Abstract
Background
Antineutrophil cytoplasmic autoantibodies against neutrophil granule bactericidal/permeability‐increasing protein (BPI‐ANCA) has been found in many inflammatory diseases, such as COPD, which can reduce the killing effect of BPI on Gram‐negative bacteria. This study was aimed to assess the clinical significance of BPI‐ANCA detecting in COPD patients with Pseudomonas aeruginosa (P aeruginosa) colonization.
Methods
A total of 216 COPD patients with lung P aeruginosa colonization, 244 patients with P aeruginosa infection from June 2015 to June 2018, and 100 healthy individuals were included. Serum BPI‐ANCA, tumor necrosis factor (TNF)‐α, and interleukin (IL)‐6 and IL‐1β levels were detected by ELISA, and the lung function of the patients was measured at stable clinical stages. Patients with COPD were grouped according to BPI‐ANCA detection and GOLD criteria, and serum TNF‐α, IL‐6, and IL‐1β levels and indices reflecting lung function were compared and analyzed between groups.
Results
Positive rate of BPI‐ANCA in COPD patients with P aeruginosa colonization was 48.15%; and compared with BPI‐ANCA(‐) group, FEV1%pred and FEV1/FVC(%) in BPI‐ANCA(+) patients were significantly decreased, while TNF‐α, IL‐6, and IL‐1β levels were elevated. There were 31.73% and 36.54% BPI‐ANCA(+) patients with severe and very severe airflow limitation, respectively, which was significantly higher than that in the BPI‐ANCA(‐) group. FEV1%pred and FEV1/FVC(%) were negatively correlated with TNF‐α, IL‐6, IL‐1β, and NEU%. C‐reactive protein (CRP) was negatively correlated with FEV1%pred, yet not significantly correlated with FEV1/FVC(%).
Conclusion
BPI‐ANCA positivity is associated with inflammatory status in COPD patients with pulmonary P aeruginosa colonization and can be used as a potential biomarker assessing disease severity.
Keywords: antineutrophil cytoplasm autoantibodies, bactericidal/permeability‐increasing protein, chronic obstructive pulmonary disease, cytokines, Pseudomonas aeruginosa
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common chronic inflammatory disease impairing lung function. Lower respiratory microbial colonization often occurs during stable stage of this disease, of whom Pseudomonas aeruginosa (P aeruginosa) is the main colony.1 Unlike bacteria infecting body in an acute way, P aeruginosa colonizing in the body usually cannot be easily killed and cleaned by bactericidal/permeability‐increasing (BPI) protein—a kind of protein presented by neutrophil (NEU),2 and on the contrary, it contributes to the production of autoantibody against BPI, unable it to clean pathogenic microorganism and inducing inflammatory responses.3 The current study was retrospectively designed to evaluate pulmonary disease severity of COPD patients with P aeruginosa chronic colonization, via comparing the lung function and serum tumor necrosis factor (TNF)‐α, interleukin(IL)‐6 and IL‐1β, and C‐reactive protein (CRP) levels between patients seropositive for BPI‐ANCA and those seronegative to explore the association of BPI‐ANCA with patient's lung inflammation levels and explore the clinical significance of serum BPI‐ANCA detection in COPD combined with P aeruginosa colonization.
2. MATERIALS AND METHODS
2.1. Participants
We continuously collected 2053 outpatient and inpatient patients with COPD definitely diagnosed in the Second Affiliated Hospital of Nanchang University from June 2015 to June 2018, 460 of whom were enrolled eventually according to inclusion and exclusion criteria. All patients enrolled with COPD were diagnosed based on the GOLD Global Initiative4 for COPD and in relative stable disease stages and with completed and detailed medical records. Patients combined with metabolic abnormalities, autoimmune diseases, malignant tumors, infection of other pathogens (such as, Klebsiella Pneumoniae, Escherichia coli, Acinetobacter baumannii, Staphylococcus aureus, Streptococcus hemolyticus), those suffering from surgery, severe stress, neuropsychiatric disorders that cannot cooperate to complete pulmonary function test because of any causes, and patients diagnosed with other lung diseases except for COPD including bronchiectasis, interstitial lung disease, asthma, tuberculosis, lung cancer, and sleep apnea syndrome were excluded from this study. Details of patients’ inclusion and exclusion criteria are shown in Figure 1. COPD patients with P aeruginosa isolated from sputum culture, in whose course of 6 months, P aeruginosa was isolated in three or more consecutive sputum culture for at least 1 month, were considered to be P aeruginosa chronic colonization5 (P aeruginosa colonization group), and P aeruginosa infection (P aeruginosa infection group) otherwise. P aeruginosa colonization group includes 152 males and 64 females, with an average age of 60.02 ± 11.17 years old. P aeruginosa infection group includes 182 males and 62 females, with an average age of 61.04 ± 13.23 years old. Another 100 healthy individuals from the medical center of the Second Affiliated Hospital were selected as healthy control, who were in good condition, without any evidence of malaise according to a thorough physical inspection and consultation. Detailed general and medical history information of each participant, including gender, age, height, weight, illness duration, smoking history, past medical history, and detailed physical examination data, was collected by medical record reviewing or telephone follow‐up and is shown in Table 1. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University, and written informed consents were obtained from all eligible participants.
