Abstract
Background
Ulcerative Colitis (UC) is an Inflammatory Bowel Disease (IBD) characterized by uncontrolled immune response, diarrhoea, weight loss and bloody stools, where sustained remission is not currently achievable. Dextran Sulphate Sodium (DSS)-induced colitis is an animal model that closely mimics human UC. Ultrasound (US) has been shown to prevent experimental acute kidney injury through vagus nerve (VN) stimulation and activation of the cholinergic anti-inflammatory pathway (CAIP). Since IBD patients may present dysfunctional VN activity, our aim was to determine the effects of therapeutic ultrasound (TUS) in DSS-induced colitis.
Methods
Acute colitis was induced by 2% DSS in drinking water for 7 days and TUS was administered to the abdominal area for 7 min/day from days 4–10. Clinical symptoms were analysed, and biological samples were collected for proteomics, macroscopic and microscopic analysis, flow cytometry and immunohistochemistry.
Findings
TUS attenuated colitis by reducing clinical scores, colon shortening and histological damage, inducing proteomic tolerogenic response in the gut during the injury phase and early recovery of experimental colitis. TUS did not improve clinical and pathological outcomes in splenectomised mice, while α7nAChR (α7 nicotinic acetylcholine receptor - indicator of CAIP involvement) knockout animals presented with disease worsening. Increased levels of colonic F4/80+α7nAChR+ macrophages in wild type mice suggest CAIP activation.
Interpretation
These results indicate TUS improved DSS-induced colitis through stimulation of the splenic nerve along with possible contribution by VN with CAIP activation.
Fund
Intramural Research Programs of the Clinical Centre, the National Institute of Biomedical Imaging and Bioengineering at the NIH and CAPES/Brazil.
Keywords: Therapeutic ultrasound, Dextran Sulphate sodium, Cholinergic anti-inflammatory pathway, Inflammatory bowel disease, Acute ulcerative colitis, α7 nicotinic acetylcholine receptor
Graphical abstract
Research in context.
Evidence before this study
Previous studies have shown the attenuation of acute kidney injury through the therapeutic use of imaging ultrasound and reported that activation of the vagus nerve and the cholinergic anti-inflammatory pathway were the main mechanisms for symptomatic, pathological and immunological amelioration. Considering there is no cure for Ulcerative Colitis and that patients have a poor quality of life, a non-invasive method that has been used for decades and easily applied to the clinic would be an interesting option. Moreover, IBD patients have been shown to present with a dysfunctional vagus nerve, demonstrating one more time how this technique would be of great importance in this context. The literature search was mainly done using the Pubmed platform, including but not limited to terms such as: therapeutic ultrasound, cholinergic anti-inflammatory pathway, α7 nicotinic acetylcholine receptor, dextran sulphate sodium, vagus nerve, splenic nerve, mucosal immunity, ulcerative colitis, inflammatory bowel disease, acetylcholine, norepinephrine, macrophages.
Added value of this study
Our finding that therapeutic ultrasound attenuates Dextran Sulphate Sodium (DSS)-induced colitis through activation of the cholinergic anti-inflammatory pathway adds a valuable treatment option for Inflammatory Bowel Disease (IBD) patients. As a non-invasive and easily accessible technique, therapeutic ultrasound was shown here to change the immunological profile of a murine IBD model, leading to clinical and pathological changes that attenuated DSS-induced acute colitis.
Implications of all the available evidence
Including all evidence available, the future of IBD treatment may include valuable novel options. The TUS treatment here used could be easily translated to the clinic, considering it has been used for decades. The development of a multiple transducer system that could be worn by patients over their abdominal area could significantly improve their quality of life. As a non-invasive low intensity ultrasound treatment, for the first time is reported here that TUS becomes an alternative treatment for IBD patients.
Alt-text: Unlabelled Box
1. Introduction
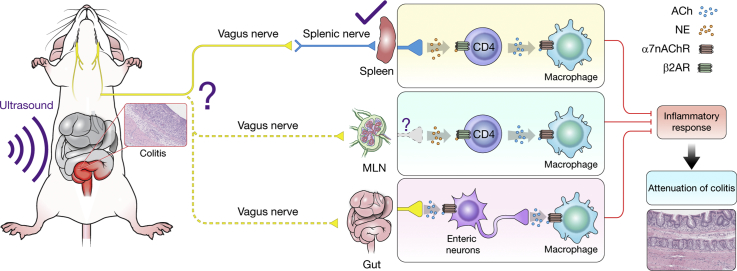
Ulcerative Colitis (UC) is an inflammatory bowel disease (IBD) that affects the colon and rectum, characterized by a disease course that includes diarrhoea, weight loss, fatigue, anaemia and blood in the stools. There is no specific cause for UC; however, genetic, environmental and microbiome factors altogether are known to unbalance the immune system, eventually leading to IBD [[1], [2], [3]]. IBD incidence has been increasing rapidly over the last few decades in newly industrialized countries, whereas the highest prevalence is reported in Europe and North America [4]. To date, there is no cure for UC, a disease that results in poor quality of life, increased risk of colorectal cancer, and morbidity/mortality associated with colectomy for possible symptomatic relief [5,6]. Despite recent advances and the development of biological therapies, a major fraction of patients does not respond to treatment. There is currently no drug available to provide sustained remission of IBD [7,8]. Therefore, a possible non-pharmacological approach to mitigate UC would be vagal nerve stimulation (VNS), which leads to activation of the cholinergic anti-inflammatory pathway (CAIP) and an anti-TNFα response [9]. It has been previously reported that UC patients may present with vagal nerve (VN) dysfunction, regardless of disease activity and previous colectomy history [10], making VNS a possible adjunct or alternative to pharmacological or biological therapeutic approaches.
Activation of CAIP through VNS acts on the spleen [11,12] by stimulating the splenic nerve (SN) that causes the secretion of norepinephrine (NE). When released, NE binds to β2 adrenergic receptors on T cells and stimulates the secretion of acetylcholine (ACh) [13]. ACh has been shown to bind to α7 nicotinic acetylcholine receptors (α7nAChR) on macrophages, thereby decreasing inflammation through inhibition of pro-inflammatory cytokine release. Moreover, VNS of enteric neurons can induce the release of ACh and bind to α7nAChR on intestinal macrophages, thus decreasing inflammation in the gut [[14], [15], [16]]. VNS has been recently shown to reduce intestinal inflammation by activation of cholinergic enteric neurons, suggesting a possible direct effect to the gut environment [17,18]. Furthermore, previous studies have stimulated the VN in IBD animal models and have shown amelioration of the disease [[19], [20], [21]]. It has also been reported that the administration of acetylcholinesterase (AChE) inhibitor in the dextran sulphate sodium (DSS)-induced colitis mouse model decreased disease severity through activation of the efferent VN, while vagotomy or splenectomy abolished CAIP therapeutic effects [22]. Moreover, stimulation of the VN has been used for clinical treatment of depression and epilepsy [23]. A pilot study using VNS in Crohn's Disease (CD) patients has also demonstrated clinical-biological-endoscopical remission [24] (clinicaltrials.gov, NCT01569503).
VNS is a highly invasive neurosurgical procedure that needs to be performed with caution due to VN proximity to the jugular vein and external carotid artery [25]. Non-invasive methods have been sought to stimulate the VN, such as low power Therapeutic Ultrasound (TUS), in order to activate CAIP. TUS has been used in physical therapy since the 1950s, in which ultrasound oscillation and pressure are capable of inducing biological effects through heating, radiation forces and other mechanotransducive effects [26]. It has been previously reported that diagnostic US to the left kidney and spleen can result in the activation of the cholinergic anti-inflammatory pathway in an ischemic reperfusion acute kidney injury (AKI) model [27]. In a recent study, peritoneal macrophages were activated by US or VNS, contributing to the protective effect seen in murine kidney injury model [28]. The application of US to the VN and the SN while sonicating the kidney led to the activation of CAIP in the spleen, which prevented AKI in the mouse model [27,29]. Therefore, the application of TUS to the abdomen may provide a non-invasive low risk alternative to the VNS along with the SN in IBD.
This study evaluated the therapeutic effects of TUS in DSS-induced acute colitis. Our results demonstrate that TUS attenuated DSS-induced colitis when clinical symptoms were present. The changes in colitis were determined by decreased clinical scores, colon shortening and histological damage, in addition to changes in the proteomic and immune cell profiles aiming disease resolution. Splenectomy resulted in abolishment of the effects of TUS, while α7nAChR knockout animals presented with worsening of the disease. Furthermore, increased levels of F4/80+α7nAchR+ in the colon suggest activation of CAIP. These results indicate that TUS can attenuate DSS-induced acute colitis through stimulation of the splenic nerve and consequent activation of CAIP while likely involving the VN.
2. Materials and methods
2.1. Study approval
This study was approved by the Animal Care and Use Committee at the Clinical Centre, National Institutes of Health, Bethesda, MD, USA, under the protocol number LDRR 16-02.
2.2. Animals
Wild type (WT) and splenectomised female C57BL/6 mice 10–12 weeks old from Taconic Biosciences (Rensselaer, NY) were used in our experiments. Female C57BL/6 WT and α7nAChR homozygous knockout (KO) mice (B6.129S7-Chrna7tm1Bay/J) purchased from Jackson Laboratories (Bar Harbor, ME) were used at 7–11 weeks old (mice were backcrossed to C57BL/6 for approximately 8 generations prior to donation to JAX). Mice were housed in controlled 12-12 h dark-light cycles, under specific pathogen-free conditions and regulated humidity and temperature.
2.3. DSS colitis model and ultrasound treatment
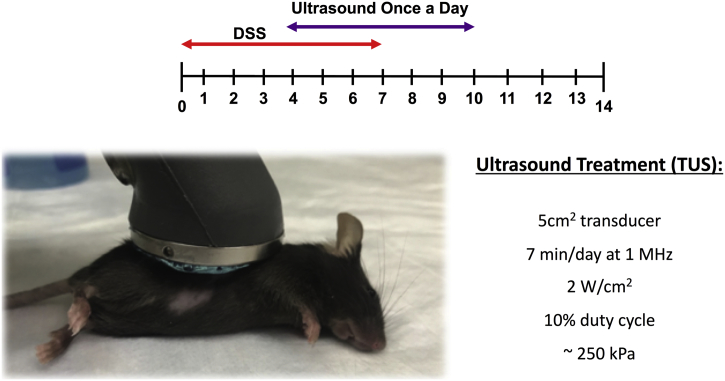
Acute colitis was induced by adding DSS (36,000–50,000 Da – MP Biomedicals, Solon, OH) to the mice drinking water at 2% (wt/vol) ad libitum for 7 days. Control animals received sterilized tap water ad libitum. Mice were distributed in 3 groups: Control, 2% DSS and 2% DSS + TUS, including C57BL/6 WT, C67BL/6 splenectomised and α7nAChR KO mice. Mice were shaved over the abdomen prior to TUS, then placed on a 37 °C heating pad for TUS treatment. Water-based ultrasound gel was applied to the shaved abdominal area and the animals were treated with a 5 cm2 transducer at 1 MHz, 10% duty cycle and intensity of 2 w/cm2 (Mettler 740×; Anaheim, CA) for 7 min from days 4–10, under isoflurane anaesthesia (Fig. 1). The transducer was held over the right side of the abdomen with a clamp holder, so no movement would interfere with the treatment. Considering that a 5cm2 transducer was used on the mouse, the area treated was the entire right side between the axilla and the hip. TUS treatment is not focused; therefore, the actual volume of tissue treated or how each organ was affected is undetermined. Calibration of the ultrasound transducer was performed by measuring the effective transducer output utilizing a needle-type hydrophone (HNA series; Onda, Sunnyvale, CA) in degassed water at RT (Supplementary Fig. 1). Mice receiving DSS alone were anesthetized on days 4–10 using the same protocol but without TUS. Mice were euthanized at different time points through isoflurane anaesthesia and cervical dislocation for the collection of the spleens, mesenteric lymph nodes (MLN) and colons for further analysis.
Fig. 1.
Schematics of the methods section. Mice were shaved over the abdomen and a water-based ultrasound gel was applied. The 5 cm2 transducer was placed on top of the gel and held motionless by a clamp holder while the animal was under isoflurane anaesthesia. The treatment lasted for 7 min at 1 MHz (2 W/cm2, 10% duty cycle, ~250 kPa) and was repeated once a day from days 4 to 10. Mice received 2% DSS in drinking water from days 0 to 7.
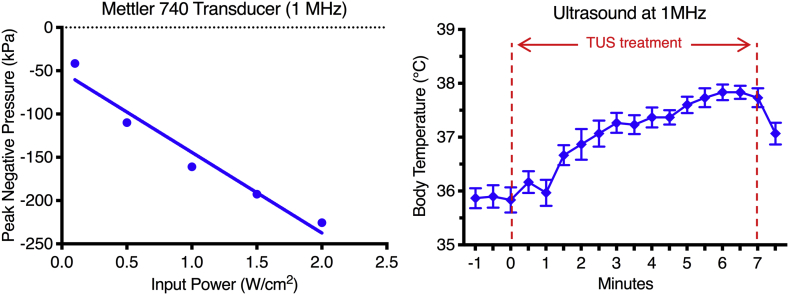
Supplementary Fig. 1.
Ultrasound transducer calibration and temperature changes in mice. (A) Calibration of the ultrasound transducer was performed by measuring the effective transducer output utilizing a needle-type hydrophone in degassed water, reported as Peak Negative Pressure (kPa) vs Input Power (W/cm2). (B) Temperature changes in mice under TUS treatment over the abdomen for 7 min at 1 MHz, 10% duty cycle and 2 w/cm2. Results demonstrate a change of ~2 °C over 7 min, with a decrease when TUS is turned off. N = 3. Results are presented as mean ± SD for body temperature changes.
