Abstract
Background
The diagnoses of active smear negative PTB, remains difficult. As a result, treatment is often carried out empirically relaying on clinical criteria. The distribution and magnitude of smear negative PTB, smear negative MDR-TB and associated factors in the same day diagnosis strategy are not clearly known in the study area. Therefore, this study aimed to determine the prevalence of TB, MDR-TB and associated risk factors among presumptive smear negative pulmonary tuberculosis patients in Addis Ababa, Ethiopia.
Methods
Analytic cross sectional study design was used. A total of 418 smear negative presumptive pulmonary TB patients were enrolled from selected health facilities since August 01, 2017 to January 5, 2018. Sputum samples were examined by Ziehl Neelsen microscopy, Xpert MTB/RIF assay and Culture. Drug susceptibility testing was performed by line probe assay and BACTEC MGIT 960 system. These laboratory tests were performed in Ethiopian Public Health Institute, National TB Reference Laboratory. Data was analyzed by SPSS Ver.20.
Results
From the total of 418 enrolled patients, 27 (6.5%) were Xpert MTB/ RIF and 26 (6.4%) were culture confirmed smear negative PTB patients. The positivity rate among male and female was 10.2 and 3.5% (p = 0.005) respectively. From 26 culture positive isolates 3 (11.54%) were MDR TB; from MDR-TB confirmed isolates 2/23 (8.7%) were among new and 1/3 (33.3%) was among retreatment smear negative presumptive pulmonary TB patients. All Rifampicin resistant smear negative pulmonary TB isolates by Xpert MTB/ RIF assay were found to be MDR TB and 7/26 (26.9%) isolates were INH mono resistant. History of migration found to be a potential factor for developing smear negative pulmonary TB.
Conclusion
In this study a significant proportion of smear negative pulmonary TB was diagnosed. Furthermore, a high smear negative multi drug resistant (MDR) TB and other mono drug resistant TB prevalence was confirmed. Due to the limitations of smear microscopy which is used as a primary diagnostic tool, these TB strains are missed to be diagnosed and transmission continues in the community.
Keywords: Prevalence, Smear negative PTB, Smear negative MDR TB, Xpert MTB/RIF assay
Background
Tuberculosis (TB) continues as a challenging bacterial disease. In 2017 alone, 10 million people have developed TB disease [1]. TB is the leading cause of death in the world among infectious diseases [1]; 1.3 million died among HIV negative and 300, 000 among HIV positive people due to TB by the year 2017 [1]. One person dies every 15 s in the world by TB and it infects one person every second of every day. If treatment is not initiated, a person with TB disease will infect an average of 10–15 other people every year [2]. In addition, emergency of drug-resistant TB makes the global TB control program more difficult. An estimated 3.5 and 18% are multidrug-resistant (MDR)/rifampicin resistant (RR) TB among new and previously treated TB patients respectively [1]. Moreover, about 9% of MDR-TB patients also have extensively drug-resistant TB (XDR-TB) [3]. Ninety-five percent of all TB diseases and 99% of deaths occur in developing countries, with the greatest burden in sub Saharan Africa [4]. Ethiopia is one of the 30 high TB; TB/HIV and MDR TB burden countries with an estimated TB incidence of 164 per 100,000 population of drug susceptible TB [1]. In addition, the burden of MDR-TB in Ethiopia is increasing. In 2005 the national prevalence of MDR-TB was 1.6% among new patients and 11.8% among previously treated patients [5]. However, in 2014 the national prevalence of MDR-TB was 2.3% among new and 17.8% among previously treated patients [6].
WHO recommends smear microscopy as an initial TB diagnostic tool. However, it cannot detect 43% of TB patients due to its poor sensitivity [7]. Its sensitivity is further reduced in patients with extra pulmonary TB, children and in those who are co infected with HIV. Furthermore, it cannot distinguish drug-susceptible from drug-resistant strains [8]. TB culture confirmation has a long turnaround time and needs high safety requirement [8]. On the other hand, molecular methods show variable sensitivity [8]. Due to the above reasons TB diagnosis faces a huge challenge. In particular, the diagnosis of smear negative pulmonary TB (SNPT) and smear negative drug resistant TB (SNDRT) is the most challenging in under developed countries [9]. Therefore, as a result this treatment is often given empirically using clinical criteria which leads to miss diagnosis of the patients, unnecessary costs, toxicities, delayed response and poor treatment outcome [10].
In 2017, 3.6 million TB patients left undiagnosed and only 25% of the estimated MDR/RR-TB was reported [1]. Smear negative pulmonary TB (SNPT) is a critical clinical and public health problem [11, 12]. This SNPT prevalence can reach 36 to 45% in United States of America [13], 65.2% in Brazil among high HIV prevalence setting (41.1%) [9], 68.1% in Italy [14], 55% in Belarus (from this 47% were smear negative MDR TB) [15]. Studies in Ethiopia have shown different evidences of SNPT prevalence. A study conducted in Adama, in 2013, indicated that from 232 sputum smear negative presumptive pulmonary tuberculosis, 28 (12.1%) prevalence of smear negative pulmonary tuberculosis [16]. Similarly, a study in North Shoa, Ethiopia has also shown a high prevalence (23.9%) smear negative pulmonary TB [17]. MDR TB among smear negative presumptive pulmonary TB patients is not reported yet in Addis Ababa. A study conducted in St. Peter TB specialized hospital Addis Ababa between 2004 and 2005 didn’t find MDR TB from smear negative TB isolates [18]. The mortality rate of SNPT can reach 25% in populations with high prevalence of HIV infection, which may be largely related to delay in diagnosis [12, 19, 20]. Furthermore, 10–20% of TB transmission at the population level is attributable from SNPT patients [21, 22]. Patients with smear-negative pulmonary TB were found to be less infectious and to have a lower mortality but a significant proportion (50–71%) progressed to active TB disease [21, 23].
Different risk factors have different impacts on the occurrence and diagnosis of SNPT. Studies have shown different risk factors for the occurrence of SNPT [9, 24, 25]. But, there is limited information about factors associated with SNPT in the study area.
There is a limited data on the prevalence of smear negative pulmonary tuberculosis, smear negative multidrug resistance tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. Therefore, the aim of this study was to determine the prevalence of tuberculosis, multidrug resistance tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in the above study area.
Methods
Study design, study site and population
This is an analytic cross sectional study which was conducted in Addis Ababa, Ethiopia from August 2017 to January, 2018. Addis Ababa is the Federal Capital of Ethiopia. It is located between 8055′ and 9005′ North Latitude and between 38040′ and 38050′ East Longitude. The population is more than 3 million [26] (Fig. 1).
Fig. 1.
Map of Addis Ababa
The study was conducted in 16 governmental and private health facilities included in directly observed treatment short course (DOTS) program in Addis Ababa region by the year 2017. A list of all DOTS sites was prepared on excel sheet and rand command was used to select health facilities from the list. Then by using simple random sampling technique 20% of the sites were selected out of the lists. Simple random sampling was used to select study sites since there were similar characteristics among governmental and private DOTS sites.
Sputum smear negative presumptive pulmonary tuberculosis patients who were equal or greater than the age of 2 (two) years visiting the selected health facilities during the study period were included in the study. Whereas, those who were taking anti-TB drugs for greater than 1 week by any means and patients incapable of producing sputum were excluded.
Sample size determination
Sample size calculation was performed for both objectives; prevalence of smear negative pulmonary TB and MDR TB by using single population proportion formula with corrected. Then the larger sample size was taken to represent the other. For prevalence of smear negative pulmonary tuberculosis, the sample size was 264 and for prevalence of SNMDR TB, it was 413 in 95% confidence interval. Therefore the 413 was the total sample size for this study [6].