Figure 1.
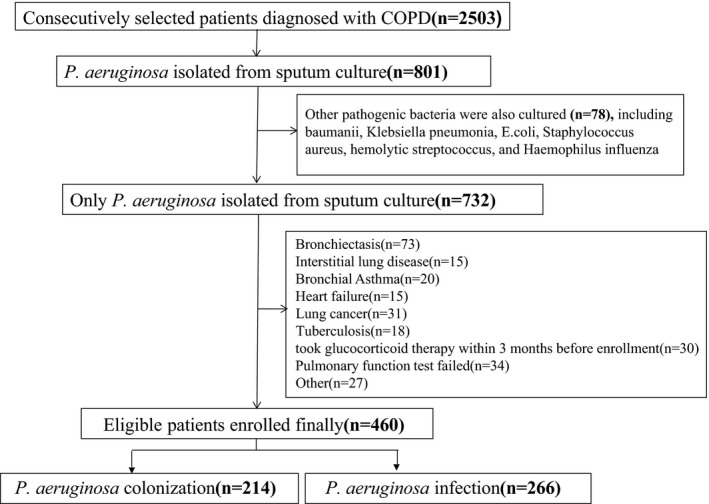
Flowchart of participants' inclusion and exclusion criteria
Table 1.
General information of subjects and IgG‐BPI‐ANCA detection rate
| Parameters | Pseudomonas aeruginosa colonization group | Pseudomonas aeruginosa infection group | HC group | P‐value |
|---|---|---|---|---|
| Age (y) | 60.03 ± 11.17 | 61.04 ± 13.23 | 58.63 ± 12.05 | 0.245† |
| Sex (n, %) | ||||
| Male | 152 (70.37%) | 182 (74.59%) | 62 (62.00%) | 0.066‡ |
| Female | 64 (29.63%) | 62 (25.41%) | 38 (38.00%) | |
| BMI (kg/m2) | 25.20 ± 3.22 | 24.65 ± 2.82 | 24.56 ± 3.41 | 0.101† |
| Smoking status (n, %) | ||||
| Never smoked | 58 (26.85%) | 74 (30.33%) | 21 (21.00%) | 0.054‡ |
| Past smoking | 119 (55.09%) | 115 (47.13%) | 48 (48.00%) | |
| Current smoking | 39 (18.06%) | 57 (23.37%) | 31 (31.00%) | |
| Smoking history (pack year) | 43.06 ± 13.10 | 41.90 ± 12.68 | 40.14 ± 13.44 | 0.174† |
| Illness duration (y) | 12.32 ± 5.23 | 10.77 ± 4.95 | ‐ | 0.001† |
| Hospital admission within 6 mo | ||||
| Yes | 116 (53.70%) | 107 (43.85%) | ‐ | 0.035‡ |
| No | 100 (46.30%) | 137 (56.15%) | ‐ | |
| Family oxygen therapy | ||||
| Yes | 18 (8.33%) | 20 (8.20%) | ‐ | 0.958‡ |
| No | 198 (91.67%) | 224 (91.80%) | ‐ | |
| Taken glucocorticoid | ||||
| Yes | 128 (59.26%) | 142 (58.20%) | ‐ | 0.817‡ |
| No | 88 (40.74%) | 102 (41.80%) | ‐ | |
| BPI‐ANCA (n, %) | ||||
| (+) | 104 (48.15%) | 6 (2.46%) | 0 (0%) | 0.000‡ |
| (−) | 112 (51.85%) | 238 (97.54%) | 0 (0%) | |
Abbreviations: BMI, body mass index; BPI‐ANCA, antineutrophil cytoplasmic autoantibodies against neutrophil granule bactericidal/permeability‐increasing protein; HC, healthy control.
