Abstract
Background
In Ethiopian, the prevalence of anemia among preschool aged children widely varied across regions. Since anemia adversely affects the cognitive and physical development of the children, it is important to determine its burden for implementing appropriate measurements. Therefore, this study was aimed at determining the anemia prevalence and associated factors among preschool aged children.
Method
A community based cross-sectional study was conducted on a total of 432 preschool children in Menz Gera Midir district from January to May, 2017. A multi stage sampling procedure was applied to select the target groups. Hemocue analyzer for Haemoglobin determination; anthropometric measurements for assessment nutritional status, structured questionnaires for socio-demographic and economic variables were used for data collection. The morphological appearance of red blood cell was assessed microscopically to determine type of anemia. Descriptive statistics were employed to summarize the data and binary logistic regression was used for inferential statistics. A p value less than 0.05 was considered as statistically significant.
Result
The overall prevalence of anemia was 123 (28.5%); of which 38 (30.9%) and 85 (69.1%) were moderate and mild, respectively. Morphologically about 50.4, 37.4 and 12.2% were microcytic hypochromic, normocytic normochromic and macrocytic anemias, respectively. Child age 6-11 months (COR: 5.67, 95% CI: 2.2, 14.86), child age 12–23 months (COR: 5.8, 95% CI: 2.3, 14.7), wasting (COR: 3.5, 95% CI: 1.2, 9.8), stunting (COR: 3.8, 95% CI: 1.92, 7.77), underweight (COR: 2.12, 95% CI: 1.07, 4.38), MUAC measurement below 13 cm (COR: 5.6, 95% CI: 2.83, 11.15), household headed by female (COR: 3.24, 95% CI: 1.1, 9.63), maternal anemia (COR: 4, 95% CI: 2.2, 7.23) and household food insecurity (COR: 2.12, 95% CI: 1.09, 4.12) were significantly associated with anemia.
Conclusion
The prevalence of anemia among the children was found to be high and associated with child age group, child nutritional status, house hold headed by female, maternal anemia and household food insecurity. Further studies on nutritional anemia, community based nutritional education, iron supplementation to children at risk should be promoted.
Keywords: Anemia, Pre-school aged children, Menz Gera Midir, Ethiopia
Background
Anemia is a condition where hemoglobin concentration is decreased in the blood. It is a serous public health problem worldwide that affects both wealthy and poor countries with a significant adverse health consequences as well as adverse impacts on social and economic development [1–3]. Anemia is considered as severe, moderate, mild and no public health problem, if its prevalence is ≥40%, 20–39.9%, 5–19.9 and < 5%, respectivel in the community [1].
According to the 2008 WHO report, 56.3% of world’s preschool aged children reside in developing countries including sub-Saharan Africa where anemia is a severe public health problem [4–6]. Golobally, the 2015 WHO report showed, 42.6% anemia prevalence among children aged 6–59 months in 2011. The WHO Africa, South East Asia and Eastern Mediterranean regions account the highest prevalence. These three regions account 62.3, 53.8 and 48.6% anemia prevalence, respectively. The report also revaled that 50% anemia prevalence among children aged 6–59 months in Ethiopia [1]. Evidences also revealing that its prevalence showed a rising in children compared to other age groups [7–9]. Since the demand of iron is higher in preschool children for rapid growth, anemia prevalence is higher. It affects children both physically and mentally. As a result, working capacity and educational performance of children will be decreased [2, 4, 10, 11].
According to the Ethiopia Demographic and Health Survey (EDHS) reports of 2005, 2011 and 2016, the national prevalence of anemia was estimated to 53.5%,44.2 and 56% in preschool aged children, respectively and its prevalence in Amhara regional state was 54, 35.1 and 41%, respectively [7, 12, 13]. In Ethiopian, its prevalence among preschool aged children widely varied across regions that have been ranged from 33% (Addis Ababa) to 75.2% (Afar region) and disparity of prevalence also observed between urban and rural children, which is higher in rural children [7]. Usually, this high prevalence of anemia is associated with food insecurity resulted from floods and drought [14]. These disasters are common problem in Ethiopia including the study area, Menz Gera Midir district. The floods and drought causes rugged terrain and degraded lands which causes a decline in agricultural land productivity [15]. This inturn cuases food insecurity and micronutrient deficiency anemia [3, 16, 17]. Therefore, determining the prevalence and associated risk factors of anemia have paramount importance for policy makers in order to ensure sustainable improvements. But in the study area, which is suffer from food insecurity with an incidence of 84.7% [14], there is limited information regarding the anemia prevalence and its predictive factors. Thus, this study was aimed to assess the prevalence and predictive factors of anema among pre-school aged children in the Menz Gera Midir district, Eastern Amhara, North Shewa zone, Ethiopia.
Methods
Study setting and population
A community based cross-sectional study was conducted on a total of 432 preschool children in Menz Gera Midir district from January to May, 2017. The district is located in Semien Shewa Zone, Eastern Amhara state, Ethiopia. The administrative center of this district is Mehal Meda town which is located 295 km away from Addis Ababa and 165 km from its zonal town (Debre Birhan). The town is also elevated 3037 m above sea level with latitude/longitude of 10018′17″ N/390 39′ 31″E. According to the population projection of Ethiopia from 2014 to 2017, this district has a total population of 138,708 with 16,361(11.8%) urban inhabitants [18]. Children aged 6–59 months reside in the selected kebeles for at least 6 months and whose parents/guardians are willing to fully participate in the study were included. While, children with active hemorrhage, history of blood transfusion within the last 2 months, history of surgery within the last 2 months were excluded from the study.
