Abstract
Background
Obesity leads to the chronic inflammation in the whole body and triggers the macrophage polarization to the pro-inflammatory phenotype. Targeting macrophage polarization provides a promising therapeutic strategy for obesity-related metabolic disorders and inflammation. Here, we show that SO1989, a derivative of natural occurring compound oleanolic acid, restores the balance between M1-polarized and M2-polarized macrophages in high fat diets (HFD)-induced obese mice resulting in the improvement of adipose inflammation and the metabolic dysfunctions.
Methods
Histological analysis, magnetic cell sorting and FACS, in vitro cell model of adipose inflammation, Western blotting, HFD mice model.
Findings
SO1989 exhibits similar or even stronger activity in inhibiting inflammation and M1 polarization of macrophages both in vitro and in vivo compared to its analogue CDDO-Me, previously known as a powerful anti-inflammation chemical small molecule. In addition, SO1989 can significantly increase the level of fatty acid oxidation in macrophages which can efficiently facilitate M2 polarization of macrophages. Unlike CDDO-Me, SO1989 shows less adverse effects on obese mice.
Interpretation
Taken all together, our findings identify SO1989 as a modulator in macrophage polarization and a safer potential leading compound for pro-resolution of inflammation treatment in metabolic disorders.
Fund
Supported by grants from the National Key Research and Development Plan (2017YFA0506000, 2017YFA0205400) and National Natural Science Foundation of China (81673439) and Natural Science Fund project in Jiangsu Province (BK20161408).
Keywords: Inflammation, Macrophage polarization, SO1989, Fatty acid oxidation, Adverse effects
Research in context.
Evidence before this study
Obesity related chronic inflammation leads to various metabolic dysfunctions. The pharmacological intervention of macrophage polarization to attenuate inflammation is an effective strategy to help prevent or reverse chronic disorders. CDDO-Me (Bardoxolone Methyl) has been studied in various chronic disorders models, such as obesity, neurodegenerative diseases and cancers etc. However, few studies investigated the efficiency of CDDO-Me and its derivatives in regulating macrophage functions in obesity. Besides, the clinical application of CDDO-Me in diabetic nephropathy, a kind of chronic inflammatory symptoms, caused several adverse effects, which directly led to the termination of its clinical investigation. Thus, further efforts are needed in the structural optimization of CDDO-Me to develop new and safer compounds in modulation of macrophage functions in obesity.
Added value of this study
This study demonstrated that SO1989, a derivative of CDDO-Me, is a promising modulator in efficiently ameliorating obesity-related inflammation and metabolic dysfunctions. In our investigation, we first synthesized SO1989, which possessed a unique structure that containing a double-bound in D ring. It exhibited similar or even higher activity than CDDO-Me in inhibition pro-inflammatory phenotype of macrophages and inflammation attenuation. Importantly, SO1989 significantly restored the M2 polarization of macrophages in peritoneal macrophages (PMs) and adipose tissue macrophages (ATMs) impaired by high fat diet (HFD) and improved chronic inflammation state in HFD mice. Moreover, SO1989 profoundly enhanced mitochondrial biogenesis and increased the expression levels of fatty acid oxidation related genes, which facilitated the lipid catabolism to help the M2 polarization in macrophages. At last, SO1989 reduced metabolic syndromes significantly with less adverse effects which were observed in CDDO-Me.
Implications of all the available evidence
Our findings expanded current knowledge of synthetic oleanane triterpenoids, showing SO1989 might serve as a safer molecule in curing chronic disorders. Moreover, SO1989 could be a drug candidate for treating chronic inflammation because of its highly efficiency in modulating macrophage polarization. The characteristics of SO1989 also lay the foundation for its further structural optimization.
Alt-text: Unlabelled Box
1. Introduction
Obesity disturbs the normal energy balance, turning adipose into inflamed tissues and further leading to chronic metabolic related inflammation, which is correlated with the metabolic syndrome closely. The continuous chronic inflammation causes the occurrence of dysfunctional regulation in metabolic organs or tissues [1]. Thus, to attenuate the inflammation has been thought as an effective strategy to prevent or reverse metabolic related diseases [2]. Certain drugs such as steroids, NSAIDs and anti-cytokine antibodies have been developed for inhibiting such chronic inflammation. However, the side effects exhibited by these drugs have been noticed in clinical application [3]. Further efforts are needed to develop newer and safer drug candidates to ameliorate obesity-related inflammation and reverse metabolic disorders.
Macrophages have been well documented to play a vital role in inflammation progression and resolution, which are highly heterogeneity and plasticity in tissue microenvironment. The different polarization states of macrophage are correlated with their functional diversity [4,5]. There are two major subpopulations of macrophages in obesity-related inflammation: the M1 polarized and the M2-polarized macrophages. M1 cells produce inflammatory cytokines such as IL-1β, TNFα, IL-6 and ROS together with NO to promote the inflammation progression. By contrast, the M2 cells secrete high-level Th2-type cytokines such as IL-10 and IL-4, maintaining the homeostasis of local tissues and facilitating the resolution of inflammation [6]. The M2 macrophages are dominant in lean adipose tissue which play against to inflammatory response and maintain adipose homeostasis [7,8]. In obesity state, inflamed tissues or organs, particularly adipose tissues could release numerous pro-inflammatory chemokines like CCL2, which recruit the monocytes to inflamed site and differentiate to M1 macrophages consequently, aggravating both local and systemic inflammation. The tissue-resident macrophages can also polarized to M1-like cells. [2,8]. Herein, inhibiting monocytes infiltration and modulating phenotypic transformation of local macrophages will be an effective strategy for treating obesity-related inflammation [9].
CDDO-Me is a type of synthetic oleanane triterpenoids (SOs) deriving from naturally occurring pentacyclic triterpenoids oleanolic acids [10]. It has been demonstrated as an inhibitor of IKKβ, the kinase that phosphorylates IκBα, and causes the blockade of NF-κB activation and inflammation attenuation [11]. CDDO-Me is also the activator of Nrf2, which elicits a protective response against the inflammation and oxidation-induced stresses [12]. Though its effects on macrophage phenotypes have not been well defined, CDDO-Me is implicated in preventing fat deposition and mitigating the chronic inflammation in obesity [13,14]. In clinical investigation, CDDO-Me exhibited the anti-diabetic nephropathy (DN) effects. However, due to the increasing risk of estimated glomerular filtration rate (eGFR), albuminuria and heart failure in patients, CDDO-Me treatment were terminated in phase III DN clinical trial [15,16]. Therefore, safer and more efficacious derivatives of CDDO-Me need to be developed in obesity-related inflammation therapies.
Here, we synthesized SO1989, a novel derivative of oleanolic acid [17], and demonstrated that it could attenuate obesity-related metabolic dysfunctions and inflammation with lower side effects than that in CDDO-Me. SO1989 possessed a more efficient in regulating macrophages phenotype in obese mice by restoring M2 phenotype of peritoneal macrophages (PMs) and adipose tissue macrophages (ATMs), while CDDO-Me could only inhibit the pro-inflammatory M1 polarization. Furthermore, we discovered SO1989 significantly increased the expressions of fatty acid oxidation related genes and promoted the mitochondria biogenesis, which could be an approach to support the M2 polarization of macrophages.
2. Materials and methods
2.1. Reagents
CDDO-Me (Bardoxolone methyl, PubChem CID: 400769) was kindly provided by Prof. Sun Hongbin (China pharmaceutical University). The chemical structure of the two compounds were shown in Fig. 1a. All compounds were dissolved in 100% DMSO. The final DMSO concentration in cell culture did not exceed 0.1% throughout the study. DMEM and RPMI1640 media were purchased from Gibco (Grand Island, NY). Penicillin and streptomycin, HRP-conjugated Goat Anti-Mouse IgG (H + L), HRP-conjugated Goat Anti-Rabbit IgG (H + L) are from Beyotime (Haimen, Jiangsu, China). TNF-α, CCL2 and IL-1β ELISA assay kits were purchased from eBioscience (San Diego, CA). Alanine/aspartate aminotransferases (ALT/AST) assay kits, TC, TG, glucose and insulin kit were from Jiancheng Biology Institution (Nanjing, Jiangsu, China). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). PPRE-Luc plasmid and dual-luciferase reporter assay systems were from Promega (Madison, WI, USA). Urine protein quantification kit and serum creatinine quantification kit were from Jiancheng Biology Institution (Nanjing, Jiangsu, China).
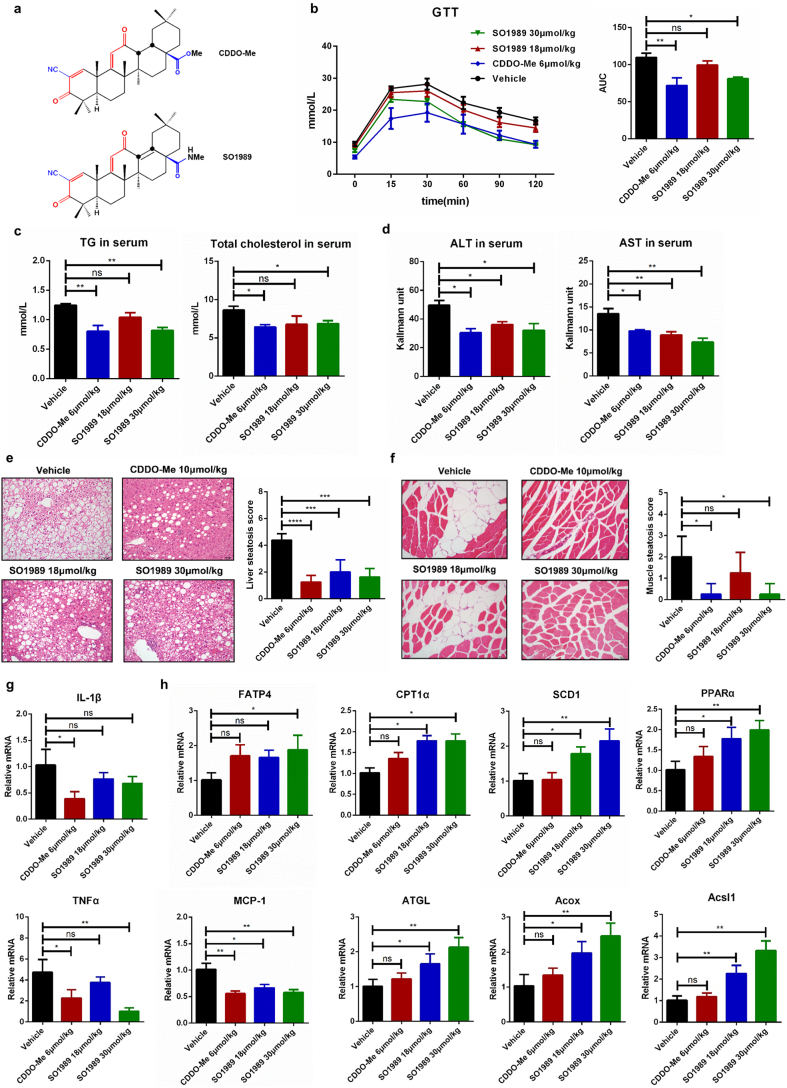
Fig. 1.