Tested by one‐way ANOVA test
Tested by chi‐square test
2.2. Detection of serum BPI‐ANCA, TNF‐α, IL‐6, and IL‐1β
Three milliliter fasting venous blood was collected to detect serum IgG‐BPI‐ANCA, TNF‐α, IL‐6, and IL‐1β using ELISA, with reagents supplied by EUROIMMUN Medical Laboratory Diagnostics Stock Company (IgG‐BPI‐ANCA) and R&D Systems (Minneapolis, MN, USA) (TNF‐α, IL‐6, and IL‐1β). For IgG‐BPI‐ANCA detection, optical density (OD) was determined by Multiskan Mk3 Enzyme labeled meter (Thermo Fisher Scientific, Shanghai, China), at the end of antigen and antibody reaction, and cutoff value was defined as 0.2 × ODcalibration. When ratio (ODsample to cutoff)>1, BPI‐ANCA was considered positive, and negative otherwise. All assay aforementioned were conducted in strict accordance with manufacturers’ protocols and standard operating procedure (SOP) of the Second Affiliated Hospital of Nanchang University, and repeated twice with included blinded quality controlled samples, and the inter‐ and intra‐batch CVs were lower than 5%.
2.3. Measurement of lung function
Pulmonary function tests were performed using a German‐born Master Screen Diffusion Pulmonary Function Tester. Lung ventilation function tests were performed in strict accordance with the quality control standards of the American Thoracic Society. Lung volume measurements and flow rate capacity curves were performed to evaluate patients’ pulmonary function. Each item was conducted three times repeatedly, and the best value of FEV1%pred and FEV1/FVC(%) was taken as the measurement result. The severity of COPD airflow limitation was classified according to the GOLD guidelines6: mild (FEV1 ≥ 80% predicted); moderate (50% ≤ FEV1 < 80% predicted); severe: (30% ≤ FEV1 < 50% predicted); and very severe: (FEV1 < 30% predicted).
2.4. Statistical analysis
The analysis was performed using SPSS 23.0 software. The normality and variance homogeneity of the measurement data were analyzed by K‐S test and Levene's test. The results of normal distribution measurement data were expressed as mean ± SD. Differences in measurement data among multiple groups were compared by one‐way ANOVA test followed by post hoc LSD test, and independent data t test was used to compare data between the two groups. Count data were expressed as percentage, and the data were compared by chi‐square tests. Pearson correlation analysis was performed to assess correlations between indices. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Characteristics and anthropometrics of participants
Positive rate of BPI‐ANCA was 48.15% in 216 COPD patients with P aeruginosa colonization, highest compared to those with P aeruginosa infection and healthy controls, and perinuclear antineutrophil cytoplasmatic antibodies (p‐ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c‐ANCA) were negative (not listed in the results). And illness duration and numbers of patients admitted to hospital within 6 months before enrollment was also significant between groups. Differences in general information such as age, gender, and smoke exposure among groups were not statistically significant. See details in Table 1.
3.2. Anthropometric comparison between COPD patients with P aeruginosa colonization positive for BPI‐ANCA and negative
A total of 216 COPD patients with P aeruginosa colonization were divided into BPI‐ANCA (+) group (n = 104) and BPI‐ANCA(−) group (n = 112) according to serum BPI‐ANCA test results, with general data compared between groups. Results showed that there was no significant difference in baseline data between groups. Lung function in BPI‐ANCA(+) patients was worse than the BPI‐ANCA(−) group, FEV1%pred and FEV1/FVC(%) being lower (50.31 ± 7.94 vs 52.91 ± 7.14, P = 0.012 and 48.35 ± 8.95 vs 50.61 ± 6.27, P = 0.037, respectively). See Table 2.
Table 2.