Sample size determination
To determine the required sample size for study, a single population proportion formula was used as denoted below
Where z = Z score for 95% confidence interval, which is 1.96
p = expected prevalence of anemia, which was 25% taken from Menz Keya [19].
d = tolerable error between the sample and true population, which is 5%
Considering affordable resources for investigations, a design effect of 1.5 for sampling error was taken and 288*1.5 = 432 children were included.
Data collection procedures
A multi-stage sampling procedure was used; at the first stage, sample was determined to collect from one fourth of the total kebeles; out of 28 kebeles, seven kebeles (1 urban and 6 rural) were selected randomly. Kebele (neighbourhood) is the smallest administrative unit in Ethiopia. At the second stage, the number of households included from each kebeles were proportionally allocated. Then a systematic sampling method was used to select each household (Fig. 1). The total numbers of households in each kebele was taken from each administrative kebele and used to calculate the sampling interval (K) which was 26. After the first household randomly selected, households every 26th interval were approached. If a household was with two or more eligible childern, only one of them was chosen randomly by lottery method. On the otherhand, when the selected household was closed even after revisit or child was not eligible, the next household was included.
Fig. 1.
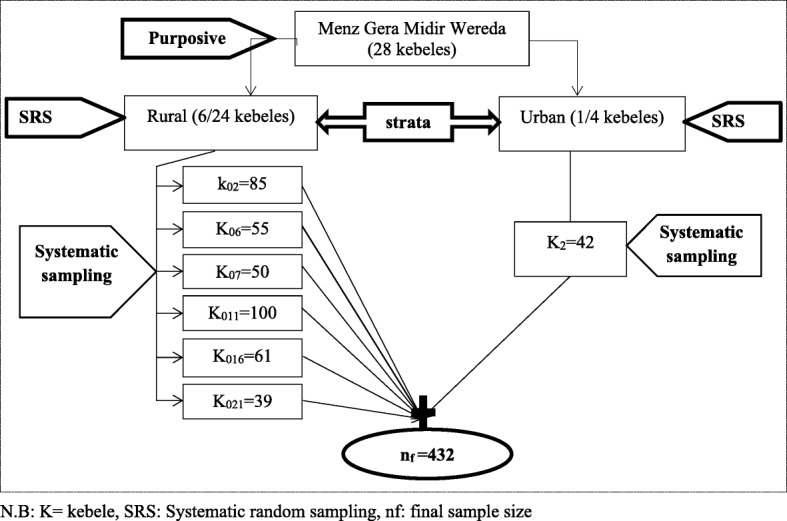
Schematic representation of sampling procedure. N.B: K = kebele, SRS: Systematic random sampling, nf: final sample size
Household demographic and socio-economic data collection
A pretested structured questionnaire which was prepared based on the national survey questionnaire and accordingly modified based on the reviewed literature [20] was used. The questionnaire consisted of socio-demographic characteristics of the child and their parents, household food security status (HFSS), child feeding practices, food consumption pattern and health condition of the children. Household food security (HHFS) status was assessed by using the standardized questionnaire developed by Food and Nutritional Technical Assistance (FANTA) [21, 22]. Food consumption pattern and dietary diversity scores (DDS) were determined by using a modified Helen Keller International Food Frequency Questionnaire (FFQ) and a 24-h dietary recall, respectively [22–24]. Altitude for each kebele was measured using an accurate altimeter app android installed on smart phone.
Wealth index determination
Wealth index was determined to assess inequalities in household characteristics, in the use of health and other services. It used as an indicator of level of household wealth. The index was determined using household assets and type of house. A principal component analysis (PCA) was used to produce a common factor score for each household. Variables were coded between 0 and 1, entered and analyzed. Then variables with communality values of > 0.5 were used to produce factor scores. Factor scores were summed and categorized into three relative measures of socio economic status of households as low, medium and rich [22].
Household food insecurity assessment
The Food and Nutritional Technical Assistance (FANTA) scale guideline questions were used to assess household food security status. The questionnaire was adapted from household food insecurity access scale and validated for developing countries. A household was considered as food secured if it had experience of less than the first 2 food insecurity indicators from the 27. Each question was responded as never, rarely, sometimes, or often. Households were considered as “food secure,” if they “never” or “rarely” worried about the deficiency of food in their households [21, 25].
Dietary assessment
Food frequency was assessed with a questionnaire consisted of ten groups of food items was used. The food items were grouped in to cereals, legumes, meat, egg, vegetables, fruits, dairy products, fish and sea foods, sweet foods made with sugar, honey, oil, fat, or butter and any other foods, such as condiments, coffee, tea. Then dietary diversity scores (DDS) was calculated from these food groups and categorized as high (DDS ≥ 7), medium (DDS = 4–6), and low (DDS ≤ 3) [22].
Anthropometric measurements
Child age, weight, height and mid-upper arm circumference (MUAC) were measured according to the 2008 WHO recommendation for nutritional status assessment [26]. All Childrens’ weight was measured while wearing light-weight cloth with a portable weight scale to the nearest 0.1 kg. The weighing scale was calibrated using the standard calibration weight of 2 kg iron bars. Child height was measusered using a locally manufactured wooden standiometre with a sliding head bar to the nearest 0.1 cm in Frankfurt position (head, shoulder, buttocks, knee, and heals touch the vertical board). Children height aged below 24 months was taken while lying down and for those older children heights were measured at standing position. Measurements of weight and height were taken twice and the average was recorded. Then the data were entered into WHO Anthro 3.2.2.1 for the calculation of weight-for-age (WAZ), weight-for-height (WHZ) and height-for-age (HAZ) standard Z-scores. Children were classified as stunted, underweight and wasted when HAZ, WAZ and WHZ scores were < −2SD, respectively [27].
