Abstract
Background
Isolated methylmalonic acidemia/aciduria (MMA) is an ultra-rare, serious, inherited metabolic disorder with significant morbidity and mortality. Exogenously delivered mRNA encoding human methylmalonyl-CoA mutase (hMUT), the enzyme most frequently mutated in MMA, is a potential therapy to produce functional MUT enzyme in liver.
Methods
Two 12-week repeat-dose studies were conducted to evaluate the efficacy and safety of intravenously-administered hMUT mRNA encapsulated in lipid nanoparticles in two murine models of MMA.
Findings
In MMA hypomorphic mice, hMUT mRNA treatment resulted in dose-dependent and reproducible biomarker responses after each dose. Enzymatically-active MUT protein was produced in liver in a dose-dependent manner. hMUT mRNA was well-tolerated with no adverse effects, as indicated by the lack of clinical observations, minimal changes in clinical chemistry parameters, and histopathology examination across all tissues. In severe MMA mice, hMUT mRNA led to substantially improved survival and growth and ameliorated biochemical abnormalities, all of which are cardinal clinical manifestations in severely affected patients.
Interpretation
These data demonstrate durable functional benefit of hMUT mRNA and support development of this new class of therapy for a devastating, pediatric disorder.
Fund
This work was funded by Moderna, Inc.
Keywords: Enzyme replacement, Liver targeted therapy, Methylmalonyl-CoA mutase, Organic acidemia, Systemic mRNA therapy
Research in context.
Evidence before this study
Isolated MMA is a pediatric disorder with significant morbidity and mortality. The disorder is primarily caused by a defect or deficiency in the mitochondrial enzyme, MUT. There are no approved therapies that address the underlying defect of the disorder. Despite dietary and supportive management, the overall long-term outcomes for MMA patients remain poor. Thus, there remains a dire unmet medical need in this population. Previous preclinical in vitro and short-term in vivo studies demonstrated proof-of-concept for hMUT mRNA as a novel therapeutic approach to restore functional enzyme in liver.
Added value of this study
We describe a comprehensive preclinical evaluation of the long-term efficacy, safety and tolerability of hMUT mRNA therapy in two separate 12-week studies in two different murine models of MMA that represent the spectrum of MUT deficiency. These studies demonstrate sustained pharmacology and durable functional benefit for hMUT mRNA in relevant murine models of disease. Additionally, hMUT mRNA therapy was well-tolerated with no adverse side effects in both mouse models.
Implications of all the available evidence
These data collectively support the clinical development of this investigational mRNA medicine for a devastating, pediatric, inherited metabolic disorder with no other effective treatment options other than solid organ transplantation.
Alt-text: Unlabelled Box
1. Introduction
Isolated methylmalonic acidemia/aciduria (MMA) is an ultra-rare, devastating, life-threatening inherited metabolic disorder with no approved therapies that address the underlying defect. It is primarily caused by a defect or deficiency in the vitamin B12-dependent mitochondrial enzyme, methylmalonyl-coenzyme A (CoA) mutase (MUT, EC 5.4.99.2) [1,2]. The deficiency in MUT causes a metabolic block in the propionate metabolism pathway, resulting in marked accumulation of toxic metabolites such as methylmalonic acid (the name-giving organic acid) [1,[3], [4], [5]]. MMA due to MUT deficiency (OMIM #251000) can be further classified into defects without (mut0) or with residual (mut−) enzyme activity.
MMA is a pediatric disorder with significant morbidity and mortality. Despite dietary and supportive management, the overall long-term outcomes for MMA patients remain poor, particularly for mut0 patients [[4], [5], [6]]. Patients suffer from numerous complications, including growth retardation, chronic renal failure, neurologic complications, and intermittent life-threatening metabolic decompensations [[4], [5], [6]]. Elective liver transplantation (LT), or combined liver-kidney transplantation (LKT) for patients with renal failure, have emerged as potential treatment options for severely affected individuals. Transplanted patients display substantial reductions in circulating methylmalonic acid and markedly fewer metabolic decompensations [[7], [8], [9], [10], [11], [12]]. While the transplant experience highlights liver as a major metabolic organ for the disorder, LT/LKT as a treatment option is limited by the risks of the procedure itself and the availability of donors. Thus, there remains a dire unmet medical need in this population.
Exogenously delivered mRNA therapy is emerging as a new therapeutic modality with the potential to treat myriad disorders including mitochondrial enzymopathies like MMA. Systemic administration of mRNA encapsulated in lipid nanoparticle (LNP) primarily induces protein expression in liver [[13], [14], [15], [16], [17]]. Low levels of expression of protein have also been reported in spleen [17]. Within the liver, hepatocytes are the primary target cell type, where dose-dependent protein expression has been observed following systemic administration of a reporter mRNA encoding enhanced green fluorescent protein (eGFP) encapsulated in LNPs [17]. LNP-encapsulated mRNA is internalized by hepatocytes via opsonization of LNPs by apolipoprotein E (ApoE) followed by receptor-mediated endocytosis [18].
Full realization of the potential of systemic mRNA therapy requires the ability to achieve long-term efficacy and safety upon repeated systemic dosing of mRNA, due to the limited duration of mRNA-encoded proteins. Although preclinical proof-of-concept studies have evaluated mRNA-encoded intracellular and secreted therapeutic proteins in various animal models of disease with liver as the target organ [17,[19], [20], [21], [22], [23], [24], [25]], the ability to chronically, effectively and safely dose exogenous mRNAs with consistent and sustained pharmacology in long-term preclinical studies has not been reported to date. Furthermore, additional long-term studies evaluating hMUT mRNA are required to support the clinical development of this novel therapy in a primarily pediatric population. Here, we describe a comprehensive preclinical evaluation of the long-term efficacy, safety and tolerability of hMUT mRNA therapy in two separate 12-week studies in two murine models of MMA that represent the spectrum of MUT deficiency. These studies underscore the durable functional benefit of mRNA therapy to potentially treat this devastating pediatric disorder.
2. Materials and methods
2.1. mRNA production and formulation
mRNA was synthesized and formulated in LNPs as described previously [[26], [27], [28]]. The same biodegradable, ionizable LNP described in our previous studies [19] was used in the current set of studies. The full sequences of hMUT mRNA and eGFP mRNA are specified in Table S1 and Table S2 respectively. Briefly, mRNA was synthesized in vitro by T7 RNA polymerase-mediated transcription with 5-methoxy UTP in place of UTP. The linearized DNA template incorporates the 5′ and 3′ untranslated regions (UTRs) and the poly-A tail. After purification, the mRNA was diluted in 50 mM sodium acetate (pH 5) and mixed with lipids dissolved in ethanol (50:10:38.5:1.5; ionizable: helper: structural: polyethyleneglycol) at a ratio of 3:1 (mRNA:lipids). The final product was filtered through a 0.22 μm filter and stored in pre-sterilized vials frozen until use. All formulations were tested for particle size, RNA encapsulation, and endotoxin and were found to be <100 nm in size, with >80% encapsulation, and <10 EU/mL endotoxin.
