Abstract
Prematurity is associated with diverse developmental abnormalities, yet few studies relate cognitive and neurostructural deficits to a dimensional measure of prematurity. Leveraging a large sample of children, adolescents, and young adults (age 8–22 years) studied as part of the Philadelphia Neurodevelopmental Cohort, we examined how variation in gestational age impacted cognition and brain structure later in development. Participants included 72 preterm youth born before 37 weeks’ gestation and 206 youth who were born at term (37 weeks or later). Using a previously-validated factor analysis, cognitive performance was assessed in three domains: (1) executive function and complex reasoning, (2) social cognition, and (3) episodic memory. All participants completed T1-weighted neuroimaging at 3 T to measure brain volume. Structural covariance networks were delineated using non-negative matrix factorization, an advanced multivariate analysis technique. Lower gestational age was associated with both deficits in executive function and reduced volume within 11 of 26 structural covariance networks, which included orbitofrontal, temporal, and parietal cortices as well as subcortical regions including the hippocampus. Notably, the relationship between lower gestational age and executive dysfunction was accounted for in part by structural network deficits. Together, these findings emphasize the durable impact of prematurity on cognition and brain structure, which persists across development.
Keywords: anatomical, development, executive functioning, prematurity
Prematurity is defined by the World Health Organization as the delivery of an infant before 37 weeks’ gestation (World Health Organization 2012), and is associated with diverse neurodevelopmental deficits (Foulder-Hughes and Cooke 2003; Peterson et al. 2003; Inder et al. 2003, 2005; Taylor et al. 2004, 2006; Hintz et al. 2015; Rogers et al. 2017). Most studies on the effects of premature birth adopt a case-control approach that compares those born preterm (usually those born very (<32 weeks) or extremely preterm (<28 weeks)) to those born term (Peterson et al. 2000, 2003; Nosarti et al. 2002; Delobel-Ayoub et al. 2009; Karolis et al. 2017; Kroll et al. 2017). Given that those born moderately (32–34 weeks) or late preterm (34–37 weeks) comprise 80% of preterm births (Ritchie et al. 2015; Cheong et al. 2017) and show evidence of cognitive deficits (Hodel et al. 2017), a dimensional approach that incorporates the spectrum of prematurity may be complementary to the traditional case-control approach.
The effects of premature birth span the developmental period. Neurocognitive impairments associated with prematurity have been shown to persist throughout childhood (Peterson et al. 2000; Foulder-Hughes and Cooke 2003; Taylor et al. 2004, 2006; Delobel-Ayoub et al. 2009; Cheong et al. 2017), adolescence (Nosarti et al. 2004, 2005; Taylor et al. 2011; Cheong et al. 2013), and young adulthood (Hack et al. 2002; Allin et al. 2006; Nosarti et al. 2014a). Compared with full term peers, infants born at extreme to late preterm gestations show lower IQ (Peterson et al. 2000; Hack et al. 2002; Pharoah et al. 2003; Cooke 2005; Allin et al. 2006; Cheong et al. 2013; Nosarti et al. 2014a; Kroll et al. 2017), impaired language development (Taylor et al. 2004; Nosarti et al. 2008; Cheong et al. 2017), reduced motor skills (Foulder-Hughes and Cooke 2003; Taylor et al. 2004; Cheong et al. 2017), and executive function deficits (Curtis et al. 2002; Taylor et al. 2004; Nosarti et al. 2007, 2008, 2014a; Wehrle et al. 2016; Kroll et al. 2017) Additionally, children, adolescents, and young adults born prematurely show greater emotional and behavioral difficulties than full term comparisons including reduced social competence (Delobel-Ayoub et al. 2009; Ritchie et al. 2015; Cheong et al. 2017), and greater behavioral problems such hyperactivity/inattention (Foulder-Hughes and Cooke 2003; Nosarti et al. 2005; Delobel-Ayoub et al. 2009). In addition to cognitive and behavioral deficits associated with prematurity, an accumulating body of work using in vivo neuroimaging indicates that prematurity is associated with abnormalities of structural brain development.
Abnormal neuroanatomical development may underlie the observed differences in neurocognitive outcomes of prematurely-born infants. A meta-analysis of structural magnetic resonance imaging (MRI) studies demonstrated that children and adolescents born before 32 weeks’ gestation showed reduced gray matter volumes in multiple brain regions (de Kieviet et al. 2012). Specifically, structural imaging studies have demonstrated that compared with full term comparators, those born preterm showed distributed gray matter volume deficits in orbitofrontal, temporal, and parietal cortices as well as in subcortical regions including caudate, hippocampus, amygdala, and thalamus (Nosarti et al. 2002, 2008; Nagy et al. 2009; Cheong et al. 2013; Cismaru et al. 2016; Keunen et al. 2016; Botellero et al. 2017; Karolis et al. 2017; Tseng et al. 2017). Furthermore, structural abnormalities have been linked to deficits in intelligence, motor, and academic abilities (Anderson et al. 2017), to increased psychiatric symptoms (Botellero et al. 2017), and to impaired memory performance (Tseng et al. 2017) in those born very preterm. However, studies seeking to link structural brain abnormalities and neurocognitive impairment in preterm youth often use a case-control design rather than a dimensional approach, and many of these studies restrict their analyses to those born very or extremely premature (Nosarti et al. 2002, 2008, 2014b; de Kieviet et al. 2012; Cheong et al. 2013; Anderson et al. 2017; Karolis et al. 2017; Tseng et al. 2017). Given evidence of smaller cerebral volumes in moderately preterm infants (Niwa et al. 2017), studies are needed that examine the impact of gestational age on brain structure and cognition across the full range of prematurity.
Most prior studies of brain structure have examined brain volume within specific regions or across hundreds of thousands of voxels using voxel-based morphometry (VBM). However, these approaches have limitations. On one hand, focused regional analyses are limited by their narrow scope. In contrast, whole-brain VBM studies are limited by either reduced power (or conversely a high risk of false positives) due to the large number of multiple comparisons. Recent investigations have instead attempted to describe configurations of structural covariance between selected brain regions, highlighting patterns of coordinated development (Mechelli 2005; Evans 2013). Structural covariance analyses take into account the tendency for brain volumes to vary consistently across both regions and individuals, and thus allow brain structure to be modeled as a complex network (Zielinski et al. 2010; Alexander-Bloch et al. 2013). Existing studies have described differential patterns of structural covariance in several regions in preterm adolescents, including the caudate, thalamus, and several other cortical and subcortical regions (Nosarti et al. 2011) as well as within the bilateral temporal and inferior frontal lobes in young adults (Scheinost et al. 2017). The functional significance of altered structural covariance networks is just beginning to be explored. For example, in adolescents born at less than 31 weeks’ gestation, differential patterns of covariance within the bilateral temporal lobes, inferior frontal lobes, and caudate were correlated with worse performance on measures of language development, including phonological processing (Scheinost et al. 2017). However, these prior studies used a seed-based approach that requires choosing a restricted number of seed regions a priori.
One recently-developed alternative to such seed-based covariance analyses is non-negative matrix factorization (NMF; Sotiras et al. 2015). Originally used for computer vision research (Lee and Seung 1999), NMF is a machine learning technique that can delineate structural covariance networks over the entire brain. As NMF is not limited to a small number of pre-defined anatomical seeds, it provides a more comprehensive description of covariance networks. Networks derived from NMF align well with functional brain networks, are highly reproducible, and maximize statistical power by limiting multiple comparisons (Sotiras et al. 2017). Thus far, no prior studies have used NMF to examine structural covariance networks in preterm youth.
