Abstract
Background
The inhibitors of nuclear factor kappa-B kinase subunit epsilon (IKBKE) and TANK-binding kinase 1 (TBK1) are important members of the nonclassical IKK family that share the kinase domain. They are important oncogenes for activation of several signaling pathways in several tumors. This study aims to explore the expression of IKBKE and TBK1 and their prognostic role in stage I non-small cell lung cancer (NSCLC).
Patients and methods
A total of 142 surgically resected stage I NSCLC patients were enrolled and immunohistochemistry of IKBKE and TBK1 was performed.
Results
IKBKE and TBK1 were expressed in 121 (85.2%) and 114 (80.3%) of stage I NSCLC patients respectively. IKBKE expression was significantly associated with TBK1 expression (P=0.004). Furthermore, multivariate regression analyses showed there was a significant relationship between patients with risk factors, the recurrence pattern of metastasis and IKBKE+/TBK1+ co-expression (P=0.032 and P=0.022, respectively). In Kaplan–Meier survival curve analyses, the IKBKE+/TBK1+ co-expression subgroup was significantly associated with poor overall survival (P=0.014).
Conclusions
This is the first study to investigate the relationship between IKBKE and TBK1 expression and clinicopathologic characteristics in stage I NSCLC patients. IKBKE+/TBK1+ co-expression was significantly obvious in patients with risk factors and with recurrence pattern of distant metastasis. Furthermore, IKBKE+/TBK1+ is also an effective prognostic predictor for poor overall survival.
Keywords: IKBKE, TBK1, NSCLC, prognosis, cancer
Introduction
Lung cancer is the leading cause of cancer-related death worldwide. Non-small cell lung cancer(NSCLC) accounts for 80–85% of all lung cancer cases.1,2 The primary treatment for stage I NSCLC is surgical resection, but at least 30% of patients experienced recurrence within 5 years.3 Adjuvant chemotherapy is recommended for patients with risk factors, such as poor histologic differentiation, vascular invasion, wedge resection, tumors >4 cm, incomplete lymph node sampling, and visceral pleural invasion.4 Even after receiving adjuvant chemotherapy, the 5-year survival rate is only 60–90% due to local recurrence or distant metastasis.5,6 Therefore, we defined high-risk patients as tending to local recurrence or distant metastasis. It is challenging to screen true high-risk patients for adjuvant chemotherapy, and to avoid unnecessary adjuvant chemotherapy in low-risk patients to reduce the survival reduction caused by treatment-related toxicity.
The IKK family contains five protein factors. IKKα, IKKβ, and IKKγ belong to canonical group, and the other two (IKBKE and TBK1) belong to non-canonical group.7 The amino acid sequence analysis confirmed that IKBKE has 67% homology with TBK1.8 IKBKE is highly expressed in human normal pancreatic tissue, thyroid tissue, spleen, and peripheral blood leukocytes, and can be up-regulated by multiple cytokines such as tumor necrosis factor alpha (TNF-α), IL-1, IL-6, and interferon-γ.9 TBK1 is constitutively expressed in immune cells, brain, lungs, gastrointestinal tract, and reproductive organs.10 Studies have demonstrated that IKBEK and TBK1 need to bind to scaffold proteins to form a protease complex to activate downstream protein factors, thereby activating the NF-κB pathway. TNAK (TNF receptor-associated factor family member-associated NF-κB activator), NAP1 (NAK associated protein 1), and SINTBAD (similar to NAP and TBK1 adaptor) are the main scaffold proteins.11–17 Several studies have shown that IKBKE was overexpressed in breast cancer, glioma, gastric cancer, pancreatic cancer, ovarian cancer, renal cancer, and lung cancer and was associated with poor prognosis.18–24 IKBKE and TBK1 may play important roles in tumorigenesis, proliferation, and angiogenesis.25,26 Nevertheless, the association between IKBKE and TBK1 expression and clinicopathological characteristics and its role in the prognosis of stage I NSCLC has not been reported.
