Significance
The origin and evolution of Earth’s nitrogen is often discussed by comparing the large variation of N-isotopic compositions among Earth’s building blocks (chondrites) to the signatures of various terrestrial reservoirs. Here, we demonstrate that planetary differentiation processes, such as core formation, may have significantly modified the N-isotopic composition of the proto-Earth. During core–mantle differentiation, a significant amount of isotopically light N entered Earth’s core, producing an isotopic fractionation much larger than has been observed for other geochemical tracers of core formation. The magnitude of N-isotopic fractionation varies significantly as a function of the redox history of the early Earth. Therefore, distinct N-isotopic ratios among Earth’s reservoirs or between planetary bodies may reflect different planetary evolution processes as opposed to different N sources.
Keywords: nitrogen isotopes, fractionation, core formation, speciation, ion probe
Abstract
The present-day nitrogen isotopic compositions of Earth’s surficial (15N-enriched) and deep reservoirs (15N-depleted) differ significantly. This distribution can neither be explained by modern mantle degassing nor recycling via subduction zones. As the effect of planetary differentiation on the behavior of N isotopes is poorly understood, we experimentally determined N-isotopic fractionations during metal–silicate partitioning (analogous to planetary core formation) over a large range of oxygen fugacities (ΔIW −3.1 < logfO2 < ΔIW −0.5, where ΔIW is the logarithmic difference between experimental oxygen fugacity [fO2] conditions and that imposed by the coexistence of iron and wüstite) at 1 GPa and 1,400 °C. We developed an in situ analytical method to measure the N-elemental and -isotopic compositions of experimental run products composed of Fe–C–N metal alloys and basaltic melts. Our results show substantial N-isotopic fractionations between metal alloys and silicate glasses, i.e., from −257 ± 22‰ to −49 ± 1‰ over 3 log units of fO2. These large fractionations under reduced conditions can be explained by the large difference between N bonding in metal alloys (Fe–N) and in silicate glasses (as molecular N2 and NH complexes). We show that the δ15N value of the silicate mantle could have increased by ∼20‰ during core formation due to N segregation into the core.
The bulk silicate Earth is the largest nitrogen reservoir on Earth, containing 3 to 11 times more N (27 ± 16 × 1018 kg N) than the present-day atmosphere (4 × 1018 kg N or the present atmospheric nitrogen abundance [PAN]) (1). In particular, Earth’s mantle is estimated to contain 6 PAN (up to 24 × 1018 kg N) (1), which is 12 times more than the continental crust (0.5 PAN) (1). The 2 stable isotopes of nitrogen, 14N and 15N (∼99.6% and ∼0.4% of present atmospheric N2, respectively), are used to trace fluxes between surface and deep-Earth reservoirs. N stable isotopic compositions are conventionally normalized to the present atmospheric value and reported as follows:
| [1] |
The current N-isotopic distribution among terrestrial reservoirs shows an unexplained difference (2) between the 15N-depleted mantle [δ15N = −5 ± 2‰ in diamonds and midocean ridge basalts (MORBs) (3)] and the 15N-enriched surface [δ15N = +6‰ in sediments, and 0‰ (by definition) in the present-day atmosphere (4)]. Contradictory to these observations, diffusion-controlled N2 degassing (5) of the mantle is expected to preferentially segregate 14N into the atmosphere (Graham’s law), which would enrich the mantle in 15N. Equilibrium degassing of MORBs modeled by Rayleigh distillation (5, 6) enriches the residual melt in 15N, but this fractionation is limited to 1 to 1.5‰ (7). Recycling (subduction) of surficial material is expected to increase the δ15N value of the mantle because sediments and altered continental crust are enriched in 15N (3) by biological processes [i.e., N fixation pathways and N cycling within microbial communities (e.g., refs. 8 and 9)] and metamorphism-related devolatilization of N (4), respectively. Marty and Dauphas (7) proposed that the mantle’s N-isotopic signature corresponds to that of recycled Archean sediments; this hypothesis has been actively debated (10) because the δ15N values of ancient sediments are highly variable [−6.2 to +13‰ (8)]. Hence, neither degassing processes nor recycling can explain the mantle’s N-isotopic signature relative to the atmosphere (3).
An alternative explanation of the mantle’s N-isotopic signature is the heterogeneous accretion of the Earth from materials with δ15N values as low as −40‰ (11), the lowest value measured on Earth in deep diamonds (12). Indeed, the low δ15N values of enstatite chondrites [−45 to −15‰ (13)] compared with carbonaceous chondrites [+15 to +55‰ (14)] suggest an enstatite chondrite origin of Earth’s nitrogen (11, 12). After accretion, planetary differentiation processes could have modified the initial N budget and isotopic signature of the mantle. Core–mantle differentiation, magma ocean solidification/crystallization, and magma ocean degassing are key stages of early planetary evolution. These 3 processes could potentially result in N-isotopic fractionations sufficient to explain the large differences in current δ15N values among the distinct terrestrial reservoirs (3).
Recent studies have revealed that a significant fraction of N could have been segregated into the core during the late stages of core formation (15–22). Investigation of the effect of core formation on the evolution of the bulk mantle δ15N value requires knowledge of the N-isotopic fractionation factors between metal and silicate phases. Li et al. (16) measured the 15N/14N fractionation between metal and basaltic glasses (∆15Nmetal-silicate = δ15Nmetal − δ15Nsilicate) at 1.5 to 7 GPa and 1,600 to 1,800 °C. They showed that during metal–silicate segregation, the silicate melt is enriched in 15N by 3.5 ± 2.5‰ relative to the coexisting liquid Fe–C–N metal alloy. These experimental results are consistent with an equilibrium isotopic fractionation, which causes the lighter isotope to be enriched in the most reduced phase (23). However, the large uncertainties on their isotopic fractionation factors (typically 2.5‰) preclude any definitive conclusion on the effect of metal–silicate N segregation. Additionally, the relatively narrow range of oxygen fugacities (fO2, reported as the logarithmic difference between experimental fO2 conditions and the iron–wüstite buffer [IW]) explored by Li et al. [ΔIW −0.11 to −0.35 (16)] presumably corresponds only to the latest stage of core–mantle differentiation (24). Although the fO2 conditions of Earth’s core formation are still debated (e.g., refs. 25–27), recent models acknowledge that the Earth accreted from highly reduced materials (typically around ΔIW −5) and that its fO2 increased throughout the late stages of accretion and core–mantle differentiation (reaching ΔIW −2 to IW during the late stages of the magma ocean; refs. 24, 26, and 28). Recent studies have shown fO2 to be the main control on N partitioning during metal–silicate segregation (17–22) because it determines the N speciation in the silicate phase (17, 29). N speciation, in turn, is expected to dictate ∆15Nmetal-silicate (29). The magnitude of isotopic fractionations is proportional to the difference in bond stiffness and vibration modes between equilibrated phases (30, 31), i.e., ∆15Nmetal-silicate is determined by the bonding of N in the metal (as Fe–N) and silicate melt [as N–N vs. N–H complexes depending on fO2 conditions (29)]. Therefore, fO2 is expected to play a fundamental role in the evolution of the δ15N value of Earth’s mantle, and thus in the establishment of the observed difference between surficial and deep N-isotopic signatures.
In this study, we investigated the redox effect on N-isotopic fractionation during metal–silicate N partitioning in samples quenched at 1 GPa and 1,400 °C in a piston-cylinder (Laboratoire Magmas et Volcans, Clermont-Ferrand, France). We explored fO2 conditions ranging from Fe–FeO equilibrium (IW) to ΔIW −3 (i.e., 3 orders of magnitude more reducing), consistent with the inferred conditions of core formation (32). The duration of the experiments was 6 h, which is sufficient to reach compositional equilibrium (17). The starting material compositions comprised a Fe-rich haplo-basalt [similar to the Humphrey basalt composition of Dalou et al. (17) and Armstrong et al. (33)] to promote the segregation of large metal blobs under reducing conditions, and a haplo-andesite composition similar to that used by Zhang et al. (34). These mafic to intermediate compositions were chosen as analogs of planetary magma ocean compositions (35). The fO2 of the samples was changed (not controlled) by adding 0.5 to 4 wt% Si3N4 [a reducing agent (17)] to the starting compositions. To “spike” the samples in 15N, we used mixtures of Si314N4 and Si315N4 as the N source. Si314N4 was synthetized by Si nitridation using flowing N2 in a Nabertherm 1-atm furnace, whereas isotopically enriched Si315N4 (∼95% 15N) was prepared as described by Leonova et al. (36). Mixtures of Si314N4 and Si315N4 were used to obtain variable initial 15N/14N ratios (between 0.1 and 8; Table 1) in the starting materials to assess the effect of 15N spiking. Experimental procedures are described in SI Appendix, section S1.
Table 1.