Accordingly, here we investigated the impact of prematurity on cognitive performance and brain structure in a large sample of youth imaged as part of the Philadelphia Neurodevelopmental Cohort (PNC; Satterthwaite, Elliott, et al. 2014; Satterthwaite et al. 2016). In contrast to prior case-control studies, we examined the effect of gestational age on cognition and brain structure on a dimensional basis, and included youth ranging from those born extremely preterm to those born full term. We also utilized NMF to delineate structural covariance networks that are not limited to seed-based analyses. We predicted that prematurity would be associated with individual differences in cognition. To evaluate this, we examined cognitive functioning in three domains: (1) executive function and complex reasoning, (2) social cognition, and (3) episodic memory. Furthermore, we predicted that dimensionally-defined prematurity would be associated with reduced volume in frontal, temporal, and subcortical brain networks. Lastly, we predicted that reduced performance on executive tasks would be in part accounted for by the observed structural deficits. As described in the Results below, we found that deficits within structural brain networks are related to the extent of prematurity, and may in part explain the relationship between preterm birth and executive deficits in youth.
Materials and Methods
Participants
A total of 9500 youths received a comprehensive neurocognitive assessment battery (Gur et al. 2010; Moore et al. 2015) and 1601 of these participants completed multimodal neuroimaging as part of the PNC (Satterthwaite, Elliott, et al. 2014; Satterthwaite et al. 2016), a large-scale community-based study of brain development. Gestational age was determined from a retrospective review of two electronic medical records systems. We identified 345 PNC participants who completed neuroimaging and had information available about gestational age, which was defined as the number of weeks of gestation at the time of birth. Of these, 257 were born full term and 88 were born preterm (<37 weeks). From these 345 participants, 42 were excluded for: medical disorders that could impact brain functioning (n = 21), medication use that could affect brain functioning (n = 17), or substantial structural brain abnormalities (n = 8); several subjects were excluded for multiple criteria. Of the remaining participants, 21 individuals were excluded for failing to meet structural image quality assurance protocols. Four participants were missing data on maternal level of education. The final sample consisted of 278 youth (mean age = 13.26 years, SD = 3.52, range = 8–22 years, 135 males; 160 non-white). Demographics of the sample are summarized in Table 1. Notably, gestational age was not significantly related to age at time of imaging (P = 0.386) or level of maternal education (P = 0.906). Male and female participants did not differ in terms of mean gestational age (P = 0.962).
Table 1.
Sample demographics
| N | GA (weeks) | GA mean (SD) | % Female | % Caucasian | Age at scan mean (SD) | Maternal education (years) | |
|---|---|---|---|---|---|---|---|
| Extremely preterm | 7 | <28 | 25.57 (0.53) | 57 | 0 | 14.00 (4.29) | 13.43 |
| Very preterm | 10 | 28 to <32 | 29.70 (1.25) | 40 | 40 | 14.61 (4.04) | 14.50 |
| Moderately preterm | 19 | 32 to <34 | 32.37 (0.50) | 63 | 32 | 12.68 (3.31) | 13.53 |
| Late preterm | 36 | 34 to <37 | 35.29 (0.88) | 50 | 42 | 13.78 (4.00) | 14.61 |
| Early term | 50 | 37 to <39 | 37.59 (0.49) | 46 | 46 | 13.04 (3.56) | 14.28 |
| Full term | 156 | >39 | 40.04 (0.57) | 53 | 45 | 13.16 (3.37) | 14.06 |
Note: GA, gestational age; SD, standard deviation; preterm gestational age intervals are defined by the World Health Organization (2012).
Neurocognitive Battery
All participants completed a cognitive assessment as measured using the University of Pennsylvania Computerized Neurocognitive Battery (CNB; Gur et al. 2010). The tests included in the CNB have been described in detail elsewhere (Gur et al. 2010; Moore et al. 2015) and are also described in the supplemental methods. The CNB, which measures performance accuracy and response time, consisted of an hour-long battery of 14 cognitive tests of executive control, episodic memory, complex reasoning, social cognition, and sensorimotor/motor speed administered in a fixed order.
In previous work with the full-sample of 9500 participants who completed the CNB (Moore et al. 2015), an exploratory factor analysis with oblique rotation revealed that the 14 cognitive tests could be summarized into three factors: (1) executive function and complex reasoning, (2) social cognition, and (3) episodic memory. Subsequent confirmatory bifactor analyses also generated a measure for overall performance accuracy. The impact of gestational age on the scores for this general accuracy factor and the three correlated-traits cognitive dimensions (executive function, social cognition, and episodic memory) were evaluated in statistical analyses as described below.
Image Acquisition
Structural image acquisition and processing are reported in detail elsewhere (Satterthwaite, Elliott, et al. 2014; Satterthwaite et al. 2016). Imaging data were acquired on the same MRI scanner (Siemens TIM Trio 3 Tesla, Erlangen, Germany; 32-channel head coil) using the same imaging sequences for all participants. Structural brain scanning was completed using a magnetization-prepared, rapid acquisition gradient-echo (MPRAGE) T1-weighted image with the following parameters: TR 1810 ms; TE 3.51 ms; FOV 180 × 240 mm; matrix 256 × 192; 160 slices; slice thickness/gap 1/0 mm; TI 1100 ms; flip angle 9 degrees; effective voxel resolution of 0.93 × 0.93 × 1.00 mm; total acquisition time 3:28 min.
Image Processing
Structural data was measured using regional analysis of volumes examined in normalized space (RAVENS; Davatzikos et al. 2001). RAVENS maps are conceptually similar to other VBM methodologies but have been shown to be more accurate than other methods (Davatzikos et al. 2001). We used Deformable Registration via Attribute Matching and Mutual-Saliency Weighting (DRAMMS), a software package designed for image registration, to construct gray matter RAVENS maps. RAVENS maps were then registered to study-specific, population-average template. This method ensured that the template had the maximum overall similarity to all images in the dataset and did not introduce registration accuracy bias. RAVENS images were down-sampled to 2 mm and smoothed with an 8 mm full-width, half maximum Gaussian kernel prior to NMF analyses. All voxel-wise RAVENS maps were reviewed manually as part of quality assurance procedures. T1 image quality was independently assessed by three expert image analysts (for full details of this procedure see Rosen et al. 2017). Briefly, three raters were trained prior to rating images on an independent training sample of 100 images. All three raters were trained to >85% concordance with faculty consensus ratings. T1 images were rated on a 0–2-Likert scale (0 = unusable images, 1 = usable images with some artifact, and 2 = images with none or almost no artifact). All images with an average rating of 0 were excluded from analyses. These average manual quality ratings were also included in sensitivity analyses.
Non-negative Matrix Factorization
We examined structural covariance networks for two reasons. First, prior work has shown that there are inherent patterns of covariance in brain structure (Zielinski et al. 2010; Alexander-Bloch et al. 2013), and analyzing the data according to this covariance structure enhances interpretability. Second, an efficient summary of the volumetric data reduces the vulnerability to false positive results, which are more likely to occur when conducting inference tests over hundreds of thousands of voxels as in typical analyses (Eklund et al. 2016). Accordingly, we used NMF to identify structural networks. NMF is a data-driven method for extracting structural networks where volume co-varies consistently across all participants (Sotiras et al. 2015). NMF is advantageous because it produces parts-based representations of imaging data and yields networks that are more interpretable and reproducible than other decomposition techniques such as Principal Component Analysis, Independent Component Analysis, and other related methods (Sotiras et al. 2015).