2.4. Clinical activity
Mice (n = 10–15/group) were daily evaluated for clinical symptoms, as previously described [30,31]. Briefly, animals were clinically evaluated for weight loss, stool consistency and blood in the stool, where which parameter varied from a score of 0–4, totalizing a Disease Activity Index (DAI) of 12 when presenting severe colitis. The specific criteria for DAI are presented in Table 1. Upon euthanasia, colons were collected, cleaned with PBS (Phosphate Buffered Saline) 1×, weighted and measured before processed for histological and proteomic analysis. Spleens were weighted before further processing for histology and flow cytometry analysis, and MLN was collected for histological analysis. Clinical Activity assessment was not masked to treatment cohorts; therefore, the clinical data should be considered qualitative. However, it is important to note that animal weights are included in the Clinical Activity Scores and are not subjective to interpretation.
Table 1.
Disease activity index scoring. Animals were evaluated daily for weight loss, stool consistency and bleeding. Maximum scoring possible is 12 for severe colitis [30,31].
| Score | Weight loss | Stool consistency | Bleeding |
|---|---|---|---|
| 0 | None | Normal stool | No bleeding |
| 1 | 1–5% | Slightly loose stool | Few blood-tinged stools |
| 2 | 5–10% | Loose stools | Slight bleeding |
| 3 | 10–15% | Watery stool | Gross bleeding |
| 4 | >15% | Severe diarrhoea | Blood filling the whole colon |
2.5. Proteomics
Colonic samples (n = 5–6/group per time point) were collected from C57BL/6 female mice receiving 2% DSS or 2% DSS + TUS. Collection was performed at days 0, 3, 5, 7, 9, 11 and 14. After PBS 1× cleaning, samples were snap frozen and processed for further proteomic analysis. Briefly, frozen colonic samples were homogenized in cell lysis buffer (1 mM EDTA, 150 mM NaCl, 0.05% Tween-20 and 20 mM Tris-HCl in ultrapure water) containing Pierce Protease Inhibitor Tablets (Thermo Scientific, Waltham, MA) and 1.0 mm Zirconium Beads. Homogenates were centrifuged at 14,000 rpm at 4 °C for 20 min and the supernatant was collected. The process was repeated two times and aliquots were stored at −80 °C. Bicinchoninic acid assay (BCA – Thermo Scientific, Waltham, MA) was used for protein quantification and samples were further diluted to 1 mg/mL of total protein. MILLIPLEX Map Mouse Cytokine/Chemokine Panel (EMD Millipore, Billerica, MA) was used for proteomic analysis of colonic homogenates according to manufacturer specifications in a Bio-Plex 200 (Bio-Rad Laboratories, Hercules, CA). The same control samples (day 0) were used for multiplex ELISA experiments in the 2% DSS and 2% DSS + TUS exposed mice. Further analysis of colonic TGFβ (Thermo Scientific, Waltham, MA) and HSP70 (R&D Systems, Minneapolis, MN) were performed using ELISA Streptavidin-HRP assay.
2.6. Flow cytometry
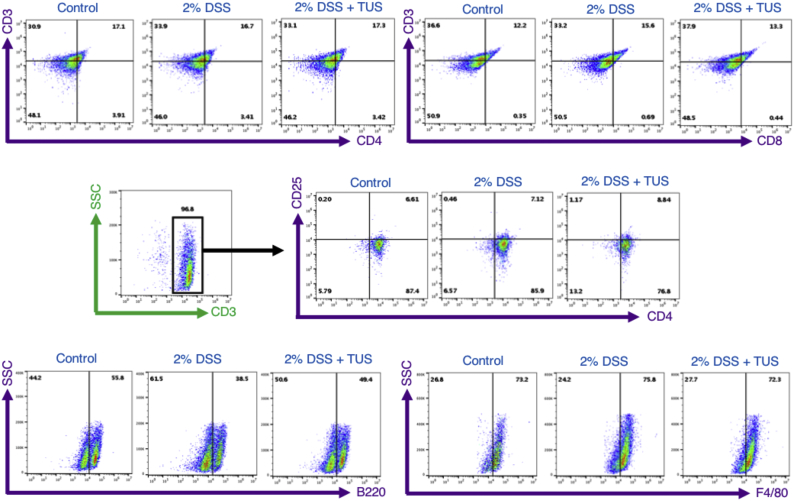
Spleens (n = 6/group) were collected at days 0 and 14 from C57BL/6 female mice receiving 2% DSS or 2% DSS + TUS. Tissue dissociation was performed in Ammonium-Chloride-Potassium (ACK) lysing buffer (Lonza, Walkersville, MD) using two frosted glass slides for cell isolation. Cells were washed in PBS 1× (1,500 rpm, 4 °C, 10 min), filtered through a 70 μm filter and washed one more time. Samples were fixed in 10% NBF (neutral buffered formalin) for 35 min at 4 °C, washed in PBS 1× and stored at 4 °C until further analysis. Cells were incubated with specific antibodies for 35 min in Stain Buffer (BD Pharmigen, San Jose, CA) on ice and protected from light, following manufacturer instructions. Flow cytometry was performed using a V-bottom 96-well plate in Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA) and analysed through FlowJo (Ashland, OR) software. Immune cell population of the spleen was characterized using the following antibodies: FITC CD3 (Rat, 0.5 mg/mL, BD Pharmingen, cat. 555274), APC CD4 (Rat, 0.2 mg/mL, BD Pharmingen, cat. 553051), PE CD8a (Rat, 0.2 mg/mL, BD Pharmingen, cat. 553033), PE CD25 (Rat, 0.2 mg/mL, BD Pharmingen, cat. 553866), FITC F4/80 (Rat, 0.5 mg/mL, eBioscience, cat. 11–4801-82) and Alexa Fluor 488 B220 (Rat, 0.5 mg/mL, Biolegend, cat. 103225). F4/80+ and CD3+CD4+CD25+ spleen populations were enriched prior to flow cytometry analysis through Magnetic Cell Separation MicroBeads (MACS - Miltenyi Biotec, Bergisch Gladbach, Germany) following manufacturer instructions. Each sample was analysed for 10,000 events and results are shown as mean ± SD percentage of the total number of cells. Isotypes were also analysed, and flow cytometry gating is represented in Supplementary Fig. 2.
Supplementary Fig. 2.
FACS gating for Immune cell profiling of the spleen. Representative gating of flow cytometry performed at days 0 (control) and 14 (2% DSS and 2% DSS + TUS) in the spleen for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. F4/80+ macrophages and CD3+CD4+CD25+ T cells were enriched through magnetic separation before flow cytometry analysis.
2.7. Histological and immunohistochemistry evaluation
Colons were collected on days 0, 7 and 14 from C57BL/6 female mice receiving 2% DSS or 2% DSS + TUS (n = 5/group per time point), and at day 14 for C57BL/6 splenectomised or α7nAChR KO female mice receiving either 2% DSS or 2% DSS + TUS (n = 10/group). Freshly collected samples were washed with PBS 1×, longitudinally cut, positioned as a Swiss Roll in 10% NBF and incubated at RT (room temperature) for 24 h. Afterwards, all tissue samples were kept in PBS 1× until embedded in paraffin. Colons were sectioned at 3 μm, deparaffinized and stained with H&E (Guills II haematoxylin and Eosin-Y) for histological grading. Samples were analysed by two independent reviewers blinded to the different cohorts, where the whole area of the colon was scored and one section per animal was processed. The analysis was performed as previously described [32], including grade of inflammation (0–3), extent within the intestine layers (0–3), regeneration (0–4), crypt damage (0–4) and percentage of involvement (0–4). Detailed scores are shown in Table 2. Images were acquired with a 20× air objective from Leica Aperio ScanScope CS (NA = 0.75, Leica Microsystems, Buffalo Grove, IL) and using Aperio ImageScope software.
Table 2.
Histological scoring. Colon samples were evaluated for inflammation, extent, regeneration, crypt damage and percent of involvement. Maximum scoring possible is 56 for severe colitis [32].
| Feature graded | Grade | Description |
|---|---|---|
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damaged | |
| 2 | Basal 2/3 damaged | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| Percent involvement | 1 | 1–25% |
| 2 | 26–50% | |
| 3 | 51–75% | |
| 4 | 76–100% |
Colons, spleens and MLNs (n = 3–4/group) collected on days 0 and 14 from all groups were evaluated through immunohistochemistry for immune cell population. Samples were cut at 3 μm of thickness using a Leica Manual Microtome, left on adhesive slides at RT overnight and baked for 1 h at 65 °C. Antigen retrieval was achieved after 40 min of incubation at 100 °C in antigen unmasking solution (citrate-based, pH = 6.0; Vector Laboratories, Burlingame, CA), followed by 15 min incubation with SuperBlock Blocking Buffer (Thermo Scientific, Waltham, MA) at RT. After blocking, samples were incubated overnight at 4 °C with the following primary antibodies: CD4 (Rabbit, 0.623 mg/mL, Abcam, cat. ab183685), CD8 (Rabbit, 1 mg/mL, Abcam, ab203035), CD25 (Goat, 0.2 mg/mL, Invitrogen, cat. PA5–46922), F4/80 (Rabbit, 0.23 mg/mL, Novus Biologicals, cat. NBP2–12506), B220 (Rat, 0.5 mg/mL, Invitrogen, cat. 14–0452-81), GFAP (Rabbit, 1 mg/mL, Abcam, cat. ab211271) and α7nAChR (Goat, 0.5 mg/mL, Abcam, cat. ab110851). Next, samples were incubated for 5 min at RT with Peroxidazed 1 (BioCare Medical, Pacheco, CA) followed by a 30 min incubation with their respective secondary HRP (Horseradish Peroxidase) antibodies. When co-staining for α7nAchR+F4/80+ cells, the secondary antibodies used were chicken anti-rabbit Alexa Fluor 488 and donkey anti-goat Alexa Fluor 594. Isotypes were also analysed. Images were acquired at 10× magnitude on a Leica Aperio ScanScope CS using a 10× air objective (NA = 0.75, Leica Microsystems, Buffalo Grove, IL) and Aperio ImageScope software. Photomicrographs were taken from the whole area of the colon, spleen or MLN (30–40 images/organ for each animal) and analysed with ImageJ software (NIH, Bethesda, MD). Results are represented as percentage following the ratio of positive cells by the total area, multiplied by 100.
2.8. Statistical analysis
Software Prism 7 (Graph Pad Inc., La Jolla, CA) was used for all statistical analysis, which was performed through two-tailed Student's t-test, two-way ANOVA followed by Sidak post-hoc test, or one-way ANOVA followed by Tukey post-hoc test. P < .05 was considered statistically significant. Data are presented as mean ± standard error of the mean (SEM), unless otherwise indicated. The data was confirmed for normal distribution in Software Prism 7 (Graph Pad Inc., La Jolla, CA).
