Abstract
Background
Leukocyte-associated immunoglobulin like receptor-1 (LAIR1) is a transmembrane inhibitory receptor that influences susceptibility to a myriad of inflammatory diseases. Our recent investigations of severe malarial anaemia (SMA) pathogenesis in Kenyan children discovered that novel LAIR1 genetic variants which were associated with decreased LAIR1 transcripts enhanced the longitudinal risk of SMA and all-cause mortality.
Methods
To characterize the molecular mechanism(s) responsible for altered LAIR1 signalling in severe malaria, we determined LAIR1 transcripts and protein, sLAIR1, sLAIR2, and complement component 1q (C1q) in children with malarial anaemia, followed by a series of in vitro experiments investigating the LAIR1 signalling cascade.
Findings
Kenyan children with SMA had elevated circulating levels of soluble LAIR1 (sLAIR1) relative to non-SMA (1.69-fold P < .0001). The LAIR1 antagonist, sLAIR2, was also elevated in the circulation of children with SMA (1.59 fold-change, P < .0001). There was a positive correlation between sLAIR1 and sLAIR2 (ρ = 0.741, P < .0001). Conversely, circulating levels of complement component 1q (C1q), a LAIR1 natural ligand, were lower in SMA (−1.21-fold P = .048). These in vivo findings suggest that reduced membrane-bound LAIR1 expression in SMA is associated with elevated production of sLAIR1, sLAIR2 (antagonist), and limited C1q (agonist) availability. Since reduced LAIR1 transcripts in SMA were associated with increased acquisition of haemozoin (PfHz) by monocytes (P = .028), we explored the relationship between acquisition of intraleukocytic PfHz, LAIR1 expression, and subsequent impacts on leukocyte signalling in cultured PBMCs from malaria-naïve donors stimulated with physiological concentrations of PfHz (10 μg/mL). Phagocytosis of PfHz reduced LAIR1 transcript and protein expression in a time-dependent manner (P < .050), and inhibited LAIR1 signalling through decreased phosphorylation of LAIR1 (P < .0001) and SH2-domain containing phosphatase-1 (SHP-1) (P < .001). This process was associated with NF-κB activation (P < .0001) and enhanced production of IL-6, IL-1β, and TNF-α (all P < .0001).
Interpretation
Collectively, these findings demonstrate that SMA is characterized by reduced LAIR1 transmembrane expression, reduced C1q, and enhanced production of sLAIR1 and sLAIR2, molecular events which can promote enhanced production of cytokines that contribute to the pathogenesis of SMA. These investigations are important for discovering immune checkpoints that could be future targets of immunotherapy to improve disease outcomes.
Keywords: Complement component 1q, Leukocyte-associated immunoglobulin like receptor-1, Leukocyte-associated immunoglobulin like receptor-2, Plasmodium falciparum malaria, Plasmodium falciparum haemozoin, Severe malarial anaemia
Abbreviations: AML, Acute myeloid leukaemia; C1q, Complement component 1q; CLL, Chronic lymphocytic leukaemia; HFRS, haemorrhagic fever with renal syndrome; HIV-1, Human immunodeficiency virus 1; IL-1β, Interleukin 1 beta.; IL-6, Interleukin 6; ITIM, immuno-tyrosine inhibition motifs; LAIR1, Leukocyte-associated immunoglobulin like receptor-1; LAIR2, Leukocyte-associated immunoglobulin like receptor-2; NF-κB, Nuclear factor-kappa beta; PBMCs, Peripheral blood mononuclear cells; P. falciparum, Plasmodium falciparum; PfHz, Plasmodium falciparum haemozoin; PCM, Pigment containing monocytes; PCN, Pigment containing neutrophils; PCR, Polymerase chain reaction; SHP-1, SH2 domain-containing tyrosine phosphatase-1; SHP-2, SH2 domain-containing tyrosine phosphatase-2; SMA, severe malarial anaemia; SLE, Systemic lupus erythematosus; TNF-α, Tumor necrosis factor alpha
Research in context.
Evidence before this study
Leukocyte-associated immunoglobulin like receptor 1 (LAIR1) encodes for a transmembrane inhibitory receptor expressed by peripheral blood mononuclear cells (PBMCs), and is required for regulation of gene pathways involved in leukocyte inflammatory mediator production and cytotoxicity. Using a combined OMICs approach in samples from children Plasmodium falciparum malaria with discrete phenotypes (i.e., mild and severe malaria), we identified LAIR1 as a novel gene that appeared to be important in the pathogenesis of severe malarial anaemia (SMA). These results are presented in a companion manuscript in this issue of EbioMedicine, along with validation of the findings in a cohort of 1512 Kenyan children in which genetic variants associated with reduced LAIR1 expression increase susceptibility to SMA and all-cause mortality during the period of naturally-acquired immunity to malaria. However, the molecular pathways contributing to suppression of LAIR1 in children with SMA remains unknown.
Added value of this study
In this study, we show that children with SMA have elevated levels of circulating sLAIR1 and sLAIR2, indicative of enhanced receptor shedding. Moreover, children with SMA have reduced levels of circulating C1, a primary ligand for activating the LAIR1 inhibitory signalling cascade. Phagocytosis of PfHz by monocytes was associated with reduced in vivo levels of LAIR1 in children with malaria. In vitro experiments confirmed that phagocytosis of PfHz by peripheral blood suppresses LAIR1 mRNA and protein, and inhibits the phosphorylation of LAIR1 ITIMS and SHP-1, events required to initiate the inhibitory signal. Reduced phosphorylation of LAIR1 signalling molecules resulted in NF-κB activation, and the consequent production of cytokines that can promote malaria pathogenesis. Collectively, these investigations describe a novel signalling pathway that becomes dysregulated in children with severe malaria.
Implications of all the available evidence
The genetic studies in the companion manuscript, along with findings presented here identify a novel signalling pathway, and the manner in which perturbations in the pathway contribute to the pathogenesis of severe malaria. Identifying therapeutic compounds that target this novel pathway to prevent blockade of the inhibitory signal may offer a promising new approach for immunotherapeutic interventions.
Alt-text: Unlabelled Box
1. Introduction
Plasmodium falciparum malaria continues to pose a significant health threat in vulnerable populations from endemic regions, particularly in children under 5 years and pregnant women [1]. The most recent data from the World Health Organization report that malaria remains responsible for the death of ~285,000 African children annually (64% of the global malaria deaths) [2]. Children under 5 years are particularly susceptible to developing severe malaria due to lack of naturally-acquired malarial immunity [2]. Severe disease manifestations vary in presentation according to transmission intensity and include cerebral malaria, metabolic acidosis, respiratory distress, and severe malarial anaemia (SMA) [2], defined as haemoglobin (Hb) < 5.0 g/dL with parasitaemia, is the most common severe malaria complication in children residing in P. falciparum holoendemic areas [[3], [4], [5]]. The pathogenesis of SMA is due to lysis of parasitized/non-parasitized erythrocytes and inadequate/dysregulated erythropoietic responses [[6], [7], [8], [9]].
Although the molecular-immunological basis of altered erythropoietic responses in children with SMA remain to be fully elucidated, our previous investigations showed that dysregulation in type 1 and type 2 innate immune responses are, at least in part, responsible [8]. For example, appropriate responses of the interleukin (IL)-12/interferon (IFN)-γ pathway predicts elevated Hb levels in children with P. falciparum, while activation of the IL-13/eotaxin pathway is associated with more profound anaemia [10]. Altered inflammatory responses in children with SMA are influenced by both parasite- and host-derived factors [8]. Phagocytosis of parasitic products, such as P. falciparum-derived haemozoin (PfHz), alters inflammatory mediator production and promotes suppression of erythropoiesis [11,12]. Our previous genetic investigations in Kenyan children demonstrate that polymorphic variability in host immune response genes can impart functional changes in inflammatory mediator production that influence susceptibility to SMA [[13], [14], [15], [16], [17], [18]]. We recently used an unbiased approach to further identify genes and gene pathways involved in the pathogenesis of SMA by performing high-throughput genotyping and whole transcriptome in a subset of Kenyan children with discrete mild and severe malaria phenotypes. The combined OMICs approach revealed that leukocyte-associated immunoglobulin like receptor-1 (LAIR1, CD305) was a high priority for further investigation. Validation of these findings showed that particular LAIR1 variants at 16231 and 18,835 are associated with altered susceptibility to malaria, SMA, and all-cause mortality over a 36-month follow-up period. In addition, ‘protective’ LAIR1 variants were associated with elevated LAIR1 transcript expression, while variants that enhanced susceptibility to SMA had reduced mRNA levels (manuscript in this issue of EbioMedicine).
LAIR1 is an inhibitory transmembrane collagen receptor involved in regulation of leukocyte inflammatory mediator production and cytotoxicity [[19], [20], [21], [22]]. LAIR1 signalling is activated by attachment of collagenous ligands to the extracellular surface of the receptor, followed by phosphorylation of the tyrosine residues in intracellular immuno-tyrosine inhibition motifs (ITIMs) through Src family kinases [23,24]. Phosphorylated LAIR1 ITIMs serve as docking sites for SH2-domain containing phosphatases, SHP-1 and SHP-2, which negatively regulate intracellular signalling pathways required for leukocyte activation [[23], [24], [25]].