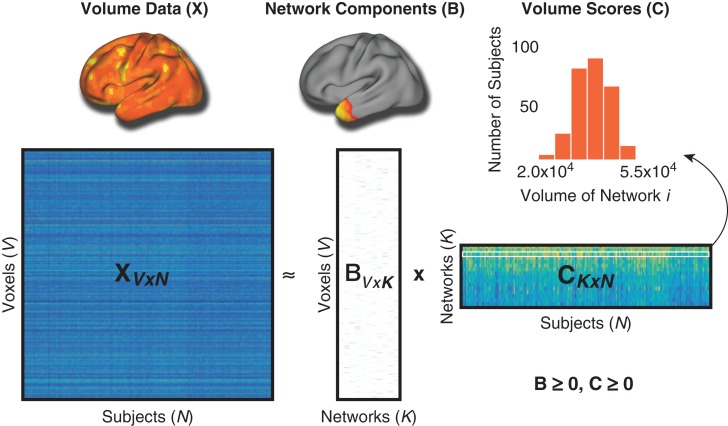
To derive NMF networks, first the NMF algorithm takes an input matrix X (“Volume Data”) containing voxel-wise RAVENS estimates (dimensions: 128,155 voxels × 278 participants), and approximates that matrix as a product of two matrices with non-negative elements: X ≅ BC (Fig. 1). The first matrix, B, is of size V × K and contains the estimated non-negative networks and their respective loadings on each of the V voxels where K is the user-specified number of networks. The B matrix, or “Network Components,” is composed of coefficients that denote the relative contribution of each voxel to a given network. These non-negative coefficients of the decomposition represent the entirety of the brain as a subject-specific addition of various parts. The second matrix, C, is of size K × N and contains subject-specific weights for each network. These subject-specific weights (“Volume Scores”) indicate the contribution of each network in reconstructing the original RAVENS map, and were evaluated for associations with gestational age as described in section Group-level statistical analyses.
Figure 1.
Non-negative matrix factorization. In this schematic, X represents the original data matrix as the product of two matrices, B and C. X contains the whole-brain volume data (RAVENS maps) for each voxel (rows) and for all subjects (columns). Above the X matrix is an example of the whole-brain volume data for one subject. B is matrix which contains the reduced number of K networks derived from NMF, and the loadings for each voxel on each of these networks. Above B is one example of NMF network loadings. C is a matrix that contains the subject-specific coefficients for volume in each network. The histogram above shows a sample row of the C matrix with scores for all subjects in one network. Importantly, both B and C are greater than or equal to 0, thus elements of the factorization are non-negative. Matrices are shown with following dimensions: V = number of voxels, N = number of participants; K = number of networks.
Consistent with prior studies using this technique (Sotiras et al. 2015, 2017), we ran multiple NMF solutions requesting 2–30 networks (in steps of two) in order to obtain a range of possible solutions for comparison. We then calculated the reconstruction error for each solution as the Frobenius norm between the RAVENS data matrix and the NMF approximation and plotted the reconstruction error for all solutions. NMF networks were visualized on the inflated Population-Average, Landmark-, and Surface-based (PALS) cortical surfaces using Caret (Van Essen et al. 2001; Van Essen 2005).
Group-level Statistical Analyses
After deriving the final solution from NMF analyses, we identified networks where volume was related to gestational age at birth (in weeks). Given that brain development is known to be a non-linear process (Giedd et al. 1999; Lenroot et al. 2007; Satterthwaite, Shinohara, et al. 2014), we modeled both linear and non-linear age effects using penalized splines within a generalized additive model (GAM; Wood 2001, 2004). The GAM was implemented to assess a penalty on non-linearity using restricted maximum likelihood (REML) in order to avoid over-fitting, and thus captures both linear and non-linear effects in a data-driven fashion. GAMs were implemented using the R package “mgcv” (https://cran.r-project.org/web/packages/mgcv/index.html). Based on prior work documenting sex differences in brain volume (Gur et al. 1999), we included sex as a covariate in the model. Furthermore, maternal level of education was added as an additional covariate as a proxy for socio-economic status.
We also performed relative likelihood ratio tests in order to test for the presence of a non-linear effect of gestational age. The parameter estimate for non-linear gestational age was fit as a random effect and tested using simulation-based likelihood ratio tests with 500 000 simulations (Scheipl et al. 2008; Wood 2011; Vandekar et al. 2015). The effect of gestational age was not found to have any significant non-linearity within our generalized additive models and, thus, non-linear gestational age was not included in the model. All models used the REML procedure, which produces unbiased estimates of variance and covariance parameters. Thus, the final model for both cognitive and image analyses was as follows (where Y is either cognitive factors or NMF networks):
First, we used this model to evaluate gestational age as a predictor of cognitive performance (as summarized by the factor scores described above). Second, we tested for associations between gestational age and volume in each of the NMF-derived structural covariance networks. To control for multiple testing across either cognitive factors or NMF-derived volume networks, we used the False Discovery Rate correction (FDR, Q < 0.05; Benjamini and Hochberg 1995).
Mediation Analyses
As a final step, we investigated whether the relationship between cognitive performance and gestational age was potentially accounted for by volume in the NMF-derived networks. We first assessed whether the structural covariance networks impacted by gestational age were similarly associated with performance on cognitive domains linked to gestational age. This was accomplished using the model described above, but in this case analyses were limited to networks where a significant association between gestational age and volume were found. Mediation analyses using the same covariates as prior were conducted using the procedures outlined by Preacher and Hayes using SPSS Statistics 22 (Preacher and Hayes 2008). Specifically, we examined the total effect of gestational age on cognitive performance (c path), the relationship between gestational age and volume (a path), the relationship between volume and cognitive performance (b path), and the direct effect of gestational age on cognitive performance after adding volume as a mediator to the model (c’ path). The indirect effect of gestational age on cognitive performance through the proposed mediator (network volume) was tested using both the Sobel test and bootstrapping procedures, which make fewer assumptions about the sampling distribution (Preacher and Hayes 2008). This procedure involves computing unstandardized indirect affects for each of 10 000 bootstrapped samples and calculating the 95% confidence interval. Multiple comparisons were accounted for using FDR corrected P-values for the Sobel tests, while the bootstrapping confidence intervals were confirmatory.
Sensitivity Analyses
We conducted separate sensitivity analyses to ensure that our results were not influenced by data quality, race, psychiatric medication use, or extremely preterm individuals. First, we added mean image quality ratings (described above) as an additional covariate in the model to ensure that image quality did not drive the observed associations between network volume and gestational age. Second, we tested whether adding race as an additional covariate in the model affected the results, given that there is a heightened risk of prematurity in under-represented groups. Third, we tested the same models after excluding the minority (12%) of participants who were taking psychiatric medications at the time of imaging. For the percentage of the sample taking each class of psychiatric medication, see Supplementary Table 1. And fourth, we removed those born extremely premature to test whether the associations were entirely driven by the extremely preterm youth.
Finally, in order to ascertain whether our choice of network dimensionality influenced the results, we conducted additional analyses. First, we conducted NMF analyses on the entire sample from the PNC (n = 1396 following medical exclusions and quality assurance) to determine whether this larger sample also suggested a 26-network solution. Second, to illustrate the stability of the 26-network solution, we conducted a split-half reliability analysis on the larger PNC sample. Third, we conducted the same gestational age analyses in the preterm sample using a 14-network solution for comparison with the 26-network solution. Lastly, we compared results from the NMF-based networks with traditional anatomically-defined regions, obtained using an advanced multi-atlas labeling approach. Specifically, 24 young adult T1 images from the OASIS dataset that were manually labeled by Neuromorphometrics, Inc. (http://Neuromorphometrics.com/) were registered to each subject’s T1 image using the top-performing SyN diffeomorphic registration (Klein et al. 2010; Avants et al. 2011). These label sets were synthesized into a final parcellation using joint label fusion (JLF; Wang et al. 2013).