SO1989 attenuate the metabolic disorders of obese mice. (a) The chemical structural of CDDO-Me and SO1989. (b) HFD-mice treated with the vehicle, indicated doses of CDDO-Me or SO1989 for 15 days. The insulin sensitivity state is evaluated by glucose tolerance tests (left), and the total area under the curve (AUC) is shown on the right, (n = 5). (c) The content of total triglyceride and cholesterol in serum were measured by using commercial kits according to manufacturer's instructions (n = 5). (d) The activities of ALT and AST in serum were measured by ALT/AST assay kits (n = 5). (e–f) Representative H&E staining showed liver/skeletal muscle morphology from HFD treated with indicated compounds, original magnification ×200. The Quantification of hepatic/muscular steatosis for five sections/200× field, five to six fields/gland/mouse, score according to the grade of lesion, slight (0.5), mild (1), moderate (2), severe (3), profound severe (4) and normal (0), (n = 5). (g–h) Liver tissues were homogenized and the total RNA was isolated by using Trizol reagent, followed by qRT-PCR with the indicated probes (n = 5). The β-actin was used as an endogenous control. All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
2.2. Mice treatment
Male C57BL/6J mice (3–4 weeks old) were purchased from Nanjing biomedical Research Institute of Nanjing University (Nanjing, China) and housed in a specific-pathogen-free (SPF) facility. Mice, starting at 3–4 weeks old, were randomly divided into four groups (n = 6 per group). Mice were fed for 16 weeks with a high-fat diet (HFD) (D12492, 60% fat, 20% carbohydrate, 20% protein, total 5.24 kcal/g; Research Diets Inc., New Brunswick, NJ). 19 weeks year-old HFD mice were grouped and administered by oral gavage with CDDO-Me (6 μmol/kg), SO1989 (18 μmol/kg and 30 μmol/kg) or vehicle alone (100% sesame oil) daily for 21 days. Mice were weighed daily until sacrificed under anesthesia using diethylether.
2.3. Glucose tolerance test (GTT)
Mice were fasted for 12 h. Then 2 g/kg glucose was intraperitoneal injected in to mice. Blood glucose levels were measured using a glucometer.
2.4. Histological analysis and scoring
H&E staining were used to score liver and skeletal muscle steatosis, adipose inflammatory cell infiltration, glomerular swelling status and cardiac pathology. The pathological score was calculated for four-five sections, five to six fields/mouse, score according to the grade of lesion, slight (0.5), mild (1), moderate (2), severe (3), profound severe (4) and normal (0).
2.5. Analysis of mitochondrial mass and activity
RAW264.7 were pre-treated with 1 mM FFA for 24 h followed by SO1989 or CDDO-Me administration for 12 h. Next, cells were incubated with 50 nM MitoTracker Red (Yeasen, China, 40739ES50) or 50 nM MitoTracker Green (Yeasen, china, 40738ES50) at 37 °C for 1 h. Cells were then washed in 1 × PBS and collected for the further FACS analysis. Flow cytometry was performed on FACScalibur. Data were analyzed by using FlowJo 10.0.7.
2.6. Isolation and purification of peritoneal macrophages
Peritoneal cells of the every male C57BL/6 J mice were obtained by peritoneal washing with 5 ml Dulbecco's PBS (D-PBS) containing 2% FBS. After centrifugation at 600 g for 5 min, red blood cells were lysed in ACK buffer and mononuclear cells were re-suspended in complete medium (DMEM, 10% FBS, 2 mM l-glutamine, 100 units/ml penicillin and 100 units/ml streptomycin) and incubated at 37 °C for 2 h in plastic culture plates. Then, the non-adherent cells were removed, and the adherent cells were harvested followed by cell surface antigens staining and FACS analysis.
2.7. SVF Isolation and the FACS analysis of adipocyte tissue macrophages
Epididymis fat was excised and minced in Hanks' Balanced Salt Solution (HBSS buffer) containing calcium, magnesium and 0.5% BSA. Collagenase (Type I; Sigma-Aldrich, St Louis, MO, C0130) was added to a final concentration of 3 mg/ml and tissue suspensions were incubated at 37 °C for 45 min with constant shaking. The resulting cell suspensions were filtered through a 100-μm filter and centrifuged at 600 g for 10 min to separate floating adipocytes from the SVC-containing pellet. The SVC was rinsed with PBS for two times and centrifuged at 600 g for 10 min. The pellet cells were resuspended in FACS buffer followed by cell surface antigens staining and FACS analysis.
2.8. Reactive oxygen species (ROS) generation
Cells were treated with 10 μM DCFH-DA probe (Beyotime, China, S0033) for 20 min at 37 °C, following 2 times washes with warmed D-PBS. The reduced DCFH-DA can be oxidized and converted into fluorescent DCF by intracellular ROS. The fluorescence signals were measured by flow cytometer (FCM, BD caliber, BD Biosciences).
2.9. Phagocytosis assay
Fluorescent red latex beads (1 μM diameter, Sigma-Aldrich, L-2778) were opsonized in 10% FBS in PRMI 1640 for 1 h at 37 °C before the experiments. Subsequently beads were added to cells at a ratio of 10:1 and incubated at 37 °C. After 2 h incubation, cells were washed three times with ice-cold sterile PBS and analyzed by flow cytometer. Totally 10,000 cells were analyzed per sample.
2.10. Cytokine assay by ELISA
Concentration of mouse IL-1β (Thermo Scientific, BMS6002), CCL2 (Thermo Scientific, BMS6005), TNFα (BD Biosciences, 88-7324-22) in 200 μl serum were determined using specific ELISA kits, in accordance with the manufacturer's instructions.
2.11. Co-culture adipocyte and macrophage
3T3-L1 adipocytes and macrophages were cultured with Transwell cell culture inserts. Briefly, 3T3-L1 pre-adipocytes were seeded into 6 well plate in DMEM and differentiated into mature adipocytes in the medium containing 0.5 mM IBMX (Sigma, I7018), 1 μM dexamethasone (Cayman chemical, 11015), 850 nM insulin (Sigma, I0320000). Two days after induction, cells were switched to the maintenance medium containing 10% FBS, 850 nM insulin. After the differentiation procedure, macrophages were seed into the corresponding insert in a well containing mature adipocytes. After 24 h, adipocytes and macrophages were collected for other assays.
2.12. Analysis of cell surface makers by flow cytometry
For cell surface protein labeling, primary peritoneal macrophages, RAW264.7 cells were stimulated with IL4 (Genescript, Z02996) or LPS (Sigma, L4130) for 24 h. Cells were collected and labeled with the fluorescein-conjugated antibodies following the FACS analysis, and corresponding isotype controls were purchased from eBioscience (San Diego, CA). The antibodies we used in present study were shown in Table S1.
2.13. Western blotting
Cells lysates were prepared with RIPA buffer (Sigma-Aldrich, R0278) containing 100× protease inhibitor cocktail (Sigma-Aldrich, K1011). The total protein (50 μg/lane) was first electrophoresed on 10% SDS-polyacrylamide gels for separation, and then electro-transferred to a poly PVDF membrane. Antibodies used were shown in Table.S1. The intensities of the bands were quantified by Image J software.
2.14. Quantitative real-time PCR
Total RNA was extracted from macrophages and purified by phenol/chloroform extraction. cDNA from each RNA sample was reverse transcribed with the 5× All-In-One RT MasterMix (abm, G490). The quantitative RT-PCR was performed using the iCycler thermocycler system software version 1.0(Life technology) and was obtained by normalizing to the endogenous control. The primers used are listed in Table S2.
2.15. PPARγ gene-reporter luciferase assay
iBMDMs were transfected with pIRES-hPPARγ/PPRE-Luc and pRL-control respectively. After 24 h treatments, cells were collected and lysed in luciferase lysis buffer, and then the luciferase activity of cells were measured using the dual-luciferase reporter assay system (Promega, E1910).
2.16. Nitric oxide (NO) assay
The concentration of NO in the culture medium was measured using a commercial NO assay kit (Beyotime, China, S0021) with Griess method. The NO levels were assayed by measuring nitrite levels through the absorbance at 520–560 nm, and calculated from a standard curve calibtated with known concentrations of sodium nitrite.
2.17. Blood brain barrier permeability
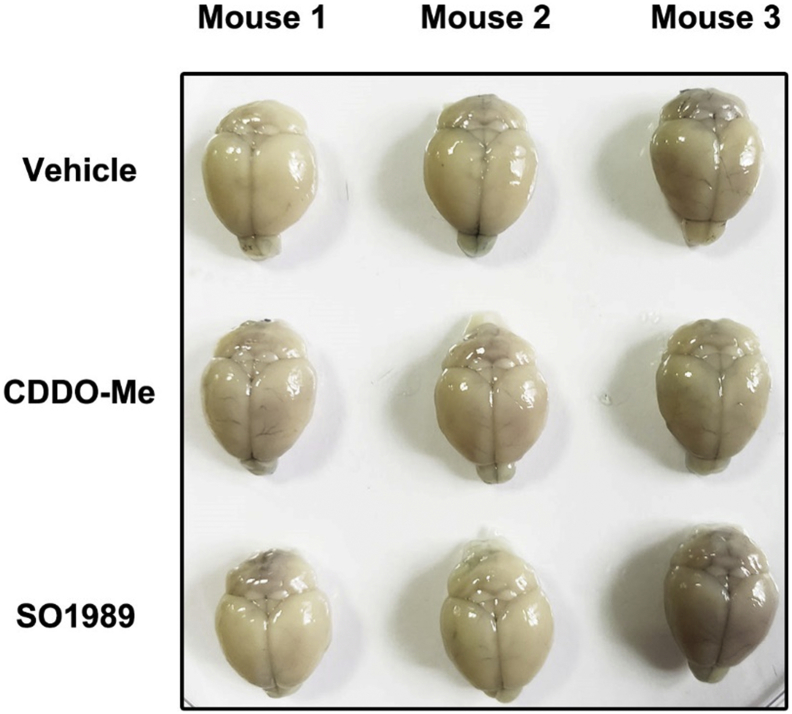
After administration of vehicle, CDDO-Me or SO1989, 2% Evans blue (10 ml/kg, Sigma-Aldrich, E2129) saline solution was injected through the tail vein. Six hours later, all mice were transcardially perfused with 40 ml saline solution from ventriculus sinister until the outflow was colorless. Then whole brain tissues were collected and photographed.
2.18. Statistical analysis
All statistical analysis was performed with Prism 5.0 software (GraphPad, San Diego, CA). The results are shown as the mean ± SEM. For the body weight change and food intake test, we used two-way ANOVA to analyze the data. The other statistical methods we used either Student's t-test (two-group comparison) or one-way ANOVA (more than two groups) followed by Bonferroni's post hoc comparison. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
2.19. Ethics statement
Animal welfare and experimental procedures were carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals (The Ministry of Science and Technology of China) and the related ethical regulations of our university. The protocol was approved by the State Key Laboratory of Pharmaceutical Biotechnology in Nanjing University, and all efforts were made to minimize suffering.