3. Results
3.1. Therapeutic ultrasound improves DSS colitis
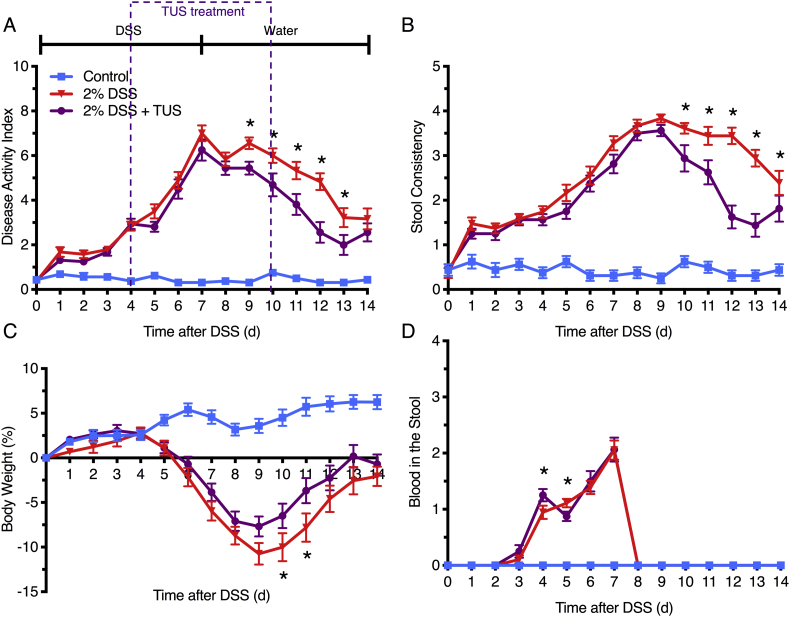
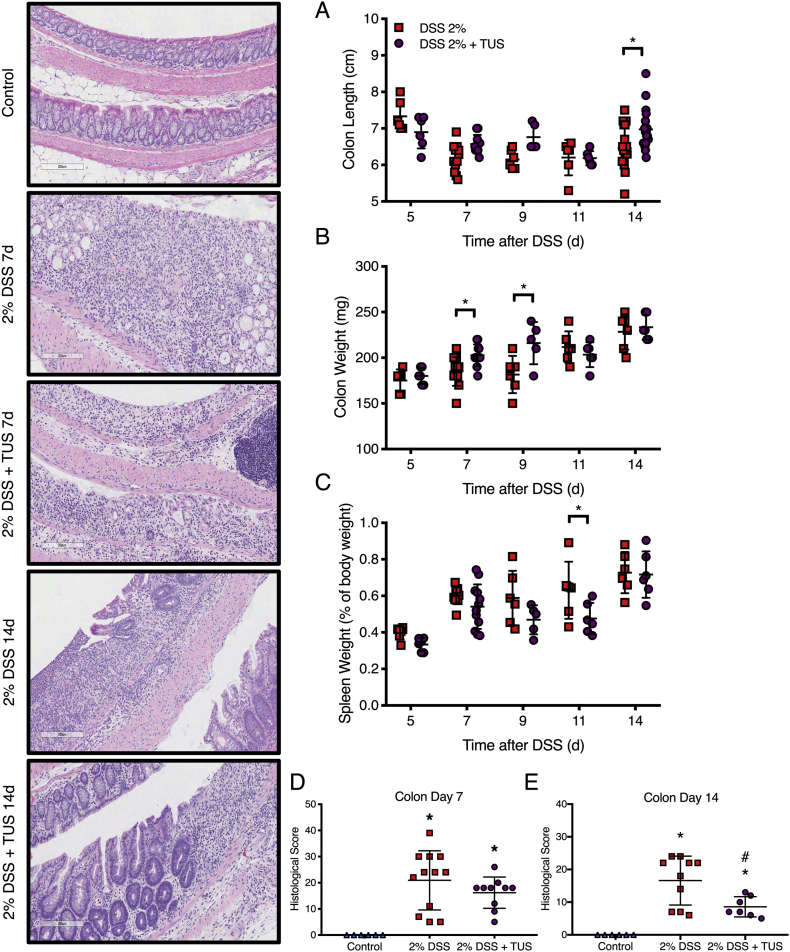
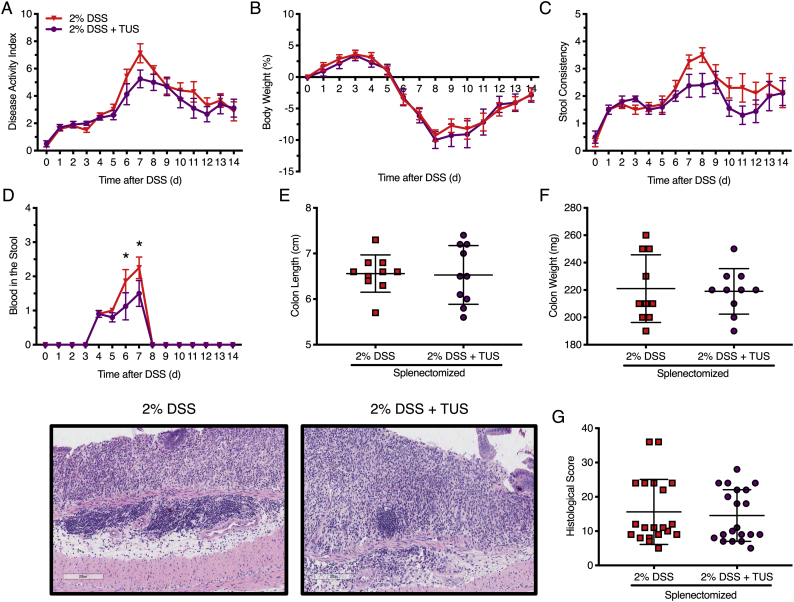
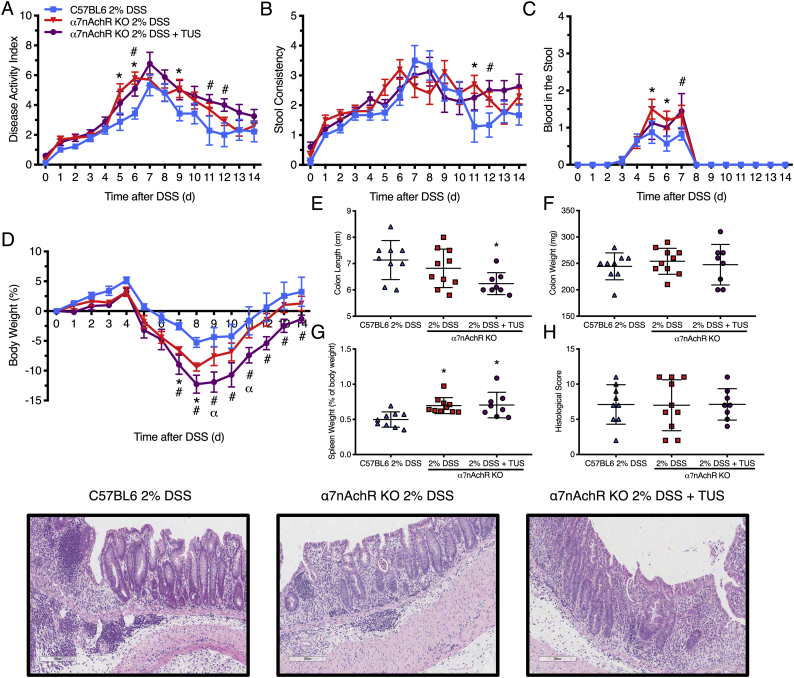
Acute colitis was induced in C57BL/6 female mice by the addition of 2% DSS in drinking water for 7 days, while TUS was initiated to the abdomen on day 4 (when animals began presenting clinical symptoms) and was continued through day 10. Clinical scores were measured daily (Fig. 2) and demonstrated improvement of colitis severity from days 9 to 13 when TUS was applied (p < .05 compared to 2% DSS). The greatest clinical improvement was observed in stool consistency from days 10 to 14, decreased weight loss at days 10 and 11, and diminished blood in the stools at day 5 (Fig. 2; p < .05 compared to 2% DSS). Of note, blood in the stool was detected by day 4 in this IBD model, prior to the administration of TUS to the abdomen. TUS decreased the amount of colon shortening, with no change in colon or spleen weights at day 14 (Fig. 3; p < .05 compared to 2% DSS). Histological scores revealed a decrease in colonic damage on day 14 with partial preservation of the epithelial barrier and goblet cells along with decreased destruction of the crypts and immune cell infiltration (Fig. 3; p < .05 compared to 2% DSS). Animals receiving TUS treatment over the abdominal area had a measured ~2 °C increase in their core temperature (Supplementary Fig. 2), assessed by rectal thermocouple (Omega Engineering Inc., Norwalk, CT). In preliminary experiments, lower power settings or shorter treatment courses for TUS did not modify colitis compared to mice receiving 2% DSS alone (data not shown).
Fig. 2.
Clinical symptoms of 2% DSS colitis mice under TUS treatment. Experimental colitis was induced by DSS for 7 days in drinking water and TUS treatment was administered from day 4 to 10 over the abdomen. TUS attenuated clinical symptoms from day 9 and forward when measuring the (A) disease activity index (DAI), including (B) amelioration of stool consistency, (C) weight loss and (D) blood in the stool at different time points. *p < .05 compared to 2% DSS + TUS. Two-way ANOVA followed by Sidak post-hoc test. N = 15/group.
Fig. 3.
Colon and spleen changes in 2% DSS colitis mice under TUS treatment. TUS attenuated (A) colon shortening and (D, E) histological damage at day 14 only, increasing (B) colon weight at days 7 and 9, while decreasing (C) spleen weight at day 11. Histological analysis demonstrated reduced tissue damage under TUS treatment at 14 days by partial preservation of the crypts, epithelial layer and goblet cells, diminishing immune cells infiltration. *p < .05 compared to control and #p < .05 compared to 2% DSS in histological comparisons. One-way ANOVA followed by Tukey post-hoc test. *p < .05 compared to 2% DSS in graphs A, B and C. Two-way ANOVA followed by Sidak post-hoc test. N = 5/group at each time point. Results are presented as mean ± SD. Images were taken with a 10× objective.
3.2. Changes in colon proteomics by ultrasound treatment
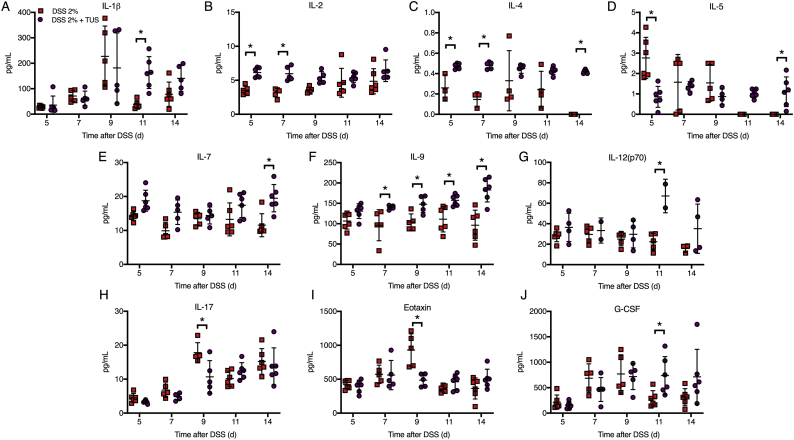
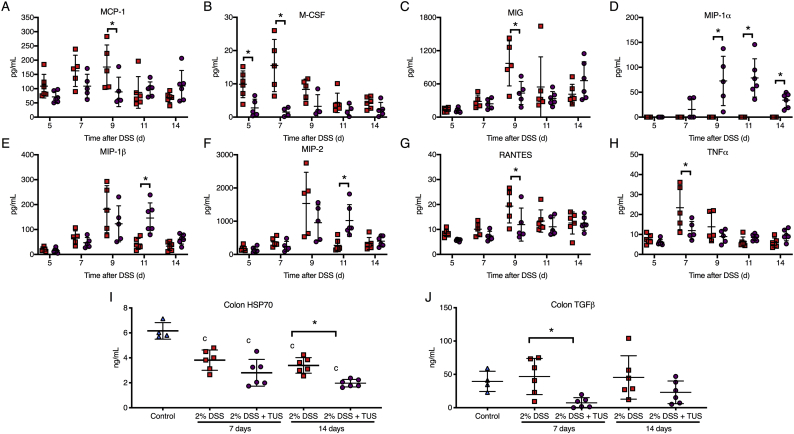
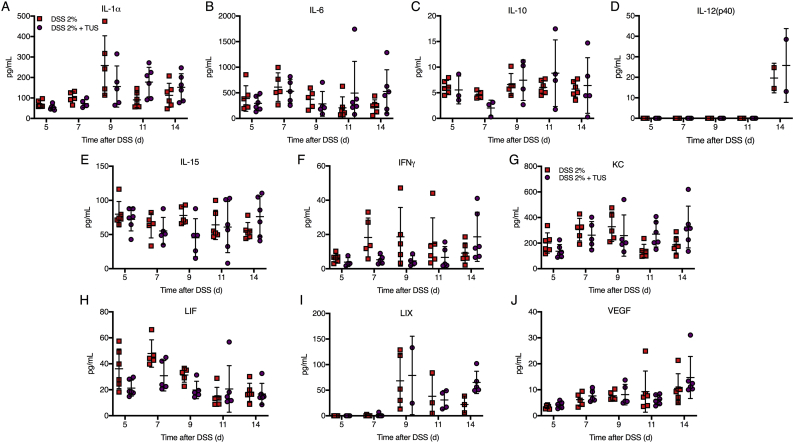
Proteomic analysis comparing cytokines, chemokines and trophic factors (CCTF) of 2% DSS and 2% DSS + TUS groups was performed at different time points over 14 days. TUS decreased the levels of the following CCTF in the colon compared to 2% DSS alone: IL-5, IL-17, Eotaxin, monocyte chemoattractant protein 1 (MCP-1), macrophage-colony stimulating factor (M-CSF), monokine induced by gamma interferon (MIG), regulated on activation, normal T cell expressed and secreted (RANTES) and tumour necrosis factor α (TNFα) (Fig. 4, Fig. 5; p < .05). TUS to the abdomen resulted in significant increases in the following compared to 2% DSS alone: IL-1β, IL-2, IL-4, IL-5, IL-7, IL-9, IL-12(p70), granulocyte-colony stimulating factor (G-CSF), macrophage inflammatory protein 1α (MIP-1α), MIP-1β and MIP-2 (Fig. 4, Fig. 5; p < .05). No differences compared to 2% DSS were observed in IL-1α, IL-6, IL-10, IL-12(p40), IL-15, interferon γ (IFNγ), keratinocyte chemoattractant (KC), leukaemia inhibitory factor (LIF), lipopolysaccharide-induced CXC chemokine (LIX) and vascular endothelial growth factor (VEGF) (Supplementary Fig. 3). Heat maps of fold changes in CCTF for each group are shown in Supplementary Fig. 4, comparing each time point to control animals (Day 0). Colonic levels of TGFβ and HSP70 were reduced by TUS treatment on days 7 and 14, respectively (Fig. 4).
Fig. 4.
Proteomic colon changes in 2% DSS colitis mice under TUS treatment. Experimental colitis was induced by DSS for 7 days in drinking water and TUS treatment was administered from day 4 to 10 over the abdomen. The colons were collected at days 5, 7, 9, 11 and 14, homogenized and later analysed by multiplex ELISA assay. Results demonstrate downregulation of colonic IL-5, IL-17 and Eotaxin, at different time points during TUS treatment (days 5 to 9); and upregulation of colonic IL-1β, IL-2, IL-4, IL-5, IL-7, IL-9, IL-12(p70) and G-CSF at different time points starting at day 5 with TUS treatment. *p < .05 compared to 2% DSS. *p < .05 compared to 2% DSS. Two-way ANOVA followed by Sidak post-hoc test. N = 5–6/group at each time point. Results are presented as mean ± SD. Heat maps of these results are presented in Supplementary Fig. 4.
Fig. 5.
Proteomic colon changes in 2% DSS colitis mice under TUS treatment. Experimental colitis was induced by DSS for 7 days in drinking water and TUS treatment was administered from day 4 to 10 over the abdomen. The colons were collected at days 5, 7, 9, 11 and 14, homogenized and later analysed by multiplex ELISA assay. Results demonstrate downregulation of colonic MCP-1, M-CSF, MIG, RANTES and TNFα at different time points during TUS treatment (days 5 to 9); and upregulation of colonic MIP-1α, MIP-1β and MIP-2 at different time points starting at day 5 with TUS treatment. In addition, results show reduction in colonic levels of TGFβ and HSP70 at days 7 and 14, respectively, under TUS treatment. *p < .05 compared to 2% DSS. cp < .05 compared to control. Two-way ANOVA followed by Sidak post-hoc test for multiplex ELISA and one-way ANOVA followed by Tukey post-hoc test for ELISA Streptavidin-HRP assay. N = 5–6/group at each time point. Results are presented as mean ± SD. Heat maps of these results are presented in Supplementary Fig. 3.