Patients and methods
Patient selection
A totlal of 142 tissue samples were retrospectively collected from patients who were pathologically diagnosed with stage I NSCLC between January 2004 and June 2012 in Shandong Cancer hospital. All of them received anatomical segmentectomy or standard lobectomy via open thoracotomy and did not receive other treatments except for surgery. This retrospective study was approved by the ethics committee at Shandong Cancer Hospital and Institute. All patients have provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry
Tumor tissue samples were obtained from 142 surgically resected stage I NSCLC patients. Surgically resected specimens were fixed in formalin and embedded in paraffin. Then, 4-μm paraffin-embedded sections were dewaxed and heated to 95°C for 20 mins for antigen retrieval in 10 mmol/L citrate buffer. Then, the sections were cleared of endogenous peroxidase activity and were blocked with 5% BSA for 30 mins in room temperature (RT). Then, all sections were incubated at 4°C overnight with primary anti-IKBKE (ab7891, Abcam, IHC 1:100, Cambridge, MA) or anti-TBK1 (ab109735, Abcam, IHC 1:400, Cambridge, MA). Then, the specimens were incubated with biotinylated secondary antibodies for 1 hr in RT. After incubation with ABC-peroxidase for 1 hr then all sections were colored using a DAB Kit (ZSGB-BIO, People’s Republic of China) and counterstained with hematoxylin. All IHC results were evaluated by two pathologists. Double-blind readings were performed by two pathologists at the unknown tumor grade, and 10 fields were randomly collected from each pathological section, and the percentage of positive cells and the intensity of staining were scored: 1) coloring cells accounted for <5% cell count is 0; 5–25% is 1 point; 26–50% is 2 points; 51–75% is 3 points; and >75% is 4 points; 2) no staining is 0 points; the light yellow is 1 point; the brown and yellow is 2 points; the sepia is 3 points; 3) the two points are multiplied to form a positive grade: 0 is negative (-), 1–4 is weakly positive (+), 5–8 is positive (++), and 9–12 is strongly positive (+++).
Statistical analysis
The categorical variables were tested using Pearson’s chi-squared tests or Fisher’s exact test, and continuous variables were expressed as means±SD and were analyzed using independent t-test. The significant (P<0.05) clinicopathological characteristics in univariate analysis were enrolled in multivariate logistic regression analysis, and multivariate logistic regression analysis was used to confirm the independent clinicopathological characteristics which were associated with IKBKE and TBK1 expression. The Kaplan–Meier was used to analyze progression-free survival (PFS) and OS, and the survival curves of four subgroups were compared by log-rank test. All analysis was two-sided, and P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 20.0).
Results
Expression of IKBKE and TBK1 and their association in stage I NSCLC patients
Among the 142 enrolled stage I NSCLC patients, 48 (33.8%) patients were squamous cell carcinoma, and 65 patients (45.8%) were adenocarcinoma. Twenty-nine patients were other types of NSCLC including 8 large cell carcinoma, 5 atypical carcinoid tumor, 5 pleomorphic carcinoma, 9 adenosquamous carcinoma, and 2 mucinous epidermoid carcinoma. IKBKE and TBK1 expression was found in 85.2% (n=121) and 80.3% (n=114) of stage I NSCLC patients, respectively. The association between IKBKE and TBK1 expression and clinicopathological characteristics is summarized in Table 1. IKBKE and TBK1 expression was associated with risk factors (P=0.021 and P=0.014). From Table 1 we found patients with risk factors showed increased IKBKE and TBK1 expression compared to patients who had no risk factors. IKBKE expression was observed in 91.3% and 77.4% of patients who had risk factors compared to patients who had no risk factors. Besides, TBK1 expression was observed in 87.5% and 71.0% in these two subgroups. In addition, TBK1 expression was significantly associated with recurrence pattern of metastasis compared to local recurrence (P<0.001) and TBK1 expression was found in 89.6% of patients with the recurrence pattern of metastasis compared to 64.0% of patients with the recurrence pattern of local recurrence. We further explored the correlation between IKBKE expression and TBK1 expression and found there was a significant correlation between IKBKE and TBK1 expression (P=0.004), as 84.3% (102/121) of tumors with IKBKE positive expression simultaneously showed TBK1 positive expression (Table 2).
Table 1.
