Abstract
Background
The seroprevalence rate of human T‐lymphotropic virus I and II (HTLV‐I/II) in Korean blood donors has been known as 0.004%, and HTLV‐I/II Ab screening test has been performed since 2008 in Korea. Korea Ministry of Food and Drug Safety (MFDS) approved two chemiluminescent microparticle immunoassays (CMIA) for testing HTLV‐I/II antibody, ABBOTT PRISM HTLV‐I/HTLV‐II and ARCHITECT rHTLV‐I/II. A multicenter performance evaluation study in Europe and Japan was carried out with the new electrochemiluminescence immunoassay (ECLIA) for HTLV‐I/II antibody detection, Elecsys HTLV‐I/II assay which launched in 2017, but not in Korea. We aimed to evaluate the clinical performance of Elecsys HTLV‐I/II assay in comparison with ARCHITECT rHTLV‐I/II for the detection of HTLV‐I/II antibody with Korean samples.
Methods
For sensitivity evaluation, 100 HTLV‐I/II‐positive Korean standards from Korean Red Cross and two HTLV‐II‐positive samples that were purchased from Seracure were used. For the specificity, 500 potential donor specimens from Korea University Hospital healthcare center were used. All the samples were simultaneously analyzed by the two HTLV‐I/II assays, Elecsys HTLV‐I/II assay and ARCHITECT rHTLV‐I/II assay.
Results
Elecsys HTLV‐I/II assay and ARCHITECT rHTLV‐I/II assay showed a complete agrement. Elecsys HTLV‐I/II assay showed 100% sensitivity (95% CI: 96.38‐100.0) and specificity (95% CI: 99.26‐100.0).
Conclusions
Elecsys HTLV‐I/II assay is as reliable as ARCHITECT rTHLV‐I/II assay, and can be used as a screening test for HTLV‐I/II in Korea.
Keywords: blood donor screening, clinical performance, HTLV‐I/II, Korean
1. INTRODUCTION
Human T‐lymphotropic virus (HTLV) was first isolated among the human retroviruses in the early 1980s.1 It is estimated that 10‐20 million people are infected with HTLV type I/II around the world.2, 3, 4 HTLV‐I/II infection has been known to be transmitted vertically between mother and child, sexually, or by blood transfusion or needles.5
In cases with blood‐borne infection which is strictly related to contaminated lymphocytes, seroconversion occurs in approximately 40% ~ 60% of the recipients in approximately 51 days.6 Although adult T‐cell leukemia (ATL) and HTLV‐I‐associated myelopathy/tropical spastic paraparesis(HAM/TSP) occur in less than 10% of HTLV‐I carriers infected by contaminated bloods, they are generally serious and often cause the patients disabled.7
Therefore, laboratory screening assays for HTLV‐I/II in blood donors have been extensively implemented in developed countries and regions with high prevalence rate.8 Screening test for HTLV‐I/II has not been considered until a case of HTLV infection due to blood transfusion was identified for the first time in 2006 in Korea9; anti‐HTLV‐I/II screening test using chemiluminescent microparticle immunoassay (CMIA) for blood donors was implemented in 2008. There are four Korea Misitry of Food and Drug Safery (MFDS)‐approved assays as of 2018: Murex HTLV‐I+II (Murex Diagnostics), MP Diagnostics HTLV‐I/II ELISA 4.0 (MP Diagnostics), ABBOTT PRISM HTLV‐I/HTLV‐II (Abbott Laboratories), and ARCHITECT rHTLV‐I/II (Abbott Laboratories). Conformité Européenne ‐marked Elecsys HTLV‐I/II assay (Roche Diagnostics) has launched recently in Asian area. However, no clinical study using Korean specimens has been performed in terms of sensitivity and specificity so far. Therefore, we aim to evaluate the clinical performance of Elecsys HTLV‐I/II assay with proven positive and presumable negative Korean specimens.
