Abstract
Background
Interleukin‐1 promotes tumor angiogenesis through VEGF production. The interleukin‐1 receptor antagonist can suppress tumors by blocking this effect.
Methods
Immunohistochemistry, WB, and gene sequencing were used to analyze the expression of IL‐1RA in esophageal cancer patients. WB was used to detect the expression of IL‐1RA and interleukin‐1α in esophageal cancer cells. Stable ESCC cell models overexpressing the IL‐1RA were constructed. Their cell functions were tested, and their effects on VEGF were examined.
Results
IL‐1RA is downregulated in primary EC tumors, and this downregulation of IL‐1RA is closely related to TNM staging and survival prognosis. The overexpression of IL‐1RA increased the proliferation of KYSE410 EC cells, which have a high level of IL‐1α expression. Overexpression of IL‐1RA in KYSE410 cells promotes a decrease in the expression of VEGF‐A. However, IL‐1RA expression did not cause any changes in EC9706 cells with low IL‐1α expression.
Conclusion
IL‐1RA acts as a tumor suppressor, and its deletion promotes tumor progression by increasing VEGF‐A expression in ESCC.
Keywords: esophageal cancer, IL‐1RA, VEGF
Acronyms
- EC
esophageal cancer
- ESCC
esophageal cancer cells
- IL‐1RA
interleukin‐1 receptor antagonist
- IL‐1RN
interleukin‐1 receptor antagonist gene
- IL‐1α
interleukin‐1α
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Esophageal cancer is the seventh most common form of malignant cancer worldwide and the sixth most common cause of cancer‐related death. Notable forms of esophageal cancer include adenocarcinoma of the esophagus and esophageal squamous cell carcinoma (ESCC).1, 2 Although current treatments, such as surgery and chemotherapy, have progressed esophageal cancer treatment, the 5‐year overall survival rate of ESCC is still low (20%‐30%) due to metastasis, recurrence, and resistance to chemoradiotherapy.3 Furthermore, the early symptoms and signs of esophageal cancer are difficult to detect; therefore, the disease is usually identified in the middle and late stages of pathogenesis. Subsequently, distant metastasis may occur, leading to poor prognosis.4, 5 Thus, exploring the mechanism of ESCC progression is critical to improve diagnosis, treatment, and prognosis.
Interleukin‐1 (IL‐1), a pro‐inflammatory chemokine, can promote the proliferation and differentiation of tumor cells via interactions with specific receptors on tumor cell membrane surfaces.6 IL‐1RA, IL‐1α, and IL‐1β are all members of the interleukin‐1 family. IL‐1α and IL‐1β bind to the same receptor and perform similar biological activities, such as promoting tumor growth and metastasis via enhanced angiogenesis. This activity is performed by the regulation of the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF).7 IL‐1RA is a pan‐physiological inhibitor of IL‐1 that prevents the activation of IL‐1 receptors by inhibiting IL‐1 binding to its receptors. IL‐1 RA is a glycosylated protein of 23‐25 KDa. Cloned IL‐1RA DNA contains an open reading frame encoding 277 amino acids, including 25 N‐terminal amino acids present in the pro‐protein and the 152 amino acids present in the mature protein. The molecular weight is 17 115 Daltons, and the isoelectric point is pH 5.2. IL‐1RA has been connected with a variety of diseases, including cardiovascular disease, type II diabetes, carcinoma, and joint diseases.8, 9, 10 IL‐1RA can inhibit angiogenesis by blocking the activation of the IL‐1α/PI3K/NF‐κB pathway in IL‐1‐producing human colon cancer cells.11 Downregulation of IL‐1RA is correlated with the metastatic potential of gastric cancer through blockage of the IL‐1α/VEGF signaling pathways.12 IL‐1RN was confirmed to be downregulated in ESCC.13 However, the mechanism and function of IL‐1RA remains unclear.
