Significance
Methicillin-resistant Staphylococcus aureus (MRSA), traditionally associated with hospitals, is increasingly circulating in the community. This imposes, in turn, a potential burden on hospital infection control due to a more frequent hospitalization of colonized patients. We developed an individual-based model, reproducing community and healthcare settings, to understand the epidemiological drivers of MRSA and the connections between the society and the healthcare institutions. We show that in Norway, a low-prevalence country, the rise of infections is driven by an increasing inflow of cases from abroad rather than by an ongoing epidemic. We demonstrate the major role played by households in transmitting MRSA and show that the burden on hospitals from the growing community circulation is still limited thanks to aggressive infection-control protocols.
Keywords: methicillin-resistant Staphylococcus aureus, individual-based model, transmission dynamics, mathematical model, antibiotic resistance
Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a primarily nosocomial pathogen that, in recent years, has increasingly spread to the general population. The rising prevalence of MRSA in the community implies more frequent introductions in healthcare settings that could jeopardize the effectiveness of infection-control procedures. To investigate the epidemiological dynamics of MRSA in a low-prevalence country, we developed an individual-based model (IBM) reproducing the population’s sociodemography, explicitly representing households, hospitals, and nursing homes. The model was calibrated to surveillance data from the Norwegian national registry (2008–2015) and to published household prevalence data. We estimated an effective reproductive number of 0.68 (95% CI 0.47–0.90), suggesting that the observed rise in MRSA infections is not due to an ongoing epidemic but driven by more frequent acquisitions abroad. As a result of MRSA importations, an almost twofold increase in the prevalence of carriage was estimated over the study period, in 2015 reaching a value of 0.37% (0.25–0.54%) in the community and 1.11% (0.79–1.59%) in hospitalized patients. Household transmission accounted for half of new MRSA acquisitions, indicating this setting as a potential target for preventive strategies. However, nosocomial acquisition was still the primary source of symptomatic disease, which reinforces the importance of hospital-based transmission control. Although our results indicate little reason for concern about MRSA transmission in low-prevalence settings in the immediate future, the increases in importation and global circulation highlight the need for coordinated initiatives to reduce the spread of antibiotic resistance worldwide.
Methicillin-resistant Staphylococcus aureus (MRSA) is a primary cause of healthcare-acquired infections (HAIs) (1, 2). In Europe, it has been estimated that, among all HAIs caused by antibiotic-resistant bacteria, MRSA is responsible for almost 44% of cases and over 20% of excess mortality (3). The large majority of countries worldwide report a proportion of more than 20% methicillin-resistant strains among Staphylococcus aureus isolates causing infections (2). As of 2016, only a few countries in Northern Europe (Norway, Sweden, Denmark, Finland, Iceland, The Netherlands, Estonia, and Latvia) reported a percentage between 1 and 5% (4), a result largely ascribed to strict infection control policies (the so-called “search and destroy” approach) (5) and antibiotic stewardship. Nonetheless, the control of MRSA in low-prevalence countries may be threatened by changes in the global epidemiology. First, its spread beyond the nosocomial environment into the community, mainly affecting young individuals without previous healthcare-related exposure (6–9); second, the continued growth of MRSA prevalence in countries with insufficient control combined with intensified international mobility, which are significantly contributing to the global spread of MRSA (9, 10). We currently have very limited knowledge of how the emerging community reservoir contributes to the local MRSA epidemiology in low-prevalence settings and to which degree it impacts the healthcare environments. The identification of the relationship between MRSA transmission within the healthcare settings and the community is of primary importance to tailor evidence-based preventive measures, which currently are largely healthcare centered.
Transmission dynamic models are increasingly used to understand the epidemiology of infectious diseases, evaluate the effectiveness of control interventions, and assess future scenarios. To date, a number of models have been applied to MRSA in the healthcare setting (11), e.g., to understand the spread of outbreaks throughout hospitals (12, 13), identify superspreading phenomena (14), decipher the relative role of specific settings in transmission (15), and assess the effectiveness and cost effectiveness of interventions (16, 17). A specific approach to modeling infections is represented by individual-based models (IBMs), which describes the individual characteristics of the members of a population (e.g., age, sex, and occupation), compared with the more traditional equation-based approach, which aims to capture the average characteristics of a limited number of population subgroups. IBMs are particularly suitable to investigate infections with a high degree of heterogeneity in infection dynamics as is the case for MRSA, but they are more complex and computationally burdensome. IBMs developed for the transmission of MRSA have predominantly focused on studying transmission within hospital wards, entire hospitals, or hospital networks (11, 12, 16, 18), and few included other settings (15, 18); due to computational constraints, many consider small populations (up to a few thousands), and none has yet been built at a national scale for MRSA.
In this study, we present an IBM of MRSA transmission in healthcare environments and community settings in Norway, distinguishing between MRSA colonizations and infections and including importation of cases from abroad. We calibrated the model to national surveillance data from the period 2008–2015 and to published household prevalence data (19) to identify the main routes of transmission, quantify transmissibility in the general population and the impact of MRSA importations, and to elucidate the interaction between community circulation and healthcare settings.
Results
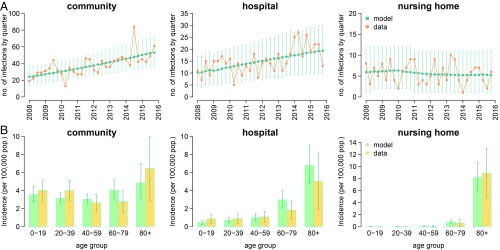
Despite the implementation of strict measures for infection prevention and control, the number of notified MRSA infections in Norway has increased in the period 2008–2015 by almost a factor of 3. The model reproduced well the rise of MRSA infections observed in Norway between 2008 and 2015 in both community and healthcare settings (Fig. 1A). The age-specific incidence of MRSA infection in different settings, which was not used during calibration, was also correctly predicted by the model thereby validating its robustness (Fig. 1B).