Hemoglobin (Hgb) measurement
Haemoglobin values of children and their mothers was determined by using HemoCue Hb 300+ analyzer. Since the study area altitude is > 3000 m above sea level, results of Hgb were adjusted to its respective sea level by subtracting 1.9 g/dL as it is recommended by WHO [28]. Anemia was defined when Hgb level is < 11 g/dL for both genders. Regarding to anemia severity Hgb value of 10 to 10.9 g/dL, 7 to 9.9 g/dL and < 7 g/dL were considered as mild, moderate and severe anemia, respectively [28].
Peripheral blood examination
The morphological characteristics of RBC were assessed by using 100x (oil immersion) high magnification power light microscope. The type of anemia then was classified based on the characteristics of RBC morphology as microcytic, normocytic and macrocytic anemias.
Stool examinations
For intestinal parasite examination, stool samples were collected from study participants and wet mount was prepared by using normal saline for direct microscopy. The remaining portion of the collected sample was preserved by using 10% formalin andconcentration techniques. Finally, examination of stool by using concentration technique for ova or parasite was performed within 24 h of collection.
Statistical analysis methods
Firstly, data were checked for completeness and coded manually. After coding, data were double entered and stored using EPI-info 7 and exported to SPSS 20 for further analysis. Descriptive statistics were used to summarize the characteristics of the study population. To determine factors associated with anemia, bivariate logistic regression analyses was done and the 95% confidence (CI) level was used determined the strength of association between the predictors and dependent variables. Those variables with a P value of < 0.2 in bivariate analysis were fitted in to multivariate logistic regression analysis. A P value < 0.05 in multivariable analysis was considered as statistically significant.
Results
Socio-demographic characteristics of children
A total of 432 preschool aged children, 390 (90.3%) from rural and 42 (9.7%) from urban were included. Of them, 227/390 (58.2%) and 27/42 (64.3%) were females from rural and urban residence, respectively. The median age of the children was 24 months with interquartile range (IQR) of 14–42 months. From 432 children, 49 (11.3%), 217 (50.2%) and 121 (28%) were wasted, stunted and underweight, respectively. During the study period, stool sample was collected from 251 children. From them about 41 (16.3%) were positive for one of the following intestinal parasites; Entamoeba histolytica, Giardia lamblia, Ascaris Lumbricoides, Hookworm and Taenia saginata. Of those children infected by intestinal parasites, 35 (85.4%) were found anemic (Table 1).
Table 1.
Characteristics of the preschool aged children
| Background characteristic | Frequency | Percentage |
|---|---|---|
| Sex of child | ||
| Male | 178 | 41.2 |
| Female | 254 | 58.8 |
| Age Group (in months) | ||
| 6–11 | 90 | 16.7 |
| 12–23 | 107 | 25.0 |
| 24–35 | 70 | 17.1 |
| 36–47 | 76 | 18.8 |
| 48–59 | 89 | 22.5 |
| Delivery Status | ||
| Term | 425 | 98.4 |
| Preterm | 7 | 1.6 |
| History of illness in the past 2 weeks | ||
| No | 415 | 96.1 |
| Yes | 17 | 3.9 |
| Place of Child Birth | ||
| Health Facility | 297 | 68.8 |
| Home | 136 | 31.2 |
| Intestinal parasites (n = 251) | ||
| Positive | 41 | 16.3 |
| Negative | 210 | 83.7 |
| Vaccination Status | ||
| No vaccinated | 5 | 1.2 |
| Partially Vaccinated | 58 | 13.4 |
| Fully Vaccinated | 369 | 85.4 |
| Wasting | ||
| Yes | 49 | 11.3 |
| No | 383 | 88.7 |
| Stunting | ||
| Yes | 217 | 50.2 |
| No | 215 | 49.8 |
| Underweight | ||
| Yes | 121 | 28.0 |
| No | 311 | 72.0 |
| MUAC | ||
| ≥ 13.5 cm | 167 | 38.7 |
| < 13.5 cm | 265 | 61.3 |
Parental socio-demographic and economic characteristics
About 420 (97.2%) of the care givers were female and 414 (95.8%) of them were mothers in relationship to the child. Majority, 402 (93%), of the mother respondents were married. Regarding paternal educational status, more than one third of 158 (36.6%) of the mothers cannot read and write, and 411 (95.2%) of them were housewives. The median household size in rural and urban was 5 (IQR: 4–6) and 4 (IQR: 3–5) person per household respectively. Economically, nearly half of the households categorized under lower socio-economic class. Around 72.9% of the households were suffering from food insecurity (Table 2).
Table 2.
