Abstract
Latent HIV reservoir is the main obstacle that prevents a cure for HIV-1 (HIV). While antiretroviral therapy is effective in controlling viral replication, it cannot eliminate latent HIV reservoirs in patients. Several strategies have been proposed to combat HIV latency, including bone marrow transplantation to replace blood cells with CCR5-mutated stem cells, gene editing to disrupt the HIV genome, and “Shock and Kill” to reactivate latent HIV followed by an immune clearance. However, high risks and limitations to scale-up in clinics, off-target effects in human genomes or failure to reduce reservoir sizes in patients hampered our current efforts to achieve an HIV cure. This necessitates alternative strategies to control the latent HIV reservoirs. This review will discuss an emerging strategy aimed to deeply silence HIV reservoirs, the development of this concept, its potential and caveats for HIV remission/cure, and prospective directions for silencing the latent HIV, thereby preventing viruses from rebound.
Keywords: HIV latency, Shock and kill, HIV reservoirs, Deep latency, Transcription
1. Introduction
With a stable and long-lived reservoir, HIV-1 (HIV) remains a global health problem as the proviral HIV DNA is transcriptionally suppressed in peripheral blood of patients, but is capable of producing infectious particles when antiretroviral therapy (ART) is interrupted [1]. HIV-infected individuals do not achieve a complete immunologic reconstitution during ART [2]. While residual replication in deep tissue sites may account for a viral rebound after therapy interruption [3], chronical immune activation-induced reactivation of latent HIV reservoirs could be involved, as HIV infection causes mucosal damage, leading to translocation of bacterial products from lumen in the gut into the systemic circulation [4]. This immune activation cannot be resolved by ART. Attempts to intensify the therapy have failed to reduce the reservoir size, creating another obstacle to achieve a cure [5,6]. Under ART, the replication of HIV is effectively suppressed, thereby low to no viral particles are actively produced. Even though some viral components can be made by defective proviruses in patients under ART, they may not be effectively detected for clearance by a compromised host immune surveillance in patients [7,8]. The inability of ART to eradicate HIV from latent viral reservoirs necessitates the need to develop novel therapeutic approaches to eradicate the virus from infected individuals in order to discontinue ART. The latent HIV reservoirs are created after CD4+ T cells encounter the virus where some infected CD4+ T cells revert to a resting state and persist as memory T cells; these memory T cells remain quiescent while harboring the stable HIV proviral DNA [1]. This reservoir is established during the primary HIV infection after CD4+ T cells are activated and proliferated to generate effector cells that clear the pathogens [1]. These latently infected CD4+ T cells are stable and long-lived due to their long half-lives (44 months). Because of this, it may take over 73 years of ART to eradicate latent HIV since the low turnover rate of these memory cells [[9], [10], [11]]. The longevity and persistence of viral reservoirs and their capabilities of producing infectious particles upon ART interruption poses a major challenge to the current treatment strategies. Therefore, approaches to eradicate the latent reservoirs in patients are urgently needed [12].
2. Molecular basis underlining current HIV cure strategies
Two opposite strategies are currently under development to attack HIV latency, “Shock and Kill” and deep latency, by manipulating the same signaling pathways that are essential for latency establishment. In “Shock and Kill”, the latent HIV is reactivated by latency reversal agents (LRAs) followed by an immune clearance. In deep latency strategy, the goal is to induce the proviral HIV towards a deeply silenced state so that HIV would not rebound or it is significantly delayed even when the ART is discontinued. HIV latency is established and maintained through multiple cellular and molecular mechanisms that exploit cellular and viral factors, which modulate the viral promoter, i.e. the 5′ long terminal repeat (LTR), thereby suppressing the transcription of the virus into latency. These mechanisms include: epigenetic silencing by chromatin remodeling at the viral promoter, such as histone deacetylation, histone methylation, DNA methylation and possibly the newly defined histone decrotonylation [[13], [14], [15], [16]], low level expression or sequestration of essential transcription factors for HIV expression, suppression of the viral trans-activator Tat, and post-transcriptional mRNA splicing [17,18]. It has been shown that HIV proviral DNA integrates into an actively transcribed host genome [19] in resting CD4+ T cells in HIV-infected patients receiving suppressive ART [20]. This viral integration could be involved in transcriptional interference that linked to HIV latency [21,22]. Epigenetic silencing occurs due to chromatin modifications at the viral promoter [17]. This alters the physical structure of the chromatin on nucleosomes by epigenetic modulators such as histone deacetylases (HDACs) and histone methyltransferases (HMTs). Nuclear factor kappa B (NF-κB)/CBF1, Myc/Sp1, YY1/LSF, and AP4 in the nucleus recruit HDACs or HMTs, such as Suv39h1, EZH2 or G9a, into the HIV LTR, resulting in deacetylation or methylation of histone tails at the HIV LTR. These modifications of histone tails inhibit the transcription initiation of HIV to induce HIV latency [17]. Sequestration of cellular cofactors required for HIV expression in the cytoplasm is another important mechanism for the establishment and maintenance of latency. Transcription factors such as NF-κB and the nuclear factor of activated T cells (NFAT) are crucial for initiating HIV transcription at the 5′-LTR. However, it was shown that NF-κB plays a bigger role in HIV transcription than NFAT [23]. In addition to this canonical NF-κB signaling pathway, the non-canonical NF-κB pathway emerged recently as an important player that may be involved in HIV replication and/or latency reversal. This pathway is triggered by NF-κB inducing kinase (NIK), which phosphorylates IKKα, the latter further phosphorylates p100, leading to its cleavage into p52 and nuclear translocation of RelB/p52 to drive transcription of its target genes. In cancer cells, this pathway is inhibited by cIAPs, which can degrade NIK via its E3 ubiquitin ligase activity, thereby turning off the non-canonical NF-κB pathway [24]. Interestingly, although not clear, cIAPs can negatively regulate HIV transcription. SMAC mimetic (SMACm) binds to cIAPs, triggering ubiquitination signaling to degrade cIAPs protein, reducing the degradation of NIK, and allowing NIK to activate the non-canonical NF-κB signaling pathways for reactivation of latent HIV [25]. In conclusion, the establishment of HIV latency is complicated and involves multiple layers of signaling pathways. This poses an immense challenge to find a cure for HIV.