Fig. 1.
Model fit and validation. (A) Quarterly time series of the number of infections reported in the community, hospitals, and nursing homes from 2008 to 2015 with 95% CIs; green: model output (average and 95% CI); orange: data from the Norwegian national registry. (B) Age-specific yearly incidence of infections, averaged over the study period; light green: model (average and 95% CI); yellow: data from the Norwegian national registry.
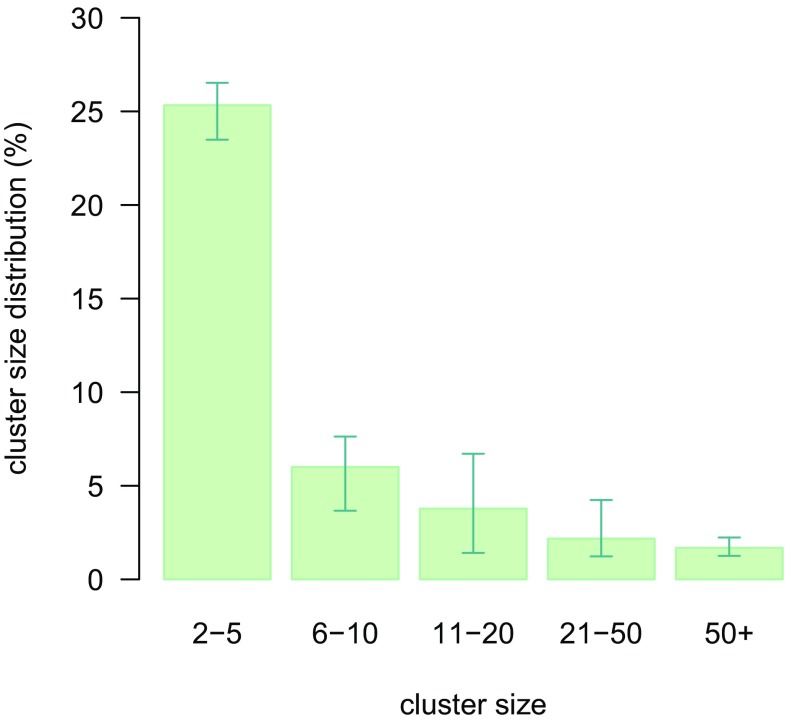
The observed growth of MRSA infections over time is worrying as it may be interpreted as a signal of containment failure. However, model results were not suggestive of this possibility as the estimated effective reproduction number for MRSA colonization was Re = 0.68 (95% CI 0.47–0.90), remaining stable over time. In a fully closed population, an effective reproduction number below one would result in a gradual disappearance of the pathogen; therefore, the increasing trend in domestic infections is explained as the result of a growing inflow of individuals acquiring MRSA abroad rather than as an ongoing epidemic. At the same time, the value of Re is sufficiently close to the epidemic threshold to allow for the occurrence of sporadic outbreaks of considerable size, which are indeed observed in Norway (10). Defining a cluster as the group of colonized persons directly or indirectly generated by a single index case, the model estimated that about 2% of all index cases resulted into a cluster involving more than 50 individuals (Fig. 2). On the other hand, about 60% of imported cases in the model were singletons, i.e., did not transmit MRSA to other persons; this finding is corroborated by genotype analyses on MRSA isolates collected in Norway, showing that about 50% of them have unique spa types (20). The estimated reproduction number per hospital admission was also below the epidemic threshold Ra = 0.25 (95% CI 0.21–0.29).
Fig. 2.
Estimated distribution of cluster size. Singletons (i.e., clusters of size one with no secondary transmission) are not shown.
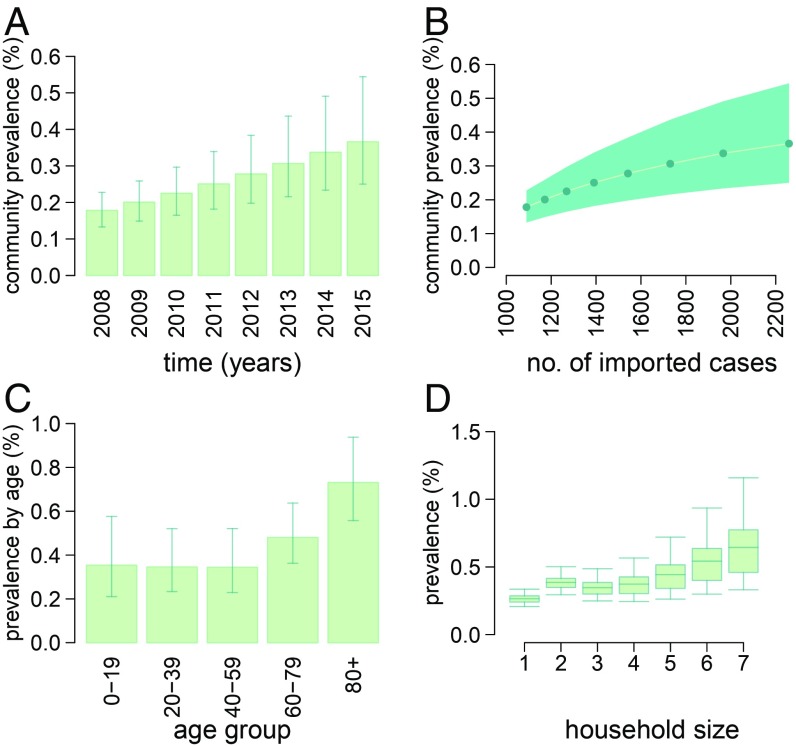
The model is able to provide surrogate estimates by setting of transmission for the prevalence of MRSA carriage in Norway in the absence of large-scale population studies. We determined that the growth of imported MRSA over time resulted in an increase in the prevalence of carriage in the general population from 0.18% (95% CI: 0.13–0.22%) in 2008 to 0.37% (95% CI: 0.25–0.54%) in 2015 (Fig. 3 A and B). These numbers are compatible with results from a cross-sectional study suggesting a prevalence around 0.2% (range: 0–0.4%) in Sweden and the Netherlands in 2009–2010 (21). The prevalence of MRSA carriage among individuals with immigrant backgrounds was twice as high than among Norwegians (0.64% vs. 0.31% at the end of 2015), a consequence of the differential influx of MRSA carriers from abroad in the two subpopulations. Prevalence in the community setting was estimated to be higher among the elderly compared with other age groups (Fig. 3C). The model predicted a slight increase in the prevalence of carriage with household size (Fig. 3D). The circulation of MRSA in the community was found to affect hospital settings: The prevalence of carriage among inpatients was estimated to rise from 0.65% (95% CI: 0.45–0.89%) to 1.11% (95% CI: 0.79–1.59%) over the study period. For what concerns nursing homes, the prevalence remained stable at about 1.7% [a value that compares well to the 1–24% range estimated for European countries (22)]. The constant level in nursing homes contrasts with the rise in other settings and may be attributed to the revision of control guidelines introduced since 2010. This change in the infection-control measures, implemented as a reaction to the rising frequency of MRSA outbreaks in nursing homes during previous years, was included in the model.