Reduced expression of LAIR1 has been observed in chronic lymphocytic leukaemia (CLL), systemic lupus erythematous (SLE), and rheumatoid arthritis (RA), whereby membrane bound LAIR1 levels were down-regulated in patients with severe disease [[26], [27], [28]]. Down-regulation of LAIR1 expression profiles were associated with elevated levels of soluble LAIR1 (sLAIR1) and soluble LAIR2 (sLAIR2, CD306), both of which antagonize membrane bound LAIR1 expression through competition for collagenous ligands, and decrease cellular levels of the receptor [[26], [27], [28]]. Soluble LAIR1 is produced upon shedding of the collagen binding extracellular region of membrane bound LAIR1 [28,29]. As such, sLAIR1 levels reflect the amount of LAIR1 on cell membranes and because it retains affinity for collagen, similar to the cognate transmembrane receptor, sLAIR1 antagonizes binding of membrane bound LAIR1 to collagenous ligands. LAIR2 is a soluble homolog of LAIR1 that is produced upon leukocyte activation. Although the function of the LAIR2 protein is unknown, sLAIR2 has a high affinity for collagen, and also antagonizes the LAIR1-ligand interaction [30]. Ligand availability has also been shown to influence cellular LAIR1 expression and activation. For example, in vitro experiments have demonstrated a role for complement component 1q (C1q) in modulating LAIR1 expression and activation in monocytes and plasmacytoid dendritic cells [31,32].
Although we recently discovered that low transcript expression of LAIR1 was associated enhanced susceptibility to SMA, the molecular basis of this finding remains unknown. As such, we performed a series of experiments in peripheral blood from children with falciparum malaria in which we measured LAIR1 transcript levels in white blood cells, and sLAIR1, sLAIR2, and C1q in circulation. These results were then used to design in vitro experiments in peripheral blood from malaria-naïve donors to determine the underlying cell signalling events responsible for down-regulation of LAIR1 in children with severe malaria.
2. Materials and methods
2.1. Study participants
Parasitaemic children (n = 122, aged 3–36 months) selected for investigation here were part of a larger study that enroled 1512 children at Siaya County Referral Hospital (SCRH) in western Kenya at their first hospital visit for childhood illness. Investigation of the role of LAIR1 genetic variants on longitudinal clinical outcomes is presented in a companion manuscript in this issue of EbioMedicince. From that study, we assayed samples from children with non-SMA and SMA that were available in the biological repository. Children were excluded from the current investigation if they tested positive for non—P. falciparum species, were previously hospitalized (for any reason), exposed to antimalarial therapy in the two weeks prior to enrolment, and diagnosed with either cerebral malaria, bacteraemia or HIV-1. After the exclusion criteria, the remaining parasitaemic children with samples available were stratified into SMA (Hb < 5.0 g/dL; n = 53) and non-SMA (Hb ≥ 5.0 g/dL; n = 69) using haemoglobin concentrations in accordance with WHO guidelines. Written informed consent in the language of choice (English, Kiswahili, or Dhuoluo) was obtained from the parent/guardian of each child participating in the study. The study was approved by the Scientific Ethics and Research Committee of the Kenya Medical Research Institute (KEMRI) and University of New Mexico Institutional Review Board. Patients were treated according to the Ministry of Health (MOH)-Kenya guidelines.
2.2. Laboratory procedures
Venipuncture blood samples (<3.0 mL) were collected from enrolled participants before any treatment interventions. Malaria trophozoite counts were determined through microscopy of thick and thin peripheral blood smears. Complete blood counts were determined using the Beckman Coulter AcT diff2™ (Beckman-Counter Corp.). Children were given appropriate treatment and supportive therapy according to the Kenyan Ministry of Health guidelines.
2.3. Circulating LAIR1, LAIR2, and C1q measurements
Soluble LAIR1 was measured in serum of study participants using the human LAIR1 ELISA matched antibody pair, and recombinant human LAIR1 (Creative Diagnostics, 45–16 Ramsey Rd, Shirley, NY 11967; Cat No. ABPR-0519). The limit of detection was 5.0 pg/mL. Soluble LAIR2 was measured in serum using the human LAIR2 ELISA matched antibody pair and recombinant LAIR2 (Antibodies online; Cat No. ABIN2010435). The limit of detection was 3.9 pg/mL. C1q was measured in serum using matched antibody pair (Cat No. H00000712-AP21) and human recombinant C1q protein (Cat No. H00000712-P01) (Novus Biologicals, 8100 Southpark Way # A8, Littleton, CO 80120). The limit of detection was 3.0 ng/mL. Absorbance was read at 450 nm on the ELISA plate reader, BIO TEK (Bio Tek U.S. Winooski, VT 05404, USA).
2.4. LAIR1 transcript expression profiling in children
Ribonucleic (RNA) was isolated from peripheral white blood cells obtained from parasitaemic children using a combination of Trizol-based and RNEasy® mini kit (Qiagen) techniques. From 1.0 μg of total RNA, complementary DNA (cDNA) was prepared with the transcriptor first strand cDNA synthesis kit (Roche). For measurement of LAIR1 expression levels, 0.5 μg of resulting cDNA was used for gene-specific TaqMan® qPCR assays [Assay ID: Hs00253790_m1; Applied Biosystems, Inc. Foster City, CA, USA]. The constitutively expressed housekeeping gene β-actin was used as an endogenous control [Assay ID: Hs01060665_g1; Applied Biosystems, Inc. Foster City, CA, USA], to normalize the target gene expression data in a quantitative gene expression assay on the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Inc. Foster City, CA, USA). LAIR1 Ct values were normalised to β-actin Ct values, and mRNA differences were determined using the delta-delta Ct method.
2.5. Quantification of pigment-containing monocytes (PCM) and neutrophils (PCN)
Enumeration of malarial pigment (PfHz)-containing monocytes (PCMs) and neutrophils (PCNs) was performed on Giemsa-stained thin smears as per our previous methods [33,34]. Briefly, a total of 30 monocytes and 100 neutrophils were examined per slide, and the number of PCM or PCN was expressed as a percentage of the total number of monocytes or neutrophils, respectively. The number of PCM/μL and PCN/μL of blood was calculated by multiplying the percentage of PCM by the absolute number of monocytes/μL and neutrophils/μL obtained from the Coulter AcT diff2.
2.6. Haemozoin (PfHz) preparation
Crude haemozoin was isolated from in vitro cultures of P. falciparum-infected RBCs (strain PfD6) as previously described with slight modifications [35]. Briefly, cultures with parasitaemia above 5% comprising mostly late trophozoite and early schizont stages were centrifuged at 13,148g for 10 min to separate infected RBCs from culture media. The resulting RBC pellet was re-suspended for 10 min. in 20 mL of 0.01 M phosphate-buffered saline (pH 7.2) containing 15% saponin. Culture lysates were examined microscopically to ensure complete lysis of RBCs. Lysates were then centrifuged at 13,148g for 15 min. The pellet was re-suspended and washed 6× with PBS until the supernatant was clear, and then sonicated 2× to remove the lipids. The final pellet was dried overnight at 40 °C on a heat block, weighed, and re-suspended in filter-sterilized H2O at a final concentration of 1.0 mg/mL. Endotoxin level in the PfHz preparation was <0.1 EU/mL as determined by the Limulus amebocyte lysate test (Thermo Fisher).
2.7. Peripheral blood mononuclear cells (PBMCs) isolation and measurement of LAIR1 transcript expression
PBMCs were isolated using the ficoll/hypaque technique as described earlier [36]. PBMCs (2.5 × 106 cells/mL) were cultured overnight in RPMI 1640 media under physiological conditions (37 °C). This was followed by incubation with PfHz (10 μg/mL, physiological concentration) for varying durations (0.5, 1, 2, 4, 6, 12, 24, and 48 h). RNA from PBMC lysates exposed to PfHz at the 8 time-points was extracted using the RNEasy® mini kit according to the manufacturer's instructions. From 1.0 μg of total RNA, complementary DNA (cDNA) was prepared with the transcriptor first strand cDNA synthesis kit (Roche). RNA then served as template for LAIR1 transcript expression profiling through TaqMan® qPCR assays as mentioned above. For the in vitro experiments, PBMCs were isolated from malaria-naïve donors (n = 6) and measurements were performed in triplicate.
2.8. Determination of LAIR1 protein levels
PBMC (2.5 × 106 cells/mL) cultures exposed to PfHz for 12, 24, and 48 h were harvested and washed twice with cold phosphate-buffered saline and then lysed in RIPA lysis buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Resulting lysates were kept on ice for 10 min and centrifuged for 15 min at 10,000 ×g at 4 °C, and recovered supernatants were stored at −80 °C until use. Quantification of LAIR1 protein was performed by boiling supernatant in 4 × Laemmli sample buffer (Bio-Rad Laboratories) for 10 min at 70 °C. Fifty micrograms of proteins was loaded onto 4–12% Bis-Tris Plus polyacrylamide gels (Life Technologies, Carlsbad, CA) for electrophoresis. Following electrophoresis, proteins were electrotransferred to iBlot 2 nitrocellulose Mini Stacks (Life Technologies, Carlsbad, CA), and subsequently blocked with 5% blotting grade nonfat dry milk (Bio-Rad Laboratories) in PBS and 0.01% Tween 20 (PBST) at RT for 1 h. Membranes were then probed with anti-LAIR1 antibody (R&D Systems, Inc. Minneapolis, MN) dissolved in fresh blocking buffer. Blots were then washed thrice with PBST and then incubated with anti-mouse IgG HRP conjugated secondary antibody (Cell Signalling Technology, Inc. Danvers, MA). Separate immunoblots were also generated by stripping the membranes to verify equal loading of lysate proteins using anti-β-actin primary antibody (Cell Signalling Technology, Inc. Danvers, MA). This was followed by incubation with anti-mouse IgG HRP conjugated secondary antibody (Cell Signalling Technology, Inc. Danvers, MA). Chemiluminescent reagent system was added to the membranes for development using a medical film processor (Konica Minolta Imaging, Wayne. NJ).