Data collection procedure
Onsite training was given for all laboratory staffs and clinicians about the project for each respective study sites. Data was collected using structured questioner about socio-demographic information, clinical presentations (Table 5) and comorbidities/co-infections (HIV AIDS, diabetes mellitus, asthma, hypertension, heart disease, liver disease, renal failure, hormonal disease, malignancy/ cancer), behavioral and other risk factors (miners, prisoners/prisoner staffs, migrant, TB/MDR TB contact, alcohol consumption, smokers, and chewing Khat) from smear negative presumptive pulmonary tuberculosis (SNPPT) patients. Laboratory investigation data was also taken from study registration book, printouts, and culture work sheet.
Table 5.
Clinical presentation, comorbidities and associated risk factors for SNPT & SNMDR TB, August 01, 2017- January 05, 2018
| Variables | Yes/No | Negative frequency (%) | Positive frequency (%) | P-value |
|---|---|---|---|---|
| Signs and symptoms | ||||
| Cough | Yes | 385 (93.4) | 27 (6.6) | 0.554 |
| No | 05 (6.4) | 0 (0.0) | ||
| Fever | Yes | 236 (92.5) | 19 (7.5) | 0.317 |
| No | 153 (95%) | 8 (5.0) | ||
| Chest pain | Yes | 232 (92.1) | 20 (7.9) | 0.134 |
| No | 158 (95.8%) | 07 (4.2) | ||
| Loss of appetite | Yes | 222 (91.4) | 21 (8.6) | 0.034 |
| No | 168 (96.6%) | 06 (3.4) | ||
| Weight loss | Yes | 141 (87.6) | 20 (12.4) | < 0.001 |
| No | 248 (97.3%) | 07 (2.7) | ||
| Shortness of breath | Yes | 120 (89.6) | 14 (10.4) | 0.023 |
| No | 270 (95.4%) | 13 (4.6) | ||
| Joint pain | Yes | 102 (91.9) | 09 (8.1) | 0.414 |
| No | 288 (94.1) | 18 (5.9) | ||
| Abdominal pain | Yes | 48 (96.0) | 02 (4) | 0.448 |
| No | 342 (93.2) | 25 (6.8) | ||
| Swelling | Yes | 17 (94.4) | 01 (5.6) | 0.871 |
| No | 373 (93.5) | 26 (6.5) | ||
| Hemoptysis | Yes | 25 (86.2) | 04 (13.8) | 0.098 |
| No | 364 (94.1) | 23 (5.9) | ||
| Night sweating | Yes | 59 (95.2) | 03 (4.8) | 0.847 |
| No | 356 (85.2) | 24 (5.74) | ||
| Comorbidity/Co-infection | ||||
| HIV infection | Positive | 54 (94.7) | 03 (5.3) | 0.448 |
| Negative | 154 (91.7) | 14 (8.3) | ||
| Diabetes mellitus | Yes | 13 (92.9) | 01 (7.1) | 0.92 |
| No | 376 (93.5) | 26 (6.5) | ||
| Hypertension | Yes | 19 (100) | 0(0.00) | 0.24 |
| No | 370 (93.2) | 27 (6.8) | ||
| Asthma | Yes | 18 (100) | 0(0.00) | 0.253 |
| No | 371 (93.2) | 27 (6.8) | ||
| Heart Disease | Yes | 05 (100) | 0(0.00) | 0.553 |
| No | 384 (93.4) | 27 (6.6) | ||
| Liver Disease | Yes | 03 (100) | 0(0.00) | 0.647 |
| No | 386 (93.5) | 27 (6.5) | ||
| Renal failure | Yes | 01(100) | 0(0.00) | 0.792 |
| No | 388 (93.5) | 27 (6.5) | ||
| Hormonal Disease | Yes | 02 (100) | 0(0.00) | 0.709 |
| No | 387 (93.5) | 27 (6.5) | ||
| Malignancy/Cancer | Yes | 02 (100) | 0(0.00) | 0.709 |
| No | 387 (93.5) | 27 (6.5) | ||
| Other risk factors | ||||
| Miner | Yes | 04 (100) | 0(0.00) | 0.596 |
| No | 383 (93.4) | 27 (6.6) | ||
| Ex-miner | Yes | 07 (100) | 0(0.00) | 0.481 |
| No | 380 (93.4) | 27(6.6) | ||
| Current smoker | Yes | 10 (83.3) | 02 (16.7 | 0.149 |
| No | 377 (93.8) | 25 (6.2) | ||
| Ex-smoker | Yes | 10 (90.9) | 01 (9.1) | 0.727 |
| No | 377 (93.5) | 26 (6.5) | ||
| Prisoner/Prison staff | Yes | 02 (100) | 0(0.00) | 0.708 |
| No | 385(93.4) | 27 (6.6) | ||
| Migrant | Yes | 8 (72.7) | 03 (27.3) | 0.005 |
| No | 379 (94.0) | 24 (6.0) | ||
| Contact with TB patient | Yes | 25 (92.6) | 02 (7.4) | 0.847 |
| No | 362 (93.5) | 25 (6.5) | ||
| MDR-TB contact | Yes | 07 (87.5) | 01 (12.5) | 0.489 |
| No | 380 (93.6) | 26 (6.4) | ||
| Alcohol consumption | Yes, currently | 59 (86.8) | 09 (13.3) | 0.042 |
| Yes, previously | 52 (96.3) | 02 (3.7) | ||
| Not at all | 279 (94.6) | 16 (5.4) | ||
| Smokers | Yes, currently | 11 (84.6) | 02 (15.4) | 0.174 |
| Yes, previously | 11 (84.6) | 02 (15.4) | ||
| Not at all | 361 (94.0) | 23 (6.0) | ||
| Chewing Khat | Yes, currently | 22 (88.0) | 3 (12.0) | 0.038 |
| Yes, previously | 27 (84.4) | 05 (15.6) | ||
| Not at all | 341 (94.7) | 19 (5.3) | ||
This was done based on both GeneXpert and culture results
Two spot sputum specimens (in Spot-Spot sample collection strategy) were collected from, presumptive pulmonary tuberculosis patients and checked for AFB using ZN method in the health facilities. Specimen was collected for the purpose of the study from smear negative presumptive pulmonary patients and from left over samples which were collected from presumptive pulmonary TB patients as well. All patients who were smear negative were enrolled according to the inclusion criteria. Sputum sample was collected with wide mouthed, clean, 50 ml capacity, sterile, disposable containers, polypropylene centrifuge tubes, translucent, screw capped container after detail patient instruction at the health facility level. It was expected to collect sample with volume > = 3 ml; but those samples less than 3 ml was rejected, wrong sample containers, containers with leakage, breakage, and with incomplete questioner were rejected. Specimens collected was stored at 2-8oc until transported. The specimen was transported using triple packaging. Specimens arrived at the Ethiopian Public Health Institute (EPHI), National Tuberculosis Reference Laboratory (NTRL), was rechecked for fulfilling criteria and was stored again at 2-8oc in the refrigerator if it was not processed immediately.