3. Challenges of current cure strategies
Ten years ago, Timothy Brown (referred to as the Berlin Patient) was declared cured of HIV infection after receiving a bone marrow transplantation from a donor with stem cells harboring a CCR5 deletion mutation [26]. This was followed by several clinical attempts to duplicate this therapy, but unfortunately were unsuccessful. This year, a second patient (referred to as the London Patient) showed a successful outcome by the same strategy: bone marrow transplantation [27]. Despite that, CCR5 ∆32 mutation appears to reduce protection against some other viral infections such as influenza and West Nile virus [28,29]. It was estimated that an individual who is homozygous for the ∆32 allele has a 21% increase of mortality rate [30]. These studies demonstrated that, while effective for cure of HIV, bone marrow transplantation is a risky procedure and is not tolerated by most patients. Therefore, it is not a scalable treatment [31]. Another emerging strategy of HIV cure is to disrupt HIV proviruses with DNA editing. This rapidly developing gene editing technology may make gene editing of the HIV genome achievable. However, it is not known what the optimal targeted HIV genome sequences are. How can we deal with a gene editing escape during the residual replication of HIV in deep tissues? And how can we effectively deliver the editing system in vivo? Recent studies have shown that gene editing off-targets may be more common than we previously thought [32]. It may also induce wide-ranging off-target RNA edit [33]. As of now, these questions still remain and need to be resolved before the technology is tested in patients. As mentioned previously, “Shock and Kill” strategy has been proposed as a therapeutic therapy to reduce the frequency of infected cells by targeting molecular mechanisms of HIV latency. However, whether or not this strategy can achieve a virological control in the absence of ART is still under active investigation. Although clinical trials have showed that latent HIV reservoirs can be flushed out [34,35], a significant reduction of reservoir size has not been observed in patients yet, indicating that the reactivation of latent HIV reservoirs alone does not necessarily cause a reservoir clearance [34,35]. Several lines of evidence may explain this daunting obstacle. First, in vivo, the reactivation process by current LRAs may not be strong enough. Therefore, new potent LRAs need to be developed. In the last few years, numerous LRAs have been developed, including epigenetic compounds such as HDAC inhibitors, HMT inhibitors, bromodomain inhibitors, protein kinase C agonists, and TLR7 agonist [16,36]. More recently, SMAC mimetics were investigated and reported as effective latency reversal agents, some were even able to induce clearance of the reservoirs [25,37]. Furthermore, because multiple signaling pathways are involved in latent HIV establishment, a combination therapy to target two or more sites within the virus replication pathways was proposed [38]. Currently, a combination strategy to reactivate latent HIV that targets both NF-κB and p-TEFb signaling pathways was shown to be more effective than any other combination strategy [39,40]. Unfortunately, the efficacy to trigger an effective host immune response is still unknown even though the reactivation was robust. Secondly, unlike patients during acute HIV infection, in chronically HIV-infected patients under ART, the cytotoxic T lymphocytes (CTLs) function is compromised and fails to clear the resting CD4+ T cells after activation with LRAs. Some patients were found to retain a broad-spectrum viral-specific CTL response in addition to an antigen-specific stimulation prior to reactivating latent HIV-1 which can kill some infected cells [41,42]. Thirdly, the degree of the reactivation of latent HIV differs among patients. It also differs among resting CD4+ T cells within the same individual due to multiple layers of regulations of HIV latency. This was evidenced that, following the first round of reactivation in resting CD4+ T cells, a second round of PHA treatment increased the efficacy of latency reactivation [43]. Lastly, recent studies have shown that HIV integrates into the same sites of the host genome in different clones of resting CD4+ T cells within the same patient [44], suggesting that HIV-infected resting CD4+ T cell clones can be expanded while maintaining latency in patients. Although mechanistically not clear, the latent HIV in these expanded clones may be regulated by the similar epigenetics as mentioned before. Therefore, in addition to targeting epigenetics at HIV LTR, eradicating these clones may require a tool to suppress their expansion and/or cellular proliferation of the resting CD4+ T cells in patients under ART. In conclusion, HIV “sterilizing cure” is faced with many challenges to achieve an efficient reactivation and an effectively killing.