2.9. Phosphor-immunoreceptor array
PBMC (4 × 10 [6]) were treated with C1q alone (25 μg/mL) [37], PfHz alone (10 μg/mL) and a combination of PfHz (10 μg/mL) + C1q (25 μg/mL) at 37 °C in complete RPMI 1640 medium. Cell lysates were harvested after C1q incubation (1 h) and PfHz incubation (4 h), followed by lysis in RIPA buffer containing protease and phosphatase inhibitors (Thermo Scientific) for 30 min on ice. Cell lysates were added to the human phosphor-immunoreceptor array membranes according to the manufacturer's protocol (Proteome Profiler Array; R&D Systems). Phosphorylated proteins were detected by pan-antiphosphotyrosine antibody conjugated to HRP. Array data were developed with X-ray film and quantified by FluorChem8900 (Alpha Innotech). Phosphorylation levels of individual analytes were determined by the average pixel density of duplicate spots; values were obtained after subtracting background signals and were normalised to positive controls. Culture supernatants were used for measurement of IL-1β, IL-6, TNF-α, LAIR1, and C1q protein levels through ELISA (see below).
2.10. Measurement of NF-κB p65
Phosphorylated NF-κB (pNF-κB) p65 in PBMC whole cell extracts treated with C1q alone (25 μg/mL), PfHz alone (10 μg/mL) and C1q + PfHz were detected using a TransAM® NFκB p65 kit (Active Motif, 1914 Palomar Oaks Way, Suite 150 Carlsbad, CA 92008), as described by the manufacturer. Levels were determined immediately after stimulation. Absorbance was read at 450 nm on the ELISA plate reader, BIO TEK (Bio Tek U.S. Winooski, VT 05404, USA).
2.11. Measurement of IL-1β, IL-6, and TNF-α in PBMC supernatants
Supernatants from PBMC cultures exposed to C1q alone (25 μg/mL), PfHz alone (10 μg/mL) and a combination of PfHz (10 μg/mL) + C1q (25 μg/mL) were used for measurement of soluble inflammatory mediators encoded by genes with NF-κB response elements and of importance in the pathogenesis of SMA: IL-1β, IL-6 and TNF-α. ELISA were performed using OptEIA™ Sets (BD Biosciences, Pharmingen, San Diego, CA 92121) in accordance with the manufacturer's protocol. Absorbance was read at 450 nm on the ELISA plate reader, BIO TEK (Bio Tek U.S. Winooski, VT 05404, USA).
2.12. Statistical analyses
Comparisons of demographic, clinical, and laboratory characteristics between the clinical groups was analysed using SPSS, version 20.0 (SPSS Inc., Chicago, IL). Pairwise comparisons were performed using the Mann-Whitney U tests. Pearson's Chi Square (χ [2]) and Fisher's exact test were used for comparisons of proportions. Pairwise comparisons of normalised continuous variables (sLAIR1, LAIR2, and C1q) between the non-SMA and SMA groups were performed using Student's t-test. Differences in continuous variables (LAIR1 protein levels, pLAIR1, pSHP-1, NF-κB, IL-1β, IL-6, and TNF-α) across the experimental groups were determined using Analysis of variance (ANOVA) tests. The relationship between soluble levels of LAIR1, LAIR2 and C1q and Hb in parasitaemic children was analysed using Spearman rank correlation test. Graphical illustrations were prepared using GraphPad prism version 22.0 (GraphPad Software, San Diego, CA, USA). Statistical significance for all analyses was set at P ≤ .050.
3. Results
3.1. Demographic, clinical, and laboratory characteristics of children with falciparum malaria
Children infected with P. falciparum (n = 122) were stratified into two categories; non-SMA (Hb ≥ 5.0 g/dL, n = 71) and SMA (Hb < 5.0 g/dL, n = 51). The demographic, clinical, and laboratory characteristics of the study participants are presented in Table 1. The distribution of males and females was comparable between the groups (P = .079) as was age at enrolment (P = .286).
Table 1.
Demographic, clinical, and laboratory characteristics upon enrolment.
| Characteristics | Non-SMA (Hb ≥ 5.0 g/dL) |
SMA (Hb < 5.0 g/dL) |
P-value |
|---|---|---|---|
| Demographic characteristics | |||
| Sample size (n) | n = 69 | n = 53 | |
| Gender, n (%) | |||
| Male | 28 (40.60) | 30 (56.60) | 0.079a |
| Female | 41 (59.40) | 23 (43.40) | |
| Age, (months) | 11.80 (8.60) | 11.73 (15.52) | 0.286b |
| Haematological Indices | |||
| Haemoglobin (g/dL) | 8.40 (3.90) | 4.30 (1.20) | <0.001b |
| Haematocrit (Hct, %) | 26.80 (11.30) | 15.10 (4.00) | <0.001b |
| RBC (×1012/μL) | 3.93 (1.52) | 1.89 (0.72) | <0.001b |
| WBC (×103/μL) | 14.40 (8.80) | 16.30 (9.20) | 0.028b |
| Lymphocytes (×103/μL) | 6.40 (4.30) | 7.90 (5.90) | 0.042b |
| Monocytes (×103/μL) | 1.20 (0.90) | 2.30 (1.20) | 0.031b |
| Granulocytes (×103/μL) | 5.70 (5.60) | 5.90 (7.40) | 0.897b |
| Platelets (×103/μL) | 164.00 (123.00) | 142.00 (120.00) | 0.037b |
| Parasite density/μL | 56,500.00 (115,126) | 23,760.00 (77,436) | 0.026b |
| PCM (/μL) | 2.66 (17.00) | 12.67 (57.00) | 0.028b |
| PCN (/μL) | 2.94 (12.00) | 4.19 (18.00) | 0.079b |
Study participants (n = 122) were stratified into two groups; non-SMA (Hb ≥ 5.0 g/dL with any density parasitaemia and SMA (i.e., Hb < 5.0 g/dL with any density parasitaemia). Data presented are medians (interquartile range, IQR), unless otherwise stated. aStatistical significance determined by the chi-square analysis. bDifferences were determined using Mann-Whitney U tests. Bold indicates P ≤ .050.
As expected based on a priori grouping, children with SMA presented with lower Hb concentrations, haematocrit, and RBC counts (P < .001, respectively). The WBC, lymphocyte, and monocyte counts were elevated in children with SMA (P = .028, P = .042, and P = .031, respectively). Granulocyte counts were comparable between the groups (P = .897), while platelet counts were lower in children with SMA (P = .037). Peripheral parasite density was lower in children with SMA (P = .026), whereas circulating levels of PCM/μL and PCN/μL were higher in children SMA (P = .028 and P = .079, respectively).
3.2. Soluble LAIR1 is elevated in the circulation of children with SMA
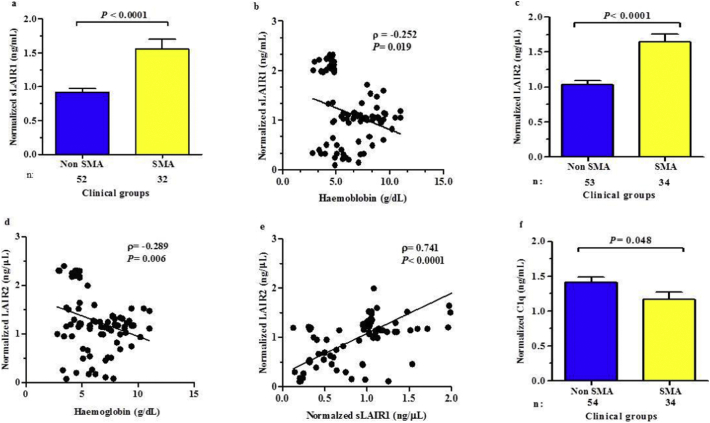
Previous studies have shown that sLAIR1 is elevated in patients with inflammatory diseases due to shedding of the extracellular portion of LAIR1 from cell membranes [26,29]. Although limited knowledge is available for sLAIR1 shedding from the membrane upon cellular activation, sLAIR1 may suppress the inhibitory signal by decreasing LAIR1 surface expression, and/or function as a receptor antagonist [26,38]. Since sLAIR1 has not been explored in individuals with malaria, levels were measured in the peripheral blood of children with non-SMA and SMA. Consistent with enhanced levels in other inflammatory diseases [[26], [27], [28]]., sLAIR1 was 1.69-fold higher in children with SMA (P < .0001, Fig. 1a). These results suggest an increased rate of cellular shedding and may offer a potential explanation for our recent finding that LAIR1 transcripts are down-regulated in circulating leukocytes from children with SMA (−3.0 fold, P = .017, companion manuscript in this issues of EbioMedicine). Correlational analyses revealed an inverse relationship between sLAIR1 and Hb concentrations (ρ = −0.252, P < .019, Fig. 1b), supporting a potential role for sLAIR1 in the pathogenesis of SMA.
Fig. 1.
Relationship between sLAIR1, sLAIR2, and C1q serum levels in children with malaria.
Differences between circulating sLAIR1 (ng/mL), sLAIR2 (ng/mL), and C1q (ng/mL) in children with non-SMA and SMA were determined using Student t-test and are presented as mean ± SEM. Relationships between sLAIR1, sLAIR2, C1q, and haemoglobin levels were determined by Spearman's correlation test.