2.2. Murine models of methylmalonic acidemia
Animals studies were approved by the Institutional Animal Care and Use Committee at Moderna. Mut−/− mice harbor a deletion of exon 3 which encodes the putative substrate-binding pocket in the MUT enzyme. This Mut allele is null and unable to produce mature RNA or protein [29,30]. Mut−/−;TgINS-MCK-Mut mice express the Mut gene under the control of a muscle-specific creatine kinase (MCK) promoter which results in the rescue of mice from neonatal lethality; however, these mice display significant mortality, severe metabolic perturbations, growth retardation, and a hepatorenal mitochondriopathy [31]. Levels of plasma methylmalonic acid, a key toxin that accumulates directly due to the causative enzymatic deficiency, are massively elevated compared to wild type littermates and are similar to levels found in severe MMA patients [31]. Mut−/−;TgINS-CBA-G715V hypomorphic mice ubiquitously express a mouse orthologue (p.G715 V) of a mutation described in MMA mut− patients (p.G717 V) under the control of an enhanced chicken β-actin (CBA) promoter in Mut−/− mice [32]. Similar to mut− patients, these mice have reduced but not complete loss of MUT enzyme activity resulting in significant elevations of disease-associated toxic metabolites such as methylmalonic acid. These mice have decreased MUT activity in all tissues and moderately increased plasma methymalonic acid concentrations in the blood, similar to MMA patients harboring the p.G717 V mutation [32]. Mut+/− mice were used as littermate controls in the current study given that their biochemical parameters are identical to Mut+/+ mice [33].
Sample sizes for the 12-week repeat dose studies in both murine models of MMA were determined from power calculations. For the 12-week study in MMA hypomorphic (Mut−/−;TgINS-CBA-G715V) mice, a sample size of n = 11/group was determined based on power calculations to detect a > 50% decrease in plasma methylmalonic acid concentrations between treated and control arms with 80% power and 5% significance level assuming a two-tailed unpaired t-test and equal variances between groups. For the 12-week study in the more severe MMA murine model (Mut−/−;TgINS-MCK-Mut), a sample size of n = 6/group was determined from power calculations using a two-tailed log-rank test to detect a ≥ 70% difference of survival rates between treatment arms assuming 15% and 85% survival rates in control and treatment arms, respectively, with 80% power and 5% significance level. All study groups were equally balanced for male and female mice. Groups in the MMA hypomorphic mouse study consisted of 4–8 week old n = 5–6 female and n = 5–6 male mice. Similarly, groups in the 12-week study in severe MMA Mut−/−;TgINS-MCK-Mut mice consisted of 3–6 week old n = 3 female and n = 3 male mice. A group of unaffected Mut+/− littermate control mice were included in both studies (n = 11 [6 female, 5 male] in the hypomorphic study, n = 6 [3 female, 3 male] in the severe MMA mouse study). A separate cohort (n = 5; 2 female, 3 male) of 6–11 week old Mut−/−;TgINS-MCK-Mut mice was utilized to evaluate endogenous tissue biomarker concentrations for the 12-week study in severe MMA mice.
All mice were randomly assigned to study groups based on age and body weight. IV injections were administered via the tail vein. In-life blood collection was via submandibular bleed and terminal blood collection was by cardiac puncture. Mice were perfused prior to tissue collection to avoid blood contamination.
2.3. Clinical observations
Clinical observations were performed and recorded at least 5 times per week in both studies. Detailed clinical observations included, but were not limited to, identification of clinical signs related to: general appearance (e.g., skin, fur, changes in eyes, eyeballs and mucous membranes; presence or absence of discharge), body position and posture (e.g., hunchback posture), autonomic nervous system function (e.g., lacrimation, piloerection, pupil diameter, respiration, excretion), motor coordination, ambulatory abnormalities, reaction to being handled and to environmental stimulation, nervous system (e.g., tremor, convulsion, muscular contractions), changes in exploratory behavior, ordinary behavior (e.g., changes in grooming, headshaking, gyration), abnormal behavior (e.g., autophagia, backward motion, abnormal vocalization) and aggression.
2.4. Quantification of plasma methylmalonic acid, 2-methylcitrate, C3 and C2 concentrations
Plasma methylmalonic acid and 2-methylcitrate were analyzed and quantified by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as described previously [34]. C3 and C2 carnitine concentrations were quantified by ion-exchange solid-phase extraction, derivatized with pentafluorophenacyl trifluoromethanesulfonate, separated by high performance liquid chromatography, and detected with an ion trap mass spectrometer as described previously [35]. The lower levels of quantification (LLOQ) for these metabolite assays are: 5 or 10 μM, depending on the dilution, for plasma methylmalonic acid, 0.5 μM for 2-methylcitrate, 0.1 μM for plasma C3, and 2 μM for plasma C2. Values below the LLOQ were imputed as 0.
2.5. Quantification of tissue methylmalonic acid and 2-methylcitrate levels
Tissue samples were homogenized using an Omni Bead Ruptor following addition of 4 eq (w/v) of 80:20 water: acetonitrile. Methylmalonic acid and 2-methylcitrate extracts were derivatized with BuOH/HCl to generate the corresponding butyl ester derivatives of each analyte respectively and analyzed and quantified by LC-MS/MS as described previously [34]. The LLOQs are: 0.1 μmol/g tissue or 0.2 μmol/g tissue, depending on the dilution, for tissue methylmalonic acid and 10 nmol/g tissue for 2-methylcitrate. Values below the LLOQ were imputed as 0. All metabolite assays for plasma and tissue matrices were conducted in a blinded fashion.
2.6. Quantification of tissue hMUT protein levels
hMUT protein concentrations in mouse tissues following hMUT mRNA administration were quantified by LC-MS/MS in a blinded fashion. Tissues were homogenized in 100 mM ammonium bicarbonate buffer with 8 M urea. Human MUT isotopically labeled signature peptides (human MUT specific peptide, IIADIFEYTAK*, in which natural carbon and nitrogen atoms on lysine were fully replaced with 13C and 15N isotopes, respectively; Thermo Pierce, Rockford, IL) were used as internal standards and spiked into each sample. Liver proteins were denatured, followed by reduction using 5 mM tris (2-carboxyethyl) phosphine hydrochloride (75,259, Sigma-Aldrich Inc., St. Louis, MO) at 37 °C for 1 h, alkylation with 10 mM iodoacetamide (I6125, Sigma-Aldrich Inc., St. Louis, MO) at 25 °C in the dark, and then digestion at 37 °C for 15 h with trypsin (trypsin:protein = 1:50 w/w; Catalog #V5280, Promega Inc., Madison, WI). The trypsin digestion reaction was stopped by adding formic acid (28,905, Thermo Fisher Scientific, Waltham, MA). Samples were desalted on the SOLA plate (60309–001, Thermo Fisher Scientific, Waltham, MA), dried, and resuspended in water with 2% acetonitrile and 0.1% formic acid (A955–1, LS120–1, Fisher Scientific, Waltham, MA). Total protein (0.25 μg) was loaded onto the column (75 μm × 15 cm column packed with Waters Acquity BEH resin 1.7 μm × 130 Å) and subjected to LC-MS/MS analysis (Thermo Easy 1000 nano-UPLC, Orbitrap Fusion Mass Spectrometer, Thermo Fisher Scientific, Waltham, MA). Water (A) and acetonitrile (B) with 0.1% formic acid were used for LC separation. The flow rate was 300 nL/min. The gradient was as follows: 2% B to 7% B in 5 min; 7% to 35% B in 45 min; 35% to 80% B in 5 min; 80% to 95%B in 2 min; and 95% B for 5 min. The mass spectrometry was performed in continuous PRM mode: spray voltage: 1900 V; S-lens RF: 60%; isolation width: 1.4 m/z; HCD: 30% collision energy; detector: orbitrap; resolution: 60,000; scan range: 100–1800 m/z; AGC: 5E4; max injection time: 200 ms. The LLOQ was 0.5 ng/mg protein and values below the LLOQ were imputed as 0.