Meanwhile, HIV can also infect and establish viral reservoirs in the central nervous system (CNS) [45]. Although it is not clear how the latency is established and which cell types harbor these reservoirs in the CNS, persistent infection in the CNS is clearly associated with neurological impairment in patients under ART, such as HIV associated neurocognitive diseases (HAND) [46]. As patients live longer under suppressive ART, the incidence of HAND is increased [46]. This is a miserable challenge for elder patients in the ART era. Unfortunately, the “Shock and Kill” strategy to flush out latent HIV may be not appropriate for eradication of HIV reservoirs in the CNS because the reactivation of latent HIV generates HIV particles or its viral components, such as Tat, which can damage neurons and are toxic to the CNS [47]. Because of these limitations, a novel way to deeply curtail the HIV proviruses in order to achieve a “functional HIV cure” is emerging.
4. Deeply silencing proviral HIV to achieve a functional HIV cure
Around 8% of the human genome consists of sequences consequent of ancient retrovirus DNA elements [48]. These human endogenous retroviruses (HERVs) are silenced unless stimulated by environmental factors or pathogenic infections. Reactivated HERVs produce envelope (Env) proteins, which are associated with the pathognomonic features of multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) by activating multiple pathophysiological signaling pathways [49]. Similarly, HERVs have the common provirus structure of coding open reading frames flanked by two long-terminal repeats [50]. Through evolution and consequences of host defense mechanisms, HERV activity is reduced in most somatic tissues. However, germline cells directly control and permit HERV expression at different levels [51]. This is achieved through epigenetic regulation via DNA methylation in normal tissues [52,53], indicating that CpG methylation is an important mechanism of silencing and turning off expression of HERV [[53], [54], [55]]. The regulation of HERVs by selective hypomethylation at HERV LTR is critical during embryonic development, such as in the placenta during the course of pregnancy [51,55,56]. The ability for host cells to manage HERV expression through epigenetic regulation suggests that this acquired neutralization strategy could be exploited for current efforts to combat latent HIV. To some extent, latent HIV resembles endogenous retroviruses that harbor in our genomes. Like HERVs, latent HIV is able to return to an active state for replication when it is in inflammatory conditions or during co-infection of pathogens in patients. Therefore, it may be possible to achieve a silence state by epigenetically shutting down or fine tuning the HIV replication machinery to a level that the interaction of latent HIV with host controls viral replication at the HIV LTR region of chromatin in the context of host immune surveillance, preventing or greatly delaying HIV rebound when ART is discontinued.
The concept of HIV deep silencing originates from some early observations that viral rebound could be prevented. In the early 1990's, studies showed that modulating HIV transcription machinery prevented virus from reactivation. In 1993, attempts to suppress viral reactivation were tested using HIV latently infected U1 cells by targeting protein kinase C signaling. Two compounds (Gö 7775 and Gö 7716) in the nanomolar levels of concentration were reported to show a strong inhibition of infectious virus particles released into the supernatant after the cells were induced by PMA or TNFα [57]. In the same year, Feorino et al. screened a broad range of antiviral compounds targeting distinct biochemical pathways to interfere with the reactivation of latent HIV in chronically HIV-infected OM-10.1 cells. Up to 58 compounds were tested, including nucleoside analogues, cytokines, steroidal and nonsteroidal anti-inflammatory agents, polyoxometalates, a Tat inhibitor, TIBO, porphyrins, oligomers and several other natural products. Among these compounds, only nucleoside 3′-fluoro3′-deoxythymidine, IFN-γ, the Tat inhibitor Ro 5-3335, and the iron chelator desferrioxamine, modestly prevented TNFα-induced reactivation of latent HIV. This was determined by measuring CD4 expression levels and the activity of reverse transcriptase of HIV [58]. While these findings were interesting, indirect evidence of HIV replication was determined by measuring CD4 levels. Therefore, it was not clear whether HIV replication or latent HIV reactivation was actually inhibited in this study. Later on, by using the same cell line model, Hashimoto K et al., reported that heat shock can induce the reactivation of latent HIV, which can be blocked by anti-TNFα antibody, the PKC inhibitor staurosporine, the NF-κB inhibitor pentoxifylline, or the HIV Tat inhibitor Ro 5-3335. Staurosporine was found to inhibit up to 90% of latency reactivation without cellular toxicity [59]. Ro 5-3335 analog, Ro 24-7429, was thought to have a potential for cure and was advanced to phase II clinical trials. While the drug was well tolerated in patients, it failed to show clinical benefit in controlling viral replication. Recently, it was shown that Ro 5-3335 is also an inhibitor of RUNX1 and was able to reactivate latent HIV in cell culture models of latency in vitro, but not in PBMCs isolated from patients under ART ex vivo [60]. Taken together, these early studies showed a promise in preventing latency reactivation by directly targeting HIV transcription mechanisms. However, this idea was not tested in proper HIV latency models, which limited further exploration.