2. MATERIALS AND METHODS
2.1. Clinical samples
2.1.1. Acquisition of clinical samples and immunohistochemical analysis
Human esophageal cancer samples and homologous esophageal tissues were collected immediately after surgical resection at Union Hospital of Fujian Medical University (Fuzhou, China) from 2009 to 2011. All samples were immediately stored at −80°C for future paraffin embedding. Clinical staging was determined according to the American Joint Committee on Cancer (AJCC) eighth edition of EC TNM staging. Immunohistochemical staining with a rabbit anti‐IL‐1RA antibody (1:3200, Abcam, ab124692) was performed on formalin‐fixed, paraffin‐embedded tissue sections of 4‐5 µm, which were cut from tissue microarrays (TMAs) and placed on glass slides. A 5‐tiered scale was used to assess the degree of nuclear or cytoplasmic staining based on the average percentage of positive staining cells (0, ≤1% positive cells; 1, 1%‐10% positive cells; 2, 11%‐50% positive cells; 3, 51%‐80% positive cells; and 4, ≥80% positive cells), which was multiplied by the staining intensity (0, no staining; 1, weak staining, light yellow; 2, moderate staining, yellow‐brown; and 3, strong staining, brown) to obtain a score ranging from 0 to 12. A score <3.5 was considered as low IL‐1RA expression, while a score >3.5 was considered as high IL‐1RA expression. All of the tissues were analyzed by two pathologists.
2.1.2. Western blot analysis
Tissues or cells were lysed in Western & IP cell lysis buffer (Beyotime) with PMSF (Amresco) for 30 minutes on ice at 4°C followed by centrifugation at 12 000 × g for 15 minutes at 4°C. The supernatants were collected, and the total protein concentration was measured using the BCA Protein Assay Kit (Thermo Scientific). Equimolar amounts of protein were loaded into each well and separated with 12% SDS‐PAGE. Then, proteins were transferred to a 0.45‐μm PVDF membrane (Amersham Hybond, GE Healthcare), which was blocked in 2% bovine serum albumin (Amresco) prior to overnight incubation overnight at 4°C with the following primary antibodies: rabbit anti‐IL‐1RA, rabbit anti‐IL‐1α (1:1000), mouse anti‐β‐actin (1:2000; Cell Signaling Technology), and VEGF‐A polyclonal antibody (1:1000, A41552). After three washes in TBST buffer lasting 10 minutes per wash, the membrane was incubated with secondary antibodies for 1 hour at room temperature. The blots were developed using enhanced chemiluminescence (Lulong Biotech).
2.1.3. 2.1.3 RNA extraction and real‐time quantitative PCR
Total RNA was extracted from cultured cells or frozen tissues using TRIzol reagent (Ambion), and 1 mg RNA was reverse transcribed using miScript Reverse Transcription Kit (Qiagen) for first complementary DNA strand synthesis. Quantitative PCR was performed using SYBR Premix EX Taq kit (Takara). Specific primers were used to detect the relative mRNA expression of IL‐1RA by the 2‐△△ Ct method. The expression level was normalized against endogenous GAPDH. All the primers were designed by BioSune Biotechnology Co., Ltd. (Shanghai).
2.2. Cell lines
Human EC cell lines KYSE410 and EC9706 were purchased from Hunan Fenghbio Biological Ltd, China. The cells were grown in RPMI‐DMEM (Gibco) medium supplemented with 10% FBS (Gibco) and incubated at 37°C in an atmosphere of 5% CO2.
2.2.1. Plasmids and generation of stable ec cell lines
The opening reading frame of the human IL‐1RA gene was PCR‐amplified and cloned into the lentivirus expression vector pCDH‐CMV‐MCS‐EF1‐RFP‐Puro (System Biosciences). Either a recombinant plasmid or an empty vector was co‐transfected with packaging plasmids pMDL, pVSVG, and pRev into 293T cells. The supernatants were collected at 48 hours post‐transfection and used to infect KYSE410 and EC9706 cells cultured in 6‐cm dishes. Puromycin‐resistant clones were expanded into cell lines as IL‐1RA overexpressing cells (KYSE410‐PIL‐1RA or EC9706‐IL‐1RA) or empty control cells (KYSE410‐pCDH or EC9706‐pCDH). The protein expression levels of IL‐1RA were evaluated by Western blot analysis.
2.2.2. Wound‐healing assay
Transfected cells were grown to 100% confluence in six‐well plates. The cell layers were scratched using a 20‐μL tip to form wound gaps, washed three times with phosphate‐buffered saline (PBS), and photographed at different time points. The cells were counted using a scale label to determine movement activity away from the original scratch location 48 hours after knockdown.
