Significance
While androgen deprivation therapy (ADT) has been the mainstay of treatment for advanced prostate cancer (PCa) leading to initial response and durable remission, incurable castration-resistant prostate cancer (CRPC) invariably develops. Importantly, androgen receptor (AR) activity remains critical for CRPC tumor growth. Despite the significant research advances in PCa biology and development of next-generation antiandrogens, there has been limited progress in the management of CRPC when direct AR-targeted therapies fail. PARP-2, which enhances AR-mediated transcription through interaction with the pioneer factor FOXA, is a druggable target. Targeting PARP-2 may potentially provide an alternative therapeutic approach for AR inhibition without involving AR ligand binding.
Keywords: PARP-2, PARP inhibitor, androgen receptor, FOXA1, prostate cancer
Abstract
Androgen receptor (AR) is a ligand-activated transcription factor and a key driver of prostate cancer (PCa) growth and progression. Understanding the factors influencing AR-mediated gene expression provides new opportunities for therapeutic intervention. Poly(ADP-ribose) Polymerase (PARP) is a family of enzymes, which posttranslationally modify a range of proteins and regulate many different cellular processes. PARP-1 and PARP-2 are two well-characterized PARP members, whose catalytic activity is induced by DNA-strand breaks and responsible for multiple DNA damage repair pathways. PARP inhibitors are promising therapeutic agents that show synthetic lethality against many types of cancer (including PCa) with homologous recombination (HR) DNA-repair deficiency. Here, we show that, beyond DNA damage repair function, PARP-2, but not PARP-1, is a critical component in AR transcriptional machinery through interacting with the pioneer factor FOXA1 and facilitating AR recruitment to genome-wide prostate-specific enhancer regions. Analyses of PARP-2 expression at both mRNA and protein levels show significantly higher expression of PARP-2 in primary PCa tumors than in benign prostate tissues, and even more so in castration-resistant prostate cancer (CRPC) tumors. Selective targeting of PARP-2 by genetic or pharmacological means blocks interaction between PARP-2 and FOXA1, which in turn attenuates AR-mediated gene expression and inhibits AR-positive PCa growth. Next-generation antiandrogens act through inhibiting androgen synthesis (abiraterone) or blocking ligand binding (enzalutamide). Selective targeting of PARP-2, however, may provide an alternative therapeutic approach for AR inhibition by disruption of FOXA1 function, which may be beneficial to patients, irrespective of their DNA-repair deficiency status.
Androgen receptor (AR)-mediated gene expression plays a key role in prostate cancer (PCa) growth and progression. Androgen deprivation therapy (ADT) is an effective treatment for advanced PCa. However, patients who initially respond to the therapy inevitably develop incurable castration-resistant prostate cancer (CRPC). The next-generation antiandrogens have shown benefits for CRPC patients; the increased length of survival, however, is measured in months (1, 2). While AR-independent mechanisms have been identified (such as glucocorticoid receptor activation and neuroendocrine transdifferentiation), the growth of therapy-resistant tumor is still largely AR-dependent due to AR amplification, point mutations, splice variants, and ligand-independent AR activation (3). Notably, AR cistrome and transcriptional activity have been studied in great detail to be prominently dictated by the pioneer factor FOXA1 (4). FOXA1 binds the enhancer regions in the genome, increases local chromatin accessibility, and facilitates the recruitment of AR (5, 6). Interestingly, genome sequencing studies have revealed that FOXA1 is one of the most frequently mutated genes in primary PCa and even more common in metastatic CRPC (7, 8). Aberrant FOXA1 function is implicated in PCa development and progression, likely through its impact on AR cistrome. Therefore, inhibition of AR through targeting FOXA1 is an attractive therapeutic approach for CRPC.
Poly(ADP-ribose) polymerase (PARP) is a family of enzymes that uses NAD+ as a substrate to synthesize and transfer ADP-ribose polymers onto target proteins. This posttranslational modification is known as poly(ADP-ribosyl)ation (PARylation), which is involved in various cellular processes, including DNA damage repair, modulation of chromatin structure, transcription regulation, and cell division (9). PARP inhibitors (PARPis) are a new type of targeted therapy, which works based on a concept of synthetic lethality. Cancer cells lacking BRCA1 or BRCA2 depend instead on PARP-regulated DNA repair and are hypersensitive to PARP inhibition (10, 11). The US Food and Drug Administration (FDA) has approved four PARPis (olaparib, niraparib, talazoparib, and rucaparib) for the treatment of advanced ovarian cancer and breast cancer patients with BRCA mutations. Recent clinical trials have also shown promising results of olaparib in the treatment of metastatic CRPC patients who have homologous recombination (HR) DNA-repair deficiency (12, 13). It should be noted that current clinically used PARPis target both PARP-1 and PARP-2. The mechanisms of action of PARPis with regard to their effects in cancer cells is largely based on PARP-1 function. PARP-2 is the closest paralog of PARP-1. Biochemical and genetic studies have provided strong evidence of key shared functions of PARP-1 and PARP-2 in response to DNA-strand breaks although PARP-2 contributes only 10% of total cellular PARP enzymatic activity (14, 15). Despite functional redundancy, studies have shown that PARP-2 has unique biological functions distinct from PARP-1. Genetic disruption of PARP-2, but not of PARP-1, affects specific differentiation processes, including adipogenesis, the survival of thymocytes, and spermatogenesis (16–18). In addition, PARP-2 is associated with different protein complexes and implicated in the maintenance of heterochromatin integrity of centromeres, telomeres, and X chromosome inactivation (19–21). It has been increasingly shown that PARP-2 is involved in transcriptional regulation. PARP-2 acts as a cofactor for several transcription factors, including nuclear receptors such as peroxisome proliferator-activated receptors (PPARs) and estrogen receptor (ER) α (16, 22, 23). Thus, it is conceivable that the clinical application of PARPi for cancer treatment will require further understanding of the specific functions of PARP-1 and PARP-2.
While studies have linked both PARP-1 and PARP-2 with PCa development and progression, the distinct roles of PARP-1 vs. PARP-2 remain unclear. It was reported that PARP-1 and the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) interact with TMPRSS2:ERG gene fusion, which is required for ERG-mediated transcription and cell invasion in PCa (24). Beyond DNA-repair function, PARP-1 was found to play a role in regulating AR target genes by promoting AR recruitment to the promoters of its target genes (25). A recent study also showed that PARP1 and PARP2 are involved in AR variant driven transcription in CRPC cells (26). In a genetic epidemiological study, we explored genetic signatures that predispose patients to aggressive PCa and revealed a link between genetic variants in DNA-repair genes (including notably PARP-2) and aggressiveness of PCa (27). This led us to study the role of PARP-2 in PCa oncogenesis. In the present study, we have discovered a functional connection between PARP-2, AR, and FOXA1 in PCa cells. We demonstrate that PARP-2 is a key component in AR-mediated transcription through interacting with the pioneer factor FOXA1 and is required for PCa growth.
