Abstract
Background
The functional quality of insulin-secreting islet beta cells is a major factor determining the outcome of clinical transplantations for diabetes. It is therefore of importance to develop methodological strategies aiming at optimizing islet cell function prior to transplantation. In this study we propose a synthetic biology approach to genetically engineer cellular signalling pathways in islet cells.
Methods
We established a novel procedure to modify islet beta cell function by combining adenovirus-mediated transduction with reaggregation of islet cells into pseudoislets. As a proof-of-concept for the genetic engineering of islets prior to transplantation, this methodology was applied to increase the expression of the V1b receptor specifically in insulin-secreting beta cells. The functional outcomes were assessed in vitro and in vivo following transplantation into the anterior chamber of the eye.
Findings
Pseudoislets produced from mouse dissociated islet cells displayed basic functions similar to intact native islets in terms of glucose induced intracellular signalling and insulin release, and after transplantation were properly vascularized and contributed to blood glucose homeostasis. The synthetic amplification of the V1b receptor signalling in beta cells successfully modulated pseudoislet function in vitro. Finally, in vivo responses of these pseudoislet grafts to vasopressin allowed evaluation of the potential benefits of this approach in regenerative medicine.
Interpretation
These results are promising first steps towards the generation of high-quality islets and suggest synthetic biology as an important tool in future clinical islet transplantations. Moreover, the presented methodology might serve as a useful research strategy to dissect cellular signalling mechanisms of relevance for optimal islet function.
Keywords: Diabetes, In vivo imaging, Synthetic biology, Vasopressin, Pseudoislet, Transplantation
Research in context.
Evidence before this study
Transplantation of pancreatic islets has the potential to cure type 1 diabetes and may also benefit patients with insulin-dependent type 2 diabetes. Besides the shortage of donor material, the success of this treatment has thus far been hampered by a suboptimal quality of islet preparations pre-transplantation contributing to poor islet graft survival and function. Genetic modification of islet cells in vitro has been shown to enhance islet function and may improve the outcome of clinical transplantations. The efficiency of available methodologies to genetically refine islet function is however suboptimal, and there is a need for a straightforward in vivo approach that could provide longitudinal information on the functional value of specific modifications of islet cells.
Added value of this study
In the current study we describe an improved protocol to genetically modify islet cell function and, as a proof-of-concept, boosted V1b receptor signalling specifically in the insulin-secreting beta cells. We demonstrate that we could thereby improve insulin secretion of individual islets upon activation of this pathway with the natural ligand vasopressin. We furthermore present an in vivo imaging platform to evaluate the function and survival of our genetically engineered islets after transplantation into mice, of importance to assess the long-term functional benefits of specific genetic alterations in beta cells.
Implications of all the available evidence
Our protocols may serve as a research strategy for other pancreatic islet researchers to dissect the function of individual signalling components within islet cells. While we amplified an existing signalling pathway in healthy islets, the described protocol may also be employed to restore impaired islet cell function and/or efficiently integrate current and future synthetic signalling pathways into islet cells, thus generating high quality islet tissue that could improve the outcome of clinical islet transplantations.
Alt-text: Unlabelled Box
1. Introduction
Pancreatic beta cell dysfunction plays an important role in the pathophysiology of both type 1 and type 2 diabetes mellitus. Impairments in glucose sensing or glucose stimulated insulin secretion, as well as glucotoxicity, lipotoxicity, increased oxidative stress and inflammation can all contribute to a suboptimal beta cell function [1,2]. For many decades, exogenous insulin injections have been one of the standard therapies for diabetes. More recently another treatment strategy is being pursued in parallel, based on the specific targeting of various cellular pathways in beta cells to increase their hormone release. One such therapeutic approach for subjects with type 2 diabetes is based on the pharmacological targeting of G protein-coupled receptors (GPCRs) expressed in the plasma membrane of beta cells, such as the GLP-1 receptor [3]. The activation of these GPCRs mediates transduction pathways that can alter intracellular levels of Ca2+, cAMP, and IP3, thus modulating insulin release [4]. However, the expression level of several GPCRs has been shown to be affected by glucose levels [[5], [6], [7]], thereby potentially hindering the efficiency of treatments targeting these receptors.
Lacking sufficient beta cell mass, most subjects with type 1 diabetes do not benefit from GPCR-targeted treatment. Instead, transplantation of pancreatic islets from cadaveric donors can help stabilize glucose levels, albeit for a specific subpopulation [8]. Several factors including variability in islet quality and limited survival of the transplanted tissue contribute to the moderate success of this treatment [[9], [10], [11], [12], [13]]. Genetic modification of islets is a promising approach to improve pre-transplant islet function, inhibit rejection and limit the rate of apoptosis [[14], [15], [16]]. In the current study, we engineered pancreatic islets with the aim of modulating their function. Instead of direct viral transduction of intact ‘native’ islets, gene transfer was achieved by adenovirus-mediated transduction of dissociated islet cells with a simultaneous controlled reconstitution into pseudoislets. The viability and function of these pseudoislets were then assessed in vitro and in vivo following transplantation into the anterior chamber of the eye [17,18].
As a proof-of-concept, we modified mouse beta cells within the islet to enhance their signalling via the V1b receptor, a GPCR member of the vasopressin-oxytocin family [19]. This receptor is naturally expressed at low levels in islet cells and stimulation with [Arg8]-vasopressin (AVP) potentiates glucose-stimulated insulin release by activation of the phospholipase C pathway and mobilization of intracellular Ca2+ [20,21]. AVP also plays an important role in glucose homeostasis by acting on various receptors of the vasopressin-oxytocin family in other tissues such as vascular smooth muscle, liver, and adipose cells [22,23]. One of the consequences of in vivo administration of AVP is the stimulation of hepatic glycogen breakdown resulting in a gradual release of glucose from the liver into the blood stream. This aspect was used to demonstrate that our engineered islets not only display an enhanced acute response to AVP but also still maintain a normal response to changes in circulating glucose concentration.
2. Materials and methods
2.1. Animals, blood glucose measurements, and STZ treatments
C57BL/6J mice were from Charles River (Germany). Ins-GCaMP3 mice were generated by crossing B6;129S-Gt(Rosa)26Sortm38(CAG-GCaMP3)Hze/j mice [24] and heterozygous B6(Cg)-Instm1.1(cre)Thor/j mice [25]. These specific Ins1(Cre) knock-in mice allow for a near complete recombination efficiency and beta cell-selectivity [25]. Mice were kept on C57BL/6 J background and have been backcrossed for >10 generations, inbred at the animal core facilities at Karolinska Institutet. Blood glucose concentrations in mice were measured using Accu-Chek Aviva monitoring system (Roche). Pancreatic beta cells were chemically ablated by intraperitoneal injection of 150 mg streptozotocin per kg body weight dissolved in citric acid buffer (pH 4·5) after 4–6 h of fasting. All experiments were performed following Karolinska Institutet's guidelines for care and use of animals in research, were approved by the animal ethics committee at Karolinska Institutet, and reported following the ARRIVE guidelines for animal studies.
2.2. Pancreatic islet isolation and generation of pseudoislets
Mouse pancreatic islets were prepared and purified from donor animals following standard procedures [26], and maintained in RPMI 1640 culture medium supplemented with l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml) and 10% fetal bovine serum (Life Technologies). Human islets were obtained from the Nordic Network for Islet Transplantation and maintained in CMRL 1066 culture medium (ICN Biomedicals) supplemented with HEPES (10 mM), l-glutamine (2 mM), Gentamycin (50 mg/ml), Fungizone (0·25 mg/ml), Ciprofloxacin (20 mg/ml), nicotinamide (10 mM) and 10% fetal bovine serum. Islets were kept at 37 °C in a humidified atmosphere with 5% CO2 for a minimum of 2 h after isolation. For the generation of pseudoislets, islets were dissociated into single cells by enzymatic digestion for 10 min at 37 °C using Accutase (Sigma-Aldrich). Cells were counted using a TC20 automated cell counter (Bio-Rad) and resuspended in serum-supplemented culture medium at a density of 62,500 cells/ml unless otherwise mentioned. 40 μl of endocrine cells in suspension were placed per well of a GravityPLUS hanging drop system (Perkin Elmer), pseudoislets were formed and collected after 7 days following the manufacturer's instructions.