2.2.3. Cell invasion assay
Cell invasion assays were performed using transwell membranes coated with Matrigel (NY, USA). Transfected cells were plated at a density of 5 × 105 cells/well in the upper chamber with a serum‐free medium. FBS (10%) was added to the lower chamber as a chemoattractant. After 48 hours of incubation, the cells were stained with crystal violet for 5‐10 minutes. Finally, the invading cells were counted in five microscopic fields (200 × magnification).
2.2.4. Colony formation assay
Stably transfected cells were harvested and seeded in six‐well plates at a density of 1 × 103 cells/well. After 2 weeks, the cells were fixed in 3% methanol for 30 minutes and stained with 1% crystal violet for 10 minutes. The number of visible colonies was then counted using a phase contrast microscope.
2.2.5. Cell proliferation assay
Cells in the logarithmic growth phase were planted in 96‐well plates at a density of 4 × 103 cells/well. The next day, a Cell Counting Kit‐8 (CCK‐8; Donjido, Kumamoto, Japan) was used according to the manufacturer's instructions. The optical density (OD) was detected using a microplate reader (BioTek, VT, USA) at a fixed time each day for 6 days.
2.3. Statistical analysis
Statistical analysis was performed using SPSS 21.0 for Windows. All data used for the analysis were expressed as the means ± SDs from three independent experiments. The association between IL‐1RA expression and the clinicopathological parameters was analyzed using Pearson's chi‐square test and Spearman's rank‐order correlation. Survival curves were plotted using Kaplan‐Meier analysis. Differences were considered significant when P‐values were <0.05.
3. RESULTS
3.1. Downregulation of IL‐1RA in EC is correlated with poor prognosis
Seventy‐six tumor samples were analyzed by immunohistochemistry (IHC) to detect the prognostic value of IL‐1RA expression. As shown in representative Figure 1 and Table 1, IL‐1RA expression was significantly lower in the EC tumors than in the adjacent normal tissues. Western blot analysis and genetic testing also confirmed the expression of both IL‐1RA protein (Figure 1A) and mRNA (Figure 1B) decreased in the esophageal tumor tissues compared with the adjacent normal tissues. Pearson's chi‐square test evaluating IL‐1RA expression and clinicopathological features showed that the level of IL‐1RA expression in EC was associated with clinical staging; low expression of IL‐1RA meant advanced T staging, N staging, and TNM staging (P = 0.011; P = 0.049; P = 0.001, respectively) (Table 1). After confirming that IL‐1RA expression was downregulated in EC (P = 0.003), Kaplan‐Meier analysis was used to investigate the relationship between IL‐1RA expression assessed by IHC and patient outcome. As shown in Figure 1D, patients with low IL‐1RA‐expressing tumors had significantly shorter 5‐year survival than patients with high IL‐1RA‐expressing tumors (P = 0.044). Altogether, these results suggest that IL‐1RA may be a tumor suppressor and that a decrease in IL‐1RA expression can promote the progression of EC.
Figure 1.