Fig. 3.
Estimates of MRSA carriage prevalence. (A) Prevalence in the community over time. (B) Relationship between the number of MRSA importations (infections and colonizations) and prevalence. (C) Age-specific prevalence by age at the end of 2015. (D) Prevalence by household size at the end of 2015.
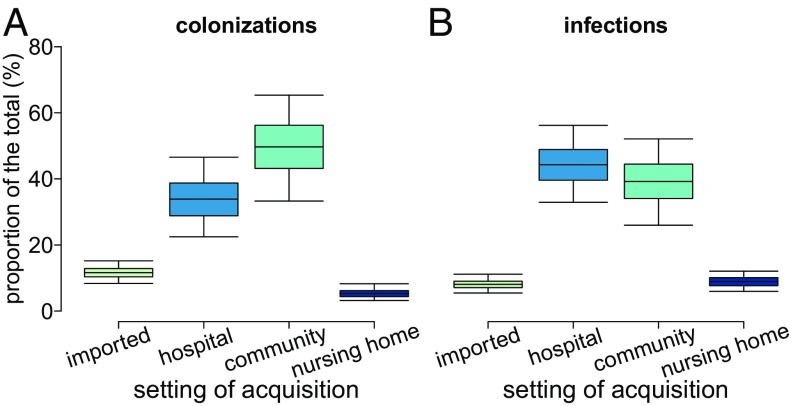
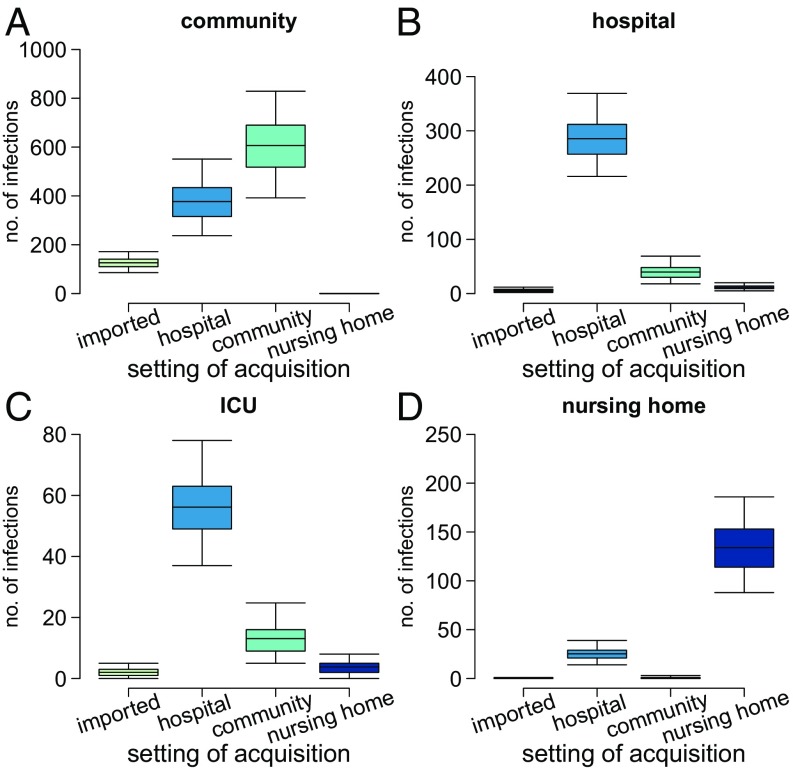
Model results also provide statistics about the place of carriage acquisition, which are very challenging to obtain through the analysis of the data collected by experimental studies. The model suggests that, despite a much higher transmission rate in the nosocomial setting with respect to households and nursing homes, household contacts accounted for the largest share (49%, 95% CI: 33–65%) of all colonization events (Fig. 4A). Hospitals followed with 34% (95% CI: 22–46%) and nursing homes with about 5%; the remaining 12% (95% CI: 8–15%) were acquired abroad. This result can be attributed to the intensive control interventions that are in place within healthcare settings and suggests the possibility of further reducing MRSA prevalence by acting on household contacts of colonized individuals. However, although community transmission represented the main route of MRSA acquisition, the largest proportion of all symptomatic infections (44%, 95% CI: 32–56%, see Fig. 4B) was developed after nosocomial transmission, followed by acquisition in the community (39%, 95% CI: 26–52%). This can be explained with the much lower rate of progression to infection estimated for the general population compared with inpatients (about 40:1, see SI Appendix, Supplementary Material), justified by increased risk factors for infection among hospitalized individuals, such as the presence of surgical wounds, pressure ulcers, or the use of catheters and other invasive devices (23). In addition, we found that nosocomial transmissions were responsible for 34% of all infections occurring in the community. This finding highlights an important interaction between the two settings and confirms recent observations that document the dissemination of hospital-associated lineages throughout the community (24). Nosocomial acquisitions were also responsible for the vast majority of infections developed in hospitals (83%) and for 16% of those developed in nursing homes (Fig. 5 and SI Appendix, Supplementary Material). On the other hand, community transmission was only directly responsible for 12% of nosocomial infections, presumably thanks to control measures which successfully identify the majority of carriers at hospital admission and prevent infection development through decolonization therapies. However, even in the context of a search and destroy policy, introduction of MRSA in hospitals may occasionally occur unnoticed; when this happens, the outbreak size can be substantial due to the large transmission rate in hospitals. Healthcare workers play an important role in the spread of MRSA, often acting as vectors for transmission (25). This dynamic is reflected in the high incidence of infections observed in healthcare workers (80 per 100,000 person years, compared with 3.5 in the general population). By estimating a high prevalence of MRSA carriage among nurses directly interacting with patients (between 2.2 and 3.8%), the model was able to correctly predict the number of infections observed in this category (SI Appendix, Supplementary Material).