2.3. Production of adenoviruses and transduction
Adenoviruses were prepared using the Gateway cloning system (Thermo Fisher Scientific). The coding sequence of the human V1b receptor together with a Strep-tag II tag was sub-cloned into the Gateway vector pENTR1A. The insulin promoter sequence RIP1 was inserted before the hV1BR coding sequence to ensure the expression in a beta cell-specific manner. Similarly, the gene encoding for the red calcium indicator R-GECO1 [27] (Addgene) was preceded by the RIP1 promoter and sub-cloned into pENTR1A. The resulting vectors pENTR1A-RIP1-hV1BR and pENTR1A-RIP1-R-GECO1 were sequenced and subjected to recombination with the promoter-less destination vector pAd/PL-DEST, for the production of adenoviruses using HEK293A cells. Adenoviruses were purified and concentrated using Fast-Trap (Millipore), following the manufacturer's instructions. Quantification was performed by real-time PCR on a QuantStudio 5 system (Thermo Fisher Scientific), using SYBR Green (Thermo Fisher Scientific) and primers specific for the adenovirus coding sequence. Transduction was performed by infecting cells with serum-supplemented culture medium containing 1000 viral particles per μl.
2.4. Functional assessment of islet cells in vitro
Prior to all functional assays islets were kept for 1 h in a buffered solution (pH 7·4, containing 125 mM NaCl, 5·9 mM KCl, 2·6 mM CaCl2, 1·2 mM MgCl2, 25 mM Hepes, and 0·1% BSA) supplemented with 3 mM glucose. In the subsequent experiments stimuli were presented in the same buffered solution. For [Ca2+]i measurements, islets loaded with Fura-2 AM (Thermo Fisher Scientific), or islets genetically expressing the GCaMP3 [Ca2+]i biosensor in their beta cells, were attached to a cover slip using PuraMatrix Hydrogel (BD Biosciences) and fluorescence was recorded using an inverted epifluorescence Axiovert 135 microscope (Zeiss, Jena, Germany) connected to a SPEX Industries Fluorolog spectrofluorometer for single- or dual-wavelength excitation fluorimetry. Islets were perfused at 37 °C with the buffered solution supplemented with either 3 mM glucose, 3 mM glucose +100 nM AVP, or 11 mM glucose, and simultaneously excited at 340 and 380 nm (for Fura-2) or at 476 nm (for GCaMP3). Fluorescence emission was recorded and values were normalized either by calculating the ratio of intensities obtained by 340 and 380 nm excitation wavelengths (for Fura-2), or to basal fluorescence intensity before stimulation (for GCaMP3). Baseline [Ca2+]i was determined as the minimum value prior to applying the stimulating buffer. For assessment of glucose-, KCl- and AVP-stimulated insulin release islets were incubated under static conditions in a buffered solution containing 11 mM glucose (for 30 min), 25 mM KCl (for 15 min), 3 mM and 7 mM glucose alone (60 min) or in combination with 100 nM AVP (for 15 min). At the end of the experiment islets were lysed in M-PER protein extraction reagent (Thermo Fisher Scientific) for assessment of DNA content using Quant-iT PicoGreen dsDNA reagent (Invitrogen). Supernatant was collected for insulin measurements using AlphaLISA immunoassay (Perkin Elmer). Amount of released insulin (in ng) was recalculated to secreted per min and normalized to islet DNA content (in ng). To measure dynamic insulin release, we used an automated perifusion system (BioRep Perifusion System, BioRep, Miami), as described elsewhere [28]. Briefly, ~100 native islets or pseudoislets were immobilised in a gel (Bio-Gel P-4, Bio-Rad) in a column-shaped chamber and stimulatory solutions were pumped through the column at a rate of 50 μl/min, perifusates with secreted insulin were collected every minute in an automatic fraction collector designed for 96-well plates. For comparison purposes, both native islets and pseudoislets were assessed for their function 8–10 days after isolation of pancreatic islets.
2.5. Transplantation of islets into the anterior chamber of the mouse eye
Animals were transplanted into the anterior chamber of the eye at 8–10 weeks of age as described previously [18]. During transplantation isoflurane was used for anaesthesia, and mice received subcutaneous injections of 2 μg Temgesic (Schering-Plough) for post-operative analgesia.
2.6. Laser scanning confocal microscopy
Laser scanning confocal micrographs were recorded at 8 bit using a Leica SP5 system equipped with 10× or 25× water-immersion objective (Leica Microsystems, Wetzlar, Germany). Fluorescence emission from GCaMP3 and MIP-GFP was obtained by excitation at 488 nm and detection between 500 and 550 nm. R-GECO1 emission was obtained by excitation at 594 nm and detection between 600 and 700 nm. Backscatter signal imaging was obtained using a 488, 561 or 633 nm laser beam as described previously [29]. In vivo imaging of islet grafts in mice anaesthetised with isoflurane was performed as previously described, 1 month after transplantation [18]. For blood vessel imaging mice received 100 μl Texas Red labelled dextran intravenously. Scanning speed and laser intensities were adjusted to avoid any cellular damage to islets in vitro, and to the mouse eye or islet grafts in vivo. All confocal images are representatives and presented as maximum intensity projections in the XY plane as indicated.
2.7. In vivo beta cell intracellular [Ca2+] measurements
Female mice were anaesthetised using Fentanyl:H2O:Midazolam mixtures at a ratio of 1:2:1, administrated intraperitoneally at a dose of 8 μl per g of mouse. The stock concentrations of midazolam (Hameln) was 5 mg/ml. Fentanyl (VetaPlasma) was bought as premixed fentanyl citrate (0·315 mg/ml) and fluanisone (10 mg/ml). Before the imaging of GCaMP3 islets, the tail vein of anaesthetised mice was cannulated with a tubing containing 100 U/ml heparin in saline. Blood glucose was measured directly before and after the [Ca2+]i recordings. A single islet in the mouse eye was continuously excited with a fluorescent lamp using a 510/40 nm bandpass filter, and GCaMP3 fluorescence emission was collected with a Hamamatsu camera installed on top of a Leica SP5 microscope chamber heated at 35 °C. A piezo element under the control of Labview software moved the 25× objective up and down every 2 s during which 60 images were collected, covering a xyz volume of 476 μm × 476 μm × 120 μm. [Arg8]-vasopressin (AVP) at a concentration of 2 μM, or glucose at 5% w/v, were prepared in saline solutions for in vivo stimulation. After recording a baseline fluorescence emission for 5 min, the stimulating solution was slowly injected intravenously at a dose of 4 μl per g of mouse body weight (during 10 s), and the islet was recorded for another 10 min.
2.8. Immunohistochemistry (IHC) and transmission electron microscopy (TEM)
For staining of tissue sections, dissected samples were fixed with formalin for 48 h at room temperature, dehydrated, and embedded in paraffin. 5-μm-thick sections were mounted on precoated microscope slides, dewaxed by xylene and progressively rehydrated before processing. Insulin and glucagon were stained using guinea-pig anti-insulin (DAKO, 1:1000) and prediluted rabbit anti-glucagon (BioGenex), followed by goat anti-guinea pig Alexa Fluor 488 (Thermo Fisher, 1:1000) and goat anti-rabbit Alexa Fluor 546 (Thermo Fisher, 1:1000) antibodies. Slides were mounted using ProLong Gold Antifade Reagent with DAPI (Thermo Fisher) and imaged with a BD Pathway 855 system (BD Biosciences). For whole mount staining, eyes were fixed in 4% paraformaldehyde (PFA) for 1·5 h on ice and washed in phosphate buffered saline (PBS), followed by dissection of single islet or pseudoislet grafts from the iris. Alternatively, non-transplanted islets or pseudoislets were directly fixed in 4% PFA for 1 h on ice and washed in PBS. Tissue samples were blocked overnight in PBS supplemented with 10% goat serum and 0·3% Triton-X, and stained overnight with primary antibodies (guinea pig anti-insulin (A0564, DAKO, 1:1000), mouse anti-Strep-tag II (34,850, Qiagen, 1:1000), rat anti-somatostatin (8330-0009, Bio-Rad, 1:500), mouse anti-glucagon (G2654, Sigma-Aldrich, 1:1000), dissolved in blocking buffer), and overnight with secondary antibodies (goat anti-guinea pig Alexa 488 (Thermo Fisher Scientific, 1:1000), goat anti-mouse Alexa 633 (Thermo Fisher Scientific, 1:1000), goat anti-mouse Alexa 546 (Thermo Fisher Scientific, 1:1000), goat anti-rat Alexa 633 (Thermo Fisher Scientific, 1:1000), dissolved in blocking buffer). Cell death was assessed using an apoptosis kit (V13240, Thermo Fisher Scientific) according to the manufacturer's instructions. Whole mounts were imaged by confocal microscopy. For in vitro comparison purposes, both native islets and pseudoislets were used for immunostaining 8–10 days after isolation of pancreatic islets. Collection, processing and imaging of individual islet grafts for TEM was performed as described previously [29].