Redox conditions, N elemental and isotopic compositions of the samples, and calculated N elemental and isotopic fractionations
| Sample | fO2 (ΔIW) | [N]silicate, ppm) | [N]metal, ppm | DNmetal-silicate | 15N/14N initial* | , ‰ | , ‰ | αmetal-silicate | ∆15Nmetal-silicate, ‰ | [H2O]silicate, wt%† |
| HB01 | −3.13 | 186 ± 2 | 28 ± 3 | 0.2 ± 0.2 | 0.09 ± 0.01 | 72 ± 10 | −171 ± 28 | 0.77 ± 0.09 | −257 ± 22 | 0.20 ± 0.04 |
| HB05 | −0.45 | 235 ± 36 | 1,571 ± 1,287‡ | 7 ± 2 | 0.67 ± 0.07 | 23 ± 3 | −27 ± 4 | 0.95 ± 0.02 | −49 ± 1 | 0.267 ± 0.002 |
| HB15 | −1.40 | 420 ± 22 | 682 ± 267 | 1.6 ± 0.8 | 8.0 ± 0.8 | 25 ± 4 | −88 ± 13 | 0.89 ± 0.05 | −116 ± 6 | 0.13 ± 0.03 |
| HB3 | −1.46 | 448 ± 5 | 1,493 ± 149 | 3.3 ± 0.2 | 0.80 ± 0.08 | 36 ± 5 | −95 ± 13 | 0.87 ± 0.01 | −135 ± 1 | 0.13 ± 0.09 |
| HB4 | −1.21 | 493 ± 12 | 1,488 ± 14 | 3.02 ± 0.05 | 0.85 ± 0.08 | 16 ± 2 | −70 ± 10 | 0.92 ± 0.01 | −89 ± 1 | 0.13 ± 0.03 |
| A05 | −2.15 | 855 ± 18 | 669 ± 126 | 0.8 ± 0.4 | 0.67 ± 0.07 | 11 ± 2 | −14 ± 2 | 0.976 ± 0.002 | −25 ± 1 | 0.19 ± 0.01 |
Reported errors are 2σ SDs.
Estimated from the addition of 14Si3N4 and 15Si3N4 to the starting material. SDs on were estimated from weighing uncertainties related to the addition of Si3N4 to the starting material, and thus are larger than αmetal-silicate and ∆15Nmetal-silicate (‰), which were directly determined from SIMS measurements.
Analyzed by SIMS (1280 HR2) following the method of Sobolev et al. (37). Details of the measurement conditions and standards are given in SI Appendix, section S1.
Large error due to C heterogeneities, likely graphite microinclusions, in the metal blobs.
Silicate- and Metal-Phase Nitrogen Analyses
Previous studies of N partitioning between metal and silicate phases (15, 17–22, 38) presented N abundance data obtained by electron probe microanalysis (EPMA). This in situ analytical method allows measuring N contents in metal alloys and silicate glasses in a polished section of an experimental charge with a detection limit as low as 0.03 wt% (17, 39) but is not adapted to isotopic measurements. Noble gas mass spectrometry enables bulk N-isotopic analyses (16) but, in our case, can only provide phase-specific isotopic compositions if the silicate glass and metal blobs are physically separated and measured independently, a challenging task depending on the sample texture (SI Appendix, Fig. S1).
We thus developed a method for in situ measurement of the N concentrations and isotopic compositions of quenched silicate and metal alloy phases by secondary ion mass spectrometry (SIMS) using the CAMECA IMS 1280 HR2 and 1270 at the Centre de Recherches Pétrographiques et Géochimiques (Nancy, France). Following the analytical protocol of Füri et al. (40), nitrogen isotopes in silicate glasses were measured with the IMS 1280 HR2 by spot analyses of 14N16O− and 15N16O− secondary molecular ions at mass numbers 30 and 31, respectively, at a nominal mass resolution m/Δm = 14,000 using a Cs+ primary ion beam with a current of ∼10 nA. A suite of reference glasses with known 14N contents (40, 41) was used to determine the nitrogen concentration of each sample based on the 14N16O− count rate normalized by the 16O2– signal (SI Appendix, section S1). The nitrogen and carbon abundances and 15N/14N and 13C/12C ratios of metal phases were measured on the IMS 1270 using a Cs+ primary ion beam with a current of ∼2.4 nA. The size of the primary beam was set to ∼5 µm to analyze metal blobs 10 to 65 µm in diameter. Carbon concentrations were measured as 12C2–, whereas nitrogen isotopes were measured as 12C14N− and 12C15N− at mass numbers 26 and 27, respectively, with a mass resolution of 8,000. Nitrogen elemental abundances were determined using the 12C14N−/12C2− ratio (42, 43). Owing to the low Si solubility in metals at the studied pressure–temperature (P–T) and fO2 conditions (17), 28Si− was analyzed to monitor for potential silicate contamination during analyses. In parallel to the SIMS measurements, the nitrogen contents and 15N/14N ratios of reference silicate glasses (40) and metal reference materials were determined independently by noble gas mass spectrometry (SI Appendix, section S1). The use of 15N-spiked samples allowed us to determine the 15N/14N ratios in the silicate and metal phases with average analytical uncertainties of 0.1‰ and 5.5‰, respectively. However, this uncertainty reached 91‰ for metal alloys in sample HB01, likely due to its highly heterogeneous C distribution.
Raman spectroscopic analyses were performed to determine N speciation in silicate glasses and metal alloy blobs (SI Appendix, section S1).
Sample Description
Experimental run products were composed of graphite-saturated mafic glass, close to the melt composition expected during a magma ocean stage (35), coexisting with variable amounts of an iron carbide metallic phase (SI Appendix, section S1 and Fig. S1). Among the experiments run, 6 samples had metal blobs of sufficient diameter (>7 µm) to permit SIMS analysis (SI Appendix, Fig. S1 and Table 1): only the data from these samples are presented herein. Silicate melts quenched to form large glass pools that were well separated from metal domains. Both silicate glasses and Fe–C–N alloys were compositionally homogeneous at the scale of EPMA analyses (∼2 μm for metals; SI Appendix, section S1 and Tables S1 and S2). Along with Fe, the Pt and Si concentrations in the metal phases of all samples were measured by EPMA, which revealed Si contents below the detection limit and low amounts of Pt (<0.7 wt%). The major element compositions of silicate glasses and metal alloys determined by EPMA are presented in SI Appendix, Tables S1 and S2, respectively.
Table 1 presents sample oxygen fugacities, expressed as log fO2 (ΔIW), as estimated from the equilibrium reaction:
| [2] |
Following this equilibrium, the fO2 of each sample was calculated according to the method described in Dalou et al. (17), i.e., using the relationship between the mole fractions of FeO in the silicate melt and Fe in the liquid metal , the associated activity coefficients ( and , respectively), and the equilibrium constant, K, of the reaction (44):
| [3] |
As described by Dalou et al. (17) and Grewal et al. (21, 22), we accounted for the effect of N and C on by extending the Wagner ε formalism (45) for nonideal interactions in liquid Fe alloys (46) via the MetalAct metal activity calculator (47), and we assumed = 1.2 (48). Moreover, to permit direct comparison with the IW buffer, which is referenced to solid Fe metal, the activity coefficient of Fe was adjusted following the method of Stanley et al. (49). Finally, the resulting fO2 was compared with the IW buffer as determined by Zhang et al. (50). This calculation was applied to our samples and all literature data presented for comparison in the figures. The fO2 of our samples spanned from ΔIW −0.45 to −3.13.
We noted a significant loss of N (up to 75%) between the N added to the starting material and the total N measured in our experimental products. Speelmanns et al. (19, 20) observed a similar N loss during their experiments and suggested that N was lost to the graphite capsules enclosing their samples. Nonetheless, no significant difference in N loss is observed in N-partitioning experiments using single graphite (this study) or double platinum–graphite capsules (22). The samples showed no signs of vapor bubbles in the silicate glasses or metal alloy blobs. Although we cannot rule out the possible presence of submicroscopic bubbles in these phases, we assume that our experiments were N-vapor saturated and that N was lost to pores in the graphite capsules during the experiments. Therefore, the measured elemental and isotopic N fractionations between the metal and silicate phases occurred in an open system. The effect of N loss on N-isotopic fractionation is discussed in the following section.
The reported N and C contents and N-isotopic ratios and their associated SDs (2σ) were averaged from 2 to 6 analyses per sample (SI Appendix, Tables S2 and S3). Nitrogen and carbon contents in metal alloys are homogeneous within most samples, with SDs below 22% and 2% (2σ), respectively. The N contents of silicate glasses in each sample were homogeneous with a SD below 9%. As observed by Dalou et al. (17) and Füri et al. (40), the glasses contained micrometer-sized graphite inclusions, causing highly variable C signals over the course of an analysis and between distinct areas. Given that silicate glass N contents and isotopic compositions were measured as 14N16O−/16O2− and 15N16O−/14N16O− instead of 12C14N−/12C2− and 12C15N−/12C14N−, this variability does not affect our N-elemental and -isotopic analyses (40). In samples for which metal blobs were large enough to perform multiple measurements (up to 4 analyses in HB05 and HB3, and 6 in HB4), homogeneous N-isotopic ratios were observed across the metal blobs, with SDs below 2% (2σ; SI Appendix, Table S3). Nitrogen contents in silicate glasses and metal alloy blobs are presented in Table 1, and carbon contents in metal phases in SI Appendix, Table S2.