Results
The Extent of Prematurity is Related to Individual Differences in Cognition
Lower gestational age at birth was associated with diminished overall cognitive accuracy (P = 0.03). In order to understand this effect, we examined each cognitive factor individually. We found that lower gestational age at birth was related to reduced executive function (P = 0.02); no significant relationship was found between gestational age and factors summarizing social cognition or episodic memory.
Non-negative Matrix Factorization Identifies Structural Covariance Networks
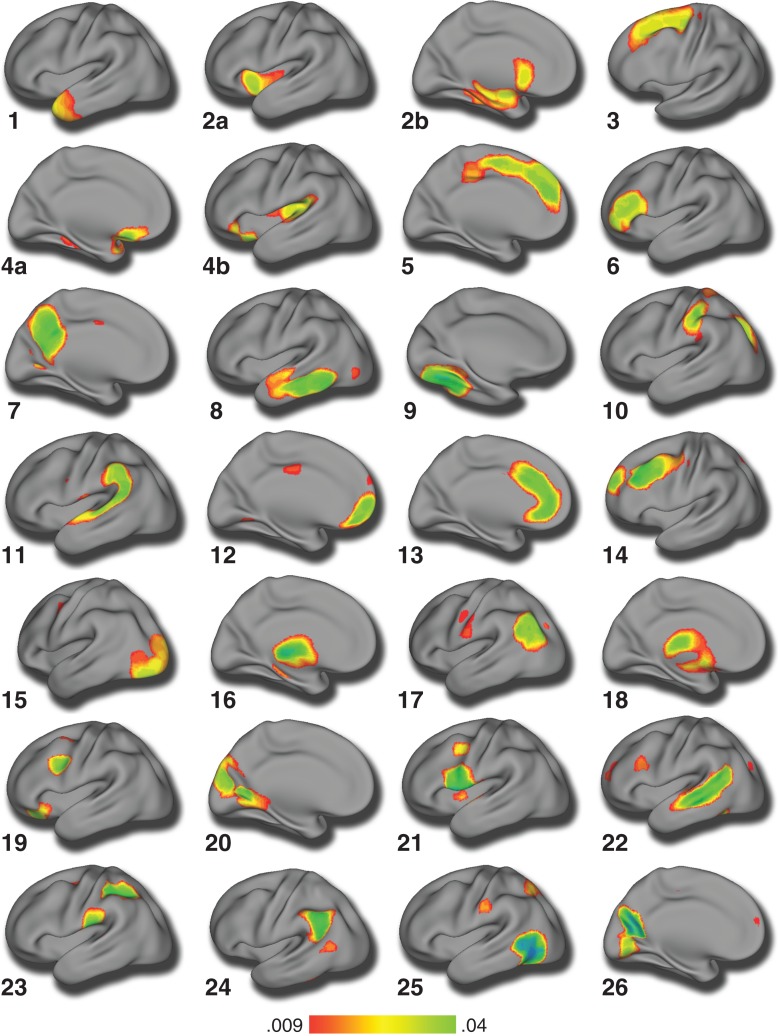
Structural covariance networks were derived using NMF, as described above. As expected, reconstruction error consistently decreased as the number of networks increased. Similar to previous applications of this method (Sotiras et al. 2015), reconstruction error stabilized at 26 networks (Supplementary Fig. 1). To validate our choice of the 26-network solution, we conducted NMF analyses on a much larger sample from the PNC (n = 1396) and found that the reconstruction error gradient plot for this large sample also suggests the 26-network solution is optimal (Supplementary Fig. 2). Additionally, to illustrate the stability of the 26-network solution, we conducted a split-half reliability analysis on the full PNC sample (n = 1396). The split-half results demonstrated a very high Adjusted Rand Index (ARI) for the 26-network solution (ARI = 0.98; see Supplementary Fig. 3), which suggests that our chosen solution is highly stable. Accordingly, the 26-network solution was used for all subsequent analyses (Fig. 2). As in prior work using NMF (Sotiras et al. 2015, 2017), the structural covariance networks identified were highly symmetric bilaterally (Supplementary Fig. 4). NMF clearly delineated networks corresponding to visual (network 15), and somoatosensory cortex (network 10). Networks within the higher-order association cortex were also represented, including the anterior cingulate cortex (network 13), posterior cingulate/precuneus (network 7), and ventromedial prefrontal cortex/orbitofrontal cortex (network 4). Medial temporal regions, the hippocampus, and the amygdala were joined in a network that also included the ventral striatum and anterior insula (network 2).
Figure 2.
Structural covariance networks delineated by NMF. Structural covariance networks are shown for the 26-network NMF solution. The spatial distribution of each network is indicated by loadings at each voxel in arbitrary units. For each network, we show the view that best captures the main area(s) of coverage; however, the loadings were generally bilateral. The anatomical coverage of each structural covariance network was a follows: (1) lateral temporal pole; (2) (a) insula, (b) caudate and hippocampus; (3) supplementary motor area; (4) (a) lateral orbitofrontal cortex and (b) posterior insula; (5) medial prefrontal cortex; (6) inferior prefrontal cortex; (7) precuneus; (8) lateral temporal cortex; (9) occipital fusiform gyrus; (10) postcentral gyrus and superior parietal cortex; (11) temporo-parietal junction; (12) frontal pole; (13) anterior cingulate cortex; (14) dorsal lateral prefrontal cortex; (15) occipital cortex; (16) thalamus; (17) lateral occipital cortex; (18) putamen; (19) orbitofrontal cortex and precentral gyrus; (20) cuneus; (21) premotor cortex; (22) superior temporal gyrus; (23) postcentral gyrus and supramarginal gyrus; (24) inferior parietal cortex; (25) lateral occipital cortex; and (26) precuneus and lingual gyrus.
Prematurity is Associated with Smaller Volumes in Multiple Structural Covariance Networks
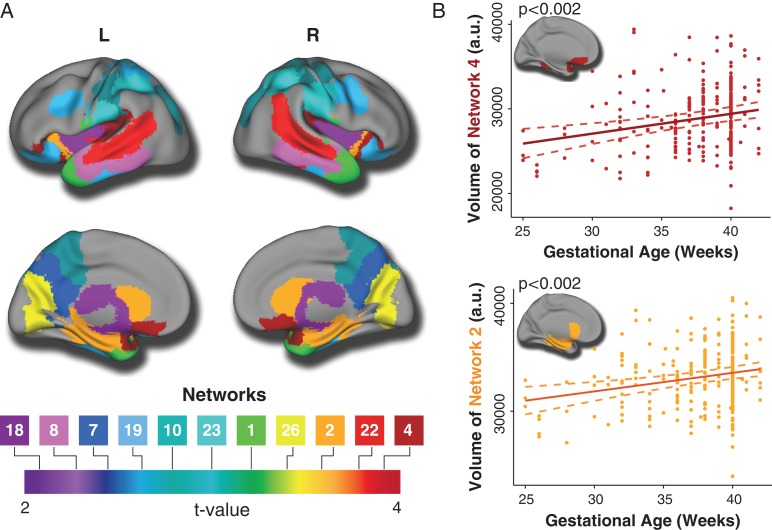
Having identified 26 structural covariance networks using NMF, we examined the associations of these networks with gestational age while controlling for sex, linear and non-linear age effects (using a penalized spline), and level of maternal education. Analyses revealed a significant association between gestational age and 11 networks following FDR correction (Fig. 3 and Table 2). In each of these networks, there was a positive association between gestational age and network volume. Notably, the strongest effects were found in networks that involved orbitofrontal, temporal, parietal cortex, as well as subcortical regions including the hippocampus.
Figure 3.