Supplementary Fig. 3.
Proteomic colon changes in 2% DSS colitis mice under TUS treatment. Experimental colitis was induced by DSS for 7 days in drinking water and TUS treatment was administered from day 4 to 10 over the abdomen. The colons were collected at days 5, 7, 9, 11 and 14, homogenized and later analysed by multiplex ELISA assay. Results demonstrate no changes of colonic IL-1α, IL-6, IL-10, IL-12(p40), IL-15, IFNγ, KC, LIF, LIX and VEGF, compared to 2% DSS. Two-way ANOVA followed by Sidak post-hoc test. N = 5–6/group at each time point. Results are presented as mean ± SD. Heat maps of these results are presented in Supplementary Fig. 4.
Supplementary Fig. 4.
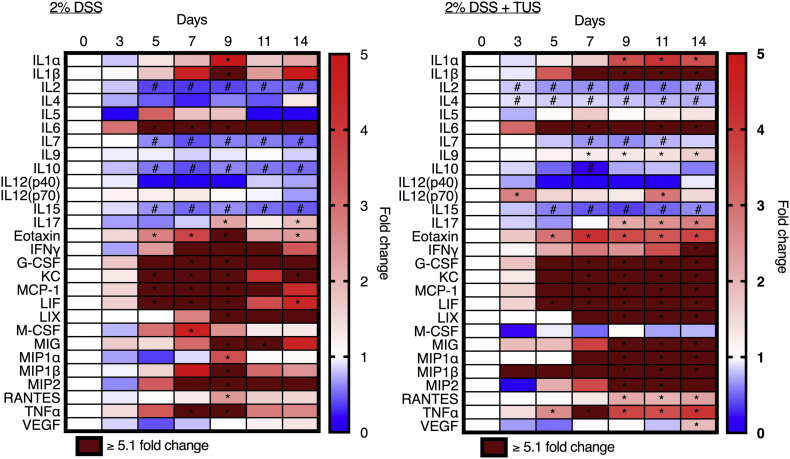
Colonic proteomic analysis in both 2% DSS and 2% DSS + TUS groups. Heat map of temporal proteomic analysis based on multiplex ELISA revealed mice receiving 2% DSS only demonstrated increased fold changes of IL-1α, IL-1β, IL-6, IL-17, Eotaxin, G-CSF, KC, MCP-1, LIF, LIX, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES and TNFα, and decreased fold changes of IL-2, IL-7, IL-10 and IL-15 at different time points, normalized to normal control colons (day 0). Mice receiving 2% DSS + TUS treatment demonstrated increased fold changes of IL-1α, IL-1β, IL-6, IL-9, IL12(p70), IL-17, Eotaxin, IFNγ, G-CSF, KC, MCP-1, LIF, LIX, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNFα and VEGF, and decreased proteomic levels of IL-2, IL-4, IL-7, IL-10 and IL-15 at different time points, compared to day 0 (control). *p < .05 fold increases compared to day 0 (control). #p < .05 fold decreases compared to day 0 (control). Two-way ANOVA followed by Sidak post-hoc test. N = 5–6/group at each time point.
3.3. Changes of immune cells populations in colon, spleen and mesenteric lymph nodes
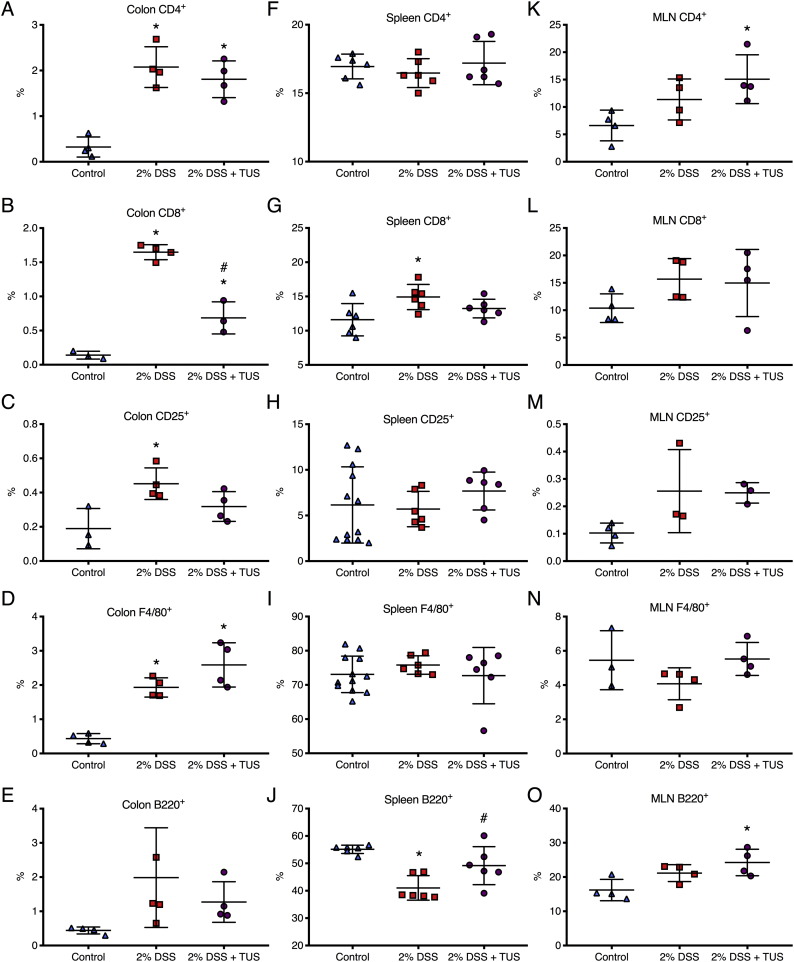
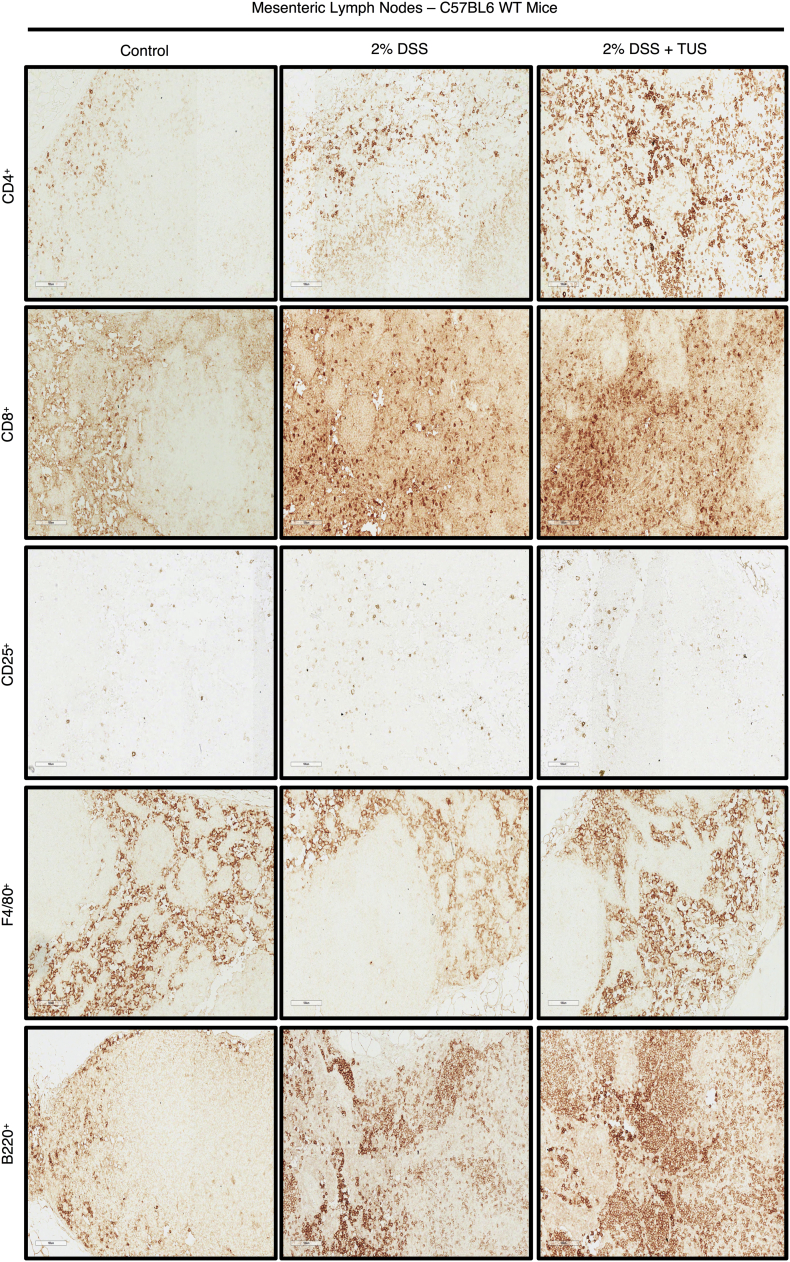
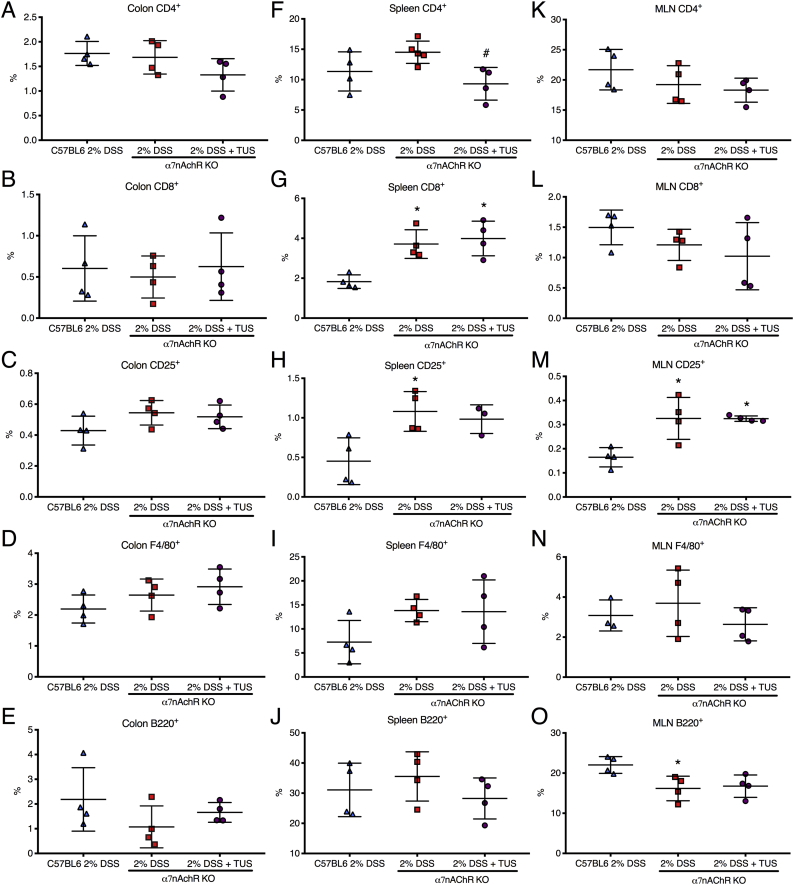
Immunohistochemistry analysis of colonic immune cell population revealed no differences when comparing 2% DSS and 2% DSS + TUS groups for CD4+, B220+ and F4/80+ cells. CD8+ T cells were downregulated in the TUS group at day 14 compared to 2% DSS alone (Fig. 6; p < .05). TUS group levels of CD25+ T cells were not different from control; however, these cells were significantly elevated in 2% DSS group compared to control (Fig. 6; p < .05). Flow cytometry analysis (FACS) of the spleen showed no differences in CD4+, CD25+ and F4/80+ cells across groups. However, CD8+ and B220+ cell levels were normalized at day 14 in 2% DSS + TUS group. The sonicated group also had increased levels of B220+ B cells compared to 2% DSS alone (Fig. 6; p < .05). MLN immunohistochemistry (IHC) analysis demonstrated no changes regarding CD8+, CD25+ and F4/80+ cells across the three groups. The percent increase in CD4+ and B220+ cells was detected in the 2% DSS + TUS group compared to controls (Fig. 6; p < .05). Representative IHC images are shown in Supplementary Fig. 5, Supplementary Fig. 6.
Fig. 6.
Immune cell population changes in colon, spleen and MLN in 2% DSS colitis mice under TUS. (A-E) Colon IHC analysis revealed no differences amongst all groups regarding B220+ B cells, increased levels of CD4+, CD8+ and F4/80+ cells in comparison to controls, while CD8+ levels were decreased when comparing TUS treated animals to 2% DSS group and CD25+ T cells were increased in 2% DSS only group. (F-G) Spleen FACS analysis demonstrated no changes for CD4+, CD25+ and F4/80+ cells. Increase percentage was seen for CD8+ T cells and decrease in B220+ B cells when comparing 2% DSS to control. In addition, TUS treatment normalized CD8+ T cells and B220+ B cells when compared to 2% DSS. (K—O) MLN IHC analysis demonstrated no difference amongst all groups for CD8+, CD25+ and F4/80+ cells. TUS treatment increased CD4+ and B220+ levels compared to control. *p < .05 compared to control. #p < .05 compared to 2% DSS. One-way ANOVA followed by Tukey post-hoc test. N = 4/group for IHC analysis and N = 6/group for FACS analysis. Results are presented as mean ± SD.