The association of IKBKE and TBK1 expression and clinicopathological characteristics
| Variable | IKBKE expression | TBK1 expression | |||||
|---|---|---|---|---|---|---|---|
| Total cases | Positive | Negative | P | Positive | Negative | P | |
| N=142(%) | N=121(85.2%) | N=21(14.8%) | N=114(80.3%) | N=28(19.7%) | |||
| Gender | 0.789 | 0.642 | |||||
| Male | 91 (64.1%) | 77 (84.6%) | 14 (15.4%) | 72(79.1%) | 19(20.9%) | ||
| Female | 51 (35.9%) | 44 (86.3%) | 7 (13.7%) | 42(82.4%) | 9(17.6%) | ||
| Age | 0.064 | 0.609 | |||||
| ≤60 | 67 (47.2%) | 61 (91.0%) | 6 (9.0%) | 55(82.1%) | 12(17.9%) | ||
| >60 | 75 (52.8%) | 60 (80.0%) | 15 (20.0%) | 59(78.7%) | 16(21.3%) | ||
| Differentiation | 0.469 | 0.940 | |||||
| Poor | 37 (26.1%) | 31 (83.8%) | 6 (16.2%) | 29(78.4%) | 8(21.6%) | ||
| Moderate | 62 (43.7%) | 51 (82.3%) | 11 (17.7%) | 50(80.6%) | 12(19.4%) | ||
| Well | 43 (30.2%) | 39 (90.7%) | 4 (9.3%) | 35(81.4%) | 8(18.6%) | ||
| Histologic type | 0.577 | 0.723 | |||||
| Squamous carcinoma | 48 (33.8%) | 43 (89.6%) | 5 (10.4%) | 37(77.1%) | 11(22.9%) | ||
| Adenocarcinoma | 65 (45.8%) | 54 (83.1%) | 11 (16.9%) | 54(83.1%) | 11(16.9%) | ||
| Large cell carcinoma | 8 (5.6%) | 7 (87.5%) | 1 (12.5%) | 6(75.0%) | 2(25.0%) | ||
| Atypical carcinoid tumor | 5 (3.5%) | 4 (80.0%) | 1 (20.0%) | 4(80.0%) | 1(20.0%) | ||
| Pleomorphic carcinoma | 5 (3.5%) | 5 (100.0%) | 0 (0%) | 4(80.0%) | 1(20.0%) | ||
| Adenosquamous carcinoma | 9 (6.4%) | 6 (66.7%) | 3 (33.3%) | 8(88.9%) | 1(11.1%) | ||
| Mucinous epidermoid carcinoma | 2 (1.4%) | 2 (100.0%) | 0 (0%) | 1(50.0%) | 1(50.0%) | ||
| Risk factors | 0.021 | 0.014 | |||||
| Yes | 80 (56.3%) | 73 (91.3%) | 7 (8.7%) | 70(87.5%) | 10(12.5%) | ||
| No | 62 (43.7%) | 48 (77.4%) | 14 (22.6%) | 44(71.0%) | 18(29.0%) | ||
| Recurrence pattern | 0.319 | <0.001 | |||||
| Local | 50 (38.7%) | 41 (82.0%) | 9 (18.0%) | 32(64.0%) | 18(36.0%) | ||
| Metastasis | 77 (54.2%) | 68 (88.3%) | 9 (11.7%) | 69(89.6%) | 8(10.4%) | ||
Table 2.
The association between IKBKE and TBK1 expression
| Variable | IKBKE expression | ||
|---|---|---|---|
| Positive (n=121) | Negative (n=21) | P | |
| TBK1 expression | 0.004 | ||
| Positive (n=114) | 102 (84.3%) | 12 (57.1%) | |
| Negative (n=28) | 19 (15.7%) | 9 (42.9%) | |
IKBKE and TBK1 co-expression in stage I NSCLC patients
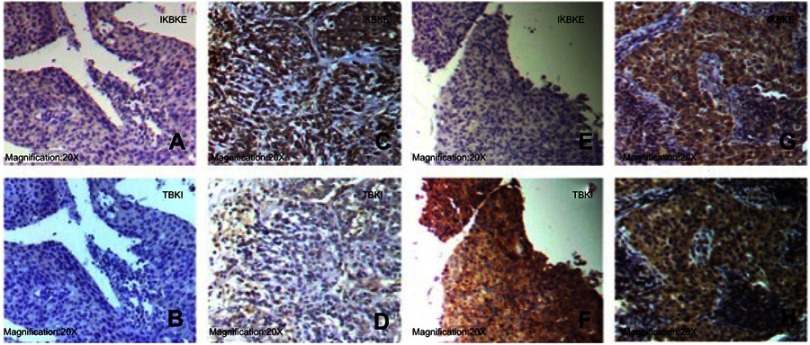
We divided all patients into four subgroups according to IKBKE and TBK1 expression as follows: IKBKE-/TBK1- (n=9, 6.3%); IKBKE+/TBK1- (n=19, 13.4%); IKBKE-/TBK1+ (n=12, 8.5%); IKBKE+/TBK1+ (n=102, 71.8%). The representative examples of four subgroups are shown in Figure 1. We then explored the association of IKBKE and TBK1 co-expression and clinicopathological characteristics (Table 3). Patients with risk factors and patients with the recurrence pattern of metastasis had more frequency in the IKBKE+/TBK1+ subgroup than in other three subgroups (P=0.042 and P=0.004, respectively). In addition, we did not find significant association between other clinicopathological characteristics and the expression of IKBKE and TBK1. In multivariate regression analysis of clinicopathological characteristics for IKBKE and TBK1 co-expression, we found patients with risk factors and the recurrence pattern of metastasis were significantly related to IKBKE+/TBK1+ co-expression (P=0.032 and P=0.022, respectively). The adjusted OR of patients with risk factors was 1.633, with a 95% CI 1.043–2.557 when compared with patients with no risk factors. The adjusted OR of patients with recurrence pattern of metastasis was 1.670, with a 95% CI 1.078–2.587 when compared with patients with recurrence pattern of local recurrence (Table 4).