It is very difficult to ensure that isotopic equilibrium is attained during isotope fractionation experiments that do not allow application of the “3-isotope method” (51). At temperatures from 1,600 °C to 1,800 °C, Li et al. (16) performed a series of experiments with increasing equilibration times between 30 and 120 min to ensure that isotopic equilibrium was attained. To promote isotopic equilibrium at 1,400 °C, we extended the run periods of our experiments to 6 h. Moreover, the homogeneity of N contents and 15N/14N ratios of the metal blobs and silicate glasses (SI Appendix, Table S3) strongly suggests that both chemical and isotopic equilibria were attained during our experiments.
fO2 Control on N Partitioning Between Metal and Silicate Phases
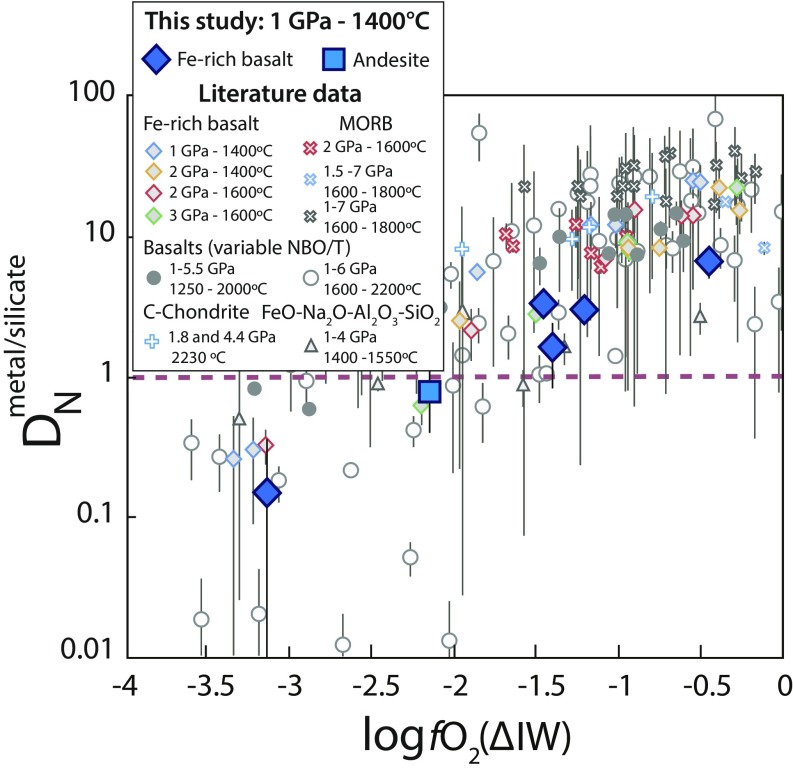
Nitrogen partition coefficients between the metal and silicate phases, DNmetal-silicate, were calculated as DNmetal-silicate = CNmetal/CNsilicate, where CNmetal and CNsilicate are the average N contents (wt%) of the metal alloys and silicate glasses, respectively (Table 1). With decreasing fO2 from ΔIW −0.45 to −3.13, DNmetal-silicate decreases from 7 ± 2 to 0.2 ± 0.2, consistent with previous experimentally determined partition coefficients (15–22, 52, 53) (Fig. 1). Despite highly variable experimental parameters between studies (melt composition, P, and T), which produce scatter in the data trend (22), fO2 remains the key parameter controlling N partitioning between the metal and silicate phases (17). DNmetal-silicate decreases with decreasing fO2 due to the stabilization of N species in more reduced silicate melts (17).
Fig. 1.
DNmetal-silicate as a function of fO2. Large symbols represent DNmetal-silicate values from this study, and small symbols are results from several previous studies: diamonds and red x’s (17), blue x’s (16), black x’s (21), gray circles (20), empty circles (22), triangles (18, 37, 50, 51), and blue crosses (15). All fO2 values were recalculated following the procedure described in the text. Following this calculation, only 2 of the 4 samples from Li et al. (16) have log fO2 < ΔIW. Error bars represent 2σ SDs. P–T conditions have only a small effect on DNmetal-silicate and explain the scatter among the overall decreasing trend with decreasing fO2. Our data measured using SIMS (Fe-rich basalt and andesite) are in agreement with previously published DNmetal-silicate values measured by EPMA.
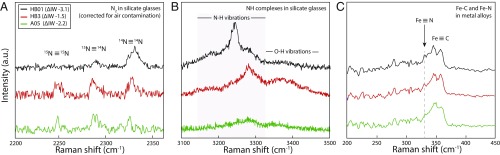
In glasses, Raman spectroscopic analysis revealed that nitrogen is dissolved as N2 and NH complexes (Fig. 2 A and B). At near-constant water content (Table 1), the ratio of NH to OH complexes (NH/OH, as determined from the Raman peak areas) increases with decreasing fO2 (54), reflecting the increased N solubility in silicate glasses at more reducing conditions (17, 53). We note that vibrations associated with NH complexes are more intense in the Raman spectra of Fe-rich basalt samples (noted HB) than in the andesite A05 (Fig. 2B). Increased melt polymerization is known to increase N solubility and decrease the proportion of NH relative to N2, i.e., the NH/N2 ratio (29, 55). Therefore, the melt structure of sample A05 (more polymerized than the Fe-rich basalt glasses) favors N dissolution in the form of N2 rather than NH complexes (Fig. 2 A and B). Overall, decreasing fO2 has 2 effects: it increases both melt polymerization (due to increasing metal precipitation; SI Appendix, section S2) and the NH/N2 ratio. Among the HB samples, we observe that the change of fO2 primarily controls the NH/N2 ratio, whereas at constant fO2, melt polymerization governs the NH/N2 ratio in silicate glasses (29).
Fig. 2.
Representative normalized Raman spectra of N species in silicate glasses and metal alloys of samples HB01, HB3, and A05: (A) N2 isotopomers in silicate glasses, (B) NH complexes in silicate glasses, and (C) Fe–N bonds in metal alloys. The Raman shifts of N species are significantly different depending on speciation (N2 versus NH complexes) and whether N is dissolved in metal alloy or silicate glass. Details of our Raman analysis are presented in SI Appendix, section S1.
Under nominally anhydrous conditions, N is assumed to chemically dissolve as nitride ions (N3−) in reduced melts (below ΔIW −1.3) at 1 atm (56). The vibration mode of Si–N (near 800 cm−1, ref. 57) is in the range of the dominant Si–O vibrations in silicate glasses. We therefore cannot ascertain the presence or absence of nitride species in our glasses by Raman spectroscopy.
Metal-Silicate N Isotopic Fractionation (∆15Nmetal-silicate)
The N-isotopic compositions of metal alloys and silicate glasses in the Fe-rich basalts and haplo-andesite were determined via SIMS. The N-isotopic ratios, , where p denotes the phase, were normalized to the initial N-isotopic composition of the starting material, , which ranged from 0.09 ± 0.01 to 8.0 ± 0.1 (Table 1). The normalized isotopic compositions are represented as follows:
| [4] |
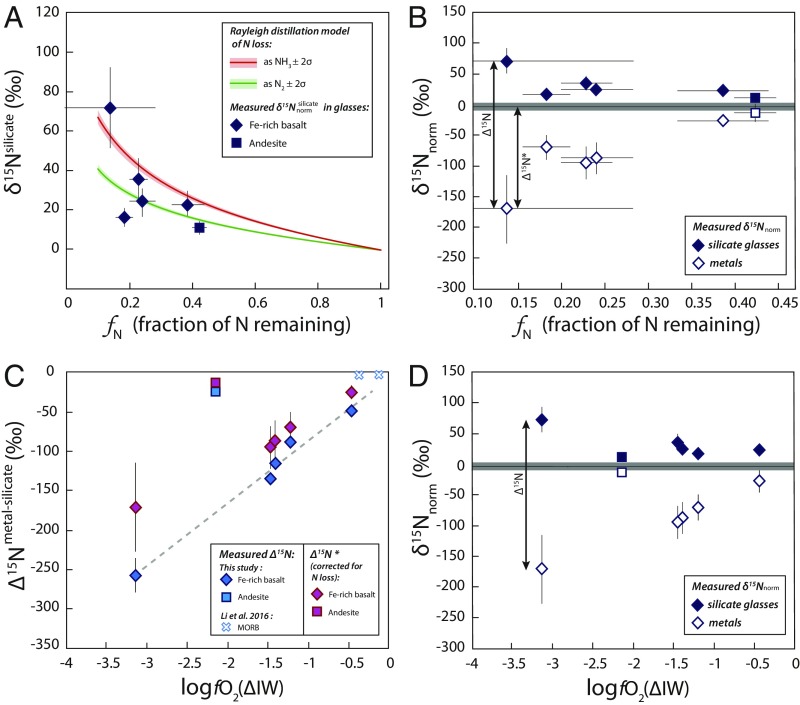
Although these normalized values were not calculated relative to the atmospheric N-isotopic composition and are not directly comparable to δ15N values measured in natural samples (40), they represent the magnitude of the observed change in the isotopic composition of each phase during the experiments. Fig. 3A reveals that N loss (in the form of N2, NH3, or both) by Rayleigh distillation during the experiments could increase the δ15Nsilicate values of the melt and may explain the positive values measured in the silicate glasses (Fig. 3B). In contrast, is clearly negative compared with the initial δ15N value (i.e., δ15Nnorm = 0‰), suggesting that the metal phase becomes enriched in 14N during its segregation (Fig. 3B). Therefore, the observed positive values may result from N loss from the experiments and/or 14N depletion during N partitioning into the metal phase.
