WT group after stimulation, suggesting that SMS1 deficiency decreased the degree of cross-linking and internalization of BCR. The correlation coefficient of BCR and LR was increased gradually and peaked at 5–10 min, and then reduced to or below baseline at 30 min. The correlation coefficient of BCR and LR at 5, 10, and 30 min were markedly reduced in SMS1 deficiency mice compared with those in WT mice (Fig. 2b). These results show that loss of SMS1 suppresses LRs moving and BCR enrichment. Furthermore, SMS1 deficiency itself did not change the expression of F-actin on B cells without stimulation, while SMS1 deficiency diminished the polymerization of F-actin to BCR upon anti-IgM F(ab’)2 activation (Fig. 2c). The correlation coefficient of BCR and F-actin was lower in SMS1 knockout group than that in WT group at 5 min, 10 min and 30 min (Fig. 2d).
Fig. 2.
SMS1 regulates BCR signaling via affecting lipid raft moving and F-actin accumulation.
Splenic B cells from WT and SMS1 KO mice were incubated with AF594-mB-F(ab)2 anti-mouse Ig(G + M) at 4 °C for 30 min to label the BCR, and then warmed to 37 °C for activation with varying lengths of time (0 min, 5 min, 10 min, 30 min). After fixation and permeabilization, cells were stained with BCR, lipid raft (LR) (a), F-actin (c), and pY/pBtk (e). The Pearson's correlation coefficients between the BCR and LR (b), F-actin (d), pY (f) and pBtk (g) were quantified using NIS-Elements AR 3.2 software. Shown are representative images from three independent experiments and in which >30 cells were individually analyzed by using NIS-Elements AR 3.2 software. (h-i) The isolated B cells from SMS1−/− and WT mice were stimulated with anti-IgM F(ab’)2 (10 μg/mL) for 5 min in vitro, the expression and the phosphorylation of Fyn and Syk were analyzed by western blotting. Data are represented as mean ± SEM and p-values are calculated using two-tailed unpaired student's t-tests, ⁎p < .05, ⁎⁎⁎p < .001.
To further determine the effect of SMS1 deficiency on BCR signaling, the levels of total phosphotyrosine (pY) and pBtk in the contact zone were detected. The colocalization between pY and BCR in SMS1 knockout B cells was reduced at 5 min, 10 min and 30 min compared to that in WT B cells. The colocalization between pBtk and BCR showed a similar distribution in both WT and SMS1 knockout groups (Fig. 2e-g). The phosphorylation of Fyn and Syk are important intracellular signal events during BCR-mediated B cell activation. After stimulation with anti-Ig(G + M) F(ab’)2, the phosphorylation levels of Fyn and Syk were increased, while both of them were reduced in SMS1 knockout group compared with WT B cells (Fig. 2h-i). These findings indicate that SMS1 is involved in BCR signaling via affecting LRs moving and F-actin accumulation.