2.9. Image processing and analysis
Laser scanning confocal microscopy images were analysed using Volocity (PerkinElmer). Determination of islet volumes was based on backscatter imaging, as previously described [29]. Transduction efficiency and apoptosis rate were determined by measuring R-GECO1 or Annexin V positive voxels, respectively, and data was presented as percentage of analysed islet volumes. Quantification of whole mount stainings were performed on two optical sections per islet or pseudoislet, at 30 μm and 50 μm below their imaged surface unless otherwise specified. For in vivo beta cell [Ca2+]i imaging, image sequences were imported in ImageJ (NIH) and average projection time series were prepared based on hyperstacks of 60 images each. After image registration, islet fluorescence intensities were measured and plotted over time after normalization to basal fluorescence intensity before stimulation.
2.10. Statistical analysis
Data processing and statistical analysis were performed using Excel (Microsoft), Prism (version 6, GraphPad) and Statistica 13·0 (Dell). Data are presented as mean ± S.E. unless indicated otherwise. The n-numbers are indicated in the figure legends. Student's unpaired or paired t-tests, Mann-Whitney tests, one-way ANOVA or mixed-design ANOVA followed by Tukey post-hoc tests were used, as appropriate. P-values < .05 were considered statistically significant.
3. Results
3.1. Endocrine cells dissociated from native islets can be reaggregated into functional pseudoislets of controllable size
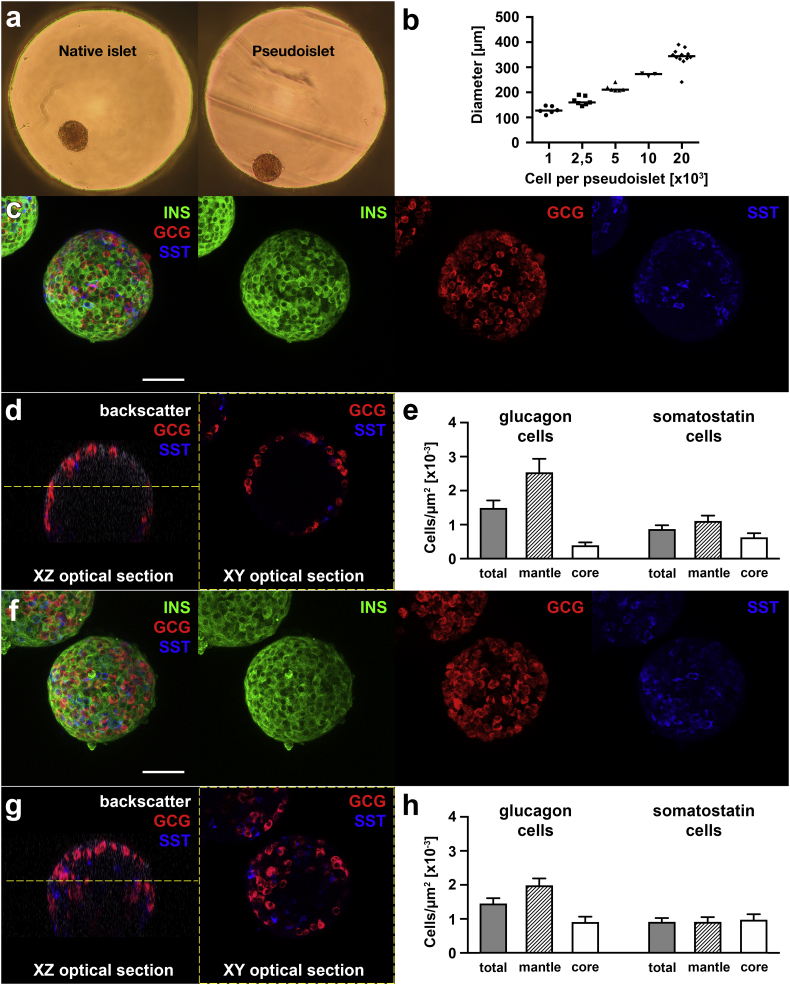
Mouse pancreatic islets were isolated using an established technique [26]. After the disaggregation of native islets, single cells were reaggregated into pseudoislets using a multi-well hanging drop system (Fig. 1a), without selecting for specific endocrine cell types or manipulating their ratio. Using this technique, the diameter of pseudoislets could be precisely controlled by altering the number of cells per droplet (Fig. 1b). Pseudoislets with a diameter of approximately 175 μm, the average diameter of native islets after isolation (Supplementary Fig. 1), were selected for further experiments. When comparing the number of different endocrine cell types as well as their localization within native islets or pseudoislets, we could confirm that the average content in alpha and delta cells was retained after pseudoislet formation (Fig. 1c–h). However, due to an apparent random relocation of various endocrine cells within pseudoislets during reaggregation, the typical mantle localization of alpha cells seen in native islets was no longer observed, alpha cells being instead intermingled with other cell types (Fig. 1d–e, g–h).
Fig. 1.
Pseudoislets can be precisely designed in terms of size and preserve the native ratio of islet endocrine cells. (a) Pseudoislets can be formed by reaggregation of dispersed islet cells in hanging droplets. Representative photograph shows a native islet placed in a hanging drop and a pseudoislet, indicating morphological resemblance of these spherical structures (approximate diameter of 175 μm). (b) The diameter of pseudoislets can be precisely adjusted depending on the number of endocrine cells used for their formation. Whole mount immunostaining of native islets (c–e) and pseudoislets (f–h) for insulin (INS), glucagon (GCG) and somatostatin (SST) served to investigate the content of different endocrine cell types as well as their architectural location. While the analysis shows more intermingling of alpha and delta cells in pseudoislets (h) as compared to native islets (e), no significant difference was seen in their total content in glucagon or somatostatin cells (n = 11 native islets, 12 pseudoislets). Confocal images are representative and shown as maximum intensity projections (c, f) or as optical sections (d, g). Yellow dashed line in XZ optical sections indicate the position of XY optical sections, 50 μm below the imaged surface of the islet or pseudoislet. Scale bars represent 50 μm. Data presented as mean ± S.E. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Pseudoislets were next assessed for their function in terms of glucose-induced increase in cytoplasmic free Ca2+ concentration ([Ca2+]i) and insulin release (Fig. 2). Pseudoislets displayed a slightly lower basal [Ca2+]i, a faster response to a rise from 3 mM to 11 mM glucose with a larger amplitude of [Ca2+]i increase (Fig. 2c), but otherwise exhibited a similar pattern of [Ca2+]i responses as compared to native islets (Fig. 2a, b). Dynamic insulin release experiments showed proper kinetics in response to 11 mM glucose and KCl from pseudoislets, comparable to that of native islets (Fig. 2d, e). Static incubations allowed more precise measurements of the amount of insulin secreted per (pseudo)islet, revealing higher basal insulin release at 3 mM glucose and stronger response to 11 mM glucose in pseudoislets as compared to native islets (Fig. 2f).
Fig. 2.
In vitro functional comparison between pseudoislets and native islets. (a–c) Native islets and pseudoislets were loaded with the fluorescent [Ca2+]i indicator Fura-2 and incubated for 1 h at 3 mM glucose concentration. Changes in [Ca2+]i in response to an increase in glucose concentration and to KCl induced plasma membrane depolarization were then monitored overtime, showing a similar response pattern for pseudoislets (b) as compared to native islets (a). Traces are representative (n ≥ 7 per group). (c) Quantitative analyses indicate a rapid [Ca2+]i increase with high amplitude in pseudoislets in response to an increase in glucose concentration. (d–e) Native islets (d) and pseudoislets (e) demonstrate identical response patterns of insulin release when stimulated with 11 mM glucose and 25 mM KCl in a dynamic perifusion system. Approximately 100 native islets or pseudoislets were perfused with 3 mM glucose, 11 mM glucose and 25 mM KCl (data represents 3 independent experiments per group). (f) Groups of native islets and pseudoislets were incubated under static conditions with 3 mM glucose, 11 mM glucose, or 3 mM glucose together with 25 mM KCl (n ≥ 3 per condition, groups of 8–10 native islets or pseudoislets). Data presented as mean ± S.E. *p < 0·05, **p < 0·01, ***p < 0·001.
3.2. Pseudoislets are properly vascularized and functional after transplantation into the anterior chamber of the eye
To determine whether pseudoislets maintain their function in vivo, these were transplanted into the anterior chamber of the mouse eye. We could show that pseudoislet grafts get vascularized and that their endothelial cells form fenestrations reminiscent of those found in pancreatic islets (Fig. 3a–d). These properties are prerequisites for proper communication between bloodstream and the endocrine cells composing the engrafted pseudoislets. Similarly as prior to transplantation, pseudoislet grafts displayed the same content in major endocrine cells as compared to native islet grafts (Supplementary Fig. 2), however one month after transplantation an intermingling of alpha cells could be observed both in native islet grafts and in pseudoislets grafts.
Fig. 3.
