Abstract
Intracranial haemorrhage (ICH) is a life-threatening type of stroke with high mortality, morbidity, and recurrence rates. However, no effective treatment has been established to improve functional outcomes in patients with ICH to date. Strategies targeting secondary brain injury are of great interest in both experimental and translational studies. The immune system is increasingly considered to be a crucial contributor to ICH-induced brain injury because it participates in multiple phases of ICH, from the early vascular rupture events to brain recovery. Various pathobiological processes that contribute to secondary brain injury closely interact with the immune system, such as brain oedema, neuroinflammation, and neuronal damage. Hence, we summarize the immune response to ICH and recent progress in treatments targeting the immune system in this review. The emerging therapeutic strategies that target the immune system after ICH are a particular focus and have been summarized.
Keywords: Intracerebral haemorrhage, Secondary brain injury, Immune response, Therapeutic targets, Immunomodulators
1. Introduction
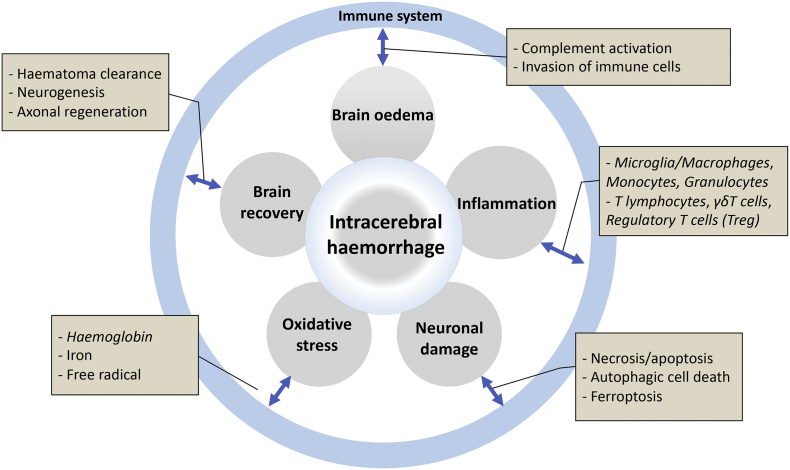
Intracerebral haemorrhage (ICH) is a severe public health issue accounts for 10–15% of all stroke types. Over the past few decades, advances have been made in the acute management of patients with ICH, such as blood pressure control, neurosurgery, and haemostasis strategies [1]. However, very little progress has been made in improving the long-term outcomes. Only 46% of patients with ICH survive for 1 year and 29% survive for 5 years [2]. Survivors suffer from severe and prolonged neurological dysfunction. After ICH, the primary injury is mainly caused by the expansion of the haematoma. Meanwhile, the haematoma itself leads to secondary brain injury (SBI). Based on accumulating evidence, the immune system is closely associated with the key pathological processes of ICH-induced SBI, including brain oedema, neuroinflammation and neuronal damage (Fig. 1). Thus, an understanding of the process and mechanism of the immune response after ICH and the identification of novel therapeutic targets are essential to harness the full therapeutic potential of the immune system and facilitate the development of new medical therapies to improve the long-term outcomes of patients with ICH.
Fig. 1.
Summary of the involvement of the immune response in the mechanism of ICH.
2. The immune response in the natural history of ICH
The immune system is activated as early as minutes after the rupture of blood vessels. After the onset of ICH, the extravasated blood components activate microglia and initiate immune responses. In addition, the damaged brain tissue releases damage-associated molecular patterns (DAMPs) that activate innate immune reactions by binding to pattern recognition receptors (PRRs). Subsequently, red blood cells lysis occurs 24 h after ICH onset and persists for several days, resulting in the release of pro-oxidative haemoglobin and its degradation products haem and iron [3]. These cytotoxic products cause brain damage and are gradually removed by macrophages/microglia via phagocytosis. SBI occurs after the development of brain oedema, inflammation, free radicals and the toxicity of the haematoma and their degraded by-products. Interestingly, brain injury in the subacute phase suppresses peripheral immune responses, although the underlying mechanisms and potential implications remain elusive. If patients survive the early stage of ICH, gradual clot resolution and brain repair occur, although the functional recovery is incomplete. Overall, the immune system plays a role in various pathobiological processes after ICH and provides potential treatment targets. Below, we discuss the role of the immune response in different stages and processes of ICH.
2.1. The immune response and primary brain injury
Depending on the haematoma expansion rate, primary brain damage occurs within minutes to hours from onset. It is primarily caused by mechanical damage associated with the haematoma mass effect [4]. The innate immune system can be triggered immediately as the extravasated blood components (including thrombin, complement components, immunoglobulins, etc.) directly activate microglia, the residential immune cells in the central nervous system [5]. DAMPs (including nucleic acids, proteins, lipid mediators, ATP, etc.) released from injured tissues also activate microglia and produce a severe cytotoxic, pro-inflammatory, and pro-oxidative insult on adjacent brain cells [6].
2.2. The immune response and secondary brain injury
2.2.1. Brain oedema
Brain oedema develops during the acute phase (1–3 day) and subacute stage (3 day to 1 month) after ICH. A reduction in brain oedema improves the survival of patients with ICH [7]. The complement system is part of the innate immune system that is activated in the brain parenchyma after ICH as a result of disruption of the blood-brain barrier (BBB). Complement activation results in neuronal damage and BBB leakage, which further increases oedema formation [8]. This vicious cycle enhances both the immune response and brain oedema. Inhibition of the complement system attenuates thrombin-induced brain oedema and improves the neurological function of an animal model of ICH [9]. Thus, treatments blocking the complement cascade may be a promising strategy to alleviate brain oedema after ICH.