3. Results
3.1. SO1989 improves obesity-induced metabolic dysfunctions
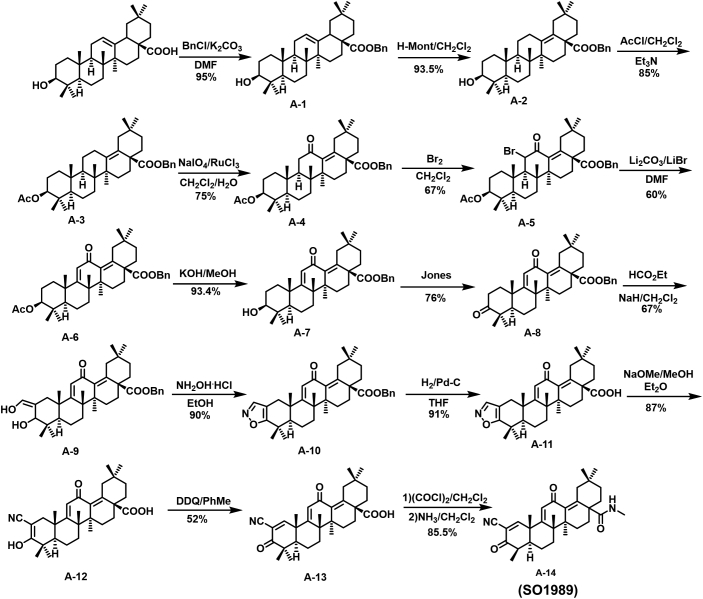
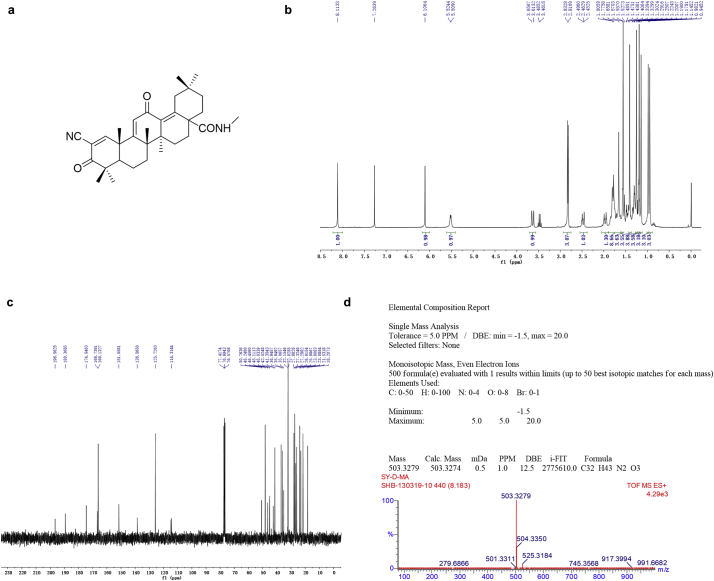
We first synthesized SO1989, the scheme of the synthesis is shown Fig. S1 and the synthetic steps was described in Supplementary methods. The identifications of SO1989 by 1HNMR, 13CNMR and HRMS were shown in Fig. S2.
In order to investigate the effects of SO1989 on obesity-related metabolic dysfunctions in vivo, 16 weeks high fat diets (HFD) male C57BL/6J mice were treated with 18 μmol/kg, 30 μmol/kg SO1989 or vehicle daily by oral gavage for 15 days. Meanwhile, mice treated with 6 μmol/kg CDDO-Me were used as a positive control. Plasma glucose concentrations during the intraperitoneal GTT were lower in 30 μmol/kg SO1989 treated mice as compared to vehicle-treated mice (Fig. 1b). Glucose tolerance in mice was improved by 30 μmol/kg SO1989 with an approximately 20–30% reduction in the AUC of glucose, whereas CDDO-Me treatment induced an approximately 25–50% reduction. Also the levels of total triglycerides (TG) and total cholesterol in serum were markedly decreased after the treatment with the higher dose SO1989 (Fig. 1c). Moreover, the histological examination of liver and muscle sections demonstrated that SO1989 efficiently alleviated the derangement of cell structures, excessive lipid accumulation and HFD-induced pathological status (Fig. 1e–f). In addition, the serum ALT and AST activities were significantly reduced by the SO1989 administration (Fig. 1d). We also observed that SO1989 profoundly inhibited the gene expressions of pro-inflammatory cytokines including IL-1β, TNFα and MCP-1 in liver tissues (Fig. 1g). However, only SO1989 enhanced the expressions of fatty acid oxidation (FAO) related genes (Fig. 1h), suggesting a beneficial impact of SO1989 on lipid metabolism of liver.
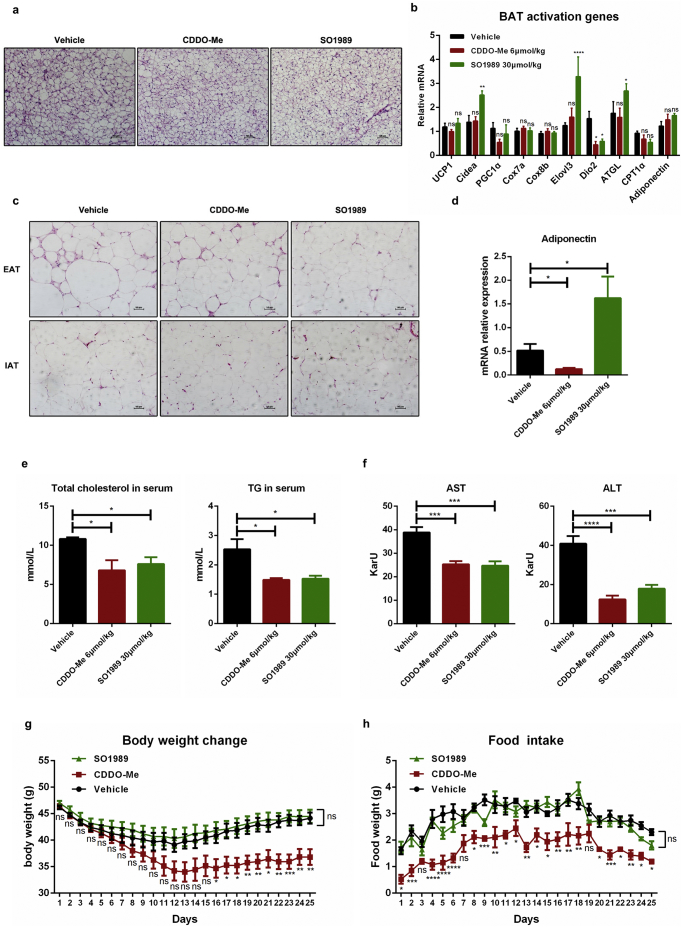
To better understand the underlying mechanism that SO1989 ameliorated HFD-induced metabolic dysfunctions, we further studied the brown adipose functions which was considered playing an important role in modulating metabolic homeostasis. The histological analysis showed that SO1989, CDDO-Me did not alter the size and content of lipid droplets in brown adipocytes compared to vehicle group (Fig. S3a). In addition, SO1989 administration only significantly upregulated the gene expressions of Cidea, Elovl3 and ATGL (BAT function specific genes). However, the expression level of UCP1, the major functional factor in BAT activation, was not impacted (Fig. S3b). These data indicated that SO1989 had little influence on BAT activation. Thus, we concluded that SO1989 improved the metabolic dysfunctions in a BAT in-dependent manner.
Collectively, these results suggest that SO1989 can effectively improve the syndromes of metabolic dysfunction in diet-induced obese mice.
3.2. SO1989 attenuates the white adipose inflammation
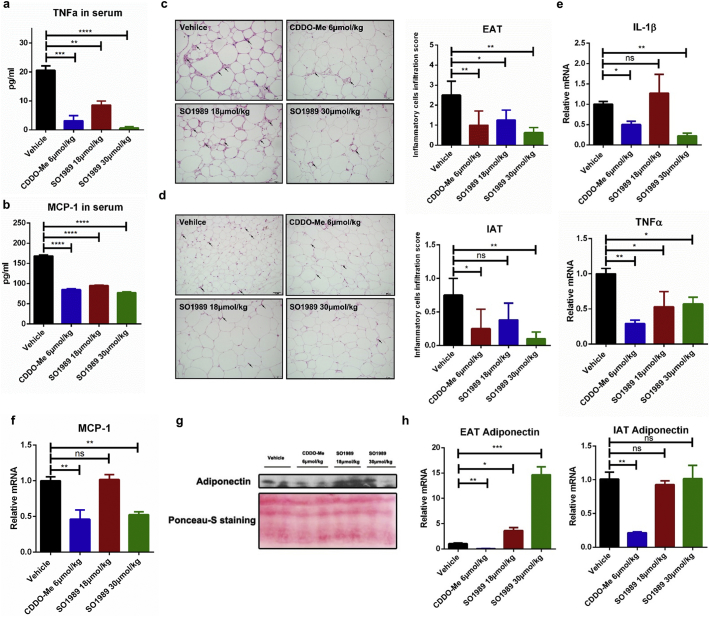
Excess fat accumulation causes chronic low-grade inflammation in white adipose tissues, which is considered a driving force of the metabolic abnormalities [18]. Herein, we further examined whether SO1989 mitigated the inflammation state in white adipose tissues. Upon the treatment with SO1989, the levels of the pro-inflammatory cytokine TNFα and chemokine MCP-1 in HFD-induced obese mice decreased in a dose-dependent manner (Fig. 2a–b). Histological analysis of two different white adipose depots indicating that epididymal adipose tissues (EAT) contained more infiltrated inflammatory cells than inguinal adipose tissues (IAT) in the obese mice, which was consistent with the fact that visceral fats were more inflammatory than subcutaneous fats thus visceral fats contributed more to the metabolic disorders [19,20]. The presented score showed that higher dosage of SO1989 inhibited more inflammatory cell infiltration comparing to CDDO-Me in both EAT and IAT (Fig. 2c–d) and significantly lowered the gene expression levels of pro-inflammatory cytokines IL-1β and TNFα as well as chemokine MCP-1 in EAT of HFD mice (Fig. 2e–f). Moreover, the level of adiponectin in serum, a key adipokine in mediating anti-inflammation and insulin sensitivity, was enhanced by SO1989 (Fig. 2g), whereas CDDO-Me failed to exhibit the similar effect. We also measured the gene expression of adiponectin in EAT and IAT and found SO1989 could up-regulate its expression in EAT but not in IAT (Fig. 2h). By contrast, CDDO-Me decreased the adiponectin mRNA level in both EAT and IAT. Together, these results indicate that SO1989 appears to be effective at alleviation for HFD-induced inflammation of white adipose in a dosage-dependent manner. Furthermore, the impaired secretion of the adiponectin secretion caused by HFD in obese mice, can be restored through SO1989 treatment.
Fig. 2.