Gestational age is associated with smaller volumes in multiple structural networks. (A) Lower gestational age is associated with diminished volumes in 11 structural covariance networks which included orbitofrontal (4), temporal (1, 8, 22), parietal (7, 10, 23), and occipital (26) regions as well as subcortical regions (2, 18) including the hippocampus. Composite network boundaries were obtained by assigning each voxel to the network which has the highest loading for that voxel (from the B matrix), across all 26 networks. Multiple comparisons were accounted for using the False Discovery Rate (Q < 0.05). (B) Scatterplots show the relationship between gestational age and volume in Networks 2 and 4, which represent hippocampus/caudate/insula and medial orbitofrontal cortex, respectively. Dotted lines represent the 95% confidence interval.
Table 2.
NMF networks significantly associated with gestational age (n = 278)
| NMF network | B | SE | t a | P fdr |
|---|---|---|---|---|
| Network 1: lateral temporal pole | 244.34 | 74.15 | 3.30 | 0.005 |
| Network 2: insula, amygdala, caudate, hippocampus | 172.00 | 46.48 | 3.70 | 0.002 |
| Network 4: lateral orbitofrontal cortex and posterior insula | 234.98 | 61.70 | 3.81 | 0.002 |
| Network 7: precuneus | 163.05 | 62.85 | 2.59 | 0.029 |
| Network 8: lateral temporal cortex | 168.23 | 70.20 | 2.40 | 0.041 |
| Network 10: postcentral gyrus and superior parietal cortex | 167.96 | 53.26 | 3.15 | 0.007 |
| Network 18: putamen | 161.09 | 67.37 | 2.39 | 0.041 |
| Network 19: orbitofrontal cortex and precentral gyrus | 147.88 | 51.54 | 2.87 | 0.014 |
| Network 22: superior temporal gyrus | 175.41 | 46.62 | 3.76 | 0.002 |
| Network 23: postcentral gyrus and supramarginal gyrus | 149.60 | 45.63 | 3.28 | 0.005 |
| Network 26: precuneus and lingual gyrus | 180.81 | 49.56 | 3.65 | 0.002 |
adf = 274.
Structural Network Abnormalities Account for Executive Deficits Associated with Prematurity
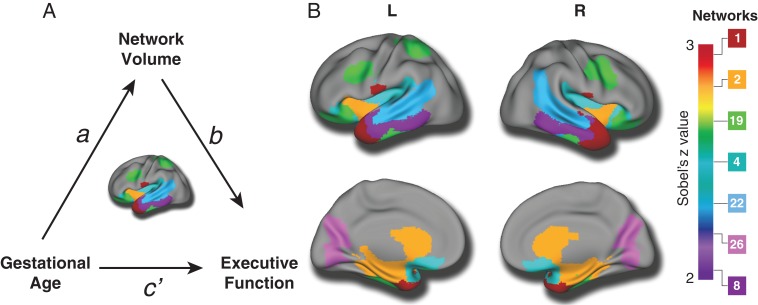
As lower gestational age was associated with both impaired executive function and smaller volumes in structural brain networks, we next evaluated whether executive functioning was also related to the magnitude of structural abnormalities in these particular networks. Impairments in executive functioning were significantly associated with smaller brain volumes in the same 11 networks identified as having a significant relationship with gestational age after FDR correction (see Table 3). These results suggest that alterations in brain structure associated with prematurity could be related to observed impairments of executive function. To test this explicitly, we conducted a mediation analysis, which revealed significant effects for seven brain networks. Networks where a significant association was present included regions such as the orbitofrontal cortex, lateral temporal cortex, and the hippocampus (Fig. 4 and Table 4). This suggests that the impact of gestational age on executive functioning may be potentially accounted for by deficits in brain structure.
Table 3.
NMF networks significantly associated with executive functioning (n = 278)
| NMF network | B | SE | t a | P fdr |
|---|---|---|---|---|
| Network 1: lateral temporal pole | 2100.01 | 310.07 | 6.77 | < 0.001 |
| Network 2: insula, amygdala, caudate, hippocampus | 1045.45 | 192.95 | 5.42 | < 0.001 |
| Network 4: lateral orbitofrontal cortex and posterior insula | 1262.37 | 263.56 | 4.79 | < 0.001 |
| Network 7: precuneus | 1271.58 | 260.42 | 4.88 | < 0.001 |
| Network 8: lateral temporal cortex | 1708.92 | 285.13 | 5.99 | < 0.001 |
| Network 10: postcentral gyrus and superior parietal cortex | 925.82 | 228.12 | 4.06 | < 0.001 |
| Network 18: putamen | 744.02 | 288.38 | 2.58 | 0.010 |
| Network 19: orbitofrontal cortex and precentral gyrus | 1181.51 | 212.88 | 5.55 | < 0.001 |
| Network 22: superior temporal gyrus | 697.41 | 194.38 | 3.59 | 0.001 |
| Network 23: postcentral gyrus and supramarginal gyrus | 782.44 | 200.50 | 3.90 | < 0.001 |
| Network 26: precuneus and lingual gyrus | 982.22 | 210.03 | 4.68 | < 0.001 |
adf = 275.
Figure 4.
Lower executive functioning in preterm youth is accounted for in part by deficits in structural covariance networks. (A) Based on the significant relationship between gestational age and both executive function and several key structural covariance networks, we tested the prediction that the association between cognitive performance and gestational age was accounted for by individual differences in structural covariance networks. (B) Mediation analyses revealed significant indirect effects for seven networks, suggesting the impact of gestational age on executive function may be driven in part by deficits in brain structure. Multiple comparisons were accounted for using the False Discovery Rate (Q < 0.05).
Table 4.
The indirect effects of the relationship between gestational age and executive functioning with NMF network as the mediator (n = 278)
| NMF network | Sobel za | P fdr | B | SE | 95% CI |
|---|---|---|---|---|---|
| Network 1: lateral temporal pole | 2.91 | 0.044 | 0.014 | 0.005 | 0.007, 0.026 |
| Network 2: insula, amygdala, caudate, hippocampus | 2.67 | 0.044 | 0.011 | 0.005 | 0.004, 0.022 |
| Network 4: lateral orbitofrontal cortex and posterior insula | 2.38 | 0.044 | 0.009 | 0.004 | 0.002, 0.019 |
| Network 8: lateral temporal cortex | 2.17 | 0.047 | 0.008 | 0.004 | 0.003, 0.018 |
| Network 19: orbitofrontal cortex and precentral gyrus | 2.39 | 0.044 | 0.009 | 0.004 | 0.003, 0.018 |
| Network 22: superior temporal gyrus | 2.32 | 0.044 | 0.009 | 0.004 | 0.002, 0.018 |
| Network 26: precuneus and lingual gyrus | 2.18 | 0.047 | 0.008 | 0.004 | 0.002, 0.017 |
adf = 272.
Sensitivity Analyses
As a final step, we conducted sensitivity analyses to ensure that our results were not influenced by data quality, race, psychiatric medication use, or extremely preterm individuals. When mean image quality (averaged across three expert raters) was added as a model covariate, the same 11 (of 26) NMF networks continued to show significant associations with gestational age after FDR correction (Supplementary Table 2). Adding race as an additional covariate resulted in the loss of only one of the 11 FDR-corrected significant networks (network 8), but this network remained significant at uncorrected levels (Supplementary Table 3). When participants taking psychoactive medication were excluded, 9 of 11 NMF networks remained significant after correction with FDR; networks 8 and 18 no longer survived correction for multiple comparisons but were significant at uncorrected levels (Supplementary Table 4). After removing those born extremely premature (<28 weeks gestation), we found that the associations between gestational age and volume size were somewhat less strong—as would be expected given the restricted range of values—but were still in the expected direction (Supplementary Table 5). In this subgroup, networks 7, 10, and 19 were no longer significant at uncorrected levels.