Comparison of general data between patients with BPI‐ANCA(+) and BPI‐ANCA(‐) of patients with COPD combined with Pseudomonas aeruginosa colonization
| Parameters |
BPI‐ANCA(+) (n = 104) |
BPI‐ANCA(−) (n = 112) |
P‐value |
|---|---|---|---|
| Age (y) | 61.08 ± 10.12 | 59.05 ± 12.02 | 0.992† |
| Sex | |||
| Male | 75 (72.12%) | 77 (68.75%) | 0.588‡ |
| Female | 29 (27.88%) | 35 (31.25%) | |
| BMI (kg/m2) | 25.49 ± 2.94 | 24.92 ± 3.26 | 0.288† |
| Smoking status (n, %) | |||
| Never smoked | 19 (24.04%) | 33 (29.47%) | 0.079‡ |
| Past smoking | 68 (59.62%) | 50 (50.89%) | |
| Current smoking | 17 (16.34%) | 30 (19.64%) | |
| Smoking history (pack year) | 44.71 ± 14.64 | 41.27 ± 11.02 | 0.052† |
| FEV1%pred | 50.31 ± 7.94 | 52.91 ± 7.14 | 0.012† |
| FEV1/FVC (%) | 48.35 ± 8.95 | 50.61 ± 6.27 | 0.037† |
Abbreviations: BMI, body mass index; BPI‐ANCA, antineutrophil cytoplasmic autoantibodies against neutrophil granule bactericidal/permeability‐increasing protein; CRP, C‐reactive protein; FEV1%pred: predicted% forced expiratory volume in 1 min; FEV1/FVC: forced expiratory volume in one second/forced vital capacity ratio; NEU, neutrophil.
Tested by independent data t test.
Tested by chi‐square test.
3.3. Comparison of serum TNF‐α, IL‐6, IL‐1β, CRP, and NEU% between patients positive for BPI‐ANCA and negative
In Table 3, serum levels of inflammatory markers (including TNF‐α, IL‐6, IL‐1β, and CRP) and NEU% of COPD patients combined with P aeruginosa colonization were compared. TNF‐α, IL‐6, IL‐1β, CRP, and NEU% levels in the BPI‐ANCA(+) group were all higher than that in the BPI‐ANCA (−) group, as shown in Table 3.
Table 3.
Serum inflammatory markers were compared between the two groups
| Parameters |
BPI‐ANCA(+) (n = 104) |
BPI‐ANCA(−) (n = 112) |
P‐value |
|---|---|---|---|
| TNF‐α (pg/mL) | 45.69 ± 19.93 | 33.31 ± 14.58 | 0.000 |
| IL‐6 (pg/mL) | 40.61 ± 12.60 | 29.25 ± 7.86 | 0.000 |
| IL‐1β (pg/mL) | 21.95 ± 7.29 | 14.37 ± 6.15 | 0.000 |
| NEU% | 64.14 ± 9.48 | 61.66 ± 8.33 | 0.042 |
| CRP (mg/L) | 8.41 ± 1.09 | 5.34 ± 1.14 | 0.000 |
Data were expressed as mean ± SD and tested by group t test.
Abbreviations: BPI‐ANCA, antineutrophil cytoplasmic autoantibodies against neutrophil granule bactericidal/permeability‐increasing protein; CRP, C‐reactive protein; IL‐1β, interleukin 1β; IL‐6, interleukin 6; NEU, neutrophil; TNF‐α, tumor necrosis factor‐α.
3.4. Association of FEV1%pred and FEV1/FVC(%) with serum inflammatory markers
Pulmonary function indicators FEV1%pred and FEV1/FVC(%) were negatively correlated with inflammatory markers: TNF‐α (r = −0.582, r = −0.589), IL‐6 (r = −0.521, r = −0.579), IL‐1β (r = −0.346, r = −0.388), NEU% (r = −0.543, r = −0.582), and CRP were negatively correlated with FEV1%pred (r = −0.159, P = 0.019), but not significantly correlated with FEV1/FVC (%), P = 0.264. See Table 4.
Table 4.
Correlation of FEV1%pred and FEV1/FVC(%) with inflammatory markers
| Parameters | FEV1%pred | FEV1/FVC(%) | ||
|---|---|---|---|---|
| r | P‐value | r | P‐value | |
| TNF‐α | −0.582 | 0.000 | −0.589 | 0.000 |
| IL‐6 | −0.521 | 0.000 | −0.579 | 0.000 |
| IL‐1β | −0.346 | 0.000 | −0.388 | 0.000 |
| CRP | −0.159 | 0.019 | −0.076 | 0.264 |
| NEU (%) | −0.543 | 0.000 | −0.582 | 0.000 |
Abbreviations: CRP, C‐reactive protein; FEV1%pred, predicted% forced expiratory volume in 1 min; FEV1/FVC, forced expiratory volume in one second/forced vital capacity ratio; IL‐1β, interleukin 1β; IL‐6, interleukin 6; NEU, neutrophil; TNF‐α, tumor necrosis factor‐α.