2.2.2. Inflammation
Inflammation is an important contributor to ICH-induced SBI. After ICH, the inflammatory response is immediately induced and is characterized by the activation of resident inflammatory cells such as microglia and astrocytes. Blood components, including red blood cells, leukocytes, macrophages and plasma proteins, are released into the cerebral parenchyma and enhance the local inflammatory processes [10]. The activated resident inflammatory cells and damaged BBB result in the infiltration and activation of systemic inflammatory cells and release of inflammatory cytokines. Both the innate and adaptive immune systems participate in ICH-induced neuroinflammation. Leukocytes infiltrate through the BBB and produce proinflammatory cytokines and MMPs, which further damage the BBB [5]. Systemic immune cells may migrate across the BBB and enter the brain. CD4+ T cells are the dominate leukocyte population that infiltrates the brain [11], but other T cell populations, such as proinflammatory γδT cells and immunosuppressive regulatory T cells, also infiltrate into the haemorrhagic brain after ICH [12]. However, the precise roles of these infiltrated immune cells remain unclear. Modulation of adaptive immunity exerts neuroprotective effects on the ischemic brain [11], which suggested the prospect of targeting the immune system in patients with ICH.
2.2.3. Neuronal damage
Secondary brain injury is caused by a cascade of events triggered by the primary injury, the response to the haematoma, and the release of clot components. The mode of neuronal injury was recently shown to vary in patients with ICH, from the canonical necrosis and apoptosis pathways to autophagic cell death and ferroptosis.
Necrotic or apoptotic cells release DAMPs after stroke, which consist of a various substances, such as purines (ATP and UTP), high mobility group binding protein 1 (HMGB1), heat shock proteins (HSPs), peroxiredoxins and mitochondrial-derived N-formyl peptides [6,13]. DAMPs activate microglia and peripheral innate immune cells by binding to specific PRRs on the cell surface [14]. In addition, the activated complement cascade triggers the formation of the membrane attack complex (MAC), which forms a pore in the cell membrane and leads to cell lysis. The MAC causes RBC lysis and the release of haemoglobin and iron, resulting in perihaematomal brain damage [4].
Autophagic cell death is morphologically defined as cell death accompanied by large-scale autophagic vacuolization in the cytoplasm. Multiple immune-related molecules regulate autophagy and therefore autophagic cell death. On the one hand, autophagy is induced by various PRRs, DAMPs, and pathogen receptors after ICH [15]. On the other hand, cytokines and molecules such as Bcl-2, NF-κB, and T helper 2 (TH2) cytokines inhibit autophagy [16]. Nevertheless, the precise role of the immune system in regulating autophagy and autophagic cell death remains poorly understood.
Ferroptosis is an iron-dependent form of regulated cell death associated with the accumulation of lipid hydroperoxides and is characterized by cell shrinkage and an increased mitochondrial membrane density [17,18]. Zille et al. observed the pathological and molecular features of ferroptosis in neurons after experimental ICH [19]. Consistent with these findings, ferroptosis occurs in a mouse ICH model and contributes to neuronal death, whereas the inhibition of ferroptosis protects the haemorrhagic brain [20]. In the mouse model of kidney and brain disease, ferroptotic cells activated the innate immune system by secreting pro-inflammatory DAMPs, which may contribute to or exacerbate the organ injury [21].
2.2.4. Haemoglobin, iron and free radicals
Haemoglobin and iron released from haematomas have been widely considered as major contributors to ICH-induced brain injury. The potential mechanism by which haemoglobin and iron cause brain damage is through the generation of free radicals [4]. Free radical-mediated tissue damage has been observed in subjects with ICH, and the clearance of free radicals alleviates ICH-induced injury in animal models [22]. Therefore, free radicals may have important clinical implications in the control of haemolysis and scavenge haemoglobin, haem and iron. Haemoglobin and haem are endocytosed by microglia/macrophages through the scavenger receptors CD163 and CD91, respectively, while iron is sequestrated within phagocytes by iron-binding proteins such as hemosiderin or ferritin [5]. Treatments that enhance the phagocytic function of microglia/macrophages may be a promising strategy to reduce the toxicity of haemolysis products and prevent oxidative brain damage.
2.3. Immune response in brain recovery
2.3.1. Haematoma resolution
Haematoma resolution gradually occurs in the first few days after ICH and might contribute to the recovery of neurological function. Microglia and blood-derived macrophages participate in the clearance of apoptotic, dislocated, and damaged cells through phagocytosis [23]. CD36 is a well-known cell surface receptor expressed on microglia/macrophage that mediates the phagocytosis of apoptotic and damaged cells. Administration of PPARγ agonists (rosiglitazone and pioglitazone), which increased CD36 expression, enhanced haematoma resolution and improved functional recovery after ICH [23].
2.3.2. Neurogenesis
Strategies that enhance NSC neurogenesis represent a potential treatment for neurological diseases, including ICH. Studies of ischemic stroke models have shown that endogenous NSCs migrate to the infarcted site and differentiate into astrocytes, which contribute to the formation of glial scar tissue [24]. Resident immune cells in the central nervous system and peripheral immune cells participate in the mechanism regulating adult neurogenesis. Microglia were first suggested to influence the differentiation of neural precursor cells in 2001, as soluble factors released from microglia not only induced neural precursor cells to differentiate into a neuronal phenotype but also directed their migration [25]. Moreover, microglia regulate neuronal differentiation and adult neurogenesis at the post-transcriptional level, as they express higher levels of microRNAs, such as miR-124 and miR-22 [26]. Systemic depletion of CD4+ T lymphocytes significantly decreases hippocampal neurogenesis and BDNF expression [27]. Moreover, activated Tregs also promote neurogenesis in the subventricular zone of normal and ischemic mice by synthesizing IL-10 [28].
2.3.3. Axonal regeneration
Axonal injury and demyelination have been reported in rat models of ICH and the possible pathophysiological mechanisms were reviewed in detail by Tao [29]. Strategies that accelerate axon regeneration are an important approach to restoring the integrity of white matter and enhancing functional recovery after ischaemic stroke [30]. The role of the immune response in axonal regeneration has frequently been studied in subjects with neurotrauma, particularly in spinal cord injury (SCI) models. Microglia and macrophages are recruited to injured regions and indirectly promote axon regeneration by neutralizing myelin-associated inhibitors and removing myelin debris [31]. However, those activated immune cells may exert detrimental effects (e.g., attacking cells by releasing pro-inflammatory cytokines or forming glial scars), depending on the context [32].