Laboratory investigations
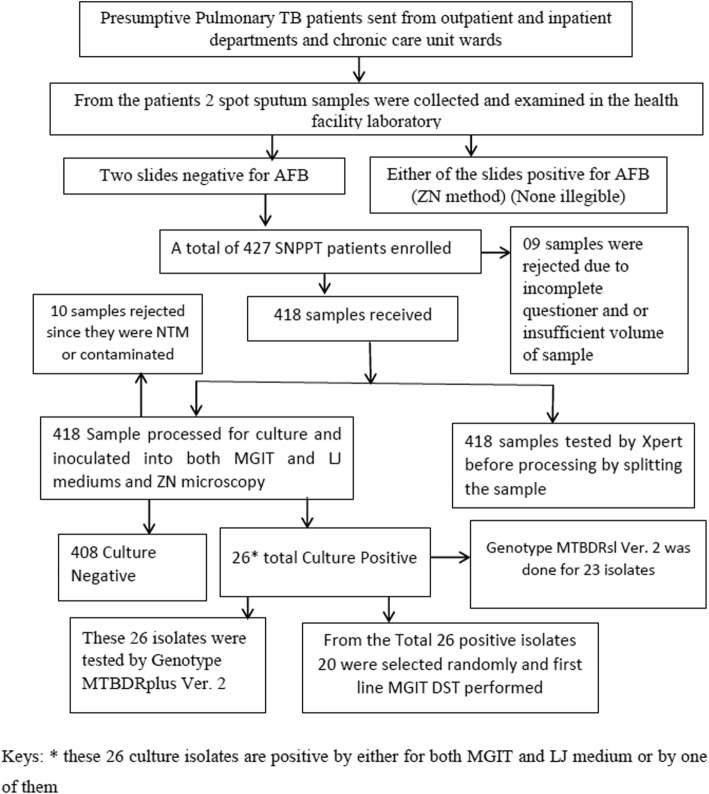
From the specimen collected, AFB microscopy [27], Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) (directly from the sample before processing for culture), Liquid (BACTEC MGIT 960 (Becton-Dickinson and Company, Sparks MD)) and Solid culture (LJ media) were performed in the EPHI, NTRL. Drug susceptibility testing was performed here for the culture isolates. Any result found positive was reported to the physician requested the test for further patient management. The samples were splited in to two; one for culture and the other for Xpert MTB/ RIF assay. For Xpert MTB/ RIF assay, sample was mixed with sample reagent buffer in 1:2 (sample: sample reagent buffer) volume ratio. Then, closing it tightly, vortexed for 15 s and allowed to stand at room temperature for 10 min. It was vortexed again after 10 min and allowed to stand for 5 min, totally for 15 min. Using the Pasteur pipette provided with the kit > 2 ml of the (just above 2 ml mark on pipette) processed sample was put into the Xpert MTB/RIF cartridge. After that, the cartridge with the specimen was loaded to the GeneXpert machine. Finally, results were collected from the GeneXpert computer after 2 h [28]. Samples for culture were processed with 3% N-Acetyl-L-Cysteine–Sodium Hydroxide (NALC-NaOH) decontamination method. Equal volume of NALC-NaOH-sodium citrate solution was added to the sample, vortexed and kept stand at room temperature for 15 min to let the NaOH decontaminate normal flora present in the sputum with the help of the mucolytic agent. Then, this solution was neutralized (effect of NaOH stopped) by 6.8 pH phosphate buffer solution (PBS), concentrated the freed bacilli in the tube by centrifugation at relative centrifugal force of 3,000 x g for 15 min using safety centrifuge at a temperature of 4–8 °C. After this the supernatant removed, smear made from the sediment and sediment resuspended by adding 2 ml of PBS. Making the sediment uniform by vortexing, 0.5 ml and 2–4 drops were added to the MGIT tube and LJ tube respectively. The MGIT tube was enriched with 0.8 ml of a mixture of growth supplement which contains Middle brook OADC enrichment and PANTA (lyophilized mixture of antimicrobial agents) prior to the inoculation of the tube with the sediment. After all, the LJ medium incubated in the incubator at 37 °C and the MGIT tube loaded to the BACTEC MGIT 960 machine. For positive culture results smear prepared and blood agar/ brain heart infusion agar inoculated for positive MGIT tube and smear prepared and examined for positive LJ for identification mycobacterium TB complex (MTBC) from other non-tuberculous mycobacteria and contaminants. Negative results reported after 42 days for MGIT 960 system and 56 days for LJ medium [29]. SD BIOLINE TB Ag MPT 64® (STANDARD DIAGNOSTICS, INC, Republic of Korea) test was used for the confirmation of MTBC [30]. Genotypic and phenotypic drug susceptibility testing was performed using world health organization recommended methods: line probe assay (MTBDR plus Ver.2 for first line anti-TB drugs, MTBDRsl Ver.2 for second line anti TB drugs) and BACTEC MGIT 960 system (for first line drugs using proportion method) respectively (Fig. 2).
Fig. 2.
Flow chart of the study
Quality assurance and quality control
Reagents, and culture media prepared within the laboratory or purchased as readymade which have effect on the test result was checked for sterility and performance characteristics using well characterized control slide or strains such as; a positive and negative control smear slides were included in each batch of new lots of reagents prepared, 2% of a batch of tubes of MGIT Culture media was tested with different dilutions of M. tuberculosis (H37Rv). Specimens which had breakage or leakage, incomplete patient information and mislabeling, delayed specimen more than 5 days after collection, inappropriate container, and insufficient volume of sample (less than 3 ml) were rejected. Processing reagents (NaOH, Phosphate buffer) was sterilized before use. Each batch of samples that was processed for culture used start and end controls. All laboratory investigations were done by experienced laboratory technologists in the National Tuberculosis Reference Laboratory, EPHI. Each of the culture and identification procedures was performed in a certified class II Bio-Safety Cabinet. Equipment preventive maintenance, temperature monitoring, monitoring of critical microbiological practices, and instrument function checkups was done. The laboratory performance is also continuously monitored by Proficiency Testing (PT) for all test methods. All laboratory analysis was done based on Ethiopian national TB, HIV and leprosy management guideline and WHO approved methods.
Data entry and analysis methods
The data collected from each questionnaire and the laboratory investigation was doubly entered and cleaned by using Epi -data statistical software version 3.0 and exported to IBM SPSS software version 20.0 for analysis. By using central measurement, dispersion and frequency distribution descriptive statistics was done to characterize the study participants and the result of the study was also presented by using tables and figures. The prevalence of SNPT and SNMDR TB was calculated with its 95% CI. Binary logistic regression also done to determine the association between dependent variables and possible risk factors. Variables which show significance at P-value of 0.2 during univariate analysis were selected for multivariable analysis. The strength of association was measured by using odds ratio and P-value of less than 0.05 was considered as statistically significant during multivariable analysis.
Results
Socio demographic characteristics of participants
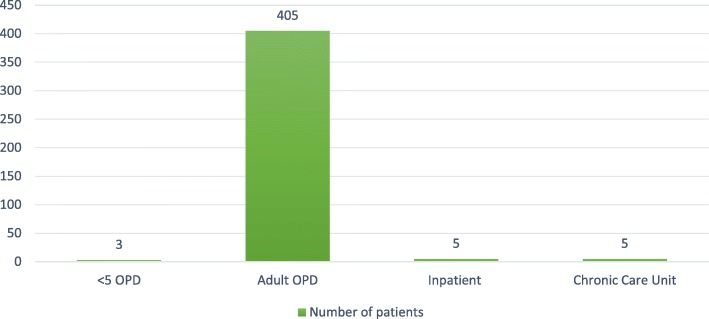
A total of 418 patients’ epidemiological and bacteriological data were analyzed. Most of the patients were from adult out-patient-department (OPD) (Fig. 3). Majority, 231(55.3%) of the patients were females. The mean age of participants was 35.68 (± 17.12) years. Most of the participants, 125 (29.8%) were in the age group of 25–34 years, and children under 15 years were 20 (4.8%). The predominant religion was Orthodox Christians, 323 (77.3%), followed by Muslims, 57 (13.6%). Among all participants, 228 (nearly 55%) were below grade 7. The participants had different occupation; the most frequent was house wife, 87 (21.1%) and the second frequent work was daily laborer, 76 (18.4%). Two hundred eighteen (52.3%) participants were married whereas 159 (38.1%) were single (Table 1).
Fig. 3.
A bar graph showing study participant proportion by department
Table 1.