Figure 1.
Representative examples of the four subgroups. A and B, E and F, G and H were moderately differentiated squamous cell carcinoma. C and D were large cell lung cancer. IKBKE and TBK were cytoplasmic stain. A and B: IKBKE+/TBK+. C and D: IKBKE+/TBK-. E and F: IKBKE-/TBK+. G and H: IKBKE-/TBK-.
Table 3.
The association of IKBKE and TBK1 co-expression and clinicopathological characteristics
| Variable | IKBKE-/TBK1- IKBKE+/TBK- IKBKE-/TBK+ | IKBKE+/TBK1+ | P | ||
|---|---|---|---|---|---|
| N=9 (6.3%) | N=19 (13.4%) | N=12 (8.5%) | N=102 (71.8%) | ||
| Gender | 0.961 | ||||
| Male | 6 (6.6%) | 13 (14.3%) | 8 (8.8%) | 64 (70.3%) | |
| Female | 3 (5.9%) | 6 (11.8%) | 4 (7.8%) | 38 (74.5%) | |
| Age | 0.114 | ||||
| ≤60 | 1 (1.5%) | 11 (16.4%) | 5 (7.5%) | 50 (74.6%) | |
| >60 | 8 (10.7%) | 8 (10.7%) | 7 (9.3%) | 52 (69.3%) | |
| Differentiation | 0.937 | ||||
| Poor | 3 (8.1%) | 5 (13.5%) | 3 (8.1%) | 26 (70.3%) | |
| Moderate | 4 (6.4%) | 8 (12.9%) | 7 (11.3%) | 43 (69.4%) | |
| Well | 2 (4.7%) | 6 (14.0%) | 2 (4.6%) | 33 (76.7%) | |
| Histologic type | 0.548 | ||||
| Squamous carcinoma | 4 (8.3%) | 7 (14.6%) | 1 (2.1%) | 36 (75.0%) | |
| Adenocarcinoma | 3 (4.6%) | 8 (12.3%) | 8 (12.3%) | 46 (70.8%) | |
| Large cell carcinoma | 1 (12.5%) | 1 (12.5%) | 0 (0%) | 6 (75.0%) | |
| Atypical carcinoid tumor | 0 (0%) | 1 (20.0%) | 1 (20.0%) | 3 (60.0%) | |
| Pleomorphic carcinoma | 0 (0%) | 1 (20.0%) | 0 (0%) | 4 (80.0%) | |
| Adenosquamous carcinoma | 1 (11.1%) | 0 (0%) | 2 (22.2%) | 6 (66.7%) | |
| Mucinous epidermoid carcinoma | 0 (0%) | 1 (50.0%) | 0 (0%) | 1 (50.0%) | |
| Risk factors | 0.042 | ||||
| Yes | 3 (3.7%) | 7 (8.8%) | 5 (6.2%) | 65 (81.3%) | |
| No | 6 (9.7%) | 12 (19.3%) | 7 (11.3%) | 37 (59.7%) | |
| Recurrence pattern | 0.004 | ||||
| Local | 6 (12.0%) | 12 (24.0%) | 3 (6.0%) | 29 (58.0%) | |
| Metastasis | 1 (1.3%) | 7 (9.1%) | 8 (10.4%) | 61 (79.2%) | |
Table 4.
Multivariate regression analysis of clinicopathological characteristics for IKBKE and TBK1 co-expression
| Variables | P | OR | 95% CI |
|---|---|---|---|
| Risk factors | 0.032 | 1.633 | 1.043–2.557 |
| Recurrence pattern | 0.022 | 1.670 | 1.078–2.587 |
The prognostic significance of IKBKE and TBK1 co-expression in stage I NSCLC patients
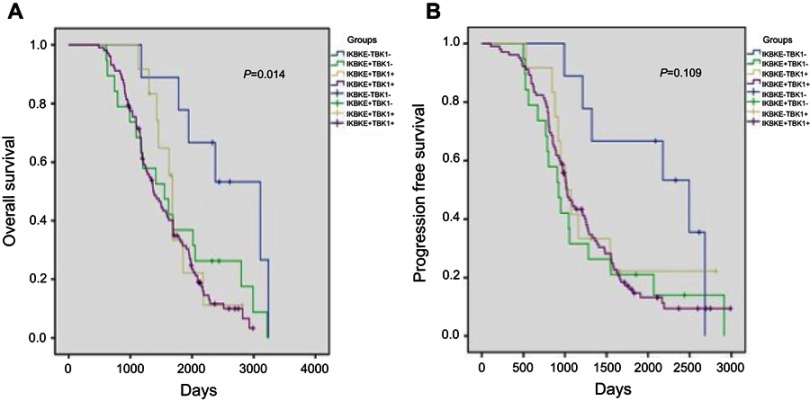
The median follow-up period for OS and PFS was 1,442 days (range 488–3,239 days) and 1,021 days (range 107−2,989 days), respectively. During the follow-up period, the recurrence rate was 89.4% (n=127). Survival was analyzed using the Kaplan–Meier method with stratification of IKBKE and TBK1 expression. The results showed that IKBKE+/TBK1+ co-expression subgroup was significantly associated with poor OS (median 1,351 days; 95 CI% 1,178–1,565; P=0.014) than those in IKBKE-/TBK1- subgroup (median 2,378 days; 95 CI% 1,924–4,291), IKBKE+/TBK1- subgroup (median 1,554 days; 95 CI% 959–2,148), and IKBKE-/TBK1+ subgroup (median 1,643 days; 95 CI% 1,529–1,836). However, the IKBKE+/TBK1+ co-expression subgroup did not show significant association with poor PFS than the other three subgroups (P=0.109), although the Kaplan–Meier survival curve showed this tendency (Figure 2).