3.5. TNF‐α, IL‐6, and IL‐1β levels of COPD patients with different severity of airflow limitation
The distribution of the number of COPD patients with different severity of airflow limitation in the BPI‐ANCA(+) group and BPI‐ANCA (−) group is shown in Table 5. The number of patients with very severe airflow limitation in the BPI‐ANCA(+) group (n = 104) was significantly higher than that in the BPI‐ANCA(−) group [38 (36.54%) vs 22 (19.64%)], and the difference was statistically significant (P = 0.018). Serum TNF‐α (65.73 ± 14.45 pg/mL), IL‐6 (54.18 ± 11.88 pg/mL), and IL‐1β (3.97 ± 1.08 pg/mL) levels in patients with very severe airflow limitation were significantly higher than those in the mild, moderate, and severe groups (P < 0.001).
Table 5.
Serum levels of TNF‐α, IL‐6, and IL‐1β at different levels of COPD airflow limitation
| Mild | Moderate | Severe | Very severe | P‐value | |
|---|---|---|---|---|---|
| BPI‐ANCA group (n,%) | |||||
| (+) | 11 (10.58%) | 22 (21.15%) | 33 (31.73%) | 38 (36.54%) | 0.018† |
| (−) | 21 (18.75%) | 35 (31.26%) | 37 (30.35%) | 22 (19.64%) | |
| TNF‐α (pg/mL) | 10.81 ± 6.05 | 28.5 ± 10.25 | 42.10 ± 9.04 | 65.73 ± 14.45 | <0.000‡ |
| IL‐6 (pg/mL) | 9.63 ± 4.77 | 18.81 ± 8.76 | 32.75 ± 8.95 | 54.18 ± 11.88 | <0.000‡ |
| IL‐1β (pg/mL) | 3.97 ± 1.08 | 6.49 ± 2.49 | 13.96 ± 6.93 | 27.28 ± 10.43 | <0.000‡ |
Abbreviations: BPI‐ANCA, antineutrophil cytoplasmic autoantibodies against neutrophil granule bactericidal/permeability‐increasing protein antibodies; IL‐1β, interleukin 1β; IL‐6, interleukin 6; TNF‐α, tumor necrosis factor‐α.
Tested by chi‐square test.
Tested by one‐way ANOVA test.
4. DISCUSSION
Chronic obstructive pulmonary disease is a kind of obstructive lung disease characterized by long‐term and chronic breathing problems and airflow limitation and usually gets worse over time. Tobacco smoking, air pollution, and occupation exposure are primary causes of chronic inflammation.7 P aeruginosa is a common opportunist, colonizing in the body for long time without inflammatory responses while causing serious infection during existing disease—for example, COPD.8 As a chronic disease, COPD is, P aeruginosa colonizing in patients usually have enough time to form a biofilm, which helps it escape from body immune system, ensuring its long‐term colonization and even probably resulting in patients’ acute exacerbation of COPD(AECOPD) during certain condition.9 NEU can differentiate into antigen‐presenting cell functioning in tumor microenvironment and some chronic inflammatory environments, expressing MHC class II molecules and related costimulatory molecules involved in adaptive immune response regulation.10 On the other hand, a large number of NEU and macrophages infiltrating in the airway and releasing inflammatory factors impair lung function in patients with COPD as well. BPI, a cationic antimicrobial peptide, presenting in human and mammalian NEU, can directly exert toxic effects on Gram‐negative bacteria (GNB) and neutralize free lipopolysaccharide (LPS).11 When P aeruginosa invades the body, BPI can neutralize endotoxin and help kill intracellular and extracellular bacteria related to P aeruginosa's being quickly cleaned from body; while P aeruginosa is chronically colonized in the lung, BPI and microbial antigen are simultaneously engulfed and in the same way presented to MHC class II molecules on the surface of cells, the body then producing both protective antibodies against microbial antigens and autoreactive antibodies against BPI.12 BPI, like other ANCA antigens, originates from polymorphonuclear leukocyte (PMN) and is exposed on the surface of PMN cells once released; binding of ANCA to this membrane‐bound antigen activates PMN and leads to the release of pro‐inflammatory cytokines, such as TNF‐α, IL‐6, and IL‐1β, which destroy lung structure and promote the inflammatory response of NEU.13 Currently, the impact of BPI‐ANCA on the clinical manifestations of chronic airway infections and elevated levels of cytokines in the serum of patients with COPD, and their relationship to disease severity remains unknown.