Supplemental analyses also showed high correspondence between the 26-network solution and an alternative 14-network solution in terms of brain regions associated with gestational age. In particular, 8 of the 14 networks showed significant associations with gestational age, including regions such as the lateral temporal pole, orbitofrontal cortex, insula, precuneus, superior temporal gyrus, and putamen. And finally, we used structural regions of interest (ROIs) derived from a JLF parcellation for comparison to NMF. The JLF ROIs showed similar regions associated with gestational age including the orbitofrontal and temporal cortices, as well as the insula, amygdala, caudate, hippocampus, and precuneus (Supplementary Fig. 5).
Discussion
The current study evaluated the dimensional impact of gestational age on long-term cognitive outcomes and structural brain networks in a large sample of youth. We identified three inter-related results that increase our understanding of the impact of prematurity on neurodevelopment. First, lower gestational age was associated with lower overall cognitive accuracy, and specifically with deficits in executive function. Second, lower gestational age was associated with smaller brain volumes in several structural brain networks involving the orbitofrontal, temporal, and parietal cortices as well as subcortical structures including the hippocampus. Third, the relationship between lower gestational age and executive deficits was accounted for in part by smaller brain volume in these structural networks. Taken together, these results emphasize that prematurity is a dimensional, rather than categorical, risk factor for neurodevelopmental abnormalities across development.
The results of the current study were facilitated by the use of an advanced image analysis technique, NMF (Sotiras et al. 2015), which allowed us to delineate structural covariance networks in a data-driven fashion. NMF is particularly effective at partitioning high-dimensional MRI data into more meaningful networks. In comparison to the compact, positive signed networks produced by NMF, Principal Component Analysis and other techniques produce widely-dispersed networks that have both positive and negative directions, often limiting straightforward interpretations. NMF also improves statistical power compared to standard mass-univariate analyses typical of VBM studies, as correction for multiple comparisons occurs over a small number of structural networks, rather than hundreds of thousands of individual voxels. Using NMF, we found that lower gestational age was associated with smaller volumes in a number of cortical and subcortical networks, representing regions that undergo substantial structural and functional changes in utero. The results of this study suggest that structural covariance networks derived from NMF are sensitive to the effects of premature birth. The impact of prematurity on structural covariance networks has been shown in studies using seed-based structural connectivity (Nosarti et al. 2011; Scheinost et al. 2017), suggesting that developmental changes following preterm birth are related to complex interrelations across brain regions. However, NMF is advantageous over seed-based covariance analyses because it is not limited to a small number of anatomical seed regions chosen a priori.
Our findings are consistent with previous studies showing that premature birth is associated with smaller volumes in both cortical and subcortical regions (Nosarti et al. 2002, 2008; Nagy et al. 2009; Cheong et al. 2013). Previous studies have described long-lasting structural brain alterations following preterm birth, including smaller gray and white matter volumes (Nosarti et al. 2008; Nagy et al. 2009), as well as regional volumetric differences within structures such as the hippocampus (Isaacs et al. 2000; Nosarti et al. 2002; Beauchamp et al. 2008) and amygdala (Cismaru et al. 2016). The preterm brain is vulnerable to impaired neurodevelopment, as the majority of brain development occurs in the third trimester. The third trimester of pregnancy represents a time of rapid growth and elaboration of the fetal brain, characterized by extensive neuron production, migration and differentiation, as well as the formation of gyri and sulci (Stiles and Jernigan 2010; Kersbergen et al. 2016). Thus, prematurity during this important period may have profound effects. The current study extends prior work by examining prematurity dimensionally across the continuum from extremely preterm to full term.
We provide evidence for both executive dysfunction and structural brain abnormalities across development that scale with the extent of prematurity. Executive function has been described as a collection of interdependent abilities and skills that are responsible for goal-oriented behavior (Taylor and Clark 2016; McKenna et al. 2017). Preterm individuals demonstrate poorer executive functioning across a range of tasks including verbal fluency, cognitive flexibility, inhibition, working memory, switching, and concept generation (Marlow et al. 2007; Nosarti et al. 2007; Aarnoudse-Moens et al. 2009; Loe et al. 2015; Delane et al. 2016; Wehrle et al. 2016; Kroll et al. 2017). The neurocognitive battery utilized in the current study included executive tests of abstraction and mental flexibility, vigilance and visual attention, and working memory (Gur et al. 2010). Consistent with case-control studies, we found that lower gestational age was associated with poorer performance on these tasks. Furthermore, prior studies of brain regions and networks underlying executive functioning implicate the prefrontal/orbitofrontal cortices, as well as temporal and parietal cortices (Miller and Cohen 2001; Alvarez and Emory 2006; Houdé et al. 2010; Ikkai and Curtis 2011; Taylor and Clark 2016). Likewise, we found executive functioning deficits were associated with smaller volumes in the orbitofrontal, temporal, and parietal cortices across the range of prematurity. These results were attenuated when those born extremely premature (<28 weeks) were excluded, supporting the notion that deficits associated with prematurity exhibit a gradation of severity. Together, our results suggest that the spectrum of prematurity has important implications for maturation of brain regions associated with executive functioning.
Interestingly, the current study found that cognitive deficits were primarily observed in the domain of executive function, consistent with prior work (Anderson et al. 2004; Taylor et al. 2004; Marlow et al. 2007; Nosarti et al. 2007; Aarnoudse-Moens et al. 2009; Wehrle et al. 2016; Kroll et al. 2017), rather than social cognition or episodic memory. In contrast, one previous study showed poorer episodic memory performance in adolescents born with very low birthweights (Isaacs et al. 2000). A number of studies show impairments in social functioning related to prematurity, including difficulty establishing relationships, greater shyness and behavioral inhibition, and lower social competence (Hille et al. 2008; Schmidt et al. 2008; Ritchie et al. 2015). Furthermore, studies have investigated the correlation between structural alterations and impairments in social functioning in the preterm population. Nosarti and colleagues described an association between smaller caudate volumes and poorer social adjustment in preterm youth; however, this did not persist into adolescence (Nosarti et al. 2005). Another study showed that poorer social cognition performance was associated with abnormalities of structural connectivity in those born extremely preterm (Fischi-Gómez et al. 2015). However, these prior studies focused on social functioning, while the social tasks probed by the current study assessed the abilities to identify facial expressions of emotion, to decode the intensity of emotional facial expressions, and to identify the age of a face (Gur et al. 2010). Although emotion identification may be a prerequisite for higher-order social cognitive skills, tasks that assess emotion identification are not identical to social functioning (e.g., parent/peer relationships, behavioral inhibition, social competence, etc.). Thus, given the tasks used, it may not be surprising that the current study did not find an association between gestational age and social cognition. Impairments in the ability to adequately identify emotions have not been widely reported in relation to premature birth; therefore, this specific skill may be less affected by prematurity than broader measures of social functioning.
The current study has both strengths and limitations. Despite leveraging a large sample of youth imaged through the PNC, data regarding gestational age was not available through electronic medical records for the majority of the participants who completed neuroimaging. Additionally, although we controlled for variables such as sex and level of maternal education, other potentially informative variables were not available such as medical comorbidities, neonatal complications, and steroid exposure, which may impact neurocognitive outcomes (Kersbergen et al. 2016; Taylor and Clark 2016; Young et al. 2016). Although the large age range in the current sample could be viewed as a limitation, we have shown previously that the covariance of these brain structures is stable over the developmental age range (Sotiras et al. 2017) and we also controlled for age effects in the model. Furthermore, a larger sample of premature youth could be used in future work to determine whether the structural covariance networks themselves differ between preterm and full term youth. Additionally, our study was limited to emotion identification tasks; therefore, future work would benefit from examining tasks designed specifically to measure social cognition. Lastly, we caution that causal inferences regarding brain structure and cognitive function cannot be made from our cross-sectional mediation analyses. Moving forward, longitudinal designs with prospective data collection are needed to account for the temporal precedence between these variables.