(a) Normalised sLAIR1 levels in children with non-SMA and SMA. Children with SMA had elevated sLAIR1 levels relative to non-SMA.
(b) Normalised sLAIR1 vs. haemoglobin concentrations in children with non-SMA and SMA. Circulating sLAIR1 levels were inversely correlated with haemoglobin levels.
(c) Normalised sLAIR1 levels in children with non-SMA and SMA. sLAIR2 serum levels were elevated in children with SMA relative to non-SMA.
(d) Normalised sLAIR2 vs. haemoglobin concentrations in children with non-SMA and SMA. Normalised sLAIR2 vs. haemoglobin. sLAIR2 levels were inversely correlated with haemoglobin concentrations.
(e) Normalised sLAIR1 vs. normalised sLAIR2 in children with non-SMA and SMA. Circulating sLAIR1 and sLAIR2 levels were positively correlated.
(f) Circulating C1q levels in children with non-SMA and SMA. Circulating C1q levels were lower in children with SMA.
3.3. Soluble LAIR2 is elevated in the circulation of children with SMA
To further explore potential molecular events that could explain reduced leukocyte LAIR1 transcript expression in severe malaria, we measured circulating levels of sLAIR2 in children with non-severe and severe malaria. Although the function of LAIR2 is largely unknown, elevated levels of sLAIR2 have been have been associated with decreased LAIR1 receptor expression in other inflammatory diseases [[26], [27], [28]]. Consistent with these findings, circulating sLAIR2 was elevated in children with SMA (1.59 fold-change, P < .0001, Fig. 1c), and showed an inverse correlation with Hb levels, (ρ = −0.289, P = .006, Fig. 1d). Consistent with the notion that cellular activation promotes the release of sLAIR1 and sLAIR2, which can act in concert to antagonize the LAIR1 transmembrane receptor, sLAIR1 and sLAIR2 were positively correlated (ρ = 0.741, P < .0001, Fig. 1e).
3.4. Circulating C1q is reduced in children with SMA
Since C1q is an important component of complement activation in the classical pathway for elimination of malaria parasites, and C1q alters LAIR1 expression and activation in monocytes [31,32], we compared circulating C1q levels in the clinical groups and found that C1q was 1.21-fold lower in children with SMA (P = .048, Fig. 1f). These results suggest that there is limited C1q ligand availability for LAIR1 signalling in children with SMA, a potential mechanism for enhanced LAIR1 shedding and reduced receptor expression on the surface of leukocyte cell membranes. Previous investigations in a small group of Thai adolescents and adults showed that P. falciparum infections accelerated the plasma half-life of C1q, but did not correlate with clinical complications [39]. However, there was a positive relationship between circulating C1q and Hb concentrations in our study participants (ρ = 0.216, P = .042), indicating that consumption of C1q is most pronounced during severe anaemia, the hallmark severe clinical sign of severe disease in the population studied.
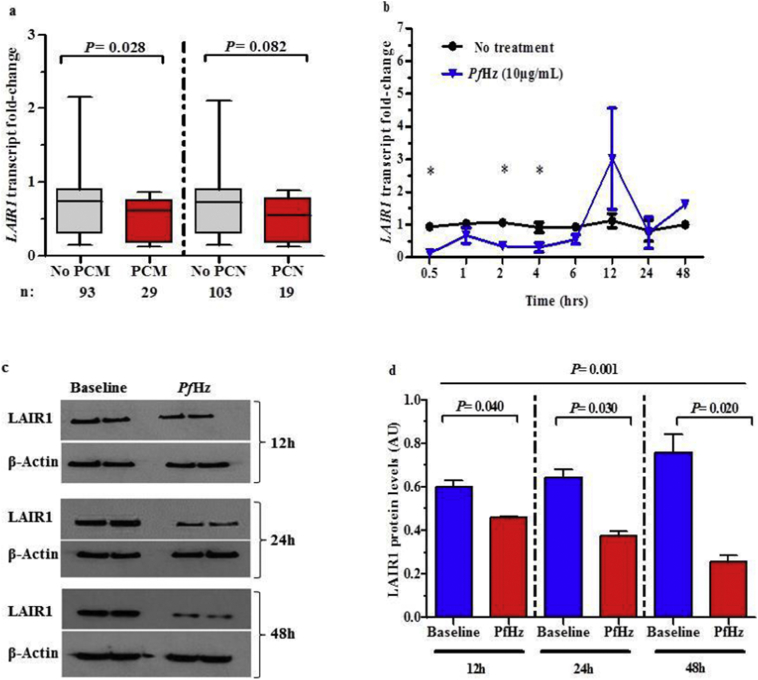
3.5. Suppression of LAIR1 in children with falciparum malaria is associated with phagocytosis of PfHz by monocytes
Our previous investigations have shown that phagocytosis of PfHz is an important cause of altered inflammatory mediator production, and hence, enhances anaemia in children with malaria [8,33]. To determine if phagocytosis of PfHz by leukocytes could be a potential mechanism for reduced LAIR1 transcript expression in children with SMA, LAIR1 transcript levels were compared in children with and without PfHz in their monocytes (PCM) and neutrophils (PCN). There was a significant reduction in LAIR1 transcript levels in children with PCM compared to those without (P = .028, Fig. 2a). However, LAIR1 transcript levels did not significantly differ between absence/presence of PCN (P = .082, Fig. 2a). These results suggest that monocytic uptake of PfHz during P. falciparum infections may be an important mechanism for down-regulation of LAIR1 expression.
Fig. 2.
Relationship between LAIR1 expression and intraleukocytic haemozoin.
(a) Pairwise comparisons of LAIR1 transcript levels between children with and without pigment-containing monocytes (PCM) and pigment-containing neutrophils (PCN) in the non-SMA and SMA groups were determined using Mann-Whitney U test. Data are presented as box-plots, where the box represents the interquartile range, the line through the box is the median, and whiskers show the 10th and 90th percentiles. LAIR1 transcript levels were lower in children with PCM compared to those without, while LAIR1 transcript levels were comparable between absence/presence of PCN.
(b) Temporal kinetics of LAIR1 transcript levels in response to PfHz treatment of PBMC from malaria-naïve donors (n = 6, measured in triplicate). Pairwise comparisons were determined using Student t-test. Significant (P < .05) differences in transcript levels between no treatment and PfHz treatment (10 μg/mL) groups represented by *at 0.5, 2, and 4 h time points. Data represent average of individuals (n = 3) with each condition performed in triplicate (error bars represent SEM).
(c) Immunoblot analysis of non-treated (baseline) and PfHz-treated (10 μg/mL) PBMC lysates for 12, 24, and 48 h.
(d) Densitometric analysis of normalised cellular LAIR1 protein production presented as mean ± SEM. Across group comparisons analysed using ANOVA. Pairwise comparisons analysed using Student t-test. Data represent average of individuals (n = 6) with each condition performed in triplicate (error bars represent SEM). LAIR1 protein levels were lower in PfHz-treated PBMC lysates compared to no treatment at 12, 24, and 24 h.
3.6. Phagocytosis of PfHz suppresses LAIR1 transcripts and protein in cultured peripheral blood
To confirm the in vivo findings, the effect of PfHz treatment (10 μg/mL; physiological dose) on LAIR1 gene expression was determined in cultured PBMCs at various time points (0.5, 1, 2, 4, 6, 12, 24 and 48 h). Based on the lack of an association between LAIR1 transcript levels and PCN, PfHz treatment of cultured neutrophils was not explored. Stimulation with PfHz caused a rapid and time-dependent suppression of LAIR1 transcript and protein levels (Fig. 2b, c, and d). LAIR1 protein levels in the experimental groups did not significantly differ between 0.5 h and 6 h (data not shown). Taken together, phagocytosis of PfHz can directly suppress LAIR1 expression.
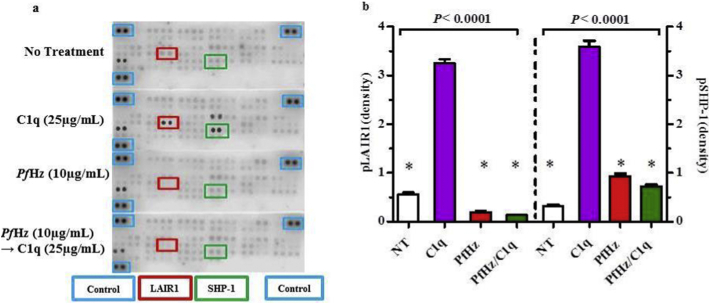
3.7. PfHz inhibits phosphorylation of LAIR1 ITIMs and SHP-1
LAIR1 ligation by collagenous ligands such as C1q, leads to phosphorylation of ITIMs and recruitment of SHP-1/2 phosphatases, which in turn, inhibit downstream processes required for leukocyte activation [31,32]. Because LAIR1 transcripts were down-regulated in children who had elevated PCM, and phagocytosis of PfHz in cultured PBMCs also suppressed LAIR1 expression, we extended our investigations to determine the impact of PfHz on leukocyte inhibitory signalling by examining phosphorylation of LAIR1 ITIMs, SHP-1, and SHP-2 in response to C1q stimulation using the human phosphor-immunoreceptor assay. Treatment with C1q (25 μg/mL, 1 h) increased LAIR1 phosphorylation 5.80-fold above baseline levels (no treatment, P < .001), whereas phagocytosis of PfHz (alone, 10 μg/mL) reduced pLAIR1–2.95-fold below baseline levels (no treatment, P = .0003, Fig. 3a and b). Relative to C1q (alone), pLAIR1 was markedly reduced (−13.79-fold) in cells treated with PfHz (alone, P < .001, Fig. 3a and b). Stimulation of haemozoin-treated cells (4 h pre-incubation) followed by the addition of C1q (1 h) failed to abrogate the blockade of LAIR1 phosphorylation induced by PfHz (P < .0001 vs. C1q alone, Fig. 3a and b). A similar trend was observed for SHP-1 phosphorylation in which C1q treatment induced an 11.25-fold increase in pSHP-1 relative to baseline (P < .0001, Fig. 3a and b). PfHz treatment caused a slight increase in pSHP-1 compared to baseline (2.93 fold, P < .001), but was −3.83-fold lower than C1q treatment (P < .0001). As with pLAIR1, stimulation of PfHz-treated cells with C1q failed to induce SHP-1 phosphorylation (P < .0001, −4.96-fold vs. C1q alone, Fig. 3a and b). Phosphorylation of SHP-2 was undetectable across the different treatment groups. Collectively, these findings demonstrate that phagocytosis of PfHz inhibits ITIM-dependent leukocyte signalling, even in the presence of strong activating signals such as C1q.