Parents’ socio- demographic and economic characteristics
| Background characteristic | Frequency | Percentage |
|---|---|---|
| Sex of the child care giver | ||
| Female | 420 | 97.2 |
| Male | 12 | 2.8 |
| Residence | ||
| Rural | 390 | 90.3 |
| Urban | 42 | 9.7 |
| Relationship with care giver | ||
| Mother | 414 | 95.8 |
| Father | 12 | 2.8 |
| Others | 6 | 1.4 |
| Marital Status | ||
| Married | 402 | 93.0 |
| Divorced | 25 | 5.8 |
| Single | 5 | 1.2 |
| Sex of the head of the household | ||
| Male | 406 | 93.99 |
| Female | 26 | 6.01 |
| Occupation of Mother | ||
| Housewife | 411 | 95.2 |
| Government employed | 14 | 3.2 |
| Merchant | 7 | 1.6 |
| Father’s Occupation | ||
| Farmer | 365 | 84.5 |
| Governmental Employee | 22 | 5.1 |
| Labor | 19 | 4.4 |
| Merchant | 26 | 6.0 |
| Maternal Educational status | ||
| No Education | 158 | 36.6 |
| Primary School | 155 | 35.9 |
| Secondary completed | 98 | 22.7 |
| Higher Education Completed | 21 | 4.9 |
| Father’s Educational status | ||
| No Education | 94 | 21.8 |
| Primary School | 191 | 44.2 |
| Secondary completed | 125 | 28.9 |
| Higher Education complete | 22 | 5.1 |
| Household Wealth quintile | ||
| Higher | 64 | 14.8 |
| Medium | 164 | 38.0 |
| Lower | 204 | 47.2 |
| Household dietary Diversity Score (HDDS) | ||
| Low | 188 | 43.5 |
| Medium | 214 | 49.5 |
| High | 30 | 7.0 |
| Family size in the household | ||
| ≤ 3 | 98 | 22.7 |
| 4–6 | 275 | 63.7 |
| ≥ 7 | 59 | 13.6 |
| Household Food Security | ||
| Food Secured | 117 | 27.1 |
| Food Insecured | 315 | 72.9 |
Prevalence of anemia
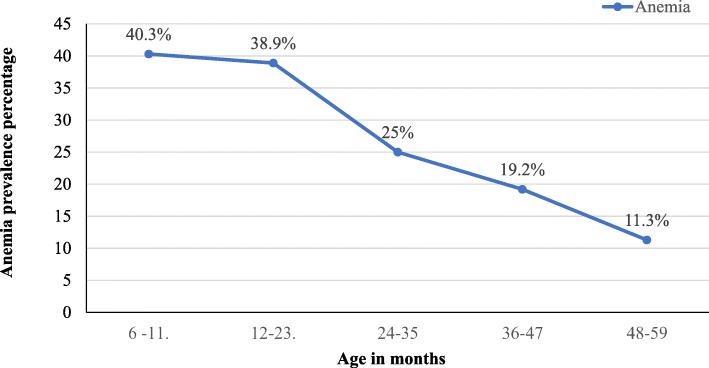
The mean (± SD) value of Hgb level was 11.3 ± 1.1 g/dL with values ranging from 7.3 g/dL to 14.8 g/dL. The overall prevalence of anemia was 123 (28.5%) (95% CI: 24.2–32.7) with 116 (29.7%) and 7 (16.7%) magnitude among rural and urban children, respectively. Of the 123 anemic children, 38 (30.9%) and 85 (69.1%) were moderately and mildly anemic, respectively. Morphologically, about 50.4, 37.4 and 12.2% of anemic cases were microcytic-hypochromic, normocytic-normochromic and were macrocytic, respectively. There was no significant difference in the prevalence of anemia between females and males which was 29.5 and 27.0%, respectively. The prevalence of anemia among wasted, stunted, underweight and MUAC children was 67.3, 36.4, 40.5 and 40%, respectively. The prevalence of anemia was higher among younger children (40.3%) in (6–11 months), and it had shown decline as the child’s age increased (Fig. 2).
Fig. 2.
Anemia distribution among preschool aged children by their age
Factors associated with anemia
Table 3 shows bivariate and multivariate analysis of factors associated with anemia. In bivariate analysis, child and maternal related variables such as child age group, vaccination status of the child, wasting, stunting, underweight, MUAC, sex of the household head, maternal education, maternal anemia and household food insecurity showed association with anemia. Then those variables having p-value less than 0.2 were subjected to multivariable binary logistic regression. In multivariable analysis of factors associated with anemia, child age 6–11 months (COR: 5.67, 95% CI: 2.2, 14.86) and 12–23 months (COR: 5.8, 95% CI: 2.3, 14.7), wasting (COR: 3.5, 95% CI: 1.2, 9.8), stunting (COR: 3.8, 95% CI: 1.92, 7.77), underweight (COR: 2.12, 95% CI: 1.07, 4.38), MUAC measurement < 13 cm (COR: 5.6, 95% CI: 2.83, 11.15), household headed by female (COR: 3.24, 95% CI: 1.1, 9.63), maternal anemia (COR: 4, 95% CI: 2.2, 7.23) and household food insecurity (COR: 2.12, 95% CI: 1.09, 4.12) were significantly associated with anemia. On the other hand child sex, residence, vaccination status, intestinal parasite, maternal age, maternal occupation, delivery status, history of illness with in last 2 weeks and place of birth did not show any statistically significant association with anemia.
Table 3.