2.7. Quantification of total MUT enzyme activity in liver
MUT activity was determined by methods described previously [36]. Briefly, lysates were incubated with 5′-deoxyadenosylcobalamin (200 μM, C0884, Sigma-Aldrich Inc., St. Louis, MO) and a racemic mix of methylmalonyl-CoA (1 mM; M1762, Sigma-Aldrich Inc., St. Louis, MO) at 37 °C for 15 min. MUT enzyme reactions were terminated by the addition of 100 g/L trichloroacetic acid (2.5%; T6399 Sigma-Aldrich Inc., St. Louis, MO) with vortexing. Samples were centrifuged at 13,000g for 5 min. Chromatographic separation and quantification were accomplished with HPLC. Supernatants (20 μL) were injected and separated on a Poroshell EC-C18 120 HPLC column (695975–302, Agilent Technologies, Santa Clara, CA) equilibrated with100 mM acetic acid (A6283, Sigma Aldrich Inc., St. Louis, MO) in 100 mM sodium phosphate (AC343815000, Fisher Scientific, Waltham, MA) buffer, pH 7.0 (Solvent A). Solvent B was prepared by addition of 18% v/v methanol in Solvent A. Elution was performed with a linear methanol gradient: Solvent B increased from 0 to 95% from 0 to 15 min (95% Solvent B) and then stayed at 95% from 15 to 25 min (95%) with a flow rate of 0.5 mL/min.
2.8. Clinical chemistry panel
Blood samples were collected into a syringe and then transferred into serum separator tubes and allowed to clot for at least 20 min (not to exceed 1 h), then centrifuged at room temperature for at least 15 min. The resulting serum samples were frozen on dry ice as soon as possible until stored in a freezer set to maintain −20 °C until shipment to IDEXX (3 Centennial Dr., North Grafton, MA 01536) for sample analysis. Clinical chemistry parameters were measured in a blinded manner by a standard chemistry analyzer as described by IDEXX.
2.9. Histopathology
Histopathological evaluation was performed by an experienced veterinary pathologist on a full panel of tissues identified in Table S5 from all mice at the end of the 12-week study in MMA hypomorphic mice. Tissues were collected and embedded in paraffin, sectioned, mounted on glass slides, and stained with hematoxylin and eosin. A complete gross pathological examination was performed in addition to a detailed microscopic evaluation of all tissues.
2.10. Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical analyses and descriptive statistics were performed using Prism 7 (GraphPad) or SAS version 9.4. The D'Agostino and Pearson normality test was performed to ensure the data met the normality assumptions of statistical tests. A repeated measures ANOVA was performed to analyze plasma disease biomarkers from the 12-week study in MMA hypomorphic mice. Continuous variables assessed at the end of the study (e.g. tissue biomarkers concentrations, liver hMUT protein and enzyme activity, clinical chemistry) were analyzed with a one-way ANOVA followed by Dunnett's or Tukey's post-hoc pairwise comparisons tests or its nonparametric equivalent. Categorical variables were analyzed using Fisher's exact test. The log-rank test was used to compare survival curves between study groups in the 12-week study in severe MMA mice. Plasma disease biomarkers in severe MMA mice were compared to pre-treatment baseline levels using paired t-tests. Two-tailed p values <.05 were considered statistically significant.
3. Results
3.1. Dose-dependent amelioration of biochemical abnormalities in a 12-week study in MMA hypomorphic mice
A 12-week repeat dose study was conducted in a hypomorphic murine model of MMA MUT deficiency (Mut−/−;TgINS-CBA-G715V) to evaluate the long-term efficacy, safety and tolerability of hMUT mRNA formulated in LNPs. Baseline (pre-treatment) characteristics of age, sex, body weight (Table S3) and plasma biomarkers (Fig. 1a–c) were similar across all study groups in MMA hypomorphic mice.
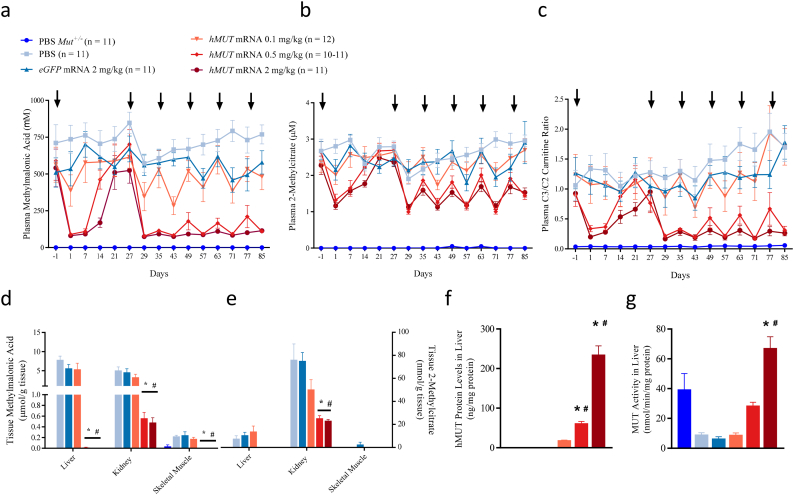
Fig. 1.
hMUT mRNA treatment ameliorates disease biomarkers and restores functional MUT protein in liver in MMA hypomorphic mice.
(a–c) Mut−/−;TgINS-CBA-G715V hypomorphic mice received repeat IV injections of either PBS control, eGFP mRNA control (2 mg/kg), or hMUT mRNA (0.1, 0.5 or 2 mg/kg) formulated in LNPs (n = 10–12/group) and were monitored for plasma methylmalonic acid (a), 2-methylcitrate (b) and C3/C2 carnitine ratio (c). An additional cohort of unaffected Mut+/− mice received repeat IV injections of PBS. Arrows denote timing of IV administration of test and control articles (weeks 0, 4, 6, 8, 10, 12).
(d–e) Methylmalonic acid (d) and 2-methylcitrate (e) concentrations were assessed in key disease tissues (liver, kidney, and skeletal muscle) at the end of the 12-week study (24 h after last dose, n = 10–12/group).
(f–g) hMUT protein concentrations (f) and total MUT enzyme activity (g) were assessed in liver at the end of the 12-week study (24 h after the last dose, n = 10–12/group).
Data presented as mean ± SEM. *p < .05 compared to PBS-injected Mut−/−;TgINS-CBA-G715V group, #p < .05 compared to eGFP mRNA-injected Mut−/−;TgINS-CBA-G715V group from Tukey's pairwise comparison test following a one-way ANOVA (D-G).
To determine the optimal dosing interval, plasma biomarkers were monitored for 4 weeks following a single IV injection of PBS control, eGFP control mRNA formulated in LNPs (2 mg/kg), or hMUT mRNA formulated in LNPs (0.1, 0.5 or 2 mg/kg; n = 11–12/group). Plasma methylmalonic acid concentrations were substantially decreased (>80% mean decrease compared to baseline) 1 week following the first IV dose of 0.5 or 2 mg/kg hMUT mRNA in MMA hypomorphic mice (Fig. 1a). A smaller reduction (31%) was observed in the 0.1 mg/kg hMUT mRNA group 1-day post-dose. A dose-dependent rebound to near-baseline concentrations was observed with the 0.1, 0.5, and 2.0 mg/kg hMUT mRNA dose groups at 1, 2, and 3 weeks post-dose, respectively. In contrast, no metabolic response in plasma methylmalonic acid was observed in either control group (PBS and eGFP mRNA). Similar plasma biomarker responses were observed for 2 additional primary disease-associated toxic metabolites, 2-methylcitrate and C3/C2 carnitine ratio (Fig. 1b–c). Based on these data, a dosing interval of every 2 weeks was chosen for the remainder of the study. All hypomorphic mice received 5 additional IV bolus injections of PBS, eGFP mRNA, or hMUT mRNA every 2 weeks for the next 8 weeks. Similar to the first 4 weeks of the study, a dose-dependent biomarker response to hMUT mRNA was observed over the remainder of the study in contrast to control groups (PBS and eGFP mRNA) (Fig. 1a–c). Collectively these plasma biomarker data demonstrate the dose-dependent bioactivity of hMUT mRNA throughout the entirety of the 12-week study in MMA hypomorphic mice.