Fig. 4.
Disaggregation of events by setting of acquisition. (A) Colonizations. (B) Infections.
Fig. 5.
Disaggregation of infections by setting of acquisition. (A) Infections developed in the community. (B) Infections developed in general wards. (C) Infections developed in intensive care units (ICUs). (D) Infections developed in nursing homes.
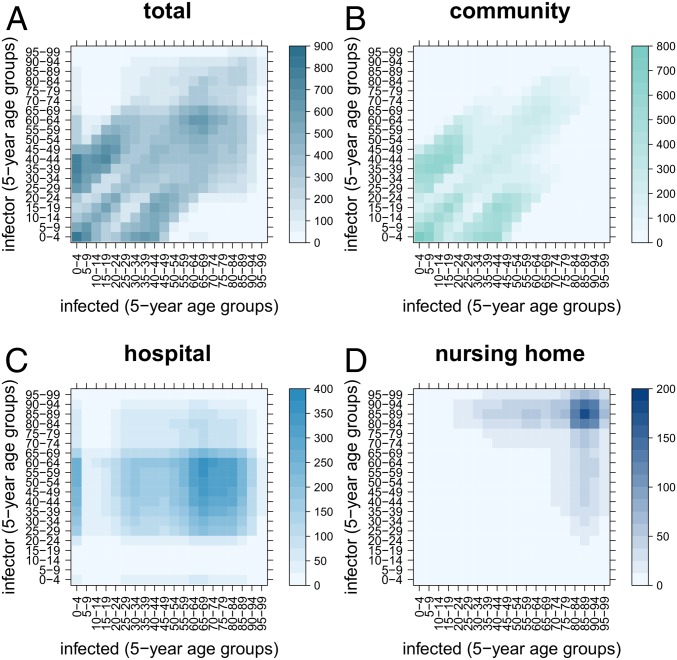
The model-estimated age-specific transmission matrices are presented in Fig. 6. The overall matrix derives from the superposition of the transmission matrix in the community, hospitals, and nursing homes. The community transmission matrix shows the characteristic diagonals due to contacts between spouses and siblings, and between parents and children within households (26, 27). Transmission in the hospital matrix is mainly concentrated in very young and older age groups (50–85 y old) where hospitalization rates are high.
Fig. 6.
Average number of transmission events by age of the infector and of the infected (transmission matrix). (A) Overall. (B) Within the community. (C) Within hospitals. (D) Within nursing homes.
Discussion
We have studied the transmission of MRSA in Norway within and between the community and the healthcare facilities using a stochastic IBM with a realistic representation of the community, hospitals, and nursing homes. To our knowledge, this paper represents a comprehensive modeling effort to understand the interplay between importations and transmission in community, hospitals, and nursing homes on the national scale. As a country with one of the lowest MRSA prevalence levels, high-quality national registry data, and aggressive control policies, Norway represents an optimal setting for studying the effect of increasing epidemiological pressure from abroad.
The model estimated an effective reproductive number of about 0.7, suggesting that circulation of MRSA in Norway was not self-sustained but maintained by the rising import of carriers from abroad. By identifying importations as a primary driver of MRSA epidemiology in a low-prevalence country, our study confirms the critical role of travel in spreading resistance (9, 28, 29). We estimated that the rise in importation has fueled an almost twofold increase in the prevalence of carriage in the general community from 2008 to 2015 up to a value of 0.37%. The reproduction number Re reflects nationwide average transmission in the community since ∼97% of the transmission events generated by imported cases occurred outside healthcare settings. The reproduction number per hospital admission was estimated to be smaller (Ra = 0.25) in the range of previous values reported in low-endemic countries, such as the Netherlands and Denmark, also found to be below the epidemic regime (30, 31). This lower value is likely a consequence of the fact that patients have a much shorter length of stay in hospitals, typically a few days, compared with the average duration of MRSA colonization (32).
