Abstract
Neuron cells consist of the soma or cell body, axons, dendritic arbor with multiple branches, and dendritic spines which are the substrates for memory storage and synaptic transmission. Detriments in neuron morphology are suggested to play a key role in cognitive impairments following brain irradiation. Multiple molecular mechanisms are involved in the regulation and stability of neuron morphology, while the effects of radiation on these processes have not been studied extensively. In this report, we consider possible biological targets in neurons for energy deposition (ED) by charged particles that could lead to neuron morphology detriments, and the resulting dose and radiation quality dependence of such detriments. The track structures of heavy ions including high charge and energy (HZE) particles consists of core of high-ED events and a penumbra of sparse ED from δ-ray electrons produced in ionization of target molecules. We consider the role of track structure relative to possible targets causative in the degradation of morphology.
Studies of cranial irradiation (IR) with low-linear energy transfer (LET) irradiation and HZE particles have revealed disruption of cognitive functions as well as drastic inhibition of hippocampal proliferation and neurogenesis(1–3). Irradiation also impacts the medial prefrontal cortex (mPFC) that affects executive function and decision making(4). A possible mechanism contributing to the cognitive detriments experienced following cranial irradiation is the persistent reduction in the structural complexity of mature neurons throughout the brain. Cranial irradiation reduces dendritic complexity and spine density, and alters the density of specific spine types(5–8).
Heavy ion irradiation at low to moderate doses occurs in exposures to cancer patients(9) near the periphery of the tumor volume and for chronic cosmic ray exposures during space missions(10). For medical radiation exposures reducing normal tissue damage to acceptable tolerances is the major consideration, while radiation protection both on Earth and in space is based on principles of risk justification and limitation. Deterministic effects occur above dose thresholds after a significant number of cells are damaged within a tissue, with a severity that increases with dose, including an inverse correlation between dose and latency. Dose limits for deterministic effects including risks to the skin, blood forming system, and lens are based on avoiding all risk with limits set at estimated doses below a likely threshold for clinically significant effects. The increased incidence of cataracts observed after the low space radiation doses of past space missions(11, 12), where only a small fraction of cells in a tissue are damaged, suggests that new paradigms for deterministic effects should be considered for HZE particles. Cognitive changes following low dose irradiation likely involve distinct biological factors compared to other tissues, including changes to synapse plasticity and distinct modes of oxidative damage.
Dendritic complexity influences many aspects of neuronal function, including action potential propagation and information processing. Alterations in neuronal branching and dendritic spine morphology, including the shape, size and number of spines, has been found in several brain disorders, suggesting that dendritic spines may serve as a common factor in the pathogenesis of neurodegenerative disorders. Studies in mice and rats provide some evidence for the dose and radiation quality dependence of cognitive changes; however, their relationship to morphological changes is poorly understood. The larger ED in small volumes representative of neuron structures from high LET radiation suggests distinct biological effects may occur between photons and HZE particles. In this report, we consider possible targets for ED in neuron cells that could be linked to changes in neuron morphology and aid in understanding cognitive detriments.
NEURON MORPHOLOGY
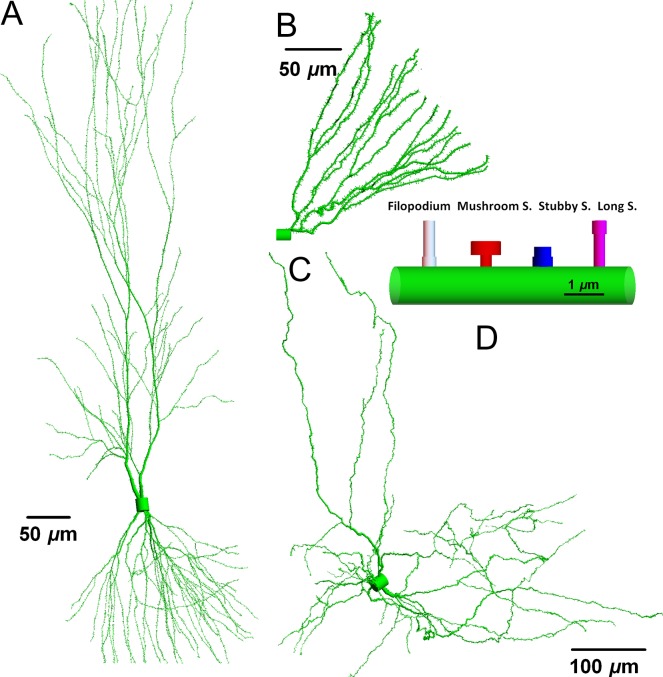
Neuron morphology can show great variability in different brain regions and in general this structural variability can be attributed to dendritic complexity. The cell body or soma containing the nucleus will have variable volume between different types of neurons. The soma diameter can be as small as 7 μm in cerebral granule cells that are the smallest and most numerous of neurons in the brain, 15 μm in dentate granule cells(13) in the hippocampus and 20 μm or larger for pyramidal cells in the hippocampus and cortex(14). The number of main dendritic branches is also a characteristic of different types of neurons. Figure 1 displays three types of neurons reconstructed from the public depository www.neuromorpho.org(15). Dendrites like the branches of a botanical tree are continuous structures. In general, dendrites have larger radius close to soma (~3 μm), become thinner as the path-length distance from the soma increases with diameters less than ~0.5 μm at the distal tips. A proximal dendrite gives rise to two daughters, distal dendrites at the dendritic bifurcation points. A main dendritic branch can have multiple branch points and branch order is a directional counting order of such bifurcation points along a dendritic path. Human neurons are typical longer in length than neurons of rodent models such as mice.