Results
Overexpression of PARP-2 Is Associated with PCa Aggressiveness.
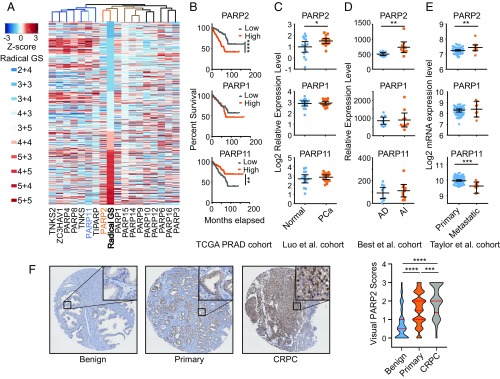
To explore the role of PARP proteins in PCa oncogenesis, we performed a metaanalysis on the mRNA expression levels of all 17 PARP members in primary PCa tumor samples from The Cancer Genome Atlas (TCGA) dataset (28). An unsupervised hierarchical clustering analysis showed that the expression of PARP-2 had the highest correlation with radical prostatectomy Gleason scores (R = 0.382, P = 1.07e−18) (Fig. 1A), followed by the expression of PARP-1 (R = 0.201, P = 6.09e−6). Interestingly, the expression of PARP-11 (R = −0.227, P = 3.02e−7) had a negative correlation with the aggressiveness of PCa. Survival analysis showed that overexpression of PARP-2, but not PARP-1, was significantly associated with PCa biochemical recurrence (Fig. 1B). In contrast, overexpression of PARP-11 was significantly associated with a better clinical outcome, indicating a tumor-suppressing role. In line with TCGA data, the PARP-2 mRNA levels were significantly elevated in PCa tumors compared with normal controls and further increased in androgen-independent primary tumors and in metastatic tumors using several publicly available PCa datasets (Fig. 1 C–E) (29–31). We further applied immunohistochemistry (IHC) assays to measure PARP-2 protein expression in a set of PCa tissue microarrays (TMAs) containing 1,129 tissue cores (Fig. 1F and SI Appendix, Fig. S1). Significantly higher PARP-2 protein expression was observed in primary PCa tumors compared with benign prostate tissues. While the PARP-2 protein levels were relatively similar between various Gleason groups among primary PCa tumors, we found much higher signal intensity of PARP-2 in the CRPC group. These results strongly support that PARP-2 plays an important role in PCa transformation and progression in contrast to other PARP members.
Fig. 1.
PARP-2 is overexpressed in PCa. (A) Association of the mRNA expression of PARP family members with PCa radical prostatectomy Gleason score (GS) in TCGA dataset (n = 499). Each row represents an individual tumor sorted by GS. The gene expression levels are presented by Z-score values. (B) Kaplan—Meier plot of biochemical recurrence-free survival proportion of all patients in TCGA dataset with low (n = 245) or high (n = 246) expression levels of PARP-1, PARP-2, and PARP-11. (C–E) The relative mRNA expression levels of PARP-1, PARP-2, and PARP-11 in normal prostate tissues (n =15) vs. PCa tumors (n = 15); androgen-dependent (AD) (n = 10) vs. androgen-independent (AI) (n =10) tumors; and primary (n = 76) vs. metastatic (n = 9) tumors. Data represent mean ± 95% CI. (F) immunohistochemistry (IHC) analysis of PARP-2 protein expression on tissue microarrays (TMAs) composed of benign prostate tissues (n = 232), primary PCa (n = 819), and CRPC (n = 78) tumor cores. Representative IHC images (original magnification 4×; Insets, original magnification 20×) are presented (Left). The expression levels of PARP-2 were quantified by visual scoring and shown in a violin plot (Right). Red solid lines represent the median, and dashed lines are the 25th and 75th percentile. Statistical significance was determined by Kruskal–Wallis test followed by Tukey’s test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
PARP-2 Is Required for the Growth of PCa Cells In Vitro and In Vivo.
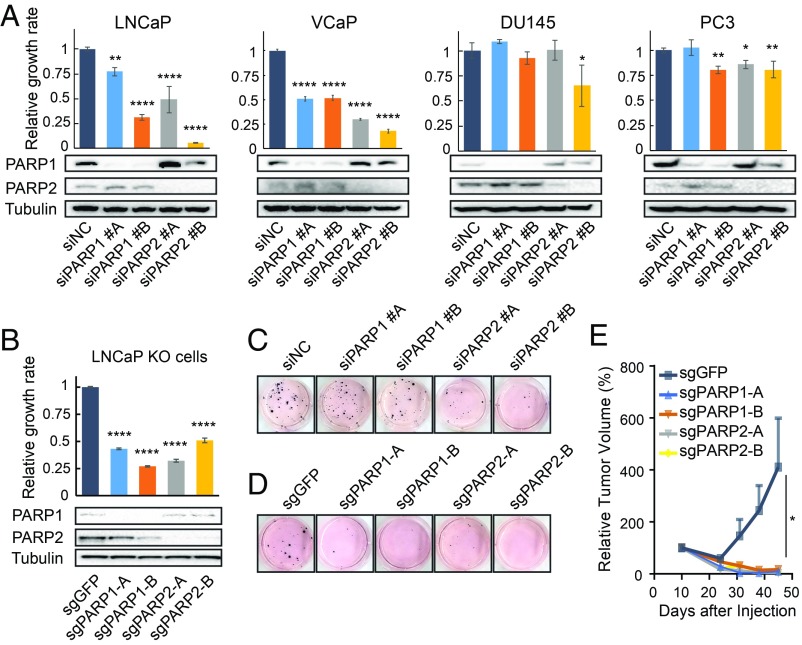
Next, we assessed the impact of PARP-2 on the growth of four PCa cell lines in comparison with PARP-1 using genetic approaches. Transient siRNA knockdown (KD) of either PARP-1 or PARP-2 markedly suppressed the growth of AR-positive LNCaP and VCaP cells but had a limited effect on AR-negative DU145 and PC-3 cells (Fig. 2A). We then established PARP-1 and PARP-2 knockout (KO) LNCaP cell lines using clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) gene editing. Consistently, KO of PARP-1 or PARP-2 significantly inhibited LNCaP cell growth (Fig. 2B). This was further confirmed in colony formation assays (SI Appendix, Fig. S2). In addition, the loss of PARP-1 or PARP-2 in LNCaP cells evidently decreased their anchorage-independent growth (Fig. 2 C and D). We observed a greater inhibitory effect from PARP-2 KD compared with PARP-1 KD using gene-specific siRNAs. To determine the oncogenic role of PARP-2 in vivo, we implanted the PARP-1 and PARP-2 KO LNCaP cell lines subcutaneously (s.c.) into immunodeficient mice and found that depletion of either PARP-1 or PARP-2 gene completely diminished tumorigenic potential of LNCaP cells (Fig. 2E).