Household transmissions accounted for about half of all colonizations acquired within the Norwegian territory. This result is likely an effect of the aggressive infection control policies in the healthcare setting as well as of the high prevalence of MRSA carriage (around 40%) detected among household members during contact tracing studies in low-prevalence countries (19, 24, 33). The proportion of community transmission may even be underestimated due to our assumption of a negligible impact of other community settings in MRSA transmission. This assumption, however, was substantiated by preliminary analyses on an extended version of the model, including school, workplace, and distance-dependent random contacts (SI Appendix, Supplementary Material). Although some transmission events, including occasional MRSA outbreaks, can be traced to specific contexts, such as contact sports associations (8), an evaluation of their contribution was not possible given the lack of information at this level of granularity. Nonetheless, it is unlikely that these settings play a major role in the overall epidemiology of MRSA in Norway.
Among persons carrying MRSA, symptoms may arise several months after the acquisition of MRSA carriage. The model was helpful in discriminating between the settings where transmissions occur from the setting where clinical infections develop. Disentangling the place of infection development vs. the place of carriage acquisition, we estimated that the largest proportion of the burden of clinical disease was attributed to nosocomial acquisitions because of the increased risk factors for infection characterizing inpatients. Importantly, about one-third of symptomatic infections developed in the community were acquired in the hospital; in contrast, only 12% of nosocomial infections originated from community acquisition, thanks to the screening and decolonization protocols at the admission of patients. However, the occasional admission of asymptomatic carriers may still result in widespread transmission in hospital wards. For example, this may happen when colonized patients with no record of MRSA in their family and no history of travel abroad in the previous year are hospitalized since these patients are not subject to screening at admission (34).
Several assumptions and simplifications were taken in this study. An important limitation is that we did not include a hospital referral network representing the movements of patients between hospital institutions. Consequently, our model may overestimate within-hospital transmission. Findings from a study in the United Kingdom have shown that MRSA transmissions related to patients' movements between hospitals was mainly associated with a noneffective communication between the source and the destination institutions (35). The national MRSA guidelines in Norway require the notification of MRSA carriage when patients are transferred between institutions. In addition, patient movements between Norwegian hospitals is overall very limited and mostly related to short-term ambulatory visits. Thus, the impact of patient transfer on transmission may be limited. The model also does not incorporate patient movements between wards, based on Norwegian hospitalization data showing that over 90% of patients visit only one ward and less than 0.2% more than three wards during the same hospitalization.
We assumed complete adherence to national MRSA guidelines across all hospitals. The actual effort put into the control measures has likely changed over time within and across different hospitals, depending on available resources and on exceptional situations, such as the management of outbreaks. Although this issue should not affect the overall transmission dynamics at the country level, variations in control activities could impact the local dynamics of hospital outbreaks. Data on control activities in healthcare settings, such as the number of performed screenings over time, are currently not available but could be used to improve this aspect of MRSA modeling and provide a better simulation of the implemented infection control measures.
Because of the asymptomatic nature of MRSA carriage, it is hard to have an accurate estimate of the total number of imported colonizations. In our study, we assumed a baseline colonization-to-infection ratio of 10:1, based on published estimates (36). Additional simulations performed with ratios of 5:1 and 20:1, resulted in substantially equivalent conclusions compared with the main analysis presented here (see SI Appendix, Supplementary Materials).
Our model does not consider genotype diversity. For example, the strain USA300 seems to have a much shorter duration of colonization but a similar, if not superior, ability to colonize individuals compared with strains with longer infectious periods (37). Considering the heterogeneity in the dynamics of different strains is challenging; first, the natural history parameters of different strains are mostly unknown [e.g., duration of carriage, transmissibility, rate of progression to infection; but also the preferred sites of colonization or specific subpopulations that may be more prone to infection with one strain (38)]; second, ecological interactions, such as competition, cooperation, and coexistence mechanisms across strains, should be studied; third, the number of different strains is potentially very high. Thus, the inclusion of strain heterogeneity would add a practically intractable level of model complexity. Previous analyses (20) have shown that, in Norway, the dominant circulating genotypes are the same for the community and healthcare settings, and they have not changed over the past decade. This relatively stable landscape of genetic diversity supports our choice of representing MRSA as if it was a single strain pathogen. On a related note, we did not include in our study zoonotic transmission to humans, mainly occurring among livestock workers, which has increasingly become a matter of concern in many areas of the world (39). In Norway, livestock associated MRSA remains low and mainly confined to farms (40). Therefore, the noninclusion of this aspect in the Norwegian setting does not represent a significant limitation for the understanding of the general transmission dynamics. The model also does not consider possible ecological interactions between MRSA and methicillin-susceptible S. aureus (MSSA). This is a common choice adopted by the majority of mathematical models developed to investigate MRSA epidemiology (11). The study of MRSA as a specific pathogen, independent from MSSA, is justified by its evolutionary history, characterized by a successful diffusion in hospital settings and, more recently, in community settings, and by its capacity to become endemic in many areas of the world (41). Furthermore, previous analyses in Norway have shown a growth in the MRSA level in relation to a constant incidence of MSSA (42), suggesting independent epidemiological dynamics between the two. However, the within-host interactions between susceptible and resistant strains as well as other microbiota are important and challenging research topics (43, 44). The inclusion of this additional level of complexity in modeling studies might help to refine our understanding of transmission of antibiotic-resistant pathogens.