3.3. SMS1 deficiency ameliorates lupus-like autoimmunity in pristane-induced lupus mice
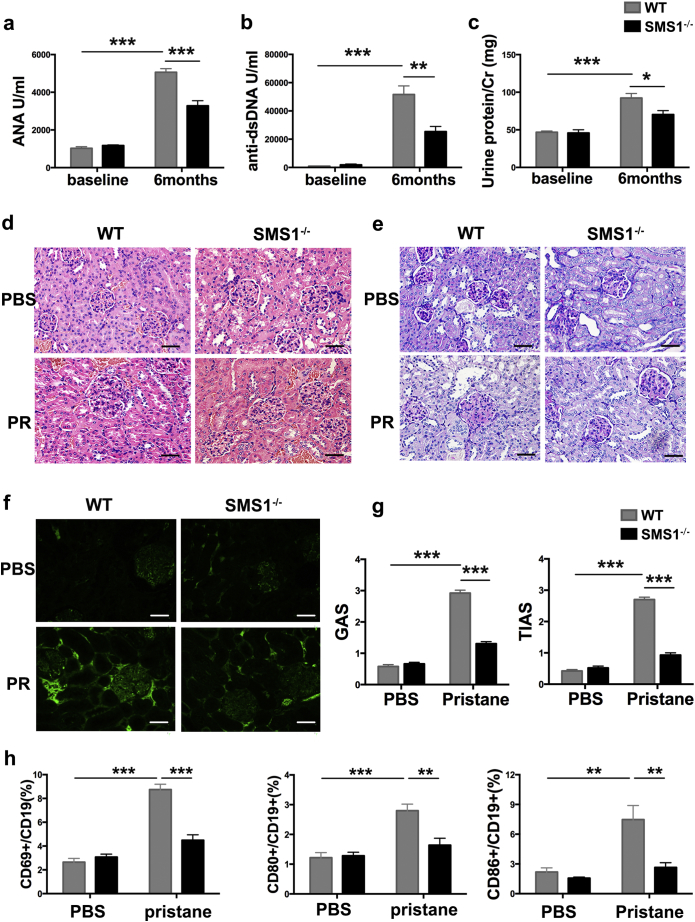
To determine the role of SMS1 in B cell-associated lupus-like autoimmunity, wide-type (WT) and SMS1 knockout mice were used in pristane-induced lupus model. No differences in ANAs and anti-dsDNA titers, urine protein excretion, and kidney anatomical structures were observed between WT and SMS1−/− group under naïve condition (Fig. 3a-g). The titers of ANAs/anti-dsDNA and urine protein excretion were increased at 6 months after pristane induction, accompanied by kidney pathological changes. SMS1 knockout resulted in less increases of ANAs and anti-dsDNA compared with WT group (Fig. 3a-b). The ratio of total urine protein to creatinine (Cr), used to estimate the 24 h urinary protein excretion, was also decreased at 6 months with SMS1 knockout (Fig. 3c). H&E staining revealed that the pristane-induced glomeruli hyperplasia was significantly alleviated in SMS1−/− mice (Fig. 3d). SMS1 deficiency also ameliorated the vacuolar degeneration of tubular epithelial cells (Fig. 3e), accompanied with decreased IgG depositions in glomeruli (Fig. 3f) and a lower glomerular/tubulointerstitial activity score (GAS/TIAS) (Fig. 3g). Simultaneously, the percentages of CD69+, CD80+ and CD86+ cells in B cells were increased after pristane induction in WT, but not in SMS1−/− mice (Fig. 3h).
Fig. 3.
SMS1 deficiency ameliorates lupus-like autoimmunity in pristane-induced lupus-like model.
(a-b) The serum titers of ANA and anti-dsDNA in each group (n = 8 per group) after pristane induction. (c) The 24-h urinary protein excretion (urine protein/Cr) at the time of baseline and 6 months post pristane induction. The pathological changes of kidney glomeruli detected by H&E staining (d) and PAS (e), original magnification×400, scale bar: 50 μm. (f) The IgG deposition in glomeruli, immunofluorescence figures are representative mice from each group. Original magnification×400, scale bar: 50 μm. (g) The glomerular activity score (GAS) and the tubulointerstitial activity score (TIAS) in WT group (n = 9) and SMS1−/− group (n = 7). (h) The percentage of CD69, CD80 and CD86 from splenic B cells were analyzed by flow cytometry, respectively. Cr, creatinine; PR, pristane; H&E staining, hematoxylin and eosin staining; PAS staining, Periodic Acid-Schiff staining. Data are represented as mean ± SEM and p-values are calculated using two-tailed unpaired student's t-tests. ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001.