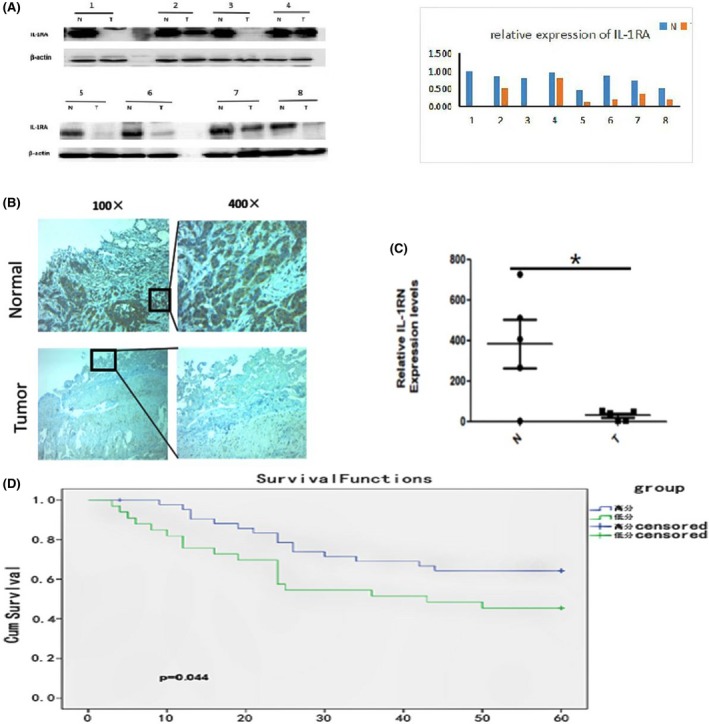
Clinical study: A, The expression of IL‐1RA was detected by Western blotting cancerous and paracancerous tissues from eight groups of patients. The results show that the expression of IL‐1RA in esophageal cancer tissues decreased compared to paracancerous tissue. B, Immunohistochemical staining of 76 tumor samples was performed to investigate the expression of IL‐1RA in paracancerous tissues and adjacent tissues and showed that the expression of IL‐1RA was significantly decreased in esophageal cancer patients (P = 0.000). C, The expression of the IL‐1RN gene in esophageal cancer and adjacent tissues was detected by RT‐PCR. The expression of IL‐1RN gene decreased in esophageal cancer tissues (P = 0.0031). D, Kaplan‐Meier survival analysis showing that downregulation of IL‐1RA in the 76 EC samples was associated with poorer overall survival (P = 0.044)
Table 1.
Clinicopathological characteristics of 76 EC patients according to IL‐1RA
| Item | IL‐1RA | P Valuea | |
|---|---|---|---|
| Normal vs Cancer | Low expression(n = 43) | High expression (n = 33) | |
| Normal | 20 | 56 | 0.000a |
| Cancer | 43 | 33 | |
| Age | |||
| <60 | 24 | 19 | 1.000 |
| ≥60 | 19 | 14 | |
| Gender | |||
| Females | 8 | 7 | 0.780 |
| Males | 35 | 26 | |
| TNM classification | |||
| Ⅰ | 11 | 1 | 0.001a |
| Ⅱ | 19 | 8 | |
| Ⅲ | 13 | 24 | |
| T classification | |||
| T1 | 9 | 2 | 0.011a |
| T2 | 11 | 2 | |
| T3 | 20 | 26 | |
| T4 | 3 | 3 | |
| N classification | |||
| N0 | 19 | 8 | 0.049a |
| N1 | 8 | 15 | |
| N2 | 4 | 16 | |
| N3 | 2 | 4 | |
| Tumor differentiation | |||
| High | 23 | 16 | 0.845 |
| Middle | 13 | 10 | |
| Low | 7 | 7 | |
P value was determined using Pearson's chi‐square test.
3.2. IL‐1RA overexpression suppressed EC cell proliferation
To investigate the biological functions of IL‐1RA in regulating ESCC, we first detected the expression of IL‐1RA and IL‐1α in EC cell lines (Figure 2A). Then, we used gene overexpression strategies to specifically increase the amount of IL‐1RA protein in the EC cell lines KYSE410 and EC9706. Stable overexpression of IL‐1RA in these cells was confirmed by Western blot analysis (Figure 2B). The overexpression of IL‐1RA did not produce any changes in the rate of migration of KYSE410 and EC9706 cells in vitro, as evaluated by wound‐healing assay (Figure 2C). Then, we compared the effect of IL‐1RA on cell proliferation using CCK‐8 and colony formation assays. As shown in Figure 2D and Figure 2E, proliferation was suppressed by overexpression of IL‐1RA in KYSE410 cells, which express IL‐1α. No difference was observed in EC9706 cells.
Figure 2.