Finally, we did not consider the emergence of resistance in patients caused by antimicrobial therapies. These cases would behave in a way that is indistinguishable from importation since they appear in the population independent of transmission. We have shown that model conclusions are robust with respect to assumptions about the number of importations. Furthermore, the low consumption of antibiotics and the decreasing use reported in recent years in Norway (45) suggest that the emergence of resistance after antimicrobial therapies likely has a minor effect on the epidemiology of MRSA.
Conclusion
The transmission dynamics of MRSA worldwide is changing with the community playing an increasingly central role in its spread. Data-driven modeling studies that include community environments in addition to healthcare settings can help frame this complex epidemiology, and it is necessary to support evidence-based planning and adaptation of integrated control measures. On one hand, our findings highlight the primary role of community transmission, pinpointing households as a potential target of preventive measures for the transmission control in the general population; on the other hand, they reemphasize the critical role of hospitals in controlling disease burden and the importance of stringent infection control measures in healthcare settings to keep the epidemiological pressure from the community at bay. Reassuringly, the model excluded the possibility of an ongoing MRSA epidemic, highlighting the drive exerted by the inflow of carriers from other countries. In Norway, a low-incidence country with below-threshold transmission, the movement of pathogens across borders represents a primary cause of the increasing number of observed MRSA infections. This result underscores the importance of coordinated global actions to tackle the rising burden of antibiotic resistance worldwide.
Materials and Methods
Data.
We considered data from the national infection registry (the Norwegian Surveillance System for Communicable Diseases [MSIS]) that contains all of the laboratory-confirmed MRSA cases reported to the Norwegian Institute of Public Health (46) between January 2008 and December 2015. The dataset reports various individual-level information, such as age, ethnic background, place of detection, and information about the hospitalization status of the patient at the time of testing. The number of symptomatic infections over time was used to calibrate the model, considering the place of MRSA detection to separate the infections in the community, hospital, or nursing home.
Individual-level data from the Norwegian Patient Registry (NPR) on episodes of hospital care within 2012 in the South-Eastern Norway health region were used to define age-specific hospitalization rates and length of stay distributions. The study of the NPR data was approved by the Regional Committees for Medical and Health Research Ethics—South-East Norway (project number 2013/1004).
Census data from the Statistical Office of the European Commission (Eurostat) were used to inform the sociodemographic structure of the model (household size distribution, typology of household, and age profile of the population). Public data from Statistics Norway (SSB) were used to define the hospitals and their size and to profile the number of people with an immigrant background.
Data on population density were obtained from the Gridded Population of the World version 3 (47) produced by the Center for International Earth Science Information Network of the Earth Institute at Columbia University.
Model Structure.
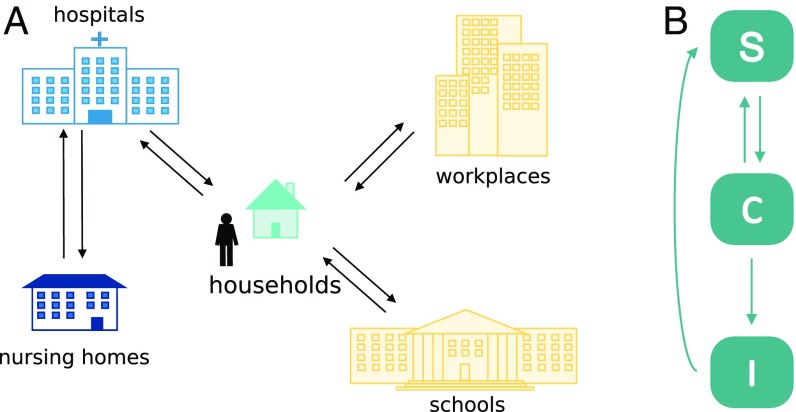
We developed a stochastic IBM to study the transmission of MRSA in the community and the healthcare setting (Fig. 7A), building on a previously published sociodemographic model (27). A grid of 4978 geolocated cells represents the Norwegian territory. The model consists of a synthetic population, whose characteristics are based on census sociodemographic data of Norway. Individuals are assigned to households distributed on the grid in accordance with the Norwegian population density (48) and age distribution. Except for unemployed, retired, and infants, persons are associated with workplaces or schools, depending on their age. The assignation to specific places is performed through a random process based on the commuting distance distribution, modeled on observed human mobility patterns (48). In this paper, we expanded the model by adding hospitals, structured into wards, and nursing homes with long-term patients. Large hospitals also include ICUs. Individuals can become hospitalized with age-dependent probabilities according to NPR data and assigned to hospital wards with a length of stay sampled from a distribution matching available data, also from NPR. Control measures recommended in healthcare institutions by the national MRSA guidelines (34) are implemented in the model, including: risk-based screening at hospital admission; screening of hospital wards and healthcare workers in case of unexpected discovery of MRSA carriers; isolation of carriers and infected patients; work restrictions for MRSA-positive healthcare workers; screening of household members of positive healthcare workers; decolonization therapies for identified MRSA carriers. Further details are reported in the SI Appendix, Supplementary Material.
Fig. 7.
Schematic of the IBM. (A) Representation of the settings included in the model. (B) Epidemiological model. S: susceptible individuals; C: colonized (asymptomatic carriers); I: infected (individuals with symptoms).