Fig. 3.
Effect of N loss (A and B) and fO2 (C and D) on N-isotopic fractionation ∆15Nmetal-silicate. (A) Modeled effect of N loss by Rayleigh distillation on the δ15Nnorm values of silicate glasses as a function of fN, the fraction of N remaining in the sample at the end of the experiments compared with the initial N content of the starting materials. The values represent measured 15N/14Nsilicate ratios normalized to the initial 15N/14N ratio of the starting material (see text and Table 1). Rayleigh distillation was modeled for N2 loss (green curve) and NH3 loss (red curve) as , where α is the fractionation factor approximated here by = 1.02 for N2 loss and by = 1.03 for NH3 loss. The positive δ15N values measured in our silicate glasses can be explained by the Rayleigh distillation model, suggesting that N loss may have increased the δ15N values of the silicate glasses. (B) Evolution of δ15N in the silicate glasses (closed symbols) and in the metal blobs (open symbols) as a function of fN. The δ15Nnorm values represent measured 15N/14Np ratios (p representing the metallic or silicate phase) normalized to the initial 15N/14N ratio of the starting material (see text and Table 1), and the gray line at 0‰ represents the initial δ15Nnorm value. The difference between the δ15N values measured in the metal and the silicate phases (δ15Nmetal − δ15Nsilicate) approximates the calculated ∆15Nmetal-silicate values. Considering that positive values may have been produced by N loss, the difference between the initial (0‰) and the values corresponds to ∆15N*, the N-isotopic fractionation corrected for N loss. (C) ∆15Nmetal-silicate as a function of fO2, showing both calculated ∆15Nmetal-silicate (blue symbols) and ∆15N* (purple symbols). The small blue x's are reproduced from Li et al. (16), with fO2 values recalculated following the procedure described in the text. Error bars represent 2σ SDs. Whereas the uncertainties on ∆15Nmetal-silicate are determined from the SDs on 15N/14N ratios measured by SIMS, the larger uncertainties on fN, δ15Nnorm values, and ∆15N* are derived from the weighing uncertainties associated with the addition of Si314N4 and Si315N4 to the starting materials. The dashed line represents a linear regression (r2 > 0.9) on the ∆15Nmetal-silicate values measured in Fe-rich basalt. ∆15Nmetal-silicate data describe 2 trends that can be explained by compositionally dependent differences in N speciation in the silicate glasses (see text). (D) Evolution of δ15Nnorm in the silicate glasses and metal blobs as a function of fO2. This plot shows that positive values can also be produced by N-isotopic fractionation during metal segregation from the melt via the formation of 14N–Fe bonds in the metal from 14N in NH complexes in the melt. This process becomes more important as the fO2 decreases due to the increasing stabilization of NH complexes in the melt under more reducing conditions.
The N-isotopic fractionations between metal and silicate in the samples (Table 1 and Fig. 3C) were calculated from the and ratios measured by ion probe as follows:
| [5] |
They decrease from −49 ± 1‰ to −257 ± 22‰ with decreasing fO2 from ∆IW −0.45 to −3.13. Such negative N-isotopic fractionations are in accord with equilibrium fractionation, during which the lightest isotopes favor bonds in the most reduced phase (23, 56), here as Fe–N (Fig. 2C). As the metal segregates from the silicate melt at isotopic equilibrium, it more favorably forms 14N–Fe bonds than 15N–Fe bonds, thereby depleting the silicate melt in 14N; this process may increase the values. Alternatively, assuming that Rayleigh distillation alone produced the positive values observed in Fig. 3 A and B, then, when corrected for this process, can be assumed to remain at 0‰ during N partitioning into the metal phase. In this scenario, N-isotopic fractionations corrected for N loss, expressed as with corrected to 0‰ (Fig. 3B), decrease from −27 ± 4‰ to −171 ± 28‰ with decreasing fO2 from ∆IW −0.45 to −3.13 (Fig. 3C). Following this reasoning, ∆15Nmetal-silicate and ∆15N* represent the maximum and minimum bounds on metal–silicate N-isotopic fractionation in our experiments, respectively.
The ∆15Nmetal-silicate and ∆15N* values from this study are significantly lower than those obtained by Li et al. [∆15Nmetal-silicate between 1.1 ± 2.8‰ and −5.5 ± 2.3‰ (16)]. This difference may be explained by the different P–T conditions of the 2 studies, although the results of Li et al. (16) show no pressure or temperature dependence over 1.5 to 7 GPa and 1,600 to 1,800 °C. It is, however, questionable that they do not observe any temperature effect on ∆15Nmetal-silicate since, by definition, fractionation factors are inversely proportional to temperature (e.g., refs. 23 and 58). In addition, our large N-isotopic fractionations cannot be explained by 15N spiking of our samples. As has been shown for H isotopic fractionation (59), ∆15Nmetal-silicate values are not dependent on the initial 15N/14N ratios of the experiments (Table 1). Instead, fO2 is the key parameter differing between the 2 studies. The fO2 of the samples of Li et al. (16) were between ∆IW −0.11 and −0.35, whereas the fO2 of our samples varies from ∆IW −0.45 to −3.13. This observation implies an effect of fO2 on ∆15Nmetal-silicate.
These large N-isotopic fractionation values are comparable to estimates of H metal–silicate isotopic fractionation [between −55 and −285‰, modeled based on chondritic and mantle-like D/H values and H solubility in metal and silicate phases (60)], although, to our knowledge, no experimental data on metal–silicate fractionation are available for hydrogen or carbon isotopes. There are, however, experimental data available on H- and C-isotopic fractionations during other high-temperature magmatic processes. For instance, hydration or dehydration of silicate melts and nominally anhydrous minerals produces δD variations of up to 550‰ at 1,000 °C (61). During aqueous fluid–silicate melt interaction, H- and C-isotopic fractionations can reach 150‰ at 750 °C (56, 62–64). Among other isotope tracers of core formation, i.e., isotopes of heavier siderophile elements such as Si, Fe, Mo, Cr, Cu, and Ni (65), sulfur is the most comparable to N in terms of mass, solubility in reduced melts (66), and volatile behavior. However, experimentally determined metal–silicate S isotopic fractionation factors, ∆34Smetal-silicate, are only on the order of 0.1 to 1.2‰ between ∆IW −0.6 and −4.1 (67). Although the mass difference between N isotopes is 2.5 times larger than that between S isotopes, this difference is insufficient to explain the vast difference between the metal–silicate isotopic fractionations of N and S.
The Effects of fO2 and N Speciation on ∆15Nmetal-silicate
Following Bigeleisen and Mayer (30) and Urey (31), mass-dependent N-isotopic fractionation can be expressed as follows:
| [6] |
where m14 and m15 are the atomic masses of 14N and 15N, respectively, Δm is their mass difference, is the difference between the force constants acting on the isotopes in the metallic phase and the silicate melt (, with and the effective force constants that depend on the chemical bond stiffness of N bonds in the metallic phase and silicate glass, respectively), and T is temperature in kelvin. In this study, at constant T, is the only variable in the above equation acting on the N-isotopic fractionation between metal alloy and silicate melt, along with fO2 variations. This parameter is related to vibrational frequency, vN, (30, 51), following:
| [7] |
where vN was determined from the Raman vibration modes of N species in the metallic phases and the silicate glasses (Fig. 2) and µN is the reduced mass of the N-bearing molecule, calculated for N–H as µ(N–H) = [mN × mH/(mN + mH)], with mN and mH being the atomic masses of N and H, respectively. More details on the principles and factors controlling stable isotopic fractionations can be found in Schauble (23) and Young et al. (51). Nitrogen dissolves in silicate glasses as molecular nitrogen (N2) and NH complexes, and as Fe–N in metal alloys. N2 has 3 isotopomers, 15N≡15N, 15N≡14N, and 14N≡14N, with vibration modes in silicate glasses at 2,251, 2,287, and 2,326 cm−1, respectively (Fig. 2A) (55), whereas the vibration mode of gaseous 14N≡14N is at 2,331 cm−1 (68). The vibration modes of NH complexes are between 3,180 and 3,350 cm−1 (Fig. 2B) (e.g., refs. 41 and 69). Fe–N vibrations in metal alloys are distinctly different, occurring at 338 cm−1 (Fig. 2C) (70). In comparison, S dissolves in metal alloys as Fe–S with vibration modes at 310 and 360 cm−1, and in silicate melts as Fe–, Mg–, and Ca–S with vibration modes between 285 and 350 cm−1 (66). Although the vibration modes of complexes involving N or S have been determined for quenched silicate and metal, their equivalents in silicate melts and liquid metals at HP–HT are expected to vary only by a few cm−1, as shown by Mysen (71) for the N2 vibration mode in silicate melts and aqueous fluids. The observed vibration modes are directly related to the reduced masses µN and µS of the bonds formed by N and S, respectively, in the metal and silicate phases. As the relevant N bonds have very different reduced masses [µ(N–H) = 1 a.m.u., µ(N≡N) = 7 a.m.u., and µ(Fe–N) = 11 a.m.u.], the bonding of N with ions of very different masses directly produces large differences in the force constants (i.e., ∆Fmetal-silicate) acting on N isotopes in the 2 phases. In comparison, the smaller ∆Fmetal-silicate values for S isotopes should result from the similar reduced masses of S bonds [µ(Mg–S) = 14 a.m.u., µ(Ca–S) = 18 a.m.u., and µ(Fe–S) = 20 a.m.u.]. Therefore, we conclude that the observed large N-isotopic fractionations relative to those of S are a consequence of the distinct N speciation between the metallic phase and the silicate melt.