Socio demographic characteristics of study participants in Addis Ababa, August 01, 2017- January 05, 2018
| Variables | Frequency | Percent (%) |
|---|---|---|
| Sex | ||
| Male | 187 | 44.7 |
| Female | 231 | 55.3 |
| Total | 418 | 100 |
| Age Group | ||
| 0–14 | 20 | 4.8 |
| 15–24 | 94 | 22.4 |
| 25–34 | 125 | 29.8 |
| 35–44 | 61 | 14.6 |
| 45–54 | 53 | 12.6 |
| 55–64 | 27 | 6.4 |
| > 65 | 38 | 9.3 |
| Total | 418 | 100 |
| Religion | ||
| Orthodox | 323 | 77.3 |
| Muslim | 57 | 13.6 |
| Protestant | 34 | 8.1 |
| Catholic | 2 | 0.5 |
| Other | 2 | 0.5 |
| Total | 418 | 100 |
| Level of Education | ||
| None(No Formal Education) | 112 | 26.9 |
| Standard grade 1–7 | 116 | 27.9 |
| Standard grade 8–10 | 88 | 21.2 |
| Standard grade 11–12 | 53 | 12.7 |
| Diploma(level 1–4 Certificate) | 26 | 6.3 |
| First Degree | 21 | 5.0 |
| Second Degree & above | 0.0 | 0.0 |
| Total | 416 | 100 |
| Work status | ||
| Farmer | 31 | 7.5 |
| Daily Laborer | 76 | 18.4 |
| House wife | 87 | 21.1 |
| Driver | 10 | 2.4 |
| Teacher | 7 | 1.7 |
| Student | 46 | 11.2 |
| Merchant | 14 | 3.4 |
| Health Care Worker | 3 | 0.7 |
| Government employee | 35 | 8.5 |
| Self-employed | 60 | 14.6 |
| Other | 43 | 10.4 |
| Total | 412 | 100 |
| Marital Status | ||
| Single | 159 | 38.1 |
| Married | 218 | 52.3 |
| Divorced | 22 | 5.3 |
| Widowed | 16 | 3.8 |
| Separated | 2 | 0.5 |
| Total | 417 | 100 |
Clinical presentation, comorbidities and associated risk factors
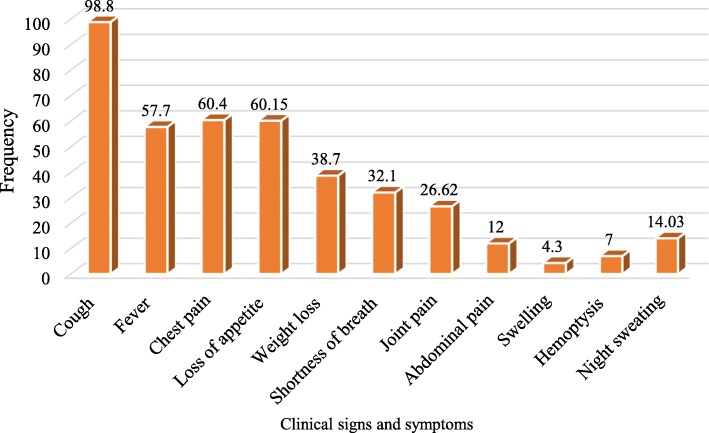
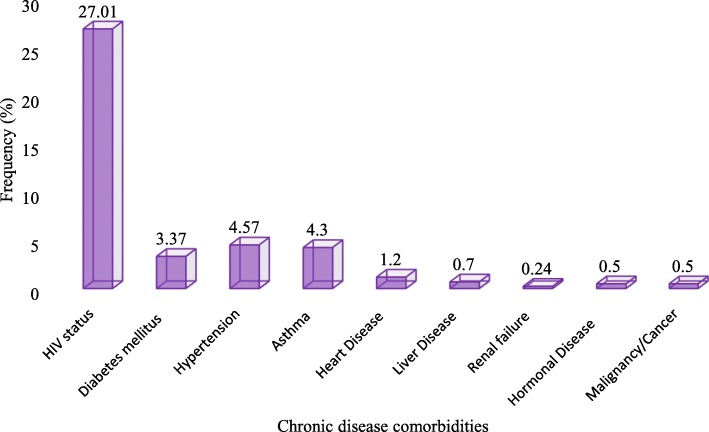
Signs and symptoms, comorbidity (self-reported by the patient not confirmed) and other risk factors were assessed in the study. From all the participants, 367 (88%) had cough as a chief compliant, and 112 (30.5%) of them with productive cough. HIV status was known only for 211 patients. HIV positivity rate was 27%. Fourteen (3.37%) participants were known to have diabetes mellitus (DM). Thirty five (8.4%) patients had TB contact history (Figs. 4 and 5, Table 2).
Fig. 4.
Clinical presentations among study participants
Fig. 5.
A bar graph demonstrating frequency of chronic comorbidities among study participants
Table 2.
Behavioral and other risk factors, Addis Ababa, Ethiopia, August 01, 2017- January 05, 2018
| Risk factors | Frequency | Percent (%) |
|---|---|---|
| Miner | 11 | 2.7 |
| Prisoner/Prisoner staff | 2 | 0.5 |
| Migrant | 11 | 2.7 |
| TB/MDR TB contact | 35 | 8.45 |
| Alcohol Consumption | 122 | 29.3 |
| Smokers | 26 | 6.3 |
| Chewing Khat | 57 | 13.7 |
Prevalence of smear negative pulmonary tuberculosis (SNPT)
SNPT prevalence among presumed smear negative PTB patients was 6.5% (95% C.I, 4.103–8.816) by Xpert MTB/RIF assay. Twenty six patients (6.4%) were smear negative and culture confirmed / by either of the culture techniques (LJ and MGIT), SNPT patients (Table 3).
Table 3.
Detection of TB using different diagnostic tools among presumptive smear negative pulmonary TB patients, August 01, 2017- January 05, 2018
| Diagnostic Method | Negative Result No. (%) | Positive Result No. (%) | Total No. (%) | Contaminated (%) | NTM (%) |
|---|---|---|---|---|---|
| Xpert MTB/RIF assay | 391 (93.5) | 27 (6.5) | 418 (100) | NA | NA |
| MGIT culture result | 354 (92.65) | 24 (6.35) | 378 (100) | 34 (8.25) | 06 |
| LJ culture result | 376 (94.47) | 22 (5.53) | 398 (100) | 18 (4.3) | 01 |
| LJ and MGIT culture result | 382 (93.6) | 26 (6.4) | 408 (100) | 03 (0.73) | 07 |
More TB was detected in male than female patients. The positivity rate among male and female was 10.2 and 3.5%, respectively. There was significant difference in TB prevalence among male and female (p = 0.005). High rate of TB detection was observed in the age range 15–24 years, 8 (8.5%) and 25–34 years, 11 (8.9%). All positive SNPT were detected from the age groups between 15 and 64 years. SNPT prevalence was, 20 (6.2%) among Orthodox Christians, 5 (8.8%) and 2 (6.0%) among Muslims and Protestants respectively. Majority, 21 (80.77%) of the total SNPT were found to be positive in those who were below grade 12, but it was not statistically significant (P = 0.496). From the total of 411 participants responded, the most common work status, 87 (21.1%) was house wife; however SNPT prevalence was low, 01 (1.2%). The second most frequent work, 76 (18.4%) was daily laborer with (7.9%) SNPT prevalence, and the least frequent, 03 (0.7%) was health worker, with the highest SNPT prevalence 01(33.3%) and there was an association with the SNPT (P < 0.006). The odds of having SNPT was 68.7 times higher in health care workers as compared to farmers, (AOR 68.7; 95% C.I, 2.447–1929.15, p = 0.013). From 417 participants interviewed, 218 (52.3%) were married, from which 11 (5.04%) was TB positive, 159 (38.1%) were single from which 14 (8.8%) was TB positive, (p = 0.460) (Table 4).