The temporal step of the model is set to 1 d. The age of individuals is updated on a yearly basis; according to their age, they can upgrade their school level, be hired for work, or retire. Age-specific rates define the mortality of the population. The population of each cell is assumed constant so that to each death corresponds a birth in a household with suitable parental age in the same cell. At each time step, a number of infections and colonizations is imported from abroad, randomly sampling from time-dependent Poisson distributions varying by age and ethnic background. The distributions are parameterized by Poisson regression models fitted separately to the monthly number of infections registered as acquired abroad in the national registry data (9). For all details on model implementation, see the SI Appendix, Supplementary Materials.
Epidemiological Model.
The epidemiological model adopted in this study has three disease states: susceptible, colonized (i.e., asymptomatic carriers), or infected (individuals with symptoms) (Fig. 7B). Susceptible individuals can acquire MRSA and become colonized by contact with other colonized or infected persons. Within each setting (e.g., a household or a hospital ward), we assumed homogeneous mixing of individuals. Colonization with MRSA is acquired from close contact with carriers; following preliminary analyses excluding a role for schools and workplaces, we only considered transmission within households in the community. MRSA carriers can become infected with a rate depending on whether they are currently not hospitalized, hospitalized in a general ward, hospitalized in an ICU, or living in a nursing home. Colonized and infected persons may return to the susceptible state after decolonization treatments; asymptomatic carriers may also undergo natural decolonization.
Model Calibration.
The model comprises the following set of free parameters: the rates of colonization in households, hospitals, and nursing homes, the rate of infection in the community (i.e., for individuals currently not hospitalized), and the three initial values of MRSA prevalence in the community, nursing homes, and hospital healthcare workers. We calibrated the model to the yearly MRSA infections reported to the Norwegian national registry between 2008 and 2015 and to the prevalence of colonization in household contacts of MRSA carriers (19). We explored the parameter space with Latin Hypercube Sampling, computed a likelihood score for each sampled parameter set, and selected the optimal parameter values via a multistep calibration procedure fully described in the SI Appendix, Supplementary Materials. Model results presented in this paper are based on simulations performed by running 50 stochastic realizations of the model for each of the 100 most likely parameters sets.
Key Estimated Epidemiological Indicators.
The calibrated model was used to estimate a number of measures relevant for MRSA epidemiology. Taking advantage of the individual-based structure of the model, which enabled to track the infector in each single transmission event, we computed the effective reproductive number Re as the average number of MRSA transmissions directly generated by a new imported carrier. Similarly, we computed the reproduction number associated with nosocomial transmissions linked to hospital admissions of colonized patients Ra. We defined a transmission cluster as the set of primary transmissions generated by the index case and all of the transmissions generated by his offspring; the size of the cluster is given by the number of persons being part of the cluster. The prevalence of MRSA was defined as the proportion of carriers in a given population at a specified point in time. The age-specific transmission matrix was estimated by reporting the mean number of colonization events, disaggregated by the ages of the infector and of the infected (ages were grouped by 5 y).
The study was approved by the Regional Committees for Medical and Health Research Ethics—South-East Norway (project number 2011/2456).
Supplementary Material
Acknowledgments
This study was funded by the South-Eastern Norway Regional Health Authority (project number 2014002). We thank Petter Elstrøm of the Norwegian Institute of Public Health for useful discussions on the MRSA epidemiology in Norway.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900959116/-/DCSupplemental.
References
- 1.Lowy F. D., Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Stefani S., et al. , Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 39, 273–282 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Köck R., et al. , Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Euro Surveill. 15, 19688 (2010). [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control , “Antimicrobial resistance surveillance in Europe 2015. Annual report of the European Antimicrobial Resistance Surveillance Network” (EARS-Net, Stockholm, 2017; https://ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2015).
- 5.Spicer W. J., Three strategies in the control of staphylococci including methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 5 (suppl. A), 45–49 (1984). [DOI] [PubMed] [Google Scholar]
- 6.Otter J. A., French G. L., Community-associated meticillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated infection. J. Hosp. Infect. 79, 189–193 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Chambers H. F., Deleo F. R., Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David M. Z., Daum R. S., Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23, 616–687 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Ruscio F., Bjørnholt J. V., Leegaard T. M., Moen A. E. F., de Blasio B. F., MRSA infections in Norway: A study of the temporal evolution, 2006-2015. PLoS One 12, e0179771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elstrøm P., et al. , Meticillin-resistant Staphylococcus aureus in Norway, a low-incidence country, 2006-2010. J. Hosp. Infect. 80, 36–40 (2012). [DOI] [PubMed] [Google Scholar]