Association of characteristics of study subjects with anemia
| Characterstics of study subjects | Anemic | Non-anemic | COR (95% CI) | AOR (95%CI) |
|---|---|---|---|---|
| Sex of child | ||||
| Female | 75 (29.5) | 179 (70.5) | 1 | |
| Male | 48 (27.9) | 130 (73.0) | 0.881 (0.58–1.35) | |
| Child age group (months) | ||||
| 6–11 | 47 (52.2) | 43 (47.8) | 5.27 (2.41–11.55) | 5.67 (2.20–14.86)a |
| 12–23 | 33 (30.8) | 74 (69.2) | 4.98 (2.40–10.40) | 5.80 (2.30–14.7)a |
| 24–35 | 21 (30.0) | 49 (70) | 2.61 (1.14–5.93) | 2.14 (0.78–5.84) |
| 36–47 | 16 (21.1) | 60 (78.9) | 2.99 (1.36–6.61) | 1.71 (0.22–4.367) |
| 48–59 | 6 (6.7) | 83 (93.3) | 1 | 1 |
| Residence | ||||
| Urban | 7 (16.7) | 35 (83.3) | 1 | 1 |
| Rural | 116 (29.7) | 274 (70.3) | 2.17 (0.91–4.90) | 1.833 (0.524–6.407) |
| Delivery status | ||||
| Preterm | 3 (42.9) | 4 (57.1) | 1.91 (0.33–10.240 | |
| Term | 120 (28.2) | 305 (71.8) | 1 | |
| History of illness in the past 2 weeks | ||||
| Yes | 5 (29.4) | 12 (70.0) | 1.05 (0.362–3.04) | |
| No | 118 (28.4) | 297 (71.6) | 1 | |
| Place of Birth | ||||
| Home | 35 (25.9) | 100 (74.1) | 0.83 (0.52–1.32) | |
| Health Facility | 88 (29.6) | 209 (70.4) | 1 | |
| Intestinal Parasites (n = 251) | ||||
| Positive | 35 (85.4) | 6 (14.6) | 0.55 (0.218–1.38) | |
| Negative | 160 (76.2) | 50 (23.8) | 1 | |
| Vaccination Status | ||||
| Not Complete | 26 (41.3) | 37 (58.7) | 1.97 (1.13–3.42) | 0.682 (0.294–1.584) |
| Complete | 97 (26.3) | 272 (73.7) | 1 | 1 |
| Wasted | ||||
| Yes | 33 (67.3) | 16 (32.7) | 6.72 (3.53–12.76) | 3.5 (1.20–9.8)a |
| No | 90 (23.5) | 293 (76.5) | 1 | 1 |
| Stunted | ||||
| Yes | 79 (36.4) | 138 (63.8) | 2.23 (1.45–3.42) | 3.8 (1.92–7.77)a |
| No | 44 (20.5) | 171 (79.5) | 1 | 1 |
| Under Weight | ||||
| Yes | 49 (40.5) | 72 (59.5) | 2.18 (1.40–3.41) | 2.12 (1.07–4.38)a |
| No | 74 (23.8) | 237 (76.2) | 1 | 1 |
| MUAC | ||||
| ≥13.5 cm | 17 (10.2) | 150 (89.8) | 1 | 1 |
| < 13.5 cm | 106 (40.0) | 159 (60.0 | 5.88 (3.37–10.28) | 5.6 (2.83–11.15)a |
| Maternal Age | ||||
| < 30 years | 91 (30.7) | 205 (69.3) | 1.44 (0.91–2.30) | 1.043 (0.504–2.155) |
| > = 30 Years | 32 (23.5) | 104 (76.5) | 1 | 1 |
| Sex of the Household head | ||||
| Female | 8 (66.7) | 4 (33.3) | 5.3 (1.57–17.9) | 3.24 (1.10–9.63)a |
| Male | 115 (27.4) | 305 (72.6) | 1 | 1 |
| Maternal Education | ||||
| No Education | 53 (33.5) | 105 (66.5) | 4.80 (1.08–21-36) | 1.16 (0.897–72.53) |
| Primary School | 39 (25.2) | 116 (74.8) | 3.20 (0.71–14.34) | 5.85 (0.759–45.07) |
| Secondary School | 29 (29.6) | 69 (70.4) | 3.9 (0.87–18-26) | 7.21 (0.898–57.86) |
| Higher Education | 2 (9.6) | 19 (90.4) | 1 | 1 |
| Maternal Anemia | ||||
| Yes | 56 (56.6) | 43 (43.4) | 5.64 (3.46–9.22) | 4.0 (2.20–7.23)a |
| No | 57 (18.8) | 247 (81.2) | 1 | 1 |
| Household food insecurity | ||||
| Secured | 23 (19.7) | 94 (80.3) | 1 | |
| Insecured | 100 (31.7) | 215 (68.3) | 1.90 (1.13–3.18) | 2.12 (1.09–4.12)a |
| Household dietry diversity score (HDDS) | ||||
| Low | 62 (33) | 126 (67) | 1.97 (0.76–5.06) | |
| Medium | 55 (25.7) | 159 (74.3) | 1.38 (0.54–3.56) | |
| High | 6 (20) | 24 (80) | 1 | |
aindicated statistically significant variables
Discussion
The findings of this study showed 28.5% overall prevalence of anemia, denoting a moderate public health problem according to WHO classification [1]. This result was in agreement with a study conducted in the neighboring district, Menz Keya and higher than Menz Lalomma [19]. On the other hand the observed prevalence was lower when compared to the national and regional prevalence reported by EDHS 2005, 2011 and 2016 [7, 12, 13]. The possible reasons for inconsistencies in the prevalence of anemia across studies might be related to variation of study time in which the studies were conducted, geographical variability of risk factors, differences in socioeconomic status of the populations and implementation of different strategies to minimize the burden of anemia in the region where these studies have been conducted.
The study showed that most of the children had mild type of anemia 85 (69.1%) and only 38 (30.9%) had moderate anemia. There was no severe anemia which was as expected because it is a community based study, not a hospital based. This is supported by a study conducted in Northwestern Uganda [29]. There was no significant difference in the prevalence of anemia between females and males which was 29.5 and 27.0%, respectively. Half (50.4%) of the type of anemia was microcytic-hypochromic anemia followed by normocytic-normochromic (37.4%) and macrocytic anemias (12.2%). This might be due the fact that the community in the study area consume cereals which have low iron content; thus, microcytic hypochromic anemia is dominantly observed type of anemia. On the other hand, vitamin B12 and/or folate deficiency may cause the development of macrocytic anemia.
From the study result, age was remained significantly associated with anemia and this finding has been reported in several other studies conducted in northern Ethiopia [5, 30], Ghana [31] and Nigeria [32]. As the child getting older, the prevalence of anemia decreased from 40.5% (in the age group of 6–11 months) to 11.3% (in the age group of 48–59 moths) which meant that children at younger age were the most vulnerable group for anemia. The likelihood of being anemic for the children in the age group of 6–11 and 12–23 months was about six fold greater as compared to children in the age group of 48–59 months.