3. From bench to bedside: emerging therapeutic targets for ICH associated with the immune system and promising immunomodulatory drugs
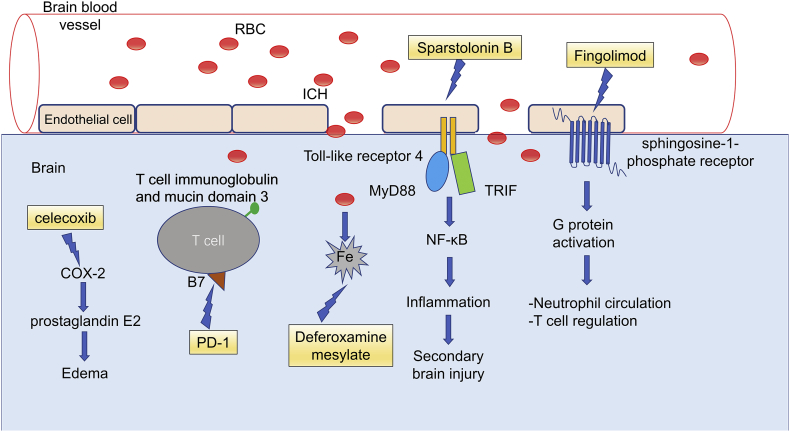
Molecular targets related to the immune system have been widely studied in preclinical studies of animal models of ICH animal and even in a few clinical trials, which are summarized in Table 1. The extensively studied molecules and pathways involved in the responses are illustrated in Fig. 2.
Table 1.
Summary of promising immunomodulatory drugs.
| Drug | Target | Study design | Intervention (dosing, route of drug administration, and duration) | Treatment initiation time after onset | Outcome | Phase | Reference |
|---|---|---|---|---|---|---|---|
| Clinical trial | |||||||
| Fingolimod (FTY720) | S1PR | Open label (n = 23) | 0.5 mg, orally for 3 consecutive days | <72 h | Safe, reduced perihaematomal oedema, attenuated neurologic deficits, and promoted recovery | Phase 2 | Fu et al. [7] |
| Minocycline | Multiple | Open label (n = 16) | 400 mg of intravenous minocycline, followed by 400 mg of oral minocycline daily for 4 days | <24 h | A 400 mg dose of minocycline was safe and achieved neuroprotective serum concentrations | Phases 1 and 2 | Fouda et al. [83] |
| Celecoxib | COX-2 | Multicentre randomized controlled trial (n = 20) | 400 mg, orally (twice a day) for 14 days | <24 h | The administration of celecoxib in the acute stage of ICH was associated with a smaller expansion of perihaematomal oedema than in controls | Phase 2 | Lee et al. [60] |
| Deferoxamine Mesylate | iron | Multicentre randomized controlled trial (n = 144) | 32 mg/kg, for 3 consecutive days | <24 h | Deferoxamine mesylate was a safe ICH treatment | Phase 2 | Selim, M. et al. (2019) [61] |
| Siponimod (BAF312) | S1PR | Randomized controlled trial | Not available | <24 h | Not available | Phase 2 (Recruiting) | Kevin N. Sheth et al. [57] |
| Experimental trial | |||||||
| RP101075 | S1PR1 | Mouse model | 0.6 mg/kg daily oral gavage for 3 consecutive days | 30 min | Significantly attenuated neurological deficits and reduced brain oedema | Pre-clinical | Sun et al. [47] |
| PD-1 | Multiple | Mice | 50 μg, one intraperitoneal injection | 1 h | Attenuated neurological deficits, reduced brain oedema, and decreased haemorrhage volume | Pre-clinical | Han et al. [73] |
| Sparstolonin B | Multiple | Mice | 5 mg/kg, intraperitoneal injection, once daily for 3 consecutive days | 2 h | Stimulates short-term neurobehavioral recovery and reduces neurological deficits | Pre-clinical | Wang et al. [41] |
| TAK-242 | TLR 4 | Mice | 3 mg/kg, intraperitoneal injection, once daily for 5 successive days | 6 h | Significantly reduced the brain water content, neurological deficit scores, and levels of inflammatory factors | Pre-clinical | Wang et al. [42,84,85] |
| Sheng-Di-Da-Huang decoction | TLR 4 | Rat | 16, 8 or 4 g/kg/day, orally, once daily for 14 successive days | <24 h | Remarkably improved neurological function and reduced the brain water content | Pre-clinical | Cai et al. [44] |
| Ligustilide and senkyunolide H | TLR4/NF-κB signalling | Mice | 10 mg/kg, intraperitoneal injection, 24 h and 48 h after ICH for the next 2 days | 24 h and 48 h | Neuroprotective effects | Pre-clinical | Han et al. [43] |
| Anti-B7-1 antibody | B7-1 (CD80)/B7-2 (CD86) signalling pathway | Mice | 8 mg/kg, inner canthus veniplex injection, 10 min and 24 h after ICH | 10 min and 24 h | Reduced long-range brain damage by reversing the immune imbalance | Pre-clinical | Ma et al. [74] |
Fig. 2.
Molecular mechanisms of the therapeutic targets for ICH.
3.1. Toll-like receptor 4
3.1.1. Toll-like receptor 4
Toll-like receptor 4 (TLR4) belongs to the TLR family, which consists of 13 transmembrane receptors (TLR1–13). TLRs play critical roles in the innate immune response to pathogens by inducing immunological and inflammatory responses [33]. TLR4 is primarily expressed in microglial cells, and its activation leads to microglial cell activation and the release of pro-inflammatory signals [34]. Upregulation of TRL4 and increased NF-κB activity were observed in the brain tissue of a rat ICH model, suggesting that the TLR4 signalling pathway is involved in ICH-induced brain injury. TLR4-deficient mice exhibit less perihaematomal inflammation after ICH, which was associated with a reduced recruitment of neutrophils and monocytes, fewer microglia, and improved functional outcomes [35]. In patients with ICH, increased expression of TLR4 is associated with poor functional outcomes at 3 months after onset [36].