SO1989 improves the white adipose inflammation. (a–b) The concentration of TNFα and MCP-1 in serum of HFD mice treated with vehicle, indicated doses of CDDO-Me or SO1989 for 15 days were quantified by ELISA assay according to manufacturer's instructions (n = 5–6). (c–d) Representative H&E staining showed adipose tissue morphology of the HFD-fed mice treated with the vehicle (0.1% DMSO), indicated doses of CDDO-Me or SO1989 for 15 days, original magnification ×200, (n = 5–6). Arrows indicated the inflammatory cells in adipose tissue. The Quantification of inflammatory cells infiltration for five sections/200× field, five to six fields/gland/mouse. (e–f) epididymal adipose tissues were homogenized and the total RNA was isolated by using Trizol reagent, followed by qRT-PCR with the indicated probes (n = 5). The 36B4 was used as an endogenous control. (g) The serum was collected and the protein concentration was quantified by using BCA assay kit. Every lane contains 30 μg total protein from three mice. Ponceau-S staining served as a loading control (n = 6). (h) Gene expression assay of adiponectin in epididymal adipose tissues (EAT) and inguinal adipose tissues (IAT) by qRT-PCR with the indicated probes (n = 6). All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
3.3. SO1989 inhibits pro-inflammatory macrophage functions in vitro and in vivo
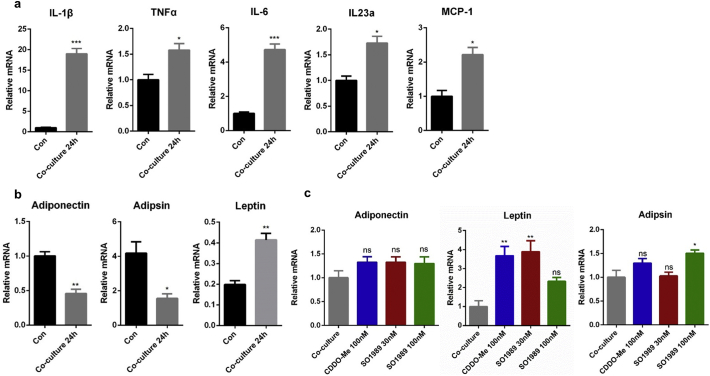
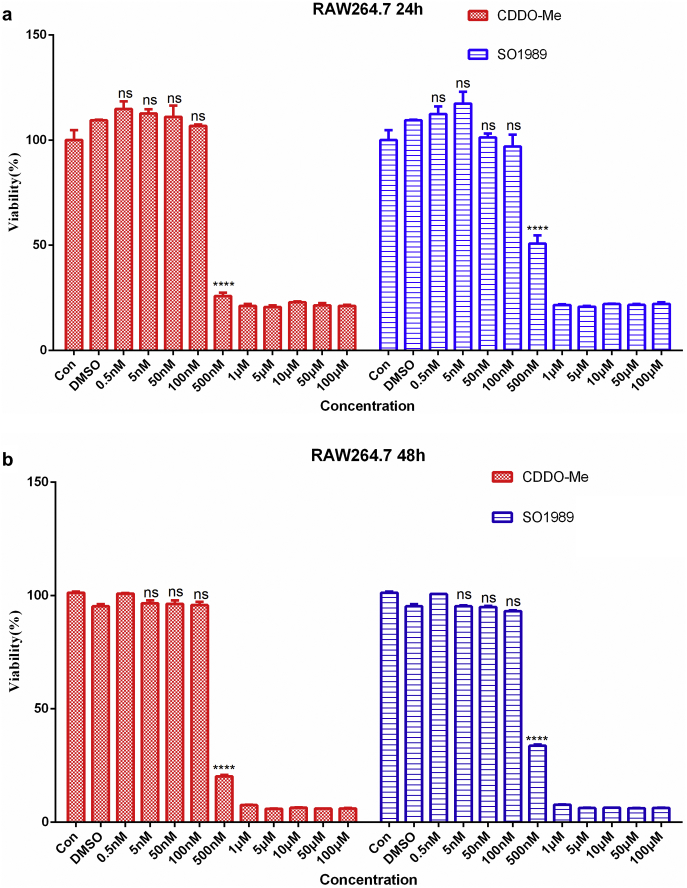
To further dig out the exact effects of SO1989 on adipose inflammation modulation, we use a co-culture model of adipocytes and macrophages to mimic the microenvironment in adipose tissue. The 3 T3-L1 cells were differentiated into mature adipocytes followed by the co-culturing with RAW264.7 cells [21]. The co-cultured RAW264.7 cell group showed a remarkable up-regulation of pro-inflammatory gene expression in RAW264.7 compared with control cells (Fig. S4a), and co-cultured 3T3-L1 adipocytes displayed gene expression dysregulation including upregulation of leptin and downregulation of both adiponectin and adipsin (Fig. S4b). These results demonstrated the pro-inflammatory cross-talk occurred between macrophages and adipocytes in the co-culture model. Meanwhile, we tested the effects of SO1989 on RAW264.7 cell viability and proliferation. The data indicated that 100 nM SO1989 is a suitable dose on cell model in vitro (Fig. S9). When treated with 100 nM SO1989, the gene expressions of several pro-inflammatory cytokines in co-cultured RAW264.7 such as IL-1β, TNFα, IL-6, were largely inhibited, while the same dosage of CDDO-Me had little effect (Fig. 3a). However, the adiponectin expression in 3T3-L1 was unchanged in contrast to the obvious enhancement of adiponectin expression in white adipose upon the SO1989 treatment in vivo (Fig. S4c). These results suggested that SO1989 suppressed the pro-inflammatory ATM phenotype in vitro, which might be a mechanism to explain the attenuation of white adipose inflammation by SO1989 treatment in HFD mice.
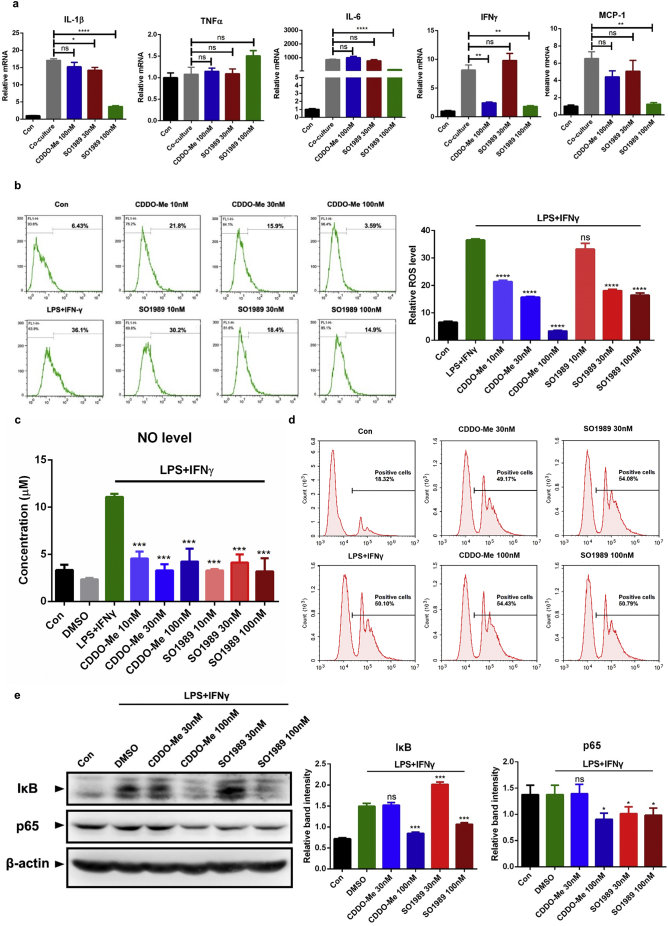
Fig. 3.
SO1989 inhibits the pro-inflammatory phenotype of RAW264.7 in vitro. (a) The indicated doses of CDDO-Me or SO1989 were added into the established co-culture model and incubated for 24 h. The expression levels of pro-inflammatory genes in RAW264.7 cells were analyzed by qRT-PCR. The β-actin was used as an endogenous control. (b) The RAW264.7 cells were stimulated by LPS and IFNγ with or without the incubation of indicated doses of CDDO-Me or SO1989 for 24 h. FACS analysis intracellular ROS levels of RAW264.7 cells and the quantification of FACS analysis. (c) The NO levels in the groups were analyzed by NO assay kit. (d) The phagocytosis of RAW264.7 cells examined by the uptake of microspheres and the quantification of FACS analysis. (e) The levels of IκB and p65 in RAW264.7 cells were analyzed by western blotting. The β-actin protein was shown as control. The quantification of relative band intensity was analyzed by Image J software. All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
In addition to releasing the pro-inflammatory cytokines, M1 polarized macrophages in obese adipose tissue can even phagocyte the hypertrophic adipocytes and release high level of ROS and NO to enhance the pro-inflammatory function. Therefore, we next evaluated the efficacy of SO1989 on inhibiting the pro-inflammatory function of M1 polarized macrophages. We pre-treated the RAW264.7 with different doses of CDDO-Me or SO1989 respectively for 3 h followed by the LPS and IFNγ stimulations for 24 h. The combination of LPS and IFNγ robustly triggered the NO release and the generation of endogenous ROS in RAW267.4 cells. SO1989 significantly inhibited ROS generation (Fig. 3b). The concentration of NO in cell culture supernatant were also markedly decreased with the treatment of SO1989 (Fig. 3c). However, neither CDDO-Me nor SO1989 could inhibit macrophage phagocytic activity trigged by LPS and IFNγ (Fig. 3d). Moreover, the protein levels of IκB and p65 in RAW264.7 cells was significantly decreased by SO1989 treatment (Fig. 3e), indicating the ability of SO1989 on inhibition of NF-κB pathway, a classical pro-inflammatory signaling in macrophages.
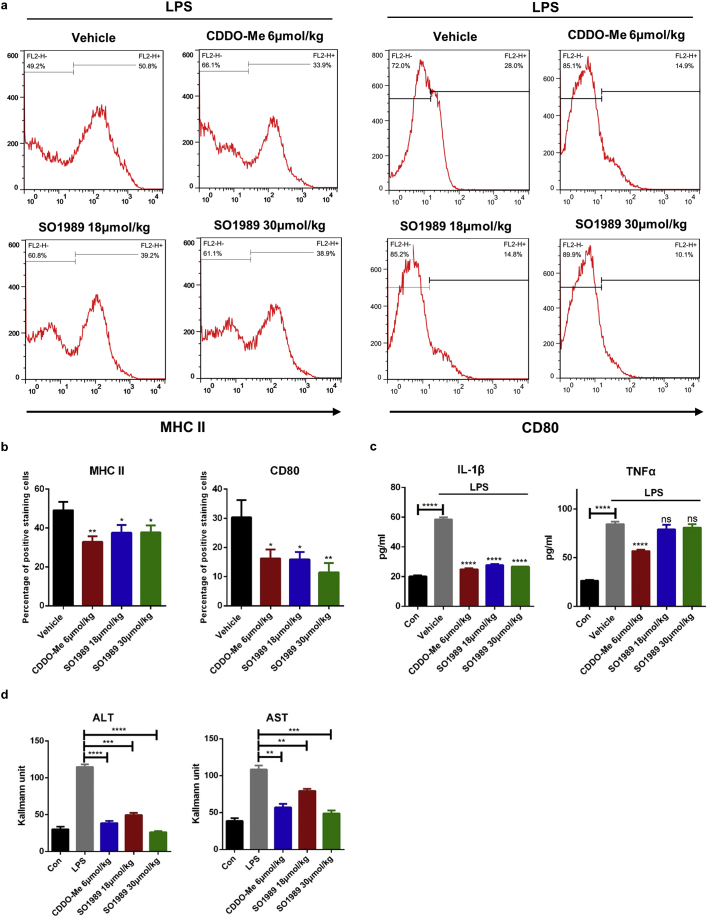
Next, to assess the efficiency of SO1989 on acute inflammation and macrophage polarization in vivo, balb/c mice were injected with LPS and the two compounds respectively. We observed that in both groups of mice, the peritoneal macrophages were resistant to LPS-induced M1 polarization (Fig. S5a–b). This phenomenon was associated with reduced levels of pro-inflammatory cytokines IL-1β and TNFα (Fig. S5c), as well as ALT and AST activity in serum (Fig. S5d), which are known as two classic indicators of liver injury. Thus, we concluded that SO1989 were capable of inhibiting LPS-induced pro-inflammatory macrophage function and attenuating acute inflammation in mice.