3.4. SMS1 deficiency alleviates lupus-like autoimmunity in bm12 inducible lupus model
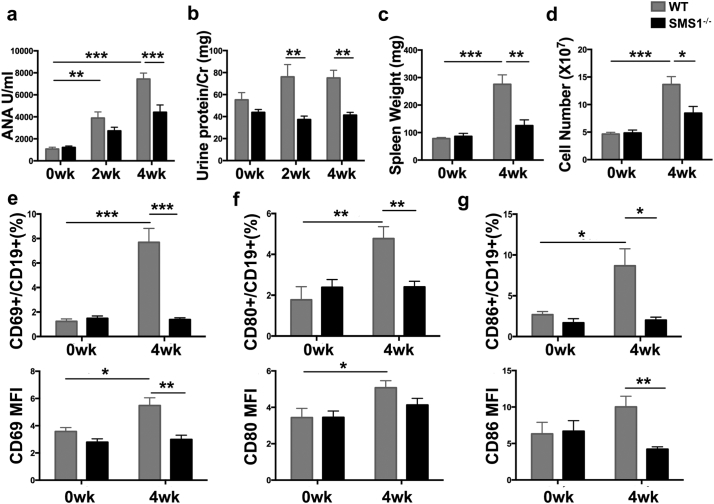
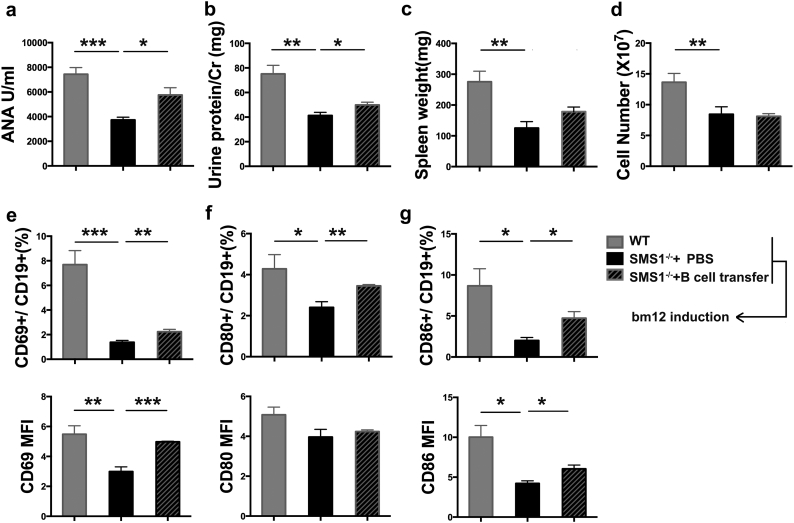
Bm12 inducible chronic GVHD (cGVHD) model, another commonly used lupus-like autoimmunity model, was also used to determine the effect of SMS1. As illustrated in Fig. 4a, the serum ANA titers were increased in WT mice after bm12 induction. The ANA titer at 4w was lower in SMS1−/− group compared with WT group. SMS1 deficient group also exhibited a decreased 24-h urinary protein levels at 2w or 4w post induction (Fig. 4b). Moreover, SMS1 deficiency ameliorated splenomegaly as quantified by spleen weight and cells number (Fig. 4c-d). The amount of ascites, a characteristic of lupus model, was also decreased in SMS1 knockout lupus group (data not shown). Accordingly, the expression of CD69, CD80 and CD86 on B cells, represented the recipient B cells activation, were increased at 4w after cGVHD induction in WT group, while SMS1 knockout downregulated the expression of CD69 and CD86 (Fig. 4e-g). These results indicate that SMS1 is involved in lupus-like autoimmunity.
Fig. 4.
SMS1 knockout alleviated the lupus-like autoimmunity in bm12 inducible lupus mice.
WT (n = 7) and SMS1−/− (n = 7) recipients were injected i.p. with 7 × 107 single cell suspensions from spleens and lymph nodes in bm12 donors. (a) The serum ANA titers were detected by ELISA. (b) The urine protein/Cr was calculated as 24 h urinary protein excretion. (c-d) Weight and lymphocytes number of spleens in recipients were measured at 4 weeks after bm12 induction. (e-g) The percentage and MFI of CD69, CD80 and CD86 on splenic B cells from recipients were analyzed by flow cytometry at 4 weeks after bm12 induction, respectively. wk., week, data were expressed as mean ± SEM and p-values are calculated using two-tailed unpaired student's t-tests.. ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001.
3.5. Adoptive transfer of WT B cells enhances the lupus-like autoimmunity in SMS1 deficiency mice
To further confirm the role of B cells in SMS1-participated lupus-like autoimmunity, an adoptive transfer of B cells from WT mice was performed. As shown in Fig. 5a-b, adoptive transfer of WT B cells increased ANA titers and 24-h urinary protein excretion in bm12-induced SMS1 knockout mice at 4w, although the ANA titers and 24-h urinary protein excretion were still lower in B cell-transferred SMS1 knockout mice compared with those in bm12-induced WT group with no adoptive transfer. The B cell transfer did not alter the spleen weight and spleen cell number in SMS1−/− group (Fig. 5c-d). The expression of CD69 and CD86 on B cells were decreased in SMS1 knockout bm12 inducible mice, and this decreased expression were partially recovered with B cells transfer, although they did not reach to the levels in WT bm12 inducible group (Fig. 5e-g). These data demonstrate that SMS1-mediated lupus-like autoimmunity depends on B cells to some extent.
Fig. 5.