Supplementary Fig. 5.
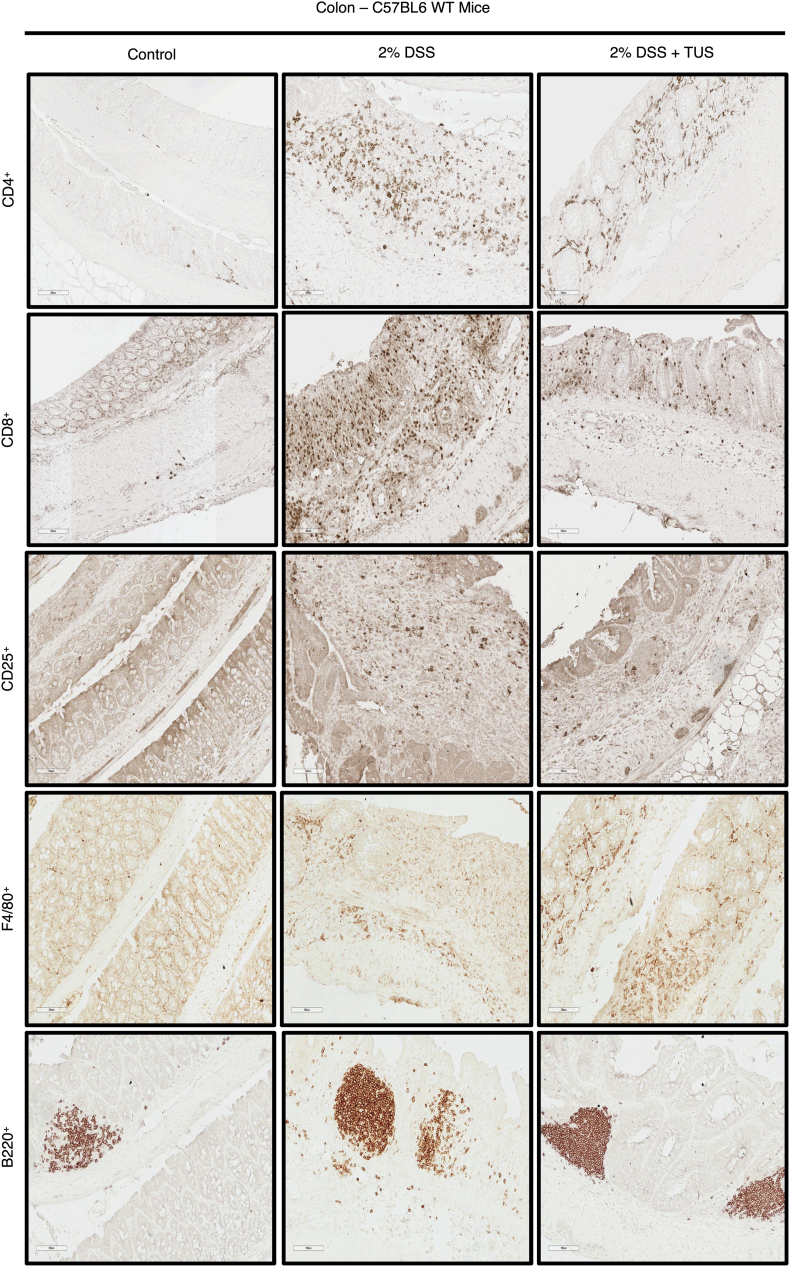
IHC staining for Immune cell profiling of the colon. Representative images of IHC analysis performed at days 0 (control) and 14 (2% DSS and 2% DSS + TUS) in the colon of C57BL6 WT mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
Supplementary Fig. 6.
IHC staining for Immune cell profiling of the MLN. Representative images of IHC analysis performed at days 0 (control) and 14 (2% DSS and 2% DSS + TUS) in the MLN of C57BL6 WT mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
3.4. TUS treatment in splenectomised mice
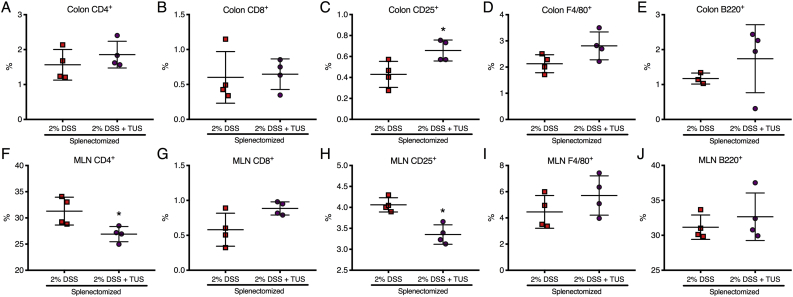
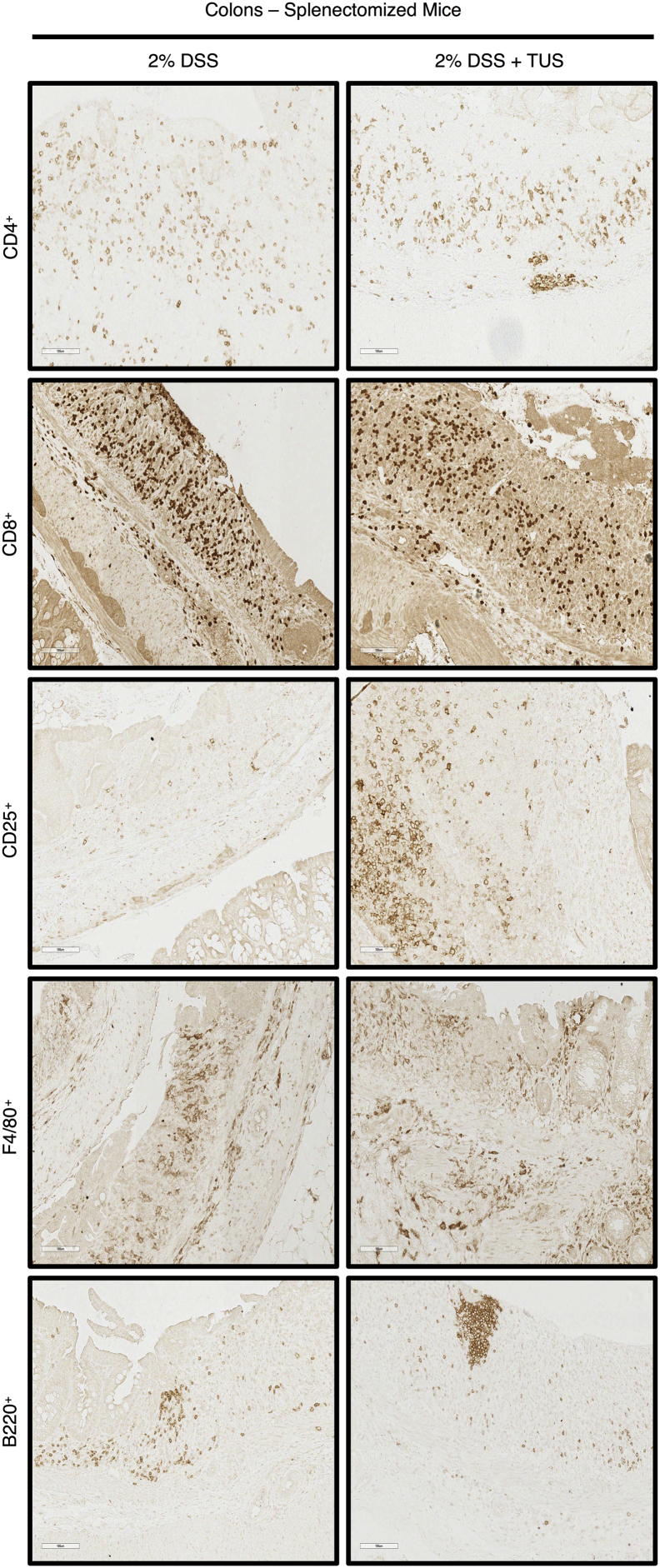
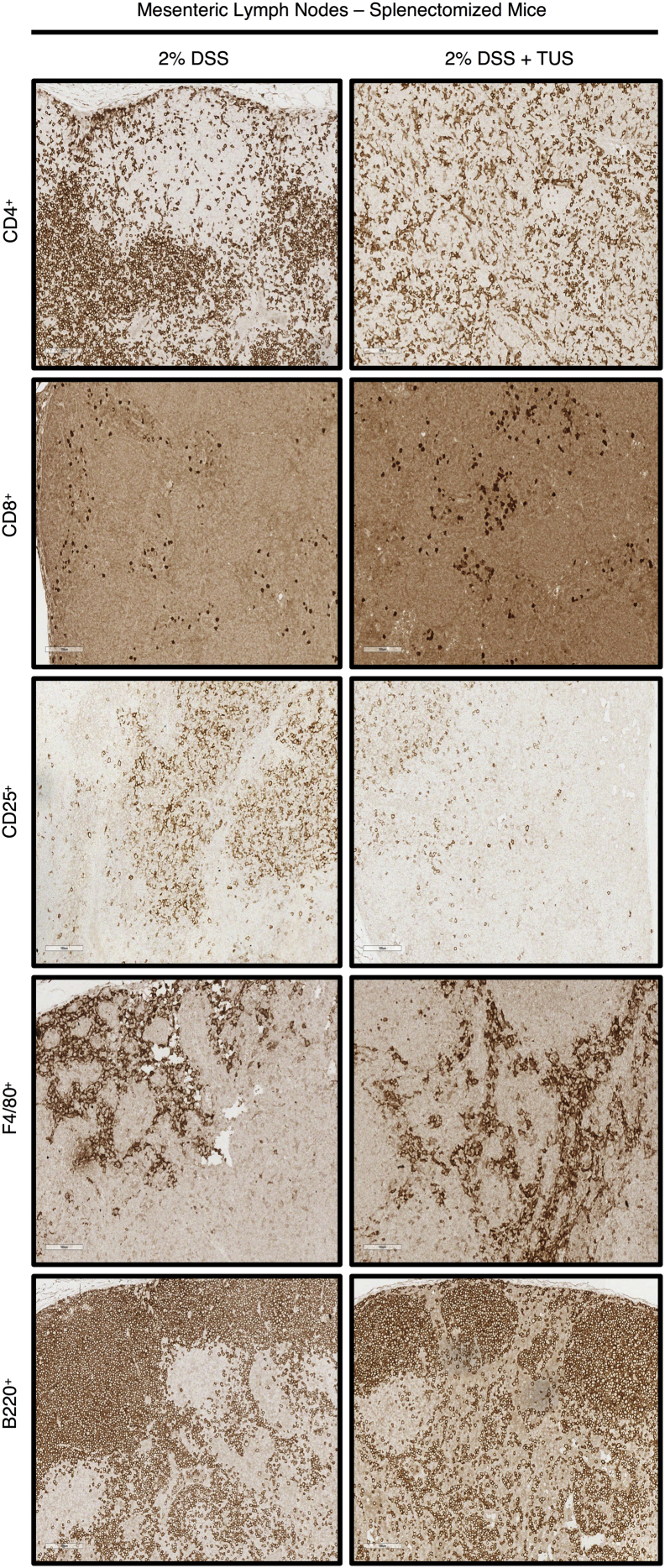
Acute DSS colitis was induced in splenectomised C57BL/6 mice by adding 2% DSS in their drinking water for 7 days, and the animals were treated with TUS from days 4 to 10. There was no overall difference between 2% DSS and 2% DSS + TUS splenectomised groups when evaluating disease activity index, weight loss and stool consistency. However, TUS did diminish the presence of blood in the stools on days 6 and 7 compared to 2% DSS cohort (Fig. 7; p < .05). There was no difference observed between the groups when analysing colon length, colon weight and histological scores (Fig. 7). There were also no differences between the two groups in CD4+, CD8+, B220+ and F4/80+ cells in the colon. A percent increase in colonic CD25+ T cells by day 14 was detected in 2% DSS + TUS compared to 2% DSS group, while a decrease was observed in the MLN (Fig. 8; p < .05). In addition, CD4+ T cells were decreased in the MLN under TUS treatment, and no difference was seen between the groups in CD8+, B220+ and F4/80+ (Fig. 8). Representative IHC images are shown in Supplementary Fig. 7, Supplementary Fig. 8.
Fig. 7.
Clinical and histological analysis of splenectomised mice. There was no difference between the groups in (A) disease activity index, (B) stool consistency, (D) weight loss, (E) histological colonic damage, (F) colon length and (G) colon weight at day 14. TUS decreased the amount of (C) blood in the stools on days 6 and 7. H&E staining of the colons demonstrated destruction of the crypts, loss of the epithelial barrier, loss of goblet cells and high immune cell infiltration for both groups. *p < .05 compared to 2% DSS + TUS. Two-way ANOVA followed by Sidak post-hoc test for clinical analysis. Student's t-test for histological scores and macroscopic measurements. N = 10/group. Results are presented as mean ± SD for histological scores. Images were taken with a 10× objective.
Fig. 8.
Colon and MLN immune cell changes in splenectomised mice. There was no difference between the groups when analysing the colons for (A) CD4+, (B) CD8+, (D) F4/80+ and (E) B220+ cells. TUS induced an increase in (C) colonic CD25+ T cells while decreasing the percentage of (H) CD25+ T cells in MLN. Furthermore, (F) CD4+ T cell levels were decreased in the MLN, while no difference was seen regarding (G) CD8+, (I) F4/80+ and (J) B220+ cells. *p < .05 compared to 2% DSS. Student's t-test. N = 4/group. Results are presented as mean ± SD.
Supplementary Fig. 7.
IHC staining for Immune cell profiling of the colon of splenectomised mice. Representative images of IHC analysis performed at day 14 in the colon of splenectomised mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
Supplementary Fig. 8.