Figure 1.
Neurons and neuronal compartments. The neuronal morphology and arborization are distinct for pyramidal (A), dentate granule cell (B) and dopaminergic (C) neurons. However, the underlying geometries are similar in different neuron types with filopodia and long, mushroom and stubby spines (D). Neuromorpho ID numbers(15) corresponding to structures shown are (A) NMO 00207, (B) NMO 07642 and (C) NMO 09579. Total dendritic lengths and number of branches: (14 793, 2491, 10 539) μm and (181, 29, 417), respectively.
Dendritic spines are the membrane protrusions on the dendritic branches and house specialized molecular machinery including intracellular proteins, membrane inserted ion channels, receptors and adhesion proteins. The size, morphology and predicted function of spines also show variability. First, immature spines, called filopodia are more motile membrane invaginations directed by fast polymerization and de-polymerization processes searching for adhesion sites on other neurons or synapses on afferent fibers. Established spines can change morphology as young, long spines to more mature spines with large head volume connected to a dendrite with thin spine neck due to the addition of pre- and post-synaptic proteins. Stubby spines are the morphological characterization of short and stalky spines as illustrated in Figure 1 for mouse neurons. Spine density including filopodia can be as high as 2.7 per μm on some dendritic branches(9) and up to 15 000 spines can form on a pyramidal cell as in Figure 1A.
Computer models(16–18) of neurons can be developed from morphological data repositories such as Neuromorpho(15) with data for different animal species and neuron types. Structural parameters to be considered define dendritic complexity without and with IR through quantification of neuronal cell bodies (NN), total dendritic length (DLT), the number of dendritic branch points (BPT), branch number (BNL) and dendritic spines normalized to DLT. Alternatively in silico neurons can be formed from Monte-Carlo based algorithms(19) which reproduce average properties (BPT, DLT, BNL) with stochastic variation and used to investigate the effects of radiation damage.
Experimental techniques to visualize morphological changes have been reported using single cell methods as well as the use of scoring of population of cells in tissues from brain slices. Single neuron observations can be achieved by neuro-tracers(20) or traditional Golgi staining(21). Tissues prepared with green fluorescent protein (GFP) have the advantage of presenting a population of neurons on thin slices (~60 μm depth) of brain sections(9, 22), however, the dose dependence of soma and dendrite damage induced apoptosis can modify the number of neurons in a slice(18). In contrast, Scholl analysis(23) considers a single neuron, while measuring the contribution of DLT and branch points in concentric circles centered at the soma thus providing a quantitative method to compare damaged and control neuron morphologies.
HEAVY ION TRACK STRUCTURE
The term ‘track-structure’ refers to the description of the position of excitations and ionization of target molecules from the passage of ions through biomolecules, cells or tissues. Originating from the primary particle track are the energetic secondary electrons, denoted as δ-rays. Ionization and excitation processes caused by the ion and δ-rays lead to a stochastic cascade of biological damage events. It is these initial insults from particles interacting with biomolecules that lead to all biological damage from radiation.