Fig. 2.
PARP-2 is required for PCa growth. (A) Relative growth rate of PCa cells transfected with two independent PARP-1 or PARP-2 siRNAs compared with nonspecific control (NC) siRNA (Upper). Gene knockdown (KD) efficiency was determined by Western blot (Lower). (B) Effect of PARP-1 or PARP-2 gene knockout (KO) on LNCaP cell proliferation using two independent sgRNAs (Upper). Gene KO was confirmed by Western blot (Lower). (C) Anchorage-independent growth of LNCaP cells after PARP-1 or PARP-2 KD. (D) Anchorage-independent growth of LNCaP cells after PARP-1 or PARP-2 KO. (E) Relative tumor volume of PARP-1 and PARP-2 KO LNCaP xenografts in vivo (10 mice per group). The GFP KO LNCaP cell line was used as a control. All data represent the mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons to the control group. *P < 0.05; **P < 0.01; ****P < 0.0001.
PARP-2 Is Critical for AR-Mediated Transcription.
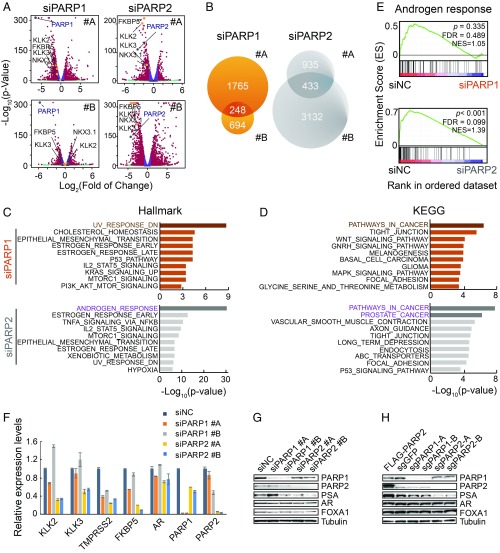
Although PARP-1 and PARP-2 account for ∼90% and ∼10% of total cellular enzymatic activity (or PARylation), respectively (14, 15), depletion of PARP-2 had comparable inhibitory effects, if not better, on LNCaP and VCaP cell growth in contrast to depletion of PARP-1. The inconsistency between their biological outcomes and enzymatic activity suggested that PARP-2 acts in a way distinct from PARP-1. To reconcile the mechanistic differences between these two proteins, we analyzed global gene expression changes after PARP-1 and PARP-2 KD in LNCaP cells using RNA-sequencing (RNA-seq). As shown in the volcano plot, well-characterized AR target genes, such as KLK2, KLK3/PSA, FKBP5, and TMPRSS2, topped the genes that were significantly suppressed after PARP-2 but not PARP-1 KD (Fig. 3A). We defined PARP-1– and PARP-2–regulated genes, respectively, by overlapping the differentially expressed genes generated from two independent siRNAs (Fig. 3B) (Dataset S1). Only a small fraction of genes were coregulated by both PARP-1 and PARP-2.
Fig. 3.
PARP-2 is critical for AR-mediated transcription. (A) Gene expression profiling of LNCaP cells after PARP-1 or PARP-2 siRNA KD for 72 h with two independent siRNAs. Differentially expressed genes with fold change >1.75 and FDR <0.01 are labeled in red; four classic AR target genes are highlighted in orange; PARP-1 and PARP-2 are marked in gray. (B) PARP-1– and PARP-2–regulated genes are identified by overlapping the differentially expressed genes generated from the two siRNAs. (C) Enrichment of Hallmark signatures for PARP-1– and PARP-2–regulated genes. (D) Enrichment of KEGG signatures for PARP-1– and PARP-2–regulated genes. (E) GSEA of RNA-seq data showing enrichment of androgen response gene signature in PARP-1 or PARP-2 KD LNCaP cells. (F) Relative mRNA expression levels were determined by RT-qPCR in LNCaP cells transfected with either PARP-1 or PARP-2 siRNAs compared with nonspecific control (NC) siRNA. The mRNA expression data represent the mean ± SD of three technical replicates. (G and H) Western blot analyses depict protein levels of genes as indicated in PARP-1 and PARP-2 KD (G) or KO (H) LNCaP cells. The tubulin level serves as a protein loading control. All experiments except the RNA-seq were repeated at least three times.
We next examined gene expression signatures enriched in PARP-1– or PARP-2–regulated genes using the Molecular Signature Database (MSigDB) (32, 33). Using “Hallmark” gene signatures, we revealed a significant enrichment of “UV-response” and “p53” pathway genes in PARP-1–regulated genes (Fig. 3C), which was consistent with the notion that PARP-1 is critical in repairing DNA damage and restoring genome stability. In contrast, PARP-2–regulated genes were primarily involved in androgen response. Overlapping with Kyoto Encyclopedia of Gene and Genomes (KEGG) gene sets showed that PARP-2–regulated genes were highly enriched in “prostate cancer” in particular (Fig. 3D). We further employed the gene set enrichment analysis (GSEA) using the “androgen response” signature and revealed significant repression of androgen-regulated genes in PARP-2 but not in PARP-1 KD cells (Fig. 3E). Using RT-qPCR, we confirmed that KD of PARP-2 markedly repressed the expression of AR target genes (KLK2, KLK3/PSA, TMPRSS2, and FKBP5) (Fig. 3F). KD of PARP-1 inhibited TMPRSS2 expression but had little effect on KLK2, KLK3/PSA, and FKBP5 expression. The protein level of PSA was also decreased after PARP-2 KD or KO (Fig. 3 G and H). Notably, AR expression remained unchanged, indicating that the suppression of AR target genes was not due to the alteration of AR expression. Interestingly, we found that dual KD of PARP-1 and PARP-2 did not affect the expression of AR target genes (SI Appendix, Fig. S3). This is likely because KD of PARP-1 predominantly induced p53-mediated cellular responses, which might overshadow the inhibitory effect on AR signaling by KD of PARP-2 at the same time. Taken together, our results suggest that PARP-2 is involved in PCa tumorigenesis, likely through transcriptionally modulating AR-mediated gene expression, which is functionally differentiated from the DNA damage repair protein PARP-1.
Selective PARP-2 Inhibitor UPF-1069 Suppresses the Expression of AR Target Genes.