IHC staining for Immune cell profiling of the MLN of splenectomised mice. Representative images of IHC analysis performed at day 14 in the MLN of splenectomised mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
3.5. TUS treatment in α7nAchR KO mice
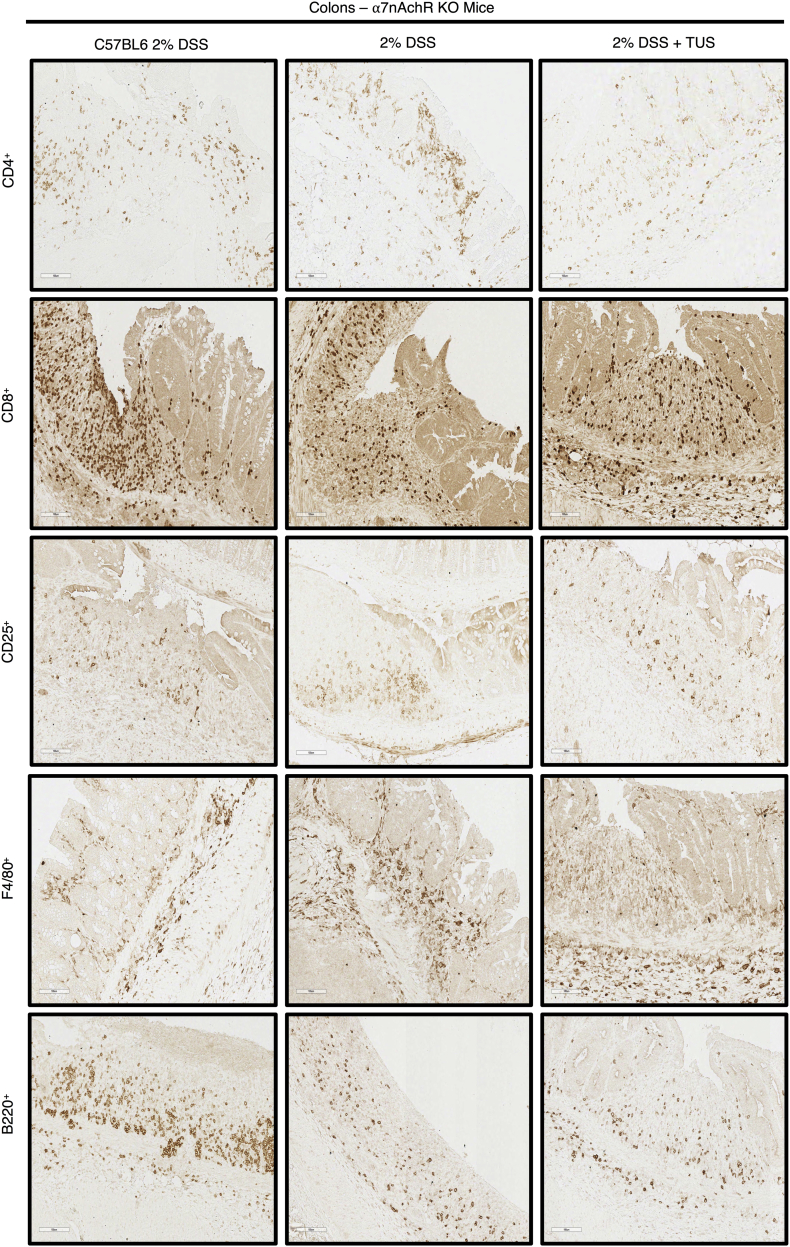
Acute DSS colitis was induced in WT C57BL/6 and α7nAchR KO mice, where one KO group received TUS treatment from days 4 to 10. There was worsening of disease activity index, weight loss, blood in the stools and stool consistency at different time points in the KO mice 2% DSS and 2% DSS + TUS groups when comparing to WT 2% DSS alone group (Fig. 9; p < .05). In addition, TUS treatment resulted in shorter colons at day 14 when compared to WT 2% DSS (Fig. 9; p < .05). No difference was seen amongst all groups for colon weight and histological scores, whereas spleen weight was increased in both KO groups compared to WT 2% DSS (Fig. 9; p < .05). No difference was observed in CD4+, CD8+, CD25+, F4/80+ and B220+ cells in the colon (Fig. 10). Splenic levels of CD4+ were decreased in 2% DSS + TUS KO group (compared to 2% DSS KO; p < .05). CD25+ T cells levels were not different from WT animals and were elevated in 2% DSS KO (compared to WT 2% DSS; p < .05). CD8+ T cells were increased in both KO 2% DSS and KO 2% DSS + TUS groups when compared to WT 2% DSS animals (Fig. 10; p < .05). MLN levels of CD25+ T cells were increased in both KO 2% DSS and KO 2% DSS + TUS groups. Decreased B220+ levels were seen in the KO 2% DSS group only (compared to WT 2% DSS; p < .05; Fig. 10). Representative IHC images are shown in Supplementary Fig. 9, Supplementary Fig. 10, Supplementary Fig. 11.
Fig. 9.
Clinical and histological analysis of α7nAChR KO mice. The absence of α7nAChR induced worsening of the disease in both 2% DSS and 2% DSS + TUS groups (compared to 2% DSS WT mice) at different time points regarding (A) disease activity index, (B) stool consistency, (C) blood in the stools and (D) weight loss. 2% DSS + TUS α7nAChR KO group resulted in worsening of (D) weight loss when compared to 2% DSS α7nAChR KO mice at days 9 and 11. There was no difference amongst all groups when analysing the colons for (E) histological damage and (G) colon weight. Both KO groups presented with (H) increased spleen size and TUS (F) worsened colon shortening, when compared to 2% DSS WT mice. H&E images of the colons reveal partial destruction of the crypts, partial loss of goblet cells and infiltration of immune cells. *p < .05 comparing 2% DSS α7nAChR KO and 2% DSS WT groups. #p < .05 comparing 2% DSS + TUS α7nAChR KO and 2% DSS WT groups. α p < .05 comparing 2% DSS α7nAChR KO and 2% DSS + TUS α7nAChR KO groups. Two-way ANOVA followed by Sidak post-hoc test for clinical analysis and one-way ANOVA followed by Tukey post-hoc test for histological scores and macroscopic measurements. N = 10/group. Results are presented as mean ± SD for histological scores and macroscopic measurements. Images were taken with a 10× objective.
Fig. 10.
Colon, spleen and MLN immune cell changes in α7nAChR KO mice. There was no difference amongst all groups when analysing the colons for (A-E) CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Splenic levels of (F) CD4+ were decreased (compared to 2% DSS) and (H) CD25+ T cells levels were not different from controls with TUS treatment. (G) CD8+ T cells were increased in both 2% DSS and 2% DSS + TUS KO groups. MLN levels of (M) CD25+ T cells were increased in both 2% DSS and 2% DSS + TUS KO groups, while decreased (O) B220+ levels were seen in the 2% DSS KO group only. There was no difference regarding splenic (I) F4/80+ and (J) B220+ cells, and no difference in MLN (K) CD4+, (L) CD8+ and (N) F4/80+ cells. *p < .05 compared to 2% DSS WT. #p < .05 compared to 2% DSS KO. One-way ANOVA followed by Tukey post-hoc test. N = 4/group for IHC analysis. Results are presented as mean ± SD.
Supplementary Fig. 9.
IHC staining for Immune cell profiling of the colon of WT or α7nAChR KO mice. Representative images of IHC analysis performed at day 14 in the colon of WT or α7nAChR KO mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
Supplementary Fig. 10.
IHC staining for Immune cell profiling of the MLN of WT or α7nAChR KO mice. Representative images of IHC analysis performed at day 14 in the MLN of WT or α7nAChR KO mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
Supplementary Fig. 11.
IHC staining for Immune cell profiling of the spleen of WT or α7nAChR KO mice. Representative images of IHC analysis performed at day 14 in the spleen of WT or α7nAChR KO mice for CD4+, CD8+, CD25+, F4/80+ and B220+ cells. Images were taken with a 20× objective.
3.6. TUS activation of CAIP
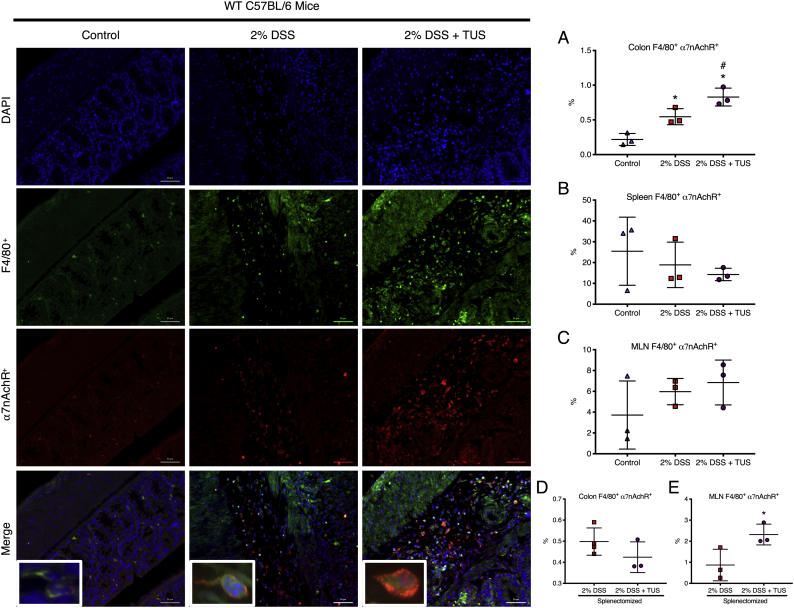
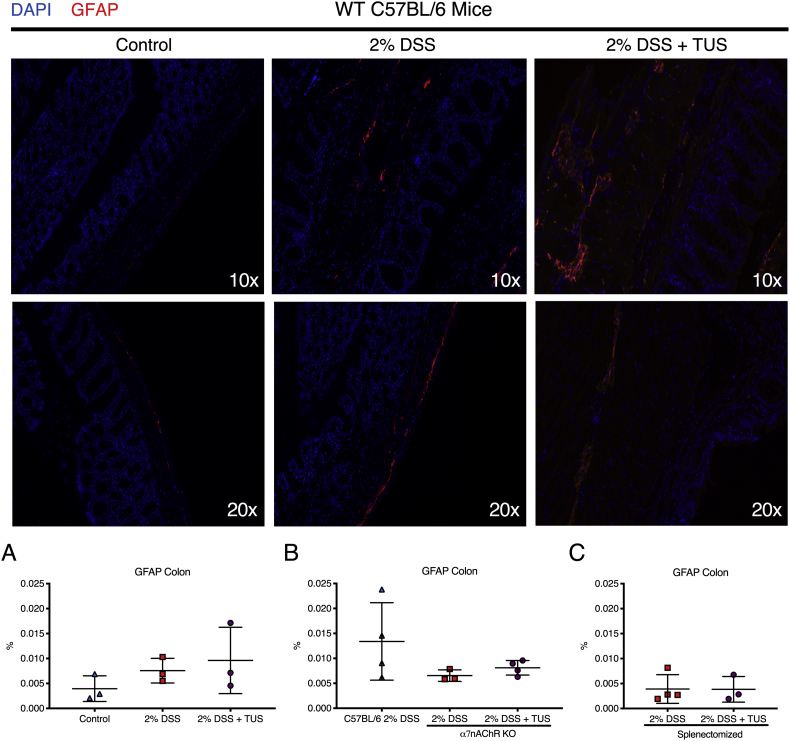
To confirm the involvement of the cholinergic anti-inflammatory pathway, co-staining of histological sections for α7nAchR+F4/80+ macrophages was performed in the spleen, MLN and colons for control, 2% DSS and 2% DSS + TUS animals (C57BL/6 WT). There was no difference amongst all groups regarding the spleen and MLN. However, TUS upregulated the levels of α7nAchR+F4/80+ cells in the colon at day 14 compared to control and 2% DSS exposed mice (Fig. 11; p < .05). This difference was not observed in colons of splenectomised animals, whereas the levels of α7nAchR+F4/80+ cells in the MLN were increased under TUS treatment (Fig. 11; p < .05). Moreover, to verify the possible activation of enteric glial cells, the levels of GFAP were measured in the colon of all groups, and no statistical difference was seen (Fig. 12).
Fig. 11.
Colon, spleen and MLN analysis for F4/80+α7nAChR+ cells. Photomicrographic images revealed (A) increased levels of F4/80+α7nAChR+ cells in the colons of 2% DSS and 2% DSS + TUS, and even higher levels at the 2% DSS + TUS mice. No difference was seen across all groups in the (B) spleen and (C) MLN, in addition to the colons from (D) splenectomised animals. However, (E) TUS increased the levels of F4/80+α7nAChR+ cells in the MLN of splenectomised animals. Images show staining for the nuclei (blue), F4/80 macrophages (green) and 〈7nAChR (red). Merged images demonstrate co-staining of F4/80+α7nAChR+ macrophages in orange (insert). *p < .05 compared to control. #p < .05 compared to 2% DSS. One-way ANOVA followed by Tukey post-hoc test for WT C57BL/6 groups. Student's test for splenectomised animals. N = 3/group. Results are presented as mean ± SD. Images were taken with a 20× objective, whereas inserts were taken with a 63× objective. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 12.
Colon analysis for GFAP+ enteric glial cells. IHC analysis revealed (A-C) no difference amongst all groups when comparing GFAP levels. Images show staining for the nuclei (blue) and GFAP (red). One-way ANOVA followed by Tukey post-hoc test or Student's t-test. N = 3–4/group. Results are presented as mean ± SD. Images were taken with a 10× and a 20× objective. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
IBD are chronic gastrointestinal disorders that have been increasing rapidly in worldwide prevalence [4]. Despite recent advances, improvements in therapeutic options are needed since definitive remission is currently not achievable [7,8]. Previous studies have explored VNS using invasive approaches as a treatment for UC [[19], [20], [21], [22],25]. Our study explored the effects of TUS to the abdomen as a potential non-invasive treatment for DSS-induced acute colitis through activation of CAIP. We demonstrate that the application of TUS attenuated the severity of colitis by improving clinical symptoms, colon shortening and histological damage that was dependent upon the response of the spleen and α7nAChR+ macrophages.