Recently, this concept was renewed as the efforts to eradicate latent HIV for an HIV cure was accelerated. In 2014, Ian Anderson et.al, discovered that the heat shock protein 90 (HSP90) was required for HIV gene expression. HSP90 acted on the NF-κB pathway upstream of IkBα by binding to IKKγ and recruiting Cdc37, keeping the IKK complex functional [61]. The inhibition of HSP90 reduced degradation of IkBα and blocked nuclear translocation of transcription factor p65/p50, thereby suppressing the NF-κB pathway [61]. The inhibition of HSP90/NF-κB signaling with specific HSP90 inhibitors, such as 17-(N-allylamino)-17-demethoxygeldanamycin and AUY922, prevented the expression of HIV from reactivating in CD4+ T cells [61]. These data indicated that selectively targeting HSP90 may provide a powerful approach to suppress HIV reactivation from latency. Some HSP90 inhibitors are tested in phase II clinical trials to treat cancers, neuro-degenerative diseases and cystic fibrosis. Soon after, Murry et al., reported that chemical inhibitors of the sulfonation pathway also prevented virus reactivation in vitro and ex vivo by blocking the initiation of HIV transcription [62]. In 2015, Zhu's group reported that the innate immune protein IFI44 suppressed HIV replication via its recruitment into HIV LTR and prevented reactivation of latent HIV [63]. Taken together, these studies suggested that by targeting the transcription machinery of HIV, reactivation of latent HIV could be blocked. However, a concept called “Block and Lock” to directly prevent latency reactivation (block) and induce provirus silencing (lock) as a tool for functional HIV cure was not proposed until recently.
In 2015, Valente's group reported that the HIV Tat inhibitor called didehydro-cortistatin A (dCA), an analog of the natural steroidal alkaloid to specifically bind to the unstructured basic region of Tat to block Tat/Tar interaction [64], effectively reduced the residual levels of viral transcription in several HIV latency models by breaking the Tat-mediated transcriptional feedback loop to establish a nearly permanent state of latency. This was based on their previous findings that dCA suppressed Tat-dependent HIV transcription [65]. Unfortunately, dCA failed to effectively prevent or delay viral rebound in an in vivo humanized mouse model of latency [66]. However, these are the important studies which are found among the initial attempts to test the idea that targeting HIV Tat along with ART can possibly delay or even halt viral replication, reactivation, or replenishment of the latent viral reservoirs in order to achieve a functional HIV cure.
It is worth noting that while the recent development of long-acting antiretroviral drugs will greatly improve patient life (Conference on Retroviruses and Opportunistic Infections in Seattle, March 7th, 2019), latent HIV reservoirs have to be eliminated in order to have a cure for HIV. Similar to drugs used in the conventional ART, these long-acting medicines are as effective as daily pills to inhibit HIV replication, but cannot purge HIV. Due to this reason, the viruses will rebound when the therapy is discontinued. The ultimate goal of deep latency is to epigenetically silence viral replication to a low level so that HIV won't significantly rebound, even ART is disrupted.