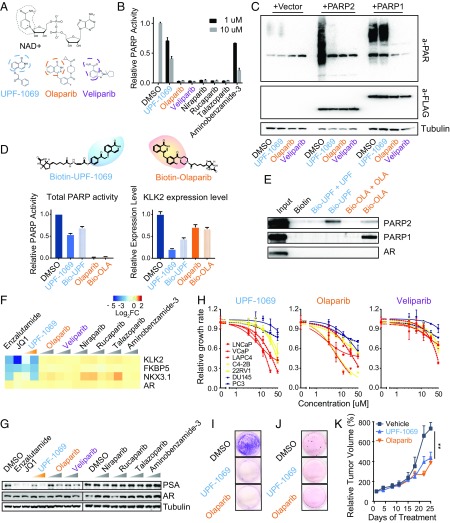
We then asked whether the PARPi could interfere with AR-mediated transcription as well. We assembled four FDA-approved PARPis (olaparib, niraparib, talazoparib, and rucaparib) and two PARPis under preclinical investigation (veliparib and 3-aminobenzamide). These pan-PARPis inhibit both PARP-1 and PARP-2 enzymatic activity. In addition, we employed the compound UPF-1069, a selective PARP-2 inhibitor with ∼27-fold selective over PARP-1 (34, 35). The structures of pan-PARPis (olaparib and veliparib) and selective PARP-2 inhibitor (UPF-1069) contain a key pharmacophore mimicking nicotinamide moiety that competes with NAD+ (Fig. 4A). To assess the inhibitory potency of PARPi, we measured total PARP enzymatic activity from LNCaP whole cell lysates after treatment with PARPi using an in vitro PARP universal colorimetric assay. As expected, all pan-PARPis, except 3-aminobenzamide, completely abolished PARP activity (Fig. 4B). In contrast, UPF-1069 suppressed the total PARP activity down to 40% at 10 µM, suggesting that UPF-1069 is not a potent pan-PARPi. To determine the specificity of UPF-1069, we measured in vivo PARylation using antibody against poly(ADP-ribose). We detected modest levels of endogenous PARylation (largely attributed to PARP-1 activity) in LNCaP cells, which was abolished by olaparib and veliparib, but not by UPF-1069 (Fig. 4C). We then overexpressed PARP-1 and PARP-2 in LNCaP cells. We showed that UPF-1069 completely inhibited the PARP-2–induced PARylation but had no effect on PARP-1. In contrast, olaparib and veliparib completely blocked the PARylation derived from both PARP-1 and PARP-2. To further determine whether UPF-1069 specifically interacts with PARP-2, we linked UPF-1069 and olaparib with a biotin moiety to allow their application in a pull-down assay (SI Appendix, Fig. S4). Biotinylated UPF-1069 and olaparib have similar inhibitory effects on total PARP activity and AR target gene KLK2 expression compared with unmodified UPF-1069 and olaparib (Fig. 3D). Biotinylated UPF-1069 and olaparib were then immobilized on streptavidin beads and incubated with whole cell lysates extracted from LNCaP cells overexpressed with PARP-1 and PARP-2. As shown in the Western blot, UPF-1069 could selectively bind to PARP-2 (Fig. 4E). As a pan-PARPi, olaparib interacted with both PARP-1 and PARP-2 although the binding with PARP-2 was weaker than UPF-1069. These interactions were outcompeted by unmodified UPF-1069 and olaparib, respectively. Taken together, our data demonstrated UPF-1069 as a selective PARP-2 inhibitor.
Fig. 4.
Selective PARP-2 inhibitor UPF-1069 suppresses AR target gene expression and AR-positive PCa cell growth. (A) Chemical structures of NAD+, UPF-1069, olaparib, and veliparib. Similar moieties shared by the compounds are circled. (B) LNCaP cells were treated with PARPi as indicated. Total PARP activity in cell lysates after the treatment was determined by PARP Universal Colorimetric Assay. (C) LNCaP cells were transfected with vector, FLAG-tagged PARP-1, or PARP-2 for 48 h before being treated with PARPi (10 µM) as indicated for another 4 h. The expression level of total PARylated proteins in cell lysates was determined by Western blot using antibody against poly(ADP-ribose) (PAR). Anti-FLAG was used to determine the expression levels of PARP-1 and PARP-2. The tubulin level serves as a protein loading control. (D) Synthesis of biotinylated UPF-1069 and olaparib. (Upper) The chemical structures of biotinylated small molecules with UPF-1069 and olaparib highlighted in blue and orange, respectively. The efficacy of biotinylated UPF-1069 (Bio-UPF) and olaparib (Bio-OLA), in comparison with unmodified PARPi, was determined by their inhibitory effects on total PARP activity (Lower Left) and KLK2 expression levels (Lower Right) after treatment for 24 h. (E) Western blot analyses of protein eluates from biotinylated UPF-1069 or olaparib coated beads after incubation with LNCaP cell lysate in the presence or absence of unmodified UPF-1069 and olaparib. (F) A heat map shows expression alteration of AR and AR target genes in LNCaP cells after treatment with Enzalutamide (10 µM), JQ1 (0.5 µM), or PARPi (1 and 10 µM) for 24 h. (G) Protein expression levels of PSA, AR, and tubulin in LNCaP cells after treatment with small molecule inhibitors as indicated for 24 h. (H) Inhibition of PCa cell proliferation by UPF-1069, olaparib, and veliparib. Red curves, androgen-dependent PCa cell lines; yellow, AR-positive CRPC cell lines; blue, AR-negative CRPC lines. (I and J) Clone-forming ability of LNCaP cells after treatment with UPF-1069 (10 µM), olaparib (10 µM), or DMSO was determined by clonogenic assays (I) and soft agar assays (J). (K) Relative tumor volume of VCaP xenografts in mice treated with vehicle, UPF-1069 or Olaparib for 3.5 wk (7 mice per group). All experiments except for animal experiment were repeated at least three times. All data represent the mean ± SD. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons to the control group. **P < 0.01.
Next, we examined the mRNA levels of three AR target genes (KLK2, FKBP5, and NKX3.1) in LNCaP cells treated with a panel of PARPis (Fig. 4F). As controls, enzalutamide (AR antagonist) and JQ1 (Bromodomain and Extra-Terminal motif [BET] inhibitor) (36) were included. Of all PARPis tested, only UPF-1069 significantly suppressed all three AR target genes in a dose-dependent manner. The PSA protein level was also significantly decreased after UPF-1069 treatment (Fig. 4G). Only minor or no effect was observed after treatment with other PARPis. Notably, PARPi did not change the expression of AR significantly, indicating that UPF-1069 does not regulate AR target genes through modulation of AR expression. In addition, we found that both olaparib and UPF-1069 had little effect on the expression and activity of SIRT1 (SI Appendix, Fig. S5), a NAD+-dependent enzyme, which is regulated by PARP-2 as previously reported (22). Thus, the inhibition of AR target gene expression by UPF-1069 cannot be attributed to its impact on NAD+ bioavailability and SIRT1 transcription in PCa cells.