3.1.2. TLR4/MyD88 and TLR4/TRIF signalling pathways
TLR4 is activated by various endogenous proteins that function as “danger signals” in the setting of injury. Many of these endogenous TLR4 ligands, including haem, fibrinogen, HSP70, hyaluronan, and high-mobility group box proteins, are present in the brain after ICH [35]. After activation by its ligands, TLR4 predominantly signals through two downstream proteins, myeloid differentiation primary response gene 88 (MyD88) and Toll/IR-1 domain containing adaptor protein inducing interferon-beta (TRIF). Both signals ultimately lead to the activation of transcription factors, such as NF-κB, and the subsequent expression of various proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [37]. The establishment of an ICH model in transgenic mice showed that MyD88 and TRIF deficiencies improve neurological deficits and reduce cytokine release and macrophage infiltration. These results were similar to the findings Lin et al. reported from TLR4 knockout mice. The reduced expression of MyD88 and TRIF in TLR4 knockout mice observed in response to ICH injury provide direct evidence of the involvement of MyD88 and TRIF in the TLR4 signalling pathway after ICH [37]. More recently, the activation of TLR4/MyD88 signalling pathway was observed in patients with ICH, as the expression of TLR4, MyD88 and NF-κB began to increase within 6 h after ICH, peaked from 24 to 72 h, and decreased after 72 h [38]. On the other hand, disrupted iron metabolism in the brain after ICH results in oxidative brain injury and cognitive impairments. Xiong et al. showed the blockade of TLR4 may be beneficial not only in reducing inflammatory injury but also in alleviating the accumulated iron in the brain caused by oxidative injury after ICH [39].
3.1.3. Sparstolonin B, TAK-242, Ligustilide, Senkyunolide H and sheng-Di-Da-Huang decoction
Several compounds targeting TLR4 exert a protective effect on ICH. Sparstolonin B (SsnB) is a natural compound extracted from the Chinese herb Scirpus yagara that blocks TLR4-triggered inflammatory signalling by inhibiting the binding of MyD88 to TLR4. Zhong et al. reported improved neurological outcomes after ICH in mice treated with SsnB [40]. SsnB ameliorates brain oedema and neurological deficits in mice with ICH by inhibiting the formation of the TLR2-TLR4 heterodimer. SsnB exhibits high liposolubility and has a low molecular weight, which allows it to cross the BBB and attain a high concentration in the brain [41]. TAK-242 is a TLR4 antagonist. Mice treated with TAK-242 show a decreased inflammatory response, less brain oedema, the downregulation of several downstream inflammatory mediators and improved neurological outcomes [42]. Ligustilide and senkyunolide H, two bioactive compounds of Chinese medicine, exert protective effects on haemorrhagic stroke. Both compounds inhibit TLR4 via the NF-κB signalling pathway, reduce immune/inflammatory injury and finally suppress neurological deterioration in an experimental haemorrhagic stroke model [43]. Sheng-Di-Da-Huang decoction, a Chinese medicine, reduces inflammatory reactions after ICH by inhibiting inflammation-mediated microglial activation and reducing TLR4 expression [44].
3.2. Sphingosine-1-phosphate receptor 1 (S1PR1)
3.2.1. S1PR1
S1PR1 is a member of the sphingosine-1-phosphate receptor family, which includes S1PR1 to S1PR5. S1PRs are a class of G protein-coupled receptors that are targets of the lipid signalling molecule sphingosine-1-phosphate (S1P). S1P is a bioactive sphingolipid mediator that is involved in many physiological processes, including angiogenesis and immune responses [45]. S1PR1 is involved in immunomodulation by regulating immune cell trafficking and differentiation [46]. S1PR1 is expressed on lymphocytes, vascular endothelial cells, neurons, and glia. Notably, the protective effects of different S1PR1 agonists on experimental ICH models have been documented [47]. Another S1PR, S1PR2, was detected in the microvessels and cerebrovascular endothelium of mice with ischemic stroke [48], indicating that S1PR2 plays a crucial role in decreasing the cerebrovascular integrity after ischemia-reperfusion injury. S1PR2 inhibition decreases the activity of matrix metalloproteinase 9 (MMP-9), resulting in increased vascular permeability.
3.2.2. Fingolimod, RP101075 and siponimod (BAF312)
Fingolimod (FTY720, Gilenya) is an S1P analogue that targets four of the five known S1P receptors (S1PR1, 3, 4, and 5) [49]. This drug was initially used to treat multiple sclerosis, based on its immunosuppressive activity. It inhibits S1PR1-dependent lymphocyte egress by downregulating S1PR1 on T cells. W. B. Rolland et al. first reported the neuroprotective effect of fingolimod on a mouse model of ICH. In their study, the administration of 1 mg/kg fingolimod to mice 1 h after ICH induction reduced brain oedema and improved neurological functions [50]. This team subsequently observed reduced cerebral lymphocyte infiltration and lower expression of intercellular adhesion molecule-1 (ICAM-1), interferon-γ (IFN-γ) and interleukin-17 (IL-17) in ICH mice treated with fingolimod. Therefore, the authors concluded that fingolimod reduces the number of T lymphocytes that migrated into the brain, thereby ameliorating cerebral inflammation, which ultimately improved neurobehavioral and cognitive outcomes [51]. In contrast, Schlunk et al. recently reported a lack of beneficial effects of fingolimod on short-term outcomes in ICH mice [52]. The reasons for the discrepancies in the results from different groups are not yet clear. In 2014, a 2-arm study of 23 patients with supratentorial ICH reported that oral FTY720 reduced perihaematomal oedema and improved functional outcomes if administered within 72 h [7]. As shown in the study by Li, Y. J. et al., fingolimod decreases the numbers of circulating CD4+ T, CD8+ T, CD19+ B, NK, and NKT cells, and the numbers recovered quickly after the drug was stopped. The plasma ICAM level was decreased, and IL-10 was increased by fingolimod [53]. Fingolimod significantly decreases T lymphocyte infiltration and improves BBB integrity compared with the vehicle control [54]. However, the adverse effects of fingolimod limit its use in patients with stroke. Because of its off-target interactions with other S1PR subtypes, particularly with S1PR3, many adverse events have been reported, including hypertension, macular oedema, pulmonary toxicity, and hepatotoxicity [55].