The difference in vibrational frequencies and reduced masses between the metal and silicate phases is higher when N is dissolved as N–H complexes (e.g., NH3) than as molecular N2. The basic equation for equilibrium isotopic fractionation for stable isotopes, derived from Bigeleisen and Mayer (30), Urey (31), and Young et al. (51), can be expressed as follows:
| [8] |
where h is Planck’s constant (in joules per kelvin), kb is the Boltzmann constant (in joules per hertz), T is temperature in kelvin, and and denote the Raman vibration modes (in hertz) for the metal and silicate phases, respectively. From Eq. 8, we estimate to be −4 to −24‰ for N dissolved as N2 or NH3 in the silicate glass, respectively. Eq. 8 only considers N speciation differences between phases and assumes the absence of N interactions between molecular groups within each metal or silicate phase (e.g., different structural units). In general, however, this assumption is not valid for silicate melts because strong intermolecular bonds influence isotopic exchanges, thereby leading to a significant underestimation of the isotopic fractionation between distinct phases (e.g., refs. 59, 67, 72, and 73). However, this calculation shows that larger N-isotopic fractionations between the metal and silicate phases are produced depending on whether N is dissolved as N–H complexes or as molecular N2. Accordingly, we can assume that higher NH/N2 ratios in the melt produce larger metal–silicate N-isotopic fractionations than when N is present in the melt mainly as N2. The increasing stabilization of NH complexes with decreasing fO2 (Fig. 2B) therefore increases the observed metal–silicate N-isotopic fractionation among HB samples (Fig. 3C and SI Appendix, Fig. S5). In contrast, the ∆15Nmetal-silicate value of A05, −25 ± 1‰, is significantly smaller than those of the Fe-rich basalts at equal fO2 values (Fig. 3). This discrepancy may be explained by the lower NH/N2 ratio of the andesite compared with that of the Fe-rich basalts.
Although not observed in our glasses, N dissolution as nitrides in the silicate melt would lead to smaller metal–silicate N-isotopic fractionation than for N dissolved as N2 or N–H complexes, given the small difference in vibration modes between Fe–N in metals and Si–N in silicates ( 462 cm–1).
Nitrogen Isotopic Fractionation during Core Formation
N partitioning data (this study; refs. 15–22, 38, 52, and 53) show that, during terrestrial core–mantle differentiation, a fraction of N must have been removed from the magma ocean into the core-forming metals (Fig. 1). This N segregation produced an isotopic fractionation that depleted the metallic phase and enriched the silicate mantle in 15N and was fundamentally dependent on the fO2 conditions of the proto-mantle. Recent models (24, 28, 74) propose that the Earth accreted from small and highly reduced materials (∆IW −5) during the first stages of its formation (60 to 70% of its mass), then from larger and more oxidized bodies (∆IW −2) toward the end of accretion. Thus, the studied fO2 range (∆IW −3.1 to −0.5) represents plausible conditions of metal–silicate equilibration during the later stages of accretion and core formation (24, 74). During progressive oxidation from ∆IW −3.1 to −0.5, while the fraction of Earth’s N increased in core-forming materials, the N-isotopic fractionation between the segregating metal and the silicate mantle would likely have decreased. These concurrent processes can be modeled using an equilibrium model of Earth’s core–mantle differentiation (16). We adapted an equilibrium model of core formation that assumes complete equilibrium between the silicate mantle and the forming core (16) to account for progressive metal–silicate equilibration. This progressive equilibration is represented by the ratio of the masses of the silicate mantle and the bulk Earth (BE), , which varied between 1 (precore) and 0.67 (present-day Earth). The total mass of N in the silicate mantle or BE is calculated from the following:
| [9] |
where is the N concentration in the mantle or BE, with
| [10] |
The evolution of the N-isotopic composition of the mantle during core formation is expressed as follows:
| [11] |
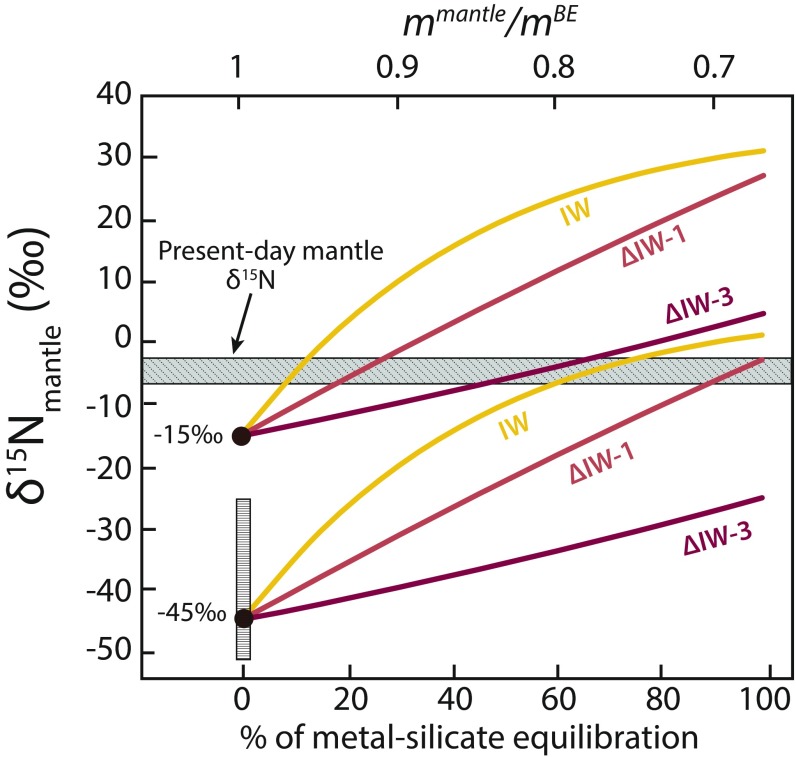
This model is primarily dependent on the N-isotopic composition of the BE, , and, to a lesser extent, , necessitating assumptions regarding the nature of the accreting bodies that formed the Earth. Considering an enstatite chondrite origin for Earth’s N (11) and assuming no N was lost during accretion, the value of the proto-Earth was 160 ppm (75). For , we chose to explore the 2 most extreme δ15N values measured in enstatite chondrites: −45 and −15‰ (13). Over the fO2 range ∆IW −3.1 to −0.5 (i.e., using the N partition coefficients and isotopic fractionation values derived from our experiments), a proto-Earth with a value of −45‰ would result in a value between −25 and +1‰ at the end of core formation depending on the fO2, whereas a value of −15‰ would result in a value between +5 and +31‰ (Fig. 4). The modeled trend is linear at ∆IW −3 but logarithmic at IW, such that the difference between the 2 trends increases to a maximum of ∼27‰ at ∼60% metal–silicate equilibration, and then decreases to ∼20‰ at 100% metal–silicate equilibration (Fig. 4). This simple model demonstrates that the present-day mantle’s δ15N value of −5‰ could have been produced by core–mantle differentiation, assuming that enstatite chondrites are Earth’s primary building blocks and the source of terrestrial N (or if was between −51 and −25‰; Fig. 4). Nevertheless, the outcome of this model is mainly controlled by the value of (Fig. 4) and therefore the source of Earth’s N, which remains a matter of debate (5, 76).
Fig. 4.
Modeled δ15N values of the silicate proto-mantle during core formation as a function of core–mantle equilibration and fO2 conditions of the magma ocean. We explored 2 different values: the lowest (−45‰) and highest (−15‰) values measured in enstatite chondrites (13). The respective values used for DNmetal-silicate and ∆15Nmetal-silicate were 7 and −49‰ at IW, 3.02 and −89‰ at ∆IW −1, and 0.2 and −257‰ at ∆IW −3.1 (Table 1). The present-day value of the mantle is −5 ± 2‰ (gray bar; the thickness of the bar represents the 2σ SD), based on a compilation from diamonds and MORBs (3). This model assumes that ∆15Nmetal-silicate was not affected by Rayleigh distillation during the experiments, and thus only considers that the mantle’s value was modified by core–mantle fractionation without accounting for the effect of degassing. After 30% core–mantle (metal–silicate) equilibration, the modeled fO2 variations account for a difference in the mantle δ15N value of up to 27‰. The vertical bar between −51 and −25‰ represents the plausible range of δ15N values of Earth’s building blocks necessary to produce the present-day δ15Nmantle value of −5 ± 2‰ at fO2 conditions between ∆IW −3 and −0.5, assuming that core formation is the only process that fractionated N isotopes during the Earth’s early history. At ∆IW −3.1, assuming a value of −25‰ for Earth’s building blocks, complete metal–silicate equilibration is required to reach the present-day δ15Nmantle value. In contrast, at IW, conditions under which N is siderophile, the present-day δ15Nmantle value is attained at 60% metal–silicate equilibration from any source of N with a ≥ −45‰.