Table 4.
Socio demographic characteristics of study participants and level of Association (Chi square), August 01, 2017- January 05, 2018
| Variables | Positive (n = 27) | Negative (n = 391) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 19 | 167 | 0.005 |
| Female | 8 | 223 | |
| Total | 27 | 390 | |
| Age Group | |||
| 0–14 | 0.0 | 20 | 0.237 |
| 15–24 | 8 | 86 | |
| 25–34 | 11 | 113 | |
| 35–44 | 05 | 56 | |
| 45–54 | 01 | 52 | |
| 55–64 | 02 | 25 | |
| > 65 | 0.0 | 38 | |
| Total | 27 | 390 | |
| Religion | |||
| Orthodox | 20 | 303 | 0.935 |
| Muslim | 5 | 52 | |
| Protestant | 2 | 31 | |
| Catholic | 0.0 | 02 | |
| Other | 0.0 | 02 | |
| Total | 27 | 390 | |
| Level of Education | |||
| None(No Formal Education) | 06 | 106 | 0.496 |
| Standard grade 1–7 | 04 | 112 | |
| Standard grade 8–10 | 06 | 82 | |
| Standard grade 11–12 | 05 | 48 | |
| Diploma(level 1–4 Certificate) | 03 | 23 | |
| First Degree | 02 | 18 | |
| Second Degree & above | 0.0 | 0.0 | |
| Total | 26 | 389 | |
| Work status | |||
| Farmer | 01 | 30 | 0.006 |
| Daily Laborer | 06 | 70 | |
| House wife | 01 | 86 | |
| Driver | 0.0 | 10 | |
| Teacher | 0.0 | 07 | |
| Student | 0.0 | 46 | |
| Merchant | 0.0 | 14 | |
| Health Care Worker | 01 | 02 | |
| Government employee | 02 | 32 | |
| Self-employed | 07 | 53 | |
| Other | 07 | 36 | |
| Total | 25 | 386 | |
| Marital Status | |||
| Single | 14 | 145 | 0.460 |
| Married | 11 | 206 | |
| Divorced | 02 | 20 | |
| Widowed | 0.0 | 16 | |
| Separated | 0.0 | 02 | |
| Total | 27 | 389 | |
This was done based on both GeneXpert and culture results
Of 417 participants, 345 (82.73%) were new presumptive TB patients and 23 (6.67%) had confirmed TB. On the other hand, 72 (17.27%) participants were retreatment presumptive TB patients and 4 (5.56%) of them were positive for TB. There was significant difference in TB detection among new and retreatment presumptive TB patients (p < 0.001).
Among clinical signs and symptoms this study investigated; weight loss (p < 0.001), shortness of breath (p = 0.034) and loss of appetite (p = 0.023), having association with the SNPT in univariate analysis. However, in the multivariate analysis these symptoms are not statistical significant; loss of appetite (AOR 0.778; 95% C.I, 0.248–2.438, P = 0.666), weight loss (AOR 0.340; 95% C.I, 0.108–1.069, p = 0.065) and shortness of breath (AOR 0.493; 95% C.I, (0.181–0.1.343), p = 0.167). Known chronic malignancies (Table 5) and comorbidities were not significantly associated with SNPT depending on these study findings. HIV-TB co-infection was 5.3%. From behavioral and other risk factors that showed association in the univariate model, only being migrant were statistically significant factor for the SNPT (AOR 0.121; 95% C.I, (0.20–0.730) p = 0.021) (Table 6).
Table 6.
Showing adjusted odds ratio of those socio demographic factors, clinical presentation and risk factors having association in the univariate analysis, August 01, 2017- January 05, 2018
| Variables | Resultsa | Adjusted Odds Ratio | P-value | |
|---|---|---|---|---|
| Positive Number (%) | Negative Number (%) | AOR (95% C.I) | ||
| Occupation | ||||
| Farmer | 01 (3.2) | 30 (96.8) | 1 | |
| Daily Laborer | 06 (7.9) | 70 (92.1) | 2.407 (0.255–22.723) | 0.443 |
| House wife | 01 (1.1) | 86 (98.9) | 0.635 (0.033–12.243) | 0.763 |
| Driver | 0 (0.00) | 10 (100) | 0 (0.00) | |
| Teacher | 0 (0.00) | 07 (100) | 0 (0.00) | |
| Student | 0 (0.00) | 46 (100) | 0 (0.00) | |
| Merchant | 0 (0.00) | 14 (100) | 0 (0.00) | |
| Health Care Worker | 01 (33.3) | 2 (66.7) | 68.7 (2.447–1929.15) | 0.013 |
| Government employee | 02 (5.9) | 32 (94.1) | 2.38 (0.188–30.159) | 0.503 |
| Self-employed | 07 (11.7) | 53 (88.3) | 3.031 (0.318–28.886) | 0.335 |
| Other | 07 (16.3) | 36 (83.7) | 6.311 (0.662–60.122) | 0.109 |
| Sex | ||||
| Male | 19 (10.2) | 167 (89.8) | 1 | |
| Female | 8 (3.5) | 223 (96.5) | 0.321 (0.097–1.062) | 0.063 |
| Loss of Appetite | ||||
| Yes | 21 (8.6) | 222 (91.4) | 1 | |
| No | 6 (3.4) | 168 (96.6) | 0.778 (0.248–2.438) | 0.666 |
| Weight Loss | ||||
| Yes | 20 (12.4) | 141 (87.6) | 1 | |
| No | 07 (2.7) | 248 (97.3) | 0.340 (0.108–1.069) | 0.065 |
| Shortness of Breath | ||||
| Yes | 14 (10.4) | 120 (89.6) | 1 | |
| No | 13 (4.6) | 270 (95.4) | 0.493 (0.181–0.1.343) | 0.167 |
| Migrant | ||||
| Yes | 03 (27.3) | 08 (72.7) | 1 | |
| No | 24 (06) | 379 (94) | 0.121 (0.20–0.730) | 0.021 |
| Alcohol Consumption | ||||
| Yes, currently | 09 (13.2) | 59 (86.8) | 1 | |
| Yes, previously | 02 (13.7) | 52 (96.3) | 0.254 (0.046–1.399) | 0.116 |
| Not at all | 16 (5.4) | 279 (94.6) | 0.956 (0.311–2.939) | 0.937 |
| Chewing Khat | ||||
| Yes, currently | 03 (12) | 22 (88) | 1 | |
| Yes, previously | 05 (15.6) | 27 (84.4) | 1.324 (0.221–7.922) | 0.759 |
| Not at all | 19 (5.3) | 341 (94.7) | 0.570 (0.127–2.565) | 0.464 |
Key; a = the sum of GeneXpert and culture results
Prevalence of smear negative multidrug resistant tuberculosis
A total of 26 smear negative, culture confirmed TB was detected. All clinical isolates were tested by using Genotype MTBDR plus Ver.2, first- line MGIT DST and Genotype MTBDRsl Ver.2. Among these isolates, 03 (11.54%) were MDR-TB (resistant to both isoniazid and rifampicin) and 07 (26.9%) were resistant to isoniazid. All rifampicin resistant (RR) TB by GeneXpert were found to be MDR-TB by Genotype MTBDR plus Ver. 2 and MGIT DST methods. Twenty three SNPT were isolated from new presumptive TB patients and two (8.7%) of them were rifampicin resistant. The remaining three SNPT were detected from previously treated patients and one (33.3%) of the isolates was rifampicin resistant. From 23 new SNPT isolates, 06 (26.08%) were resistant for isoniazid. From 03 (11.54%) isolates diagnosed from retreatment presumptive TB patients, 01 (33.33%) was resistant for isoniazid (p = 0.79). All of SNMDR TB 03/26 were detected from men (p = 0.264) and in the age group of 15–34 years old (p = 0.814). Drug susceptibility testing was performed for randomly selected 23 (88.46%) of the isolates for second line anti-TB drugs using Genotype MTBDRsl Ver.2, to check for any resistance but all the isolates were susceptible (Table 7).