Fig. 3.
Effect of intraleukocytic PfHz on LAIR1 and SHP-1 phosphorylation.
Temporal kinetics of the signalling pathway in response to the different treatment conditions was determined in PBMCs from malaria-naïve donors (n = 6, measured in triplicate).
(a) Human Phospho-immunoreceptor array results showing LAIR1 and SHP-1 phosphorylation upon no treatment (baseline), C1q (25 μg/mL), PfHz (10 μg/mL), and PfHz + C1q. Phosphorylation (spots) in the different conditions with control represented by blue boxes, LAIR1 by red boxes, and SHP-1 by green boxes.
(b) Densitometric analysis of human phosphor-immunoreceptor array data. Data presented as (mean ± SEM). Across group comparisons analysed using ANOVA. *indicates significant differences (P < .05) determined by Student t-test in pLAIR1 and pSHP-1 compared to C1q treatment. Phagocytosis of PfHz resulted in a reduction of pLAIR1 relative to C1q (alone) treatment. Similarly, ingestion of PfHz also caused a marked decrease in pSHP-1 levels relative to C1q treatment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
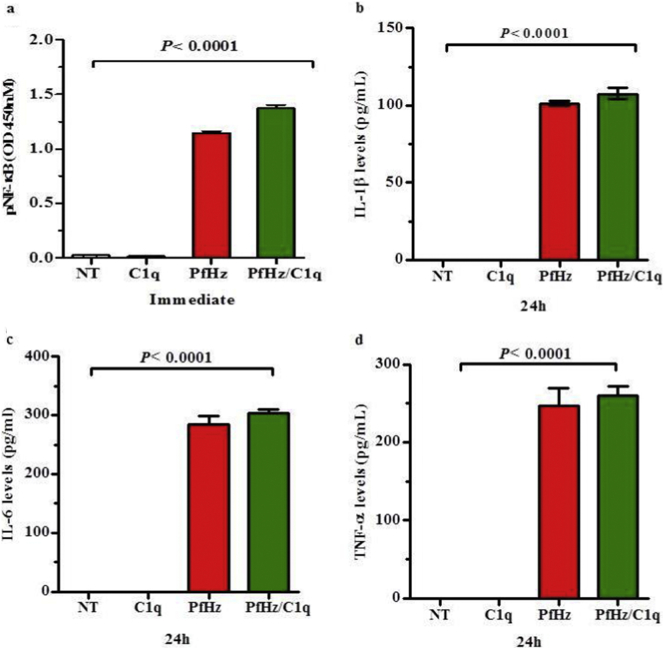
3.8. Blockade of the LAIR1 inhibitory signal by PfHz is associated with enhanced NF-κB p65 activation and cytokine production
NF-κB p65 activation and subsequent nuclear translocation is an important mechanism for leukocyte activation and inflammatory mediator production [40]. Binding of C1q to LAIR1 inhibits cellular activation and prevents the production of cytokines containing NF-κB and IRAF promoter response elements [41]. Our previous studies demonstrate that treatment of PBMCs with PfHz induces NF-κB activation and cytokine production [42]. Since phagocytosis of PfHz decreased LAIR1 transcription in cultured PBMCs, and blocked the induction of pLAIR1 by C1q, we determined the effect of C1q on pNF-κB p65 in PfHz-treated cells. Stimulation of PBMCs with C1q resulted in lower pNF-κB p65 (0.01 ± 0.01) compared to baseline levels (no treatment, 0.02 ± 0.02, P = .029, Fig. 4a). Phagocytosis of PfHz increased pNF-κB p65 (1.15 ± 0.01) above baseline (no treatment, P < .0001), and C1q (alone) exposure (P < .0001, Fig. 4a). The addition of C1q to PfHz pre-treated PBMCs caused a further increase in NF-κB p65 phosphorylation (1.37 ± 0.03) relative to PfHz alone (1.14 ± 0.02, P = .003, 4ig. 5a). These results indicate that the inhibitory effect of C1q on pNF-κB p65 is abrogated by phagocytosis of PfHz, a process that could culminate in overproduction of cytokines known to enhance the pathogenesis of SMA.
Fig. 4.
Effect of haemozoin on NF-κB activation and cytokine production.
Measurements ndwere determined in PBMCs from malaria-naïve donors (n = 6, measured in triplicate). Data presented as mean ± SEM. Across group and pairwise comparisons determined using Student t-test and Anova analyses, respectively.
(a) Comparisons of phosphorylated NF-κB immediately after stimulation of PBMCs with no treatment (NT), C1q (25 μg/mL), PfHz (10 μg/mL), and PfHz + C1q. NF-κB phosphorylation levels were elevated in the PfHz and PfHz + C1q treatment groups relative to no treatment (denoted by *P < .05).
(b) IL-1β levels (pg/mL) were elevated in culture supernatants from PBMCs treated with PfHz and PfHz + C1q.
(c) IL-6 levels (pg/mL) were elevated in culture supernatants from PBMCs treated with PfHz and PfHz + C1q.
(d) TNF-α levels (pg/mL) were elevated in culture supernatants from PBMCs treated with PfHz and PfHz + C1q.
To test this hypothesis, we used an identical experimental paradigm and determined the impact of C1q on the production of cytokines which contain NF-κB transcription elements, and influence the pathogenesis of SMA (i.e., IL-1β, IL-6, and TNF-α [10,14]. Culture supernatant levels of IL-1β, IL-6, and TNF-α were undetectable in control (non-treated) and C1q-treated PBMCs at 24 h (Fig. 4b, c, and d). However, the addition of PfHz increased IL-1β (101.2 ± 1.1), IL-6 (284.8 ± 9.4), and TNF-α (247.2 ± 15.8) relative to baseline and C1q treatment (Fig. 4b, c, and d). The addition of C1q to PfHz-treated PBMCs failed to significantly increase IL-1β (107.6 ± 2.6, P = .153), IL-6 (303.4 ± 4.8, P = .220), and TNF-α (260.2 ± 8.6, P = .547, Fig. 4b, c, and d). Taken together, these results suggest that phagocytosis of PfHz suppresses LAIR1 transcripts, and blocks LAIR1 (ITIMs) and SHP-1 phosphorylation, events which promote NF-κB activation and enhanced production of inflammatory mediators which enhance the pathogenesis of SMA.
4. Discussion
The novel findings presented here describe molecular mechanisms that may explain reduced LAIR1 transcript expression in white blood cells from children with SMA. Our recent investigations using a combined OMICs approach (high-throughput genotyping and global gene expression) in discrete phenotypes of Kenyan children with non-severe and severe malarial anaemia identified LAIR1 as a potential novel pathway in malaria pathogenesis. Validation of these results revealed that novel LAIR1 genetic variants that were associated with decreased LAIR1 mRNA enhanced the cross-sectional (enrolment) longitudinal (36-month follow up) risk of SMA, and all-cause mortality (manuscript in this issue of EbioMedicine). Although novel in the context of malaria, an association between down-regulation of LAIR1 expression and severe forms of other inflammatory diseases such as CLL, SLE, and RA have been reported [[26], [27], [28],38]. However, reduced cellular LAIR1 expression and enhanced disease severity does not appear to be universal as exemplified by investigation in hepatocellular carcinoma, epithelial ovarian cancer, and acute myeloid leukaemia (AML) [[43], [44], [45]].
It is well known that many transmembrane receptors including LAIR1, are shed from the cell surface and released into the circulation in soluble forms when immune cells are activated [[46], [47], [48], [49]]. Shedding of transmembrane receptors is a mechanism that regulates expression and activation of membrane-bound receptors by reducing the abundance of receptors at the cell membrane, and through competition for ligands since many soluble receptors retain their affinity for ligands. Enhanced shedding of membrane bound LAIR1 has clinical relevance in CLL, SLE, RA, haemorrhagic fever with renal syndrome (HFRS), and transplant rejection [26,28,29,48]. In our cohort of children with falciparum malaria, we also found elevated levels of sLAIR1 levels in the circulation of children with severe disease. This finding implies that there is a significantly higher rate of LAIR1 shedding from the surface of leukocytes in SMA, and offers a potential mechanism through which membrane bound LAIR1 expression is reduced in severe malaria.