Figure 2.
The Kaplan–Meier overall survival (A) and progression free survival (B) curves for stage I NSCLC according to IKBKE and TBK1 co-expression stratification.
Abbreviations: OS, overall survival; PFS, progression free survival.
Discussion
Our study tried to investigate IKBKE and TBK1 expression in stage I NSCLC and the association between their expression and clinicopathological characteristics. We found that IKBKE and TBK1 expressed in 85.2% and 80.3% of stage I NSCLC, respectively, and IKBKE and TBK1 co-expressed in 71.8% of all cases. Identifying high-risk patients who need postoperative adjuvant chemotherapy to improve OS and reduce the toxicity of unnecessary chemotherapy is a key element in the whole treatment management of early-stage NSCLC. Our study demonstrated that IKBKE and TBK1 might play an important role in tumorigenesis and proliferation in stage I NSCLC. IKBKE and TBK1 may be good molecular targets for combination therapy.
Targeted therapy is the treatment of precise targeting molecular “targets” by small molecule drugs that specifically bind to these targets. Protein kinases are important signal messengers to modulate cell life activities and play an important role in cell proliferation, survival, apoptosis, metabolism, transcription, and differentiation. IKBKE and TBK1 are serine/threonine protein kinases which is part of IKK family.9 Ma et al demonstrated the activity of TBK1 is strongly promoted by phosphorylation at Ser 172.27 In breast cancer, as an oncogene, IKBKE is regulated by K63-linked polyubiquitination at lysine 30 and lysine 401 and controlled by the cIAP1/cIAP2/TRAF2 E3 ligase complex ubiquitinates itself.28 Although IKBKE and TBK1 were characterized as activators of NF-κB pathway, several studies indicated non-canonical IKKs (IKBKE and TBK1) were sufficient, but not essential for NF-κB activation.18,29 NF-κB pathway plays a pivotal role in the development of various tumors. In addition to NF-κB pathway, IKBKE and TBK1 also activated AKT by direct phosphorylation of AKT to promote cell proliferation and survival.30 In addition, Saxton et al31 found mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) controls metabolic processes and phosphorylates key substrates to promote oncoprotein-induced cell proliferation and survival. Yu et al32 showed TBK1 can regulate AKT-mTORC1 signaling axis. Bodur et al33 recently demonstrated TBK1 can directly phosphorylate mTORC1 at Ser 2159 to promote its tumorigenic activity. Recent studies have confirmed that IKBKE can directly phosphorylate signal transducers and activators of transcription 3 (STAT3), knocking down IKBKE can significantly reduce the content of STAT3 and phosphorylated STAT3, and affect the proliferation and progression of lymphoma through STAT3 signaling pathway.34 Furthermore, several studies have explored the tumorigenic function of IKBKE and TBK1 in various cancers. Qin et al35 indicated silencing IKBKE using synthetic siRNA can reduce focus formation potential and clonogenicity in human breast cancer cells. Li et al36 verified that IKBKE overexpression in glioma is positively correlated to the grade of glioma. Silencing IKBKE can significantly inhibit tumor cell proliferation and invasion,and lead to cell cycle arrest. Lu et al demonstrated IKBKE plays an important role in regulating cell proliferation, invasion and epithelial-mesenchymal transitionof glioblastoma cells in vitro and in vivo.37 Besides, Niederberger et al38 found IKBKE/TBK1-sensitive acute myeloid leukemia (AML) cells tend to have MYC oncogenic signatures and inhibition of IKBKE/TBK1 can downregulate the MYC oncogenic pathway in AML cell lines. Accumulating evidence indicates the oncogenic potential of IKBKE and TBK1 is an important basis for them as targets in targeted therapy. Recent studies have shown that CYT387, a small molecule inhibitor that is traditionally thought to inhibit JAK/STAT signal transduction pathway, could inhibit IKBKE kinase activity and become a newly discovered small inhibitor of IKBKE. Experiments demonstrated CYT387 could significantly inhibit the proliferation of NSCLC lines in vitro, block the development and progression of NSCLC induced by IKBKE and TBK1 mediated KRAS-driven tumorigenesis.39 Considering the high positive expression rate of IKBKE and TBK1 in stage I NSCLC, targeted therapy targeting IKBKE and TBK1 might be a potential strategy to suppress cancer development.