Methylmalonic acid and 2-methylcitrate concentrations were assessed in key disease tissues (liver, kidney, and skeletal muscle) at the end of the 12-week study. Concentrations of both biomarkers were significantly lower (p < .05 from Tukey's pairwise comparison following a one-way ANOVA) in all key disease tissues in hypomorphic mice treated with 0.5 and 2 mg/kg hMUT mRNA compared to PBS and eGFP mRNA controls (Fig. 1d). Indeed, hypomorphic mice treated with 0.5 and 2 mg/kg hMUT mRNA had nearly undetectable levels of these biomarkers in liver, similar to unaffected Mut+/− littermate mice.
3.2. Dose-dependent restoration of functional MUT enzyme in liver due to hMUT mRNA in MMA hypomorphic mice
hMUT mRNA treatment resulted in dose-dependent increases in hepatic hMUT protein and enzyme activity at the end of the 12-week study. Hepatic hMUT protein concentrations, quantified with an LC-MS/MS method using a human-specific detection peptide, were 18.1 ± 3.3, 61.2 ± 15.3 and 234.6 ± 75.6 ng/mg protein in mice treated with 0.1, 0.5 and 2 mg/kg hMUT mRNA, respectively (Fig. 1f). Similarly, liver MUT enzyme activity levels were 8.8 ± 5.4, 28.5 ± 7.4 and 67.1 ± 25.7 nmol/min/mg protein in mice treated with 0.1, 0.5 and 2 mg/kg hMUT mRNA, respectively (Fig. 1g).
To determine the bio-distribution of mRNA expressed hMUT protein, a separate single dose study was performed. Mut−/−;TgINS-CBA-G715V mice received a single IV bolus dose administration of LNP-encapsulated hMUT mRNA (n = 18) or eGFP mRNA (n = 12) at 0.5 mg/kg and were sacrificed at 24 h. hMUT protein concentrations in six tissues (liver, spleen, kidney, heart, lung and lymph node) were measured by LC-MS/MS. hMUT protein concentrations were 65.3 ± 40.8 and 0.6 ± 0.4 ng/mg protein in liver and spleen, respectively, and not detectable in the remaining tissues (kidney, heart, lung, lymph node) in mice administered hMUT mRNA (Fig. S3). hMUT protein was undetectable in all evaluated tissues in eGFP mRNA-injected mice.
3.3. hMUT mRNA therapy was well-tolerated in MMA hypomorphic mice
To evaluate the safety and tolerability of hMUT mRNA therapy in MMA hypomorphic mice, clinical observations and body weights were monitored throughout the 12-week study. Clinical observations were performed at least 5 times per week. No hMUT mRNA-related clinical findings were identified throughout the entirety of the study. Body weights and body weight gains were similar between all groups throughout the study, including unaffected Mut+/− littermate mice (Fig. S1).
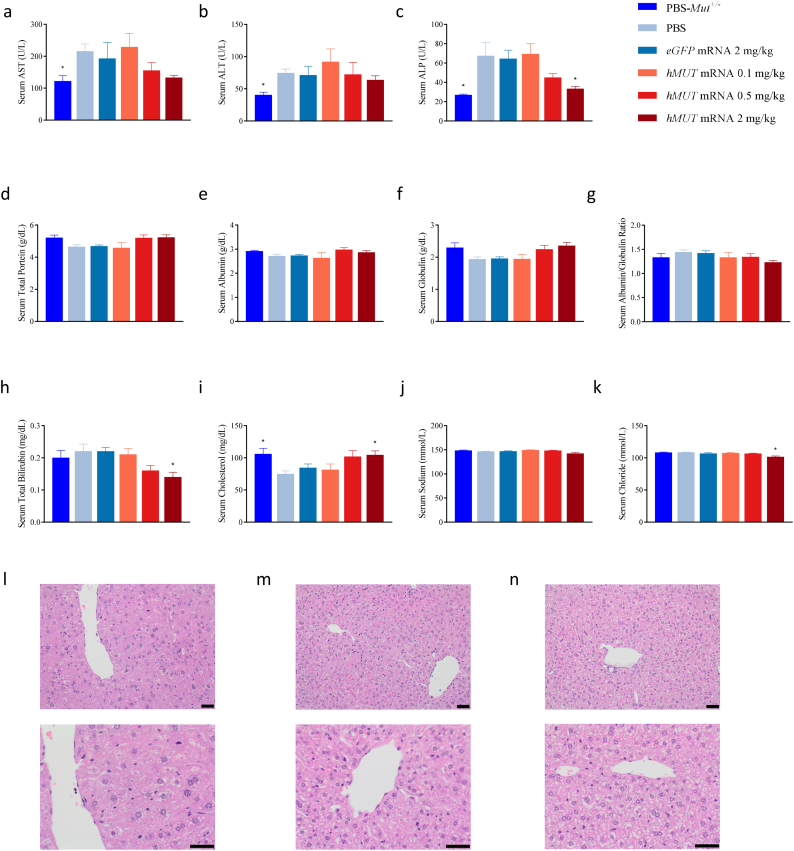
Clinical chemistry was additionally evaluated at the end of the study in all mice (Fig. 2 & Table S4). Across all clinical chemistry parameters, no toxicologically-relevant findings were observed. Importantly, no increase in markers of liver toxicity was observed in hMUT mRNA treated mice compared to PBS-injected hypomorphic mice (Fig. 2a–h and Table S4). When compared to phenotypically normal Mut+/− mice, a slight yet statistically significant increase of liver enzymes (serum AST, ALT, ALP) was observed in PBS-injected MMA hypomorphic mice suggesting mild liver changes in affected mice (Fig. 2a–c). This is further supported by mild decreases in total protein, albumin, globulin and cholesterol in PBS-injected hypomorphic mice compared to unaffected Mut+/− mice (Fig. 2d–f and i). In contrast, treatment with 0.5 and 2 mg/kg hMUT mRNA led to dose-dependent reductions in all liver enzymes and increases in total protein, albumin, globulin, and cholesterol, suggesting improved liver health in MMA hypomorphic mice due to mRNA therapy. Additionally, minimal decreases in serum albumin/globulin ratio, sodium, and chloride were observed in hMUT mRNA-treated versus PBS-injected hypomorphic mice (Fig. 2g and j–k).
Fig. 2.
Minimal changes in clinical chemistry parameters and improved liver histopathology in MMA hypomorphic mice after repeated hMUT mRNA treatment.
(a–i) Select clinical chemistry (serum AST, ALT, ALP, total protein, globulin, albumin, albumin/globulin ratio, total bilirubin, and cholesterol) in Mut+/− and PBS, eGFP mRNA and hMUT mRNA-treated hypomorphic mice (n = 10–12/group).
(j–k) Select clinical chemistry electrolytes (serum sodium and chloride) in Mut+/− and PBS, eGFP mRNA and hMUT mRNA-treated hypomorphic mice (n = 6–9/group).
(l–n) Histology of the liver was examined and representative images of Mut−/−;TgINS-CBA-G715V mice injected with PBS (l) and hMUT mRNA 0.5 mg/kg (m) and Mut+/− (n) are presented.