Similarly, sLAIR2 levels were also higher in children with SMA relative to those with non-SMA. These results offer another potential explanation for decreased LAIR1 transcripts in children with SMA and parallel previous studies in other inflammatory diseases in which increased sLAIR2 levels were associated with decreased LAIR1 receptor expression [[26], [27], [28]]. These results also support previous findings in patients with RA where the presence of LAIR2 in circulation was associated with increased shedding of membrane bound LAIR1 [26]. Based on the significant positive correlation between sLAIR1 and sLAIR2 in children with malaria, we hypothesize that infection with P. falciparum promotes enhanced release of these two proteins into circulation which then act to antagonize the LAIR1 receptor in peripheral mononuclear cells. As shown in the current study, levels of the soluble proteins are higher in severe malaria, suggesting that their release into circulation may have direct clinical relevance. This hypothesis is consistent with the data showing that elevation of both sLAIR1 and sLAIR2 are associated more profound anaemia.
Since the availability of natural ligands for LAIR1 such as C1q can influence cellular expression of LAIR1 in leukocytes [31,32], we also investigated the relationship between C1q production and susceptibility to SMA. C1q levels were significantly lower in the sera of children with SMA, suggesting that there is limited C1q ligand available to activate LAIR1 expression and downstream signalling. However, this does not exclude the fact that other than C1q, LAIR1 may recognize and bind to ligands that possess the conserved Gly-Pro-Hyp collagen repeats, which could potentially be present during P. falciparum infections [31,50,51]. Additional binding to the LAIR1 receptor during a P. falciparum infection may also occur through interactions with RIFIN-binding antibodies that contain LAIR1 inserted into their variable regions [52]. These antibodies appear to be produced in individuals infected with P. falciparum, and may have evolved to escape immune surveillance by binding to inhibitory receptors such as LAIR1 [52,53]. Since RIFINs are a family of clonally variant proteins derived from P. falciparum expressed on the surface of infected red blood cells [54], there appears to be multiple mechanisms through which the LAIR1 signalling pathway can be altered.
In the current study, there was a positive correlation between circulating C1q and haemoglobin concentrations, indicating low C1q levels during severe disease. Lower circulating C1q has previously been observed in Thai adolescents and adults with severe malaria, a process that the authors attributed to higher C1q consumption during complement activation [39]. However, that study (n = 18) did not identify any relationships between C1q levels and clinical complications, including anaemia. This can be explained by the mixed phenotype of disease presentations in the small group (e.g., disseminated intravascular dissemination manifestations organ dysfunction, and central nervous system features). Another study in children from Mali (6–60 mos.) found that C1q was elevated in severe malaria, as determined by microarray analysis [55]. However, these data may not be in contrast to the studies reported here since the severe disease phenotypes appear to differ (SMA versus cerebral malaria), and increased C1q gene expression may be a physiological response to consumption of C1q. However, since circulating C1q was not measured in that study, the potentially differing results remain to be determined.
We have previously shown that children with SMA have elevated numbers of circulating phagocytes loaded with PfHz, particularly monocytes [33,56,57]. Intraleukocytic PfHz is a significant predictor of SMA and modulates inflammatory mediator production through activation of leukocyte receptors such as NOD-like receptor containing pyrin domain 3 (NLRP3) and Toll-like receptor 9 (TLR9) [[58], [59], [60]]. Based on these previous investigations, we determined if phagocytosis of PfHz could be a source of the reduced LAIR1 expression observed in children with SMA. The experiments revealed that children with PCM had significantly lower LAIR1 transcript levels than children without PCM. This in vivo finding promoted us to perform assays in cultured PBMCs to determine if phagocytosis of a physiological concentration of PfHz could drive suppression of LAIR1 transcripts and protein. Consistent with our previous studies showing that phagocytosis of PfHz by cultured PBMCs offers important information about the potential in vivo mechanisms which elicit changes in inflammatory mediator genes, and their products [33,61,62], phagocytosis of PfHz caused a time-dependent decrease in LAIR1 mRNA and protein. Taken together, the in vivo and in vitro findings suggest that phagocytosis of PfHz has a direct effect LAIR1 expression, and that LAIR1 suppression in children with SMA can be explained, at least in part, through accumulation of PfHz within circulating mononuclear cells.
Next, we sought to determine if phagocytosis of PfHz could alter the LAIR1 signalling pathway. The inhibitory signalling for leukocytes through the LAIR1 receptor is dependent upon phosphorylation of LAIR1 ITIM, and SHP-1 and SHP-2 phosphatases [23,63,64]. Phagocytosis of PfHz decreased phosphorylation of both LAIR1 ITIM and SHP1 relative to C1q stimulation. Suppression of the LAIR1 signalling pathway by PfHz (i.e., low phosphorylation of LAIR1 ITIM and SHP-1) could not be rescued by stimulation with C1q. This finding is consistent with previous studies in murine macrophages in which phagocytosis of haemozoin can dephosphorylate/inactivate protein tyrosine phosphatases [65]. Of note, SHP-2 phosphorylation levels were undetectable at baseline and after C1q stimulation, hence we were unable to determine effect of PfHz treatment on SHP-2 phosphorylation.
To further investigate the effects of PfHz on the LAIR1 signalling pathway, we examined the functional implications of LAIR1 and SHP-1 inactivation on downstream events i.e., NF-κB activation and the subsequent production of cytokines. In PfHz-treated cells in which the LAIR1 signalling pathway was inhibited, there was increased activation of NF-κB. Although stimulation with C1q should initiate the LAIR1 signalling pathway and decrease activation of NF-κB, this was not the case: pNF-κB was slightly higher in haemozoin-treated cells upon stimulation with C1q. These findings provide further evidence of the antagonistic effect of PfHz on LAIR1 inhibitory signalling. The effects of impaired LAIR1 ITIM and SHP-1 phosphorylation, mediated by PfHz, were also reflected at the level of soluble cytokine expression. In comparison to C1q treatment, PfHz resulted in significant over expression of IL-1β, IL-6 and TNF-α. Enhanced production of these inflammatory mediators parallels our previous in vivo findings of cytokine levels in children with SMA [8].
Taken together, the novel findings presented here describe how blockade of the LAIR1 leukocyte inhibitory signalling pathway impacts on the pathogenesis of SMA. Based on our investigations, we propose that reduced levels of C1q in children with SMA limit ligand availability, and thereby, decrease LAIR1 inhibitory activities. Enhanced levels of sLAIR1 and sLAIR2 indicate increased receptor shedding in severe malaria, events that contribute to reduced signalling through the LAIR1 pathway. In addition, phagocytosis of PfHz results in low levels of phosphorylation of LAIR1 ITIMs and SHP-1, as well as down-regulation of LAIR1 transcripts and protein. Collectively, these events result in blockade of the inhibitory signal through the LAIR1 receptor which causes activation of NF-κB and overproduction of cytokines that promote enhanced pathogenesis of SMA (see Fig. 5). Based on our recent work showing that genetic variation in LAIR1 impacts on the longitudinal acquisition of SMA and all-cause mortality, along with the molecular data presented here showing that the LAIR1 signalling pathway is dysregulated in children with SMA, we propose that LAIR1, and SHP-1 may be a promising target for future studies aimed at utilizing immunotherapeutic approaches to treat malaria.
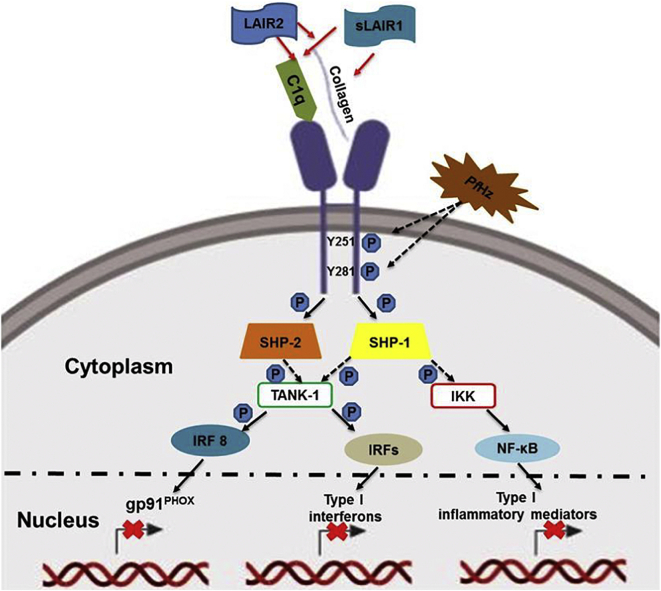
Fig. 5.
LAIR1 signalling pathway in malaria.