In our study, we explored the correlation between the following clinicopathological characteristics, including gender, age, differentiation, histologic type, risk factors, and recurrence pattern and IKBKE/TBK1 expression status. We found IKBKE+/TBK1+ expressed in 71.8% of all cases, suggesting that IKBKE and TBK1 may play a pivotal role in NSCLC initiation. The multivariate regression analysis was applied to select prediction characteristics for IKBKE+/TBK+ expression in stage I NSCLC and the multivariate analysis demonstrated patients with risk factors and the recurrence pattern of metastasis were significantly related to IKBKE +/TBK1+ co-expression (P=0.032 and P=0.022, respectively).
In addition, we did not find significant association between other clinicopathological characteristics and the expression of IKBKE and TBK1. The stage I NSCLC patients with risk factors were optimal candidates to receive adjuvant chemotherapy, whereas the locoregional or systemic recurrence was still high in these patients.5 Even in stage I NSCLC patients with no risk factors, the locoregional or systemic recurrence also could not be ignored. Therefore, how to explore effective treatments to prolong the OS time in high-risk patients remains challenging. Our research provides a new perspective for screening high-risk patients. We found stage I NSCLC patients with risk factors were significantly related to IKBKE+/TBK1+ co-expression (P=0.032). Furthermore, we found the recurrence pattern of metastasis was also significantly related to IKBKE+/TBK1+ co-expression (P=0.022). Recently, studies have shown IKBKE and TBK1 could phosphorylate AKT to activate AKT pathway,30,40 and AKT1 regulates pathological angiogenesis, vascular maturation, and permeability.41 All these may explain why the patients with IKBKE+/TBK1+ co-expression were prone to recurrence with metastasis in our study and the IKBKE+/TBK1+ subgroup has shorter OS than other subgroups (P=0.014), whereas we did not find statistically significant PFS differences between the four subgroups (P=0.109). Li et al showed IKBKE and TBK1 dual inhibitors could suppress AKT activation and reduce VEGF expression, leading to impaired angiogenesis and inhibition of tumor growth.42 Furthermore, several studies have shown that the correlation between high expression of IKBKE and chemotherapy resistance. Guo et al22 verified that IKBKE is upregulated on mRNA and protein levels in 63 ovarian cancer tissues of 96 ovarian cancer patients, and elevated IKBKE obviously enhances the ability of ovarian cancer cells to resist cisplatin. Besides, Zhang et al43 demonstrated that PLK4 decreases temozolomidesensitivity by regulating the IKBKE/NF-κB axis which indicates IKBKE could influence glioblastoma proliferation and chemosensitivity. In addition to ovarian cancer and glioblastoma, Guo et al24 reported that the expression of IKBKE was increased in 98 cases of NSCLC, and the sensitivity of NSCLC cell lines to chemotherapy drugs was increased after silencing IKBKE, suggesting that IKBKE plays a key role in lung cancer and tumor resistance. Zhang et al44 also demonstrated that TBK1 contributes to tumor invasion and might be a driving factor for metastatic spread of breast cancer. Thus, IKBKE/TBK1 dual inhibitors combined with adjuvant chemotherapy may be a potential strategy for high-risk stage I NSCLC patients.
Apparently, our study has several limitations that should be considered. The retrospective character of this study exists inevitable bias. Considering the limited amount of tissue, we cannot test as many as possible relevant proteins. Moreover, when the patient relapsed, we were unable to obtain the specimen, but only analyzed the specimen that was surgically removed at the initial diagnosis, which limited our understanding of the changes in tumor molecular characteristics.
In conclusion, the current study provides further insights for tumor targeted therapy and the landscape of kinase proteins IKBKE and TBK1 in various cancers. To our knowledge, our study is the first study to investigate the relationships between expression of IKBKE and TBK1 and clinicopathologic characteristics in stage I NSCLC patients and their predictive significance on patients’ survival. We found IKBKE+/TBK1+ co-expression was significantly obvious in patients with risk factors and the recurrence pattern of metastasis, and is an effective prognostic predictor for poor OS. Of note, further investigation is required to identify optimal patients who likely obtain benefit from IKBKE and TBK1 dual inhibitors therapy.