Table 7.
Genotypic and phenotypic drug susceptibility testing pattern of SNPT isolates, August 01, 2017- January 05, 2018
| Drug susceptibility testing | Drugs | Status (Resistant/Sensitive) | ||
| Resistant No. (%) | Sensitive No. (%) | |||
| Genotype MTBDRplus Ver. 2 | Rif | 03 (11.54) | 23 (88.46) | |
| INH | 07 (26.9) | 19 (73.1) | ||
| MDR TB | 03 (11.54) | 23 (88.46) | ||
| Drugs | Final drug concentration tested | Drug status (Sensitive/ Resistant) | ||
| Resistant No. (%) | Sensitive No. (%) | |||
| First line MGIT DST | STR | 1.0 μg/ml | 03 (15%) | 17 (85%) |
| INH | 0.1 μg/ml | 03 (15%) | 17 (85) | |
| RIF | 1.0 μg/ml | 02 (10%) | 18 (90%) | |
| ETM | 5.0 μg/ml | 01 (5%) | 19 (95%) | |
| MDR TB | 02 (10%) | 18 (90) | ||
First Line MGIT DST; the reason for variation with LPA is that 06 isolates from which 1 MDR Isolate, 03 were INH resistant by LPA was not finalized
Operationalized definitions
1. Smear Negative Pulmonary Tuberculosis (SNPT):- Is PTB that was initially negative for two (spot-spot) consecutive sputum smear examinations and later diagnosed positive by other advanced TB diagnostic tools [11]
2. Smear Negative Presumptive Pulmonary Tuberculosis (SNPPT) patients: - Are patients who have signs and symptoms and are presumptive to be diagnosed with pulmonary tuberculosis but two of their consecutive sputum samples are microscopically negative [37]
3. Alcohol consumption: is when a person takes alcohol every day and who was taking alcohol previously, but he/ she stopped drinking now
4. Smokers: are persons who smokes cigarettes every day and who was smoking previously, but he/ she stopped now
5. Contact with TB patient: according to WHO, these are people who have close contact with infectious TB and have high risk for infection with TB
Discussion
According to WHO, more than 9 million people who become ill with TB each year; more than 3 million are not diagnosed, treated, or officially registered by national TB programmes. The emergence of MDR TB makes the situation worse. Collectively, these “missed” million TB patients are a global public health failure [7]. Similarly, diagnosis of MTB in developing countries largely depends on smear microscopy which has poor sensitivity [31], resulting in increased smear negative pulmonary tuberculosis (SNPT) that remains undiagnosed, or delay in diagnosis and treatment [10]. SNPT and SNMDR TB prevalence in Addis Ababa was not well known. Therefore, determining the magnitude and distribution of SNPT and smear negative MDR TB in different settings can contribute to the national efforts for fighting TB.
Our study findings showed that the prevalence of smear negative pulmonary tuberculosis among smear negative presumptive pulmonary tuberculosis patients was 27/418 (6.5%) (95% C.I, 4.103–8.816), which was Xpert MTB/ RIF confirmed and 26/408 (6.4%) %)(95% C.I, 4.003–8.743), by the collaborative diagnostic performance of BACTEC MGIT 960 and LJ culture. The difference between GeneXpert and culture results might be due to cross contamination during Xpert MTB/RIF sample processing or Xpert MTB/ RIF assay might detect dead remnant DNA from retreatment patients or harsh decontamination during culture processing. From the total, majority (55%) of the study participants were female, but more than two times SNPT (10.2%) were detected from men (p = 0.005) which is in line with the fact that TB is more common in men than women [1]. The present study revealed higher smear negative PTB prevalence than a report from Vietnam which was 5.6% (smear negative culture positive PTB) [32]. Our study reveals lower prevalence, as compared to a finding by a study conducted in 8 prisons in Amhara region, Ethiopia in the year 2013 that the overall prevalence of culture and/or Xpert test positive smear-negative pulmonary TB in the study was 8% [33]. Another study in Karamara hospital, Jigjiga, Ethiopia, conducted from December 2013 to May 2014, shown 7.9% SNPT prevalence; this result was the combined result of Xpert MTB/ RIF and culture-positive [34]. A study conducted in Adama, Ethiopia in 2013, also showed a higher SNPT prevalence that was 12.1% [16] than our study results and this study is similar with our study finding in that men were more affected by SNPT than females. Other study conducted in St. Peter TB specialized hospital Addis Ababa, Ethiopia in the year between November 2004 and October 2005, confirmed a higher prevalence that is 12.46% pulmonary TB isolated from smear negative pulmonary TB patients [18] than the present study. These differences may be due to different reasons like geographical variation, change in sample collection strategy, difference in time that the study conducted, change in TB diagnostic and treatment algorithm and diagnostic method differences. Another justification for these differences might be, our study tried to cover a wide range of facilities including private sites.
Although the study included all age groups, above two years, all (100%) of the SNPT were detected in the age group 15–64 years’ participants which are the economically productive age group of the population. This finding is in line with WHO estimation that states TB affects mostly adults in the economically productive age groups; around two-thirds of are estimated to occur among people aged 15–59 years [1].
According to the findings from the 26 isolates from smear negative presumptive pulmonary TB patients 03 (11.54%) were MDR TB. From these SNMDR TB isolates 02/23 (8.7%) were among new smear negative presumptive TB patients and 01/03 (33.3%) was from retreatment presumptive SNPT patients. Our study showed higher SNMDR TB prevalence in comparison with a study in Iran among tuberculosis patients in between the year 2011–2013, which was 4.3% (2.38% in the new TB treatment group and 23.1% in the retreatment groups) [35]. The present study demonstrated low MDR TB prevalence as compared to a finding by a study in Belarus which was 47% SNMDR-TB: 33% among new and 73% among previously treated patients [15]. But, our study showed high prevalence of SNMDR TB in comparison with a study in India that was conducted among newly diagnosed sputum-positive pulmonary tuberculosis between February 2008 and December 2009 demonstrated very low prevalence that was 1.13% MDR-TB [36]. The present study shows a very high SNMDR-TB prevalence even compared to the national MDR TB prevalence in Ethiopia which was performed among smear positive patients [6]. This is the first report in Addis Ababa. For instance, there was no smear negative MDR TB detected in a study conducted in St. Peter hospital, Addis Ababa, Ethiopia [18]. In our study among 27 Xpert MTB/ RIF confirmed Mycobacterium tuberculosis complex (MTBC) strains, 03 (11.11%) were Rifampicin resistant (RR) which is a bit higher compared to a study conducted in Karamara hospital, Jigjiga, Ethiopia which was 3/38 (7.9%); RR strains were identified from smear-negative culture-positive samples [32]. These variations of prevalence of SNMDR TB, and RR TB from our study result may be due to geographical location, population difference, time, study settings and method variation. Using Genotype MTBDRplus ver.2, Isoniazid (INH) mono resistance was 07/26 (26.9%). All RR SNPT isolates that Xpert MTB/ RIF assay diagnosed as RR were found to be MDR TB from which 01 (33.3%) MDR TB patients had TB contact history. Drug susceptibility testing was performed for 23 (88.46%) of the isolates for second line anti-TB drugs using Genotype MTBDRsl ver.2, to check for any resistance, but all isolates were susceptible. This study is different from other study conducted in Kenya, which showed resistance to first line and second line anti-TB drugs, but no MDR or XDR TB was detected [38].