RP101075 is a selective S1PR1 agonist with a superior cardiovascular safety profile. It displays high S1PR1 selectivity (>100-fold over S1PR5 and >10,000-fold over S1PR 2, 3, and 4). An RP101075 treatment attenuates neurological deficits and brain oedema in a mouse ICH model. RP101075 reduces the number of brain-infiltrating immune cells, enhances BBB integrity and attenuates cell death after ICH [47]. It represents an alternative agent to fingolimod in patients who have experienced severe side effects.
Siponimod (BAF312), an analogue of FTY720, binds to 2 of 5 S1P receptor isoforms: S1PR1 and S1PR5. Siponimod reduces the infiltration of lymphocytes into the brain, thus slowing the progression of inflammation. A siponimod treatment reduces brain infiltration but not lesion volumes in middle-aged mice with transient middle cerebral artery occlusion [56]. Therefore, siponimod is a drug that might potentially limit brain inflammation after ICH and thereby improve neurological outcomes for patients with haemorrhagic stroke. Currently, a randomized, placebo-controlled, subject- and investigator-blinded trial of BAF312 is ongoing in patients with ICH to study its efficacy, safety, and tolerability [57].
3.3. Cyclooxygenase 2 (COX-2)
Celecoxib is a selective inhibitor of COX-2 that has been widely used as an anti-inflammatory agent. Celecoxib reduces perihaematomal oedema, inflammation and cell death, and improves functional recovery in a rodent model of ICH [58]. A retrospective analysis showed the potential benefit of celecoxib in patients with ICH [59]. A phase 1/2 multicentre trial confirmed that the early administration of celecoxib was safe and efficacious in the treatment of primary ICH. After 1 week of treatment, the expansion of perihaematomal oedema volume was reduced in patients treated with celecoxib compared with controls who received standard management [60].
3.4. Deferoxamine mesylate
Deferoxamine mesylate is the mesylate salt of an iron-chelating agent that binds free iron in a stable complex, preventing it from engaging in chemical reactions. Selim et al. reported the safety of deferoxamine mesylate in a prospective, multicentre, futility-design, randomized, placebo-controlled, double-blind, phase 2 clinical trial at 40 hospitals in Canada and the USA. However, based on the findings of this study, a phase 3 efficacy trial of deferoxamine mesylate was recommended because the expected result is that the treatment will significantly improve the possibility of patients with ICH achieving a good clinical outcome after 90 days [61].
3.5. T cell immunoglobulin and mucin domain 3 (Tim-3)
Tim-3 is a new immunoregulatory molecule that was discovered in 2002. Originally, it was described as a specific cell surface marker of activated T helper 1 cells [62,63]. Tim-3 has been detected on other immune cells, including monocytes/macrophages, mast cells, CD8+ T cells, NK cells and dendritic cells [[64], [65], [66]]. Tim-3 is referred to as a checkpoint receptor and exerts both positive and negative effects, partially depending on the specific cell type and immune response course. For instance, Tim-3 expressed on T helper type 1 cells inhibits the T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance [63]. In patients with spontaneous ICH, Tim-3 expression was significantly downregulated on peripheral CD3+ T cells and CD8+ T cells, while Tim-3 expression was increased on CD14+ monocytes and CD16+ CD56+ NK cells. Furthermore, Tim-3 expression on peripheral CD8+ cells was negatively correlated with the inflammatory response, the disease severity and the outcomes of patients with ICH [67]. A subsequent study observed a remarkable increase in the expression of the Tim-3 mRNA in peripheral blood mononuclear cells from patients with ICH that peaked on day 3, and Tim-3 expression was positively correlated with the concentrations of the TNF-α, IL-1β, and S—100B proteins. Moreover, increased expression of Tim-3 on CD14+ monocytes is associated with systemic inflammatory reactions and brain injury in patients with spontaneous ICH [68]. The possible mechanisms underlying the dysregulation of Tim-3 expression after ICH were analysed in a mouse model. Increased expression of Tim-3 aggravates brain inflammation by regulating the function of microglia/macrophages after ICH [69]. In addition, Tim-3 increases brain inflammation by promoting macrophage polarization, which produces destructive proinflammatory factors and exacerbates brain injury in ICH mice [70].
3.6. Programmed death 1(PD-1)
PD-1, also known as CD279, is an important immune checkpoint receptor. PD-1 is expressed in a variety of activated immune cells, including CD4+ and CD8+ T cells, NKT cells, B cells, monocytes and certain subsets of dendritic cells. PD-1 binds to its ligand PD-L1 or PD-L2 to attenuate infectious immunity and tumour immunity, resulting in chronic infections and tumour progression [71]. Therefore, PD-1 blockade has been proven to be an effective therapy for certain cancers. The role of PD-1 in ICH is a new topic that has not been widely studied. In contrast to the scenario in cancer, PD-1 exerts a protective effect on ICH because it attenuates macrophage-mediated inflammation. In the mouse model, ICH promotes PD-1 expression in macrophages and increases the concentrations of inflammatory cytokines and Fgl-2. PD-1-deficient mice presented significantly higher levels of the inflammatory cytokines that induce Fgl-2 expression than their wild-type littermates. As a result, higher levels of macrophage activation and cerebral oedema and higher neurological deficit scores were observed in PD-1-deficient mice [72]. PD-L1 reduces the number of brain-infiltrating immune cells, alleviates the inflammatory milieu, inhibits cell death and strengthens the BBB integrity after ICH. As a result, a PD-L1 treatment attenuates neurological deficits, brain oedema, and the haemorrhage size in a mouse model of ICH. [73]. To date, only the two studies mentioned above have explored the association between PD-1 and ICH. The mechanism underlying the protective effect of PD-L1 requires further study.
