Abstract
Objectives:
The purpose of the present study was to validate the Apathy Evaluation Scale, self-rated version (AES-S), and assess the severity of apathy in a cognitively healthy middle-aged cohort at risk for Alzheimer’s disease (AD).
Method:
Three hundred and sixteen middle-aged adults were selected to represent a subset of the Wisconsin Alzheimer’s Disease Research Center Clinical Core: the Investigating Memory in People At-risk, Causes and Treatments cohort.
Results:
An exploratory factor analysis (EFA) with varimax rotation identified 3 subscales: apathy, disinterest, and social withdrawal factors. Confirmatory factor analysis confirmed the EFA findings. Results indicated acceptable convergent and discriminant validity. The AES-S is a reliable instrument to quantify apathy in cognitively healthy middle-aged individuals at risk for AD.
Discussion:
This study demonstrates the AES-S is a psychometrically sound measurement tool for assessing levels of apathy in a cognitively healthy middle-aged cohort at risk for AD.
Keywords: apathy, Apathy Evaluation Scale, Alzheimer’s disease, cognitively healthy middle-aged individuals, preclinical Alzheimer’s disease
The pathophysiological process of Alzheimer’s disease (AD) starts years before the clinical diagnosis. 1,2 About 47 million Americans had preclinical AD in 2017. 3 The preclinical phase of AD, 2 that is, the initial yet active phase of disease without apparent clinical symptom manifestation, is considered the optimal time to implement preventative strategies and intervene with the disease progression before any significant brain damage occurs. 3 Therefore, to fully characterize the preclinical phase, it is important to consider not only traditional AD biomarkers including cerebrospinal fluid (CSF) analyses and neuroimaging data but also behavioral markers altered during the preclinical phase.
Apathy, defined as loss of motivation or interest, is an alarming behavioral symptom associated with a number of neuropsychiatric conditions, including several types of dementia. 4,5 Apathy symptoms in cognitively normal older adults and individuals with mild cognitive impairment (MCI) were examined, and results suggested apathy was a robust predictor of the progression from MCI to AD dementia. 6 Others have identified apathy as a risk factor for the progression to dementia in persons with MCI. 7 Administering psychometrically sound clinical tools to measure behavioral and psychological symptoms, including apathy, may be a cost-effective and noninvasive method to identify preclinical signs of AD.
Given the potential of neuropsychiatric symptoms, such as using apathy as an indicator for risk of AD, 8,9 identifying valid measures of apathy in a preclinical population is a critical step toward the development of behavioral disease markers. Previously, scales were developed to measure levels of apathy in healthy people (eg, Dimensional Apathy Scale, Apathy Motivation Index). 10,11 Besides, Marin et al 5 developed the Apathy Evaluation Scale (AES) to quantify apathy in a clinical sample comprising participants who were depressed, poststroke, or diagnosed with probable AD. The scale has 3 versions: self-rated (AES-S), clinician-rated (AES-C), and informant-rated (AES-I). Using factor analysis, 3 factors were identified for the scale, including general apathy, disinterest/amotivation, and lack of concern. 5 The AES has been validated in various clinical cohorts, including individuals with AD, 12 individuals with severe mental illness, 13 and older individuals with significant cognitive deficits referred to a dementia assessment clinic. 14 However, its usefulness and applicability in clinically normal populations has not been established, due to the lack of effective clinical assessment tools to evaluate apathy in middle-aged individuals in the current literature. Furthermore, little is known about the levels and nature of apathy in cognitively healthy middle-aged individuals at risk for AD.
Therefore, the current study utilizes the AES-S to assess apathy in cognitively healthy middle-aged individuals at risk for AD. This study examines (1) the factorial structure of the AES-S, (2) the internal consistency of the AES-S, (3) the convergent and discriminant validity of the AES-S using well-known clinical measures, and (4) group differences based on risk for AD (ie, apolipoprotein E [APOEe4] and parental history) in apathy scores.
Methods
Participants
Analyses included data from 316 middle-aged adults (age range = 45-65) enrolled in a subset of the Wisconsin Alzheimer’s Disease Research Center’s Clinical Core: Investigating Memory in People At Risk, Causes and Treatments (IMPACT) cohort. Participants were included in the analysis if: (1) they completed the AES-S and (2) did not have a diagnosis of AD or MCI. Study partners of IMPACT participants completed the AES-I. Participants were required to identify someone who knew them well as a study partner. Study partners were typically spouses, siblings, or close friends.