Pseudoislets are functional in vivo after their transplantation into the anterior chamber of the eye. (a) Photograph of an eye transplanted with a few reporter pseudoislets. (b) In vivo imaging by confocal microscopy 1 month after transplantation shows that pseudoislets are engrafted and vascularized (vessels are shown in red, observed after intravenous injection of fluorescently-labelled dextran). (c) Immunohistochemistry on paraffin sections of pseudoislet grafts indicates the presence of distinct endocrine cell types (green, insulin; red, glucagon; blue, DAPI). (d) Electron microscopy imaging shows that endothelial cells forming intra-pseudoislet blood vessels are fenestrated (examples shown with red arrowheads), permitting proper communication between endocrine cells and blood plasma. (e) To visualize beta cell function in vivo, pseudoislets were prepared using islets originating from transgenic mice expressing the [Ca2+]i sensitive fluorescent biosensor GCaMP3 in beta cells. One month after transplantation, pseudoislets were imaged in vivo to assess their response to an increase in blood glucose concentration. After a 5 min baseline intensity imaging, mice were injected intravenously with a 5% glucose solution in saline and islets were imaged for an additional 10 min. The increase in GCaMP3 fluorescence intensity upon glucose administration is reflective of a proper [Ca2+]i response in beta cells, similar to that of native islets (see Supplementary Fig. 4). Our setup permits the imaging of one single pseudoislet graft over time per mouse. (f) In order to assess the ability of transplanted pseudoislets to regulate blood glucose levels, mice received streptozotocin (STZ) treatment to ablate endogenous pancreatic beta cells and induce diabetes. Transplantation (Tx) of approximately 100 pseudoislets into the anterior chamber of the eye of mice reversed the hyperglycaemic condition after a few weeks, while non-transplanted mice remained hyperglycaemic (n = 3–7). Images are representative (n ≥ 3). Confocal images are shown as maximum intensity projection (b) and average intensity projections (e) of single pseudoislet grafts. Scale bars represent 50 μm (b, c) and 1 μm (d). *p < 0·05, **p < 0·01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Beta cell function in vivo was next assessed by transplanting pseudoislets prepared from islets originating from Ins-GCaMP3 mice. One month after transplantation, we imaged in vivo the fluorescence intensity of the GCaMP3 biosensor, reflecting [Ca2+]i in pseudoislet beta cells. The intravenous administration of glucose during imaging resulted in a rapid and transient increase in GCaMP3 intensity, demonstrating the proper communication between engrafted pseudoislets and bloodstream, and the adequate functional response of their beta cells (Fig. 3e, Supplementary Movie 1). Although a difference in timing of response to glucose could be observed in vitro prior to transplantation between these Ins-GCaMP3 pseudoislets and Ins-GCaMP3 native islets (Supplementary Fig. 3), no difference could be detected 1 month after transplantation under in vivo conditions (Supplementary Fig. 4).
To further ensure that the beta cells within pseudoislets function in vivo and contribute to the maintenance of blood glucose homeostasis by timely release of adequate insulin amounts, mice were first treated with streptozotocin (STZ) to ablate their in situ pancreatic beta cells. A few days after intraperitoneal administration of STZ, mice became hyperglycaemic. The transplantation of approximately 100 pseudoislets into the anterior chamber of the eye normalized blood glucose levels in these mice, demonstrating that pseudoislets are functional and are able to maintain their physiological role in vivo (Fig. 3f and Supplementary Fig. 5).
3.3. Optimization of virus-mediated gene transfer by engineering pseudoislets from disaggregated islets
Genetic regulation of beta cell function requires an optimal gene transfer methodology. When using native islets, adenovirus transduction methodology is relatively inefficient in reaching cells beyond the peripheral cell layers. To improve gene transfer into beta cells, we disaggregated islets into single cells, and reaggregated them into engineered pseudoislets in the presence of an adenovirus (Fig. 4a). To evaluate the transduction efficiency, we prepared an adenovirus encoding a fluorescent biosensor for beta cell function, thereby allowing for an optical appreciation of gene transfer effectiveness. For selective expression in pancreatic beta cells the red [Ca2+]i biosensor R-GECO1 [27] was placed under the rat insulin promoter 1 (RIP1). The analysis of images acquired by confocal microscopy demonstrated that this methodology resulted in a significant increase in efficiency for cells transduced during reaggregation into pseudoislets as compared to transduced native islets (Fig. 4b–c). The transduction efficiency was then further optimized by the use of four different virus concentrations (ranging from 0·2 to 25 million virus particles per ml). An increase in virus concentration positively correlated with increased transduction efficiency and the number of cells that could be detected (Fig. 4d). However, concentrations >5 million particles per ml led to morphological impairments and increased cell death resulting in smaller pseudoislets (Table 1, Fig. 4e–f). A virus concentration of 1 million particles per ml was therefore chosen for all further experiments. In addition, we successfully applied this synthetic expression protocol to engineer pseudoislets derived from human islet cells, with an equivalent transduction efficiency as for mouse islets while retaining a high cell viability (Supplementary Fig. 6). This indicates that our strategy can be applied to endocrine cells independently of their species of origin.
Fig. 4.
Transduction during reaggregation of cells into pseudoislets improves gene transfer. (a) Schematic representation of the procedure aiming at improving gene transfer by dispersion of islet cells prior to virus transduction and simultaneous reaggregation into pseudoislets. (b) Dispersed endocrine islet cells or intact islets were incubated for 7 days in a hanging droplet in the presence of 1 or 5 million R-GECO1 virus particles per ml, followed by confocal microscopy imaging. A dose-dependent relationship could be observed between the concentration of virus particles and the amount of fluorescence. (c) Quantification of the R-GECO1 positive volume showed that transduction during pseudoislet formation improved the efficiency as compared to transduction of intact islets with both virus concentrations (n ≥ 10 islets per condition). (d) Analysis of the fluorescence intensity of single cells within the islet (n = 3 per condition) demonstrates that higher concentrations of virus allow detection of more cells with a larger range of intensities. Dotted line shows lower limit of the detection threshold. (e) Backscatter imaging of pseudoislets prepared with different virus concentrations revealed that high virus concentrations affect pseudoislet morphology. (f) After 7 days of culture in the presence of different concentrations of the virus, pseudoislets contain a small number of dead or apoptotic cells. Fluorescence staining for apoptosis revealed that pseudoislet formation in the presence of virus concentrations exceeding 1 million particles per ml induces increased cell death and apoptosis in endocrine cells (n = 6–8 islets per condition). (g–i) Pseudoislets were made from dispersed islets originating from transgenic Ins-GCaMP3 mice (carrying a beta cell specific [Ca2+]i sensitive fluorescent biosensor GCaMP3) and reaggregated in the presence of 1 million particles per ml of R-GECO1 virus. (g) Confocal imaging demonstrates the presence of both biosensors in beta cells within the pseudoislet. (h–i) Optical sections by confocal imaging displaying (h) GCaMP3 and (i) R-GECO1 signal intensities at 3 mM glucose (“before”) and 4·5 min post-stimulation with 15 mM glucose (“after”). Dot plots show increased fluorescence of individual cells upon stimulation, demonstrating the proper glucose responsiveness of transduced pseudoislets (n = 6 and 3 islets for h and i, respectively). Data presented as mean ± S.E. (c), median ± interquartile range (d) or mean ± S.D. (f, h, i). Confocal images are shown as maximum intensity projections of z-stacks (b, g) or as single planes (h, i). Scale bar represents 50 μm (b, g–i) or 250 μm (e). **p < 0·01; ****p < 0·0001.
Table 1.
Comparative transduction efficiency and viability per virus concentration in mouse pseudoislets. Transduction efficiency and apoptosis was quantified as R-GECO1 or Annexin V positive voxels, respectively, and presented as percentage of islet volume (n = 5–9).
| Virus concentration [×106 particles/ml] | R-GECO1 positive voxels [% of islet volume] | Islet cell apoptosis [% of islet volume] | Islet volume [×106 μm3] |
|---|---|---|---|
| 0·2 | 2·2 ± 0·2 | 2·6 ± 0·7 | 1·41 ± 0·03 |
| 1 | 12·7 ± 2·0 | 3·2 ± 0·7 | 1·36 ± 0·07 |
| 5 | 24·3 ± 4·0 | 7·2 ± 0·6 | 1·24 ± 0·06 |
| 25 | 35·2 ± 8·1 | 15·9 ± 1·7 | 0·60 ± 0·06 |
To assess beta cell function in transduced pseudoislets, we next turned to islets from the Ins-GCaMP3 transgenic mouse, expressing a fluorescent reporter for [Ca2+]i in its islet beta cells. By similarly preparing R-GECO1-expressing pseudoislets from Ins-GCaMP3 islets we obtained two spectrally distinct biosensors for beta cell function that could be cross-validated, allowing to ensure a proper beta cell signalling upon stimulation (Fig. 4g–i). Activation of Ins-GCaMP3 pseudoislets transduced with R-GECO1 could be efficiently measured, with a 2- or 3-fold increase in fluorescent intensity upon stimulation with high glucose in GCaMP3 positive cells or R-GECO1 positive cells, respectively (Fig. 4h, i). Together, these results demonstrate that transduced pseudoislets maintain their function in terms of glucose-induced beta cell signalling and thus that our methodology for synthetic expression is not detrimental to the function of beta cells.
