Sir:
Classical Hodgkin lymphoma (CHL) is characterised by an inflammatory background and Hodgkin/Reed–Sternberg (HRS) cells. The HRS cells show abundant amphophilic cytoplasm, large nuclei, vesicular chromatin, and prominent inclusion-like nucleoli. HRS-like cells may be seen in disorders other than CHL. The HRS cells express CD30 in 90–96% of cases, and CD15 in 75–85% of cases. Pan-B-cell markers are expressed variably, the most consistent being PAX5.1 The variability in the morphology and immunophenotype poses diagnostic difficulty, and other disorders may be mistaken for CHL.
A review of the archives of the haematopathology consultation service at the National Cancer Institute from 2014 to 2017 identified four cases of lymphoid hyperplasia with atypical dendritic/Langerhans cell proliferations, which were initially interpreted as CHL by the referring institutions. Clinical data on all four cases were collected. Immunohistochemistry with antibodies against CD3, CD20, PAX5, CD30, CD15, CD4, MUM1, S100, CD1a, langerin, CD68, CD163 and CD45 was performed on formalin-fixed paraffin-embedded sections by the use of standard immunoperoxidase procedures with an automated immunostainer (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturer’s instructions. Epstein–Barr virus-encoded small RNA (EBER) in-situ hybridisation was performed with an EBER1 DNP probe supplied by Ventana on an automated stainer (Ventana-Benchmark XT, Tucson, AZ, USA), with previously published methods.2 Polymerase chain reaction (PCR) for T-cell receptor (TCR) γ gene and immunoglobulin gene rearrangement was performed with previously published methods.3,4 Mutation analysis for BRAF V600E (c.1799C>A) was performed with a Bio-Rad (Bio-Rad Inc, Hercules, CA, USA) QX200 Droplet Digital PCR System (ddPCR) and two allele-specific competitive probes, one for the wild-type sequence c.1799T and a second for the mutant sequence c.1799A.
Patient demographics, clinical details and pathological findings are summarised in Table 1. All four patients had leukocytosis and/or peripheral blood eosinophilia.
Table 1.
Patient demographics, clinical details and pathological findings
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years) | 69 | 40 | 58 | 63 |
| Gender | Male | Male | Male | Male |
| Biopsy site | Cervical LN | Left axillary LN | Left inguinal LN | Right axillary LN |
| Extent of adenopathy | Cervical, axillary, and inguinal | Isolated | Diffuse adenopathy | Axillary, inguinal, and external iliac |
| Clinical characteristics | Extreme fatigue and B symptoms | NA | History of RA on long-term steroids | Recurrent skin rash, treated with prednisone. History of MAI |
| Pertinent laboratory findings | Eosinophilia | Leukocytosis | Leukocytosis, neutrophilia, and eosinophilia | Eosinophilia, elevated IgE |
| Extranodal mass lesions | None | None | Lung nodules and skin lesions | Multiple small lung nodules |
| Initial diagnosis | CHL NS | CHL | CHL NS | CHL |
| Initial treatment | Two cycles of ABVD | ABVD | ABVD | ABVD |
| Response | Poor | None | Poor | None |
| IHC | ||||
| S100 | Positive | Positive | Positive | Positive/weak |
| CD1a | Negative | Negative | Negative | Negative |
| CD30 | Positive | Positive | Positive | Positive |
| CD15 | Negative | Negative | Negative | Negative |
| PAX5 | Negative | Negative | Negative | Negative |
| Langerin | Negative | Positive | Negative | Negative |
| CD4 | Positive/negative | Positive/negative | Positive | Positive |
| EBER | Negative | Negative | Negative | Negative |
| PCR | Poly B and T | Poly T | Poly B and T | Poly B and T |
| BRAF V600E | Negative | Negative | Negative | Negative |
| New treatment | Steroids | Clofarabine | Rituximab, prednisone, and etoposide | Steroids |
| Follow-up | Complete clinical response | Developed severe neuropathy with diplopia and paraplegia, and died in December 2017 | Response as of June 2016 | Good initial response, but lost to follow-up |
ABVD, Doxorubicin, Bleomycin, Vinblastine, Dacarbazine; CHL, Classical Hodgkin lymphoma; EBER, Epstein–Barr virus-encoded small RNA; IHC, Immunohistochemistry; LN, Lymph node; MAI, Mycobacterium avium-intracellulare; NA, Not available; NS, Nodular sclerosis; RA, Rheumatoid arthritis; PCR, Polymerase chain reaction.