Adoptive transfer of B cells drives the bm12 inducible lupus-like response in SMS1 deficiency mice.
The WT and SMS1 knockout mice were transferred i.v. with 10 million purified B cells from C57BL/6 mice and control group was injected with 300 μL PBS. Six hours after transfer, the three groups were induced for cGVHD lupus-like model. (a) Serum ANA titers, and (b) the urine protein/Cr were detected at the 4 weeks after induction. (c-d) Weight and lymphocytes number of spleens were measured. (e-g) The percentage and MFI of CD69, CD80 and CD86 of B-lymphocytes were assessed by flow cytometry, respectively. Data presented as mean ± SEM and p-values are calculated using unpaired student's t-tests, two-tailed. ⁎p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001.
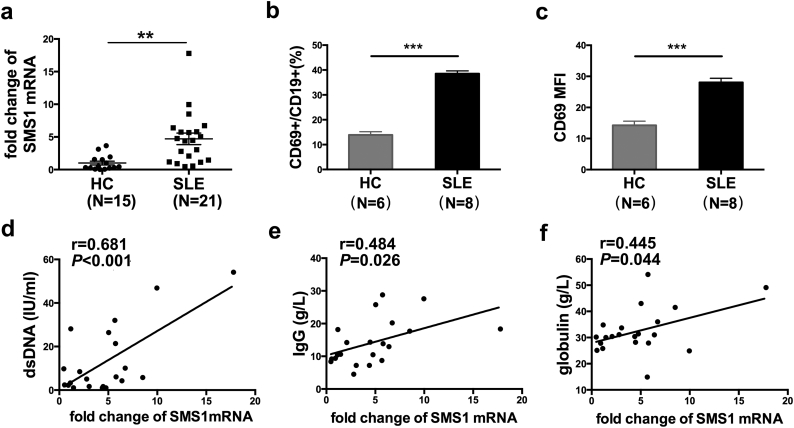
3.6. The levels of SMS1 mRNA were increased in B-lymphocytes from SLE patients and positively correlated with the serum anti-dsDNA, IgG and globulin levels
We further detected the levels of SMS1 mRNA in isolated peripheral blood B cells from SLE patients. The characteristics of the patients were summarized (Supplemental Table 1) and the purity of isolated B cells was about 95% (Supplemental Fig. 1). The quantity of SMS1 mRNA from patients was elevated compared with that in healthy control (HC) group (Fig. 6a), and the percentage and MFI of CD69 on B cells of SLE patients were also higher than those in HC group (Fig. 6b and c). Furthermore, there were positive correlations between the SMS1 mRNA level and serum anti-dsDNA level (r = 0.681, p < .001, Fig. 6d), IgG content (r = 0.484, p = .026, Fig. 6e) as well as globulin titers (r = 0.445, p = .044, Fig. 6f), although we didn't find a correlation between SMS1 mRNA expression and SLE disease activity index (SLEDAI). These data suggest that SMS1 is associated with B cell hyperactivity in SLE.
Fig. 6.
The correlation analysis between SMS1 mRNA in B cells and anti-dsDNA, IgG as well as globulin of SLE patients.
(a) The mRNA level of SMS1 in isolated B-lymphocytes from 21 SLE patients and 15 healthy controls. Data was expressed as mean ± SEM. (b-c) The percentage and MFI of CD69 on B cells from SLE patients and HC group. (d-f) The correlation between SMS1 mRNA level and anti-dsDNA (d), IgG level (e), and globulin levels (f). In Fig a-c, data presented as mean ± SEM and p-values are calculated using unpaired student's t-tests, two-tailed. In Fig d-e, data were analyzed using two-tailed Pearson's correlation and p value <.05 was set as statistically significance.
⁎⁎p < .01, ⁎⁎⁎p < .001.
4. Discussion
SMS1 is the major synthetase for SM, an important component of lipid rafts regulating cell signal transduction and immune activation. Our previous work has shown that SMS1 knockout mice exhibited reduced liver damage in a Concanavalin A (ConA)-induced hepatitis model, due to the membrane SM deficiency-induced suppression of cellular proliferation and signal transduction in CD4+ T cells [23]. However, whether SMS1 contributes to the pathogenesis of SLE and how SMS1 participates in BCR signaling remains unknown. The present research indicates a critical role of SMS1 in the pathogenesis of SLE. It revealed conclusively that SMS1 participates in the lupus-like autoimmune response via affecting BCR signal transduction, and thus regulates B cells activation and differentiation. Moreover, the effect of SMS1 on BCR signaling was associated with the lipid graft moving and the polymerization of F-actin to BCR.