Track-structure descriptions are needed in theoretical models of biological responses to understand and extrapolate limited radiobiology data to other radiation qualities and doses. However, unlike progress that has been made over many years in modeling DNA damage, mutations and cell survival using track-structure models, the use of such models and possible biological targets for degradation of neuron morphology have not been considered until recently(16–19).
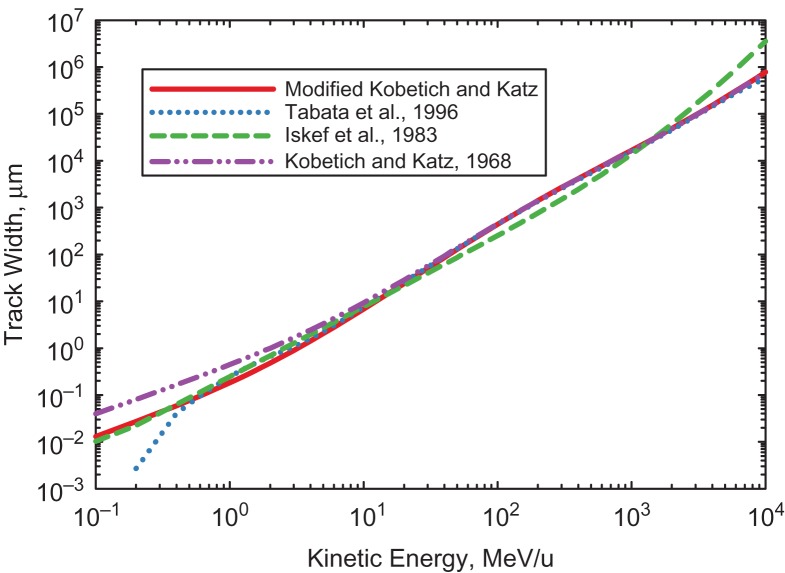
A challenge for computational models of neuron damage is the large radial and longitudinal extensions of a particles track due to δ-rays relative to the spatial extent of a neurons dentate arbor (1000s of microns depending on neuron types and animal species). Defining a δ-ray range is complicated by the large angular scattering that occurs in their interaction with atomic electrons. Common approaches(24, 25) are to use extrapolated range or range defined by a low value of transmission (~5%). Figure 2 shows predictions of various models(24–26) for the track-width defined by the maximum range of δ-rays with the ion velocity determining the kinematic constraint on the corresponding maximum δ-ray kinetic energy(27, 28). The Kobetich and Katz formula allows for convenient analytic formulas for electron extrapolated-range, LET, and inverse energy-range(28).
Figure 2.
Predictions of track width in water media from δ–ray emission. Prediction from the Kobetich and Katz(24), Tabata et al.(25) and Iskef et al.(26) models are compared. The Tabata et al.(25) and Iskef et al.(26) formula are plotted outside of published lower and upper energy ranges, respectively. The modified Kobetich and Katz model adjusts the parameters of the original formula to agree with other determinations for ions with kinetics energies <20 MeV/u.
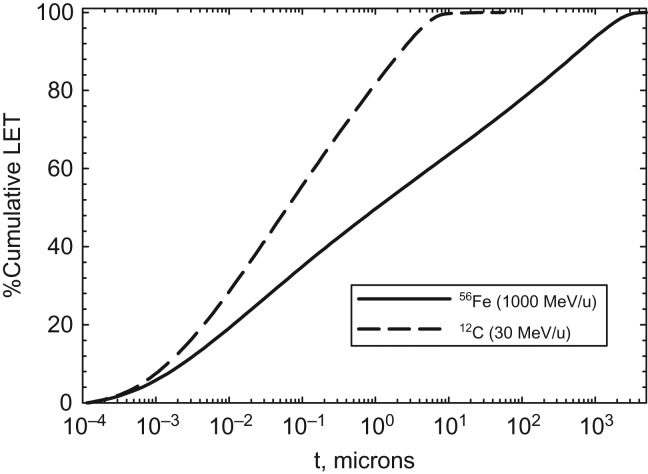
Radial dose models can be built from analytic formula leading to accurate predictions(27, 28), which is an advantage compared to Monte-Carlo codes(18, 29) that have slow convergence at large radial distances from the track for HZE particles. The integration of radial dose overall all radial distance yields the LET. Figure 3 shows the %-cumulative LET versus radial distance in water media for representative ions in therapy (12C at 30 MeV/u) and cosmic rays (56Fe at 1000 MeV/u). Similar results were found for radial frequency distributions(30). This comparison indicates heavy ions can deposit significant amounts of energy in multiple spines and dendritic branches along its track. Interestingly because of the large sizes of mouse or human neurons dendritic arbors, a significant number of ions in therapy or space exposures will deposit all of their energy within the volume containing a single neuron (with sizes of a 1000-μm or more) as indicated by the range and track width data in Table 1. Significant clusters of ionization including multiple ionizations in a molecule, and production of reactive oxygen species (ROS) will occur along the track core and for stopping ions within individual arbors.