The LAIR1 pathway is activated by attachment of collagen and collagenous ligands (C1q) to LAIR1 extracellular surface. This results in phosphorylation of intracellular LAIR1 ITIM tyrosine residues by Src family kinases. Phosphorylated ITIMs serve as docking sites for recruitment of SHP-1 and SHP-2 phosphatases. SHP-1 and SHP-2 become localized to phosphorylated ITIMs through their regulatory SH2 domains, subsequently inducing their phosphatase activity. Activated SHP-1 has been shown to block activation and nuclear translocation of nuclear factor nuclear factor-kappa beta (NF-κB) through de-phosphorylation of inhibitor of kappa-beta kinase complex (IKK). SHP-1 phosphatase activity also inhibits activation and translocation of IRFs from the cytoplasm to the nucleus by preventing TANK-binding kinase 1 (TANK-1) phosphorylation of interferon regulatory factors (IRFs). These events subsequently block transcription of inflammatory mediator encoding genes with response elements for IRFs and NF-κB. SHP-2 inhibits activation of IRF 8 and blocks expression of phagocyte NADPH oxidase (gp91PHOX). LAIR1 inhibitory signalling is regulated by soluble LAIR1 and LAIR2 (soluble homolog of LAIR1) through competition for collagenous ligands. Children with SMA had increased circulating levels of sLAIR1 and sLAIR2 indicative of enhanced receptor shedding. C1q was reduced in children with SMA, thereby, limiting ligand availability. Phagocytosis of PfHz antagonizes LAIR1 signalling through down-regulation of LAIR1 ITIM and SHP-1 phosphorylation, but does not alter SHP-2 phosphorylation. Leukocytic ingestion of PfHz also decreases LAIR1 transcripts and protein. These events result in NF-κB activation and the consequent production of pro-inflammatory mediators that enhance the pathogenesis of SMA. Red arrows represent ligand-receptor interaction, black solid arrows represent activation, and dashed black arrows represent blockade. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Acknowledgments and funding
The authors gratefully acknowledge the assistance of the University of New Mexico-Kenya Research Team (Nicholas Otieno Ondiek, Vincent Odhiambo Otieno, Anne A Ong'ondo, Chrispine Wasonga Ochieng, Everlyne A Modi, Joan L A Ochieng, Joseph Oduor, Moses Ebungure, Moses Lokorkeju, Rodney B Mongare, and Vincent Omanje). We are also grateful to all of the parents, guardians and children who participated in the study.
The work was supported by National Institutes of Health (NIH) Research Grants R01AI130473, R01AI51305 and D43TW05884 (DJP), and the University of New Mexico Health Sciences Clinical and Translational Science Center (UL1TR001449). The content is solely the responsibility of the authors and the funders did not have any role in study design, data collection, data analysis, interpretation, or writing of the report.
Ethics approval and consent to participate
The study was approved by the ethical and scientific review committees at the, the University of New Mexico and the Kenya Medical Research Institute.
Declaration of interests
The authors declare that they have no competing interests.
Authors' contributions
AOA: conducted experiments, performed data analyses, and manuscript writing.
BG: technical advice on study design, and manuscript review and editing.
QC: technical support, and manuscript review and editing.
JMO: project supervision, and manuscript review and editing.
CO: project supervision, and manuscript review and editing.
CGL: data analyses, and manuscript review and editing.
DJP: designed clinical and experimental studies, data analyses, manuscript writing and editing, provided clinical samples and data, and supplied reagents, materials, and analyses tools.
References
- 1.UNICEF. Malaria in Africa. 2018. [Google Scholar]
- 2.WHO. World Malaria (Report. http://appswhoint/iris/bitstream/handle/10665/259492/9789241565523-engpdf;jsessionid=7C4AC215FEF706839BD95175ADE65ABA?sequence=1) 2017.
- 3.Obonyo C.O., Vulule J., Akhwale W.S., Grobbee D.E. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg. 2007;77(6 Suppl):23–28. [PubMed] [Google Scholar]
- 4.Ong'echa J.M., Keller C.C., Were T., Ouma C., Otieno R.O., Landis-Lewis Z. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg. 2006;74(3):376–385. [PubMed] [Google Scholar]
- 5.Pullan R.L., Gitonga C., Mwandawiro C., Snow R.W., Brooker S.J. Estimating the relative contribution of parasitic infections and nutrition for anaemia among school-aged children in Kenya: a subnational geostatistical analysis. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awandare G.A., Kempaiah P., Ochiel D.O., Piazza P., Keller C.C., Perkins D.J. Mechanisms of erythropoiesis inhibition by malarial pigment and malaria-induced proinflammatory mediators in an in vitro model. Am J Hematol. 2011;86(2):155–162. doi: 10.1002/ajh.21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K.H., Tam M., Stevenson M.M. Inappropriately low reticulocytosis in severe malarial anemia correlates with suppression in the development of late erythroid precursors. Blood. 2004;103(10):3727–3735. doi: 10.1182/blood-2003-08-2887. [DOI] [PubMed] [Google Scholar]
- 8.Perkins D.J., Were T., Davenport G.C., Kempaiah P., Hittner J.B., Ong'echa J.M. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7(9):1427–1442. doi: 10.7150/ijbs.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickramasinghe S.N., Abdalla S.H. Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol. 2000;13(2):277–299. doi: 10.1053/beha.1999.0072. [DOI] [PubMed] [Google Scholar]
- 10.Ong'echa J.M., Davenport G.C., Vulule J.M., Hittner J.B., Perkins D.J. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79(11):4674–4680. doi: 10.1128/IAI.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casals-Pascual C., Kai O., Cheung J.O., Williams S., Lowe B., Nyanoti M. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108(8):2569–2577. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 12.Awandare G.A., Kremsner P.G., Hittner J.B., Keller C.C., Clark I.A., Weinberg J.B. Higher production of peripheral blood macrophage migration inhibitory factor in healthy children with a history of mild malaria relative to children with a history of severe malaria. Am J Trop Med Hyg. 2007;76(6):1033–1036. [PubMed] [Google Scholar]
- 13.Kempaiah P., Anyona S.B., Raballah E., Davenport G.C., Were T., Hittner J.B. Reduced interferon (IFN)-alpha conditioned by IFNA2 (−173) and IFNA8 (−884) haplotypes is associated with enhanced susceptibility to severe malarial anemia and longitudinal all-cause mortality. Hum Genet. 2011;131(8):1375–1391. doi: 10.1007/s00439-012-1175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouma C., Davenport G.C., Awandare G.A., Keller C.C., Were T., Otieno M.F. Polymorphic variability in the interleukin (IL)-1beta promoter conditions susceptibility to severe malarial anemia and functional changes in IL-1beta production. J Infect Dis. 2008;198(8):1219–1226. doi: 10.1086/592055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouma C., Davenport G.C., Garcia S., Kempaiah P., Chaudhary A., Were T. Functional haplotypes of fc gamma (Fcgamma) receptor (FcgammaRIIA and FcgammaRIIIB) predict risk to repeated episodes of severe malarial anemia and mortality in Kenyan children. Hum Genet. 2012;131(2):289–299. doi: 10.1007/s00439-011-1076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouma C., Davenport G.C., Were T., Otieno M.F., Hittner J.B., Vulule J.M. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet. 2008;124(5):515–524. doi: 10.1007/s00439-008-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munde E.O., Okeyo W.A., Anyona S.B., Raballah E., Konah S., Okumu W. Polymorphisms in the fc gamma receptor IIIA and toll-like receptor 9 are associated with protection against severe malarial anemia and changes in circulating gamma interferon levels. Infect Immun. 2012;80(12):4435–4443. doi: 10.1128/IAI.00945-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munde E.O., Raballah E., Okeyo W.A., Ong'echa J.M., Perkins D.J., Ouma C. Haplotype of non-synonymous mutations within IL-23R is associated with susceptibility to severe malaria anemia in a P. falciparum holoendemic transmission area of Kenya. BMC Infect Dis. 2017;17(1):291. doi: 10.1186/s12879-017-2404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maasho K., Masilamani M., Valas R., Basu S., Coligan J.E., Borrego F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol Immunol. 2005;42(12):1521–1530. doi: 10.1016/j.molimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Merlo A., Tenca C., Fais F., Battini L., Ciccone E., Grossi C.E. Inhibitory receptors CD85j, LAIR-1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin Diagn Lab Immunol. 2005;12(6):705–712. doi: 10.1128/CDLI.12.6.705-712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83(4):799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 22.Meyaard L., Hurenkamp J., Clevers H., Lanier L.L., Phillips J.H. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol. 1999;162(10):5800–5804. [PubMed] [Google Scholar]
- 23.Verbrugge A., Ruiter Td T., Clevers H., Meyaard L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int Immunol. 2003;15(11):1349–1358. doi: 10.1093/intimm/dxg134. [DOI] [PubMed] [Google Scholar]
- 24.Xu M., Zhao R., Zhao Z.J. Identification and characterization of leukocyte-associated Ig-like receptor-1 as a major anchor protein of tyrosine phosphatase SHP-1 in hematopoietic cells. J Biol Chem. 2000;275(23):17440–17446. doi: 10.1074/jbc.M001313200. [DOI] [PubMed] [Google Scholar]
- 25.Verbrugge A., de Ruiter T., Geest C., Coffer P.J., Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79(4):828–836. doi: 10.1189/jlb.0705370. [DOI] [PubMed] [Google Scholar]
- 26.Olde Nordkamp M.J., van Roon J.A., Douwes M., de Ruiter T., Urbanus R.T., Meyaard L. Enhanced secretion of leukocyte-associated immunoglobulin-like receptor 2 (LAIR-2) and soluble LAIR-1 in rheumatoid arthritis: LAIR-2 is a more efficient antagonist of the LAIR-1-collagen inhibitory interaction than is soluble LAIR-1. Arthritis Rheum. 2011;63(12):3749–3757. doi: 10.1002/art.30612. [DOI] [PubMed] [Google Scholar]