5. Outstanding questions raised from early studies
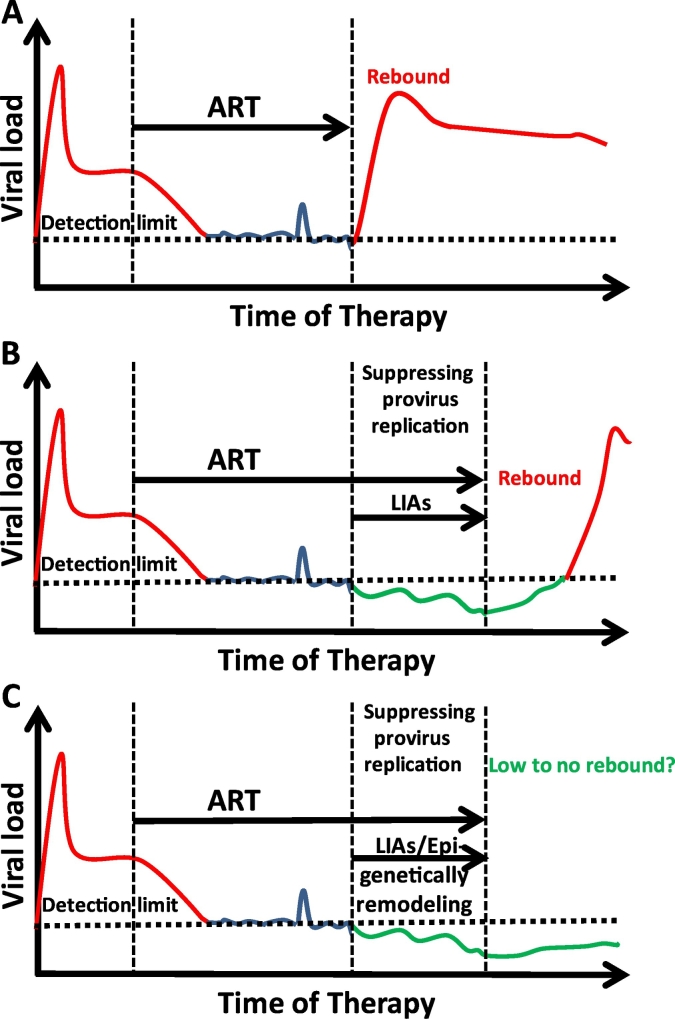
Current studies of the new “Block and Lock” strategy were developed based on the ideas of preventing the reactivation of latent HIV or viral rebound and are advocating for a possible complete silencing for a remission of HIV. Such studies are moving forward as more discoveries are being reported [63,67], which indicate that compounds with the capability to prevent a reactivation of latent HIV and cure HIV may be attained. One of the most attractive targets to suppress HIV and prevent its rebound is the inhibition of Tat protein by using dCA. In addition to dCA, several Tat inhibitors were shown to inhibit Tat expression, either transcriptionally or translationally [68]. The development of specific and potent Tat inhibitors is urgently needed. Nevertheless, limitations to this approach do exist. One of these limitations is that these inhibitors exert their effects on viral protein Tat. This might cause the virus to acquire a drug resistance during the residual replication such as the case with ART drugs, meaning the treatment might fail. To avoid this outcome, host cellular proteins, such as NFAT, NF-κB and p-TEFb could be exploited for this purpose. Fortunately, a number of small molecules are available to inhibit these host signaling pathways, which can be evaluated for their efficacy for the induction of deep latency [69]. Like the “Shock and Kill” strategy, a combination therapy to target multiple signaling pathways is probably required in order to achieve an effective silencing of latent HIV. However, it is not known if the outcome of preventing the viral rebound can be maintained for an extended period of time. Also, it is not clear to what extent the reduction of HIV provirus expression can be achieved so that HIV may not rebound and the induced silencing can be effectively maintained. Therefore, the real challenges for the deep latency strategy are: 1) how to effectively induce silencing to prevent the viral rebound (block); 2) after the deep silencing is induced, how to maintain the complete silenced state in reservoirs (lock). Without a real “lock” to prevent a reactivation, proviral HIV will rebound as we routinely see in patients after ART is discontinued. To achieve these goals, firstly, an epigenetic remodeling has to be induced in order to maintain the deep latency. It may be possible to epigenetically modify the local chromatin environment at HIV LTR into a suppressive state during the induction of deep latency. This prospective scenario is a reminder of how genomes of HERVs achieved their dormancy after its integration into the human host genome. Essentially, an epigenetic regulation of HIV LTR is a dynamic and flexible process, as we have seen during the latency reversal. When latent HIV is disrupted by LRAs, its epigenetic marks are also reversed. Inhibition of HIV transcription machinery may eventually be able to transform an active histone epigenetic environment into a repressive histone modification environment, such that after long-term inhibition of HIV transcription machinery, the temporary suppressive chromatin could be maintained at HIV LTR (Fig. 1). While no data have shown an effectively epigenetic lock during induction of deep latency, a recent study does indicate a potential lock by formations of heterochromatin-like environment at HIV LTR when HIV Tat is targeted for deep silencing [70]. Secondly, host immune recovery may be needed to facilitate a prevention of viral rebound when the residual viral replication is controlled by deep silencing. This viscous feedback may reinforce the epigenetic silence. Similar to the “Shock and Kill” strategy, a combination tool could be included to enhance the immune response during the inactivation of provirus in patients.
Fig. 1.
Strategy to induce silencing of latent HIV reservoirs. A, ART suppresses viral expression, but HIV quickly rebounds when ART is discontinued. B, Suppressing HIV transcription machinery prevents provirus from reactivation however fails to maintain the suppression of viral rebound. When treatment is discontinued, HIV comes back. C, Re-programming chromatin environment during provirus suppression could maintain the deep suppression during the induction of deep silencing, therefore preventing viral rebound when ART is disrupted. This process could be strengthened by enhancing the host immune recovery. LIAs: latency inducing agents.
6. Conclusion and prospective
Although initially doubted, with tremendous support from both the government, community, and patients along with extensive research efforts, exciting progress has been made in both understanding HIV latency and clinical practice for an HIV cure. This is particularly evidenced by the substantial benefit of early ART to substantially reduce reservoir sizes in HIV-infected patients and the successful clinical practice in the Berlin patient, the London Patient, the Mississippi baby cases, and many others. Undoubtedly, we are in the infancy stage of anti-latency studies, but with the discovery of new mechanisms of HIV latency, the numerous strategies under investigations, and the hundreds of different classes of compounds that are available for testing, we may be closer to curing HIV and/or HIV remission. Time will tell whether a strategy of deep latency can help us achieve the ultimate goal during this treacherous journey.