In summary, these results suggest that the extent of prematurity is significantly related to both neurocognitive and structural brain deficits across development. Furthermore, abnormalities in specific orbitofrontal, temporal, parietal, and subcortical brain networks may in part explain the relationship between lower gestational age and executive impairment. The finding that executive deficits associated with the degree of prematurity persist throughout development has important implications for identifying individuals at higher risk of long-term neurodevelopmental impairment. Perhaps most importantly, the current data suggests that interventions that reduce the incidence of prematurity may have a substantial benefit to public health.
Supplementary Material
Footnotes
We thank the acquisition and recruitment team, including Karthik Prabhakaran and Jeff Valdez. Thanks to Chad Jackson for data management and systems support, to Dr Monica E. Calkins for phenotyping expertize, and to Dr Elizabeth E. Foglia for her comments on the manuscript. Dr Shinohara has received legal consulting and advisory board income from Genentech/Roche. All other authors (Dr Nassar, Dr Kaczkurkin, Mr Xia, Dr Sotiras, Dr Pehlivanova, Dr Moore, Mr Garcia de La Garza, Dr Roalf, Mr Rosen, Dr Lorch, Ms Ruparel, Dr Davatzikos, Dr R.E. Gur, Dr R.C. Gur, and Dr Satterthwaite) reported no biomedical financial interests or potential conflicts of interest. Conflict of Interest: None declared.
Funding
This work was supported by grants from the National Institute of Mental Health including a Research Supplement to Promote Diversity in Health-Related Research (NIMH; grant numbers: R01MH107703 to T.D.S., R01MH113550 to TDS, R01NS085211 to R.T.S., R01MH112847 to R.T.S. and T.D.S., R01MH107235 to R.C.G.); the Dowshen Program for Neuroscience, and the Lifespan Brain Institute at the Children’s Hospital of Philadelphia and Penn Medicine. The PNC was funded through RC2 grants MH089983 and MH089924 to R.E.G. from the NIMH. Support for developing statistical analyses (R.T.S. and T.D.S.) was provided by a seed grant from the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Support for developing multivariate pattern analysis software (A.S. and T.D.S.) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Support was also provided by a NARSAD Young Investigator Award (ANK) as well as a Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) grant (K12 HD085848) and Penn PROMOTES Research on Sex and Gender in Health grant at the University of Pennsylvania (ANK).
References
- Aarnoudse-Moens CSH, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas-Kuperus N. 2009. Executive function in very preterm children at early school age. J Abnorm Child Psychol. 37:981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, Raznahan A. 2016. Subtle in-scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum Brain Mapp. 37:2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E. 2013. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin M, Rooney M, Griffiths T, Cuddy M, Wyatt J, Rifkin L, Murray R. 2006. Neurological abnormalities in young adults born preterm. J Neurol Neurosurg Psychiatry. 77:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. 2006. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 16:17–42. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group . 2004. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 114:50–57. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Treyvaud K, Neil JJ, Cheong JLY, Hunt RW, Thompson DK, Lee KJ, Doyle LW, Inder TE. 2017. Associations of newborn brain magnetic resonance imaging with long-term neurodevelopmental impairments in very preterm children. J Pediatr. 187:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. 2008. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 131:2986–2994. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 57:289–300. [Google Scholar]
- Botellero VL, Skranes J, Bjuland KJ, Håberg AK, Lydersen S, Brubakk AM, Indredavik MS, Martinussen M. 2017. A longitudinal study of associations between psychiatric symptoms and disorders and cerebral gray matter volumes in adolescents born very preterm. BMC Pediatr. 17:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JLY, Anderson PJ, Roberts G, Burnett AC, Lee KJ, Thompson DK, Molloy C, Wilson-Ching M, Connelly A, Seal ML, et al. 2013. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PLoS One. 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JL, Doyle LW, Burnett AC, Lee KJ, Walsh JM, Potter CR, Treyvaud K, Thompson DK, Olsen JE, Anderson PJ, et al. 2017. Association between moderate and late preterm birth and neurodevelopment and social–emotional development at age 2 years. JAMA Pediatr. 171:2–7. [DOI] [PubMed] [Google Scholar]
- Cismaru AL, Gui L, Vasung L, Lejeune F, Barisnikov K, Truttmann A, Borradori Tolsa C, Hüppi PS. 2016. Altered amygdala development and fear processing in prematurely born infants. Front Neuroanat. 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RWI. 2005. Perinatal and postnatal factors in very preterm infants and subsequent cognitive and motor abilities. Arch Dis Child - Fetal Neonatal Ed. 90:F60–F63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA. 2002. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain. 125:1646–1659. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Genc A, Xu D, Resnick SM. 2001. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. NeuroImage. 14:1361–1369. [DOI] [PubMed] [Google Scholar]
- de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. 2012. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 54:313–323. [DOI] [PubMed] [Google Scholar]
- Delane L, Bayliss DM, Campbell C, Reid C, French N, Anderson M. 2016. Poor executive functioning in children born very preterm: using dual-task methodology to untangle alternative theoretical interpretations. J Exp Child Psychol. 152:264–277. [DOI] [PubMed] [Google Scholar]
- Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, Burguet A, Roze J-C, Matis J, Picaud J-C, et al. , EPIPAGE Study Group . 2009. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE study. Pediatrics. 123:1485–1492. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. 2013. Networks of anatomical covariance. NeuroImage. 80:489–504. [DOI] [PubMed] [Google Scholar]
- Fischi-Gómez E, Vasung L, Meskaldji D-E, Lazeyras F, Borradori-Tolsa C, Hagmann P, Barisnikov K, Thiran J-P, Hüppi PS. 2015. Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cereb Cortex. 25:2793–2805. [DOI] [PubMed] [Google Scholar]
- Foulder-Hughes LA, Cooke RW. 2003. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol. 45:97–103. [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2:861–863. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. 2010. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 187:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 19:4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. 2002. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 346:149–157. [DOI] [PubMed] [Google Scholar]
- Hille ETM, Dorrepaal C, Perenboom R, Gravenhorst JB, Brand R, Verloove-Vanhorick SP. 2008. Social lifestyle, risk-taking behavior, and psychopathology in young adults born very preterm or with a very low birthweight. J Pediatr. 152:793–800. [DOI] [PubMed] [Google Scholar]
- Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA, Das A, Tyson JE, Stevenson DK, Carlo WA, et al. 2015. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 135:e32–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Senich KL, Jokinen C, Sasson O, Morris AR, Thomas KM. 2017. Early executive function differences in infants born moderate-to-late preterm. Early Hum Dev. 113:23–30. [DOI] [PubMed] [Google Scholar]
- Houdé O, Rossi S, Lubin A, Joliot M. 2010. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci. 13:876–885. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. 2011. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 49:1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. 2005. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 115:286–294. [DOI] [PubMed] [Google Scholar]
- Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. 2003. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 143:171–179. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG. 2000. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 47:713–720. [DOI] [PubMed] [Google Scholar]
- Karolis VR, Froudist-Walsh S, Kroll J, Brittain PJ, Tseng CEJ, Nam KW, Reinders AATS, Murray RM, Williams SCR, Thompson PM, et al. 2017. Volumetric grey matter alterations in adolescents and adults born very preterm suggest accelerated brain maturation. NeuroImage. 163:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersbergen KJ, Makropoulos A, Aljabar P, Groenendaal F, de Vries LS, Counsell SJ, Benders MJNL. 2016. Longitudinal regional brain development and clinical risk factors in extremely preterm infants. J Pediatr. 178:93–100. [DOI] [PubMed] [Google Scholar]
- Keunen K, Išgum I, van Kooij BJM, Anbeek P, van Haastert IC, Koopman-Esseboom C, Fieret-van Stam PC, Nievelstein RAJ, Viergever MA, de Vries LS, et al. 2016. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J Pediatr. 172:88–95. [DOI] [PubMed] [Google Scholar]