2. MATERIALS AND METHODS
2.1. Specimens
This study was conducted at the Department of Laboratory Medicine, Korea University Hospital in Seoul, Korea. The protocol of the study was approved by the institutional review board (IRB: 2017AN0001). For sensitivity evaluation, 100 Korean MFDS‐type standards, which were Western blot‐confirmed anti‐HTLV‐I/II‐positive, were obtained from Korean Red Cross (KRC). Two additional anti‐HTLV‐II‐positive plasma from SeraCare were purchased additionally. For specificity evaluation, leftover serum samples of randomly chosen 500 visitors at Korea University Hospital healthcare center were used. The samples for specificity evaluation had previously been proven as negative for HBs Ag, anti‐HIV, and anti‐HCV. The samples were excluded if there were precipitates or turbidity, or the volume was less than 1 mL.
2.2. Anti‐HTLV‐I/II assays and confirmatory tests
Elecsys HTLV‐I/II assay detects anti‐HTLV‐I/II against the HTLV‐specific recombinant antigens HTLV‑I gp21 and HTLV‑II p24. The test was run on Cobas 8000 modular analyzer (Roche), a fully automated electrochemiluminescence immunoassay (ECLIA) analyzer. ARCHITECT rHTLV‐I/II assay (Abbott Laboratories) was a comparator assay, which used synthetic peptides and recombinant antigens derived from gp46 and gp21 proteins of HTLV‐I/II to capture antibodies. This test was run on ARCHITECT i2000 immunoassay analyzer (Abbott Laboratories), a fully automated CMIA analyzer. All samples were simultaneously tested with the two assays according to the manufacturers’ recommendations. For the specimens with discrepant or positive results by either one of the two, both assays were repeated. Only for the discrepant samples, a confirmatory test using MP Diagnostics HTLV Blot 2.4. (MP Biomedicals) was applied.
2.3. Data analysis
Clinical sensitivity was defined as the percentage of HTLV‐I/II‐positive samples correctly identified as reactive by Elecsys HTLV‐I/II assay and calculated with the following formula: Clinical sensitivity (%) = 100 × [True‐positive number/(True‐positive number + False‐negative number)]. Clinical specificity was defined as the percentage of HTLV‐I/II‐negative samples correctly identified as nonreactive by Elecsys HTLV‐I/II assay and calculated with the following formula: Clinical specificity (%) = 100 × [True‐negative number/(True‐negative number + False‐positive number)]. For the comparison of S/CO value, Deming regression and Pearson correlation coefficient (r) were calculated by MedCalc software version 14.8.1 (MedCalc).
3. RESULTS
A total of 102 anti‐HTLV‐positive samples were tested simultaneously using both anti‐HTLV‐I/II assays. Two of the anti‐HTLV‐positive Korean standards from KRC were excluded from the sensitivity analysis according to the study protocol in which denoted that the comparator assay, ARCHITECT rHTLV‐I/II‐negative specimen, should be excluded for the sensitivity evaluation. Although Elecsys HTLV‐I/II showed positive result (S/CO = 1.05) for one of the two samples, it was not included for the sensitivity calculation. Therefore, data from the 100 samples composed of 98 Korean standards and two commercial HTLV‐II‐positive plasma samples purchased from SeraCare (Milford) were incorporated into the sensitivity calculation.
The clinical sensitivity of Elecsys HTLV‐I/II was 100.00% (n = 100, 95% CI 96.38‐100.00). The correlation of the mean S/CO values obtained by duplicates of 100 positive samples in both assays is shown in Figure 1. Pearson correlation coefficient between the two anti‐HTLV assays was 0.5479.
Figure 1.
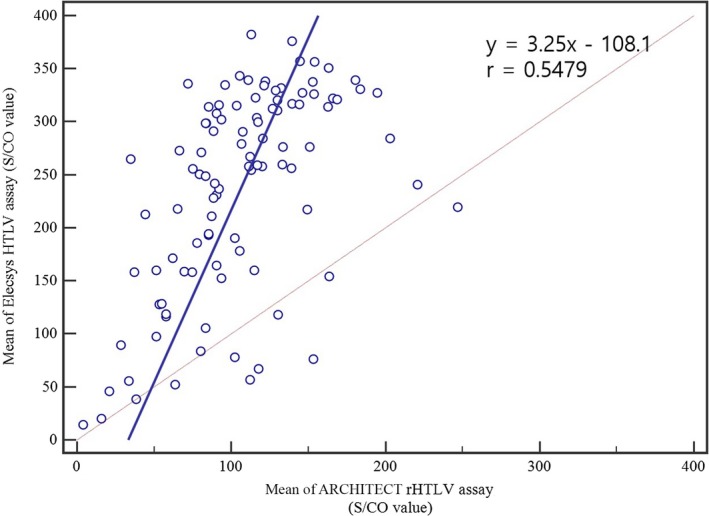
Comparison of the two anti‐HTLV assays, Elecsys HTLV‐I/II and ARCHITECT rHTLV‐I/II, with proven anti‐HTLV‐positive specimens (n = 100)
The clinical specificity of the Elecsys HTLV‐I/II was also 100.00% (n = 500, 95% CI 99.26‐100.00) since all specimens showed nonreactive results (S/CO < 1). The distribution pattern of S/CO values of 500 potential blood donors with the two assays is shown in Figure 2. ARCHITECT rHTLV‐I/II demonstrated a narrower distribution pattern of S/CO values than that of Elecsys HTLV‐I/II (P<0.001).