UPF-1069 Inhibits PCa Cell Growth In Vitro and In Vivo.
To determine whether UPF-1069 can inhibit PCa cell growth, we performed cell proliferation assays on a panel of seven PCa cell lines (Fig. 4H). We found that UPF-1069 inhibited the growth of both androgen-dependent PCa cells (LAPC4, LNCaP, and VCaP) and AR-positive CRPC cells (C4-2B and 22RV-1) although androgen-dependent PCa cells showed greater sensitivity. In contrast, AR-negative CRPC cells (PC3 and DU145) showed resistance. No clear pattern was noticeable after treatment with olaparib or veliparib although PCa cells were more sensitive to olaparib than veliparib, indicating differential efficacy between PARPis even with similar catalytic inhibitory potency. Moreover, UPF-1069 and olaparib markedly suppressed the colony formation and anchorage-independent growth of LNCaP cells (Fig. 4 I and J). In agreement with in vitro proliferation results, UPF-1069 significantly inhibited VCaP tumor growth in vivo (Fig. 4K). No weight loss was observed in UPF-1069–treated mice (SI Appendix, Fig. S6). Similar tumor suppression was also achieved with olaparib treatment. Our data indicate that selective targeting of PARP-2 with a pharmacological agent is sufficient to suppress PCa growth in preclinical models.
UPF-1069 Abrogates AR Transcriptional Activity in PCa Cells.
To explore the molecular mechanism by which PARPis suppress PCa cell growth, we analyzed the global gene expression changes in LNCaP cells after treatment with UPF-1069, olaparib, and veliparib using RNA-seq. As shown in the volcano plot (Fig. 5A), UFP-1069 had a much greater effect on gene expression (895 differentially expressed genes) compared with olaparib (144 genes) and veliparib (92 genes) (Dataset S1). In accordance with PARP-2 KD results, pharmaceutical inhibition of PARP-2 dramatically altered AR-mediated gene expression, with classical AR target genes (KLK2, KLK3/PSA, FKBP5, NKX3.1, and TMPRSS2) being on the top of the differentially expressed gene list. However, broadly quenching PARP activity using either olaparib or veliparib was not able to affect these genes. Only a small number of commonly altered genes were identified between three different treatments (Fig. 5B). Analyses of MSigDB showed that UPF-1069–altered genes were highly enriched for “androgen response” hallmark while olaparib-altered genes were involved in “p53” and “apoptosis” pathways (SI Appendix, Fig. S7).
Fig. 5.
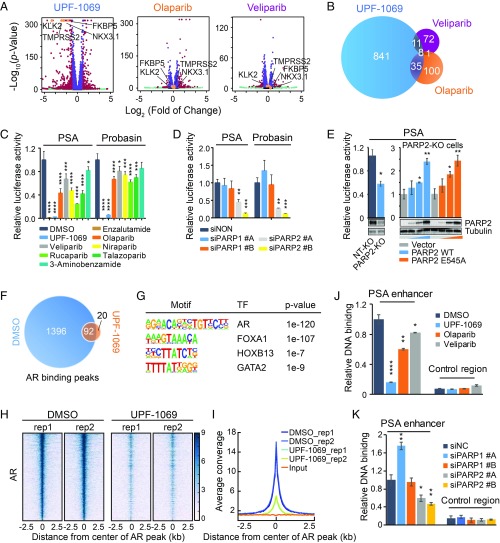
Inhibition of PARP-2 abrogates genome-wide AR recruitment. (A) Gene expression profiling of LNCaP cells after treatment with UPF-1069 (10 µM), olaparib (10 µM), or veliparib (10 µM) for 24 h. Differentially expressed genes with fold change >2 and FDR <0.01 are labeled in red. Four classic AR target genes are highlighted in orange. (B) Overlap between UPF-1069–, olaparib-, and veliparib-altered genes. (C) PSA and Probasin luciferase reporter plasmids containing highly conserved androgen response elements were transfected into LNCaP cells. The treatment with PARPi (10 µM) as indicated started 4 h after transfection. Luciferase activity was measured 24 h after reporter transfection. (D) PARP-1 and PARP-2 siRNA KD was conducted in LNCaP cells through reverse transfection 48 h before luciferase reporter plasmid transfection. Luciferase activity was measured 24 h after reporter transfection. (E) A PARP-2 KO cell line and a nontargeting (NT) control line were generated through ribonucleoprotein (RNP) delivery approach as described in Materials and Methods. KO efficiency was confirmed by Western blot. PSA luciferase report activity was decreased in PARP-2 KO cells but rescued after overexpression of PARP-2 wild-type (WT) or E545A mutant plasmid in a dose-dependent manner (0, 50, 100, 200 ng per well). PARP-2 overexpression was examined by Western blot. (F) Venn diagram illustrating the overlap of AR binding sites identified by AR ChIP-seq in LNCaP cells between DMSO and UPF-1069 (10 µM) treatments for 16 h. (G) Motifs enriched in AR binding sites. (H) A heat map showing AR ChIP-seq signal density in a region of 2.5 kb flanking AR binding summits. Two replicates (rep) for each treatment are presented. (I) A summary plot of AR average signal density (reads per genomic coverage) across all AR-binding sites. (J and K) Relative AR binding determined by ChIP-qPCR at the PSA enhancer and the control region in LNCaP cells after treatment with PARPi for 16 h (J) or 2 d after siRNA KD (K). All data represent the mean ± SD of three technical replicates. All experiments except for RNA-seq and ChIP-seq were repeated at least three times. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons to the control group. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To further examine the impact of PARPi on AR activity, we performed androgen response element driven luciferase report assays and showed that UPF-1069 completely abolished the PSA and Probasin reporter activity while pan-PARPis had a minor to moderate effect (Fig. 5C). Interestingly, pan-PARPis showed a similar inhibition on WNT and E2F signaling (SI Appendix, Fig. S8), indicating a general effect on transcriptional regulation. Consistently, the PSA and Probasin reporter activity was significantly inhibited after PARP-2 KD (Fig. 5D) while KD of PARP-1 had no effect. Furthermore, we generated a PARP-2 KO LNCaP cell line through a Ribonucleoprotein (RNP) delivery approach, which has no DNA integration into the genome and fewer off-target effects. We found that the PSA reporter activity was significantly decreased in PARP-2 KO cells compared with nontarget (NT) control cells (Fig. 5E). Notably, both wild-type PARP-2 and enzymatically inactive E545A mutant PARP-2 (SI Appendix, Fig. S9) could restore the PSA reporter activity, indicating that PARP-2 enzymatic activity is not required for modulating AR transcriptional activity. Taken together, our data suggest that UPF-1069 suppresses PCa cell growth by attenuating AR signaling while olaparib induces p53-dependent apoptosis to achieve a similar effect. Importantly, the effect of PARPis on gene expression is not correlated with their inhibitory potency on PARP enzymatic activity.