3.4. Impaired alternative polarization of macrophages can be restored by SO1989
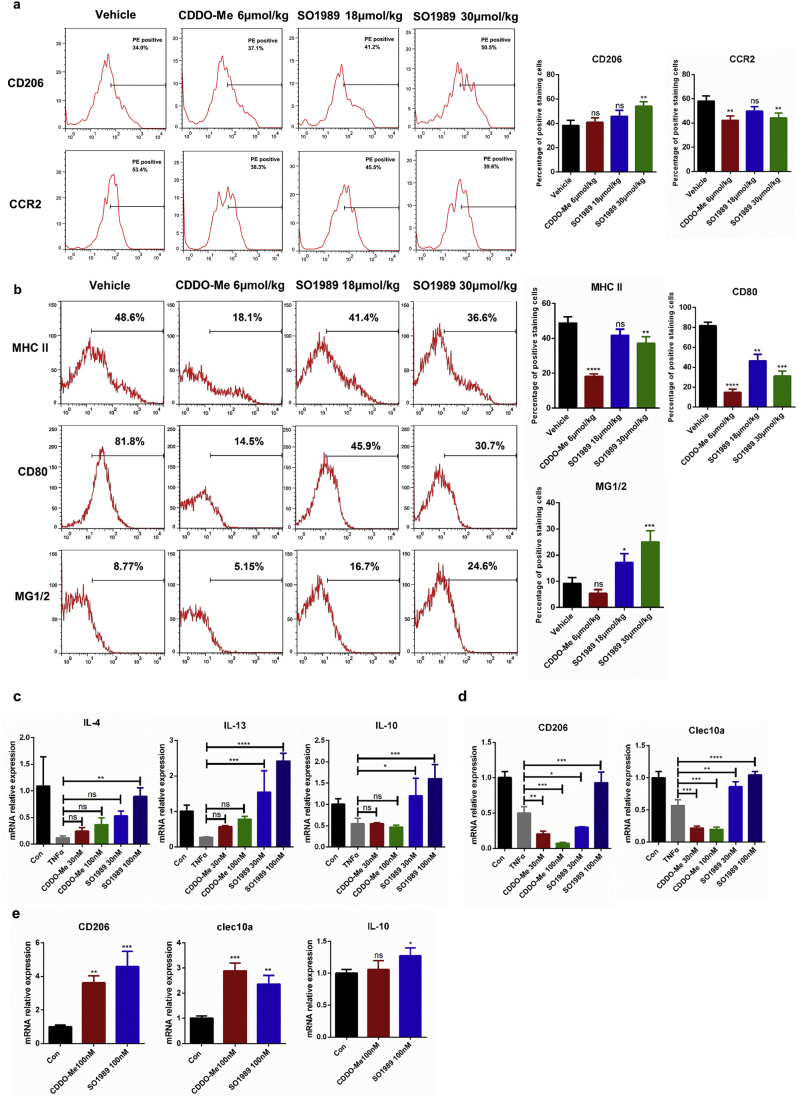
FACS analysis was used here to evaluate the polarization state of ATMs in SO1989-treated obese mice. Upon CDDO-Me and SO1989 administration, we observed significant decline of CCR2 expression, a M1 macrophage maker which was the receptor for the inflammatory chemokine CCL2, in the ATMs from epididymal adipose of both CDDO-Me and SO1989 treated mice. However, marked increase in CD206 expression, a M2 macrophage maker, was only noted in SO1989 treated mice (Fig. 4a). Similarly, the polarization state of PMs in these groups were also been evaluated by analyzing the expression of M1 polarized maker MHC II and CD80, and M2 polarized maker MG1/2. Consistently, we found that the proportion of M2 polarized PMs had remarkably increased after the administration of SO1989, while the CDDO-Me could only suppress the M1 polarization phenotype (Fig. 4b). Taken together, these results demonstrate that, besides the capacity of reducing inflammatory phenotype of macrophages, SO1989 is able to restore the HFD-induced malfunction of M2 macrophage polarization in vivo.
Fig. 4.
SO1989 regulates macrophage polarization in vivo and in vitro. (a) HFD-mice treated with the vehicle, indicated doses of CDDO-Me or SO1989 for 15 days. ATMs were sorted by both CD11b and F4/80 positive staining in SVF of EAT. The expression levels of surface markers (CD206 and CCR2) in ATMs were analyzed by FACS (n = 6). The statistical analysis of positive staining cells was based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM. (b) The peritoneal macrophages were isolated by CD11b magnetic beads and the M1/M2 surface markers (MHC II, CD80 and MG1/2) were analyzed by FACS (n = 6). The statistical analysis of positive staining cells was based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM. (c–d) The bone marrow derived macrophages (BMDMs) were isolated and differentiated by the previous protocol (n = 3). The BMDMs were pre-treated by 20 ng/ml TNFα for 12 h, then they were incubated with the indicated doses of CDDO-Me or SO1989 for 24 h. The gene expression levels of M1/M2 makers were analyzed by qRT-PCR with the indicated probes. (e) SVF cells were isolated from EAT, and the gene expression of M2 makers were quantified by qRT-PCR (n = 3). All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
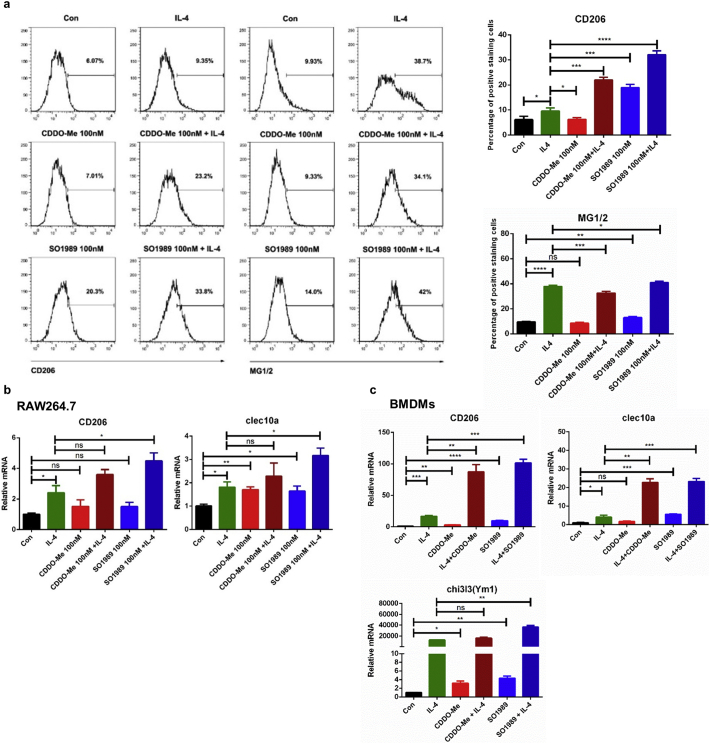
Obesity is characterized by elevated circulating and local levels of pro-inflammatory cytokines,such as TNFα and IL-6 etc. To further confirm whether SO1989 directly regulated the polarization state in macrophages upon the inflammation state, bone marrow derived macrophages (BMDMs) were pre-treated with TNFα for 24 h, followed by administration of SO1989 or CDDO-Me. We then analyzed the M2 phenotype gene expression of BMDMs to evaluate the efficiency of the two compounds on rescuing M2 phenotype polarization in vitro. As is shown in Fig. 4c, gene expressions of Th2 type cytokines, such as IL-4, IL-10, IL-13, were increased by SO1989 treatment in a dose-dependent manner. Typically, as a major cytokine playing a key role in subsiding inflammation, IL-10 expression could only be rescued and even enhanced in SO1989-treated group compared with the control group, but not in CDDO-Me-treated group. Although CDDO-Me upregulated the gene expressions of IL-4 and IL-13 stimulated by TNFα, the expression levels of the two cytokines were still lower than the control group. Despite of the concurrent enhanced expression levels of Th2 type cytokines by both drugs, the impairment of CD206 and clec10a gene expression, which are surface markers of M2 macrophages, could only be rescued by SO1989 treatment (Fig. 4d). Similar results were also obtained in primary SVCs (Stromal vessel cells), which were separated from adipose tissue of HFD mice and contained a large number of inflamed ATMs (Fig. 4e). Thus, these results according with the FACS results of HFD mice in vivo, proved that SO1989 directly restored the damaged M2 polarization of macrophages more efficiently than CDDO-Me. Interestingly, macrophage-like ANA-1 cells treated with SO1989 alone were already capable of inducing the M2 polarization effectively, while CDDO-Me treatment did not elicit such a response (Fig. S6a). Furthermore, in BMDMs and RAW264.7, SO1989 could further enhance the efficiency of IL-4 on inducing M2 polarization (Fig. S6b–c). Collectively, these data demonstrated that impaired alternative polarization of macrophages could be restored by SO1989 in vitro and in vivo, and the IL-4-induced M2 polarization in macrophages is also able to be further enhanced by SO1989.
3.5. SO1989 promotes mitochondria biogenesis and fatty acid oxidation to enhance M2 polarization in macrophages
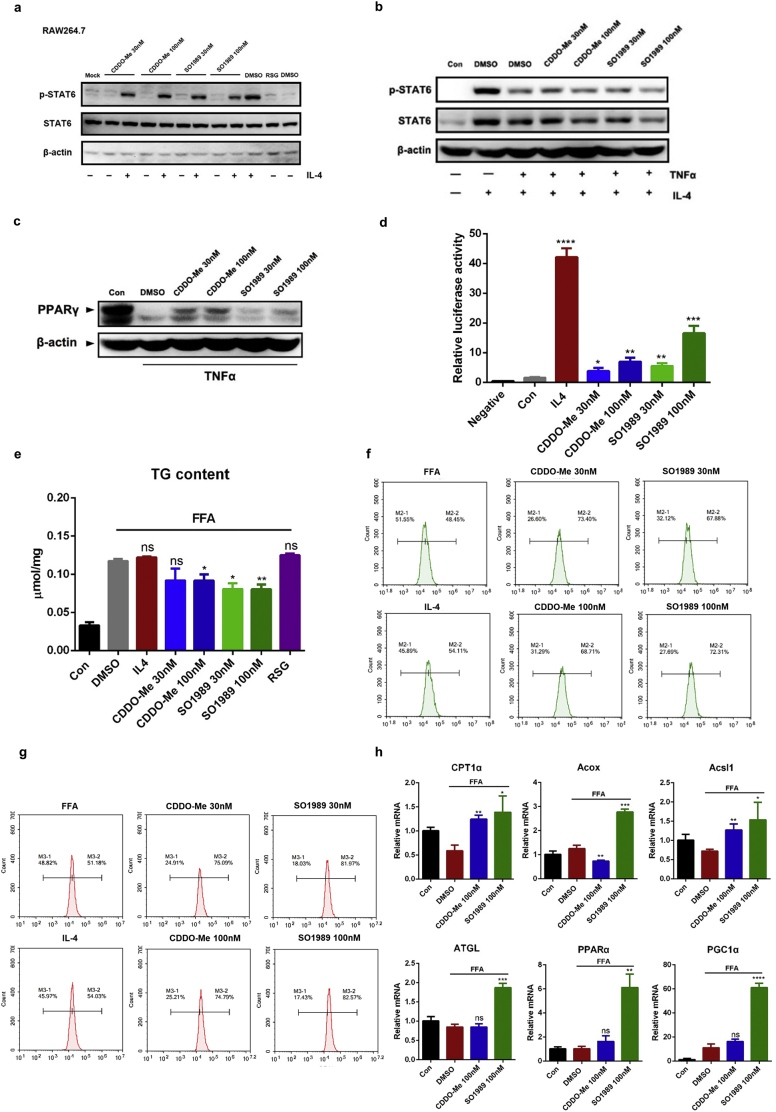
To elucidate the mechanism for an enhanced M2 polarized phenotype in macrophages in vitro and in vivo upon the SO1989 treatment, we first investigated the activation of major pathway associated with M2 polarization in macrophage: IL-4/STAT6 pathway. In RAW264.7 cells, the Y641 phosphorylation of STAT6, a maker of STAT6 activation, could only occur by IL-4 stimulation. Neither CDDO-Me nor SO1989 induced the STAT6 phosphorylation whether upon IL4 administration or not (Fig. 5a). In the TNFα-induced M1 polarization model, CDDO-Me and SO1989 failed to redeem STAT6 activity inhibited by TNFα (Fig. 5b). This suggested that the M2 polarization in macrophages by SO1989 treatment was unconnected with IL4/STAT6 pathway. Nevertheless, we did, find the restoration of PPARγ level upon the TNFα stimulation, which is another effector for IL-4/IL-13 and modulates the M2 polarization of macrophages, in both CDDO-Me and SO1989-treated group (Fig. 5c). Based on the previous studies showing that several derivatives from oleanolic acid are the ligands of PPARγ [22,23], we wondered whether SO1989 could initiate the transcriptional activity of PPARγ. Hence, we conducted the luciferase reporter gene assay in iBMDMs, a macrophage cell line derived from the BMDMs and easier to be transfected. We observed that SO1989 administration upregulated PPARγ transcriptional activity in a dose-dependent manner (Fig. 5d). This observation confirmed that the activation of PPARγ signaling pathway could be achieved through SO1989 treatment.