Figure 2.
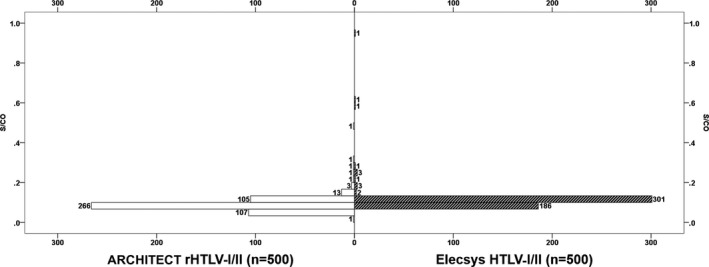
Frequency histogram for the S/CO results obtained from the potential blood donor screening (n = 500). S/CO values (mean ± SD) for Elecsys HTLV‐I/II and ARCHITECT rHTLV‐I/II are 0.107 ± 0.052 and 0.0854 ± 0.033, respectively
The total agreement between Elecsys HTLV‐I/II and ARCHITECT rHTLV‐I/II was 100%.
4. DISCUSSION
This study intended to evaluate the clinical sensitivity and specificity of Elecsys HTLV‐I/II from specimens of Korean population through agreement with ARCHITECT rHTLV‐I/II, which was the popular existing assay. The results of the present study demonstrated that Elecsys HTLV‐I/II assay has a good performance with 100% clinical sensitivity and specificity. The results of Elecsys HTLV‐I/II for a total of 600 specimens showed a perfect agreement with the results of the comparator assay, ARCHITECT rHTLV‐I/II.
These findings correspond well with those reports from the prior multicenter study with the same anti‐HTLV assay.10 In the previous study, clinical sensitivity of Elecsys HTLV‐I/II was demonstrated at 100% (n = 1149) using clinical specimens from Japan, the United States, Europe, and the Middle East. The clinical specificity was 99.95% (n = 11 575) from blood donors and 99.83% (n = 2399) from routine diagnostic samples, including pregnancies and specimens requested for hepatitis, HIV, HSV, EBV, and rubella virus testing. In addition, the performance of Elecsys HTLV‐I/II was equivalent to that of several commercially available anti‐HTLV assays. Since HTLV antibody assays were developed in the mid‐1980s, a third‐generation assay is now available. For the first‐ and second‐generation assays, an indirect format was applied using viral lysate as an antigen. From the third generation, the recombinant proteins and synthetic peptides were used to capture antibodies by double‐antigen sandwich method.11 These technological advances have demonstrated that the previous comparative assessments showed higher sensitivity and specificity with the third generation than with the first and second generation.12, 13, 14
In 2015, the nationwide data of anti‐HTLV prevalence reported by the Korean Red Cross.15 Between 2009 and 2015, a screening test for blood donor was performed with ABBOTT PRISM HTLV‐I/II assay. As a confirmatory test, immunoblot and nucleic acid amplification test were performed simultaneously. Screening positive rate was 0.027%, and the confirmed prevalence rate was 0.0039% (499/ 12,923,854).
The limitation of this study was a low sample size for the specificity evaluation. It would have been great if we could collect more samples because the HTLV prevalence was very low in Korea. This might result in overestimation of the specificity of Elecsys HTLV‐I/II assay.