Figure 3.
Predictions of %Cumulative LET versus radial distance, t for 56Fe (1000 MeV/u) and 12C(30 MeV/u) ions.
Table 1.
Average properties of protons and several heavy ions in water equivalent media.
| Particle (MeV/u) | LET, keV/μm | Range, μm | Track-width, μm |
|---|---|---|---|
| 1H (5) | 7.9 | 362 | 2 |
| 12C (30) | 68 | 2950 | 50.8 |
| 12C (300) | 13 | 1.7 × 105 | 2701 |
| 56Fe (1000) | 150 | 2.7 × 105 | 1.7 × 104 |
DAMAGE MECHANISMS
Experimental studies of possible damage mechanisms causative of the degradation of neuron morphology following low or high LET radiation are extremely sparse. Here, we briefly describe several possible mechanisms, and also consider results from non-radiation studies of morphology detriments that can help guide experimentation with charged particles while focusing theoretical studies of ED in possible neuronal or other biological targets.
Direct Damage to Dendrites and Spines
In vivo studies of neuron morphology at early time-points (minutes to hours post-irradiation) have not been reported with most studies using time-points several weeks to months after irradiation. In vitro studies of neurite outgrowth assays using neuronal cell cultures suggest rapid changes within hours after low to intermediate doses of X-ray, carbon beam or neutron radiation(31, 32). These in vitro studies showing rapid degradation are consistent with UV-laser studies(33) where neurites were severed in Drosophila neurons demonstrating rapid clearance of damaged segments within 24-h. Severing of dendrites with femtosecond lasers in neurons of Caenorhabditis elegans showed rapid fusion of ends or severing of the distal end of the neurons(34). Cisplatin has been shown to cause early morphological changes (<1-d) in rat hippocampal neuron cell cultures(35). Cisplatin induces a wide range of ROS, leading to DNA adducts and a mitochondria-dependent ROS response.
Several studies suggest molecular programs for dealing with neurite injury are distinct from that of developmental pruning(36, 37). In Drosophila(38), there are two widely known cellular mechanisms of dendritic pruning that have been observed: branch retraction and local degeneration or fragmentation. The latter is observed to be the mechanism in proximal dendrites while the former occurred in distal branches and in proximal dendrites after fragmentation. Both mechanisms involve destabilization of microtubule cytoskeleton after the severing event, followed by microtubule thinning and then phagocyte aided fragmentation and/or retraction(34, 36).
These observations of early changes suggest that ionization and excitation of molecules within dendrites and spines as important damage sites, with the high-ED events from the track core of heavy ions possibly leading to more severe effects at low to moderate doses compared to X-rays or γ-rays because overlapping electron tracks will be rare for photon irradiation in small volumes (<20 nm) typical of proteins for absorbed doses below 100 Gy, but quite common for the track core in heavy ion irradiation at any absorbed dose. In modeling studies of neuron degradation(18, 19), we assumed that radiation-induced changes in neuronal morphology are caused by ‘snipping’ via dendritic fragmentation. Damaged ends may repair or lead to a snipped site at a ED location as suggested by the laser induced damage experiments(33, 34).
Activated Microglial Cells
Activated microglial cells are a key component of neuroinflammation. Radiation studies have shown that the activation of microglia is dose dependent with a delayed response increasing over several weeks, while being sustained for several months post-irradiation(2, 37–39). A variety of insults can lead to activation including ROS, cells undergoing apoptosis, and damaged dendrites. Activated microglia cells interact with damaged neuron cells and remove synapses leading to their description as synaptic strippers(40) and have been suggested to eliminate entire dendritic trees in damaged neurons(40). Because microglia cells can become over activated(40) releasing cytotoxic factors such as superoxide, nitric oxide (NO) and TNFα, a damage cascade may occur leading to a neurodegenerative state.