Fig. 5.
SO1989 promotes mitochondria biogenesis and fatty acid oxidation as well as activates the PPARγ activity. (a) RAW264.7 cells were treated by CDDO-Me or SO1989 for 24 h with or without the IL-4 administration. The levels of Y641 phosphorylation of STAT6 induced by IL-4 and endogenous STAT6 expression were analyzed by Western blotting. The β-actin protein was shown as control. (b) BMDMs were pre-treated by IL-4 with or without the TNFα stimulation for 12 h, then the cells were incubated with CDDO-Me or SO1989 for 24 h. The levels of Y641 phosphorylation of STAT6 induced by IL-4 and endogenous STAT6 expression were analyzed by Western blotting. The β-actin protein was shown as control. (c) The BMDMs were pre-treated by TNFα for 12 h, then the cells were incubated with CDDO-Me or SO1989 for 24 h (n = 4). The protein levels of PPARγ in all groups were detected by Western blotting. (d) Transcriptional activation of PPARγ in cells treated with the indicated dosages of CDDO-Me or SO1989. iBMDMs were transfected with pIRES-mPPARγ/PPRE and pRL-control using Lipofectamine 2000. Then cells were pre-treated with CDDO-Me or SO1989 for 24 h. Luciferase activities were measured by using the dual-luciferase reporter assay system. (e) RAW264.7 cells were pre-treated by 1 mM FFA for 24 h, the cells were then incubated in indicated dose of CDDO-Me or SO1989 for 12 h, and the concentration of triglyceride (TG) in cells were analyzed by TG detection kit. The contents of TG were normalized to the protein concentration of cell lysis. (f-g) RAW264.7 cells were pre-treated by 1 mM FFA for 24 h, the cells were then incubated in indicated dose of CDDO-Me or SO1989 for 12 h, and the mitochondrial mass (f) or membrane potential (g) were analyzed by FACS. (h) The expression levels of FAO-related genes were analyzed by qRT-PCR. All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
The phenomenon that SO1989 highly increased the FAO related genes in liver tissues indicated the profoundly effects of SO1989 on lipid catabolism. In fact, enhanced mitochondrial fatty acid oxidation (FAO) and oxidative phosphorylation contributed to the IL-4 triggered M2 polarization of macrophages and had benefits for insulin resistance in mouse model [24]. These findings suggest FAO plays a vital role in modulating macrophages polarization and response to inflammatory stimulation, so we speculate that SO1989 is more efficient than CDDO-Me to promote FAO. To investigate the effects of SO1989 on FAO in macrophages, RAW264.7 cells were pre-treated with FFA for 24 h to mimic the lipid metabolic dysregulation of ATMs in obese mice, and then incubated with CDDO-Me or SO1989 for 24 h. We began with the measurement of triglyceride (TG) content of RAW264.7 to analyze the FAO improvement in macrophages. As is shown in Fig. 5e, SO1989 reduced the TG content in FFA-stimulated RAW264.7 with the similar efficacy of CDDO-Me. Next, we performed the mitochondrial biogenesis assay by measuring mitochondrial mass alteration We observed CDDO-Me and SO1989 induced mitochondrial biogenesis to a similar degree (Fig. 5f), which displayed a negative correlation to the TG content. We then examined the mitochondrial activity by assessing membrane potential in the same model. SO1989 reinforced the mitochondrial activity and the enhancement of SO1989 was significantly higher than CDDO-Me (Fig. 5g), suggesting that SO1989 was likely to further strengthen the FAO in macrophages compared to CDDO-Me. To confirm the role of SO1989 in FAO enhancement, we continued analyzing the specific FAO gene expressions in macrophages. The expressions of β-oxidation genes, such as Acox1, Acsl1 and Cpt1a, were higher in SO1989-treated groups than CDDO-Me-treated group. In addition, mRNA levels of lipolysis enzyme (ATGL), FAO-related transcriptional factor (PPARα) and the co-activator (PGC1α) were all more increased by SO1989 treatment than CDDO-Me treatment (Fig. 5h). Taken together, these data indicates that SO1989 promotes M2 polarization of macrophages via activing PPARγ signaling pathway. The FAO enhancement by SO1989 in macrophages may further contribute to promotion of M2 polarization.
3.6. SO1989 exhibits less adverse effects than CDDO-Me
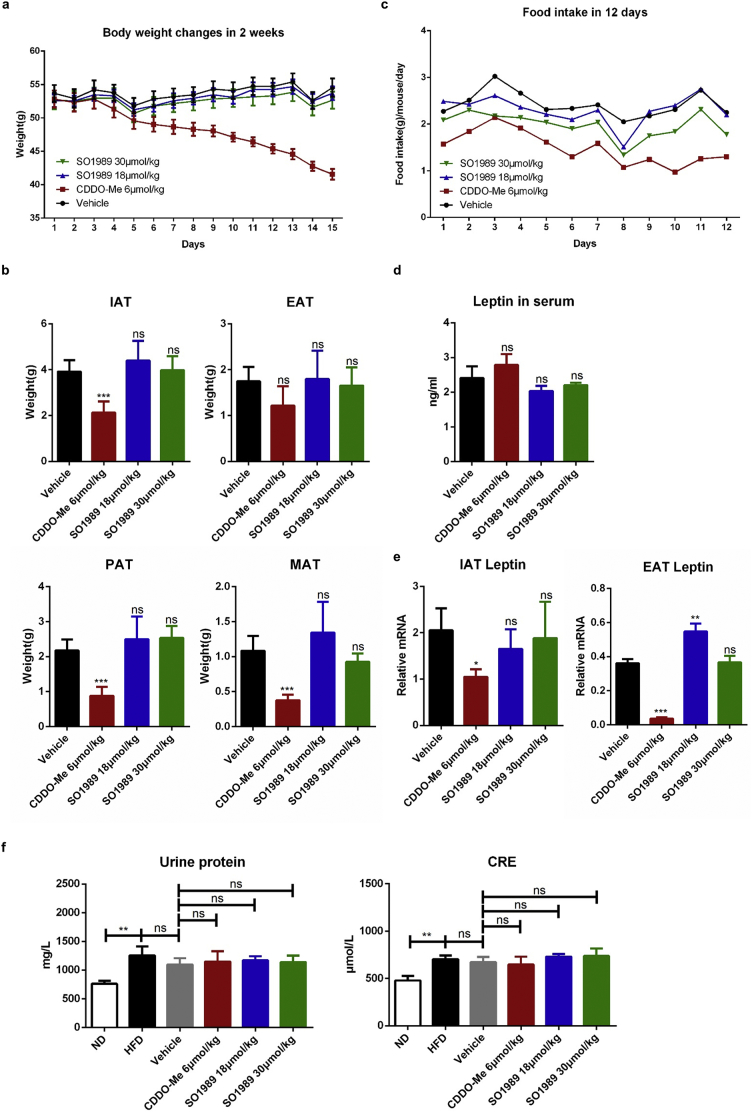
During the period of the treatment with CDDO-Me or SO1989 in vivo, we observed some side effects of CDDO-Me on HFD-induced obese mice. Administration of CDDO-Me led to noteworthy weight loss of HFD mice (Fig. 6a, Fig. S3g). In consistent, the mass of various white adipose tissues significantly decreased after the CDDO-Me treatment (Fig. 6b). The daily average food intake of each mouse in CDDO-Me–treated group was continuously inhibited during the period of the treatment (Fig. 6c, Fig. S3h). In contrast to CDDO-Me, SO1989 treatment did not cause obvious changes in body weight, fat mass and the appetite. However, the level of leptin in serum, an adipokine secreted by adipocyte to act on hypothalamus and inhibit the appetite, was unchanged under the treatment of CDDO-Me (Fig. 6d), though it even inhibited the gene expression of leptin in WAT (Fig. 6e). This indicated that the appetite inhibition by CDDO-Me was not attribute to the leptin's functions in central nervous system (CNS). We wondered whether CDDO-Me could alter the mouse BBB permeability, which might be related with compound crossing BBB. By utilizing Evans blue (EB) labeling, we tested blood-brain-barrier (BBB) intactness [25,26]. The staining intensity revealed that the mouse brains of different experimental groups failed to be stained by EB (Fig. S7), which indicated that neither CDDO-Me nor SO1989 could impact the BBB integrity. Thus, there should be an alternative mechanism involving in the appetite inhibition by CDDO-Me.
Fig. 6.
SO1989 did not exhibit obvious side effects on adipose and appetite in HFD mice in comparison with CDDO-Me. (a–b) HFD-mice treated with the vehicle, indicated doses of CDDO-Me or SO1989 for 15 days, the effect of CDDO-Me or SO1989 on body weight (a) and adipose weight (b) (n = 6). IAT:inguinal adipose tissue, EAT:epididymal adipose tissue,MAT:Mesenteric adipose tissue, PAT: perirenal adipose tissue. (c) The effect of CDDO-Me or SO1989 on food intake in HFD mice. For each mouse, average daily food intakes were calculated (n = 6). (d) The leptin levels in serum upon the CDDO-Me or SO1989 administration were detected by ELISA kit (n = 6). (e) The expression levels of leptin in EAT and IAT were analyzed by qRT-PCR (n = 6). (f) The mice were placed in metabolic cages and urine was collected. The levels of urine protein and CRE in serum were detected by commercial kit (n = 6). All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
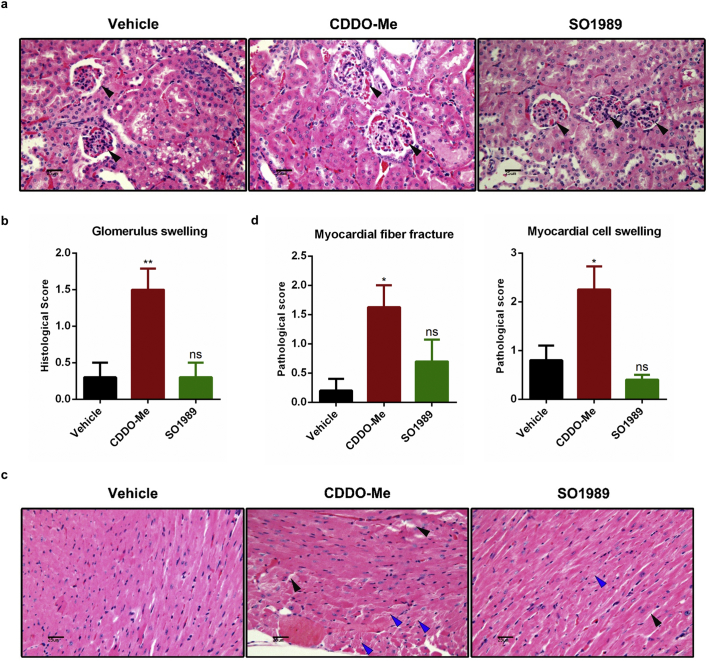
We further assess the renal and cardiac adverse effects of SO1989, which have been observed in clinical trials as CDDO-Me. Compared with the vehicle group, the concentrations of urine protein and creatinine in serum, the biological markers of kidney damage, were not changed after treatment with SO1989 and CDDO-Me (Fig. 6f). Next, we analyzed the histopathological patterns in kidney tissue sections. The data indicated that SO1989 treatment did not increase the glomerulus swelling in HFD mice, which was observed in CDDO-Me group (Fig. S8a–b). The pathological score of heart sections in SO1989 treatment group was also lower than that in CDDO-Me group (Fig. S8d). SO1989 caused fewer myocardial fiber fracture and myocardial cell swelling than CDDO-Me (Fig. S8c). The results indicated that SO1989 showed less cardiotoxicity and renal toxicity than CDDO-Me.