3.7. The B7–1 (CD80)/CD28 signalling pathway, interferon regulatory factor 3 (IRF3) and interleukin-27 (IL-27)
In addition to the targets mentioned above, other less frequently reported immune system-related proteins and pathways may also have potential value in the treatment of ICH. Blockade of the B7-1 (CD80)/CD28 signalling pathway with an anti-B7-1 antibody promotes spatial memory recovery. The underlying mechanisms may be the combined effect of B7-1 (CD80) on regulating inflammatory and immune responses, including reversing the Th1/Th2 imbalance and downregulation of IFN-γ and oedema, rather than regulating programmed cell death through autophagy and apoptosis [74]. The IRF3 protein might exert pro-apoptotic functions in neurons after ICH. IRF3 is mainly expressed in neurons and is expressed at low levels in astrocytes [75]. IRF3 levels adjacent to the hematoma are increased following ICH compared with the cortex of the sham group. IL-27, a heterodimeric cytokine that functions in innate immunity, is produced by activated dendritic cells, macrophages and astrocytes [76]. Zhao et al. observed IL-27 production in the rodent brain in response to ICH, which improves ICH outcomes by reducing oedema and increasing iron and haemoglobin clearance from the ICH-affected brain [77].
4. Future directions
Most evidence showing the therapeutic potential of immune modulators in ICH has been obtained from preclinical studies using animal models. The discrepancy between animal experiments and patient studies in the clinic imposes a translational uncertainty of the immune-based drugs. Clinical trials of immunomodulators with solid preclinical evidence are recommended to evaluate their safety and efficacy on patients with ICH. Among the targets and drugs discussed in this review, strategies that reduce perihaematomal oedema and target the sphingolipid signalling pathway are the most promising, as they are supported by relatively adequate clinical evidence. Moreover, several key considerations for immune interventions are required before their applications in clinical practice, such as the treatment time window, safety, patient selection and outcome measurements.
4.1. Promising drugs
Only celecoxib, a COX-2 inhibitor, exerted beneficial effects on reducing the perihaematomal oedema volume in patients with ICH in multicentre clinical trials [60]. Based on this strong clinical evidence, celecoxib is currently the most promising drug. However, other clinical trials have not reached the same conclusion. Another multicentre clinical trial showed that deferoxamine mesylate was safe for patients with ICH, but this study failed to determine a method to increase the therapeutic efficacy of this drug for ICH [61]. Nevertheless, the useful treatment duration should be examined in future studies.
In experimental models, FTY720 appears to exert beneficial effects on preventing the progression of secondary brain injury and promoting recovery in subjects with ICH [7]. However, only one 2-arm proof-of-concept study confirmed these effects of FTY720 on patients with ICH [7]. Hence, additional solid evidence should be obtained from randomized controlled trials before FTY720 is routinely used to treat patients with ICH. FTY720 targets S1P and modulates the sphingolipid signalling pathway. Interestingly, a clinical trial studying the effects of an analogue of FTY720 named siponimod on ICH is currently recruiting patients [57], indicating that SP1R is a promising molecular target. Additional studies aiming to explore a drug more specific for this target should be performed to reduce the side effects.
In this review, we summarize twelve drugs targeting different molecules that exert effects on ICH recovery. Theoretically, inventions targeting multiple cellular components and pathways may generate synergistic effects [78], but currently no study has explored this hypothesis.
4.2. Treatment time window
Although increasing evidence has confirmed that the immune system plays an essential role in the pathological process of ICH, the features of respondents at different phases and the mechanisms remain largely unclear. In the acute phase, the activated immune system facilitates the formation of the pro-inflammatory milieu and promotes brain damage, whereas inhibition of the immune response in this phase reduces brain injury. In the subacute phase, however, the injured brain tissue may suppress the peripheral immune responses and increase the infection risk [79]. The time window in which immune modulation might inhibit acute brain damage and reduce the associated complications is determined by the time at which the immune deficiency develops. A potential strategy is to administer fast-acting immune-suppressors to exert anti-inflammatory effects on the acute phase and to cease the treatment when the patient enters the subacute stage [78]. The identification of an appropriate time window is very important for the immune-based treatment of patients with ICH.
4.3. Safety
Despite the general risks associated with immunosuppressive drugs, such as increased risks of infection and malignancy, the long-term use of immunomodulators in patients with multiple sclerosis has been associated with rare but fatal adverse effects, including progressive multifocal leukoencephalopathy and herpes virus encephalitis [80]. The adverse effects of these drugs are less likely to occur when they are used in patients with ICH because they are more likely designed for short-term use [78]. However, a better understanding of the immune environment of the CNS after ICH is essential to identify the risks and reduce the adverse effects.
4.4. Patient selection
Researchers have assumed that patients with largest haematoma volume were the most likely to benefit from the immune interventions because more severe neuronal inflammation is observed in these patients [78]. One study of ICH showed that fingolimod produced greater benefits in patients with larger haematoma volumes [7]. However, no controlled perspective clinical study has tested this hypothesis.
4.5. Outcome measurements
The ICH score is a valid clinical grading scale for measuring both short-term (30 days) and long-term (12 months) outcomes after ICH. The scoring system has helped researchers stratify likelihood of favourable functional outcomes and to elucidate the course of improvement [81]. In addition, perihaematomal oedema is an independent predictor of mortality after ICH, which is also an important treatment target for strategies designed to improve patient outcomes. It is readily monitored by measuring the oedema extension distance growth rate using semiautomated planimetry based on computed tomographic scans [82].
5. Conclusions
The immune response after ICH is a dynamic process that involves various cells and molecules, which exert both destructive and regenerative effects on the brain tissue. Based on the current evidence, strategies targeting immune responses represent a viable approach to rescue brain injury and improve outcomes after ICH. Various compounds or drugs have been tested in preclinical studies using animal models. Nevertheless, only two clinical trials using immunomodulatory therapy have been conducted. More effort should be devoted to selecting promising modulators for clinical trials. In addition, compounds from Chinese medicine may offer new ideas for exploring potential drugs.