Measures
Apathy Evaluation Scale
Apathy was assessed with the self-rated (AES-S) and informant (AES-I) versions of the AES. 5 The AES consisted of 18 items, each with a 4-point Likert response scale ranging from 1 = not at all true to 4 = a lot true. Scores for each item are summed; higher scores indicate greater levels of apathy. All but 3 items (items 6, 10, and 11) are reverse-scored in order to be consistent with total scale scoring; such higher scores correspond to higher levels of apathy. The primary scale used in this study was the AES-S. The AES-I was used to test validity. The reported internal consistency reliability coefficient (Cronbach’s α) was 0.86 for the AES-S and 0.94 for AES-I. 5 The reported test–retest reliability for the AES-S was 0.76. 5
Center for Epidemiological Studies Depression Scale
Depressive symptoms were assessed with the Center for Epidemiological Studies Depression Scale (CES-D). 15 The scale includes 20 items; each is self-rated for frequency over the past 2 weeks with a 4-point Likert response scale ranging from 0 = rarely or none of the time to 3 = most or all of the time. Responses are summed over the 20 items to produce a CES-D total score, which ranges from 0 to 60. Higher scores correspond to higher levels of depressive symptomatology.
Mini-Mental State Examination
Global cognitive status was assessed with the Mini-Mental State Examination (MMSE), 16 a 30-point clinician-administered scale measuring 5 areas of cognitive function: (1) orientation, (2) registration, (3) attention and calculation, (4) recall, and (5) language. The MMSE has been validated and extensively applied in both clinical practice and research to estimate global cognitive abilities.
Neuropsychiatric Inventory Questionnaire
The Neuropsychiatric Inventory Questionnaire 17 is a brief version of the Neuropsychiatric Inventory (NPI). 18 This clinician-administered evaluation uses an algorithm to assess neuropsychiatric symptoms in persons with dementia. The presence of symptoms, including apathy, depression, and delusion, is first identified, and the severity of presenting symptoms is then evaluated. Severity is ranked on a 3-point scale, ranging from 1 = mild to 3 = severe. Clinicians were naive to questionnaire and cognitive data when collecting NPI data. In the current research, 3 items (ie, apathy, depression, and delusions) were used to assess convergent and discriminant validity of the AES-S, and the Neuropsychiatric Inventory Questionnaire (NPI-Q) was administered to family/friend informants during a face-to-face interview.
Parental history and APOEe4
Parental history of AD was obtained from participant self-report in interview. The APOEe4 status was determined from blood samples. We categorized APOEe4 status as either a carrier (e2/4, e3/4, e4/4) or noncarrier (e2/2, e2/3, e3/3).
Data and Statistical Analysis
Descriptive analyses were conducted to characterize the study sample. An exploratory factor analysis (EFA) and a subsequent confirmatory factor analysis (CFA) were conducted with the Statistical Packages for the Social Sciences (version 24) and the analysis of moment structures (version 23). Cronbach α was calculated to determine the internal consistency of each identified factor. To investigate the convergent and discriminant validity of the AES-S, correlational analyses with clinical variables of interest were conducted, and an independent-samples t test and 1-way analysis of variance were used to examine the relationship between scores on the apathy factor of the AES-S with genetic risk factor APOEe4 status and parental history.
Results
Description of Participants
Table 1 shows demographic and clinical characteristics of the sample. Mean age was 57.23 years, and participants were highly educated. The majority of participants were female and white. Risk for AD due to a genetic risk factor and parental history was overrepresented in this sample. Two hundred twenty-six (71.5%) the participants endorsed a parental history of AD, and 112 (35.5%) participants were either hetero- or homozygous APOEe4 allele carriers.
Table 1.
Demographic and Clinical Characteristics of Participants.a,b,c
| Variables | Participants, Mean (SD) | Range Statistic (Min-Max) |
|---|---|---|
| Age (years) | 57.23 (5.18) | 20.70 (45-65) |
| Education (years) | 15.97 (2.49) | 16 (9-25) |
| Apathy Evaluation Scale–self-rated | 24.85 (5.94) | 44 (18-62) |
| Apathy Evaluation Scale–informant | 24.17 (6.97) | 54 (18-72) |
| Mini-Mental State Examination | 29.28 (1.1) | 9 (21-30) |
| Center for Epidemiologic Studies Depression Scale | 7.52 (7.07) | 43 (0-43) |
| n (%) | ||
| Gender | ||
| Male | 94 (29.7) | |
| Female | 222 (70.3) | |
| Race | ||
| White | 285 (90.2) | |
| Black | 31 (9.8) | |
| Marital status | ||
| Married | 242 (76.6) | |
| Widowed | 6 (1.9) | |
| Divorced | 30 (9.5) | |
| Separated | 3 (0.9) | |
| Never married | 20 (6.3) | |
| Living as married | 11 (3.5) | |
| Other | 4 (1.3) | |
| APOEe4 status | ||
| No allele e 4 | 192 (60.8) | |
| Heterozygous 4 | 95 (30.1) | |
| Homozygous 4 | 17 (5.4) | |
| Genotype 24 | 10 (3.2) | |
| Missing | 2 (0.6) | |
| Parental history of AD | ||
| Negative parental history | 62 (19.6) | |
| Positive parental history | 226 (71.5) | |
| Unknown | 28 (8.9) |
Abbreviation: APOE, apolipoprotein E.
a Apathy Evaluation Scales–self-rated or informant rated: total score = 72.
bMini-Mental State Examination: total score = 30/30. Scores above 26/30 are within normal limits.
c Center for Epidemiologic Studies Depression Scale: total score = 60.