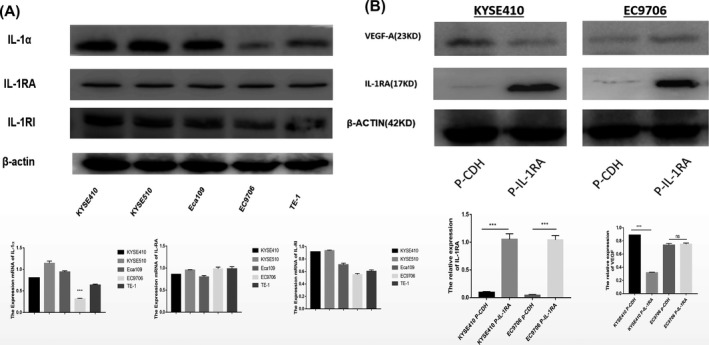
Cell study A, Western blotting (WB) detected the expression of IL‐1RA and IL‐1α in different esophageal squamous carcinoma cells. IL‐1RA is expressed in all esophageal squamous carcinoma cells, and IL‐1α is expressed at low levels in EC9706 cells. B, WB verified cells stably overexpressing IL‐1RA in esophageal squamous cell carcinoma. Changes in VEGF‐A expression were observed to correlate with the change in IL‐1RA expression. The results showed that for KYSE410 cells, the expression of VEGF‐A decreased with an increase in IL‐1RA expression, while for EC9706 cells, this result was not the case. In this case, the expression was not obvious
3.3. IL‐1RA suppressed ESCC proliferation via the IL‐1α/VEGF signaling pathway
To understand the molecular mechanisms underlying IL‐1RA‐induced inhibition of EC proliferation, we assessed the expression of VEGF‐A protein. VEGF‐A protein levels decreased in KYSE410 cells when IL‐1RA was overexpressed, but the VEGF‐A levels did not change in EC9706 cells, possibly due to the low expression of IL‐1α (Figure 2A, 2B). These data imply that IL‐1RA may suppress tumors by regulating the IL‐1α/VEGF signaling pathway in ESCC cells (Figure 3).
Figure 3.
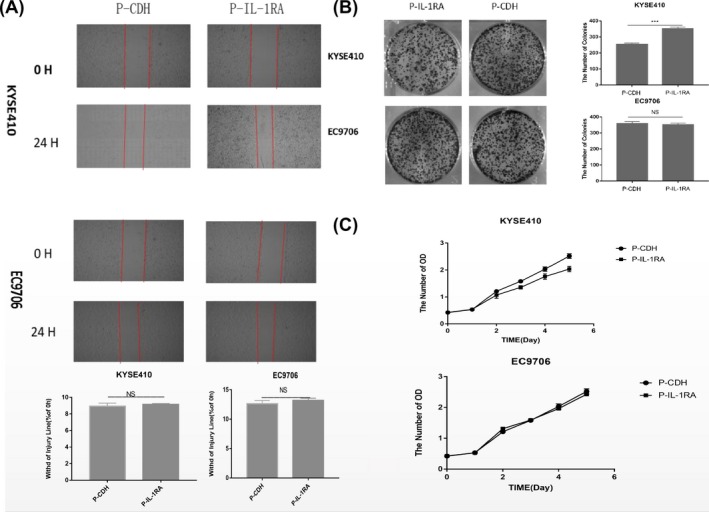
A, Cell scratch test to detect cell migration ability. IL‐1RA expression had no significant effect on cell migration ability. B, Cell plate clone assay to detect cell proliferation ability. IL‐1RA overexpression significantly reduced the proliferation of KYSE410 cells, but no effect was observed for the EC9706 cells. C, CCK‐8 assay to detect cell proliferation function. IL‐1RA overexpression significantly reduced the proliferation of KYSE410 cells (P<0.05), but no effect was observed for EC9706 cells
4. DISCUSSION
The results reported in this study provide evidence that the expression levels of IL‐1RA affect the behavior of EC. IL‐1RA is downregulated in primary EC tumors, and this downregulation of IL‐1RA is associated with advanced tumor stage, grade progression, and worse survival outcomes. In the cell models, the overexpression of IL‐1RA increased the proliferation of KYSE410 EC cells, which have high IL‐1α expression. Furthermore, the overexpression of IL‐1RA in the KYSE410 cells promotes a decrease in the expression of VEGF‐A. However, IL‐1RA does not increase the proliferation and expression of VEGF‐A in EC9706 cells with low IL‐1α expression. These results indicate that IL‐1RA acts as a tumor suppressor, and its deletion promotes tumor progression by increasing VEGF‐A expression.