The greatest prevalence of anemia in the age group of 6–11 and 12–23 months might be attributable to different factors. This is may be due to the transition from feast to famine of iron store due to the rapid growth and expanding of blood volume during the first 2 years. As studies conducted in India [10], Nigeria [32] and Sub-Saharan Africa [4] showed, children within this age group require large amount of iron to maintain a near steady mean hemoglobin concentration within their body. Since the iron stores in their body has usually been depleted and exclusive breast feeding might not contain adequate iron, the initiation of complementary foods with high iron content is mandatory to satisfy their high iron demand. Even though complementary diet is a crucial source of iron during this time, the major source of diet in the study area was cereal groups which have low iron content [33]. In addition to this, after the age of 24 months, they use more diversified diet which in turn increases the quality and quantity of foods. Moreover, the amount of iron required per body kilogram relatively decreases due to the slowdown of growth rate as the child gets older [2].
Maternal anemia during the time of survey was another factor that statistically associated with childhood anemia. The odds of anemia for children whose mother was anemic was about four times higher as compared to children whose mothers were non-anemic. This association has also been reported by studies conducted in Cameroon [34], Northern Ethiopia [5] and Eastern Cuba [9]. The possible reason for this association might be that both mothers and children mostly share a common home environment, which involves mutual exposure to a common set of physical, socioeconomic, and dietary conditions. Furthermore, maternal anemia might be associated with poor birth outcomes such as low birth weight and prematurity of the child. Thus, hemoglobin level of the child is strongly dependent on the level of maternal hemoglobin concentration in their blood. In addition to this, maternal anemia might led to limited fetal iron store and the amount of iron secreted by the breast milk might be insufficient for daily iron requirement of the child. Therefore, due to these consequences of maternal anemia, the possibility of childhood anemia is greater in children whose mothers were anemic. Thus, it is important to screen pregnant women for anemia and regular nutrient supplementation during their follow-ups.
Wasting, stunting and underweight were significantly associated with anemia in preschool aged children. This association was supported by a studies conducted in Kilte Awlalo zone Northern Ethiopia [35], Tigray Province [5], Nigeria [32], United Republic of Tanzania [36] and Pakistan [37]. Children who were wasted, stunted, underweight and whose MUAC measurement below 13 cm were 3.5, 3.8, 2.12 and 5.6 times more likely to have anemia compared to children with who were not undernourished, respectively. Stunting is an indicator of chronic malnutrition whereas underweight is a combination of both chronic and acute malnutrition. Usually, anemia and undernutrition often share common causes because both these factors are aggravated by poverty and food insecurity. Undernourished children are more suffered from inadequate bioavailability of micronutrients such as iron, B12 and folate in their body which are important for the formation of blood cells. Therefore, those children who are undernourished cannot form adequate blood cells as many as required; consequently the this leads to the development of nutritional deficiency anemia which is common especially in developing countries [38].
Household food insecurity was also identified as an associated factor for childhood anemia which implies children from food insecured household were 2.34 times greater at risk of developing anemia. This finding is supported by similar studies conducted in Tigray province and rural Cameroon [36]. From this study nearly three forth (72.9%) of the household were food insecured which is almost similar with previous data conducted in the area [7, 14]. The higher prevalence of anemia in those children living in food insecured households might be due to inadequate intake of diets, consumption of a limited variety of food groups with low iron content and coping mechanism during food shortage [21, 29]. The coping mechanisms during food shortage were factor that might be attributed to poor nutritional status, and thus for the development of anemia. During food shortage, the household planned to limit the portion of food size at meal time and reducing frequency of meals per day, feeding only working family members, and consuming less preferred or inexpensive food were the common coping mechanisms in the area to prevent early used up of reserved foods.
Another finding in this study was that, anemia was significantly associated with living in a household headed by females. Children from female headed household were 3.24 times at greater risk for anemia. This association can be explained by the time allocated for child care and household food security level. Most of the involvements to improve the child health and nutritional status rely on the behavior of their caregiver, often the mother. When females become the head of the household with no husband in the household, every task in and outside the house were on the shoulder of the women. In these circumstances, women focus on outside home activities which are both time and labor intensive tasks. The time allocated to care their children in the house is minimized. Therefore, initiation of complementary foods, close follow up of their children and timely breast feeding had been compromised. Consequently, the child become more vulnerable either for chronic or acute malnutrition which leads to the development of nutritional anemia [39].
As a strength, this study is a community based and tried to assess different factors of anemia among preschool aged children in rural part of eastern Amhara, northeast Ethiopia. The findings could be used as a base line information to conduct large scale study that determine the cause and effect relationship. There were also some limitations to this study ought to be taken into account when interpreting the results of this study. The first major limitation is being cross-sectional nature that does not allow us to observe causality in the relationship between anemia and its associated factors. Secondly, we were also unable to measure serum concentration of micronutrients to characterize anemia.
Conclusion
The prevalence of anemia among preschool aged children in Menz Gera Midir district was a moderate public health problem. As shown by the study, children younger than 24 months of age were more likely to be affected by anemia than older age groups. Mild.and microcytic-hypochromic anemia were the dominant type of anemias observed. Nutritional status of the child (wasting, stunting, under weight and MUAC < 13 cm), maternal anemia, household food insecurity and female headed households were the contributing factors for the development of childhood anemia in the district. From the finding, it is recommended that community based nutritional education, nutritional support programs for children living in households with food insecured should be implemented to reduce the burden of anemia in the community. Households, those in food insecure status should be identified and included in safety net programs. Children living in those households should be included in school feeding programs. In addition, maternal hemoglobin. Finaly We recommend further studies that include larger sample size and assessment of micronutrients to characterize as iron, B12 and folate deficiency anemia should be conducted.