On the Origin(s) and Evolution of Terrestrial N
Because the N-isotopic signatures of solar system objects and reservoirs are highly variable (77), N isotopes are key tracers of the source of terrestrial volatiles. Among chondrites, only enstatite chondrites have negative δ15N values [−45 to −15‰ (13)]. This fact led Javoy et al. (5) to suggest that enstatite chondrites are the source of Earth’s N. An enstatite chondrite-like δ15N value of the proto-Earth is consistent with the scenario presented by Li et al. (16) and herein: N segregation into the core during proto-Earth differentiation could have increased the δ15N value of the silicate mantle from very negative values (as low as −45‰) to −5‰. In addition, the range of δ15N values in Archean diamonds is similar to that of present-day diamonds and MORBs (11), suggesting that the mantle acquired its δ15N value of −5‰ before the Archean (16). This would support the idea of a very negative proto-Earth δ15N signature, similar to that of enstatite chondrites, and the subsequent enrichment of the mantle in 15N by an early process, for instance core formation (16), possibly combined with magma ocean degassing and/or N recycling (7).
Nitrogen degassing (as N2 or NH3; Fig. 3A) from the magma ocean to the primitive atmosphere could also have increased the δ15Nmantle value from an enstatite chondrite N-isotopic signature to the present-day value. Considering the highest reported δ15N value for enstatite chondrites (−15‰), only 30% of magma ocean degassing is required to increase δ15Nmantle by 10‰ to the present-day value of −5‰ assuming a Graham’s law relationship (Fig. 3A). On the other hand, from an enstatite chondrite δ15N value of −45‰, more than 75% of the initial N of the Earth needs to be degassed to increase δ15Nmantle by 40‰ to the present-day value (Fig. 3A). Considering the low solubility of N2 at low pressure under reduced conditions [<0.2 ppm at 1 atm between ΔIW −2 and IW (56)], large fractions of the proto-Earth’s initial N content could have been degassed from a reduced magma ocean (e.g., refs. 20 and 35). However, these considerations are dependent on N solubility variations with fO2 and fH2 (which control the stabilization of NH complexes and thus N solubility) in mafic to ultramafic melts, for which data are sparse. In comparison, N recycling via subduction is insufficient to increase δ15Nmantle by more than 5‰, even if subduction was initiated 4 Ga (78). As both magma ocean degassing and core formation may have increased the δ15N value of the magma ocean, the source of terrestrial N might have had a N-isotopic signature significantly more negative than −45‰.
The very low δ15N values of some Archean diamonds [−25 to −39‰, compared with −12 to +6‰ in most other Archean diamonds (12, 79)] have been suggested to represent relics of primordial N that have been preserved in an undegassed reservoir. The existence of such a primitive, undegassed mantle reservoir has been proposed to explain the low 4He/3He and 21Ne/22Ne [i.e., low (U+Th)/3He and (U+Th)/22Ne] values of ocean island basalts (OIBs) [see review by Moreira (80)], as well as the preservation of a chondritic Xe component in the deep mantle (81). However, OIBs, typically associated with mantle plumes, exhibit high δ15N values (around +2‰) akin to surficial N-isotopic compositions (7), suggesting that at least some of the N in OIBs is derived from recycled surficial N. The low δ15N signature of the inferred undegassed reservoir responsible for Archean diamonds must have somehow been maintained during metal–silicate fractionation and core formation. The stable isotopic (i.e., δ13C) signature of diamonds can vary widely as a result of the redox state of the C–O–H–N fluids/melts from which they form (82, 83). Therefore, the extremely low δ15N values of some Archean diamonds (12, 79) may similarly reflect fO2 heterogeneities in the Archean mantle, although the effects of fO2 on N speciation and isotopic fractionation in diamonds remain unknown (82).
Another possible origin of terrestrial N arises from the combined study of N isotopes and noble gases among solar system bodies. Based on the solar-like Ne isotopic composition of the mantle, Marty (76) discussed the possibility that the mantle has preserved some solar N. This scenario implies that precursor grains accreted during Earth’s formation were first irradiated by the solar wind and preserved their solar volatile isotopic signature during Earth’s accretion and differentiation [δ15NIFSW ≤ −240‰; IFSW: implantation-fractionated solar wind (84, 85)]. Alternatively, a primordial solar atmosphere could have been captured by Earth’s gravity and dissolved into the early magma ocean [δ15Nsolar = −383‰ (e.g., ref. 86)]. Assuming a solar-like source of N for the proto-Earth, the mantle’s N must have been significantly enriched in 15N by N-isotopic fractionation during core–mantle differentiation, during magma ocean degassing (as N2 and/or NH3; Fig. 3A), and/or by the delivery of carbonaceous chondrites [δ15NCC = +15 to +55‰ (14)] during late accretion and the addition of the late veneer.
Conclusions
We studied metal–silicate N-isotopic fractionation over a fO2 range consistent with the inferred conditions of Earth’s core formation. Our results reveal large N-isotopic fractionations over fO2 conditions spanning 3 orders of magnitude. Such large fractionations can be explained by the vastly different N bonds formed in metal and silicate phases. The particularity of N, compared with other isotope tracers of core formation, is that it forms N2 molecules or NH complexes in silicate melts, whereas it bonds as Fe–N in metal alloys. The strong dependence of N-isotopic fractionation on fO2 conditions is a consequence of the increased solubility of NH complexes in silicate melts under increasingly reducing conditions. We also demonstrated that the NH/N2 ratio and the N-isotopic fractionation depend on melt composition. Planetary magma oceans are expected to be mafic (close to our Fe-rich basalts) to ultramafic. Ultramafic melts (SiO2-poor and MgO-rich) are difficult to quench from HP–HT to ambient conditions. Therefore, to advance our understanding of the effect of melt composition on N speciation and isotopic fractionation for extrapolation to such ultramafic melts at core-forming reduced conditions, experimental studies need to be performed using silicate melts spanning a large range of polymerization degrees.
Metal–silicate N partitioning coefficients increase significantly with increasing P–T conditions (22) because N solubility in metal blobs increases (15). How P–T conditions affect N2 and NH solubility in silicate melts, and thereby metal–silicate N-isotopic fractionation, remains to be experimentally constrained over a wide range of P–T conditions. Although isotopic fractionations decrease with increasing temperature [Δ15N ∝ 1/T2 (20, 21)], an increase of pressure over tens of gigapascals should increase isotopic fractionations by increasing bond stiffness (51). However, how pressure affects bond stiffness in silicate glasses versus in metal alloys remains to be studied. At P–T conditions more representative of core–mantle equilibration in the terrestrial magma ocean [0 to 40 GPa and 2,000 to 3,000 K (87)], the magnitude of N-isotopic fractionation is expected to be smaller than determined in this study since the effect of T should dominate that of P. Thanks to our analytical developments, the N-content and -isotopic ratios of small phases (<15 µm in diameter) can now be measured in high-pressure (>10-GPa) experimental samples, which will allow the determination of N-isotopic fractionations at P–T conditions more representative of core–magma ocean equilibration conditions.
We have highlighted the fundamental effect of fO2 on the evolution of the mantle’s δ15N value during core formation. We showed that fO2 variations of 3 log units can produce a δ15N variation of >20‰ in the silicate mantle after only 30% metal–silicate equilibration (Fig. 4). The magnitude of the N-isotopic fractionation observed here during metal–silicate segregation, larger fractionations under more reducing conditions, suggests that large N-isotopic fractionations could have also occurred during other high-temperature stages of Earth’s formation, such as accretion and magma ocean crystallization and/or degassing.
Supplementary Material
Acknowledgments
We thank Etienne Deloule, Nordine Bouden, and Laurent Zimmermann for their assistance and support during SIMS and noble gas mass spectrometry analyses. We also thank Mathieu Roskosz, Laurent Tissandier, Guillaume Paris, and Guillaume Caro for fruitful discussions, and Robert Dennen for English editing. We appreciate the insightful comments by 2 anonymous reviewers, which helped us to improve the manuscript. This work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (Grant Agreement 715028). M.L. was supported by the French Government Laboratory of Excellence Initiative ANR-10-LABX-0006 (ClerVolc Contribution 355). This is Centre de Recherches Pétrographiques et Géochimiques–CNRS Contribution 2713.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820719116/-/DCSupplemental.