To sum up, SO1989 appears to be a safer modulator with lower adverse effects compared to CDDO-Me.
4. Discussion
CDDO-Me (Bardoxolone methyl), an activator of Keap1-Nrf2, was used as one of antioxidant inflammation modulators (AIMs). This compound had entered stage III clinical trial as an anti-DN (diabetic nephropathy) drug candidate and failed eventually due to its severe adverse effects [15,27]. Afterward, scientists have been trying to develop the novel derivatives of oleanolic acid in order to get powerful drug candidates with less side effects [[27], [28], [29]]. During drug development, an interesting discovery is that δ-oleanolic acid possesses higher bioactivity than oleanolic acid. This result suggests that δ-oleanolic acid can be a promising precursor compound for further structural optimization of CDDO-Me [30,31]. Given the fact that the δ-oleanolic acid content is much lower than oleanolic acid in plants, we established an efficient synthetic strategy for preparation of δ-oleanolic acid and its derivatives including SO1989 in our previous work [17]. SO1989 had been proven exerting an action on macrophage. The minimum IC50 value against NO release of RAW 264.7 induced by IFN-γ is 1.8 nM, suggesting that SO1989 can regulate macrophage's function. It is worthy to be mentioned that δ-oleanolic acid contains a double-bound in the D ring, which confers SO1989 defined characteristic and biological factions.
For figuring out the specific functions of SO1989, we evaluated the SO1989 efficiency in attenuating metabolic dysfunctions in HFD mice model based on a bunch of preliminary experimental outcomes. We found that SO1989 exhibited the different activity from that of CDDO-Me in alleviating hepatic steatosis. Unlike CDDO-Me, SO1989 highly upregulated the gene expression level associated with fatty acid oxidation in fat mice liver [14]. Moreover, SO1989 restored the adiponectin level in white adipose tissues and serum in HFD mice, although it reduced adipocyte hypertrophy slightly. These intriguing results provided us a hint that SO1989 has a different biological capacity compared with CDDO-Me. Generally, hypertrophic adipocytes have been thought producing less adiponectin and metabolic unhealthy [32]. However, in some cases, despite adipocytes is hypertrophy, the adipose is still healthy and keeps normal adiponectin level. The adipose microenvironment and genetic background of adipocytes are involved in regulating this biological process [33,34]. The mechanisms underlying the adiponectin generation is comprehensive. For instance, Khan T etc. reported that extracellular matrix (ECM) collapse could release the mechanical stress of adipocyte, leading to the adipocyte healthy expansion and released more adiponectin in both HFD and ob/ob mice [35]. The correlation between adipocyte size and adipokine secretion patterns still remains elusive. Here, we think that SO1989 reduced the inflammatory cell infiltration in adipose, which could probably be a cause for restoring adiponectin level. Besides, the appetite control of CDDO-Me is an accompanying phenomenon we have observed. We wondered at the first time whether CDDO-Me could alter the mouse BBB permeability, which might be related with compound crossing BBB. Thus, we performed an experiment to estimate BBB permeability by utilizing the Evans blue (EB) labeling method, which is the most common dye in evaluating the BBB integrity [25,26]. The result showed a negative outcome, suggesting that the BBB intactness was not impacted by CDDO-Me. Although this data is preliminary, it reminds us that there should be an intriguing mechanism involving in the food intake control by CDDO-Me, which needs next study to explore how CDDO-Me influences central nutrition sensing, whereby, regulating appetite regulation.
Macrophages are now regarded as prominent players in metabolic disorder and related diseases. Their sub-populations (M1-like and M2-like macrophages) may have either deleterious or protective functions towards inflammatory regulation depending on certain conditions of various inflammatory microenvironments [36]. In obese state, inflamed tissues produce more pro-inflammatory cytokines, triggering the tissue-resident macrophages polarize from M1-like to M2-like phenotype. The highly secretion of chemokines from inflammatory sites recruits monocytes to further differentiate to M1 macrophages, aggravating the local inflammation. Other studies have indicated that M2 activation ameliorates obesity-related inflammation and related insulin resistance [37,38]. There evidences indicated that M1/M2 status of macrophages represents one of the pivotal characteristics of the inflammatory state [39]. Thus, functionally regulating the balance between two subpopulation in tissues, for example, inhibiting pro-inflammatory functions of M1 macrophages and activating inflammation-resolving functions of M2 macrophages, is an efficient therapeutic strategy in treating metabolic inflammation [40,41]. In our investigation, we demonstrated the link between SO1989 and macrophages phenotype regulation in vitro and in vivo. SO1989 served a dual function in regulating macrophages: promoting the M2 polarization and inhibiting the M1 polarization. For ATMs, both SO1989 and CDDO-Me decreased proportion of CCR2 positive subpopulation in epididymal adipose tissue, suggesting the infiltration of monocyte-derived M1 polarized macrophages was inhibited. In the contrary, CD206 positive subpopulation, which represented the major adipose resident macrophages [42], was restored only by SO1989. Similarly, SO1989 highly rescued the M2 polarized PMs. These indicated that SO1989 could be more effective in regulating inflammation-resolving function of macrophages than CDDO-Me in obese state. In fact, the proresolution therapies has been considered superior to traditional anti-inflammatory strategy [3]. Accordingly, SO1989 could be a more effective modulator in immune cells, suggesting an advantages for using it in metabolic disorder therapy.
Although we can come up the conclusion that SO1989 enhances M2-prone polarization of macrophages both in vivo and in vitro, the underlying mechanisms remain elusive. Taking associated pathways in regulating M2 polarization into consideration [43,44], CDDO-Me and SO1989 similarly recovered the level of PPARγ after inflammatory stimulation and initiated PPARγ transcriptional activity in macrophages. Nevertheless, the explanations for the outcome that SO1989 appears higher activity in M2 polarization regulation are unknown. Metabolic remodeling lays the foundation for phenotype transformation of macrophages and associated functions in both physiological and pathological states [45]. The lipid metabolism plays a vital role in controlling the polarization state in response to exogenous stimulation, providing a clue that the metabolic regulation of lipid might explain the effects of SO1989 on M2 polarization [24]. Previous reports have shown that fatty acid oxidation (FAO) and oxidative phosphorylation are enhanced by the IL-4 treatment, contributing to M2 polarization in vitro and in vivo [46,47]. Accordingly, we performed quantitative analysis of triglyceride (TG) and free fatty acid (FFA) in macrophages in vitro to investigate whether SO1989 is involved in FFA oxidation modulation. As expected, SO1989 was more efficient in promoting TG breakdown than CDDO-Me in macrophages. The mitochondrial biogenesis, an indicator for FAO [48], and mitochondrial activity [49] were also enhanced by SO1989 treatment. These suggested that SO1989 was capable of remodeling the balance between lipid components and mitochondrial dynamics. In addition to mitochondrial remodeling, lipid catabolism still requires the modulation by related key regulators including metabolic enzymes, vital transcriptional factors and co-factors etc. Administration with SO1989 highly improved the expressions of β-oxidation, lipolysis enzyme associated genes. In addition, the mRNA levels of PPARα and the PGC1α, two key factors in regulating lipid catabolism profiling [[50], [51], [52]], were also strongly enhanced by SO1989 treatment. However, CDDO-Me induced expressions of FAO-related genes slightly. Thus, in macrophages, greater extent in mitochondria remodeling and lipid catabolism induction by SO1989 might be the reason for the M2 polarization enhancement in vitro and in vivo.
In summary, SO1989 is a promising modulator in efficiently ameliorating obesity-related inflammation and metabolic dysfunctions with lower side effects compared with CDDO-Me. Moreover, we explored the possible mechanism underlying the anti-inflammatory properties of SO1989 in aspect of modification in macrophage functional polarization. We also found that SO1989 was more effective than CDDO-Me in modulating macrophages polarization by a possible way of lipid catabolism regulation and mitochondrial remodeling. These finding indicated that SO1989 could be a prospective drug candidate for the treatment of obesity-related inflammation and related comorbidities, thereby providing a reasonable strategy for optimizing and developing more δ-oleanolic acid derivatives that shift macrophage polarization.
The following are the supplementary data related to this article.
Fig. S1.
Synthetic Route of SO1989.
Fig. S2.
Analytical data of SO1989
Fig. S3.
SO1989 improved the BAT and WAT functions in obese mice after 25 days treatment. (a) Representative H&E staining showed brown adipose tissue morphology of the HFD-fed mice treated with the vehicle (0.1% DMSO), indicated doses of CDDO-Me or SO1989 for 25 days, original magnification ×200. (b) The expressions levels of BAT activation genes in all three groups were analyzed by qRT-PCR. The 36B4 was used as an endogenous control. (c) Representative H&E staining showed EAT and IAT morphology of the HFD-fed mice treated with the vehicle (0.1% DMSO), indicated doses of CDDO-Me or SO1989 for 25 days, original magnification ×200. (d) The mRNA levels of adiponectin were quantified by real time PCR. The 36B4 was used as an endogenous control. (e) The activities of ALT and AST in serum were measured by ALT/AST assay kits. (f) The content of total triglyceride and cholesterol in serum were measured by using commercial kits according to manufacturer's instructions. (g-h) The body weight change and the food intake during the treatment period. (vehicle n = 5, CDDO-Me n = 4, SO1989 n = 5, in this figure). All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
Fig. S4.
Establishment of co-culture model of adipocytes and macrophages in vitro. (a) RAW264.7 cells were co-incubated with differentiated 3 T3-L1 adipocytes for 24 h. The expression levels of pro-inflammatory genes in macrophages were analyzed by qRT-PCR. The β-actin was used as an endogenous control. (b) The gene expressions of adipokines in 3 T3-L1 adipocytes were quantified and normalized to the 36B4 endogenous gene. The statistical analysis in (a-b) is based on unpaired Student's t-test. (c) Cultures were stimulated with CDDO-Me or SO1989 for one more 24 h, and the gene expression levels of adiponectin, leptin and adipsin were analyzed by qRT-PCR. The 36Β4 was used as an endogenous control. The statistical analysis in (c) is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
Fig. S5.