Acknowledgments
The authors would like to acknowledge Mehal Meda Hospital laboratory, the state of Amhara Health Bureau and all the study participants.
Abbreviations
- AOR
Adjusted odds ratio
- COR
Crude odds ratio
- DDS
Dietary diversity scores
- EDHS
Ethiopian demographic and health survey
- FANTA
Food and Nutritional Technical Assistance
- FFQ
Food Frequency Questionnaire
- HAZ
Height for Age Z score
- HFSS
Household food security status
- Hgb
Hemoglobin
- MUAC
Mid-upper arm circumference
- PCA
Principal component analysis
- RBC
Red blood cells
- RDT
Rapid diagnostic test
- WAZ
Weigh for Age Z score
- WHO
World Health Organization
- WHZ
Weigh for Height Z score
Authors’ contributions
GE, MM and BE involved in the study design, statistical analysis and draft the manuscript. GE & AY involved in laboratory analysis and result interpretation. GE, ZG & FA involved in data collection, and drafting of the manuscript along with BE & MM. All authors read and approved the final manuscript.
Funding
The author(s) received no specific funding for this work.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical clearance and approval was granted from University of Gondar, Collage of Medicine and Health Sciences, School of Biomedical and Laboratory Sciences Ethical Review Committee. Permission letter was also obtained from district health office and each village administrative offices. The purpose of the research was explained to the study subjects and written informed consent was obtained from the mother or guardian of the child and then those who were willing to participate included in the study. Participation was fully voluntarily, refusal at any time during data collection was permitted. Confidentiality was kept. Any abnormal findings were linked to the nearby health center. Additionally, when haemoglobin level of the children was below 11 g/dL, their mothers/care givers were informed to take them to a health facility for follow up care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Getabalew Engidaye, Email: getabaleweng@gmail.com.
Mulugeta Melku, Email: mulugeta.melku@gmail.com.
Aregawi Yalew, Email: yalewaregawi@gmail.com.
Zegeye Getaneh, Email: zegeyegetaneh91@gmail.com.
Fikir Asrie, Email: fikirie2000@gmail.com.
Bamlaku Enawgaw, Email: bamlak21@gmail.com.
References
- 1.WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015. Available at: http://apps.who.int/iris/bitstream/10665/177094/1/9789241564960_eng.pdf. Accessed Nov 2016.
- 2.Brabin BJ, Premji Z, Verhoeff F. Iron-deficiency anemia : reexamining the nature and magnitude of the public health problem an analysis of anemia and child mortality. J Nutr. 2001;131(2):636S–648S. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee A, Bosch RJ, Kupka R, Hunter DJ, Msamanga GI, Fawzi WW. Predictors and consequences of anaemia among antiretroviral-naïve HIV-infected and HIV-uninfected children in Tanzania. Public Health Nutr. 2010;13(02):289–296. doi: 10.1017/S1368980009990802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanou D, Ngnie-Teta I. Risk factors for anemia in preschool children in sub-Saharan Africa: INTECH Open Access Publisher; 2012. Available at: https://www.researchgate.net/publication/272830110_Risk_factors_for_anemia_in_preschool_children_in_developing_countries.
- 5.Adish A, Esrey SA, Gyorkos TW, Johns T. Risk factors for iron deficiency anemia in preschool children in northern Ethiopia. Public Health Nutr. 1999;2(03):243–252. doi: 10.1017/S1368980099000336. [DOI] [PubMed] [Google Scholar]
- 6.Zein Z. Hematocrit levels and anemia in Ethiopian children. East Afr J Med. 1991;68(6):412–419. [PubMed] [Google Scholar]
- 7.Central Statistical Agency [Ethiopia] and ORC Macro. Ethiopia Demographic and Health Survey 2005. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ORC Macro; 2006.
- 8.Kale V, Aftab AR. Diagnostic Evaluation of Anaemia, Gastroenterology Department. Ireland: Institute-for-New-Technologies Open Access Publisher; 2012. [Google Scholar]
- 9.Pita GM, Jiménez S, Basabe B, García RG, Macías C, Selva L, et al. Anemia in children under five years old in eastern Cuba, 2005–2011. Med Cent Rev. 2011;16(1):16–23. doi: 10.37757/MR2014.V16.N1.5. [DOI] [PubMed] [Google Scholar]
- 10.Das P. Can we eliminate nutritional anemia in the near future? Kolkata, West Bengal, India. South East Asia J Public Health. 2015;5(1):1–3. doi: 10.3329/seajph.v5i1.24844. [DOI] [Google Scholar]
- 11.Ekwochi U, Osuorah D, Odetunde O, Egbonu I, Ezechukwu C. Prevalence of iron deficiency anaemia in anaemic under-5 children in Enugu south East Nigeria. Niger J Paediatr. 2014;41(2):129–132. doi: 10.4314/njp.v41i2.10. [DOI] [Google Scholar]
- 12.Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey 2011. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International; 2012.
- 13.Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016
- 14.Tadesse A. Household food insecurity and coping Mechansms in Menz -Gera District of Amhara regional state, Central Ethiopia; 2016.
- 15.Behaylu A, Bantider A, Tilahun A, Gashaw T. The role of rural land registration and certification program in ensuring tenure security in menz Gera midir district, Amhara state, Ethiopia. Int J Agric Ext Rural Dev Stud. 2008;2(2):44–52. [Google Scholar]
- 16.Magalhães RJS, Clements AC. Archie CA Clements: spatial variation in childhood anaemia in Africa. Bull World Health Organ. 2011;89(6):459–468. doi: 10.2471/BLT.10.083568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.USAID. Ethiopia: Nutrition Profile. 2018. Available at: https://www.usaid.gov/sites/default/files/documents/1864/Ethiopia-Nutrition-Profile-Mar2018-508.pdf.