Overall, the results of comparing the Elecsys HTLV‐I/II with the ARCHITECT rHTLV‐I/II showed a total agreement of 100.00%, and the clinical sensitivity and specificity of Elecsys HTLV‐I/II both came to 100.00%, suggesting the assay's excellent performance. Since Elecsys HTLV‐I/II showed the excellent performance as a screening assay for anti‐HTLV with Korean samples, Elecsys HTLV‐I/II can be considered as a reliable anti‐HTLV assay that can be used in a low‐HTLV seroprevalence setting.
ACKNOWLEDGMENT
Roche Korea supported this study through financial, instrumentation, and reagent contribution. The funding organization played no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the report for publication. The authors would like to acknowledge the technical support provided by Hyuk‐Jun Kim who performed the tests at Korea University Hospital.
Yun SG, Kim S‐W, Sohn JY, Cho Y. Evaluation of Elecsys HTLV‐I/II assay in comparison with ARCHITECT rHTLV‐I/II assay with Korean samples. J Clin Lab Anal. 2019;33:e22909 10.1002/jcla.22909
REFERENCES
- 1. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T‐cell lymphoma. Proc Natl Acad Sci USA. 1980;77(12):7415‐7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall WW, Takahashi H, Liu C, et al. Multiple isolates and characteristics of human T‐cell leukemia virus type II. J Virol. 1992;66(4):2456‐2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cleghorn FR, Manns A, Falk R, et al. Effect of human T‐lymphotropic virus type I infection on non‐Hodgkin's lymphoma incidence. J Natl Cancer Inst. 1995;87(13):1009‐1014. [DOI] [PubMed] [Google Scholar]
- 4. de The G, Bomford R. An HTLV‐I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9(5):381‐386. [DOI] [PubMed] [Google Scholar]
- 5. Goncalves DU, Proietti FA, Ribas JG, et al. Epidemiology, treatment, and prevention of human T‐cell leukemia virus type 1‐associated diseases. Clinical Microbiol Rev. 2010;23(3):577‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manns A, Hisada M, La Grenade L. Human T‐lymphotropic virus type I infection. Lancet (London, England). 1999;353(9168):1951‐1958. [DOI] [PubMed] [Google Scholar]
- 7. Cook LB, Elemans M, Rowan AG, Asquith B. HTLV‐1: persistence and pathogenesis. Virology. 2013;435(1):131‐140. [DOI] [PubMed] [Google Scholar]
- 8. Murphy EL. Infection with human T‐lymphotropic virus types‐1 and ‐2 (HTLV‐1 and ‐2): Implications for blood transfusion safety. Transfus Clin Biol. 2016;23(1):13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim TY. The outbreak of transfusion‐transmitted HTLV infection by one blood donor with HTLV in Korea. Vox Sang. 2007;93:116. [Google Scholar]
- 10. Laperche S, Sauleda S, Piron M, et al. Evaluation of sensitivity and specificity performance of Elecsys HTLV‐I/II assay in a multicenter study in Europe and Japan. J Clin Microbiol. 2017;55(7):2180‐2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kapprell HP, Stieler M, Oer M, et al. Evaluation of a new third‐generation ARCHITECT rHTLV‐I/II assay for blood screening and diagnosis. Diagn Microbiol Infect Dis. 2010;67(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 12. Andersson S, Thorstensson R, Ramirez KG, et al. Comparative evaluation of 14 immunoassays for detection of antibodies to the human T‐lymphotropic virus types I and II using panels of sera from Sweden and West Africa. Transfusion. 1999;39(8):845‐851. [DOI] [PubMed] [Google Scholar]
- 13. Andersson S, Gessain A, Taylor GP. Pooling of samples for seroepidemiological surveillance of human T‐cell lymphotropic virus types I and II. Virus Res. 2001;78(1–2):101‐106. [DOI] [PubMed] [Google Scholar]
- 14. Vrielink H, Reesink H, Habibuw M, Schuller M, van der Meer C, Lelie P. Comparison of four HTLV‐I and HTLV‐I + II ELISAs. Vox Sang. 1999;76(3):187‐191. [DOI] [PubMed] [Google Scholar]
- 15. Youn KW, Kang JW, Kwon S‐Y, Oh DJ. Consideration of the Improvement of the Confirmatory Assay for the Anti‐HTLV Positive Blood Donation. Korean J Blood Transfus. 2015;26(3):300‐308. [Google Scholar]