Inhibition of PARP-2 Attenuates Genome-Wide AR Recruitment.
We then asked whether inhibition of PARP-2 by UPF-1069 affects AR binding to the genome. We performed chromatin immunoprecipitation-sequencing (ChIP-seq) with an antibody against AR in LNCaP cells. Surprisingly, the number of AR binding peaks identified after UPF-1069 treatment dramatically decreased (Fig. 5F). As expected, all AR binding peaks were enriched with AR and its coregulator (FOXA1, HOXB13, and GATA2) motifs (Fig. 5G). In line with the global effect of UPF-1069 on AR-mediated gene expression, a striking reduction of global AR binding was observed (Fig. 5 H and I). ChIP-qPCR assays confirmed that AR binding was diminished by UPF-1069 and less so by olaparib and veliparib at the PSA (Fig. 5J) and other AR-targeted loci (SI Appendix, Fig. S10). KD of PARP-2 also attenuated AR binding, supporting that AR binding is largely modulated by PARP-2 but not PARP-1 (Fig. 5K and SI Appendix, Fig. S10).
PARP-2 Modulates the Pioneer Factor FOXA1 Function.
Given the genome-wide regulatory effect of PARP-2 on AR occupancy, we hypothesized that PARP-2 may physically interact with AR and function as a coactivator. We performed a coimmunoprecipitation (Co-IP) assay in LNCaP cells. Surprisingly, no interaction was detected between AR and either PARP-1 or PARP-2 (Fig. 6A). Instead, we observed that the pioneer factor FOXA1 strongly interacted with PARP-2 but not with PARP-1. Neither PARP-1 nor PARP-2 could bind to HOXB13, another critical AR coregulator (37). Using an in vitro GST pull-down assay, we further demonstrated that PARP-2 directly interacted with FOXA1 (Fig. 6B). However, FOXA1 was not modified or PARylated by PARP-2 (SI Appendix, Fig. S11). Instead, we found that both wild-type and E545A mutant PARP-2 could interact with FOXA1 (SI Appendix, Fig. S12), in line with the notion that PARP-2 modulates AR activity through interacting with FOXA1 independent of its enzymatic activity. Importantly, the interaction between PARP-2 and FOXA1 could be blocked by UPF-1069 (Fig. 6C). Interestingly, pan-PARPis seem to enhance the PARP-2/FOXA1 interaction, likely due to PARP trapping to chromatin induced by pan-PARPi (38).
Fig. 6.
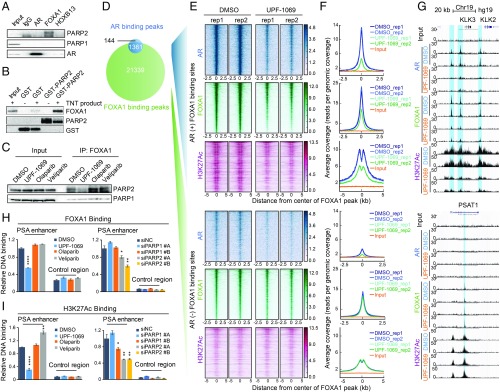
PARP-2 modulates FOXA1 function. (A) Co-IP showing association of endogenous PARP-1/PARP-2 with AR/FOXA1/HOXB13 in LNCaP cells. (B) GST pull-down showing direct interaction between purified GST-PARP-2 protein and FLAG-FOXA1 in vitro translation product (TNT product). (C) Co-IP showing Interaction between PARP-1/PARP-2 and FOXA1 in LNCaP cells after treatment with PARPi (10 µM) for 6 h. (D) Venn diagram showing the overlap of AR and FOXA1 binding sites identified by ChIP-seq in LNCaP cells. (E and F) Inhibition of PARP-2 disrupts FOXA1 binding and function at AR binding sites. Heat maps (E) and average signal density plots (F) showing AR, FOXA1, and H3K27Ac binding at the AR-occupied (+) (Upper) and non-AR-occupied (−) (Lower) FOXA1 binding sites (2.5 kb flanking FOXA1 binding summits) after treatment with DMSO or UPF-1069 (10 µM) for 16 h. (G) AR, FOXA1, and H3K27Ac ChIP-seq signals at KLK2, KLK3, and PSAT1 loci. (H and I) ChIP-qPCR results showing FOXA1 (H) and H3K27Ac (I) binding at the PSA enhancer and the control region in LNCaP cells after treatment with PARPi as indicated or PARP-1/PARP-2 siRNA KD. All experiments except for ChIP-seq were repeated at least three times. The ChIP-qPCR data represent the mean ± SD of three technical replicates. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons to the control group. *P < 0.05; **P < 0.01; ****P < 0.0001.
We then tested whether selective inhibition of PARP-2 could impair the FOXA1 chromatin association and function. We employed ChIP-seq with antibodies against FOXA1 and an enhancer histone mark, histone H3 lysine 27 acetylation (H3K27Ac), in LNCaP cells after treatment with UPF-1069. In agreement with previous studies (5, 37), AR binding sites were largely overlapped with FOXA1 binding sites (>90%) (Fig. 6D). We divided FOXA1 binding sites into two groups, AR-occupied and non–AR-occupied regions. Strikingly, UPF-1069 treatment not only abrogated AR recruitment but also diminished FOXA1 binding in the AR-occupied regions (Fig. 6 E–G). Accordingly, H3K27Ac was also decreased within these regions, suggesting impaired enhancer activity. These changes were not observed in the non–AR-occupied FOXA1 binding sites. ChIP-seq results were further validated by ChIP-qPCR at the PSA enhancer (Fig. 6 H and I). In agreement with pharmaceutical inhibition, KD of PARP-2 also led to a reduction of FOXA1 and H3K27Ac binding to the PSA enhancer. KD of PARP-1, however, showed no effect on FOXA1 binding and a mild H3K27Ac decrease by only one of the siRNAs. Taken together, our findings suggest that inhibition of PARP-2 disrupts FOXA1 binding and function at the AR enhancers, which in turn attenuates AR-mediated transcription (Fig. 7).
Fig. 7.