- 18.Central Statistical Agency [Ethiopia]. Population projection of Ethiopia for all regions at wereda level from 2014–2017. Addis Ababa, Ethiopia, Central Statistical Agency, 2013. Available at: https://www.scribd.com/document/343869975/Population-Projection-At-Wereda-Level-from-2014-2017-pdf.
- 19.Aweke K, Habtamu F, Akalu G. Nutritional status of children in food insecure households in two districts of north showa zone, Ethiopia. Afr J Food Agric Nutr Dev. 2012;12(2):5915–5927. [Google Scholar]
- 20.Hall A, Kassa T, Demissie T, Degefie T, Lee S. National survey of the health and nutrition of schoolchildren in Ethiopia. Tropical Med Int Health. 2008;13(12):1518–1526. doi: 10.1111/j.1365-3156.2008.02168.x. [DOI] [PubMed] [Google Scholar]
- 21.Coates J, Swindale A, Bilinsky P. Household food insecurity access scale (HFIAS) for measurement of food access: indicator guide. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 22.Getaneh Z, Enawgaw B, Engidaye G, Seyoum M, Berhane M, Abebe Z, et al. Prevalence of anemia and associated factors among school children in Gondar town public primary schools, Northwest Ethiopia: a school-based cross-sectional study. PLoS One. 2017;12(12):e0190151. doi: 10.1371/journal.pone.0190151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amare B, Moges B, Moges F, Fantahun B, Admassu M, Mulu A, et al. Nutritional status and dietary intake of urban residents in Gondar, Northwest Ethiopia. BMC Public Health. 2012;12(1):1. doi: 10.1186/1471-2458-12-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belachew T, Lindstrom D, Gebremariam A, Hogan D, Lachat C, Huybregts L, et al. Food insecurity, food based coping strategies and suboptimal dietary practices of adolescents in Jimma zone Southwest Ethiopia. PLoS One. 2013;8(3):e57643. doi: 10.1371/journal.pone.0057643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebreyesus SH, Lunde T, Mariam DH, Woldehanna T, Lindtjørn B. Is the adapted household food insecurity access scale (HFIAS) developed internationally to measure food insecurity valid in urban and rural households of Ethiopia? BMC Nutr. 2015;1:2. doi: 10.1186/2055-0928-1-2. [DOI] [Google Scholar]
- 26.WHO, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a Joint Statement by the World Health Organization and the United Nations Children’s Fund. Geneva, World Health Organization, 2008. [PubMed]
- 27.WHO, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children, A Joint Statement by the World Health Organization and the United Nations Children’s Fund. Geneva,World Health Organization, 2009. [PubMed]
- 28.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011 (WHO/NMH/NHD/MNM/11.1). Available at: https://www.who.int/vmnis/indicators/haemoglobin.pdf.
- 29.Legason ID, Atiku A, Ssenyonga R, Olupot-Olupot P, Barugahare JB. Prevalence of Anaemia and associated risk factors among children in North-Western Uganda: a cross sectional study. BMC Hematol. 2017;17(1):10. doi: 10.1186/s12878-017-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebreselassie H. Iron supplementation and malaria infection: results from a randomized controlled field trial. Montreal: McGill University; 1999. [Google Scholar]
- 31.Ewusie JE, Ahiadeke C, Beyene J, Hamid JS. Prevalence of anemia among under-5 children in the Ghanaian population: estimates from the Ghana demographic and health survey. BMC Public Health. 2014;14(1):1. doi: 10.1186/1471-2458-14-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekwochi U, Osuorah D, Odetunde O, Egbonu I, Ezechukwu C. Prevalence of iron deficiencyanaemia in anaemic under-5 children in Enugu south East Nigeria. Niger J Paediatr. 2014;41(2):129–132. doi: 10.4314/njp.v41i2.10. [DOI] [Google Scholar]
- 33.Hoddinott J, Yohannes Y. Dietary diversity as a household food security indicator. Food and nutrition technical assistance project. Academy for educational development. 2002. [Google Scholar]
- 34.Sop MMK, Mananga M-J, Tetanye E, Gouado I. Risk factors of anemia among young children in rural Cameroon. Int J Curr Microbiol App Sci. 2015;4(3):925–935. [Google Scholar]
- 35.Habte D, Asrat K, Magafu MG, Ali IM, Benti T, Abtew W, et al. Maternal risk factors for childhood Anaemia in Ethiopia. Afr J Reprod Health. 2013;17(3):110–118. [PubMed] [Google Scholar]
- 36.Leal LP, Batista Filho M, PICd L, Figueiroa JN, Osório MM. Prevalence of anemia and associated factors in children aged 6-59 months in Pernambuco, Northeastern Brazil. Rev Saude Publica. 2011;45(3):457–466. doi: 10.1590/S0034-89102011000300003. [DOI] [PubMed] [Google Scholar]
- 37.Tazhibayev S, Dolmatova O, Yessimsiitova Z, Bazarbayeva Z, Muratbekova N, Beisbekova A, et al. Prevalence of anaemia in under five-years-old children in three counties of Kazakhstan. Eur J Pub Health. 2014;24(Supplementt 2):eku165–eku076. [Google Scholar]
- 38.de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005 WHO Global Database on Anaemia. Geneva, World Health organization, 2008. Available at: https://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf?sequence=1.
- 39.Malagi U, Reddy M, Naik RK. Evaluation of national nutritional anaemia control programme in Dharwad (Karmataka) J Hum Ecol. 2006;20(4):279–281. doi: 10.1080/09709274.2006.11905939. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.