Acknowledgment
This work was financially supported by a research grant from the Construction and Verification of Precise Medical Diagnosis and Treatment System for Non-small Cell Lung Cancer (2016CYJS01A03).
Ethical statement
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.D’Addario G, Felip E; Group EGW. Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:68–70. doi: 10.1093/annonc/mdp132 [DOI] [PubMed] [Google Scholar]
- 3.Santarpia M, Altavilla G, Pitini V, Rosell R. Personalized treatment of early-stage non-small-cell lung cancer: the challenging role of EGFR inhibitors. Future Oncol. 2015;11:1259–1274. doi: 10.2217/fon.14.320 [DOI] [PubMed] [Google Scholar]
- 4.Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Risk factors for tumor recurrence in patients with early-stage (stage I and II) non-small cell lung cancer: patient selection criteria for adjuvant chemotherapy according to the seventh edition TNM classification. Chest. 2011;140:1494–1502. doi: 10.1378/chest.10-3279 [DOI] [PubMed] [Google Scholar]
- 5.Custodio AB, Gonzalez-Larriba JL, Bobokova J, et al. Prognostic and predictive markers of benefit from adjuvant chemotherapy in early-stage non-small cell lung cancer. J Thorac Oncol. 2009;4:891–910. doi: 10.1097/JTO.0b013e3181a4b8fb [DOI] [PubMed] [Google Scholar]
- 6.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623 [DOI] [PubMed] [Google Scholar]
- 7.Verhelst K, Verstrepen L, Carpentier I, et al. IkappaB kinase epsilon (IKKepsilon): a therapeutic target in inflammation and cancer. Biochem Pharmacol. 2013;85:873–880. doi: 10.1016/j.bcp.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien Y, Kim S, Bumeister R, et al. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127(1):0–170. doi: 10.1016/j.cell.2006.08.034 [DOI] [PubMed] [Google Scholar]
- 9.Shimada T, Kawai T, Takeda K, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357 [DOI] [PubMed] [Google Scholar]
- 10.Larabi A, Dev JM, Ng SL, et al. Crystal structure and mechanism of activation of TANK-binding kinase 1. Cell Rep. 2013;3:734–746. doi: 10.1016/j.celrep.2013.01.034 [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Chau TL, Gioia R, Gatot J-S, et al. Are the IKKs and IKK-related kinases TBK1 and IKK-? similarly activated? Trends Biochem Sci. 2008;33(4):171–180. doi: 10.1016/j.tibs.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 13.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. Embo J. 1999;18(23):6694–6704. doi: 10.1093/emboj/18.23.6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura F, Kawai T, Nakanishi K, Akira S. NF-kappaB activation through IKK-i-dependent I-TRAF/TANK phosphorylation. Genes Cells. 2000;5(3):191–202. [DOI] [PubMed] [Google Scholar]
- 15.Gatot JS, Gioia R, Chau TL, et al. Lipopolysaccharide-mediated interferon regulatory factor activation involves TBK1-IKKϵ-dependent Lys63-linked polyubiquitination and phosphorylation of TANK/I-TRAF. J Biol Chem. 2007;282(43):31131. doi: 10.1074/jbc.M701690200 [DOI] [PubMed] [Google Scholar]
- 16.Fujita F, Taniguchi Y, Kato T, et al. Identification of NAP1, a regulatory subunit of IκB kinase-related kinases that potentiates NF-κB signaling. Mol Cell Biol. 2003;23(21):7780–7793. doi: 10.1128/mcb.23.21.7780-7793.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. Embo J. 2007;26(13):3180–3190. doi: 10.1038/sj.emboj.7601743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eddy SF, Guo S, Demicco EG, et al. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65(24):11375. doi: 10.1158/0008-5472.CAN-04-4557 [DOI] [PubMed] [Google Scholar]
- 19.Guan H, Zhang H, Cai J, et al. IKBKE is over-expressed in glioma and contributes to resistance of glioma cells to apoptosis via activating NF-κB. J Pathol. 2011;223(3):436–445. doi: 10.1002/path.2815 [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Hong M, Cho J, et al. IKKε and TBK1 expression in gastric cancer. Oncotarget. 2016;8(10):16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng A, Guo J, Henderson-Jackson E, Kim D, Malafa M, Coppola D. Iκb kinase ε expression in pancreatic ductal adenocarcinoma. Am J Clin Pathol. 2011;136(1):60–66. doi: 10.1309/AJCP2JJGYNIUAS2V [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo JP, Shu SK, He L, et al. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am J Pathol. 2009;175(1):324–333. doi: 10.2353/ajpath.2009.080767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghatalia P, Yang ES, Lasseigne BN, et al. Kinase gene expression profiling of metastatic clear cell renal cell carcinoma tissue identifies potential new therapeutic targets. PLoS One. 2016;11(8):e0160924. doi: 10.1371/journal.pone.0160924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Kim D, Gao J, et al. IKBKE is induced by STAT3 and tobacco carcinogen and determines chemosensitivity in non-small cell lung cancer. Oncogene. 2013;32(2):151–159. doi: 10.1038/onc.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Seo SI, Song SY, Kang MR, et al. Immunohistochemical analysis of NF-kappaB signaling proteins IKKepsilon, p50/p105, p52/p100 and RelA in prostate cancers. Apmis. 2009;117:623–628. doi: 10.1111/j.1600-0463.2009.02506.x [DOI] [PubMed] [Google Scholar]