3.4. Synthetic expression of the V1b GPCR in beta cells as a proof-of-concept for the modulation of islet signalling
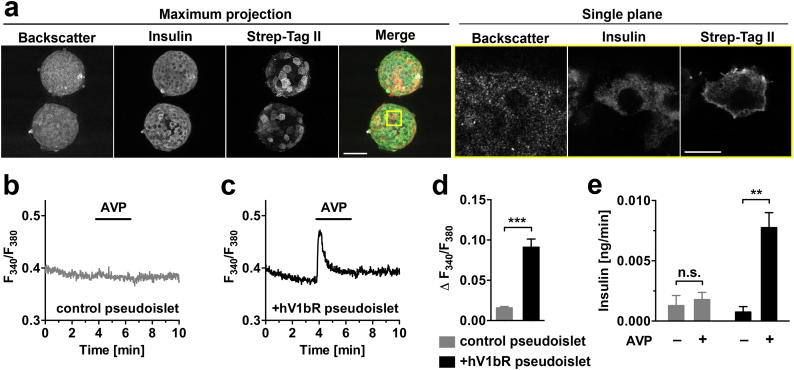
To investigate the effects of changes in gene expression levels on islet function, pseudoislets were engineered for an increased expression of a specific GPCR in beta cells. As an example, we aimed at amplifying the signalling pathway of the vasopressin V1b receptor by increasing its expression level in vitro (Fig. 5). The optimized gene transfer methodology led to a beta cell-specific expression of the receptor, which appeared to be properly located at the cell membrane (Fig. 5a). The proper functioning of this synthetically expressed receptor was confirmed by functional experiments, demonstrating an increase in beta cell [Ca2+]i as well as in islet insulin release upon AVP stimulation, as compared to non-modified pseudoislets (Fig. 5b–e). Similarly as for native islets endogenously expressing the V1b receptor [23,30,31], insulin release from engineered pseudoislets upon AVP stimulation was glucose dependent, the V1b receptor signalling contributing to a greater extent to insulin release at 7 mM glucose as compared to 3 mM glucose (Supplementary Fig. 7).
Fig. 5.
Equipping beta cells with the human V1b receptor allows for specific [Ca2+]i signalling in pseudoislets in vitro. (a) Mouse islet cells were transduced for 7 days with an adenovirus encoding for the human V1b receptor (hV1bR) during reaggregation into pseudoislets. After fixation, pseudoislets were co-stained by immunohistochemistry to visualize beta cells and localization of the synthetically expressed receptor. Representative confocal images show an overview of two pseudoislets as maximum intensity projection, and as single optical plane at higher magnification (corresponding region marked with yellow square). The receptor was expressed in beta cells, with a main localization at the plasma membrane. (b–e) Functional responses of non-fixed pseudoislets to AVP stimulation. (b) Non-transduced pseudoislets have a low background level of vasopressin-induced [Ca2+]i increase. (c) The overexpression of hV1bR in pseudoislet beta cells leads to AVP-induced activation of the receptor and rapid [Ca2+]i increase. (d) Quantitative comparison of responses to AVP in terms of [Ca2+]i mobilization demonstrates an increased activation of the hV1bR signalling pathway in transduced as compared to non-transduced pseudoislets (n = 5–7). (e) Groups of pseudoislets were incubated under static conditions in a buffered solution containing 3 mM glucose with or without AVP. Pseudoislets overexpressing the hV1bR responded to AVP with an increase in insulin release, while control pseudoislets showed no response (n = 3, groups of 20 islets). Scale bars = 50 μm and 10 μm for maximum intensity projection and single plane images, respectively. Data presented as mean ± S.E. **p < 0·01; ***p < 0·001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With the aim of investigating the possibility to modify islet function in vivo we increased the expression level of the V1b receptor in Ins-GCaMP3 pseudoislets prior to transplantation into the anterior chamber of the mouse eye, leading to their increased response to AVP in vitro (Supplementary Fig. 8). Intravital microscopy was performed one month after transplantation, a time point at which the pseudoislets were properly vascularized (Fig. 6a–e). V1b receptor expression was still present in several beta cells within the pseudoislet graft, although there was an approximate two-fold reduction in the number of overexpressing beta cells one month after transplantation (Supplementary Fig. 9). Online monitoring of beta cell function enabled us to show that the V1b receptor could be activated in vivo, giving a robust response to intravenous administration of vasopressin (Fig. 6f–h, Supplementary Fig. 10, Supplementary Movie 2). Interestingly, the first response due to the acute activation of the beta cell expressed V1b receptors was followed by a secondary increase in [Ca2+]i oscillations. The latter was due to the progressive increase in blood glucose levels (Fig. 6i), caused by the stimulation of hepatic glycogen breakdown upon vasopressin administration [32]. Although both primary and secondary responses could be seen in all [Ca2+]i traces in vivo, the first responses exhibited a wide range of amplitudes among the individual mice, hindering a precise comparison between overexpressing versus non-modified pseudoislets under in vivo conditions (Supplementary Fig. 10).
Fig. 6.
Pseudoislets expressing the human V1b receptor elicit [Ca2+]i responses upon stimulation with vasopressin in vivo. (a–e) Pseudoislets expressing the [Ca2+]i sensitive fluorescent biosensor GCaMP3 and engineered to overexpress the human V1b receptor were transplanted into the anterior chamber of the mouse eye, and imaged in vivo by confocal microscopy one month after transplantation. Intravenous injection of dextran-TR confirmed that the pseudoislet was vascularized. (f-h) In vivo imaging of [Ca2+]i in pseudoislet beta cells upon V1b receptor activation was performed ~4 weeks after transplantation. After imaging baseline intensity for 5 min, mice were injected intravenously with a solution containing 2 μM AVP in saline, and islets were imaged for an additional 10 min. Snapshot images from the recording (f–g) display fluorescence intensity before and after stimulation with AVP. The corresponding [Ca2+]i trace (h) shows an immediate response upon AVP administration, followed by a secondary indirect response due to increase in blood glucose levels. (i) Blood glucose levels before injection and at the end of the imaging session demonstrate the effect of AVP on glucose release from the liver in vivo. Trace and images are representative, n = 8 mice (in vivo Ca2+ imaging), and 14 mice (blood glucose measurements). Images show maximum intensity projections (b–e) and average intensity projections (f–g) of single pseudoislet grafts. Scale bar = 50 μm. ****p < 0·0001.
In combination with our in vitro results we could nevertheless show – by the use of our synthetic expression methodology – that islet function can be in principle modulated by targeted overexpression of specific GPCRs in beta cells, concomitantly maintaining normal responses to variations in blood glucose levels.
4. Discussion
Transplantation of pancreatic islets has the potential to provide a fine and continuous control of glucose levels in diabetic patients, however the success of this treatment strategy is challenged by the limited survival rate of the grafts and suboptimal functional quality of donor islets. In this study, we have developed a beta cell specific synthetic expression methodology that can be used to address these challenges. Ex vivo genetic modification of islets pre-transplantation has previously been shown to potentially improve islet function [33], or attenuate immune-mediated destruction [34] and hypoxia-related apoptosis [35] following transplantation. In an attempt to target an increased percentage of cells within the islet and enhance gene delivery, we made use of a hanging droplet system that facilitates reaggregation of dispersed islet cells [36,37]. During the reaggregation phase islet cells were incubated in the presence of an adenovirus to genetically modify the produced pseudoislets. We found that transduction efficiency can be improved while a relatively small amount of virus is needed.
While native rodent islets consist of a beta cell core surrounded by a mantle of alpha and delta cells [38,39], their dissociation into single cells followed by reaggregation into pseudoislets should in principle lead to a random spatial rearrangement of individual islet cells. Previous work on rat pseudoislets reported however that their cellular architecture was similar to that of native islets [36,[40], [41], [42], [43]], i.e. alpha cells were predominantly located in the periphery. These studies thus suggest that randomly positioned endocrine cells are subject to a spatial reorganization during or following the reaggregation procedure. In contrast, mouse pseudoislets in our present study displayed more alpha cells in the core as compared to native isolated islets, although we did not investigate whether further rearrangement could occur over a longer period of time in culture. Considering the total content in different endocrine cell types, we found that the density of alpha and delta cells within mouse pseudoislets was not different to that of native islets. Previous studies have shown either a reduction in the number of alpha and delta cell in rat pseudoislets [44], or a maintenance of that ratio [43], a discrepancy probably originating from different experimental protocols.