Evaluation of the proteomic changes induced by TUS in 2% DSS colitis mice revealed two different CCTF patterns when compared to 2% DSS mice: A) downregulation of colonic IL-5, IL-17, Eotaxin, MCP-1, LIF, M-CSF, MIG, RANTES and TNFα at different time points during TUS treatment (days 5 to 9); and B) upregulation of colonic IL-1β, IL-2, IL-4, IL-5, IL-7, IL-9, IL-12(p70), G-CSF, LIX, MIP-1α, MIP-1β and MIP-2 at different time points starting at day 5 with TUS treatment. Decreased expression of IL-5 and Eotaxin may have contributed to decrease chemoattraction of eosinophils to the colon [33,34] during the injury phase, resulting in less tissue damage and mucosal healing [35]. Moreover, TNFα levels were decreased in TUS cohort, possibly due to activation of CAIP [15]. The decrease in TNFα could contribute to functional epithelial barrier and attenuation of clinical symptoms [36,37].
The decreased expression of IL-17, MCP-1, MIG, M-CSF and RANTES in the TUS treated cohort may also contribute to the attenuated pro-inflammatory response. These CCTF (i.e., IL17, RANTES, MIG) [[38], [39], [40]] may contribute to the reduced presence of CD8+ and CD25+ T cells in the colon; along with decreased M-CSF and MCP-1 in TUS-treated mice, they may have attenuated macrophage inflammatory functions [41,42] resulting in the reduction of tissue damage. We also detected a decrease in TGFβ in the TUS cohort treatment, which could have contributed to an immunosuppressive environment in the gut. TGFβ is known to induce the differentiation of Th17 cells [43,44] and to retain lymphocytes in the gut [44,45]. The decreased levels in our study could relate to reduced levels of IL-17 at day 9 and could have contributed to decreased levels of CD8+ and CD25+ T cells in the gut, translating in attenuation of the disease.
In comparison, the increase of IL-2, IL-4 and IL-9 with TUS treatment may have contributed to a more tolerogenic response to antigens in the gut and attenuated colitis. TUS increased expression of IL-7, G-CSF, MIP-1α, MIP-1β and MIP-2 that could modulate the influx of neutrophils and macrophages [[43], [44], [45], [46], [47], [48]], resulting in improvement in colitis. G-CSF therapy has been shown to ameliorate DSS-induced colitis by increasing tropism of macrophages and stimulating a M2 macrophage phenotype [44]. Furthermore, increased levels of colonic IL-1β and IL-12 during the recovery phase could aid in the clearance of dead cells and debris [49,50], decreasing intestinal inflammation. Since the percentage of F4/80+ macrophages were not different when comparing 2% DSS and 2% DSS + TUS groups, the results suggest that the morphology and functionality of the macrophages were altered, but not necessarily their numbers. Further analysis is necessary to determine TUS induced polarization of M1 or M2 macrophages in DSS colitis. Overall, we observed an increase of pro-inflammatory cytokines/chemokines with TUS treatment towards the end of the disease course. Although counterintuitive, our results suggest that TUS treatment induced a tolerogenic response in the gut during the injury phase, whilst promoting early resolution and recovery of DSS colitis.
TUS reduced levels of CD8+ T cells, while the levels of CD25+ T cells were similar to control in the gut and spleen, contributing to decreased inflammation and epithelial damage. We have previously reported the increase of colonic CD8+ and CD25+ T cells in the DSS model of colitis related to disease worsening and increased histological damage [51], whereas both cell types were attenuated by TUS in the current study. We also observed an increase of CD4+ T cells and B220+ B cells in the MLN that may indicate a transition to a chronic state of the disease [52]. However, the upregulation of B220+ B cells in the spleen and MLN may contribute to gut homeostasis and attenuation of colitis while interacting with T regulatory cells [53]. Further studies are needed in a chronic relapsing DSS colitis model to determine if TUS treatment to the abdomen will result in decreased morbidity and durable positive clinical outcomes.
It has been previously shown that VNS ameliorated intestinal inflammation by activating CAIP directly in the gut, without involving the spleen or SN [54]. In the study of Matteoli et al. (2014), an invasive technique was used to directly electrical stimulate the right cervical vagus nerve in order to activate CAIP and decrease inflammation [54]. In comparison, the current study achieves similar downregulation of gut inflammation by applying TUS to the abdomen by activating the splenic nerve, splenocytes, vagal afferents, and/or vagal efferents resulting in elevation of the number of α7nAChR+F4/80+ in the colon. In addition, the current study demonstrated that there were no differences between the cohorts of splenectomised mice when comparing 2% DSS to 2% DSS + TUS cohorts. These observations would support the hypothesis that improvement of colitis requires the presence of the spleen and the splenic nerve, possibly involving activation of the VN. In our study, splenectomy by itself did worsen DSS-induced colitis (data not shown). This observation would suggest that CAIP is also a normal response to DSS injury in the absence of TUS. If the ultrasound treatment to the abdomen did not involve activation of CAIP, amelioration of the disease would have occurred in the DSS colitis model in splenectomised mice. The VN stimulates the secretion of ACh by enteric neurons, which in turn reduces the production of pro-inflammatory factors by macrophages and induces a tolerogenic response in the gut [15]. However, VN innervation of the colon is limited [55]. In this study, TUS treatment in splenectomised mice resulted in a lack of significant clinical response, highlighting the importance of an intact spleen and SN. Reduced levels of MLN CD4+ and CD25+ T cells may indicate their migratory movement to the gut in response to colonic inflammation.
The interdependence of the spleen and VNS for activation of CAIP in the DSS colitis model has been previously reported [22]. VNS was ineffective in the treatment of intestinal inflammation in α7nAChR KO mice [54], or it promoted worsening of the disease when inducing DSS colitis [56]. VNS activates the SN through the celiac ganglion to release norepinephrine in the spleen. T cells respond by producing ACh that binds on splenic α7nAChR+ macrophages and inhibits the secretion of pro-inflammatory cytokines, specially TNFα [55,57,58]. The absence of α7nAChR abolished TUS therapeutic effects in DSS colitis, resulting in worsening of the clinical disease, where the immune cell profile reflected no differences in the colonic damage amongst all three groups. The decrease in body weight in α7nAChR KO mice was probably related to the further shortening of colon length detected in this group compared to 2% DSS KO. We were unable to point the exact cause of the colon damage; however, these results confirm the importance of α7nAChR in the context of TUS therapeutic effect. TUS treatment resulted in increases of colonic F4/80+α7nAChR+ cells compared to controls and 2% DSS WT, indicative of CAIP activation. The increased numbers of F4/80+α7nAChR+ cells in the colon might result from splenic egress, since we observed a trend towards reduced numbers of immune cells in the spleen overtime. In splenectomised DSS colitis mice, we observed an increase in F4/80+α7nAChR+ cells in the MLN but not in the colon. Since the VN also enervates the MLN [59], it is possible that the activation of CAIP occurred in the MLN, without altering the clinical and pathological disease outcomes. Our results in the 2% DSS + TUS group agree with the previous use of TUS, in which stimulation of the VN and the SN attenuated AKI through activation of CAIP, resulting in improvement of tissue morphology and function. No improvement in AKI was detected in splenectomised mice that were exposed to US to the abdomen, confirming the need for a spleen [27,29]. Lastly, VNS may also cause the activation of enteric glial cells (GFAP+) and support/preserve the epithelial barrier function [[60], [61], [62]]. We did observe a trend for increased GFAP+ cells in the colon with TUS treatment compared to 2% DSS WT animals.
In the current study, TUS exposure would result in a mechanical radiation force or peak rarefactional pressure of ~250 KPa, equal to a mechanical index (MI) of 0.25, below the food and drug administration (FDA) MI = 1.9 [[63], [64], [65]] for diagnostic US. In addition, TUS exposure to the abdomen resulted in ~2 °C rise in core body temperature (Supplementary Fig. 1). Hyperthermia has been previously reported to ameliorate intestinal inflammation when reaching core body temperatures of 42–43 °C maintained for 5 to 20 min, mainly by upregulating heat shock proteins (HSP) like HSP70 or HSP32 [[66], [67], [68]]. Although heat shock proteins have been shown to be protective in DSS colitis [69,70], our results demonstrated decreased levels of HSP70 by day 14 with TUS treatment. Interestingly, extracellular HSP70 has been considered as a pro-inflammatory factor since it stimulates the expression of pro-inflammatory genes in innate immune cells [71,72]. Therefore, the reduced levels seen in our study may contribute to the attenuation of clinical and pathological damage with TUS treatment. Further investigations are needed to determine how TUS directly causes CCTF changes, immune cell responses and stimulation of CAIP. Moreover, the complex response of immune cells and CCTF needs to be studied in KO mouse models and IBD patients, so we can better understand how to target these dynamic responses.
There were several limitations of this study that need to be addressed. This study was performed in female mice from one vendor and it is unclear if there was a gender bias associated with the response to TUS in the DSS colitis model. Male mice are more responsive to amelioration of DSS colitis with the use of α7 agonists, possibly due to hormonal influence [56]. Therefore, TUS effects in male mice require further investigation. It is also possible that the microbiome in the same strain of mice but obtained from different vendors, or the same vendor from a different location, may alter our observed results. Further studies would be needed to determine how TUS to the abdomen may alter the gut microbiome and how that could contribute to altering the DSS colitis model. We were also limited by small sample sizes for immunohistological analysis in the spleen and MLN, resulting in lack of differences between cohorts with and without TUS. In future experiments, we plan to use fluorescently marked splenic α7nAChR+F4/80+ cells to track them during the 14 days of the disease. We would also stain the colon samples for eosinophils, which might be participating in TUS effects. We need to further investigate the beneficial effects of TUS as a function of decreased colonic epithelial barrier permeability, since the animals presented firmer stools along with decreased levels of TNFα. Reduced expression of adhesion molecules could also contribute to decrease trafficking of immune cells and future studies would be needed confirm this hypothesis. In addition, proteomic analysis in the DSS colitis model of the colon for splenectomised and α7nAChR KO mice treated with TUS could also provide insight into the lack of a therapeutic response. Lastly, further experiments should be done to investigate the response of vagotomised mice to TUS, clarifying the specific involvement of the VN.
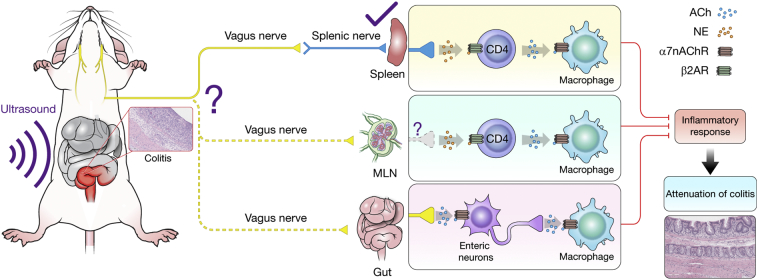
The results of this study demonstrate that TUS treatment decreased the severity of colitis most likely via stimulation of the splenic nerve, leading to CAIP activation (Fig. 13). We observed that the proteomic and immune cell profile in the gut was altered by TUS with decreased clinical symptoms and reduced histological damage during the recovery phase of DSS colitis. TUS exposures in α7nAChR KO or splenectomised mice receiving DSS confirmed that the activation of the CAIP and the spleen/splenic nerve were involved in TUS therapeutic effects. Since TUS is a non-invasive technique that has been used in the clinic for decades, it may be possible to use such an approach as an adjuvant in combination with current IBD treatments, aiming to improve clinical outcomes and reduce morbidity in IBD patients. In addition, our study is the first step to a possible future development of a multiple transducer-based system for low intensity ultrasound. The system could be potentially worn by IBD patients to stimulate changes in their immunological profile through sonication. Further investigation needs to be done to understand the involvement of the VN, the effects of TUS in chronic DSS colitis and in combination with pharmacological-biological IBD treatments.
Fig. 13.
Schematics of therapeutic ultrasound (TUS) effects on Dextran Sulphate Sodium (DSS)-induced acute colitis. TUS administration to the mouse abdomen attenuated DSS colitis through stimulation of the splenic nerve, activating the cholinergic anti-inflammatory pathway (CAIP). The release of norepinephrine (NE) in the spleen stimulates CD4+ T cells to release acetylcholine (ACh), which binds to α7 nicotinic acetylcholine receptors (α7nAChR) on macrophages and inhibits the release of pro-inflammatory cytokines. The vagus nerve (VN) could be the one initially stimulated by TUS, thus carrying out a therapeutic effect through the splenic nerve and, to a lesser extent, possibly the colon and mesenteric lymph node (MLN). In particular, enteric neurons release ACh to the muscularis macrophages in the gut, consequently carrying an anti-inflammatory effect and contributing to attenuation of experimental colitis. Further studies are needed to clarify the involvement of the vagus nerve. β2AR = β2 Adrenergic Receptor.
The following are the supplementary data related to this article
Funding sources
This work was supported by the Intramural Research Programs of the Clinical Centre, the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health, Bethesda/MD, USA and CAPES (Coordination for the Training of Higher Education Personnel Ministry of Education) from Brazil. The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Declaration of competing interests
The authors have declared that no conflict of interest exists.