Clinical features are significant in supporting the diagnosis of smear-negative TB even though it has poor sensitivity and specificity [31]. The present study to investigated clinical presentations, such as cough, fever, chest pain, loss of appetite, weight loss, shortness of breath, joint pain, abdominal pain, hemoptysis, and night sweating. But, none of this showed significant association with SNPT patients. Furthermore, cough, fever, weight loss, chest pain and night sweating are also symptoms in smear-positive patients, as it was reported in previous studies [33]. Shortness of breath, (AOR 0.493; 95% C.I, 0.181–0.1.343, p = 0.167), weight loss (AOR 0.340; 95% C.I, 0.108–1.069, p = 0.065) and loss of appetite (AOR 0.778; 95% C.I, 0.248–2.438, p = 0.666) are significant clinical features of SNPT patients in our study in the univariate analysis, but doesn’t have association in the multivariate model. However, another study reported that smear-negative patients were less probable to suffer from fever and weight loss than their smear-positive counterparts [39].
HIV comorbidity is a known confounder factor for the diagnosis of TB [8]. Our finding demonstrated 27% HIV positivity rate and 5.3% SNPT- HIV co-infection; but it doesn’t show a significant association and the comorbidity is much lower than the finding reported in Brazil which was 41% [9]. Our finding also showed that 7.1% SNPT among known diabetes mellitus patients and this finding is a bit higher compared to a study finding from St. Peter Hospital Addis Ababa, Ethiopia which was 6.7% [40]. This difference may be due to difference in study participants (they have done it on active TB patients), geographical coverage and time. From all demographic, behavioral and other risk factors that the study has assessed, sex (p = 005), work status or occupation (p = 0.006), being a migrant (p = 0.005), alcohol consumption (p = 0.042), chewing Khat (p = 0.038), has association with SNPT in a univariable analysis. A study conducted in Ethiopian prisons has showed alcohol consumption as a risk factor but chewing Khat was not associated with active TB disease [41]. Migrants have 0.121 times more likely to have smear negative pulmonary TB in comparison with non-migrants, (AOR 0.121; 95% C.I, 0.20–0.730, p = 0.021). The above factors needs to be further studied for the possible reasons of the association.
One limitation of the study is that chronic disease comorbidities are self-responded by the patient, not confirmed by diagnostic methods. The other one is phenotypic MGIT DST for Pyrazinamide (PZA) and for other 6 isolates for other first line drugs was not performed due to shortage of drugs and PZA sets.
Conclusion
In this study a significant proportion of smear negative pulmonary TB was diagnosed. Furthermore, a high smear negative multi drug resistant (MDR) TB and other mono drug resistant TB prevalence was confirmed. Due to the limitations of smear microscopy which is used as a primary diagnostic tool, these TB strains are missed to be diagnosed and transmission continues in the community. Therefore, considering more sensitive diagnostic tools and drug susceptibility testing facilities in the peripheral TB diagnostic laboratories is crucial. Further studies on comorbidities and risk factors of SNPT are advised to be conducted in large scale.
Acknowledgements
We would like to acknowledge Ethiopian Public Health Institute and Addis Ababa University department of Medical Laboratory Science. We are also thankful for Mr. Girum Taye, Study site staffs and study participants.
Abbreviations
- AFB
Acid fast bacilli
- AIDS
Acquired immunodeficiency syndrome
- BSC
Biosafety cabinet
- DOTS
Directly observed treatment short course
- DRS
Drug resistance surveillance or survey
- DST
Drug susceptibility testing
- EPHI
Ethiopian public health institute
- EQA
External quality assurance
- ETM
Ethambutol
- GLI
Global laboratory initiative
- HIV
Human immunodeficiency virus
- INH
Isoniazid
- LJ
Lowenstein jensen
- LPA
Line probe assay
- MDR
Multidrug resistant
- MTB
Mycobacterium tuberculosis
- NALC
N-Acetyl L-Cysteine
- NaOH
Sodium hydroxide
- NPV
Negative predictive value
- NTM
Non tuberculosis mycobacteria
- NTRL
National tuberculosis reference laboratory
- PPV
Positive predictive value
- PT
Proficiency testing
- PTB
Pulmonary tuberculosis
- RIF
Rifampicin
- RR
Rifampicin resistant
- SNMDR TB
Smear negative pulmonary multidrug resistance tuberculosis
- SNPP
Smear negative pulmonary patients
- SNPPTP
Smear negative presumptive pulmonary tuberculosis patients
- SNPT
Smear negative pulmonary tuberculosis
- SPPT
Smear positive pulmonary tuberculosis
- STR
Streptomycin
- WHO
World Health Organization
- XDR
Extensively drug resistant
- ZN
Ziehl Neelsen
Authors’ contributions
WS is the principal investigator who designed the project, organize and participate in laboratory testing, data collection and entry, analyze the result and write this manuscript. AK, AB, and ZM have given unreserved and substantial contributions starting from the design of the proposal through all the processes up to the manuscript write up. This project also finalized with the substantial support of ET, MT, BY, MA, BD, GD, AA, MG and DF, in laboratory analysis, site supervision, data collection, data entry and reporting. KD and HH have assisted in the data analysis and manuscript write up. In addition, all the authors read and approved the manuscript.
Funding
All the costs of this project were covered by Ethiopian Public Health Institute (EPHI) by availing vehicles, reagents, consumables and covering for training and data collection related costs.
Availability of data and materials
All original raw data is available with the corresponding author.
Ethics approval and consent to participate
This project was reviewed and approved by the department of Medical Laboratory Sciences research and ethics review committee (DRERC), school of allied health sciences, College of Health Sciences, Addis Ababa University (Ref.No: MLS/564/17) and by Institutional Review Board (IRB) of Ethiopian Public Health Institute (Protocol No: EPHI-IRB-021-2017). Sample was collected after receiving written consent from each participant or assent from the parents/guardians. For participants who were below the age of 18 years, permission from the parents or the guardians was taken and signed a written parental permission form. All positive SNPT/SNMDR TB patients were attached to the respective DOTS sites or TICs for treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Waganeh Sinshaw, Email: wagamels@gmail.com.
Abebaw Kebede, Email: jkabebaw@gmail.com.
Adane Bitew, Email: bitewadane@gmail.com.
Ephrem Tesfaye, Email: ephremt13@gmail.com.
Mengistu Tadesse, Email: mengistutadesse86@gmail.com.
Zemedu Mehamed, Email: zemedu2003@gmail.com.
Bazezew Yenew, Email: bazezewyenew@yahoo.com.
Misikir Amare, Email: misikiramare.ma@gmail.com.
Biniyam Dagne, Email: biniyamd16@gmail.com.
Getu Diriba, Email: diribagetu@yahoo.com.
Ayinalem Alemu, Email: ayinalemal@gmail.com.
Muluwork Getahun, Email: mimishaget@yahoo.com.
Dinka Fikadu, Email: ejeta430@gmail.com.
Kassu Desta, Email: kassudesta2020@gmail.com.
Habteyes Hailu Tola, Email: habtetola@gmail.com.
References
- 1.WHO. Global tuberculosis report. Geneva: World Health Organization; 2018.
- 2.Dye C, Lönnroth K, Jaramillo E, Williams BG. Raviglione. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87:683–91. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Multidrug-Resistant Tuberculosis (MDR-TB) report. 2013. [Google Scholar]
- 4.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 5.WHO/HTM/TB. Anti-Tuberculosis Drug Resistance in the world global report number 4. WHO. 2008;(4):394.
- 6.Lemma E, Feleke B, Kebede A, Getahun M, Yaregal Z, Fantu R. Second Round National Anti-tuberculosis Drug Resistance Surveillance in Ethiopia Report. 2014. pp. 1–52. [Google Scholar]
- 7.The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis. Geneva: World Health Organization; 2011. Available from: http://stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. Accessed 10 Mar 2017.