When transplanted into the anterior chamber of the mouse eye, pseudoislets displayed the same content in alpha and delta cells as transplanted native islets, previously shown to be reflective of in situ pancreatic islets in terms of ratio in different endocrine cell types [17]. Interestingly, one month after transplantation native islet grafts had an architecture resembling that of pseudoislet grafts marked by the presence of alpha cells in their core region, inferring a rearrangement of endocrine cells in vivo after transplantation. This has previously been documented in the case of native mouse islets engrafted into the anterior chamber of the eye [17], and in the present case results in a reduction of the architectural difference observed between native islets and pseudoislets in vitro prior to transplantation. Other studies have similarly shown that a rearrangement of alpha and beta cells occurs in vivo in human pseudoislets [45] and rat islet cell sheets [46] after transplantation into mice.
In accordance with previous studies [40,41,47,48], our data demonstrate that stimulation of mouse pseudoislets with glucose in vitro results in a cellular increase in [Ca2+]i and subsequent insulin release comparable to native islets. More detailed analyses revealed that pseudoislets display a faster response time in terms of glucose induced [Ca2+]i increase in vitro as well as augmented insulin release during static incubations at low and high glucose. The three-dimensional cytoarchitecture of the pancreatic islet has been shown to be important for its function [38,41,47], which might explain the slight differences observed in vitro between native islets and pseudoislets. Since glucagon has a potentiating effect on insulin release [[49], [50], [51], [52]], the increased interaction between alpha and beta cells within pseudoislets obtained by the intermingling of their different endocrine cell types could thus be accountable for a stronger insulin release. A similar improvement in insulin release has previously been observed by dynamic perifusion of rat pseudoislets [44], corroborating our present findings on mouse pseudoislets, and confirming their functional capacity in vitro.
Further investigating pseudoislet function under in vivo conditions, we found that pseudoislets transplanted into the anterior chamber of the eye engrafted properly and intra-pseudoislet blood vessels formed fenestrations similar to in situ pancreatic or transplanted native islets, a prerequisite allowing for proper exchange between endocrine cells and factors in the blood circulation. In contrast to in vitro experiments, we could not observe differences in glucose-induced beta cell [Ca2+]i response between native islet grafts and pseudoislet grafts, both showing similar [Ca2+]i pattern and timing of response. We next investigated the ability of engrafted islets to secrete insulin and contribute to blood glucose homeostasis in mice. Hyperglycaemia was induced by chemical ablation of in situ pancreatic beta cells. Although STZ-induced diabetes could be reverted within a month by transplantation of ~100 pseudoislets, we and others have previously observed that the transplantation of a comparable number of native mouse islets is able to normalize glycemia already within two weeks [53]. This is in line with the findings of Beger and colleagues, who compared pseudoislets (diameter 100–150 μm) and native islets following transplantation into the dorsal skinfold of Syrian hamsters, and showed a delayed revascularization in the case of pseudoislets [54]. The lack of intra-islet endothelial cells after prolonged (pseudo)islet culture [55] infers that all blood vessels need to originate from the host, which could explain the longer time necessary for complete revascularization.
The efficiency by which targeted islet cells express proteins of interest is an important factor determining the success of gene therapy. Most experiments involving the modification of islet cells are performed in a timeframe of several hours in presence of a high concentration of virus particles [34,[56], [57], [58]]. Viruses can negatively influence islet cell viability and function and therefore a thorough optimization of transduction protocols is essential. Previous studies have indeed shown a reduced beta cell function in virus-infected rat pseudoislets [59,60]. Our protocol exposed the islet cells to the virus preparation for a duration of 6–7 days, which is a relatively long period. This allowed us to use substantially lower virus concentrations compared to commonly used protocols. Despite the fact that stronger transduction efficiencies can be reached with higher virus titres, it proved to come at the cost of increased islet cell death. We took into account both positive and negative aspects to optimize our protocol and succeeded in expressing heterologous proteins while maintaining normal beta cell function and viability.
As a proof-of-concept for the regulation of pseudoislet function in vitro and in vivo, we increased the expression level of the vasopressin V1b receptor, a GPCR that is endogenously expressed at low levels in mouse beta cells [20,21]. The activation of this receptor by the natural ligand AVP revealed a potentiating effect on insulin secretion in a glucose dependent manner, exhibiting a greater effect at higher glucose concentrations in mouse islets as shown in other studies [23,30,31]. Our in vitro data demonstrate that AVP stimulation of beta cells overexpressing the V1b receptor leads to an increase in islet [Ca2+]i at 3 mM glucose, supporting evidence for a Gαq driven signalling pathway [30].
To guarantee long term application in vivo, stable expression of the transgene is essential. Our results show that the synthetically expressed V1b receptors are still present in our engineered pseudoislets one month after transplantation, allowing in principle to enhance [Ca2+]i mobilization upon AVP administration. The fact that this ligand is activating V1b receptors in the liver as well, promoting glycogenolysis and gluconeogenesis and leading to a subsequent increase in blood glucose concentration [32], permitted us to confirm that our pseudoislet grafts also responded properly to varying levels of glucose and thus maintained their primary role in the regulation of blood glucose homeostasis.
Contrary to our in vitro experiments, we could not demonstrate that the [Ca2+]i response of V1b receptor overexpressing pseudoislets was improved as compared to non-modified pseudoislets under in vivo conditions. Ex vivo immunostainings on pseudoislet grafts demonstrated that the synthetic expression of V1b receptor was maintained at least until the time of imaging, with approximately 30% of beta cells still expressing the receptor. The activation of these cells should lead to an enhanced response to AVP, and at the pseudoislet level this response should be further supported by beta cell connectivity [61]. Due to the complexity of in vivo conditions however, several additional factors could have influenced the beneficial outcome of V1b receptor overexpression. For instance the internalization of the V1b receptor [62] or the dependence of V1b receptor activation-driven [Ca2+]i increase on blood glucose levels [30] could be responsible for the large variability we observed among pseudoislet responses under in vivo conditions. Since the synthetic expression of the V1b receptor was still present at the time of imaging, our in vivo assessment approach allowed to comprehensibly determine the potential benefit of this specific genetic modification for islet graft function. The combination of our optimized methodology for genetic modification of islet cells with our in vivo transplantation and imaging approach might therefore serve as a useful strategy to evaluate the translational relevance of various islet modifications to improve the outcome of future clinical transplantations.
Since AVP is not only a ligand for the V1b receptor in islet cells but also has a number of physiological functions in other organs [23], the combined use of this ligand together with engineered pseudoislets does not represent a valid strategy for the treatment of insulin-dependent diabetes. The V1b receptor has been selected here solely as a proof-of-concept for evaluating the potential to regulate islet function in vivo by synthetic engineering, boosting a signalling pathway that is naturally present in beta cells. Ideally, a custom-designed ligand is needed to ensure specific activation of the receptor. One could also use an approach involving custom-designed ligands in combination with a designer GPCR such as presented by Guettier et al. [63]. In addition to enhancing existing signalling pathways or establishing novel synthetic pathways for the purpose of increased insulin secretion, pseudoislets should simultaneously be engineered for a protection against immune challenge and apoptosis [34,35]. In parallel, the long-term expression of constructs of interest could be further improved by the use of other viral vectors for gene delivery, such as lentiviruses [64], or by the implementation of CRISPR/Cas9 technology for precise gene insertion into the cellular genome [65,66]. The fine-tuning of specific gene expression levels should also be optimized, as strong overexpression can overload biological pathways and exhaust cellular resources for protein production and transport [67,68], thus proving detrimental to pseudoislet function. Finally, the combination of novel self-renewable sources of islet cells derived from stem cells [69] with such improved genetic engineering approaches to regulate cellular function could potentially offer high quality material for regenerative medicine in diabetes.
In summary, we present a methodology that enables the synthetic regulation of gene expression in specific islet cells, as well as different means – in vitro and in vivo – to assess the ensuing functional outcome. This methodology can be directly applied to functional studies on genetically engineered human pseudoislets designed to improve the efficiency of clinical islet transplantations, which will provide a possible paradigm shift with regard to future strategies for the treatment of diabetes.
The following are the supplementary data related to this article.
Supplementary Figures 1-10
In vivo [Ca2+]iimaging movie. Islets expressing the [Ca2+]i sensitive fluorescent biosensor GCaMP3 in beta cells were transplanted into the anterior chamber of the mouse eye, and imaged one month after transplantation. After a 5 min baseline intensity imaging, mice were injected intravenously with a solution containing 5% glucose and islets were imaged for an additional 10 min. Intravenous injections were done with injected volumes of 4 μl per g mouse. Movies are 20× accelerated and show average intensity projections of single islets.