Data presented as mean ± SEM. *p < .05 compared to Mut−/−;TgINS-CBA-G715V PBS group obtained from Dunn's multiple comparisons test following a Kruskal-Wallis test.
3.4. Histopathology examination revealed minimal microscopic findings in spleen and potential improvement in hepatic pathology due to hMUT mRNA therapy
Histopathology examination of a full set of tissues (Table S5) was performed at the end of the 12-week study to further evaluate the safety of hMUT mRNA therapy. No gross pathological findings related to hMUT mRNA were observed in any tissues. Across all examined tissues, hMUT mRNA-related microscopic findings were limited to the spleen where a dose-dependent increase in minimal to mild lymphoid depletion of the periarteriolar lymphoid sheath was observed (Table 1). This finding was characterized by a decrease in lymphocytes in the region adjacent to the central arteries (Fig. S2). In addition, a dose-dependent increase in red pulp cellularity in spleen was observed in MMA hypomorphic mice treated with hMUT mRNA (Fig. S2). These findings were likely related to the LNP as they were also observed with similar incidence in hypomorphic mice administered eGFP mRNA formulated in the same LNPs at 2 mg/kg.
Table 1.
Summary of microscopic findings in spleen and liver in 12-week study in MMA hypomorphic mice.
| Genotype |
Mut+/− |
Mut−/−;TgINS-CBA-G715V |
|||||
|---|---|---|---|---|---|---|---|
| Treatment |
PBS |
PBS |
eGFP mRNA |
hMUT mRNA |
|||
| Dose (mg/kg) |
NA |
NA |
2 |
0.1 |
0.5 |
2 |
|
| n | 11 | 11 | 11 | 12 | 10 | 11 | |
| Spleen | |||||||
| Lymphoid depletion PALSa | Minimal | 0 (0%) | 0 (0%) | 7 (63.6%) | 1 (8.3%) | 9 (90.0%) | 9 (81.8%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9.1%) | |
| Increased cellularity (red pulp) | Minimal | 0 (0%) | 0 (0%) | 8 (72.7%) | 0 (0%) | 8 (80.0%) | 10 (90.9%) |
| Liver | |||||||
| Hepatocellular hypertrophy | Minimal | 0 (0%) | 3 (27.3%) | 1 (9.1%) | 1 (8.3%) | 0 (0%) | 0 (0%) |
| Increased mitosis | Minimal | 0 (0%) | 3 (27.3%) | 0 (0%) | 3 (25.0%) | 0 (0%) | 0 (0%) |
PALS = peri-arteriolar lymphoid sheaths. Data presented as n (%).
In the liver, a minimal increase in mitotic figures and centrilobular hepatocellular hypertrophy was observed in 17.6% (6/34) of hypomorphic mice administered PBS, eGFP mRNA or hMUT mRNA at the low dose (0.1 mg/kg) (Table 1, Fig. 2l–n). These findings were not identified in unaffected Mut+/− littermate mice administered PBS nor in hypomorphic mice receiving higher, efficacious dose levels of hMUT mRNA (0.5 mg/kg and 2 mg/kg) (Table 1, Fig. 2l–n). The absence of these degenerative findings in mice treated with efficacious dose levels of hMUT mRNA is suggestive of a beneficial effect of hMUT mRNA on liver pathology.
3.5. hMUT mRNA therapy improved survival, growth, and metabolic disturbances in a 12-week study in severe MMA mice
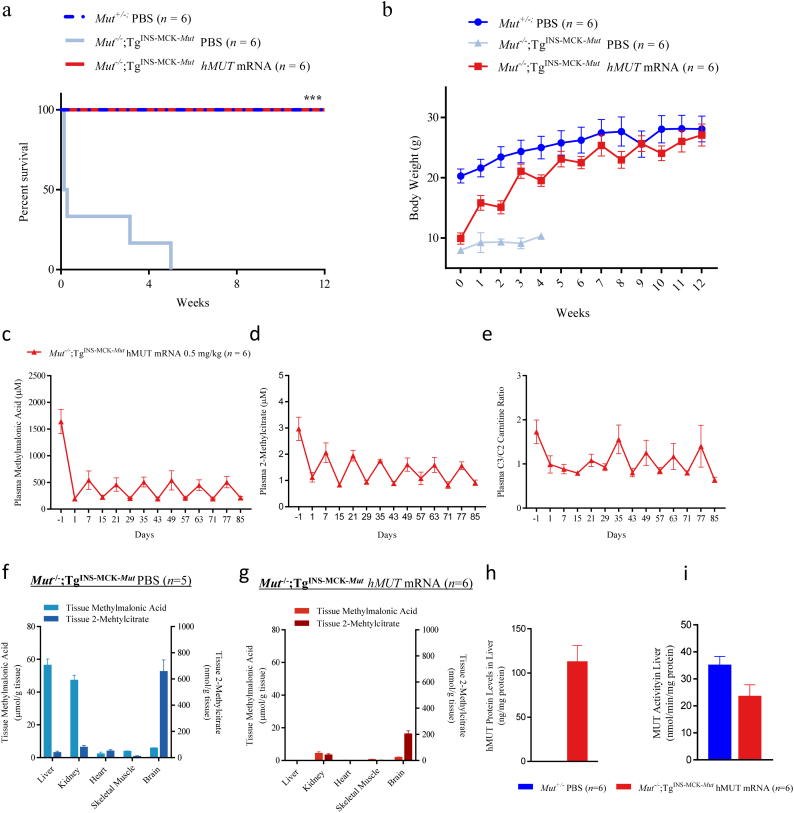
The efficacious dose level and regimen of hMUT mRNA was further evaluated in a separate 12-week pharmacology study in a severe mouse model of MMA, Mut−/−;TgINS-MCK-Mut. We have previously shown that there is no difference in survival between Mut−/−;TgINS-MCK-Mut mice that received control mRNA and untreated mice [19]. Additionally, the 12-week study in MMA hypomorphic mice showed no differences in disease biomarkers and metabolic characteristics between mice injected with eGFP mRNA vs. PBS. Therefore, we did not include a control mRNA group in the 12-week study in Mut−/−;TgINS-MCK-Mut mice. Repeat IV administration of hMUT mRNA at 0.5 mg/kg every 2 weeks resulted in a pronounced and highly significant improvement in survival in severe MMA mice (n = 6/group, p < .001 from log-rank test, Fig. 3a). All hMUT mRNA treated mice survived the entire duration of the 12-week study similar to PBS-injected unaffected Mut+/− mice (Fig. 3a). In marked contrast, PBS-injected severe MMA mice exhibited a precipitous decline in survival (median study day of death: 1.5 days).
Fig. 3.
hMUT mRNA improves survival and growth, reduces disease biomarkers and restores functional MUT enzyme in liver in severe MMA Mut−/−;TgINS-MCK-Mut mice.
(a) Kaplan-Meier survival curves of severe MMA Mut−/−;TgINS-MCK-Mut mice receiving every other week IV bolus injections of hMUT mRNA 0.5 mg/kg or PBS (n = 6/group). *** p < .001 from log-rank test. Survival curve of unaffected Mut+/− mice receiving IV injections of PBS (n = 6) additionally shown.
(b) Body weight was monitored weekly throughout the 12-week study. n = 6 for hMUT mRNA-treated Mut−/−;Tg INS-MCK-Mut and PBS-injected Mut+/− mouse groups. n = 6, 2, 2, 2, and 1 at study weeks 1, 2, 3, 4, and 5 respectively for PBS-injected Mut−/−;Tg INS-MCK-Mut group.