- Klein A, Ghosh SS, Avants B, Yeo BTT, Fischl B, Ardekani B, Gee JC, Mann JJ, Parsey RV. 2010. Evaluation of volume-based and surface-based brain image registration methods. NeuroImage. 51:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J, Karolis V, Brittain PJ, Tseng CEJ, Froudist-Walsh S, Murray RM, Nosarti C. 2017. Real-life impact of executive function impairments in adults who were born very preterm. J Int Neuropsychol Soc. 23:381–389. [DOI] [PubMed] [Google Scholar]
- Lee DD, Seung HS. 1999. Learning the parts of objects by non-negative matrix factorization. Nature. 401(6755):788–791. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe IM, Chatav M, Alduncin N. 2015. Complementary assessments of executive function in preterm and full-term preschoolers. Child Neuropsychol. 21:331–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell MA, Wolke D. 2007. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 120:793–804. [DOI] [PubMed] [Google Scholar]
- McKenna R, Rushe T, Woodcock KA. 2017. Informing the structure of executive function in children: a meta-analysis of functional neuroimaging data. Front Hum Neurosci. 11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A. 2005. Structural covariance in the human cortex. J Neurosci. 25:8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. [DOI] [PubMed] [Google Scholar]
- Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. 2015. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 29:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Ashburner J, Andersson J, Jbabdi S, Draganski B, Skare S, Bohm B, Smedler A-C, Forssberg H, Lagercrantz H. 2009. Structural correlates of preterm birth in the adolescent brain. Pediatrics. 124:e964–e972. [DOI] [PubMed] [Google Scholar]
- Niwa T, Suzuki K, Sugiyama N, Imai Y. 2017. Regional volumetric assessment of the brain in moderately preterm infants (30–35 gestational weeks) scanned at term-equivalent age on magnetic resonance imaging. Early Hum Dev. 111:36–41. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Al-Asady MHS, Frangou S, Stewart AL, Rifkin L, Murray RM. 2002. Adolescents who were born very preterm have decreased brain volumes. Brain. 125:1616–1623. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Allin MP, Frangou S, Rifkin L, Murray RM. 2005. Hyperactivity in adolescents born very preterm is associated with decreased caudate volume. Biol Psychiatry. 57:661–666. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SCR, Murray RM. 2008. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 131:205–217. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM. 2007. Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc. 13:571–581. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Mechelli A, Herrera A, Walshe M, Shergill SS, Murray RM, Rifkin L, Allin MPG. 2011. Structural covariance in the cortex of very preterm adolescents: a voxel-based morphometry study. Hum Brain Mapp. 32:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Nam KW, Walshe M, Murray RM, Cuddy M, Rifkin L, Allin MPG. 2014. a. Preterm birth and structural brain alterations in early adulthood. NeuroImage Clin. 6:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Nam KW, Walshe M, Murray RM, Cuddy M, Rifkin L, Allin MPG. 2014. b. Preterm birth and structural brain alterations in early adulthood. NeuroImage Clin. 6:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PWR, Stewart AL, Rifkin L, Murray RM. 2004. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 127:2080–2089. [DOI] [PubMed] [Google Scholar]
- Pardoe HR, Kucharsky Hiess R, Kuzniecky R. 2016. Motion and morphometry in clinical and nonclinical populations. NeuroImage. 135:177–185. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR. 2003. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 111:939–948. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, et al. 2000. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 284:1939–1947. [DOI] [PubMed] [Google Scholar]
- Pharoah POD, Stevenson CJ, West CR. 2003. General certificate of secondary education performance in very low birthweight infants. Arch Dis Child. 88:295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. 2008. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 40:879–891. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Bora S, Woodward LJ. 2015. Social development of children born very preterm: a systematic review. Dev Med Child Neurol. 57:899–918. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, Smyser CD. 2017. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J Am Acad Child Adolesc Psychiatry. 56:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa P, Cook PA, Davatzikos C, Elliott MA, Garcia de la Garza A, et al. 2017. Data-driven assessment of structural image quality. NeuroImage. 169:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, Roalf DR, Hopson R, Prabhakaran K, Behr M, et al. 2016. The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. NeuroImage. 124:1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, et al. 2014. a. Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage. 86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, et al. 2014. b. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci. 111:8643–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR. 2017. Alterations in anatomical covariance in the prematurely born. Cereb Cortex. 27:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheipl F, Greven S, Kuchenhoff H. 2008. Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models. Comput Stat Data Anal. 52:3283–3299. [Google Scholar]
- Schmidt LA, Miskovic V, Boyle MH, Saigal S. 2008. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 122:e181–e187. [DOI] [PubMed] [Google Scholar]
- Sotiras A, Resnick SM, Davatzikos C. 2015. Finding imaging patterns of structural covariance via non-negative matrix factorization. NeuroImage. 108:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, Davatzikos C. 2017. Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci U S A. 114:3527–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. 2010. The basics of brain development. Neuropsychol Rev. 20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Clark CAC. 2016. Executive function in children born preterm: risk factors and implications for outcome. Semin Perinatol. 40:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Filipek PA, Juranek J, Bangert B, Minich N, Hack M. 2011. Brain volumes in adolescents with very low birth weight: effects on brain structure and associations with neuropsychological outcomes. Dev Neuropsychol. 36:96–117. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. 2006. Consequences and risks of. J Dev Behav Pediatr. 27:459–469. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. 2004. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 10:149–163. [DOI] [PubMed] [Google Scholar]
- Tisdall MD, Reuter M, Qureshi A, Buckner RL, Fischl B, van der Kouwe AJW. 2016. Prospective motion correction with volumetric navigators (vNavs) reduces the bias and variance in brain morphometry induced by subject motion. NeuroImage. 127:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CEJ, Froudist-Walsh S, Brittain PJ, Karolis V, Caldinelli C, Kroll J, Counsell SJ, Williams SCR, Murray RM, Nosarti C. 2017. A multimodal imaging study of recognition memory in very preterm born adults. Hum Brain Mapp. 38:644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. 2005. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 28:635–662. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. 2001. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 8:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekar SN, Shinohara RT, Raznahan A, Roalf DR, Ross M, DeLeo N, Ruparel K, Verma R, Wolf DH, Gur RC, et al. 2015. Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 35:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Suh JW, Das SR, Pluta JB, Craige C, Yushkevich PA. 2013. Multi-atlas segmentation with joint label fusion. IEEE Trans Pattern Anal Mach Intell. 35:611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle FM, Kaufmann L, Benz LD, Huber R, O’Gorman RL, Latal B, Hagmann CF. 2016. Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Hum Dev. 92:37–43. [DOI] [PubMed] [Google Scholar]
- Wood SN. 2001. mgcv: GAMs and generalized ridge regression for R. R News. 1:20–25. [Google Scholar]
- Wood SN. 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 99:673–686. [Google Scholar]
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B (Statistical Methodol). 73:3–36. [Google Scholar]
- World Health Organization 2012. Born Too Soon: The Global Action Report on Preterm Birth http://apps.who.int/iris/bitstream/10665/44864/1/9789241503433_eng.pdf?ua=1
- Young JM, Morgan BR, Powell TL, Moore AM, Whyte HEA, Smith ML, Taylor MJ. 2016. Associations of perinatal clinical and magnetic resonance imaging measures with developmental outcomes in children born very preterm. J Pediatr. 170:90–96. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. 2010. Network-level structural covariance in the developing brain. Proc Natl Acad Sci. 107:18191–18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.