SLE is an autoimmune disease characterized by immune cell activation and production of autoantibodies. Abnormal immune responses with excessive autoantibody production by hyper-activated B cells play an important role in the pathogenesis of SLE. We found that the level of SMS1 mRNA was increased in B cells from SLE patient and was positively correlated with anti-dsDNA levels, serum IgG as well as globulin titers. These data are consistent with other reports. For example, the expression pattern of LRs was changed and associated with the B cells dysfunction in SLE patients [34]. The aggregation of LRs accelerated lupus pathology whereas disruption of which delayed disease progression in lupus-prone mice. B-lymphocytes isolated from SLE patients displayed increased formation of LRs, which was positively correlated with SLEDAI and anti-dsDNA titers [24,34]. Therefore, the increased formation of LRs in B cells plays a pivotal role in the development of SLE.
SM, synthesized by sphingomyelin synthase (SMS), is an essential functional component of LRs, and about 65% of membrane-associated SM was found in LRs [18]. Several studies have indicated that LRs could mediate BCR signal initiation [6,35]. In this study, we characterized the role of SMS1 in lupus-like autoimmunity by using SMS1 deficiency mice. No differences in anti-dsDNA and ANAs titers, urine protein excretion, kidney anatomical structures and the expression of CD69 on B cells were observed between WT and SMS1−/− group under naïve condition. These were consistent with our previous work [23].
Pristane-induced lupus-like animal model was used to reflect the disease of SLE in this study, as the accelerated cells death and the presentation of apoptotic debris as autoantigen are considered as the mainly mechanisms of this model [36]. Bm12- induced cGVHD model was another lupus-like autoimmunine model [37] and was also utilized in the current study to identify the effect of SMS1 on SLE-like autoimmunity. In bm12-induced lupus-like model, cognate recognition of the MHC II in recipient B cells by donor CD4+T cells results in extensive activation of host B cells and production of auto-Abs from recipient B-lymphocytes [38]. CD69 is defined as an early activation marker of lymphocytes [39,40]. We found that SMS1 deficiency ameliorates auto-Abs production, urine protein/Cr level, glomerulonephritis as well as the granulomas and adhesions in peritoneum, accompanied with impaired B cells activation. Adoptive transfer of WT B-lymphocytes into SMS1−/− mice partially recovered ANA titers, 24-h urinary protein excretion, and the expression of CD69 in SMS1 deficient mice after bm12 induction. Therefore, B cell contributes to SMS1-mediated lupus-like pathological injury and the inhibition of SMS1 may be a promising method to alleviate the lupus progression.
B cell hyper-activation is prior to the differentiation and auto-Abs secretion in the pathogenesis of SLE. BCR signaling serves key roles in directing B cells activation and differentiation into auto-Abs secreting plasma cells. Our data showed that the activation and differentiation of B cells were blocked in SMS1 deficiency condition. We then explored how SMS1 affects the BCR signaling. BCR is present in the non-lipid raft domain of plasma membrane in resting states, whereas BCR clusters rapidly and co-localization with LRs after engagement with proper antigen. LRs moving depend on dynamic accumulation of F-actin. We found that the colocalization of BCR and F-actin in SMS1−/− B cell was reduced, the colocalization of pY/pBtk in the contact zone and BCR was also disrupted in SMS1 knockouts. Fyn and Syk are positive regulators of BCR early signal transduction [41]. Our results showed that the phosphorylation of Fyn and Syk of SMS1 deficient B cells upon active stimulation were reduced, indicating that SMS1 is essential for BCR signal transduction. Nevertheless, how SMS1 affects the polymerization of F-actin in B cell activation needs further investigation.
In addition to B cells, T cells are also necessary for the development of lupus. Studies from us and others have demonstrated that SMS1 is involved in T cell proliferation and survival [19,23]. In this study, we found that SMS1 participated in lupus-like autoimmunity via regulating BCR signal transduction and subsequent B cell activation. These data suggest that SMS1 regulates lupus and B cell function may via both T cell-dependent and –independent pathways, which is a further explanation of SMS1.