References
- 1.Johnson B., Goldblatt C., The nitrogen budget of Earth. Earth Sci. Rev. 148, 150–173 (2015). [Google Scholar]
- 2.Javoy M., Pineau F., Demaiffe D., Nitrogen and carbon isotopic composition in the diamonds of Mbuji Mayi (Zaire). Earth Planet. Sci. Lett. 68, 399–412 (1984). [Google Scholar]
- 3.Cartigny P., Marty B., Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359–366 (2013). [Google Scholar]
- 4.Thomazo C., Papineau D., Biogeochemical cycling of nitrogen on the early Earth. Elements 9, 345–351 (2013). [Google Scholar]
- 5.Javoy M., Pineau F., Delorme H., Carbon and nitrogen isotopes in the mantle. Chem. Geol. 57, 41–62 (1986). [Google Scholar]
- 6.Cartigny P., Harris J. W., Javoy M., Diamond genesis, mantle fractionations and mantle nitrogen content: A study of δ13C–N concentrations in diamonds. Earth Planet. Sci. Lett. 185, 85–98 (2001). [Google Scholar]
- 7.Marty B., Dauphas N., The nitrogen record of crust–mantle interaction and mantle convection from Archean to present. Earth Planet. Sci. Lett. 206, 397–410 (2003). [Google Scholar]
- 8.Beaumont V., Robert F., Nitrogen isotope ratios of kerogens in Precambrian cherts: A record of the evolution of atmosphere chemistry? Precambrian Res. 96, 63–82 (1999). [Google Scholar]
- 9.Paris G., et al. , Nitrogen isotope record of a perturbed paleoecosystem in the aftermath of the end‐Triassic crisis, Doniford section, SW England. Geochem. Geophys. Geosyst. 11, Q08021 (2010). [Google Scholar]
- 10.Cartigny P., Ader M., A comment on “The nitrogen record of crust–mantle interaction and mantle convection from Archean to Present” by B. Marty and N. Dauphas [Earth Planet. Sci. Lett. 206 (2003) 397–410]. Earth Planet. Sci. Lett. 216, 425–432 (2003). [Google Scholar]
- 11.Javoy M., The birth of the Earth’s atmosphere: The behaviour and fate of its major elements. Chem. Geol. 147, 11–25 (1998). [Google Scholar]
- 12.Palot M., Cartigny P., Harris J. W., Kaminsky F. V., Stachel T., Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth Planet. Sci. Lett. 357, 179–193 (2012). [Google Scholar]
- 13.Grady M. M., Wright I. P., Carr L. P., Pillinger C. T., Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochim. Cosmochim. Acta 50, 2799–2813 (1986). [Google Scholar]
- 14.Kerridge J. F., Carbon, hydrogen and nitrogen in carbonaceous chondrites: Abundances and isotopic compositions in bulk samples. Geochim. Cosmochim. Acta 49, 1707–1714 (1985). [DOI] [PubMed] [Google Scholar]
- 15.Roskosz M., Bouhifd M. A., Jephcoat A. P., Marty B., Mysen B. O., Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochim. Cosmochim. Acta 121, 15–28 (2013). [Google Scholar]
- 16.Li Y., Marty B., Shcheka S., Zimmermann L., Keppler H., Nitrogen isotope fractionation during terrestrial core-mantle separation. Geochem. Perspect. Lett. 2, 138–147 (2016). [Google Scholar]
- 17.Dalou C., Hirschmann M. M., von der Handt A., Mosenfelder J., Armstrong L. S., Nitrogen and carbon fractionation during core–mantle differentiation at shallow depth. Earth Planet. Sci. Lett. 458, 141–151 (2017). [Google Scholar]
- 18.Kadik A. A., et al. , Formation of N–C–O–H molecules and complexes in the basalt-basaltic andesite melts at 1.5 GPa and 1400°C in the presence of liquid iron alloys. Geochem. Int. 55, 151–162 (2017). [Google Scholar]
- 19.Speelmanns I. M., Schmidt M. W., Liebske C., Nitrogen solubility in core materials. Geophys. Res. Lett. 45, 7434–7443 (2018). [Google Scholar]
- 20.Speelmanns I. M., Schmidt M. W., Liebske C., The almost lithophile character of nitrogen during core formation. Earth Planet. Sci. Lett. 510, 186–197 (2019). [Google Scholar]
- 21.Grewal D. S., Dasgupta R., Sun C., Tsuno K., Costin G., Delivery of carbon, nitrogen, and sulfur to the silicate Earth by a giant impact. Sci. Adv. 5, eaau3669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grewal D. S., et al. , The fate of nitrogen during core-mantle separation on Earth. Geochim. Cosmochim. Acta 251, 87–115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauble E. A., Applying stable isotope fractionation theory to new systems. Rev. Mineral. Geochem. 55, 65–111 (2004). [Google Scholar]
- 24.Rubie D. C., et al. , Heterogeneous accretion, composition and core–mantle differentiation of the Earth. Earth Planet. Sci. Lett. 301, 31–42 (2011). [Google Scholar]
- 25.Badro J., Brodholt J. P., Piet H., Siebert J., Ryerson F. J., Core formation and core composition from coupled geochemical and geophysical constraints. Proc. Natl. Acad. Sci. U.S.A. 112, 12310–12314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubie D. C., et al. , Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89–108 (2015). [Google Scholar]
- 27.Wade J., Wood B. J., The oxidation state and mass of the Moon-forming impactor. Earth Planet. Sci. Lett. 442, 186–193 (2016). [Google Scholar]
- 28.Cartier C., Hammouda T., Boyet M., Bouhifd M. A., Devidal J. L., Redox control of the fractionation of niobium and tantalum during planetary accretion and core formation. Nat. Geosci. 7, 573–576 (2014). [Google Scholar]
- 29.Mysen B. O., Fogel M. L., Nitrogen and hydrogen isotope compositions and solubility in silicate melts in equilibrium with reduced (N+H)-bearing fluids at high pressure and temperature: Effects of melt structure. Am. Mineral. 95, 987–999 (2010). [Google Scholar]
- 30.Bigeleisen J., Mayer M. G., Calculation of equilibrium constants for isotopic exchange reactions. J. Chem. Phys. 15, 261–267 (1947). [Google Scholar]
- 31.Urey H. C., The thermodynamic properties of isotopic substances. J. Chem. Soc. 1947, 562–581 (1947). [DOI] [PubMed] [Google Scholar]
- 32.Hirschmann M. M., Magma ocean influence on early atmosphere mass and composition. Earth Planet. Sci. Lett. 341, 48–57 (2012). [Google Scholar]
- 33.Armstrong L. S., Hirschmann M. M., Stanley B. D., Falksen E. G., Jacobsen S. D., Speciation and solubility of reduced C–O–H–N volatiles in mafic melt: Implications for volcanism, atmospheric evolution, and deep volatile cycles in the terrestrial planets. Geochim. Cosmochim. Acta 171, 283–302 (2015). [Google Scholar]
- 34.Zhang H. L., Solheid P. A., Lange R. A., von der Handt A., Hirschmann M. M., Accurate determination of Fe3+/∑Fe of andesitic glass by Mössbauer spectroscopy. Am. Mineral. 100, 1967–1977 (2015). [Google Scholar]
- 35.Hirschmann M. M., Constraints on the early delivery and fractionation of Earth’s major volatiles from C/H, C/N, and C/S ratios. Am. Mineral. 101, 540–553 (2016). [Google Scholar]
- 36.Leonova E., Grins J., Shariatgorji M., Ilag L. L., Edén M., Solid-state NMR investigations of Si-29 and N-15 enriched silicon nitride. Solid State Nucl. Magn. Reson. 36, 11–18 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Sobolev A. V., et al. , Komatiites reveal a hydrous Archaean deep-mantle reservoir. Nature 531, 628–632 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Kadik A. A., et al. , Solution behavior of reduced N–H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature. Phys. Earth Planet. Inter. 214, 14–24 (2013). [Google Scholar]
- 39.von der Handt A., Dalou C., Quantitative EPMA of nitrogen in silicate glasses. Microsc. Microanal. 22 (suppl. 3), 1810–1811 (2016). [Google Scholar]
- 40.Füri E., Deloule E., Dalou C., Nitrogen abundance and isotope analysis of silicate glasses by secondary ionization mass spectrometry. Chem. Geol. 493, 327–337 (2018). [Google Scholar]
- 41.Mosenfelder J., et al. , Nitrogen incorporation in silicates and metals: Results from SIMS, EPMA, FTIR, and laser-extraction mass spectrometry. Am. Mineral. 104, 31–46 (2019). [Google Scholar]
- 42.Sugiura N., Ion probe measurements of carbon and nitrogen in iron meteorites. Meteorit. Planet. Sci. 33, 393–409 (1998). [Google Scholar]
- 43.Sugiura N., Zashu S., Weisberg M., Prinz M., A nitrogen isotope study of bencubbinites. Meteorit. Planet. Sci. 35, 987–996 (2000). [Google Scholar]
- 44.Médard E., McCammon C. A., Barr J. A., Grove T. L., Oxygen fugacity, temperature reproducibility, and H2O contents of nominally anhydrous piston-cylinder experiments using graphite capsules. Am. Mineral. 93, 1838–1844 (2008). [Google Scholar]
- 45.Wagner C., Thermodynamics of Alloys (Addison-Wesley, Reading, MA, 1962). [Google Scholar]
- 46.Wade J., Wood B. J., Core formation and the oxidation state of the Earth. Earth Planet. Sci. Lett. 236, 78–95 (2005). [Google Scholar]
- 47.Wood B. J., Wade J., Norris A., MetalAct Website (2017) http://norris.org.au/expet/metalact/. Accessed 19 February 2019.