In vivo [Ca2+]iimaging movie. Islets expressing the [Ca2+]i sensitive fluorescent biosensor GCaMP3 in beta cells were transplanted into the anterior chamber of the mouse eye, and imaged one month after transplantation. After a 5 min baseline intensity imaging, mice were injected intravenously with a solution containing 2 μM AVP and islets were imaged for an additional 10 min. Intravenous injections were done with injected volumes of 4 μl per g mouse. Movies are 20× accelerated and show average intensity projections of single islets.
Acknowledgments
Acknowledgements
The authors thank Lamia Mebarki for providing a plasmid encoding the human V1b receptor N-terminally tagged with Strep-Tag II sequence, and Kjell Hultenby at Karolinska Institutet's shared facility for electron microscopy for providing TEM images.
Funding sources
Human islets were provided by the Nordic Network for Clinical Islet Transplantation through the JDRF award 31-2008-416 (ECIT Islet for Basic Research program). This study was supported by funding from Karolinska Institutet, the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Diabetes Association, the Family Knut and Alice Wallenberg Foundation, Diabetes Research and Wellness Foundation, the Stichting af Jochnick Foundation, the Family Erling-Persson Foundation, Berth von Kantzow's Foundation, the Skandia Insurance Company, Ltd., ERC-2013-AdG 338936-BetaImage, the European Union's Seventh Framework Programme under grant agreements No 289932 and 613879, and the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology under grant agreement 2015R1D1A1A09057348. Funding sources were not involved in study design, in data collection, analysis and interpretation, and in the writing and decision to submit the manuscript for publication.
Declaration of Competing Interests
Dr. van Krieken, Dr. Voznesenskaya, Dr. Dicker, Dr. Xiong, Dr. Ilegems, and Dr. Berggren report grants from JDRF, Karolinska Institutet, Novo Nordisk Foundation, Swedish Diabetes Association, Family Knut and Alice Wallenberg Foundation, Diabetes Research and Wellness Foundation, Stichting af Jochnick Foundation, Family Erling-Persson Foundation, Berth von Kantzow's Foundation, Skandia Insurance Company, ERC Advanced Grant, EU FP7, during the conduct of the study. Dr. Lee reports grants from National Research Foundation of Korea during the conduct of the study. Dr. Berggren has a patent “Non-invasive in vivo imaging and methods for treating diabetes” issued to BioCrine AB. Per-Olof Berggren is founder and CEO of Biocrine AB. Erwin Ilegems is consultant for Biocrine AB. Dr. Park has nothing to disclose.
Author contributions
E.I., P.-O.B. and J.I.L. designed research; P.v.K., J.I.L., and E.I. optimized pseudoislet engineering methodology; E.I. prepared adenovirus constructs; P.v.K., A.V., E.I., and J.H.P. performed in vitro assessments of islet function; P.v.K., Y.X., and A.D. performed in vivo transplantation and islet graft collection; P.v.K. and E.I. performed immunofluorescence and in vivo imaging experiments; P.-O.B. and A.V. edited the manuscript. P.v.K. and E.I. wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Gupta D., Krueger C.B., Lastra G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr Diabetes Rev. 2012;8:76–83. doi: 10.2174/157339912799424564. [DOI] [PubMed] [Google Scholar]
- 2.Robertson R.P., Harmon J., Tran P.O.T., Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl. 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 4.Winzell M.S., Ahrén B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther. 2007;116:437–448. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Dunér P., Al-Amily I.M., Soni A. Adhesion G protein-coupled receptor G1 (ADGRG1/GPR56) and pancreatic β-cell function. J Clin Endocrinol Metab. 2016;101:4637–4645. doi: 10.1210/jc.2016-1884. [DOI] [PubMed] [Google Scholar]
- 6.Holst J.J., Gromada J., Nauck M.A. The pathogenesis of NIDDM involves a defective expression of the GIP receptor. Diabetologia. 1997;40:984–986. doi: 10.1007/s001250050779. [DOI] [PubMed] [Google Scholar]
- 7.Xu G., Kaneto H., Laybutt D.R. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- 8.de Kort H., de Koning E.J., Rabelink T.J., Bruijn J.A., Bajema I.M. Islet transplantation in type 1 diabetes. BMJ. 2011;342:d217. doi: 10.1136/bmj.d217. [DOI] [PubMed] [Google Scholar]
- 9.Corrêa-Giannella M.L., Raposo do Amaral A.S. Pancreatic islet transplantation. Diabetol Metab Syndr. 2009;1:9. doi: 10.1186/1758-5996-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsgren O., Lundgren T., Felldin M. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia. 2008;51:227–232. doi: 10.1007/s00125-007-0868-9. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson P.-O. Influence of microenvironment on engraftment of transplanted β-cells. Ups J Med Sci. 2011;116:1–7. doi: 10.3109/03009734.2010.548609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kayton N.S., Poffenberger G., Henske J. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am J Physiol Endocrinol Metab. 2015;308:E592–E602. doi: 10.1152/ajpendo.00437.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balamurugan A.N., Naziruddin B., Lockridge A. Islet product characteristics and factors related to successful human islet transplantation from the collaborative islet transplant registry (CITR) 1999-2010. Am J Transplant. 2014;14:2595–2606. doi: 10.1111/ajt.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir G.C., Bonner-Weir S. Scientific and political impediments to successful islet transplantation. Diabetes. 1997;46:1247–1256. doi: 10.2337/diab.46.8.1247. [DOI] [PubMed] [Google Scholar]
- 15.Pileggi A., Fenjves E.S., Klein D., Ricordi C., Pastori R.L. Protecting pancreatic beta-cells. IUBMB Life. 2004;56:387–394. doi: 10.1080/15216540400006469. [DOI] [PubMed] [Google Scholar]
- 16.Leibowitz G., Beattie G.M., Kafri T. Gene transfer to human pancreatic endocrine cells using viral vectors. Diabetes. 1999;48:745–753. doi: 10.2337/diabetes.48.4.745. [DOI] [PubMed] [Google Scholar]
- 17.Speier S., Nyqvist D., Cabrera O. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speier S., Nyqvist D., Köhler M., Caicedo A., Leibiger I.B., Berggren P.-O. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto T., Saito M., Mochizuki S., Watanabe Y., Hashimoto S., Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994;269:27088–27092. [PubMed] [Google Scholar]
- 20.Oshikawa S., Tanoue A., Koshimizu T.-A., Kitagawa Y., Tsujimoto G. Vasopressin stimulates insulin release from islet cells through V1b receptors: a combined pharmacological/knockout approach. Mol Pharmacol. 2004;65:623–629. doi: 10.1124/mol.65.3.623. [DOI] [PubMed] [Google Scholar]
- 21.Richardson S.B., Laya T., VanOoy M. Similarities between hamster pancreatic islet beta (HIT) cell vasopressin receptors and V1b receptors. J Endocrinol. 1995;147:59–65. doi: 10.1677/joe.0.1470059. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara Y., Hiroyama M., Sanbe A. Insulin hypersensitivity in mice lacking the V1b vasopressin receptor. J Physiol (Lond) 2007;584:235–244. doi: 10.1113/jphysiol.2007.136481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koshimizu T.-A., Nakamura K., Egashira N., Hiroyama M., Nonoguchi H., Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 24.Zariwala H.A., Borghuis B.G., Hoogland T.M. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorens B., Tarussio D., Maestro M.A., Rovira M., Heikkilä E., Ferrer J. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia. 2015;58:558–565. doi: 10.1007/s00125-014-3468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D.-S., Yuan Y.-H., Tu H.-J., Liang Q.-L., Dai L.-J. A protocol for islet isolation from mouse pancreas. Nat Protoc. 2009;4:1649–1652. doi: 10.1038/nprot.2009.150. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y., Araki S., Wu J. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molina J., Rodriguez-Diaz R., Fachado A., Jacques-Silva M.C., Berggren P.-O., Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63:2714–2726. doi: 10.2337/db13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilegems E., van Krieken P.P., Edlund P.K. Light scattering as an intrinsic indicator for pancreatic islet cell mass and secretion. Sci Rep. 2015;5 doi: 10.1038/srep10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Z.Y., Drews G., Nenquin M., Plant T.D., Henquin J.C. Mechanisms of the stimulation of insulin release by arginine-vasopressin in normal mouse islets. J Biol Chem. 1990;265:15724–15730. [PubMed] [Google Scholar]
- 31.Abu-Basha E.A., Yibchok-Anun S., Hsu W.H. Glucose dependency of arginine vasopressin-induced insulin and glucagon release from the perfused rat pancreas. Metab Clin Exp. 2002;51:1184–1190. doi: 10.1053/meta.2002.34052. [DOI] [PubMed] [Google Scholar]