7. Search strategy and selection criteria
We have searched throughout PubMed to examine the concept of deep latency or silencing for functional HIV cure on June 3, 2019. Search terms included “HIV deep latency”, “HIV deep silencing”, “HIV latency”, “latent HIV”, “HIV reservoir”, “HIV persistence”, “Shock and Kill”, “Kick and Kill”, “HIV cure”, “HIV functional cure” and “HIV rebound”. Evidence of prevention of HIV rebound and silencing of HIV were found in vitro, ex vivo, and in vivo. However, the prevention of HIV rebounding was mainly investigated in either the early 1990s or in recent years after 2010. Concept of deep latency or silencing was found after 2015.
Declaration of interests
The authors have no conflict of interests relevant to the manuscript.
Contributions
GJ had the initial concept of this article. MME, YT, DL and GJ wrote this review and approved the article.
Acknowledgements
We thank Drs. David M. Margolis and Lynn Suer for their critical pre-review. This work is supported by Qura Therapeutics funding 2019-01 and University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410) to GJ. The funder has no role in paper design, data collection, data analysis, interpretation, or writing of this paper.
References
- 1.Sengupta S., Siliciano R.F. Targeting the latent reservoir for HIV-1. Immunity. 2018;48(5):872–895. doi: 10.1016/j.immuni.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grow E.J., Flynn R.A., Chavez S.L., Bayless N.L., Wossidlo M., Wesche D.J. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature. 2015;522(7555):221–225. doi: 10.1038/nature14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Picado J., Deeks S.G. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS. 2016;11(4):417–423. doi: 10.1097/COH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zevin A.S., McKinnon L., Burgener A., Klatt N.R. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11(2):182–190. doi: 10.1097/COH.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruelas D.S., Greene W.C. An integrated overview of HIV-1 latency. Cell. 2013;155(3):519–529. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josefsson L., Dahl V., Palmer S. Can HIV infection be eradicated through use of potent antiviral agents? Curr Opin Infect Dis. 2010;23(6):628–632. doi: 10.1097/QCO.0b013e32833ff1d0. [DOI] [PubMed] [Google Scholar]
- 7.Huang S.H., Ren Y., Thomas A.S., Chan D., Mueller S., Ward A.R. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest. 2018;128(2):876–889. doi: 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack R.A., Jones R.B., Pertea M., Bruner K.M., Martin A.R., Thomas A.S. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe. 2017;21(4):494–506 e4. doi: 10.1016/j.chom.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 10.Rouzioux C., Richman D. How to best measure HIV reservoirs? Curr Opin HIV AIDS. 2013;8(3):170–175. doi: 10.1097/COH.0b013e32835fc619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crooks A.M., Bateson R., Cope A.B., Dahl N.P., Griggs M.K., Kuruc J.D. Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J Infect Dis. 2015;212(9):1361–1365. doi: 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis D.M., Garcia J.V., Hazuda D.J., Haynes B.F. Latency reversal and viral clearance to cure HIV-1. Science. 2016;353(6297):aaf6517. doi: 10.1126/science.aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He G., Margolis D.M. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol. 2002;22(9):2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richman D.D., Margolis D.M., Delaney M., Greene W.C., Hazuda D., Pomerantz R.J. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 15.Jiang G., Nguyen D., Archin N.M., Yukl S.A., Mendez-Lagares G., Tang Y. HIV latency is reversed by ACSS2-driven histone crotonylation. J Clin Invest. 2018;128(3):1190–1198. doi: 10.1172/JCI98071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang G., Dandekar S. Targeting NF-kappaB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res Hum Retroviruses. 2015;31(1):4–12. doi: 10.1089/aid.2014.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battistini A., Sgarbanti M. HIV-1 latency: an update of molecular mechanisms and therapeutic strategies. Viruses. 2014;6(4):1715–1758. doi: 10.3390/v6041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donahue D.A., Wainberg M.A. Cellular and molecular mechanisms involved in the establishment of HIV-1 latency. Retrovirology. 2013;10:11. doi: 10.1186/1742-4690-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder A.R., Shinn P., Chen H., Berry C., Ecker J.R., Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 20.Han Y., Lassen K., Monie D., Sedaghat A.R., Shimoji S., Liu X. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78(12):6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greger I.H., Demarchi F., Giacca M., Proudfoot N.J. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 1998;26(5):1294–1301. doi: 10.1093/nar/26.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinski M.K., Bisgrove D., Shinn P., Chen H., Hoffmann C., Hannenhalli S. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J Virol. 2005;79(11):6610–6619. doi: 10.1128/JVI.79.11.6610-6619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y.K., Mbonye U., Hokello J., Karn J. T-cell receptor signaling enhances transcriptional elongation from latent HIV proviruses by activating P-TEFb through an ERK-dependent pathway. J Mol Biol. 2011;410(5):896–916. doi: 10.1016/j.jmb.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Almagro M.C., Vucic D. The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol. 2012;34(3):200–211. [PubMed] [Google Scholar]