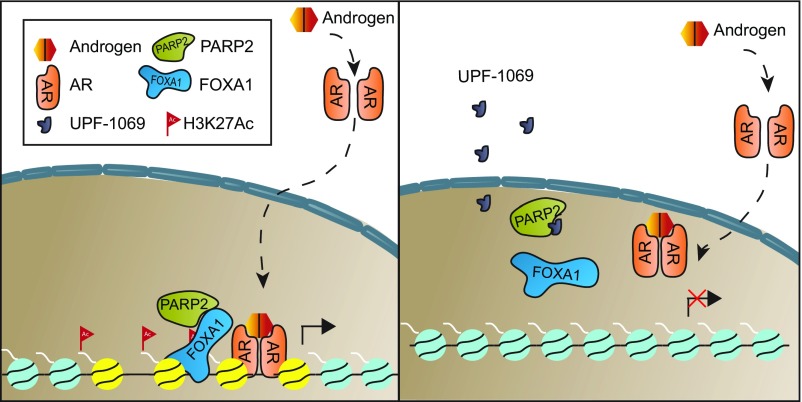
Model depicting the mechanism by which selective targeting of PARP-2 attenuates AR signaling and inhibits PCa growth. PARP-2 enhances AR transcriptional activity via physically interacting with the pioneer factor FOXA1. Selective PARP-2 inhibitor UPF-1069 blocks this interaction, diminishes the genomic occupancies of FOXA1 and H3K27Ac at AR binding sites, and, thereby, inhibits AR-mediated gene expression and PCa growth.
Discussion
Understanding the mechanisms underlying AR-mediated transcription is a critical step for the development of effective therapies for PCa. In the present study, we have identified PARP-2 as an oncogenic contributor to AR signaling through interacting with FOXA1 at prostate-specific enhancers, thereafter promoting PCa growth. Our findings offer a mechanistic insight into PARP-2 as a potential therapeutic target and shed light on a selective PARP-2 inhibitor in the treatment of PCa.
While we have demonstrated that both PARP-1 and PARP-2 are required for PCa cell growth, our analyses reveal that genetic and pharmacological inhibition of PARP-1 alters gene expression implicated in p53-mediated cellular response and apoptosis. In contrast, inhibition of PARP-2 has a significant impact on AR-mediated gene expression, which is comparable with enzalutamide treatment. It should be noted that PARP-1 is generally involved in the interplay between transcription and DNA repair since transcription has classically been considered a danger to genome integrity (39). As AR action has been linked to DNA damage and associated response (40, 41), it is not surprising that the high-abundant protein PARP-1 may somewhat interact with AR (42) or be considered as a coactivator (25). Moreover, studies have shown that the association between FOXA1 and DNA-repair complex is critical for the transcription pioneering and epigenetic reprogramming (43). Thus, it is expected that inhibition of PARP-1 may affect AR-mediated transfection to a certain extent. Nevertheless, PARP-1 likely impacts transcriptional regulation in a more ubiquitous fashion while PARP-2 is a critical component in AR signaling specifically. The oncogenic role of PARP-2 in PCa is further supported by the finding that PARP-2 is overexpressed in primary PCa and more so in CRPC tumors. Interestingly, PARP-2 is highly expressed in normal testicular tissues. KO of PARP-2 but not PARP-1 causes defect of spermatogenesis in mice (17), which is the same phenotype observed when AR is knocked out, supporting a specific role of PARP-2 in AR signaling.
Current clinically used PARPi abolishes both PARP-1 and PARP-2 catalytic activity, which raises the question of why these pan-PARPis (such as olaparib) have a limited effect on AR signaling in contrast to the selective PARP-2 inhibitor UPF-1069. Studies have shown that the efficacy of PARPis does not always rely on their catalytic inhibitory potency. For example, trapping PARP-1 and PARP-2 onto damaged DNA has been correlated with the cytotoxicity of PARPi (38), and yet PARPis with equal catalytic inhibitory potency show markedly different PARP trapping ability and cytotoxicity. Furthermore, catalytic-independent functions for both PARP-1 and PARP-2 have been reported (44, 45). Indeed, our results support the notion that PARP-2 enzymatic activity is not required for its role in AR-mediated transcription. Thus, blocking PARP-2 enzymatic activity by pan-PARPi is neither sufficient nor necessary for AR inhibition. We reason that UPF-1069 inhibits PARP-2 and abolishes AR activity, likely through its unique physicochemical properties, despite its relatively weak catalytic inhibition. We have provided compelling evidence that UPF-1069 strongly interacts with PARP-2 and disrupts the interaction between PARP-2 and FOXA1, which in turn attenuates AR recruitment. This cannot be achieved by pan-PARPi. Although efforts were made in this study to prove UPF-1069 as a selective PARP-2 inhibitor, we cannot rule out the possibility that UPF-1069 may interact with other proteins, including other PARP members. Proteome-wide profiling study has revealed PARPis to have compound-specific secondary targets, which may be involved in the therapeutic effects of these drugs (46). Considerable variability has been reported between different PARPis in the clinical development despite similar catalytic inhibition (47). Further investigation is necessary to explore other UPF-1069–associated proteins and potential off-target effects using proteomic approaches. Mechanistic characterization of each PARPi may guide a better application of these drugs under different disease settings.
PCa patients may benefit from selective targeting of PARP-2. First, pan-inhibitors normally have more side effects than selective inhibitors. As expression profiling indicates that PARP-11 may play a tumor-suppressive role in PCa development and progression, inhibition of total PARP activity may in fact denote unfavorable outcomes. Studies have even shown that inactivation of PARP-1 by gene-targeted deletion may lead to epithelial-mesenchymal transition induction toward high-grade prostate tumors (48), which may not be beneficial to patients in certain disease contexts. Second, the therapeutic outcomes of current pan-PARPis rely on whether tumors have HR deficiency. Lack of biomarkers for patient selection and quickly developed drug resistance are the major obstacles. Selective targeting of PARP-2 through the FOXA1/AR pathway may broaden the clinical application of PARP-targeted therapy in PCa management without considering HR deficiency status. Finally, selective targeting of PARP-2 may potentially provide an alternative therapeutic strategy for AR inhibition through disrupting FOXA1 binding instead of targeting AR directly.
Materials and Methods
Cell Lines and Materials.
Prostate cancer cell lines were maintained in RPMI 1640 (for LNCaP, LAPC4, C4-2B, 22Rv1, PC-3, and DU145) or DMEM (for VCaP) supplemented with 10% FBS as previously described (49). The 293T cells were obtained from ATCC and maintained in DMEM with 10% FBS. All cell lines were authenticated using high-resolution small tandem repeats (STRs) profiling at Dana-Farber Cancer Institute (DFCI) Molecular Diagnostics Core Laboratory and were confirmed Mycoplasma-free before experiments. The small molecule inhibitors, antibodies, siRNAs, sgRNAs, and PCR primers are listed in SI Appendix, Table S1.