Results of the current study showed that illness duration of P aeruginosa colonization group was longer (12.32 ± 5.23 vs 10.77 ± 4.95 year) and more patients in P aeruginosa colonization group admitted to hospital within 6 months before enrollment. As mentioned before, chronic disease characteristic of COPD was one of the requirements for P aeruginosa to form biofilm and against being cleaned, so it is reasonable that there is significant difference in illness duration between groups. And serum BPI‐ANCA positivity in the P aeruginosa colonization group (48.15%) was higher than patients with P aeruginosa infection (2.46%) in the present study. However, positive rate of BPI‐ANCA here was lower compared to what reported by Lachenal et al,14 which might cause by different sample size, illness duration and subtypes of P aeruginosa. What's more, in the current study we detected IgG‐BPI‐ANCA only, considering that it is the main type of autoantibody against BPI.
Thomsen et al15 reported that COPD patients with elevated levels of CRP and NEU count usually had higher risk of AECOPD, even those with relative moderate symptoms. The present study detected TNF‐α, IL‐6, IL‐1β, CRP, and NEU% levels in COPD patients with P aeruginosa colonization and found that inflammatory markers were higher in patients seropositive for BPI‐ANCA than those negative, indicating a link between BPI‐ANCA positivity and inflammatory status in COPD patients with P aeruginosa colonization. A 10‐year study followed 46 patients with cystic fibrosis and P aeruginosa colonization drawn conclusion basically consistent with us—it showed that 15/28 BPI‐ANCA(+) patients developed adverse outcomes (P = 0.01), while only 2/18 BPI‐ANCA(−) patients did so,16 suggesting the close association of inflammation and poor prognosis in COPD patients with P aeruginosa colonization with serum BPI‐ANCA positivity. Besides, pulmonary function test showed that indices including FEV1%pred and FEV1/FVC (%) decreased significantly in COPD patients with P aeruginosa colonization positive for BPI‐ANCA, which is basically consistent with the findings of Seema Singh et al.17
Cytokines act as the main inflammatory mediator to regulate the aggregation and activation of inflammatory cells, which will release more proteases or oxidants to further aggravate the damage of airway epithelium and lung tissue. Previous research has showed that TNF‐α was involved in the process of NEU activation during airway inflammation, resulting in a large amount of NEU infiltrating in the airway mucosa and enhancing the solubility of extracellular elastin, which is responsible for airway restructure,18 while both IL‐6 and IL‐1β played regulatory role in immune responses and participated in disease development and tissue damage.19 Zhou et al20 reported that serum IL‐1β in COPD patients was significantly elevated compared to healthy controls (43.33 ± 5.70 vs 28.60 ± 5.07 pg/mL), and Celli BR et al21 detected serum inflammatory markers for 3 years revealing that with serum IL‐6 elevated, mortality of COPD patients increased significantly. All mentioned above showed that inflammatory markers such as IL‐6 and IL‐1β were good indicator of poor outcomes for COPD patients. Correlation analysis demonstrated that TNF‐α, IL‐6, IL‐1β, and NEC% levels were negatively related to lung function indicators including FEV1%pred and FEV1/FVC(%), and CRP was correlated with FEV1%pred negatively. Considering that serum cytokine levels were susceptible to multiple factors, for example, IL‐6 levels were seriously affected by nutritional status and its levels usually elevated in patients with lower BMI or dystrophy.22 We, in the current study, selected COPD patients with P aeruginosa colonization in a relatively stable illness stage and excluded those with chronic consumptions such as heart failure, diabetes mellitus, and malignant neoplasm and those with dystrophia to minimize the influence of confounding factors.
A total of 216 patients studied currently were grouped according to severity of COPD airflow limitation based on the GOLD criteria. Results showed that the proportion of patients with very severe airway limitation in BPI‐ANCA(+) patients were 36.54%, higher than that in the BPI‐ANCA(−) group, and inflammatory indicator levels in patients with very severe airflow limitation were higher compared to other groups, suggesting that inflammation levels of COPD patients with P aeruginosa colonization and seropositive for BPI‐ANCA might be higher compared to those seronegative and should be paid more attention during disease management.