(c–e) Plasma methylmalonic acid (c), 2-methylcitrate (d), and C3/C2 carnitine ratio (e) were serially monitored weekly throughout the 12-week study.
(f–g) Methylmalonic acid and 2-methylcitrate concentrations were assessed in key disease tissues (liver, kidney, heart, brain and skeletal muscle) 24 h after a single injection of PBS (f) and at the end of 12-weeks of treatment of hMUT mRNA (g) in Mut−/−;TgINS-MCK-Mut mice (n = 5–6/group).
(h–i) hMUT protein concentrations (h) and total MUT enzyme activity (i) were assessed in liver at the end of the 12-week study (24 h after the last dose administration) in hMUT mRNA-treated Mut−/−;TgINS-MCK-Mut mice and PBS-injected unaffected Mut+/− mice (n = 6/group).
Data presented as mean ± SEM.
hMUT mRNA-treated mice thrived, as demonstrated by their substantial improvement in growth. Mean body weights of hMUT mRNA-treated mice increased 2.7-fold during the 12-week study (Fig. 3b). Indeed, the body weights of hMUT mRNA-treated mice approached the body weights of unaffected Mut+/− littermate mice. In contrast, PBS-injected severe MMA mice gained minimal weight during the period that they survived.
Correlated with the improvement in survival and growth, hMUT mRNA-treated mice showed clear and consistent decreases in all 3 plasma biomarkers 1 day and 1 week after each dose administration (Fig. 3c–e). In contrast, these biomarkers showed no metabolic response and high variability in PBS-treated MMA mice (Fig. S4).
Tissue methylmalonic acid and 2-methylcitrate concentrations were evaluated in key disease tissues (liver, kidney, skeletal muscle, heart, brain) of surviving mice at the end of the 12-week study. All PBS-injected severe MMA mice perished by week 5 of the study (Fig. 3A) and thus were not available for tissue biomarker evaluation. To determine biomarker concentrations in untreated mice, tissue biomarkers were also evaluated in a slightly younger cohort of severe MMA mice (6–11 weeks old, n = 5). Tissue methylmalonic acid and 2-methylcitrate concentrations were 45–100% lower in hMUT mRNA versus PBS-injected severe MMA mice (Fig. 3f–g). Of note, liver biomarker levels were depleted to normal levels in hMUT mRNA-treated MMA mice.
hMUT mRNA treatment restored functional MUT enzyme in liver in severe MMA mice (Fig. 3h–i). Following 12 weeks of treatment, hMUT protein and activity were detected in liver at concentrations of 113 ± 44.7 ng/mg protein and 23.6 ± 10.3 nmol/min/mg protein 24 h after the last dose (Fig. 3h–i).
Finally, in this study, mice were monitored for clinical observations throughout the study at least 5 times per week. No clinical signs were observed in hMUT mRNA-treated mice. The lack of clinical observations combined with the survival and body weight data suggest that hMUT mRNA was well-tolerated in severe MMA mice.
4. Discussion
Despite dietary and supportive management, MMA patients, particularly mut0 patients, suffer high morbidity and mortality. Conventional enzyme replacement therapy is currently not a viable treatment modality for MMA and many other inborn errors of metabolism due to technical challenges in producing complex recombinant enzymes that require specialized subcellular localizations. Exogenous mRNA, in principle, can produce any type of complex protein by using the cell's endogenous translational machinery. In previous proof-of-concept studies, we demonstrated that LNP-encapsulated hMUT mRNA encoded functional MUT enzyme in liver, reduced disease-associated metabolites, and ultimately improved survival upon repeat dosing in a 6-week study. However, comprehensive long-term efficacy, safety and tolerability studies in relevant animal models are required to support the clinical development of hMUT mRNA therapy for this pediatric population.
Here, we demonstrate sustained bioactivity and pharmacology of hMUT mRNA in two long-term repeat dose studies in two murine models of MMA. Reproducible and marked decreases in plasma methylmalonic acid, 2-methylcitrate and C3/C2 carnitine ratio were observed following each dose administration of LNP-encapsulated hMUT mRNA in both murine models. These biomarker reductions correlated with remarkable improvements in survival and growth in severe MMA mice over the span of 12 weeks. The magnitude of plasma methylmalonic acid reductions was similar to decreases observed following LT in MMA patients [10,11]. Although all plasma biomarkers were substantially decreased, they were not reduced to normal levels. Complete normalization of plasma methylmalonic acid and other biomarkers was not expected, similar to transplanted patients [10,11], due to production of toxins in other high energy organs such as skeletal muscle, heart and brain.
The liver, and more specifically the hepatocyte, is regarded as the major target for mitochondrial pathology in MMA [29]. hMUT mRNA restored functional MUT enzyme in liver and as a direct consequence, depleted toxic metabolite concentrations in livers of MMA mice. Liver from patients and murine models of MMA have a mega-mitochondrial phenotype that is readily visible using electron microscopy [29,37]. Although electron microscopy was not performed in the current study, light microscopy analysis of liver tissues revealed an increase in hepatocellular hypertrophy in control hypomorphic mice that may be due to enlarged mitochondria. Interestingly, hepatocellular hypertrophy was not observed in hypomorphic mice treated with efficacious dose levels of hMUT mRNA or in unaffected littermate control mice. Additionally, mild liver chemistry abnormalities were observed in untreated MMA hypomorphic mice compared to unaffected mice. This is consistent with published reports from the European registry and network for intoxication type metabolic diseases, in which a small fraction (<20%) of MMA patients had abnormal liver function tests [38]. Other laboratory and ultrasound measured liver abnormalities have been described in MMA patients [39]. hMUT mRNA treatment normalized most of the altered liver function parameters in a dose-dependent manner in hypomorphic mice, suggesting a potential improvement on liver health. These data, combined with the depletion of hepatic toxic metabolites and improved liver function tests, suggest an overall improvement in liver pathology due to hMUT mRNA.
Impaired mitochondrial function observed in key extrahepatic disease tissues (kidney, heart, skeletal muscle, and brain) from MMA patients may contribute to long-term complications associated with this disease [29,[40], [41], [42], [43], [44], [45], [46], [47], [48]]. The molecular mechanisms of mitochondrial dysfunction in these key disease tissues are not fully understood but are hypothesized to be partially caused by accumulation of toxic metabolites such as methylmalonic acid and 2-methylcitrate [49]. Although hMUT mRNA is a liver-focused therapy, significant decreases in tissue methylmalonic acid and 2-methylcitrate concentrations were seen in extrahepatic tissues. As there was no detectable increase in MUT enzyme produced in these extrahepatic tissues, these tissue biomarker reductions were likely due to decreases in circulating toxic metabolites. Recent single-center reports describe renal and neurological stabilization or improvement following LT in patients with MMA [10,11], however, other studies have reported renal and neurologic deterioration post-LT [9,50]. The long-term outcomes, particularly renal and neurologic progression, following LT remain to be fully described. Whether a liver-focused therapy such as hMUT mRNA has a beneficial effect on extrahepatic clinical manifestations will have to be determined in clinical trials. Early intervention in the disorder may, in theory, prevent irreversible insults to various extrahepatic organs during metabolic decompensations, although further research is warranted.