Statistical Analyses.
Quantitative measurements are graphed as mean ± SD from at least three biological replicates unless otherwise indicated. All analyses were carried out using Prism software (GraphPad). One-way ANOVA (or Kruskal–Wallis test) followed by post hoc Tukey’s test was used to calculate significance using P < 0.05.
Other Methods are described in SI Appendix, Supplementary Materials and Methods. ChIP-seq and RNA-seq data have been deposited into the Gene Expression Omnibus (GEO) database (accession no. GSE114275).
Supplementary Material
Acknowledgments
We thank Quang-De Nguyen, Kristen L. Jones, Rebecca J. Modiste, and Ruthie Jia for technical support in this study. This work was supported by Department of Defense Idea Award Grant W81XWH-17-1-0251 (to L.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All ChIP-seq and RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE114275).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908547116/-/DCSupplemental.
References
- 1.de Bono J. S., et al. ; COU-AA-301 Investigators , Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher H. I., et al. ; AFFIRM Investigators , Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Crona D. J., Whang Y. E., Androgen receptor-dependent and -independent mechanisms involved in prostate cancer therapy resistance. Cancers (Basel) 9, E67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y. A., Yu J., Current perspectives on FOXA1 regulation of androgen receptor signaling and prostate cancer. Genes Dis. 2, 144–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu B., et al. , Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 30, 3962–3976 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin H. J., Zhao J. C., Wu L., Kim J., Yu J., Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat. Commun. 5, 3972 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbieri C. E., et al. , Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedge D. C., et al. ; CAMCAP Study Group; TCGA Consortium , Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat. Genet. 50, 682–692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus W. L., PARPs and ADP-ribosylation: 50 years … and counting. Mol. Cell 58, 902–910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant H. E., et al. , Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Farmer H., et al. , Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Mateo J., et al. , DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall C. H., et al. , Differential response to olaparib treatment among men with metastatic castration-resistant prostate cancer harboring BRCA1 or BRCA2 versus ATM mutations. Eur. Urol. 10.1016/j.eururo.2019.02.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amé J. C., et al. , PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 274, 17860–17868 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Schreiber V., et al. , Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem. 277, 23028–23036 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Bai P., et al. , Poly(ADP-ribose) polymerase-2 [corrected] controls adipocyte differentiation and adipose tissue function through the regulation of the activity of the retinoid X receptor/peroxisome proliferator-activated receptor-gamma [corrected] heterodimer. J. Biol. Chem. 282, 37738–37746 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Dantzer F., et al. , Poly(ADP-ribose) polymerase-2 contributes to the fidelity of male meiosis I and spermiogenesis. Proc. Natl. Acad. Sci. U.S.A. 103, 14854–14859 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yélamos J., et al. , PARP-2 deficiency affects the survival of CD4+CD8+ double-positive thymocytes. EMBO J. 25, 4350–4360 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena A., et al. , Poly(ADP-ribose) polymerase 2 localizes to mammalian active centromeres and interacts with PARP-1, Cenpa, Cenpb and Bub3, but not Cenpc. Hum. Mol. Genet. 11, 2319–2329 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Dantzer F., et al. , Functional interaction between poly(ADP-ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol. Cell. Biol. 24, 1595–1607 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yélamos J., Schreiber V., Dantzer F., Toward specific functions of poly(ADP-ribose) polymerase-2. Trends Mol. Med. 14, 169–178 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Bai P., et al. , PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 13, 450–460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szántó M., et al. , Poly(ADP-ribose) polymerase-2: Emerging transcriptional roles of a DNA-repair protein. Cell. Mol. Life Sci. 69, 4079–4092 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner J. C., et al. , Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell 19, 664–678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiewer M. J., et al. , Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2, 1134–1149 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kounatidou E., et al. , A novel CRISPR-engineered prostate cancer cell line defines the AR-V transcriptome and identifies PARP inhibitor sensitivities. Nucleic Acids Res., gkz286 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koboldt D. C., et al. , Rare variation in TET2 is associated with clinically relevant prostate carcinoma in African Americans. Cancer Epidemiol. Biomarkers Prev. 25, 1456–1463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research Network , The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J. H., et al. , Gene expression analysis of prostate cancers. Mol. Carcinog. 33, 25–35 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Best C. J., et al. , Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin. Cancer Res. 11, 6823–6834 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor B. S., et al. , Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberzon A., et al. , The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A., et al. , Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellicciari R., et al. , On the way to selective PARP-2 inhibitors. Design, synthesis, and preliminary evaluation of a series of isoquinolinone derivatives. ChemMedChem 3, 914–923 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Moroni F., et al. , Selective PARP-2 inhibitors increase apoptosis in hippocampal slices but protect cortical cells in models of post-ischaemic brain damage. Br. J. Pharmacol. 157, 854–862 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovén J., et al. , Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pomerantz M. M., et al. , The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat. Genet. 47, 1346–1351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murai J., et al. , Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 72, 5588–5599 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Alessandro G., d’Adda di Fagagna F., Transcription and DNA damage: Holding hands or crossing swords? J. Mol. Biol. 429, 3215–3229 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Haffner M. C., et al. , Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 42, 668–675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polkinghorn W. R., et al. , Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stelloo S., et al. , Endogenous androgen receptor proteomic profiling reveals genomic subcomplex involved in prostate tumorigenesis. Oncogene 37, 313–322 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., et al. , Nucleation of DNA repair factors by FOXA1 links DNA demethylation to transcriptional pioneering. Nat. Genet. 48, 1003–1013 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Liang Y. C., Hsu C. Y., Yao Y. L., Yang W. M., PARP-2 regulates cell cycle-related genes through histone deacetylation and methylation independently of poly(ADP-ribosyl)ation. Biochem. Biophys. Res. Commun. 431, 58–64 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Liu Z., Kraus W. L., Catalytic-independent functions of PARP-1 determine Sox2 pioneer activity at intractable genomic loci. Mol. Cell 65, 589–603 e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knezevic C. E., et al. , Proteome-wide profiling of clinical PARP inhibitors reveals compound-specific secondary targets. Cell Chem. Biol. 23, 1490–1503 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jelinic P., Levine D. A., New insights into PARP inhibitors’ effect on cell cycle and homology-directed DNA damage repair. Mol. Cancer Ther. 13, 1645–1654 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Pu H., et al. , PARP-1 regulates epithelial-mesenchymal transition (EMT) in prostate tumorigenesis. Carcinogenesis 35, 2592–2601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng D., et al. , Secretory leukocyte protease inhibitor is a survival and proliferation factor for castration-resistant prostate cancer. Oncogene 35, 4807–4815 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.