- 26.Kim JY, Welsh EA, Oguz U, et al. Dissection of TBK1 signaling via phosphoproteomics in lung cancer cells. Proc Natl Acad Sci USA. 2013;110:12414–12419. doi: 10.1073/pnas.1220674110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Helgason E, Phung QT, et al. Molecular basis of tank-binding kinase 1 activation by transautophosphorylation. Proc Natl Acad Sci USA. 2012;109:9378–9383. doi: 10.1073/pnas.1121552109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou AY, Shen RR, Kim E, et al. IKK”-mediated tumorigenesis requires K63-linked polyubiquitination by a cIAP1/cIAP2/TRAF2 E3 ubiquitin ligase complex. Cell Rep. 2013;3:724–733. doi: 10.1016/j.celrep.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokavec M, Luo JL. The transient and constitutive inflammatory signaling in tumorigenesis. Cell Cycle. 2012;11:2587–2588. doi: 10.4161/cc.21139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gulen MF, Bulek K, Xiao H, et al. Inactivation of the enzyme GSK3α by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance. Immunity. 2012;37(5):800–812. doi: 10.1016/j.immuni.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Zhou X, Chang M, et al. Regulation of T-cell activation and migration by the kinase TBK1 during neuroinflammation. Nat Commun. 2015;6:6074. doi: 10.1038/ncomms7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodur C, Kazyken D, Huang K, et al. The IKK-related kinase TBK1 activates mTORC1 directly in response to growth factors and innate immune agonists. Embo J. 2018;37:19–38. doi: 10.15252/embj.201696164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Chen L, Cai SH, Cheng H. Identification of TBK1 and IKKε, the non-canonical IκB kinases, as crucial pro-survival factors in HTLV-1-transformed T lymphocytes. Leuk Res. 2016;46:37–44. doi: 10.1016/j.leukres.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin B, Cheng K, Gite S, Ostendorff HP, Narod S, Rothschild KJ. Silencing of the IKKε gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010;12(5):R74. doi: 10.1186/bcr2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Chen L, Zhang A, et al. Silencing of IKKε using siRNA inhibits proliferation and invasion of glioma cells in vitro and in vivo. Int J Oncol. 2012;41(1):169–178. doi: 10.3892/ijo.2012.1452 [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Yang Y, Guo G, et al. Ikbke regulates cell proliferation and epithelial-mesenchymal transition of human malignant glioma via the hippo pathway. Oncotarget. 2017;8(30):49502–49514. doi: 10.18632/oncotarget.17738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederberger E, Möser C, Kynast K, et al. The non-canonical IjB kinafound IKBKE/TBK1-sensitive acute myeloid leukemia (AML) cells tend to have MYC ses IKKe and TBK1 as potential targets for the development of novel therapeutic drugs. Curr Mol Med. 2012;13:7. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Z, Aref AR, Cohoon TJ, et al. Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov. 2014. doi: 10.1158/2159-8290.CD-13-0646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo JP, Coppola D, Cheng JQ. IKBKE protein activates AKT independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–37398. doi: 10.1074/jbc.M111.287433 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Chen J, Somanath PR, Razorenova O, et al. AKT1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–1196. doi: 10.1038/nm1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Huang J, Jeong JH, et al. Selective TBK1/IKKi dual inhibitors with anticancer potency. Int J Cancer. 2014;134(8):1972–1980. doi: 10.1002/ijc.28507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Wang Z, Huang K, et al. PLK4 is a determinant of temozolomide sensitivity through phosphorylation of IKBKE in glioblastoma. Cancer Lett. 2019;443:91–107. doi: 10.1016/j.canlet.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Unnithan RVM, Hamidi A, et al. TANK-binding kinase 1 is a mediator of platelet-induced EMT in mammary carcinoma cell. Faseb J. 2019;fj201801936RRR. doi: 10.1096/fj.201801936RRR [DOI] [PubMed] [Google Scholar]