Search strategy and selection criteria
Articles included in this review were identified by searching MEDLINE and PUBMED, along with the reference lists from relevant articles, using the terms “ICH”, “mechanism”, “molecular target” and “immunotherapy”. Articles included were mainly published from 2014 to 2019 in the English language in peer-reviewed journals.
Conflict of interests
All authors state that there is no conflict of interest.
Acknowledgements
This work was funded by China Postdoctoral Science Foundation (2017M612010) and National Natural Science Foundation of China (81701144, 81870916).
Contributor Information
Anwen Shao, Email: 21118116@zju.edu.cn.
Shizhong Zhang, Email: zhangshizhong@smu.edu.cn.
References
- 1.Cordonnier C. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392(10154):1257–1268. doi: 10.1016/S0140-6736(18)31878-6. [DOI] [PubMed] [Google Scholar]
- 2.Poon M.T., Fonville A.F., Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(6):660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 3.Wagner K.R. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23(6):629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- 4.Keep R.F., Hua Y., Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronowski J., Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhry S.R. Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH) Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19072035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71(9):1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 8.Hua Y. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92(6):1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- 9.Gong Y. Complement inhibition attenuates brain edema and neurological deficits induced by thrombin. Acta Neurochir Suppl. 2005;95:389–392. doi: 10.1007/3-211-32318-x_79. [DOI] [PubMed] [Google Scholar]
- 10.Taylor R.A., Sansing L.H. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mracsko E. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45(7):2107–2114. doi: 10.1161/STROKEAHA.114.005801. [DOI] [PubMed] [Google Scholar]
- 12.Gao L. Transplanted neural stem cells modulate regulatory T, gammadelta T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int J Mol Sci. 2014;15(3):4431–4441. doi: 10.3390/ijms15034431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu S.W. The role of high mobility group box 1 protein in acute cerebrovascular diseases. Biomed Rep. 2018;9(3):191–197. doi: 10.3892/br.2018.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann S. Innate immunity and inflammation post-stroke: an alpha7-nicotinic agonist perspective. Int J Mol Sci. 2015;16(12):29029–29046. doi: 10.3390/ijms161226141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang D. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190(5):881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockwell B.R. Ferroptosis: a regulated cell death nexus linking metabolism. Redox Biol Dis Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21(4):648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zille M. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48(4):1033–1043. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Sanchez D. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol. 2017;28(1):218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39(2):463–469. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 24.Faiz M. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17(5):624–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Aarum J. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100(26):15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Miranda A.S. Hippocampal adult neurogenesis: does the immune system matter? J Neurol Sci. 2017;372:482–495. doi: 10.1016/j.jns.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 27.Wolf S.A. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182(7):3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 28.Wang J. Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front Cell Neurosci. 2015;vol. 9:361. doi: 10.3389/fncel.2015.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao C. White matter injury after intracerebral hemorrhage: pathophysiology and therapeutic strategies. Front Hum Neurosci. 2017;11:422. doi: 10.3389/fnhum.2017.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S. GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015;18(12):1737–1745. doi: 10.1038/nn.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gensel J.C. Achieving CNS axon regeneration by manipulating convergent neuro-immune signaling. Cell Tissue Res. 2012;349(1):201–213. doi: 10.1007/s00441-012-1425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egawa N. Mechanisms of axonal damage and repair after central nervous system injury. Transl Stroke Res. 2017;8(1):14–21. doi: 10.1007/s12975-016-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehnardt S. Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sansing L.H. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol. 2011;70(4):646–656. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Yanez M. Increased expression of toll-like receptors 2 and 4 is associated with poor outcome in intracerebral hemorrhage. J Neuroimmunol. 2012;247(1–2):75–80. doi: 10.1016/j.jneuroim.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Lin S. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gang X. Dynamic changes in toll-like receptor 4 in human perihematoma tissue after intracerebral hemorrhage. World Neurosurg. 2018;118:e593–e600. doi: 10.1016/j.wneu.2018.06.247. [DOI] [PubMed] [Google Scholar]
- 39.Xiong X.Y. Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain Iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation. 2016;134(14):1025–1038. doi: 10.1161/CIRCULATIONAHA.116.021881. [DOI] [PubMed] [Google Scholar]
- 40.Zhong Q. Interleukin-23 secreted by activated macrophages drives gammadeltaT cell production of interleukin-17 to aggravate secondary injury after intracerebral hemorrhage. J Am Heart Assoc. 2016;5(10) doi: 10.1161/JAHA.116.004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y. Sparstolonin B improves neurological outcomes following intracerebral hemorrhage in mice. Exp Ther Med. 2018;15(6):5436–5442. doi: 10.3892/etm.2018.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y.C. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke. 2013;44(9):2545–2552. doi: 10.1161/STROKEAHA.113.001038. [DOI] [PubMed] [Google Scholar]
- 43.Han L. The neuroprotective effects and probable mechanisms of Ligustilide and its degradative products on intracerebral hemorrhage in mice. Int Immunopharmacol. 2018;63:43–57. doi: 10.1016/j.intimp.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 44.Cai M. Sheng-Di-Da-Huang decoction inhibited inflammation expressed in microglia after intracerebral hemorrhage in rats. Evid Based Complement Alternat Med. 2018;vol. 2018:6470534. doi: 10.1155/2018/6470534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takabe K., Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res. 2014;55(9):1839–1846. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aoki M. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediators Inflamm. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun N. Selective sphingosine-1-phosphate receptor 1 modulation attenuates experimental intracerebral hemorrhage. Stroke. 2016;47(7):1899–1906. doi: 10.1161/STROKEAHA.115.012236. [DOI] [PubMed] [Google Scholar]