- 32.Hems D.A., Whitton P.D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973;136:705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng S., Vatamaniuk M., Lian M.-M. Insulin gene transfer enhances the function of human islet grafts. Diabetologia. 2003;46:386–393. doi: 10.1007/s00125-003-1038-3. [DOI] [PubMed] [Google Scholar]
- 34.Gallichan W.S., Kafri T., Krahl T., Verma I.M., Sarvetnick N. Lentivirus-mediated transduction of islet grafts with interleukin 4 results in sustained gene expression and protection from insulitis. Hum Gene Ther. 1998;9:2717–2726. doi: 10.1089/hum.1998.9.18-2717. [DOI] [PubMed] [Google Scholar]
- 35.Yook S., Jeong J.-H., Jung Y.S. Molecularly engineered islet cell clusters for diabetes mellitus treatment. Cell Transplant. 2012;21:1775–1789. doi: 10.3727/096368912X640628. [DOI] [PubMed] [Google Scholar]
- 36.Cavallari G., Zuellig R.A., Lehmann R., Weber M., Moritz W. Rat pancreatic islet size standardization by the ‘hanging drop’ technique. Transplant Proc. 2007;39:2018–2020. doi: 10.1016/j.transproceed.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Jo Y.H., Nam B.M., Kim B.Y. Pseudoislet of hybrid cellular spheroids from commercial cell lines. Transplant Proc. 2013;45:3113–3117. doi: 10.1016/j.transproceed.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 38.Cabrera O., Berman D., Kenyon N., Ricordi C., Berggren P.-O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2:135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopcroft D.W., Mason D.R., Scott R.S. Insulin secretion from perifused rat pancreatic pseudoislets. In Vitro Cell Dev Biol. 1985;21:421–427. doi: 10.1007/BF02620828. [DOI] [PubMed] [Google Scholar]
- 41.Halban P.A., Powers S.L., George K.L., Bonner-Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes. 1987;36:783–790. doi: 10.2337/diab.36.7.783. [DOI] [PubMed] [Google Scholar]
- 42.Matta S.G., Wobken J.D., Williams F.G., Bauer G.E. Pancreatic islet cell reaggregation systems: efficiency of cell reassociation and endocrine cell topography of rat islet-like aggregates. Pancreas. 1994;9:439–449. [PubMed] [Google Scholar]
- 43.Ichihara Y., Utoh R., Yamada M., Shimizu T., Uchigata Y. Size effect of engineered islets prepared using microfabricated wells on islet cell function and arrangement. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuellig R.A., Cavallari G., Gerber P. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. J Tissue Eng Regen Med. 2017;11:109–120. doi: 10.1002/term.1891. [DOI] [PubMed] [Google Scholar]
- 45.Lavallard V., Armanet M., Parnaud G. Cell rearrangement in transplanted human islets. FASEB J. 2016;30:748–760. doi: 10.1096/fj.15-273805. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu H., Ohashi K., Saito T. Topographical arrangement of α- and β-cells within neo-islet tissues engineered by islet cell sheet transplantation in mice. Transplant Proc. 2013;45:1881–1884. doi: 10.1016/j.transproceed.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Hauge-Evans A.C., Squires P.E., Persaud S.J., Jones P.M. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 1999;48:1402–1408. doi: 10.2337/diabetes.48.7.1402. [DOI] [PubMed] [Google Scholar]
- 48.Hilderink J., Spijker S., Carlotti F. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J Cell Mol Med. 2015;19:1836–1846. doi: 10.1111/jcmm.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crockford P.M., Porte D., Wood F.C., Williams R.H. Effect of glucagon on serum insulin, plasma glucose and free fatty acids in man. Metab Clin Exp. 1966;15:114–122. doi: 10.1016/0026-0495(66)90032-1. [DOI] [PubMed] [Google Scholar]
- 50.Huypens P., Ling Z., Pipeleers D., Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- 51.Wojtusciszyn A., Armanet M., Morel P., Berney T., Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51:1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- 52.Song G., Pacini G., Ahrén B., D'Argenio D.Z. Glucagon increases insulin levels by stimulating insulin secretion without effect on insulin clearance in mice. Peptides. 2017;88:74–79. doi: 10.1016/j.peptides.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mojibian M., Harder B., Hurlburt A., Bruin J.E., Asadi A., Kieffer T.J. Implanted islets in the anterior chamber of the eye are prone to autoimmune attack in a mouse model of diabetes. Diabetologia. 2013;56:2213–2221. doi: 10.1007/s00125-013-3004-z. [DOI] [PubMed] [Google Scholar]
- 54.Beger C., Cirulli V., Vajkoczy P., Halban P.A., Menger M.D. Vascularization of purified pancreatic islet-like cell aggregates (pseudoislets) after syngeneic transplantation. Diabetes. 1998;47:559–565. doi: 10.2337/diabetes.47.4.559. [DOI] [PubMed] [Google Scholar]
- 55.Nyqvist D., Speier S., Rodriguez-Diaz R. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60:2571–2577. doi: 10.2337/db10-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ju Q., Edelstein D., Brendel M.D. Transduction of non-dividing adult human pancreatic beta cells by an integrating lentiviral vector. Diabetologia. 1998;41:736–739. doi: 10.1007/s001250050977. [DOI] [PubMed] [Google Scholar]
- 57.Giannoukakis N., Mi Z., Gambotto A. Infection of intact human islets by a lentiviral vector. Gene Ther. 1999;6:1545–1551. doi: 10.1038/sj.gt.3300996. [DOI] [PubMed] [Google Scholar]
- 58.Csete M.E., Benhamou P.Y., Drazan K.E. Efficient gene transfer to pancreatic islets mediated by adenoviral vectors. Transplantation. 1995;59:263–268. [PubMed] [Google Scholar]
- 59.Caton D., Calabrese A., Mas C. Lentivirus-mediated transduction of connexin cDNAs shows level- and isoform-specific alterations in insulin secretion of primary pancreatic beta-cells. J Cell Sci. 2003;116:2285–2294. doi: 10.1242/jcs.00442. [DOI] [PubMed] [Google Scholar]
- 60.Kim H.J., Alam Z., Hwang J.W. Optimal formation of genetically modified and functional pancreatic islet spheroids by using hanging-drop strategy. Transplant Proc. 2013;45:605–610. doi: 10.1016/j.transproceed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Markovič R., Stožer A., Gosak M., Dolenšek J., Marhl M., Rupnik M.S. Progressive glucose stimulation of islet beta cells reveals a transition from segregated to integrated modular functional connectivity patterns. Sci Rep. 2015;5:7845. doi: 10.1038/srep07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashiwazaki A., Fujiwara Y., Tsuchiya H., Sakai N., Shibata K., Koshimizu T.-A. Subcellular localization and internalization of the vasopressin V1B receptor. Eur J Pharmacol. 2015;765:291–299. doi: 10.1016/j.ejphar.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 63.Guettier J.-M., Gautam D., Scarselli M. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callewaert H., Gysemans C., Cardozo A.K. Cell loss during pseudoislet formation hampers profound improvements in islet lentiviral transduction efficacy for transplantation purposes. Cell Transplant. 2007;16:527–537. doi: 10.3727/000000007783464948. [DOI] [PubMed] [Google Scholar]
- 65.Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9:1911. doi: 10.1038/s41467-018-04252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lino C.A., Harper J.C., Carney J.P., Timlin J.A. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kafri M., Metzl-Raz E., Jona G., Barkai N. The cost of protein production. Cell Rep. 2016;14:22–31. doi: 10.1016/j.celrep.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kintaka R., Makanae K., Moriya H. Cellular growth defects triggered by an overload of protein localization processes. Sci Rep. 2016;6 doi: 10.1038/srep31774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunha J.P.M.C.M., Gysemans C., Gillard P., Mathieu C. Stem-cell-based therapies for improving islet transplantation outcomes in type 1 diabetes. Curr Diabetes Rev. 2018;14:3–13. doi: 10.2174/1573399812666160629094031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-10
In vivo [Ca2+]iimaging movie. Islets expressing the [Ca2+]i sensitive fluorescent biosensor GCaMP3 in beta cells were transplanted into the anterior chamber of the mouse eye, and imaged one month after transplantation. After a 5 min baseline intensity imaging, mice were injected intravenously with a solution containing 5% glucose and islets were imaged for an additional 10 min. Intravenous injections were done with injected volumes of 4 μl per g mouse. Movies are 20× accelerated and show average intensity projections of single islets.
In vivo [Ca2+]iimaging movie. Islets expressing the [Ca2+]i sensitive fluorescent biosensor GCaMP3 in beta cells were transplanted into the anterior chamber of the mouse eye, and imaged one month after transplantation. After a 5 min baseline intensity imaging, mice were injected intravenously with a solution containing 2 μM AVP and islets were imaged for an additional 10 min. Intravenous injections were done with injected volumes of 4 μl per g mouse. Movies are 20× accelerated and show average intensity projections of single islets.