- 27.Perbellini O., Falisi E., Giaretta I., Boscaro E., Novella E., Facco M. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica. 2014;99(5):881–887. doi: 10.3324/haematol.2013.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombo B.M., Canevali P., Magnani O., Rossi E., Puppo F., Zocchi M.R. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Wang S., Dong H., Yi X., Zhang J., Liu X. LAIR-1 shedding from human fibroblast-like synoviocytes in rheumatoid arthritis following TNF-α stimulation. Clin Exp Immunol. 2018;192(2):193–205. doi: 10.1111/cei.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebbink R.J., van den Berg M.C., de Ruiter T., Raynal N., van Roon J.A., Lenting P.J. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J Immunol. 2008;180(3):1662–1669. doi: 10.4049/jimmunol.180.3.1662. [DOI] [PubMed] [Google Scholar]
- 31.Son M., Diamond B. C1q-mediated repression of human monocytes is regulated by leukocyte-associated Ig-like receptor 1 (LAIR-1) Mol Med. 2014;20(1):559–568. doi: 10.2119/molmed.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son M., Santiago-Schwarz F., Al-Abed Y., Diamond B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci U S A. 2012;109(46):E3160–E3167. doi: 10.1073/pnas.1212753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awandare G.A., Ouma Y., Ouma C., Were T., Otieno R., Keller C.C. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect Immun. 2007;75(1):201–210. doi: 10.1128/IAI.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouma C., Keller C.C., Davenport G.C., Were T., Konah S., Otieno M.F. A novel functional variant in the stem cell growth factor promoter protects against severe malarial anemia. Infect Immun. 2010;78(1):453–460. doi: 10.1128/IAI.00895-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller C.C., Kremsner P.G., Hittner J.B., Misukonis M.A., Weinberg J.B., Perkins D.J. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect Immun. 2004;72(8):4868–4873. doi: 10.1128/IAI.72.8.4868-4873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Were T.D.G., Yamo E.O., Hittner J.B., Awandare G.A., Otieno M.F., Ouma C. Ong'echa JM, Perkins DJ. Naturally acquired hemozoin by monocytes promotes suppression of RANTES in children with malarial anemia through an IL-10-dependent mechanism. Microbes and Infection / Institut Pasteur. 2009;11:811–819. doi: 10.1016/j.micinf.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son M., Diamond B., Volpe B.T., Aranow C.B., Mackay M.C., Santiago-Schwarz F. Evidence for C1q-mediated crosslinking of CD33/LAIR-1 inhibitory immunoreceptors and biological control of CD33/LAIR-1 expression. Sci Rep. 2017;7(1):270. doi: 10.1038/s41598-017-00290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poggi A., Catellani S., Bruzzone A., Caligaris-Cappio F., Gobbi M., Zocchi M.R. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia. 2008;22(5):980–988. doi: 10.1038/leu.2008.21. [DOI] [PubMed] [Google Scholar]
- 39.Srichaikul T., Puwasatien P., Karnjanajetanee J., Bokisch V.A., Pawasatien P. Complement changes and disseminated intravascular coagulation in Plasmodium falciparum malaria. Lancet. 1975;1(7910):770–772. doi: 10.1016/s0140-6736(75)92436-8. [DOI] [PubMed] [Google Scholar]
- 40.Baeuerle P.A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 41.Poggi A., Pellegatta F., Leone B.E., Moretta L., Zocchi M.R. Engagement of the leukocyte-associated Ig-like receptor-1 induces programmed cell death and prevents NF-ΰB nuclear translocation in human myeloid leukemias. Eur J Immunol. 2000;30(10):2751–2758. doi: 10.1002/1521-4141(200010)30:10<2751::AID-IMMU2751>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Kempaiah P., Dokladny K., Karim Z., Raballah E., Ong'echa J.M., Moseley P.L. Reduced Hsp70 and glutamine in pediatric severe Malaria Anemia: role of Hemozoin in suppressing Hsp70 and NF-kappaB activation. Mol Med. Oct 2016;22:570–584. doi: 10.2119/molmed.2016.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X., Zhang L., Zhou J., Liu L., Fu Q., Fu A. Clinicopathologic significance of LAIR-1 expression in hepatocellular carcinoma. Curr Probl Cancer. Feb 2018;43(1):18–26. doi: 10.1016/j.currproblcancer.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Kang X., Lu Z., Cui C., Deng M., Fan Y., Dong B. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat Cell Biol. 2015;17(5):665–677. doi: 10.1038/ncb3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao Q., Fu A., Yang S., He X., Wang Y., Zhang X. Leukocyte-associated immunoglobulin-like receptor-1 expressed in epithelial ovarian cancer cells and involved in cell proliferation and invasion. Biochem Biophys Res Commun. 2015;458(2):399–404. doi: 10.1016/j.bbrc.2015.01.127. [DOI] [PubMed] [Google Scholar]
- 46.Gong J., Zhu C., Zhuang R., Song C., Li Q., Xu Z. Establishment of an enzyme-linked immunosorbent assay system for determining soluble CD96 and its application in the measurement of sCD96 in patients with viral hepatitis B and hepatic cirrhosis. Clin Exp Immunol. 2009;155(2):207–215. doi: 10.1111/j.1365-2249.2008.03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Lv K., Zhang C.M., Jin B.Q., Zhuang R., Ding Y. The role of LAIR-1 (CD305) in T cells and monocytes/macrophages in patients with rheumatoid arthritis. Cell Immunol. 2014;287(1):46–52. doi: 10.1016/j.cellimm.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang W, Xue J, Liu J, Jia W, Li Z, Xie X, et al. Establishment of an ELISA system for determining soluble LAIR-1 levels in sera of patients with HFRS and kidney transplant; 2004. [DOI] [PubMed]
- 49.Jones S.A., Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2002;1592(3):251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 50.Olde Nordkamp MJ1 vEM, Urbanus RT3, Bont L4, Haagsman HP2, Meyaard L5 Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J Leukoc Biol. 2014;96(1):105–111. doi: 10.1189/jlb.3AB0213-092RR. [DOI] [PubMed] [Google Scholar]
- 51.Lebbink R.J., de Ruiter T., Adelmeijer J., Brenkman A.B., van Helvoort J.M., Koch M. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203(6):1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan J., Pieper K., Piccoli L., Abdi A., Foglierini M., Geiger R. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature. 2016;529(7584):105. doi: 10.1038/nature16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pieper K., Tan J., Piccoli L., Foglierini M., Barbieri S., Chen Y. Public antibodies to malaria antigens generated by two LAIR1 insertion modalities. Nature. 2017;548(7669):597–601. doi: 10.1038/nature23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saito F., Hirayasu K., Satoh T., Wang C.W., Lusingu J., Arimori T. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature. 2017;552(7683):101. doi: 10.1038/nature24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobota R.S., Dara A., Manning J.E., Niangaly A., Bailey J.A., Kone A.K. Expression of complement and toll-like receptor pathway genes is associated with malaria severity in Mali: a pilot case control study. Malar J. 2016;15(1):150. doi: 10.1186/s12936-016-1189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller C.C., Yamo O., Ouma C., Ong'echa J.M., Ounah D., Hittner J.B. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect Immun. 2006;74(9):5249–5260. doi: 10.1128/IAI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novelli E.M., Hittner J.B., Davenport G.C., Ouma C., Were T., Obaro S. Clinical predictors of severe malarial anaemia in a holoendemic Plasmodium falciparum transmission area. Br J Haematol. 2010;149(5):711–721. doi: 10.1111/j.1365-2141.2010.08147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shio M.T., Eisenbarth S.C., Savaria M., Vinet A.F. Bellemare M-Je, Harder KW, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS pathogens. 2009;5(8) doi: 10.1371/journal.ppat.1000559. e1000559-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalantari P., DeOliveira R.B., Chan J., Corbett Y., Rathinam V., Stutz A. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 2015;6(1):196–210. doi: 10.1016/j.celrep.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner H. Hemozoin: malaria's "built-in" adjuvant and TLR9 agonist. Cell Host Microbe. 2010;7(1):5–6. doi: 10.1016/j.chom.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Keller C.C., Hittner J.B., Nti B.K., Weinberg J.B., Kremsner P.G., Perkins D.J. Reduced peripheral PGE2 biosynthesis in Plasmodium falciparum malaria occurs through hemozoin-induced suppression of blood mononuclear cell cyclooxygenase-2 gene expression via an interleukin-10-independent mechanism. Mol Med. 2004;10(1–6):45–54. doi: 10.2119/2004-00035.perkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ong'echa J.M., Remo A.M., Kristoff J., Hittner J.B., Were T., Ouma C. Increased circulating interleukin (IL)-23 in children with malarial anemia: in vivo and in vitro relationship with co-regulatory cytokines IL-12 and IL-10. Clin Immunol. 2008;126(2):211–221. doi: 10.1016/j.clim.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu F., Xu M.J., Zhao R., Guerrah A., Zeng F., Zhao Z.J. Tyrosine phosphatases SHP-1 and SHP-2 are associated with distinct tyrosine-phosphorylated proteins. Exp Cell Res. 2002;272(1):75–83. doi: 10.1006/excr.2001.5397. [DOI] [PubMed] [Google Scholar]
- 64.Lebbink R.J., de Ruiter T., Verbrugge A., Bril W.S., Meyaard L. The mouse homologue of the leukocyte-associated Ig-like Receptor-1 is an inhibitory receptor that recruits Src homology region 2-containing protein tyrosine phosphatase (SHP)-2, but not SHP-1. J Immunol. 2004;172(9):5535. doi: 10.4049/jimmunol.172.9.5535. [DOI] [PubMed] [Google Scholar]
- 65.Jaramillo M., Plante I., Ouellet N., Vandal K., Tessier P.A., Olivier M. Hemozoin-inducible proinflammatory events in vivo: potential role in malaria infection. J Immunol. 2004;172(5):3101–3110. doi: 10.4049/jimmunol.172.5.3101. [DOI] [PubMed] [Google Scholar]