- 8.WHO . Implementing tuberculosis diagnostics: A policy framework. 2015. p. 39. [Google Scholar]
- 9.Leandro CC, Marcos VVR, Denise M, Cunha WDR. Characteristics of patients with smear negative Tuberculosis (TB) in a Region with high TB and HIV prevalence. 2015. pp. 1–10. [Google Scholar]
- 10.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. New Engl J Med. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Improving the diagnosis and treatment of smear-negative pulmonary and extra pulmonary tuberculosis among adults and adolescents. Recommendations for HIV prevalent and resource-constrained settings. 2007. Available at: http://www.who.int.
- 12.Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 13.Cavanaugh JS, Shah NS, Cain KP, Winston CA. Survival among patients with HIV infection and smear-negative pulmonary tuberculosis - United States, 1993-2006. PLoS One. 2012;7(10):e47855. doi: 10.1371/journal.pone.0047855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control/WHO Regional Office for Europe . Tuberculosis surveillance and monitoring in Europe. 2015. [Google Scholar]
- 15.Rusovich V, Kumar AMV, Skrahina A, Hurevich H, Astrauko A, de Colombani P, et al. High time to use rapid tests to detect multidrug resistance in sputum smear-negative tuberculosis in Belarus. Public Heal action. 2014;4(4):243–248. doi: 10.5588/pha.14.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adugna B, Ibrahim A, Kassu D. Prevalence of sputum smear negative, culture positive pulmonary tuberculosis among suspected patients at Adama referral hospital, Adama; 2013. Available from: http://etd.aau.edu.et/handle/123456789/3018.
- 17.Keflie TSS, Ameni G. Microscopic examination and smear negative pulmonary tuberculosis in ethiopia. Pan Afr Med J. 2014;19(162):1–10. [DOI] [PMC free article] [PubMed]
- 18.Desta K, Asrat D, Lemma E, Gebeyehu M, Feleke B. Drug susceptibility of Mycobacterium tuberculosis isolates from smear negative pulmonary tuberculosis patients, Addis Ababa: Ethiop. JHealth Dev. 2008;22(2):212–215. [Google Scholar]
- 19.Calis JCJ, Bakker ML, Elens RB, Borgdorff M, Harries AD. Mortality in smear-negative tuberculosis patients in Phalombe. Malawi Med J. 2002;14(2):13–4. [DOI] [PMC free article] [PubMed]
- 20.Macpherson P, Dimairo M, Bandason T, Zezai A, Munyati SS, Butterworth AE, et al. Risk factors for mortality in smear-negative tuberculosis suspects: a cohort study in Harare, Zimbabwe. Int J Tuberc Lung Dis. 2011;15:1390–1396. doi: 10.5588/ijtld.11.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47(9):1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]
- 22.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de LA, Daley CL, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Garduno E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59:286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samb B, Sow PS, Kony S, Maynart-Badiane M, Diouf G, Cissokho S, et al. Risk factors for negative sputum acid-fast bacilli smears in pulmonary tuberculosis: results from Dakar, Senegal, a city with low HIV seroprevalence. Int J Tuberc Lung Dis. 1999;3(4):330–336. [PubMed] [Google Scholar]
- 25.Nyagosya R, Andersen AB, van LF, Magnussen P, Apolinary M, Friis H. Risk factors for smear negative and culture positive results among pulmonary tuberculosis patients in Mwanza, Tanzania. Open Trop Med J. 2008;1(1):68–73. doi: 10.2174/1874315300801010068. [DOI] [Google Scholar]
- 26.Federal Democratic Republic of Ethiopia, Addis Ababa City Government Website. Available from: http://www.addisababa.gov.et/web/guest/city-map. Accessed 20 June 2019.
- 27.Federal Democratic Republic of Ethiopia Ministry of Health . National Comprehensive Tuberculosis, Leprosy and TB/HIV Training Manual for Health Care Workers. 2016. [Google Scholar]
- 28.Cepheid . GeneXpert Dx System Users’ manual. 2012. pp. 2–13. [Google Scholar]
- 29.Kanade S, Nataraj G, Suryawanshi R, Mehta P. Utility of MPT 64 antigen detection assay for rapid characterization of mycobacteria in a resource constrained setting. Indian JTuberc. 2012;59(2):92–96. [PubMed] [Google Scholar]
- 30.Stinson KW, Eisenach K, Kayes S, Matsumoto M, Siddiqi S, Nakashima, S. Global Laboratory Initiative Advancing TB Diagnosis: Mycobacteriology Laboratory Manual. (K. W. Stinson, Ed.). 2014. p. 147.
- 31.Hargreaves NJ, Kadzakumanja O, Phiri S, Nyangulu DS, Salaniponi FM, Harries AD. What causes smear-negative pulmonary tuberculosis in Malawi, an area of high HIV seroprevalence? Int J Tuberc Lung Dis. 2001;5:113–122. [PubMed] [Google Scholar]
- 32.Nguyen D, Nguyen H, Beasley R. Performance of clinical algorithms for smear-negative tuberculosis in HIV-infected persons in Ho Chi Minh City, Vietnam. Tuberc Res Treat. 2012;6:e360852. doi: 10.1155/2012/360852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biadglegne F, Rodloff AC, Sack U. A first insight into high prevalence of undiagnosed smear-negative pulmonary tuberculosis in northern Ethiopian Prisons implications for greater investment and quality control. PLoS One. 2014;9(9):e106869. doi: 10.1371/journal.pone.0106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geleta DA, Megerssa YC, Gudeta AN, Akalu G, Taddesse KD. Xpert MTB / RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiol. 2015;15:220. doi: 10.1186/s12866-015-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahebi Leyla, Ansarin Khalil, Mohajeri Parviz, Khalili Majid, Monfaredan Amir, Farajnia Safar, Zadeh Simin Khayyat. Patterns of Drug Resistance Among Tuberculosis Patients in West and Northwestern Iran. The Open Respiratory Medicine Journal. 2016;10(1):29–35. doi: 10.2174/1874306401610010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma SK, Kaushik G, Jha B, George N, Arora SK, Gupta D. Prevalence of multidrug-resistant tuberculosis among newly diagnosed cases of sputum-positive pulmonary tuberculosis. Indian J Med Res. 2011;133:308–311. [PMC free article] [PubMed] [Google Scholar]
- 37.WHO, Definitions and reporting framework for tuberculosis 2013, revision, updated December 2014. Available from: https://www.who.int/tb/publications/definitions/en/. Accessed 24 May 2018.
- 38.Ombura IP, Onyango N, Odera S, Mutua F, Nyagol J. Prevalence of drug resistance Mycobacterium tuberculosis among patients seen in coast provincial general hospital , Mombasa, Kenya. PLoS One. 2016;253:1–12. doi: 10.1371/journal.pone.0163994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samb B, Sow PS, Kony S, Maynart-Badiane M, Diouf G, et al. Risk factors for negative sputum acid-fast bacilli smears in pulmonary tuberculosis: results from Dakar, Senegal, a city with low HIV seroprevalence. Int J Tubrc Lung Dis. 1999;3(4):330–336. [PubMed] [Google Scholar]
- 40.Damtew E, Ali I, Meressa D. Prevalence of Diabetes Mellitus among Active Pulmonary Tuberculosis Patients at St. Peter Specialized Hospital, Addis Ababa, Ethiopia. world J Med Sci. 2014;11(3):389–96.
- 41.Ali Solomon, Haileamlak Abraham, Wieser Andreas, Pritsch Michael, Heinrich Norbert, Loscher Thomas, Hoelscher Michael, Rachow Andrea. Prevalence of Pulmonary Tuberculosis among Prison Inmates in Ethiopia, a Cross-Sectional Study. PLOS ONE. 2015;10(12):e0144040. doi: 10.1371/journal.pone.0144040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All original raw data is available with the corresponding author.