Mitochondria
Mitochondria play an important role in providing energy to neuron cells including pre-synaptic spines, and function in buffering calcium as part of electrical signaling(41). Mitochondrion are collocated with about 10% of spines/synapses(42). Other roles(41) for mitochondria include local protein synthesis which is important for the plasticity of spines and synapses, pruning of dendrites and spines, and for synaptic transmission. Mitochondria are typically 1–2 μm in length and represent a similar size target as individual spines for a charged-particle track.
Early Response Genes
Early response genes such as c-fos and zif268 are associated with memory maintenance, while activity-regulated cytoskeleton (Arc) is an activity induced gene that correlates temporally and spatially with the stimulus that induced its transcription. Arc mRNA is localized to dendrites and activated synapses. Arc increases spine density and regulates spine morphology by increasing the proportion of thin spines(43). Studies in C57Bl/6 J mice irradiated with X-rays(44) or heavy ions(45) suggests Arc protein is decreased in hippocampal neurons by 10-d post-irradiation and both Arc protein and mRNA are decreased several months post-irradiation with these changes modified by the behavioral activity of animals. In vivo studies show a correlation between neuroinflammation, Arc dysregulation and changes in hippocampus-dependent memory functions(46). Both increased and decreased Arc impairs the synaptic plasticity required for memory formation and optimal Arc levels are necessary for proper memory processes. These changes are correlated with increased activation of microglia(47).
Non-targeted Effects and Morphology Detriments
A wide variety of cognitive challenges have been show to lead to detriments in neuron morphology. Because radiation induces chronic ROS and inflammatory responses, observations of morphological changes for other insults such as chronic stress, fatigue, and sleep deprivation, suggests an indirect pathway of radiation action through signaling and changes to neuro-transmitters or receptors could impact cognition leading to morphology detriments. Aging and diseases such as Alzheimer’s disease and other dementia are also related to morphology detriments. Because aging can compromise morphology, radiation effects on cognition could be more significant at older ages, which is in contrast to cancer risks which decrease with age at exposure. An exception is likely for children and adolescents where active pruning occurs.
In rats chronic immobilization stress-induced dendritic atrophy and debranching in CA3 pyramidal neurons of the hippocampus, while chronic unpredictable stress had little effects, and differential effects were observed in amygdala neurons(47). In other studies, the hippocampal CA3 apical dendritic retraction initially occurs, while for a sufficiently long stress CA1 and dentate gyrus neurons also show retraction(48). It is possible that radiation leaves a signature of distinct changes to neuron types and anatomical regions compared to other stressors, however only a small number of neuron types have been considered to date, with additional difference in animal, time-points, particles and doses used in various studies(6–8, 49).
SUMMARY
Possible targets for energy deposition from heavy ions in degrading neuron morphology include direct damage to spines and dendrites, with qualitatively distinct effects between heavy ions and photons a possibility due to the ions track core and for stopping particles near the Bragg peak where all energy is deposited locally in a neuron. ROS plays an important role in neuronal damage, including in activating microglia cells, mitochondria modifications, changes in signaling and possible effects on neuro-transmitters and receptors. However, little is known with respect to radiation quality effects related to ROS and neuron morphology changes. Differences in ROS between water and the proteins of spines and dendrites will occur. Interactions between neurons and other cells in the tissue microenvironment need to be considered, but discussion of these areas is outside the scope of the present paper. Experimental studies comparing photons to heavy ions under the same conditions, rodent models and anatomical regions are needed and have not been undertaken in the past.
FUNDING
Supported by the National Institute of Health-National Cancer Institute (NIH-NCI) Grant 1RO1CA208526-01.