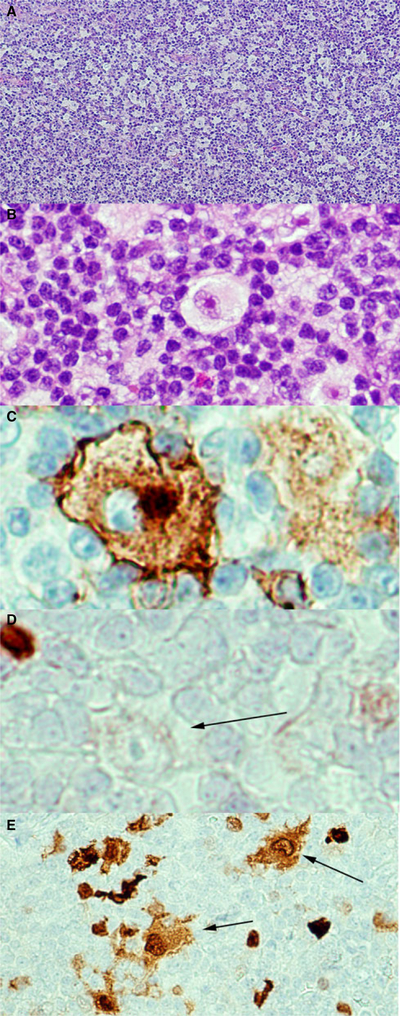
The lymph nodes showed diffuse paracortical expansion with small lymphocytes, eosinophils, histiocytes, immunoblasts, and plasma cells, and admixed scattered large cells with abundant pale eosinophilic cytoplasm and focal emperipolesis. The atypical cells had irregular, sometimes lobulated, nuclei, with finely dispersed chromatin, and variably prominent nucleoli (Figure 1). They were positive for S100, and showed variable positivity for CD30 and CD4. They were negative for CD15, CD23, PAX5, and EBER. Both the morphology and S100 positivity suggested a Langerhans cell or dendritic origin. As the atypical cells were widely scattered, the changes did not support a diagnosis of a histiocytic or dendritic neoplasm. Moreover, studies on BRAF V600E mutation gave negative results.
Figure 1.
Histological and immunohistochemical features of the atypical Langerhans/dendritic cell proliferations. A, Diffuse paracortical expansion with a mixed inflammatory cell infiltrate. Haematoxylin and eosin (H&E). B, High-power view demonstrating the large atypical cells with abundant pale cytoplasm, irregularly contoured nuclei, vesicular chromatin, and prominent nucleoli. H&E. C, CD30 shows a membranous and Golgi pattern of staining in the atypical cell. Immunohistochemistry (IHC). D, PAX5 is negative in the atypical cell (arrow). IHC. E, S100 is positive in the Langerhans cells, dendritic cells, and large atypical cells (arrows). IHC.
Unusual features were the focally strong expression of CD30, and prominent nucleoli in the atypical S100-positive population, findings that had led to an initial diagnosis of CHL in all cases. Reactive macrophages have been shown to express CD30 in certain disease conditions.5 Cells in interdigitating reticulum cell sarcoma have also been shown to express CD30.6 However, we could not find reports of benign dendritic cells expressing CD30. The atypical paracortical proliferation also raised consideration for peripheral T-cell lymphoma, but molecular studies with PCR failed to show evidence of clonal TCR γ or immunoglobulin rearrangements. All patients who received long-term steroid treatment responded clinically. These findings suggest a reactive origin for this lesion with an inflammatory/immune origin. Even though further detailed studies and follow-up will be required to determine the exact aetiology and pathogenesis of these lesions, a haematolymphoid neoplasm is highly unlikely. Awareness of the alterations in cellular morphology and immunophenotype in reactive conditions will aid in correct diagnosis, and will prevent patients from being subjected to unnecessary aggressive treatment modalities.
Acknowledgements
This study was supported by funding from the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Protocol no. 10CN074.
Footnotes
Conflicts of interest
The authors state that they have no conflicts of interest.
References
- 1.Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Martinez L. Hematopathology. 2nd ed Philadelphia, PA: Elsevier, 2017. [Google Scholar]
- 2.Dojcinov SD, Venkataraman G, Pittaluga S et al. Age-related EBV-associated lymphoproliferative disorders in the western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 2011; 117; 4726–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawnicki LC, Rubocki RJ, Chan WC, Lytle DM, Greiner TC. The distribution of gene segments in T-cell receptor gamma gene rearrangements demonstrates the need for multiple primer sets.J. Mol. Diagn 2003; 5; 82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J. Clin. Pathol 1992; 45; 770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreesen R, Brugger W, Lohr GW, Bross KJ. Human macrophages can express the Hodgkin’s cell-associated antigen Ki-1 (CD30). Am. J. Pathol 1989; 134; 187–192. [PMC free article] [PubMed] [Google Scholar]
- 6.Papadimitriou CS, Bai MC, Stephanou DG, Pavlidis NA, Athanasiadou SE. Interdigitating reticulum cell sarcoma positive immunohistochemistry for HLA-DR and two other activation antigens, CD30(Ki-1) and CD25(IL2R). Leuk. Lymphoma 1991; 4; 411–417. [DOI] [PubMed] [Google Scholar]