SO1989 alleviates the pro-inflammatory function of macrophages in vivo. (a–b) Male C57/B6J mice were pre-treated by CDDO-Me or SO1989 for 3 h, and the mice were further administrated by 25 mg/kg LPS for 12 h. The peritoneal macrophages were isolated by CD11b magnetic beads and the M1 surface markers (MHC II and CD80) were analyzed by FACS (n = 6). The statistical analysis of positive staining cells was based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM. (c) The concentrations of TNFα and IL-1β in serum were quantified by ELISA assay according to manufacturer's instructions (n = 6). (d) The activities of ALT and AST in serum were measured by ALT/AST assay kits (n = 6). All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
Fig. S6.
SO1989 enhances IL-4 induced M2 polarization in macrophages. (a) ANA-1 cells were treated with indicated dose of CDDO-Me or SO1989 for 24 h, the expression levels of surface markers (CD206 and MG1/2) in ANA-1 cells were analyzed by FACS. The statistical analysis of positive staining cells was based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM. (b) RAW264.7 cells were treated by the CDDO-Me or SO1989 for 24 h with or without IL4 administration. The expression levels of CD206, clec10a were analyzed by qRT-PCR. (c) The bone marrow derived macrophages (BMDMs) were isolated and differentiated by the previous protocol. The BMDMs were treated by IL4 for 24 h with or without the CDDO-Me or SO1989 administration (n = 3). The expression levels of CD206, clec10a and chi3l3 were analyzed by qRT-PCR. The statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
Fig. S7.
SO1989 could not impact the BBB permeability. Mice were treated with vehicle (0.1% DMSO), indicated CDDO-Me (6 μmol/kg) or SO1989 (30 μmol/kg) for 25 days, followed by the evans blue injection. The brains were isolated and the staining were observed under the bright field (n = 3).
Fig. S8.
SO1989 showed less adverse effects on kidney and hearts. (a) Representative H&E staining indicted glomerulus morphology of the HFD-fed mice treated with the vehicle (0.1% DMSO), indicated CDDO-Me (6 μmol/kg) or SO1989 (30 μmol/kg) for 25 days, original magnification ×400. Arrow represented the glomerulus. (b) The histological score of glomerulus swelling. The Quantification five sections/400× field, five to six fields/mouse, score according to the grade of swelling, slight (0.5), mild (1), moderate (2), severe (3), profound severe (4) and normal (0). (c) Representative H&E staining indicted myocardial fiber morphology of the HFD-fed mice treated with the vehicle (0.1% DMSO), indicated CDDO-Me (6 μmol/kg) or SO1989 (30 μmol/kg) for 25 days, original magnification ×400. Black arrow represented the myocardial fiber fracture and the blue arrow indicated the myocardial cell swelling. (d) The Quantification five sections/400× field, five to six fields/mouse, score according to the grade of swelling, slight (0.5), mild (1), moderate (2), severe (3), profound severe (4) and normal (0). (vehicle n = 5, CDDO-Me n = 4, SO1989 n = 5, in this figure). All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
Fig. S9.
The effects of SO1989 on cell viability in RAW264.7. (a) RAW264.7 cells were treated by the indicated dose of SO1989 or CDDO-Me for 24 h followed by the MTT test. (b) RAW264.7 cells were treated by the indicated dose of SO1989 or CDDO-Me for48h followed by the MTT test. All statistical analysis is based on one-way ANOVA. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are presented as mean ± SEM.
Antibodies used in this article.
Primers for Q-PCR.
Synthesis of SO1989.
Author contributions
S.P.P., S.H.B. and Y.N.F. designed the research and analyzed the data. Y.N.F., T.Q., Q.W.T., L.Z.Y. and G.X.Y. performed the experiments. C.X. assessed the BBB integrity. Z.W.J., W.D.D. and L.Y. provided scientific suggestions and contributed to the manuscript revision. S.P.P. wrote the manuscript and supervised the project. All authors critically reviewed the article and approved the final manuscript.
Declaration of Competing Interest
None.
Acknowledgements
We are grateful to Prof. Feng Shao (National Institute of Biological Sciences, Beijing, 102206, China) for kindly providing iBMDMs. This research was financed by grants from the National Key Research and Development Plan (2017YFA0506000, 2017YFA0205400) and National Natural Science Foundation of China (81673439) and Natural Science Fund Project in Jiangsu Province (BK20161408).
Contributor Information
Hongbin Sun, Email: hongbinsun@cpu.edu.cn.
Pingping Shen, Email: ppshen@nju.edu.cn.
References
- 1.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Brestoff J.R., Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161(1):146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fullerton J.N., Gilroy D.W. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 4.Udalova I.A., Mantovani A., Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(8):472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 5.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNelis J.C., Olefsky J.M. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15(9):639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 10.Liby K.T., Sporn M.B. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64(4):972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad R., Raina D., Meyer C., Kharbanda S., Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281(47):35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 12.Impellizzeri D., Esposito E., Attley J., Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD) Pharmacol Res. 2014;81:91–102. doi: 10.1016/j.phrs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Dinh C.H., Szabo A., Camer D., Yu Y., Wang H., Huang X.F. Bardoxolone methyl prevents fat deposition and inflammation in the visceral fat of mice fed a high-fat diet. Chem Biol Interact. 2015;229:1–8. doi: 10.1016/j.cbi.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Saha P.K., Reddy V.T., Konopleva M., Andreeff M., Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem. 2010;285(52):40581–40592. doi: 10.1074/jbc.M110.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abboud H.E. Synthetic oleanane triterpenoids: magic bullets or not? Kidney Int. 2013;83(5):785–787. doi: 10.1038/ki.2013.38. [DOI] [PubMed] [Google Scholar]
- 16.nephropathy Rossing P. Diabetic. Could problems with bardoxolone methyl have been predicted? Nat Rev Nephrol. 2013;9(3):128–130. doi: 10.1038/nrneph.2013.13. [DOI] [PubMed] [Google Scholar]
- 17.Chen D., Zhang P., Sun Y., Wang P., Zhang C., Kong L. Protonated montmorillonite-mediated highly specific isomerization of oleanolic acid esters: application to the synthesis of Delta(13(18))-CDDO-Me. Org Biomol Chem. 2016;14(47):11154–11161. doi: 10.1039/c6ob02126c. [DOI] [PubMed] [Google Scholar]
- 18.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano A., Frontini A., Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov. 2016;15(6):405–424. doi: 10.1038/nrd.2016.31. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos N., Katritsis D., Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233(1):104–112. doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 21.De Boer A.A., Monk J.M., Robinson L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chintharlapalli S., Papineni S., Konopleva M., Andreef M., Samudio I., Safe S. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol. 2005;68(1):119–128. doi: 10.1124/mol.105.011437. [DOI] [PubMed] [Google Scholar]
- 23.WWP Yongping Wang*, Suh Nanjoo, Honda Tadashi, Gribble Gordon W., Leesnitzer Lisa M., Plunket Kelli D. A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor γ. Mol Endocrinol. 2000;14(10):1550–1556. doi: 10.1210/mend.14.10.0545. [DOI] [PubMed] [Google Scholar]
- 24.Namgaladze D., Brune B. Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim Biophys Acta. 2016;1861(11):1796–1807. doi: 10.1016/j.bbalip.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Tang X., Liu K., Hamblin M.H., Xu Y., Yin K.J. Genetic deletion of Kruppel-like factor 11 aggravates ischemic brain injury. Mol Neurobiol. 2018;55(4):2911–2921. doi: 10.1007/s12035-017-0556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou Z., Rong X., Zhao E., Zhang L., Lv Y. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gut-brain axis. Cell Mol Neurobiol. 2019;39(6):883–898. doi: 10.1007/s10571-019-00687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z., Mou Y., Xu X., Zhao D., Lai Y., Xu Y. Novel derivative of bardoxolone methyl improves safety for the treatment of diabetic nephropathy. J Med Chem. 2017;60(21):8847–8857. doi: 10.1021/acs.jmedchem.7b00971. [DOI] [PubMed] [Google Scholar]
- 28.Wong M.H., Bryan H.K., Copple I.M., Jenkins R.E., Chiu P.H., Bibby J. Design and synthesis of irreversible analogues of bardoxolone methyl for the identification of pharmacologically relevant targets and interaction sites. J Med Chem. 2016;59(6):2396–2409. doi: 10.1021/acs.jmedchem.5b01292. [DOI] [PubMed] [Google Scholar]
- 29.Zheng S., Santosh Laxmi Y.R., David E., Dinkova-Kostova A.T., Shiavoni K.H., Ren Y. Synthesis, chemical reactivity as Michael acceptors, and biological potency of monocyclic cyanoenones, novel and highly potent anti-inflammatory and cytoprotective agents. J Med Chem. 2012;55(10):4837–4846. doi: 10.1021/jm3003922. [DOI] [PubMed] [Google Scholar]
- 30.Norihiro B., Toshihiro A., Harukuni T., Ken T., Yosuke T., Hiroyuki A. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull. 2005;28(10):1995–1999. doi: 10.1248/bpb.28.1995. [DOI] [PubMed] [Google Scholar]
- 31.Takashi K., Hiroyuki A., Keiichi T., Aranya M., Jiradej M., Takashi S. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem Pharm Bull. 2011;59(3):378–381. doi: 10.1248/cpb.59.378. [DOI] [PubMed] [Google Scholar]
- 32.Fasshauer M., Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin T., Deng A., Yee G., Lamendola C., Reaven G., Tsao P.S. Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia. 2010;53(2):369–377. doi: 10.1007/s00125-009-1496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaughlin T., Sherman A., Tsao P., Gonzalez O., Yee G., Lamendola C. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(8):1707–1715. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 35.Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinetti-Gbaguidi G., Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22(5):365–372. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., Morel C.R., Subramanian V., Mukundan L. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patsouris D., Li P.P., Thapar D., Chapman J., Olefsky J.M., Neels J.G. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng X., Weng D., Zhou F., Owen Y.D., Qin H., Zhao J. Activation of PPARgamma by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine. 2016;9:61–76. doi: 10.1016/j.ebiom.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szanto A., Balint B.L., Nagy Z.S., Barta E., Dezso B., Pap A. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33(5):699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S.C., Everts B., Ivanova Y., O'Sullivan D., Nascimento M., Smith A.M. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vats D., Mukundan L., Odegaard J.I., Zhang L., Smith K.L., Morel C.R. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150(3):620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho K.W., Kim Y.O., Andrade J.E., Burgess J.R., Kim Y.-C. Dietary naringenin increases hepatic peroxisome proliferators–activated receptor α protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr. 2011;50(2):81–88. doi: 10.1007/s00394-010-0117-8. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Z., Xu D., Wang Z., Wang L., Han R., Wang Z. Hepatic PPARalpha function is controlled by polyubiquitination and proteasome-mediated degradation through the coordinated actions of PAQR3 and HUWE1. Hepatology. 2018;68(1):289–303. doi: 10.1002/hep.29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besse-Patin A., Jeromson S., Levesque-Damphousse P., Secco B., Laplante M., Estall J.L. PGC1A regulates the IRS1:IRS2 ratio during fasting to influence hepatic metabolism downstream of insulin. Proc Natl Acad Sci. 2019:201815150. doi: 10.1073/pnas.1815150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibodies used in this article.
Primers for Q-PCR.
Synthesis of SO1989.