The development of new blood vessels is a key pathway in the progression of solid tumors. Angiogenesis not only provides the tumor with oxygen and essential nutrients but also promotes the spread and proliferation of cancer cells.14 Angiogenesis is a complex, multi‐step process involving extracellular matrix remodeling, migration and proliferation of endothelial cells, and capillary formation.15 VEGF is the prototypical angiogenic stimulating molecule that has been implicated in several steps of the angiogenic process. Increasing VEGF expression is directly proportional to increasing tumor growth and metastasis by promoting angiogenesis and increasing vascular permeability.16 The role of VEGF in angiogenesis was further confirmed by the effects of hypoxia and several indirect effects of pro‐angiogenic factors, which were able to further increase the synthesis of VEGF.17, 18 It is important that VEGF has also been associated with tumor progression by stimulating angiogenesis in human esophageal carcinoma.19, 20
IL‐1RA is a member of the interleukin‐1 family. In contrast to IL‐1α and IL‐1β, which promote tumor progression through inflammation, IL‐1RA possesses a tumor‐suppressing effect by blocking the binding of IL‐1 to its target receptor [11,12]. Increasing amounts of evidence have shown that the microenvironment of tumors, including inflammation, plays an important role in the development and progression of cancer. Furthermore, inflammatory cytokine–mediated signaling pathways are involved in the malignant transformation of tumor cells.21, 22 In addition, IL‐1 produced by tumor‐associated macrophages (TAMs) is an inflammatory cytokine and can lead to the expression of NF‐κB‐regulated genes, such as VEGF‐A, in chondrosarcoma cells.23, 24 Our study showed that KYSE410 cells have a high expression of IL‐1α; when IL‐1RA was overexpressed, the expression of VEGF‐A, which has been reported to promote angiogenesis, was reduced. The results of CCK‐8 and colony formation assays showed that IL‐1RA inhibits the proliferation of KYSE410 cells. Finally, no difference in VEGF‐A expression or cell proliferation was observed upon overexpression of IL‐1RA in the EC9706 cells, which naturally have low IL‐1α expression. All our experiments indicate that IL‐1RA may inhibit the angiogenesis of EC cells by inhibiting the IL‐1α/VEGF signaling pathway. In addition, anakinra (Kineret), an IL‐1 receptor antagonist that blocks the biologic activity of IL‐1, has been confirmed to be safe to inject into the human body.25, 26 Thus, anakinra has been approved as a clinical drug for the treatment of symptoms of rheumatoid arthritis and hidradenitis suppurative.27, 28 In summary, our study found that IL‐1RA acted as a tumor suppressor in EC via regulation of the IL‐1α/VEGF signaling pathway; therefore, IL‐1RA might serve as a potential molecular target for EC treatments.
Chen S, Shen Z, Liu Z, et al. IL‐1RA suppresses esophageal cancer cell growth by blocking IL‐1α. J Clin Lab Anal. 2019;33:e22903 10.1002/jcla.22903
Sui Chen and Zhimin Shen contributed equally to this work.
Funding information
This study was supported by The Fujian Young Teacher Fund (JAT160209), Sailing Fund of Fujian Medical University (2016QH036), Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant Number: 2017Y9039), Program for Innovative Research Team in Science and Technology at Fujian Province University, and Startup Fund for scientific research at Fujian Medical University (Grant Number: 2017XQ2027).
Contributor Information
Shaobin Yu, Email: 243431476@qq.com.
Mingqiang Kang, Email: mingqiang_kang@126.com.