- 25.Pache L., Dutra M.S., Spivak A.M., Marlett J.M., Murry J.P., Hwang Y. BIRC2/cIAP1 is a negative regulator of HIV-1 transcription and can be targeted by Smac Mimetics to promote reversal of viral latency. Cell Host Microbe. 2015;18(3):345–353. doi: 10.1016/j.chom.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutter G., Nowak D., Mossner M., Ganepola S., Mussig A., Allers K. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R.K., Abdul-Jawad S., McCoy L.E., Mok H.P., Peppa D., Salgado M. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568(7751):244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcon A., Cuevas M.T., Rodriguez-Frandsen A., Reyes N., Pozo F., Moreno S. CCR5 deficiency predisposes to fatal outcome in influenza virus infection. J Gen Virol. 2015;96(8):2074–2078. doi: 10.1099/vir.0.000165. [DOI] [PubMed] [Google Scholar]
- 29.Lim J.K., Murphy P.M. Chemokine control of West Nile virus infection. Exp Cell Res. 2011;317(5):569–574. doi: 10.1016/j.yexcr.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei X., Nielsen R. CCR5-32 is deleterious in the homozygous state in humans. Nat Med. Jun 2019;25(6):909–910. doi: 10.1038/s41591-019-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Peterson C.W., Kiem H.P. Lessons from London and Berlin: designing a scalable gene therapy approach for HIV cure. Cell Stem Cell. 2019;24(5):685–687. doi: 10.1016/j.stem.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Zuo E., Sun Y., Wei W., Yuan T., Ying W., Sun H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science. 2019;364(6437):289–292. doi: 10.1126/science.aav9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunewald J., Zhou R., Garcia S.P., Iyer S., Lareau C.A., Aryee M.J. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature. 2019;569(7756):433–437. doi: 10.1038/s41586-019-1161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archin N.M., Liberty A.L., Kashuba A.D., Choudhary S.K., Kuruc J.D., Crooks A.M. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott J.H., Wightman F., Solomon A., Ghneim K., Ahlers J., Cameron M.J. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10(10) doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim S.Y., Osuna C.E., Hraber P.T., Hesselgesser J., Gerold J.M., Barnes T.L. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med. 2018;10(439) doi: 10.1126/scitranslmed.aao4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell G.R., Bruckman R.S., Chu Y.L., Trout R.N., Spector S.A. SMAC Mimetics induce autophagy-dependent apoptosis of HIV-1-infected resting memory CD4+ T cells. Cell Host Microbe. 2018;24(5):689–702 e7. doi: 10.1016/j.chom.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margolis D.M., Hazuda D.J. Combined approaches for HIV cure. Curr Opin HIV AIDS. 2013;8(3):230–235. doi: 10.1097/COH.0b013e32835ef089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang G., Mendes E.A., Kaiser P., Wong D.P., Tang Y., Cai I. Synergistic reactivation of latent HIV expression by Ingenol-3-Angelate, PEP005, targeted NF-kB Signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darcis G., Kula A., Bouchat S., Fujinaga K., Corazza F., Ait-Ammar A. An in-depth comparison of latency-reversing agent combinations in various in vitro and ex vivo HIV-1 latency models identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to potently reactivate viral gene expression. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan L., Deng K., Shroff N.S., Durand C.M., Rabi S.A., Yang H.C. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng K., Pertea M., Rongvaux A., Wang L., Durand C.M., Ghiaur G. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho Y.C., Shan L., Hosmane N.N., Wang J., Laskey S.B., Rosenbloom D.I. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maldarelli F., Wu X., Su L., Simonetti F.R., Shao W., Hill S. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345(6193):179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valcour V., Chalermchai T., Sailasuta N., Marovich M., Lerdlum S., Suttichom D. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenthal J., Tyor W. Aging, comorbidities, and the importance of finding biomarkers for HIV-associated neurocognitive disorders. J Neurovirol. 2019:358–378. doi: 10.1007/s13365-019-00735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagashev A., Sawaya B.E. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013;10:358. doi: 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths D.J. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2(6) doi: 10.1186/gb-2001-2-6-reviews1017. [REVIEWS1017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X., Huang J., Zhu F. Human endogenous retroviral envelope protein Syncytin-1 and inflammatory abnormalities in neuropsychological diseases. Front Psych. 