REFERENCES
- 1. Monje M. L., Vogel H., Masek M., Ligon K. L., Fischer P. G. and Palmer T. D.. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann. Neurol. 62, 515–520 (2007). [DOI] [PubMed] [Google Scholar]
- 2. Greene-Schloesser D., Robbins M. E., Peiffer A. M., Shaw E. G., Wheeler K. T. and Chan M. D.. Radiation-induced brain injury: a review. Front. Oncol. 2, 73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rola R., Otsuka S., Obenaus A., Nelson G. A., Limoli C. L., VandenBerg S. R. and Fike J. R.. Indicators of hippocampal neurogenesis are altered by 56Fe-particle irradiation in a dose-dependent manner. Radiat. Res. 162, 442–446 (2004). [DOI] [PubMed] [Google Scholar]
- 4. Jim H. S. et al.. Cognitive functioning in breast cancer survivors: a controlled comparison. Cancer 115, 1776–1783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirai K. et al.. X irradiation changes dendritic spine morphology and density through reduction of cytoskeletal proteins in mature neurons. Radiat. Res. 179, 630–636 (2003). [DOI] [PubMed] [Google Scholar]
- 6. Chakraborti A., Allen A., Allen B., Rosi S. and Fike J. R.. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One 7, e40844 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parihar V. K. and Limoli C. L.. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 110, 12822–12827 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parihar V. K. et al.. What happens to your brain on the way to Mars? Sci. Adv. 1, e1400256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shulz-Ertner D. and Tsujii H.. Particle radiation therapy using proton and heavier ion beams. J. Clinic. Oncol. 25, 953–964 (2007). [DOI] [PubMed] [Google Scholar]
- 10. Cucinotta F. A., Alp M., Sulzman F. M. and Wang M.. Space radiation risks to the central nervous system. Life Sci. Space Res. 2, 54–69 (2014). [Google Scholar]
- 11. Cucinotta F. A. et al.. Space radiation and cataracts in astronauts. Radiat. Res. 156, 460–466 (2001). [DOI] [PubMed] [Google Scholar]
- 12. Chylack L. T. et al.. NASCA Report 1: cross-sectional study of relationship of exposure to space radiation and risk of lens opacity. Radiat. Res. 172, 10–20 (2009). [DOI] [PubMed] [Google Scholar]
- 13. Amaral D. G., Scharfman H. E. and Lavenex P.. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larkman A. U. Dendritic morphology of pyramidal neurons of the visual cortex of the rat: III. Spine distributions. J. Comp. Neurol. 306, 332–343 (1991). [DOI] [PubMed] [Google Scholar]
- 15. Ascoli G. A. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat. Rev. Neurosci. 7, 318–324 (2006). [DOI] [PubMed] [Google Scholar]
- 16. Alp M., Parihar V. K., Limoli C. L. and Cucinotta F. A.. Irradiation of neurons with high-energy charged particles: an in silico modeling approach. PLoS Comput. Biol. 11(8), e1004428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alp M. and Cucinotta F. A.. Track structure model of microscopic energy deposition by protons and heavy ions in segments of neuronal cell dendrites represented by cylinders or spheres. Life Sci. Space Res. 13, 27–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alp M. and Cucinotta F. A.. Biophysics model of heavy ion degradation of neuron morphology in mouse hippocampal granular cell layer neurons. Radiat. Res. 189, 312–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cacao E., Parihar V. P., Limoli C. L. and Cucinotta F. A.. Stochastic modeling of radiation-induced dendritic damage on in silico mouse hippocampal neurons. Sci. Rep. 8, 5494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wearne S. L., Rodriguez A., Ehlenberger D. B., Rocher A. B., Henderson S. C. and Hof P. R.. New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience 136, 661–680 (2005). [DOI] [PubMed] [Google Scholar]
- 21. Valverde F. The Golgi method. A tool for comparative structural analyses In: Contemporary Research Methods in Neuroanatomy. Nauta W. J. H. and Ebbeson S. O. E., Eds (Berlin: Springer; ) pp. 12–13 (1970). [Google Scholar]
- 22. Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H., Fukami K, Sakaue-Sawano A and Miyawaki A. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 14, 1481–1488 (2011). [DOI] [PubMed] [Google Scholar]
- 23. Sholl D. A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 87, 387–406 (1953). [PMC free article] [PubMed] [Google Scholar]
- 24. Kobetich E. J. and Katz R.. Electron energy dissipation. Nucl. Instr. Meth. 71, 226–230 (1969). [Google Scholar]
- 25. Tabata T., Pedro A. and Shinoda K.. An analytic formula for the extrapolated range of electrons in condensed materials. Nucl. Instr. Meth. Phys. Res. B 119, 463–470 (1996). [Google Scholar]
- 26. Iskef H., Cunningham J. W. and Watt D. E.. Projected ranges and effective stopping powers of electrons with energy between 20 eV and 10 keV. Phys. Med. Biol. 28, 535–545 (1983). [Google Scholar]
- 27. Katz R. and Kobetich E. J.. Particle tracks in emulsion. Phys. Rev. 189, 344–351 (1969). [Google Scholar]
- 28. Cucinotta F. A., Katz R., Wilson J. W. and Dubey R. R.. Heavy ion track-structure calculations for radial dose in arbitrary materials NASA Technical Paper 3497, Washington D.C. (1995).