The safety and tolerability of repeat IV administration of hMUT mRNA encapsulated in LNPs were evaluated in hypomorphic mice. hMUT mRNA was well-tolerated, as demonstrated by the lack of any hMUT mRNA-related clinical findings and similar body weights across treatment and control groups. Clinical chemistry and histopathology findings were limited and are likely due to a non-specific, generalized inflammatory state that is likely transient and not considered to be adverse. Due to blood volume limitations, hematology, cytokines, and complement factors were not evaluated in the current study. Nevertheless, we have previously evaluated inflammatory markers in a 5-week repeat dose study in MMA hypomorphic mice [19]. No increase of plasma cytokines (IL-6, IFN-γ, TNF-α, IL-1β) was observed in MMA hypomorphic mice following 3 and 5 weekly IV bolus injections of hMUT mRNA (0.2 mg/kg) compared to mice receiving weekly injections of PBS. Moreover, minimal elevations in inflammatory markers including complement and cytokines have been observed in repeat dose toxicology studies conducted in cynomolgus monkeys receiving mRNA encapsulated with a similar LNP (1 mg/kg) [23]. Furthermore, even if inflammatory markers were aberrant, the histopathology examination across a full panel of tissues did not identify any obvious microscopic correlate. Although anti-drug antibodies were not evaluated in the current studies, the consistent and reproducible pharmacologic response following each mRNA dose suggests that no neutralizing antibodies were developed. Furthermore, preclinical evaluation of immunogenicity has limited translation to humans and thus, anti-drug antibodies should be carefully monitored in clinical trials. Common strategies used to mitigate deleterious anti-drug antibodies such as immunomodulatory and/or premedication regimens could be considered as clinical data emerge.
As systemic hMUT mRNA therapy will require chronic, repeat IV infusions every few weeks, the clinical adoption of such a therapy remains to be determined in this disorder. However, this dosing regimen is similar to several enzyme replacement therapies prescribed for multiple lysosomal storage disorders (e.g. Fabry, Gaucher, and Mucopolysaccharidoses). Additionally, clinical implementation of a potential mRNA therapy will largely be influenced by safety and efficacy results from upcoming clinical trials in patients and ultimately, the cost-effectiveness of the therapy.
In summary, results from two long-term studies demonstrate sustained pharmacology and durable functional benefit for hMUT mRNA in two murine models of MMA representing the spectrum of MUT deficiency. hMUT mRNA therapy was well-tolerated with no adverse side effects in both mouse models. These data collectively support the clinical development of hMUT mRNA therapy in this devastating pediatric disorder with no effective treatment options other than elective solid organ transplantation.
Acknowledgments
Acknowledgements
The authors thank Dr. Charles Venditti at NHGRI/NIH for licensing the two murine models of MMA. The authors thank Katherine Kacena for performing statistical analyses.
Funding source
This work was funded by Moderna, Inc. Funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Declaration of interests
All authors are employees of, and receive salary and stock options from Moderna, Inc.
Author contributions
D.A., A.F., and L.T.G. designed the experiments. D.A., A.F., E.J., M.E., C.D., V.N. and J. Milton performed the experiments. D.A., A.F., E.J., J.M., R.L. and L.T.G. analyzed the data. L.T.G. and D.A. wrote the manuscript, and all authors edited the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.003.
Appendix A. Supplementary data
Supplementary material
References
- 1.Manoli I., Sloan J.L., Venditti C.P. Isolated Methylmalonic Acidemia. In: Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., editors. GeneReviews(R) 1993. Seattle (WA) [Google Scholar]
- 2.Forny P., Schnellmann A.S., Buerer C., Lutz S., Fowler B., Froese D.S. Molecular genetic characterization of 151 Mut-type Methylmalonic aciduria patients and identification of 41 novel mutations in MUT. Hum Mutat. 2016;37(8):745–754. doi: 10.1002/humu.23013. [DOI] [PubMed] [Google Scholar]
- 3.Zwickler T., Haege G., Riderer A., Horster F., Hoffmann G.F., Burgard P. Metabolic decompensation in methylmalonic aciduria: which biochemical parameters are discriminative? J Inherit Metab Dis. 2012;35(5):797–806. doi: 10.1007/s10545-011-9426-1. [DOI] [PubMed] [Google Scholar]
- 4.Fraser J.L., Venditti C.P. Methylmalonic and propionic acidemias: clinical management update. Curr Opin Pediatr. 2016;28(6):682–693. doi: 10.1097/MOP.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgartner M.R., Horster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horster F., Baumgartner M.R., Viardot C., Suormala T., Burgard P., Fowler B. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, Mut-, cblA, cblB) Pediatr Res. 2007;62(2):225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- 7.Hussein M.H., Hashimoto T., Suzuki T., Daoud G.A., Goto T., Nakajima Y. Children undergoing liver transplantation for treatment of inherited metabolic diseases are prone to higher oxidative stress, complement activity and transforming growth factor-beta1. Ann Transplant. 2013;18:63–68. doi: 10.12659/AOT.883820. [DOI] [PubMed] [Google Scholar]
- 8.Kamei K., Ito S., Shigeta T., Sakamoto S., Fukuda A., Horikawa R. Preoperative dialysis for liver transplantation in methylmalonic acidemia. Ther Apher Dial. 2011;15(5):488–492. doi: 10.1111/j.1744-9987.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 9.Kasahara M., Horikawa R., Tagawa M., Uemoto S., Yokoyama S., Shibata Y. Current role of liver transplantation for methylmalonic acidemia: a review of the literature. Pediatr Transplant. 2006;10(8):943–947. doi: 10.1111/j.1399-3046.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 10.Niemi A.K., Kim I.K., Krueger C.E., Cowan T.M., Baugh N., Farrell R. Treatment of methylmalonic acidemia by liver or combined liver-kidney transplantation. J Pediatr. 2015;166(6):1455–1461 e1. doi: 10.1016/j.jpeds.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Critelli K., McKiernan P., Vockley J., Mazariegos G., Squires R.H., Soltys K. Liver transplantation for propionic Acidemia and Methylmalonic Acidemia: perioperative management and clinical outcomes. Liver Transpl. 2018;24(9):1260–1270. doi: 10.1002/lt.25304. [DOI] [PubMed] [Google Scholar]
- 12.Chen P.W., Hwu W.L., Ho M.C., Lee N.C., Chien Y.H., Ni Y.H. Stabilization of blood methylmalonic acid level in methylmalonic acidemia after liver transplantation. Pediatr Transplant. 2010;14(3):337–341. doi: 10.1111/j.1399-3046.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 13.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardi N., Secreto A.J., Shan X., Debonera F., Glover J., Yi Y. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun. 2017;8 doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman K.J., Mir F.F., Jhunjhunwala S., Kaczmarek J.C., Hurtado J.E., Yang J.H. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials. 2016;109:78–87. doi: 10.1016/j.biomaterials.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 17.Ramaswamy S., Tonnu N., Tachikawa K., Limphong P., Vega J.B., Karmali P.P. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc Natl Acad Sci U S A. 2017;114(10):E1941–E1950. doi: 10.1073/pnas.1619653114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullis P.R., Hope M.J. Lipid nanoparticle Systems for Enabling Gene Therapies. Mol Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.S. Systemic messenger RNA therapy as a treatment for Methylmalonic Acidemia. Cell Rep. 2017;21(12):3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L., Berraondo P., Jerico D., Guey L.T., Sampedro A., Frassetto A. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat Med. 2018;24(12):1899–1909. doi: 10.1038/s41591-018-0199-z. [DOI] [PubMed] [Google Scholar]