REFERENCES
- 1. Freddie B, Jacques F, Cancer S et al. Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin, 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Lagergren J. Oesophageal cancer in 2014: advances in curatively intended treatment. Nat Rev Gastroenterol Hepatol. 2015;12(2):74‐75. [DOI] [PubMed] [Google Scholar]
- 3. Li B, Xu WW, Han L, et al. MicroRNA‐377 suppresses initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Oncogene. 2017;36(28):3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933‐7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(13):142‐146. [DOI] [PubMed] [Google Scholar]
- 6. England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of interleukin‐1α or interleukin‐1β depends on mechanism of cell death. J Biol Chem. 2014;289(23):15942‐15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen M, Corless CL, KräLing BM, et al. Vascular expression of E‐selectin is increased in estrogen‐receptor‐ negative breast cancer: a role for tumor‐cell‐secreted interleukin‐1α. Am J Pathol. 1997;150(4):1307‐1314. [PMC free article] [PubMed] [Google Scholar]
- 8. Kathrin M, Pavel S, Jan A, et al. Leptin modulates cell expression of IL‐1 receptor antagonist and release of IL‐1 in human islets. Proc Natl Acad Sci USA. 2004;101(21):8138‐8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bar D, Apte RN, Voronov E, Dinarello CA, Cohen S. A continuous delivery system of IL‐1 receptor antagonist reduces angiogenesis and inhibits Tumor development. Faseb journal official publication of the federation of American societies for. Experimental Biology. 2004;18(1):161‐163. [DOI] [PubMed] [Google Scholar]
- 10. Kay JD, Gouze E, Oligino TJ, et al. Intra‐articular gene delivery and expression of IL‐1Ra mediated by self‐complementary adeno‐associated virus. Journal of Gene Medicine. 2010;11(7):605‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiachi MA, Xiaowen S, Tiankang G, et al. Interleukin‐1 receptor antagonist inhibits angiogenesis via blockage IL‐1α/PI3K/NF‐κβ pathway in human colon cancer cell. Cancer Manag Res. 2017;9:481‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gong Z, Ma J, Su H, et al. Interleukin‐1 receptor antagonist inhibits angiogenesis in gastric cancer. Int J Clin Oncol. 2018;23:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Lu Z, Che Y. Immune signature profiling identified predictive and prognostic factors for esophageal squamous cell carcinoma. Oncoimmunology. 2017;6(11):e1356147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liotta LA, Kohn EC. The microenvironment of the tumour‐host interface. Nature. 2001;411:375‐379. [DOI] [PubMed] [Google Scholar]
- 15. Ma J, Sawai H, Matsuo Y, et al. Interleukin‐1 enhances angiogenesis and is associated with liver metastatic potential in human gastric cancer lines. J Surg Res. 2008;148:197‐204. [DOI] [PubMed] [Google Scholar]
- 16. Yi‐Xuan LI, Pharmacy DO. Experimental study on inhibition effect of plumbagin on the angiogenesis in colon cancer by regulating VEGF/VEGFR2 signaling pathway. Pract Pharm Clin Remedies. 2018;21(7):745‐749. [Google Scholar]
- 17. Wang Y, Jiang B, Ying W et al. Expression and clinical significance of VEGF‐A in esophageal squamous cell carcinoma. Prog Mod Biomed, 2016;15:2956‐3003. [Google Scholar]
- 18. Kong R, Ma Y, Feng J, et al. The crucial role of miR‐126 on suppressing progression of esophageal cancer by targeting VEGF‐A. Cell Mol Biol Lett. 2016;21(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ccf L, Noguti J, Araãºjo L, et al. Expression of VEGF and Cox‐2 in patients with esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2018;19(1):171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kitadai Y, Haruma K, Tokutomi T, et al. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4(9):2195. [PubMed] [Google Scholar]
- 21. Balkwill F,Mantovani A, et al Inflammation and cancer: back to virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 22. Fridman WH, Galon J, Dieunosjean MC, et al. Immune Infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344(1):1. [DOI] [PubMed] [Google Scholar]
- 23. Voronov E, Shouval DS, Krelin Y, et al. IL‐1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100(5):2645‐2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalinski T. Inflammation as molecular target in chondrosarcoma. Pathologe. 2014;35(Suppl 2):249‐253. [DOI] [PubMed] [Google Scholar]
- 25. Cohen SB. The use of anakinra, an interleukin‐1 receptor antagonist, in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30(2):365‐380. [DOI] [PubMed] [Google Scholar]
- 26. Hallegua DS, Weisman MH. Potential therapeutic uses of interleukin 1 receptor antagonists in human diseases. Ann Rheum Dis. 2002;61(11):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bresnihan B. Treatment of rheumatoid arthritis with interleukin 1 receptor antagonist. Ann Rheum Dis. 1999;58(Suppl 1):I96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedman BE, English RJ. Drug‐induced sarcoidosis in a patient treated with an interleukin‐1 receptor antagonist for hidradenitis suppurativa. Jaad Case Reports. 2018;4(6):543‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]