2018;9:422. doi: 10.3389/fpsyt.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grandi N., Tramontano E. HERV envelope proteins: physiological role and pathogenic potential in cancer and autoimmunity. Front Microbiol. 2018;9:462. doi: 10.3389/fmicb.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Montojo M., Doucet-O'Hare T., Henderson L., Nath A. Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit Rev Microbiol. 2018;44(6):715–738. doi: 10.1080/1040841X.2018.1501345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szpakowski S., Sun X., Lage J.M., Dyer A., Rubinstein J., Kowalski D. Loss of epigenetic silencing in tumors preferentially affects primate-specific retroelements. Gene. 2009;448(2):151–167. doi: 10.1016/j.gene.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtani H., Liu M., Zhou W., Liang G., Jones P.A. Switching roles for DNA and histone methylation depend on evolutionary ages of human endogenous retroviruses. Genome Res. 2018;28(8):1147–1157. doi: 10.1101/gr.234229.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavie L., Kitova M., Maldener E., Meese E., Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2) J Virol. 2005;79(2):876–883. doi: 10.1128/JVI.79.2.876-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Q., Chen H., Li J., Oliver M., Ma X., Byck D. Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues. Cell Signal. 2014;26(3):648–656. doi: 10.1016/j.cellsig.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Denner J. Expression and function of endogenous retroviruses in the placenta. APMIS. 2016;124(1–2):31–43. doi: 10.1111/apm.12474. [DOI] [PubMed] [Google Scholar]
- 57.Patzold S., Schneider J., Rudolph C., Marme D., Schachtele C. Novel indolocarbazole protein kinase C inhibitors prevent reactivation of HIV-1 in latently infected cells. Antiviral Res. 1993;22(4):273–283. doi: 10.1016/0166-3542(93)90037-j. [DOI] [PubMed] [Google Scholar]
- 58.Feorino P.M., STB T.M. Folks, Schinazi R.F. Prevention of activation of HIV-1 by antiviral agents in OM-10.1 cells. Antivir Chem Chemother. 1993;4(1):55–63. [Google Scholar]
- 59.Hashimoto K., Baba M., Gohnai K., Sato M., Shigeta S. Heat shock induces HIV-1 replication in chronically infected promyelocyte cell line OM10.1. Arch Virol. 1996;141(3–4):439–447. doi: 10.1007/BF01718308. [DOI] [PubMed] [Google Scholar]
- 60.Klase Z., Yedavalli V.S., Houzet L., Perkins M., Maldarelli F., Brenchley J. Activation of HIV-1 from latent infection via synergy of RUNX1 inhibitor Ro5-3335 and SAHA. PLoS Pathog. 2014;10(3) doi: 10.1371/journal.ppat.1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson I., Low J.S., Weston S., Weinberger M., Zhyvoloup A., Labokha A.A. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. 2014;111(15):E1528–E1537. doi: 10.1073/pnas.1320178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murry J.P., Godoy J., Mukim A., Swann J., Bruce J.W., Ahlquist P. Sulfonation pathway inhibitors block reactivation of latent HIV-1. Virology. 2014;471(473):1–12. doi: 10.1016/j.virol.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Power D., Santoso N., Dieringer M., Yu J., Huang H., Simpson S. IFI44 suppresses HIV-1 LTR promoter activity and facilitates its latency. Virology. 2015;481:142–150. doi: 10.1016/j.virol.2015.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mediouni S., Chinthalapudi K., Ekka M.K., Usui I., Jablonski J.A., Clementz M.A. Didehydro-Cortistatin a inhibits HIV-1 by specifically binding to the unstructured basic region of tat. mBio. 2019;10(1) doi: 10.1128/mBio.02662-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mousseau G., Kessing C.F., Fromentin R., Trautmann L., Chomont N., Valente S.T. The tat inhibitor Didehydro-Cortistatin a prevents HIV-1 reactivation from latency. mBio. 2015;6(4) doi: 10.1128/mBio.00465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kessing C.F., Nixon C.C., Li C., Tsai P., Takata H., Mousseau G. In vivo suppression of HIV rebound by Didehydro-Cortistatin a, a "block-and-lock" strategy for HIV-1 treatment. Cell Rep. 2017;21(3):600–611. doi: 10.1016/j.celrep.2017.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jean M.J., Hayashi T., Huang H., Brennan J., Simpson S., Purmal A. Curaxin CBL0100 blocks HIV-1 replication and reactivation through inhibition of viral transcriptional elongation. Front Microbiol. 2017;8:2007. doi: 10.3389/fmicb.2017.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mousseau G., Valente S. Strategies to block HIV transcription: focus on small molecule tat inhibitors. Biology (Basel) 2012;1(3):668–697. doi: 10.3390/biology1030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Massari S., Sabatini S., Tabarrini O. Blocking HIV-1 replication by targeting the Tat-hijacked transcriptional machinery. Curr Pharm Des. 2013;19(10):1860–1879. doi: 10.2174/1381612811319100010. [DOI] [PubMed] [Google Scholar]
- 70.Li C., Mousseau G., Valente S.T. Tat inhibition by didehydro-Cortistatin A promotes heterochromatin formation at the HIV-1 long terminal repeat. Epigenetics Chromatin. 2019;12(1):23. doi: 10.1186/s13072-019-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]