- 21.Prieve M.G., Harvie P., Monahan S.D., Roy D., Li A.G., Blevins T.L. Targeted mRNA therapy for ornithine Transcarbamylase deficiency. Mol Ther. 2018;26(3):801–813. doi: 10.1016/j.ymthe.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roseman D.S., Khan T., Rajas F., Jun L.S., Asrani K.H., Isaacs C. G6PC mRNA therapy positively regulates fasting blood glucose and decreases liver abnormalities in a mouse model of glycogen storage disease 1a. Mol Ther. 2018;26(3):814–821. doi: 10.1016/j.ymthe.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26(6):1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeRosa F., Guild B., Karve S., Smith L., Love K., Dorkin J.R. Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system. Gene Ther. 2016;23(10):699–707. doi: 10.1038/gt.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X., Yin L., Theisen M., Zhuo J., Siddiqui S., Levy B. Systemic mRNA therapy for the treatment of Fabry disease: preclinical studies in wild-type mice, Fabry mouse model, and wild-type non-human primates. Am J Hum Genet. 2019;104(4):625–637. doi: 10.1016/j.ajhg.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168(6):1114–1125 e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akinc A., Zumbuehl A., Goldberg M., Leshchiner E.S., Busini V., Hossain N. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung A.K., Tam Y.Y., Chen S., Hafez I.M., Cullis P.R. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J Phys Chem B. 2015;119(28):8698–8706. doi: 10.1021/acs.jpcb.5b02891. [DOI] [PubMed] [Google Scholar]
- 29.Chandler R.J., Zerfas P.M., Shanske S., Sloan J., Hoffmann V., DiMauro S. Mitochondrial dysfunction in Mut methylmalonic acidemia. FASEB J. 2009;23(4):1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandler R.J., Tsai M.S., Dorko K., Sloan J., Korson M., Freeman R. Adenoviral-mediated correction of methylmalonyl-CoA mutase deficiency in murine fibroblasts and human hepatocytes. BMC Med Genet. 2007;8:24. doi: 10.1186/1471-2350-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manoli I., Sysol J., Li L., Chandler R., Senac J., Hoffmann V. Muscle targeted transgene expression rescues the lethal phenotype of Mut knockout mice. Mol Genet Metab. 2011;102(3):248. [Google Scholar]
- 32.Senac J.S., Aswani V.H., Sysol J.R., Manoli I. C.P. V. Partial deficiency model of MUT Methylmalonic Acidemia (MMA) displays diet inducible disease and sensitivity to acetaminophen (APAP) Mol Ther. 2013;21(1):S107. [Google Scholar]
- 33.Chandler R.J., Sloan J., Fu H., Tsai M., Stabler S., Allen R. Metabolic phenotype of methylmalonic acidemia in mice and humans: the role of skeletal muscle. BMC Med Genet. 2007;8:64. doi: 10.1186/1471-2350-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turgeon C.T., Magera M.J., Cuthbert C.D., Loken P.R., Gavrilov D.K., Tortorelli S. Determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin Chem. 2010;56(11):1686–1695. doi: 10.1373/clinchem.2010.148957. [DOI] [PubMed] [Google Scholar]
- 35.Minkler P.E., Stoll M.S., Ingalls S.T., Yang S., Kerner J., Hoppel C.L. Quantification of carnitine and acylcarnitines in biological matrices by HPLC electrospray ionization-mass spectrometry. Clin Chem. 2008;54(9):1451–1462. doi: 10.1373/clinchem.2007.099226. [DOI] [PubMed] [Google Scholar]
- 36.Ouattara B., Duplessis M., Girard C.L. Optimization and validation of a reversed-phase high performance liquid chromatography method for the measurement of bovine liver methylmalonyl-coenzyme a mutase activity. BMC Biochem. 2013;14:25. doi: 10.1186/1471-2091-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zsengeller Z.K., Aljinovic N., Teot L.A., Korson M., Rodig N., Sloan J.L. Methylmalonic acidemia: a megamitochondrial disorder affecting the kidney. Pediatr Nephrol. 2014;29(11):2139–2146. doi: 10.1007/s00467-014-2847-y. [DOI] [PubMed] [Google Scholar]
- 38.Kolker S., Valayannopoulos V., Burlina A.B., Sykut-Cegielska J., Wijburg F.A., Teles E.L. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis. 2015;38(6):1059–1074. doi: 10.1007/s10545-015-9840-x. [DOI] [PubMed] [Google Scholar]
- 39.Imbard A., Garcia Segarra N., Tardieu M., Broue P., Bouchereau J., Pichard S. Long-term liver disease in methylmalonic and propionic acidemias. Mol Genet Metab. 2018;123(4):433–440. doi: 10.1016/j.ymgme.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 40.de Keyzer Y., Valayannopoulos V., Benoist J.F., Batteux F., Lacaille F., Hubert L. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66(1):91–95. doi: 10.1203/PDR.0b013e3181a7c270. [DOI] [PubMed] [Google Scholar]
- 41.Schuck P.F., Rosa R.B., Pettenuzzo L.F., Sitta A., Wannmacher C.M., Wyse A.T. Inhibition of mitochondrial creatine kinase activity from rat cerebral cortex by methylmalonic acid. Neurochem Int. 2004;45(5):661–667. doi: 10.1016/j.neuint.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro L.R., Della-Pace I.D., de Oliveira Ferreira A.P., Funck V.R., Pinton S., Bobinski F. Chronic administration of methylmalonate on young rats alters neuroinflammatory markers and spatial memory. Immunobiology. 2013;218(9):1175–1183. doi: 10.1016/j.imbio.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Pettenuzzo L.F., Ferreira Gda C., Schmidt A.L., Dutra-Filho C.S., Wyse A.T., Wajner M. Differential inhibitory effects of methylmalonic acid on respiratory chain complex activities in rat tissues. Int J Dev Neurosci. 2006;24(1):45–52. doi: 10.1016/j.ijdevneu.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Jafari P., Braissant O., Zavadakova P., Henry H., Bonafe L., Ballhausen D. Brain damage in methylmalonic aciduria: 2-methylcitrate induces cerebral ammonium accumulation and apoptosis in 3D organotypic brain cell cultures. Orphanet J Rare Dis. 2013;8:4. doi: 10.1186/1750-1172-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amaral A.U., Cecatto C., Castilho R.F., Wajner M. 2-Methylcitric acid impairs glutamate metabolism and induces permeability transition in brain mitochondria. J Neurochem. 2016;137(1):62–75. doi: 10.1111/jnc.13544. [DOI] [PubMed] [Google Scholar]
- 46.Wajner M., Goodman S.I. Disruption of mitochondrial homeostasis in organic acidurias: insights from human and animal studies. J Bioenerg Biomembr. 2011;43(1):31–38. doi: 10.1007/s10863-011-9324-0. [DOI] [PubMed] [Google Scholar]
- 47.Ostergaard E., Wibrand F., Orngreen M.C., Vissing J., Horn N. Impaired energy metabolism and abnormal muscle histology in Mut- methylmalonic aciduria. Neurology. 2005;65(6):931–933. doi: 10.1212/01.wnl.0000176065.80560.26. [DOI] [PubMed] [Google Scholar]
- 48.Wilnai Y., Enns G.M., Niemi A.K., Higgins J., Vogel H. Abnormal hepatocellular mitochondria in methylmalonic acidemia. Ultrastruct Pathol. 2014;38(5):309–314. doi: 10.3109/01913123.2014.921657. [DOI] [PubMed] [Google Scholar]
- 49.Morath M.A., Okun J.G., Muller I.B., Sauer S.W., Horster F., Hoffmann G.F. Neurodegeneration and chronic renal failure in methylmalonic aciduria--a pathophysiological approach. J Inherit Metab Dis. 2008;31(1):35–43. doi: 10.1007/s10545-007-0571-5. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan P., Ficicioglu C., Mazur A.T., Palmieri M.J., Berry G.T. Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol Genet Metab. 2006;88(4):322–326. doi: 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material