Collectively, the present work demonstrated that SMS1 is involved in lupus-like immune damage by regulating B cells activation and differentiation. This study furthers our understanding of the role of SMS1/SM in B cells immune response and SLE. Our data may also provide a new therapeutic target for lupus-like autoimmune responses. (Word count for the paper: 3888).
Acknowlegdgments/Funding
This work was supported by grants from National Natural Science Foundation of China (no. 31270965 and 81771754 to Lingli Dong) and Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST to Lingli Dong and Xiangping Yang.
Conflicts of interest
The authors declare no conflicts of interest.
Author contribution
L.-L. D, C.-Q. W and B.-X.M led study design and prepared the manuscript; C.-Q. W, B.-X.M and X.-F. W carried out the experiments; T. W and S.-Z. C performed statistical analysis; P. H and J.-G. T assisted in tissue sample collection; Z.T, C.-H. L, J.-X. Z, and F.Z reviewed and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.06.038.
Contributor Information
Fang Zheng, Email: zhengfangtj@hust.edu.cn.
Lingli Dong, Email: tjhdongll@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Tsokos G.C. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Martin F., Chan A.C. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20(5):517–527. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- 3.Liossis S.N., Melissaropoulos K. Molecular abnormalities of the B cell in systemic lupus erythematosus are candidates for functional inhibition treatments. Expert Opin Pharmacother. 2014;15(6):833–840. doi: 10.1517/14656566.2014.894976. [DOI] [PubMed] [Google Scholar]
- 4.Moulton V.R., Suarez-Fueyo A., Meidan E., Li H., Mizui M., Tsokos G.C. Pathogenesis of human systemic lupus Erythematosus: a cellular perspective. Trends Mol Med. 2017;23(7):615–635. doi: 10.1016/j.molmed.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsokos G.C., Lo M.S., Costa Reis P., Sullivan K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–730. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 6.Saeki K., Miura Y., Aki D., Kurosaki T., Yoshimura A. The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J. 2003;22(12):3015–3026. doi: 10.1093/emboj/cdg293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dal Porto J.M., Gauld S.B., Merrell K.T., Mills D., Pugh-Bernard A.E., Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41(6–7):599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Miller H., Song W. Use of Streptolysin O-induced membrane damage as a method of studying the function of lipid rafts during B cell activation. Methods Mol Biol. 2018;1707:235–241. doi: 10.1007/978-1-4939-7474-0_17. [DOI] [PubMed] [Google Scholar]
- 9.Cheng P.C., Cherukuri A., Dykstra M. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin Immunol. 2001;13(2):107–114. doi: 10.1006/smim.2000.0302. [DOI] [PubMed] [Google Scholar]
- 10.Pierce S.K. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2(2):96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 11.Viola A., Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7(11):889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 12.Carlile-Klusacek M., Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol. 2007;293(1):H366–H375. doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- 13.Song W., Liu C., Upadhyaya A. The pivotal position of the actin cytoskeleton in the initiation and regulation of B cell receptor activation. Biochim Biophys Acta. 2014;1838(2):569–578. doi: 10.1016/j.bbamem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C., Miller H., Orlowski G., Hang H., Upadhyaya A., Song W. Actin reorganization is required for the formation of polarized B cell receptor signalosomes in response to both soluble and membrane-associated antigens. J Immunol. 2012;188(7):3237–3246. doi: 10.4049/jimmunol.1103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C., Richard K., Wiggins M., Zhu X., Conrad D.H., Song W. CD23 can negatively regulate B-cell receptor signaling. Sci Rep. 2016;6 doi: 10.1038/srep25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone M.B., Shelby S.A., Nunez M.F., Wisser K., Veatch S.L. Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. Elife. 2017;6 doi: 10.7554/eLife.19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deckert M., Elly C., Altman A., Liu Y.C. Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J Biol Chem. 1998;273(15):8867–8874. doi: 10.1074/jbc.273.15.8867. [DOI] [PubMed] [Google Scholar]