- 11.van Kleef E., Robotham J. V., Jit M., Deeny S. R., Edmunds W. J., Modelling the transmission of healthcare associated infections: A systematic review. BMC Infect. Dis. 13, 294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee B. Y., et al. , Modeling the spread of methicillin-resistant Staphylococcus aureus (MRSA) outbreaks throughout the hospitals in Orange County, California. Infect. Control Hosp. Epidemiol. 32, 562–572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agata E. M. C., Webb G. F., Horn M. A., Moellering R. C. Jr, Ruan S., Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin. Infect. Dis. 48, 274–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temime L., et al. , Peripatetic health-care workers as potential superspreaders. Proc. Natl. Acad. Sci. U.S.A. 106, 18420–18425 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B. Y., et al. , The importance of nursing homes in the spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospitals. Med. Care 51, 205–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robotham J. V., et al. , Cost-effectiveness of national mandatory screening of all admissions to English National Health Service hospitals for meticillin-resistant Staphylococcus aureus: A mathematical modelling study. Lancet Infect. Dis. 16, 348–356 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Bootsma M. C. J., Diekmann O., Bonten M. J. M., Controlling methicillin-resistant Staphylococcus aureus: Quantifying the effects of interventions and rapid diagnostic testing. Proc. Natl. Acad. Sci. U.S.A. 103, 5620–5625 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macal C. M., et al. , Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: A dynamic agent-based simulation. J. Transl. Med. 12, 124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson P. J. H., Gustafsson E. B., Ringberg H., High prevalence of MRSA in household contacts. Scand. J. Infect. Dis. 39, 764–768 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Di Ruscio F., et al. , Epidemiology and spa-type diversity of meticillin-resistant Staphylococcus aureus in community and healthcare settings in Norway. J. Hosp. Infect. 100, 316–321 (2018). [DOI] [PubMed] [Google Scholar]
- 21.den Heijer C. D. J., et al. ; APRES Study Team , Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S aureus, in nine European countries: A cross-sectional study. Lancet Infect. Dis. 13, 409–415 (2013). Erratum in: Lancet Infect. Dis.13, 1011 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Hughes C., Tunney M., Bradley M. C., Infection control strategies for preventing the transmission of meticillin-resistant Staphylococcus aureus (MRSA) in nursing homes for older people. Cochrane Database Syst. Rev. 11, CD006354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coello R., Glynn J. R., Gaspar C., Picazo J. J., Fereres J., Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J. Hosp. Infect. 37, 39–46 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Coll F., et al. , Longitudinal genomic surveillance of MRSA in the UK reveals transmission patterns in hospitals and the community. Sci. Transl. Med. 9, eaak9745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albrich W. C., Harbarth S., Health-care workers: Source, vector, or victim of MRSA? Lancet Infect. Dis. 8, 289–301 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Mossong J., et al. , Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fumanelli L., Ajelli M., Manfredi P., Vespignani A., Merler S., Inferring the structure of social contacts from demographic data in the analysis of infectious diseases spread. PLoS Comput. Biol. 8, e1002673 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y. P., Wilder-Smith A., Hsu L.-Y., The role of international travel in the spread of methicillin-resistant Staphylococcus aureus. J. Travel Med. 21, 272–281 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Woodford N., Unwanted souvenirs: Travel and multi-resistant bacteria. J. Travel Med. 18, 297–298 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Bootsma M. C. J., Wassenberg M. W. M., Trapman P., Bonten M. J. M., The nosocomial transmission rate of animal-associated ST398 meticillin-resistant Staphylococcus aureus. J. R. Soc. Interface 8, 578–584 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetem D. J., et al. , Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus in Danish hospitals. J. Antimicrob. Chemother. 67, 1775–1780 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Smith D. L., Dushoff J., Perencevich E. N., Harris A. D., Levin S. A., Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: Resistance is a regional problem. Proc. Natl. Acad. Sci. U.S.A. 101, 3709–3714 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mollema F. P. N., et al. , Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J. Clin. Microbiol. 48, 202–207 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute of Public Health, The Norwegian Directorate of Health , “Infection control 16 MRSA-guidelines” (NIPH, Oslo, 2009).
- 35.Tosas Auguet O., et al. , Frequent undetected ward-based methicillin-resistant Staphylococcus aureus transmission linked to patient sharing between hospitals. Clin. Infect. Dis. 66, 840–848 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krämer A., Kretzschmar M., Krickeberg K., Modern Infectious Disease Epidemiology: Concepts, Methods, Mathematical Models, and Public Health (Springer Science & Business Media, New York, London, 2010). [Google Scholar]
- 37.Bootsma M. C. J., Bonten M. J. M., Unraveling the dynamics of community-associated methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 56, 1075–1077 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Popovich K. J., et al. , Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin. Infect. Dis. 56, 1067–1074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graveland H., Wagenaar J. A., Bergs K., Heesterbeek H., Heederik D., Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS One 6, e16830 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grøntvedt C. A., et al. , Methicillin-resistant Staphylococcus aureus CC398 in humans and pigs in Norway: A “one health” perspective on introduction and transmission. Clin. Infect. Dis. 63, 1431–1438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mostofsky E., Lipsitch M., Regev-Yochay G., Is methicillin-resistant Staphylococcus aureus replacing methicillin-susceptible S. aureus? J. Antimicrob. Chemother. 66, 2199–2214 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moxnes J. F., de Blasio B. F., Leegaard T. M., Moen A. E. F., Methicillin-resistant Staphylococcus aureus (MRSA) is increasing in Norway: A time series analysis of reported MRSA and methicillin-sensitive S. aureus cases, 1997-2010. PLoS One 8, e70499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Kleef E., Luangasanatip N., Bonten M. J., Cooper B. S., Why sensitive bacteria are resistant to hospital infection control. Wellcome Open Res. 2, 16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies N. G., Flasche S., Jit M., Atkins K. E., Within-host dynamics shape antibiotic resistance in commensal bacteria. Nat. Ecol. Evol. 3, 440–449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blix H. S., et al. , The Norwegian Prescription Database 2012-2016 (Norwegian Institute of Public Health, Oslo, 2017). [Google Scholar]
- 46.Norwegian Institute of Public Health, the Norwegian Surveillance System for Communicable Diseases (MSIS) http://www.msis.no/. Accessed 5 May 2016.
- 47.Center for International Earth Science Information Network (CIESIN), Columbia University, United Nations Food and Agriculture Programme (FAO), and Centro Internacional de Agricultura Tropical (CIAT), Gridded Population of the World, Version 3 (GPWv3): Population Count Grid (NASA Socioeconomic Data and Applications Center, Palisades, NY, 2005) 10.7927/H4639MPP. Accessed 12 June 2019. [DOI]
- 48.González M. C., Hidalgo C. A., Barabási A.-L., Understanding individual human mobility patterns. Nature 453, 779–782 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.