- 48.O’Neill H. S. C., Eggins S. M., The effect of melt composition on trace element partitioning: An experimental investigation of the activity coefficients of FeO, NiO, CoO, MoO2 and MoO3 in silicate melts. Chem. Geol. 186, 151–181 (2002). [Google Scholar]
- 49.Stanley B. D., Hirschmann M. M., Withers A. C., Solubility of COH volatiles in graphite-saturated martian basalts. Geochim. Cosmochim. Acta 129, 54–76 (2014). [Google Scholar]
- 50.Zhang H. L., Hirschmann M. M., Cottrell E., Newville M., Lanzirotti A., Structural environment of iron and accurate determination of Fe3+/ΣFe ratios in andesitic glasses by XANES and Mössbauer spectroscopy. Chem. Geol. 428, 48–58 (2016). [Google Scholar]
- 51.Young E. D., et al. , High-temperature equilibrium isotope fractionation of non-traditional stable isotopes: Experiments, theory, and applications. Chem. Geol. 395, 176–195 (2015). [Google Scholar]
- 52.Kadik A. A., et al. , Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO–Na2O–SiO2–Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400 °C. Geochem. Int. 49, 429–438 (2011). [Google Scholar]
- 53.Kadik A. A., et al. , Solubility of nitrogen, carbon, and hydrogen in FeO–Na2O–Al2O3–SiO2 melt and liquid iron alloy: Influence of oxygen fugacity. Geochem. Int. 53, 849–868 (2015). [Google Scholar]
- 54.Dalou C., Le Losq C., Hirschmann M. M., Jacobsen S. D., Füri E., Evolution of COHN volatile species in the magma ocean during core formation. AGU Fall Meeting Abstr. V31E-01 (2017). http://adsabs.harvard.edu/abs/2017AGUFM.V31E..01D. Accessed 12 December 2018.
- 55.Roskosz M., Mysen B. O., Cody G. D., Dual speciation of nitrogen in silicate melts at high pressure and temperature: An experimental study. Geochim. Cosmochim. Acta 70, 2902–2918 (2006). [Google Scholar]
- 56.Libourel G., Marty B., Humbert F., Nitrogen solubility in basaltic melt. Part I. Effect of oxygen fugacity. Geochim. Cosmochim. Acta 67, 4123–4135 (2003). [Google Scholar]
- 57.Soignard E., McMillan P. F., Raman spectroscopy of γ-Si3N4 and γ-Ge3N4 nitride spinel phases formed at high pressure and high temperature: Evidence for defect formation in nitride spinels. Chem. Mater. 16, 3533–3542 (2004). [Google Scholar]
- 58.Kendall C., Caldwell E. A., “Fundamentals of isotope geochemistry” in Isotope Tracers in Catchment Hydrology, Kendall C, McDonnell J. J, Eds. (Elsevier, Amsterdam, 1998), pp. 51–86. [Google Scholar]
- 59.Dalou C., Le Losq C., Mysen B. O., In situ study of the fractionation of hydrogen isotopes between aluminosilicate melts and coexisting aqueous fluids at high pressure and high temperature–Implications for the δD in magmatic processes. Earth Planet. Sci. Lett. 426, 158–166 (2015). [Google Scholar]
- 60.Wu J., et al. , Origin of Earth’s water: Chondritic inheritance plus nebular ingassing and storage of hydrogen in the core. J. Geophys. Res. Planets 123, 2671–2712 (2018). [Google Scholar]
- 61.Roskosz M., et al. , Kinetic D/H fractionation during hydration and dehydration of silicate glasses, melts and nominally anhydrous minerals. Geochim. Cosmochim. Acta 233, 14–32 (2018). [Google Scholar]
- 62.Mysen B. O., Tomita T., Ohtani E., Suzuki A., Speciation of and D/H partitioning between fluids and melts in silicate-DOHCN systems determined in-situ at upper mantle temperatures, pressures, and redox conditions. Am. Mineral. 99, 578–588 (2014). [Google Scholar]
- 63.Mysen B., Experimentally-determined carbon isotope fractionation in and between methane-bearing melt and fluid to upper mantle temperatures and pressures. Earth Planet. Sci. Lett. 445, 28–35 (2016). [Google Scholar]
- 64.Mysen B., Experimental in-situ carbon solution mechanisms and isotope fractionation in and between (C–O–H)-saturated silicate melt and silicate-saturated (C–O–H) fluid to upper mantle temperatures and pressures. Earth Planet. Sci. Lett. 459, 352–361 (2017). [Google Scholar]
- 65.Bourdon B., Roskosz M., Hin R. C., Isotope tracers of core formation. Earth Sci. Rev. 181, 61–81 (2018). [Google Scholar]
- 66.Namur O., Charlier B., Holtz F., Cartier C., McCammon C., Sulfur solubility in reduced mafic silicate melts: Implications for the speciation and distribution of sulfur on Mercury. Earth Planet. Sci. Lett. 448, 102–114 (2016). [Google Scholar]
- 67.Labidi J., et al. , Experimentally determined sulfur isotope fractionation between metal and silicate and implications for planetary differentiation. Geochim. Cosmochim. Acta 175, 181–194 (2016). [Google Scholar]
- 68.Fenner W. R., Hyatt H. A., Kellam J. M., Porto S. P. S., Raman cross section of some simple gases. J. Opt. Soc. Am. 63, 73–77 (1973). [Google Scholar]
- 69.Mysen B. O., Yamashita S., Chertkova N., Solubility and solution mechanisms of NOH volatiles in silicate melts at high pressure and temperature-amine groups and hydrogen fugacity. Am. Mineral. 93, 1760–1770 (2008). [Google Scholar]
- 70.Lei L., Yin W., Jiang X., Lin S., He D., Synthetic route to metal nitrides: High-pressure solid-state metathesis reaction. Inorg. Chem. 52, 13356–13362 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Mysen B., Silicate solution, cation properties, and mass transfer by aqueous fluid in the Earth’s interior. Prog. Earth Planet. Sci. 5, 1–18 (2018). [Google Scholar]
- 72.Wang Y., Cody S. X., Foustoukos D., Mysen B. O., Cody G. D., Very large differences in intramolecular DH partitioning in hydrated silicate melts synthesized at upper mantle pressures and temperatures. Am. Mineral. 100, 1182–1189 (2015). [Google Scholar]
- 73.Le Losq C., Mysen B. O., Cody G. D., Intramolecular fractionation of hydrogen isotopes in silicate quenched melts. Geochem. Perspect. Lett. 2, 87–94 (2016). [Google Scholar]
- 74.Morbidelli A., et al. , Source regions and timescales for the delivery of water to the Earth. Meteorit. Planet. Sci. 35, 1309–1320 (2000). [Google Scholar]
- 75.Bergin E. A., Blake G. A., Ciesla F., Hirschmann M. M., Li J., Tracing the ingredients for a habitable earth from interstellar space through planet formation. Proc. Natl. Acad. Sci. U.S.A. 112, 8965–8970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marty B., The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313, 56–66 (2012). [Google Scholar]
- 77.Füri E., Marty B., Nitrogen isotope variations in the Solar System. Nat. Geosci. 8, 515–522 (2015). [Google Scholar]
- 78.Mallik A., Li Y., Wiedenbeck M., Nitrogen evolution within the Earth’s atmosphere–mantle system assessed by recycling in subduction zones. Earth Planet. Sci. Lett. 482, 556–566 (2018). [Google Scholar]
- 79.Cartigny P., Boyd S., Harris J., Javoy M., Nitrogen isotopes in peridotitic diamonds from Fuxian, China: The mantle signature. Terra. Nova 9, 175–179 (1997). [Google Scholar]
- 80.Moreira M., Noble gas constraints on the origin and evolution of Earth’s volatiles. Geochem. Perspect. 2, 229–230 (2013). [Google Scholar]
- 81.Caracausi A., Avice G., Burnard P. G., Füri E., Marty B., Chondritic xenon in the Earth’s mantle. Nature 533, 82–85 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Cartigny P., Palot M., Thomassot E., Harris J. W., Diamond formation: A stable isotope perspective. Annu. Rev. Earth Planet Sci. 42, 699–732 (2014). [Google Scholar]
- 83.Bureau H., Remusat L., Esteve I., Pinti D. L., Cartigny P.. The growth of lithospheric diamonds. Sci. Adv. 4, eaat1602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hashizume K., Chaussidon M., Marty B., Robert F., Solar wind record on the moon: Deciphering presolar from planetary nitrogen. Science 290, 1142–1145 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Péron S., Moreira M., Agranier A., Origin of light noble gases (He, Ne, and Ar) on Earth: A review. Geochem. Geophys. Geosyst. 19, 979–996 (2018). [Google Scholar]
- 86.Marty B., Chaussidon M., Wiens R. C., Jurewicz A. J. G., Burnett D. S., A 15N-poor isotopic composition for the solar system as shown by Genesis solar wind samples. Science 332, 1533–1536 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Andrault D., et al. , Solidus and liquidus profiles of chondritic mantle: Implication for melting of the Earth across its history. Earth Planet Sci. Lett. 304, 251–259 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.