- 29. Plante I. and Cucinotta F. A.. Ionization and excitation cross sections for the interaction of HZE particles in liquid water and application to Monte-Carlo simulation of radiation tracks. New J. Phys. 10, 1–15 (2008). [Google Scholar]
- 30. Cucinotta F. A., Nikjoo H. and Goodhead D. T.. Model for radial dependence of frequency distributions for energy imparted in nanometer volumes from HZE particles. Radiat. Res. 153, 459–468 (2000). [DOI] [PubMed] [Google Scholar]
- 31. Al-Jahdari W. S. et al.. The radiobiological effectiveness of carbon-ion beams on growing neurons. Int. J. Radiat. Biol. 85, 70–79 (2009). [DOI] [PubMed] [Google Scholar]
- 32. Pani G., Verslegers M., Quintens R., Samari N., de Saint Georges L., van Oostveldt P., Baatout S and Benotmane MA. Combined exposure to simulated microgravity and acute or chronic radiation reduces neuronal network integrity and survival. PLoS One 11, e0155260 (2016) 0155260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tao J. and Rolls M. M.. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J. Neurosci. 31(14), 5398–5405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oren-Suissa M., Gattengo T., Kravtsov V. and Podbilewicz B.. Extrinsic repair of injured dendrites as a paradigm for regeneration by fusion in Caenorhabditis elegans. Genetics 206, 215–230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andres A. L., Gong X., Di K. and Bota D. A.. Low-doses of cisplatin injure hippocampal synapses: A mechanism for ‘chemo’ brain? Exp. Neurol. 255, 137–144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams D. W. and Truman J. W.. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development 132(16), 3631–3642 (2005). [DOI] [PubMed] [Google Scholar]
- 37. Rola R., Fishman K., Baure J., Rosi S., Lamborn KR, Obenaus A, Nelson GA and Fike JR. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat. Res. 169, 626–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rola R., Sarkissian V., Obenaus A., Nelson G. A., Otsuka S., Limoli CL and Fike JR. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat. Res. 164, 556–560 (2005). [DOI] [PubMed] [Google Scholar]
- 39. Parihar V. K. et al.. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 6, 34773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kettenmann H., Kirchoff F. and Verkhratsky A.. Microglia: new roles for the synaptic stripper. Neuron 77, 10–17 (2013). [DOI] [PubMed] [Google Scholar]
- 41. Devine M. J. and Kittler J. T.. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 19, 63–80 (2018). [DOI] [PubMed] [Google Scholar]
- 42. Li Z., Okamato K., Hayashi Y. and Sheng M.. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887 (2004). [DOI] [PubMed] [Google Scholar]
- 43. Peebles C. L., Yoo J., Thwin M. T., Palop J. J., Noebels J. L. and Finkbeiner S.. Arc regulates spine morphology and maintains network stability in vivo. Proc. Natl. Acad. Sci. USA 107, 18173–18178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosi S., Andres-Mach M., Fishman K. M., Levy W., Ferguson R. A. and Fike J. R.. Cranial irradiation alters the behaviorally induced immediate-early gene Arc (Activity-Regulated Cytoskeleton-Associated Protein). Cancer Res. 68, 9763–9770 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raber J., Allen A. R., Rosi S., Sharma S., Dayger C., Davis M. J. and Fike J. R.. Effects of whole body 56Fe radiation on contextual freezing and Arc-positive cells in the dentate gyrus. Behav. Brain Res. 246, 162–167 (2013). [DOI] [PubMed] [Google Scholar]
- 46. Rosi S. Neuroinflammation and the plasticity-related immediate-early gene Arc. Brain Behav. Immun. 25(Suppl 1), S39–S49 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vyas A., Mitra R., Shankaranarayana R. and Sumantra C.. Chronic stress induced contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22, 6810–6818 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conrad C. D., Ortiz J. B. and Judd J. M.. Chronic stress and hippocampal dendritic complexity: methodological and functional considerations. Physiol. Behav. 178, 66–81 (2017). [DOI] [PubMed] [Google Scholar]
- 49. Allen A., Raber J., Chakraborti A., Sharma S. and Fike J. R.. 56Fe irradiation alters spine density and dendritic complexity in the mouse hippocampus. Radiat. Res. 184, 586–594 (2015). [DOI] [PubMed] [Google Scholar]