In conclusion, our study suggests that BPI‐ANCA positivity is associated with inflammatory status in COPD patients with pulmonary P aeruginosa colonization and can be used as a potential biomarker assisting physicians in disease management.
CONFLICT OF INTERESTS
All authors declared that they have no conflict of interests.
Tian Y, Zeng T, Tan L, et al. Clinical significance of BPI‐ANCA detecting in COPD patients with Pseudomonas aeruginosa colonization. J Clin Lab Anal. 2019;33:e22908 10.1002/jcla.22908
Yongjian Tian and Tingting Zeng contributed equally.
Funding information
National Nature Science Foundation of China (81760382), Grants from Jiangxi Provincial Science and Technology Bureau (20151122070198).
REFERENCES
- 1. Hassett DJ, Borchers MT, Panos RJ. Chronic obstructive pulmonary disease (COPD): evaluation from clinical, immunological and bacterial pathogenesis perspectives. J Microbiol. 2014;52:211‐226. [DOI] [PubMed] [Google Scholar]
- 2. Choi J, Oh JY, Lee YS, et al. Pseudomonas aeruginosa infection increases the readmission rate of COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3077‐3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultz H, Weiss JP. The bactericidal/permeability‐increasing protein (BPI) in infection and inflammatory disease. Clin Chim Acta. 2007;384:12‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347‐365. [DOI] [PubMed] [Google Scholar]
- 5. Canton R, Cobos N, de Gracia J, et al. Antimicrobial therapy for pulmonary pathogenic colonisation and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect. 2005;11:690‐703. [DOI] [PubMed] [Google Scholar]
- 6. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53:128‐149. [DOI] [PubMed] [Google Scholar]
- 7. Avci E, Avci GA. Important biomarkers that play a role in the chronic obstructive pulmonary disease process. J Med Biochem. 2018;37:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Millares L, Marti S, Ardanuy C, et al. Specific IgA against Pseudomonas aeruginosa in severe COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2807‐2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non‐cystic fibrosis bronchiectasis. Mol Immunol. 2013;55:27‐34. [DOI] [PubMed] [Google Scholar]
- 10. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16‐27. [DOI] [PubMed] [Google Scholar]
- 11. Balakrishnan A, Chakravortty D. Epithelial cell damage activates bactericidal/permeability increasing‐protein (BPI) expression in intestinal epithelium. Front Microbiol. 2017;8:1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schultz H. From infection to autoimmunity: a new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI). Autoimmun Rev. 2007;6:223‐227. [DOI] [PubMed] [Google Scholar]
- 13. Konstantinov KN, Ulff‐Moller CJ, Tzamaloukas AH. Infections and antineutrophil cytoplasmic antibodies: triggering mechanisms. Autoimmun Rev. 2015;14:201‐203. [DOI] [PubMed] [Google Scholar]
- 14. Lachenal F, Nkana K, Nove‐Josserand R, et al. Prevalence and clinical significance of auto‐antibodies in adults with cystic fibrosis. Eur Respir J. 2009;34:1079‐1085. [DOI] [PubMed] [Google Scholar]
- 15. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353‐2361. [DOI] [PubMed] [Google Scholar]
- 16. Lindberg U, Carlsson M, Lofdahl CG, et al. BPI‐ANCA and long‐term prognosis among 46 adult CF patients: a prospective 10‐year follow‐up study. Clin Dev Immunol. 2012;2012:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh S, Verma SK, Kumar S, et al. Correlation of severity of chronic obstructive pulmonary disease with potential biomarkers. Immunol Lett. 2018;196:1‐10. [DOI] [PubMed] [Google Scholar]
- 18. Nunez B, Sauleda J, Garcia‐Aymerich J, et al. Lack of correlation between pulmonary and systemic inflammation markers in patients with chronic obstructive pulmonary disease: a simultaneous, Two‐Compartmental Analysis. Arch Bronconeumol. 2016;52:361‐367. [DOI] [PubMed] [Google Scholar]
- 19. Warnatsch A, Ioannou M, Wang Q, et al. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou L, Liu Y, Chen X, et al. Over‐expression of nuclear factor‐kappaB family genes and inflammatory molecules is related to chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:2131‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065‐1072. [DOI] [PubMed] [Google Scholar]
- 22. Emami AM, Zaerin O. Role of Serum Interleukin 6, Albumin and C‐Reactive Protein in COPD Patients. Tanaffos. 2015;14:134‐140. [PMC free article] [PubMed] [Google Scholar]