Authors' contributions
Frank JA, Paz AH and Nunes NS assisted in research design. Nunes NS carried out the research. Nunes NS and Frank JA analysed data and wrote manuscript. Paz AH and Visioli F assisted with histological grading. Chandran P, Sundby M and Gonçalves FC assisted with immunohistochemistry analysis. Chandran P assisted with flow cytometry analysis. Burks SR assisted with proteomic analysis. All authors reviewed and approved the manuscript.
References
- 1.Lee S.H., Kwon J.E., Cho M.L. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26–42. doi: 10.5217/ir.2018.16.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shouval D.S., Rufo P.A. The role of environmental factors in the pathogenesis of inflammatory bowel diseases: a review. JAMA Pediatr. 2017;171(10):999–1005. doi: 10.1001/jamapediatrics.2017.2571. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 4.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 5.Ryan D.P., Doody D.P. Surgical options in the treatment of ulcerative colitis. Semin. Pediatr. Surg. 2017;26(6):379–383. doi: 10.1053/j.sempedsurg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Sairenji T., Collins K.L., Evans D.V. An update on inflammatory bowel disease. Prim Care. 2017;44(4):673–692. doi: 10.1016/j.pop.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Dulai P.S., Jairath V. Acute severe ulcerative colitis: latest evidence and therapeutic implications. Ther Adv Chronic Dis. 2018;9(2):65–72. doi: 10.1177/2040622317742095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer E.A., Dubinsky M.C. Therapeutic drug monitoring in inflammatory bowel disease. History and Future Directions Pediatr Clin North Am. 2017;64(6):1309–1326. doi: 10.1016/j.pcl.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Bonaz B., Sinniger V., Pellissier S. Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 2017;282(1):46–63. doi: 10.1111/joim.12611. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren S., Stewenius J., Sjölund K., Lilja B., Sundkvist G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand. J. Gastroenterol. 1993;28(7):638–642. doi: 10.3109/00365529309096103. [DOI] [PubMed] [Google Scholar]
- 11.Cailotto C., Costes L.M., van der Vliet J., van Bree S.H., van Heerikhuize J.J., Buijs R.M. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 2012;24(2):191–200, e93. doi: 10.1111/j.1365-2982.2011.01824.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosas-Ballina M., Ochani M., Parrish W.R., Ochani K., Harris Y.T., Huston J.M. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U. S. A. 2008;105(31):11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas-Ballina M., Olofsson P.S., Ochani M., Valdés-Ferrer S.I., Levine Y.A., Reardon C. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matteoli G., Boeckxstaens G.E. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62(8):1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goverse G., Stakenborg M., Matteoli G. The intestinal cholinergic anti-inflammatory pathway. J. Physiol. 2016;594(20):5771–5780. doi: 10.1113/JP271537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson P.S., Rosas-Ballina M., Levine Y.A., Tracey K.J. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 2012;248(1):188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stakenborg N., Labeeuw E., Gomez-Pinilla P.J., De Schepper S., Aerts R., Goverse G. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2018;0:1–11. doi: 10.1136/gutjnl-2018-317263. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchida Y., Hatao F., Fujisawa M., Murata T., Kaminishi M., Seto Y. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via alpha7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60(5):638–647. doi: 10.1136/gut.2010.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun P., Zhou K., Wang S., Li P., Chen S., Lin G. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0069424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meregnani J., Clarençon D., Vivier M., Peinnequin A., Mouret C., Sinniger V. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci. 2011;160(1–2):82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Jin H., Guo J., Liu J., Lyu B., Foreman R.D., Yin J. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313(3):G192–G202. doi: 10.1152/ajpgi.00254.2016. [DOI] [PubMed] [Google Scholar]
- 22.Ji H., Rabbi M.F., Labis B., Pavlov V.A., Tracey K.J., Ghia J.E. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014;7(2):335–347. doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonaz B., Picq C., Sinniger V., Mayol J.F., Clarençon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 2013;25(3):208–221. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- 24.Bonaz B., Sinniger V., Hoffmann D., Clarençon D., Mathieu N., Dantzer C. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol. Motil. 2016;28(6):948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 25.Bonaz B. Is-there a place for vagus nerve stimulation in inflammatory bowel diseases. Bioelectron Med. 2018;4(4) doi: 10.1186/s42234-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D.L., Smith N.B., Bailey M.R., Czarnota G.J., Hynynen K., Makin I.R. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012;31(4):623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gigliotti J.C., Huang L., Ye H., Bajwa A., Chattrabhuti K., Lee S. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol. 2013;24(9):1451–1460. doi: 10.1681/ASN.2013010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T., Abe C., Kohro T., Tanaka S., Huang L., Yao J. Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Int. 2019;95(3):563–576. doi: 10.1016/j.kint.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gigliotti J.C., Huang L., Bajwa A., Ye H., Mace E.H., Hossack J.A. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J. Am. Soc. Nephrol. 2015;26(10):2470–2481. doi: 10.1681/ASN.2014080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee A., Bizzaro D., Burra P., Di Liddo R., Pathak S., Arcidiacono D. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res Ther. 2015;6:79. doi: 10.1186/s13287-015-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonçalves FaC, Schneider N., Pinto F.O., Meyer F.S., Visioli F., Pfaffenseller B. Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J. Gastroenterol. 2014;20(48):18228–18239. doi: 10.3748/wjg.v20.i48.18228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieleman L.A., Palmen M.J., Akol H., Bloemena E., Pena A.S., Meuwissen S.G. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waddell A., Ahrens R., Steinbrecher K., Donovan B., Rothenberg M.E., Munitz A. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J. Immunol. 2011;186(10):5993–6003. doi: 10.4049/jimmunol.1003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Haddad S., Riddell R.H. The role of eosinophils in inflammatory bowel disease. Gut. 2005;54(12):1674–1675. doi: 10.1136/gut.2005.072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichman H., Moshkovits I., Itan M., Pasmanik-Chor M., Vogl T., Roth J. Transcriptome profiling of mouse colonic eosinophils reveals a key role for eosinophils in the induction of s100a8 and s100a9 in mucosal healing. Sci. Rep. 2017;7(1):7117. doi: 10.1038/s41598-017-07738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.König J., Wells J., Cani P.D., García-Ródenas C.L., MacDonald T., Mercenier A. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016;7(10) doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeissig S., Bürgel N., Günzel D., Richter J., Mankertz J., Wahnschaffe U. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56(1):61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andoh A., Fujino S., Bamba S., Araki Y., Okuno T., Bamba T. IL-17 selectively down-regulates TNF-alpha-induced RANTES gene expression in human colonic subepithelial myofibroblasts. J. Immunol. 2002;169(4):1683–1687. doi: 10.4049/jimmunol.169.4.1683. [DOI] [PubMed] [Google Scholar]
- 39.Andres P.G., Beck P.L., Mizoguchi E., Mizoguchi A., Bhan A.K., Dawson T. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 2000;164(12):6303–6312. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 40.Egesten A., Eliasson M., Olin A.I., Erjefalt J.S., Bjartell A., Sangfelt P. The proinflammatory CXC-chemokines GRO-alpha/CXCL1 and MIG/CXCL9 are concomitantly expressed in ulcerative colitis and decrease during treatment with topical corticosteroids. Int. J. Color. Dis. 2007;22(12):1421–1427. doi: 10.1007/s00384-007-0370-3. [DOI] [PubMed] [Google Scholar]
- 41.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interf. Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall D., Cameron J., Lightwood D., Lawson A.D. Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflamm. Bowel Dis. 2007;13(2):219–224. doi: 10.1002/ibd.20055. [DOI] [PubMed] [Google Scholar]
- 43.Wéra O., Lancellotti P., Oury C. The dual role of neutrophils in inflammatory bowel diseases. J. Clin. Med. 2016;5(12) doi: 10.3390/jcm5120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meshkibaf S., Martins A.J., Henry G.T., Kim S.O. Protective role of G-CSF in dextran sulfate sodium-induced acute colitis through generating gut-homing macrophages. Cytokine. 2016;78:69–78. doi: 10.1016/j.cyto.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Willis C.R., Seamons A., Maxwell J., Treuting P.M., Nelson L., Chen G. Interleukin-7 receptor blockade suppresses adaptive and innate inflammatory responses in experimental colitis. J Inflamm (Lond) 2012;9(1):39. doi: 10.1186/1476-9255-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthas D., Reznichenko A., Balendran C.A., Böttcher G., Clausen I.G., Kärrman Mårdh C. Neutrophils in ulcerative colitis: a review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 2017;52(2):125–135. doi: 10.1080/00365521.2016.1235224. [DOI] [PubMed] [Google Scholar]
- 47.Tokuyama H., Ueha S., Kurachi M., Matsushima K., Moriyasu F., Blumberg R.S. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int. Immunol. 2005;17(8):1023–1034. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- 48.Ohtsuka Y., Lee J., Stamm D.S., Sanderson I.R. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49(4):526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prame Kumar K., Nicholls A.J., Wong C.H.Y. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371(3):551–565. doi: 10.1007/s00441-017-2753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nunes N.S., Kim S., Sundby M., Chandran P., Burks S.R., Paz A.H. Temporal clinical, proteomic, histological and cellular immune responses of dextran sulfate sodium-induced acute colitis. World J. Gastroenterol. 2018;24(38):4341–4355. doi: 10.3748/wjg.v24.i38.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postovalova E.A., Khochansky D.N., Zolotova N.A., Gao Y., Makarova O.V., Dobrynina M.T. Morphological changes in mesenteric lymph nodes and lymphocyte subpopulation composition in experimental ulcerative colitis. Bull. Exp. Biol. Med. 2016;160(6):835–839. doi: 10.1007/s10517-016-3322-5. [DOI] [PubMed] [Google Scholar]
- 53.Wang L., Ray A., Jiang X., Wang J.Y., Basu S., Liu X. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol. 2015;8(6):1297–1312. doi: 10.1038/mi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matteoli G., Gomez-Pinilla P.J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S.H. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 55.de Jonge W.J. The Gut's little brain in control of intestinal immunity. ISRN Gastroenterol. 2013;2013:630159. doi: 10.1155/2013/630159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.AlSharari S.D., Bagdas D., Akbarali H.I., Lichtman P.A., Raborn E.S., Cabral G.A. Sex differences and drug dose influence the role of the α7 nicotinic acetylcholine receptor in the mouse dextran sodium sulfate-induced colitis model. Nicotine Tob. Res. 2017;19(4):460–468. doi: 10.1093/ntr/ntw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chavan S.S., Tracey K.J. Essential Neuroscience in Immunology. J. Immunol. 2017;198(9):3389–3397. doi: 10.4049/jimmunol.1601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8(12):743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Browning K.N., Verheijden S., Boeckxstaens G.E. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152(4):730–744. doi: 10.1053/j.gastro.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costantini T.W., Bansal V., Krzyzaniak M., Putnam J.G., Peterson C.Y., Loomis W.H. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(6):G1308–G1318. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharkey K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Invest. 2015;125(3):918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu Y.B., Li Y.Q. Enteric glial cells and their role in the intestinal epithelial barrier. World J. Gastroenterol. 2014;20(32):11273–11280. doi: 10.3748/wjg.v20.i32.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serra C., Menozzi G., Labate A.M., Giangregorio F., Gionchetti P., Beltrami M. Ultrasound assessment of vascularization of the thickened terminal ileum wall in Crohn's disease patients using a low-mechanical index real-time scanning technique with a second generation ultrasound contrast agent. Eur. J. Radiol. 2007;62(1):114–121. doi: 10.1016/j.ejrad.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 64.Barnett S.B., Ter Haar G.R., Ziskin M.C., Rott H.D., Duck F.A., Maeda K. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med. Biol. 2000;26(3):355–366. doi: 10.1016/s0301-5629(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 65.Mullick Chowdhury S., Lee T., Willmann J.K. Ultrasound-guided drug delivery in cancer. Ultrasonography. 2017;36(3):171–184. doi: 10.14366/usg.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldhill J.M., Stojadinovic A., Kiang J., Smallridge R., Shea-Donohue T. Hyperthermia prevents functional, histological and biochemical abnormalities induced during ileitis. Neurogastroenterol. Motil. 1999;11(1):69–76. doi: 10.1046/j.1365-2982.1999.00130.x. [DOI] [PubMed] [Google Scholar]
- 67.Kokura S., Yoshida N., Okuda T., Nakabe N., Sakamoto N., Isozaki Y. Hyperthermia ameliorates 2,4,6-trinitrobenzene sulphonic acid-induced colitis in rats: the role of heat shock proteins. Int. J. Hyperth. 2007;23(1):17–28. doi: 10.1080/02656730601090223. [DOI] [PubMed] [Google Scholar]
- 68.Sakamoto N., Kokura S., Okuda T., Hattori T., Katada K., Isozaki Y. Heme oxygenase-1 (Hsp32) is involved in the protection of small intestine by whole body mild hyperthermia from ischemia/reperfusion injury in rat. Int. J. Hyperth. 2005;21(7):603–614. doi: 10.1080/02656730500188599. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka K., Mizushima T. Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int. J. Hyperth. 2009;25(8):668–676. doi: 10.3109/02656730903213366. [DOI] [PubMed] [Google Scholar]
- 70.Liao Y.F., Zhu W., Li D.P., Zhu X. Heme oxygenase-1 and gut ischemia/reperfusion injury: a short review. World J. Gastroenterol. 2013;19(23):3555–3561. doi: 10.3748/wjg.v19.i23.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnal M.E., Lallès J.P. Gut epithelial inducible heat-shock proteins and their modulation by diet and the microbiota. Nutr. Rev. 2016;74(3):181–197. doi: 10.1093/nutrit/nuv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giuliano J.S., Lahni P.M., Wong H.R., Wheeler D.S. Pediatric Sepsis - Part V: Extracellular heat shock proteins: Alarmins for the host immune system. Open Inflamm J. 2011;4:49–60. doi: 10.2174/1875041901104010049. [DOI] [PMC free article] [PubMed] [Google Scholar]