- 18.Gowda S., Yeang C., Wadgaonkar S. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L430–L440. doi: 10.1152/ajplung.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakor A.B., Taniguchi M., Kitatani K. Sphingomyelin synthase 1-generated sphingomyelin plays an important role in transferrin trafficking and cell proliferation. J Biol Chem. 2011;286(41):36053–36062. doi: 10.1074/jbc.M111.228593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi M., Tasaki T., Ninomiya H. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci Rep. 2016;6 doi: 10.1038/srep37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe M., Makino A., Hullin-Matsuda F. A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol Cell Biol. 2012;32(8):1396–1407. doi: 10.1128/MCB.06113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z.X., Huang C.R., Dong L. Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells. Int Immunol. 2008;20(11):1427–1437. doi: 10.1093/intimm/dxn100. [DOI] [PubMed] [Google Scholar]
- 23.Dong L., Watanabe K., Itoh M. CD4+ T-cell dysfunctions through the impaired lipid rafts ameliorate concanavalin A-induced hepatitis in sphingomyelin synthase 1-knockout mice. Int Immunol. 2012;24(5):327–337. doi: 10.1093/intimm/dxs008. [DOI] [PubMed] [Google Scholar]
- 24.Dong L., Hu S., Chen F. Increased expression of ganglioside GM1 in peripheral CD4+ T cells correlates soluble form of CD30 in systemic lupus Erythematosus patients. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/569053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong G.F., Zhang X., He de N., Li L., Zhang G.F. Effect of Leflunomide on the abnormal expression of lipid rafts and F-actin in B lymphocytes from patients with systemic lupus Erythematosus. J Immunol Res. 2015;2015 doi: 10.1155/2015/832916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z., Fan Y., Liu J. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(7):1577–1584. doi: 10.1161/ATVBAHA.112.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petri M., Orbai A.M., Alarcon G.S. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahlenberg J.M., Yalavarthi S., Zhao W. An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheum. 2014;66(1):152–162. doi: 10.1002/art.38225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amarilyo G., Lourenco E.V., Shi F.D., La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193(2):540–543. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 30.Ferrera F., Hahn B.H., Rizzi M. Protection against renal disease in (NZB x NZW)F(1) lupus-prone mice after somatic B cell gene vaccination with anti-DNA immunoglobulin consensus peptide. Arthritis Rheum. 2007;56(6):1945–1953. doi: 10.1002/art.22700. [DOI] [PubMed] [Google Scholar]
- 31.Klarquist J., Janssen E.M. The bm12 inducible model of systemic lupus Erythematosus (SLE) in C57BL/6 mice. J Vis Exp. 2015;(105) doi: 10.3791/53319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao W.H., Eisenberg R.A., Cohen P.L. The Mer receptor tyrosine kinase is required for the loss of B cell tolerance in the chronic graft-versus-host disease model of systemic lupus erythematosus. J Immunol. 2008;180(11):7728–7735. doi: 10.4049/jimmunol.180.11.7728. [DOI] [PubMed] [Google Scholar]
- 33.Eisenberg R.A., Via C.S. T cells, murine chronic graft-versus-host disease and autoimmunity. J Autoimmun. 2012;39(3):240–247. doi: 10.1016/j.jaut.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong G.F., Zhang X., He D.N., Li L., Zhang G.F. Effect of Leflunomide on the abnormal expression of lipid rafts and F-actin in B lymphocytes from patients with systemic lupus Erythematosus. J Immunol Res. 2015;2015 doi: 10.1155/2015/832916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller H., Castro-Gomes T., Corrotte M. Lipid raft-dependent plasma membrane repair interferes with the activation of B lymphocytes. J Cell Biol. 2015;211(6):1193–1205. doi: 10.1083/jcb.201505030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry D., Sang A., Yin Y., Zheng Y.Y., Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., Sun X., Yang W. An autoimmune disease variant of IgG1 modulates B cell activation and differentiation. Science. 2018;362(6415):700–705. doi: 10.1126/science.aap9310. [DOI] [PubMed] [Google Scholar]
- 38.Shao W.H., Gamero A.M., Zhen Y. Stat1 regulates lupus-like chronic graft-versus-host disease severity via interactions with Stat3. J Immunol. 2015;195(9):4136–4143. doi: 10.4049/jimmunol.1501353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokunaga M., Saito K., Kawabata D. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–475. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu H., Crawford R.B., Kaplan B.L., Kaminski N.E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated disruption of the CD40 ligand-induced activation of primary human B cells. Toxicol Appl Pharmacol. 2011;255(3):251–260. doi: 10.1016/j.taap.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barua D., Hlavacek W.S., Lipniacki T. A computational model for early events in B cell antigen receptor signaling: analysis of the roles of Lyn and Fyn. J Immunol. 2012;189(2):646–658. doi: 10.4049/jimmunol.1102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material