- 48.Kim G.S. Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat Commun. 2015;6:7893. doi: 10.1038/ncomms8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinkmann V. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 50.Rolland W.B., 2nd FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolland W.B. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlunk F. Treatment with FTY720 has no beneficial effects on short-term outcome in an experimental model of intracerebral hemorrhage. Exp Transl Stroke Med. 2016;8(1) doi: 10.1186/s13231-016-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y.J. Fingolimod alters inflammatory mediators and vascular permeability in intracerebral hemorrhage. Neurosci Bull. 2015;31(6):755–762. doi: 10.1007/s12264-015-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X. T lymphocytes infiltration promotes blood-brain barrier injury after experimental intracerebral hemorrhage. Brain Res. 2017;1670:96–105. doi: 10.1016/j.brainres.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 55.Ontaneda D., Cohen J.A. Potential mechanisms of efficacy and adverse effects in the use of fingolimod (FTY720) Expert Rev Clin Pharmacol. 2011;4(5):567–570. doi: 10.1586/ecp.11.46. [DOI] [PubMed] [Google Scholar]
- 56.Vogelgesang A. Siponimod (BAF312) treatment reduces brain infiltration but not lesion volume in middle-aged mice in experimental stroke. Stroke. 2019;50(5):1224–1231. doi: 10.1161/STROKEAHA.118.023667. [DOI] [PubMed] [Google Scholar]
- 57.Efficacy, Safety and Tolerability of BAF312 Compared to Placebo in Patients With Intracerebral Hemorrhage (ICH). Available from: https://ClinicalTrials.gov/show/NCT03338998
- 58.Chu K. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24(8):926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- 59.Park H.K. Effects of celecoxib on volumes of hematoma and edema in patients with primary intracerebral hemorrhage. J Neurol Sci. 2009;279(1–2):43–46. doi: 10.1016/j.jns.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Lee S.H. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicenter randomized controlled trial. Eur J Neurol. 2013;20(8):1161–1169. doi: 10.1111/ene.12140. [DOI] [PubMed] [Google Scholar]
- 61.Selim M. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18(5):428–438. doi: 10.1016/S1474-4422(19)30069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monney L. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Fueyo A. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4(11):1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 64.Nakae S. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110(7):2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson A.C. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 66.Ndhlovu L.C. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119(16):3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X. Dysregulated expression of T cell immunoglobulin and mucin domain 3 is associated with the disease severity and the outcome of patients with spontaneous intracerebral hemorrhage. Clin Biochem. 2013;46(15):1502–1508. doi: 10.1016/j.clinbiochem.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu C. Increased expression of T cell immunoglobulin and mucin domain 3 on CD14(+) monocytes is associated with systemic inflammatory reaction and brain injury in patients with spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2018;27(5):1226–1236. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 69.Xu C. Increased expression of T cell immunoglobulin and mucin domain 3 aggravates brain inflammation via regulation of the function of microglia/macrophages after intracerebral hemorrhage in mice. J Neuroinflammation. 2013;(10):141. doi: 10.1186/1742-2094-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu A. Tim-3 enhances brain inflammation by promoting M1 macrophage polarization following intracerebral hemorrhage in mice. Int Immunopharmacol. 2017;53:143–148. doi: 10.1016/j.intimp.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 71.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 72.Yuan B. Programmed death (PD)-1 attenuates macrophage activation and brain inflammation via regulation of fibrinogen-like protein 2 (Fgl-2) after intracerebral hemorrhage in mice. Immunol Lett. 2016;179:114–121. doi: 10.1016/j.imlet.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Han R. PD-L1 (Programmed Death Ligand 1) protects against experimental intracerebral hemorrhage-induced brain injury. Stroke. 2017;48(8):2255–2262. doi: 10.1161/STROKEAHA.117.016705. [DOI] [PubMed] [Google Scholar]
- 74.Ma L. Blocking B7-1/CD28 pathway diminished long-range brain damage by regulating the immune and inflammatory responses in a mouse model of intracerebral hemorrhage. Neurochem Res. 2016;41(7):1673–1683. doi: 10.1007/s11064-016-1883-3. [DOI] [PubMed] [Google Scholar]
- 75.Tao X. Up-regulation of interferon regulatory factor 3 involves in neuronal apoptosis after intracerebral hemorrhage in adult rats. Neurochem Res. 2016;41(11):2937–2947. doi: 10.1007/s11064-016-2012-z. [DOI] [PubMed] [Google Scholar]
- 76.Smits H.H. Commensal gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34(5):1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 77.Zhao X. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat Commun. 2017;8(1):602. doi: 10.1038/s41467-017-00770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu Y. Immune interventions in stroke. Nat Rev Neurol. 2015;11(9):524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi K. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol. 2018;3(1):34–41. doi: 10.1136/svn-2017-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yousry T.A. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354(9):924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hemphill J.C., 3rd, Farrant M., Neill T.A., Jr. Prospective validation of the ICH score for 12-month functional outcome. Neurology. 2009;73(14):1088–1094. doi: 10.1212/WNL.0b013e3181b8b332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu T.Y. Natural history of perihematomal edema and impact on outcome after intracerebral hemorrhage. Stroke. 2017;48(4):873–879. doi: 10.1161/STROKEAHA.116.014416. [DOI] [PubMed] [Google Scholar]
- 83.Fouda A.Y. Minocycline in acute cerebral hemorrhage: an early phase randomized trial. Stroke. 2017;48(10):2885–2887. doi: 10.1161/STROKEAHA.117.018658. [DOI] [PubMed] [Google Scholar]
- 84.Lei C. Brain recovery mediated by toll-like receptor 4 in rats after intracerebral hemorrhage. Brain Res. 2016;1632:1–8. doi: 10.1016/j.brainres.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 85.Hasegawa Y. Blockage of central sphingosine-1-phosphate receptor does not abolish the protective effect of FTY720 in early brain injury after experimental subarachnoid hemorrhage. Curr Drug Deliv. 2017;14(6):861–866. doi: 10.2174/1567201813666160907094401. [DOI] [PubMed] [Google Scholar]