Exploratory Factor Analysis
The 18 × 18 correlation matrix of the AES-S was subjected to a principal components analysis. The Kaiser-Meyer-Olkin measure of sampling adequacy of 0.86 is considered reasonable, and the Bartlett test of sphericity is significant (χ2 [153, N = 316] = 2081.46, P < .001), indicating the correlations within the data set are appropriate for factor analysis. Principal components analysis with varimax rotation identified 4 factors with an eigenvalue >1.0, accounting for 54.56% of the variance. However, following inspection of the scree plot, a 3-factor model was determined to be most meaningful. The first and the most prominent factor, “apathy” (eg, “I have motivation,” reverse-scored), comprised 11 items and accounted for 33.61% of the variance. The second factor, “disinterest” (eg, “I am interested in things.”), comprised 4 items and accounted for 8.28% of the variance. The third factor, “social withdrawal” (eg, “I have friends.”), comprised 3 items and accounted for 6.73% of the variance. Table 2 presents the results of our EFA for the AES-S.
Table 2.
Factor Structure of the AES-S Principal Components Analysis With Varimax Rotation Method.
| AES-S | Apathy | Disinterest | Social Withdrawal |
|---|---|---|---|
| 17. I have initiative | 0.67 | ||
| 16. Getting things done during the day is important to me | 0.67 | ||
| 18. I have motivation | 0.67 | ||
| 8. Seeing a job through to the end is important to me | 0.61 | ||
| 2. I get things done during the day | 0.58 | ||
| 9. I spend time doing things that interest me | 0.55 | ||
| 6. I put little effort into anything | 0.50 | ||
| 3. Getting things started on my own is important to me | 0.49 | ||
| 11. I am less concerned about my problems than I should be | 0.48 | ||
| 15. I have an accurate understanding of my problems | 0.43 | ||
| 10. Someone has to tell me what to do each day | 0.33 | ||
| 4. I am interested in having new experiences | 0.79 | ||
| 5. I am interested in learning new things | 0.79 | ||
| 1. I am interested in things | 0.55 | ||
| 7. I approach life with intensity | 0.51 | ||
| 13. Getting together with friends is important to me | 0.85 | ||
| 12. I have friends | 0.85 | ||
| 14. When something good happens, I get excited | 0.42 | ||
| Eigenvalue | 6.05 | 1.49 | 1.21 |
| % Variance | 33.61 | 8.28 | 6.73 |
| Reliability | 0.82 | 0.71 | 0.70 |
Abbreviations: AES-S, Apathy Evaluation Scale, self-rated.
Confirmatory Factor Analysis
As our sample size precluded splitting the data set into 2 samples, we conducted a CFA to confirm the EFA findings on the same data. This approach is supported by authors 19 who noted the usefulness of understanding the (lack of) fit via CFA on the same data where the factor model was derived prior to cross-validating the results on different samples in subsequent studies. As suggested by authors, 20 the goodness of fit of the measurement model was evaluated through the χ2 goodness-of-fit test, χ2/df ratio, the standardized root mean square residual (SRMR), the comparative fit index (CFI), and the goodness-of-fit index (GFI). According to these guidelines, models having a nonsignificant χ2, a relative χ2 (χ2/df) in the range of 3 to 1, and values greater than 0.90 for the GFI and CFI are considered to have an acceptable fit. In addition, a root mean square error of approximation (RMSEA) with 90% confidence interval (CI) was reported, where a value of less than 0.05 is considered a close fit and values up to 0.08 are considered reasonable errors of approximation in the population. 21
The initial 3-factor CFA model indicated a relatively poor fit for the data: χ 2 (132, N = 316) = 389.52, P < .001; χ 2/df = 2.95, SRMR = 0.07; CFI = 0.87; GFI = 0.87; and RMSEA = 0.08, 90% CI = 0.07 to 0.09. An examination of modification indexes revealed one pair of error terms, suggesting high correlation between selected questionnaire items: (1) item e17 (“I have initiative.”) with item e18 (“I have motivation.”). Correlated errors frequently occur between items using similar wording or appearing in close physical proximity to each other on the questionnaire 22 and reduce the accuracy of the model fit. Results of the respecified 3-factor CFA model indicated a very good model fit, χ 2 (131, N = 316) = 292.77, P < .001; however, χ 2/df = 2.23 was less than 3, SRMR of 0.06 was less than 0.08, CFI of 0.92 was greater than 0.90, GFI of 0.91 was greater than 0.90, and RMSEA of 0.06 (90% CI: 0.05-0.07) was below the value of 0.08; all these indexes meet the criteria of good model fit. 20 Although the χ2 was still significant, all other fit indices were within acceptable ranges. As a result, the overall CFA results suggested the 3-factor model fit the data adequately well after connecting one pair of error term. All items in the 3-factor model significantly loaded their respective factors. Figure 1 depicts the CFA for the AES-S.
Figure 1.
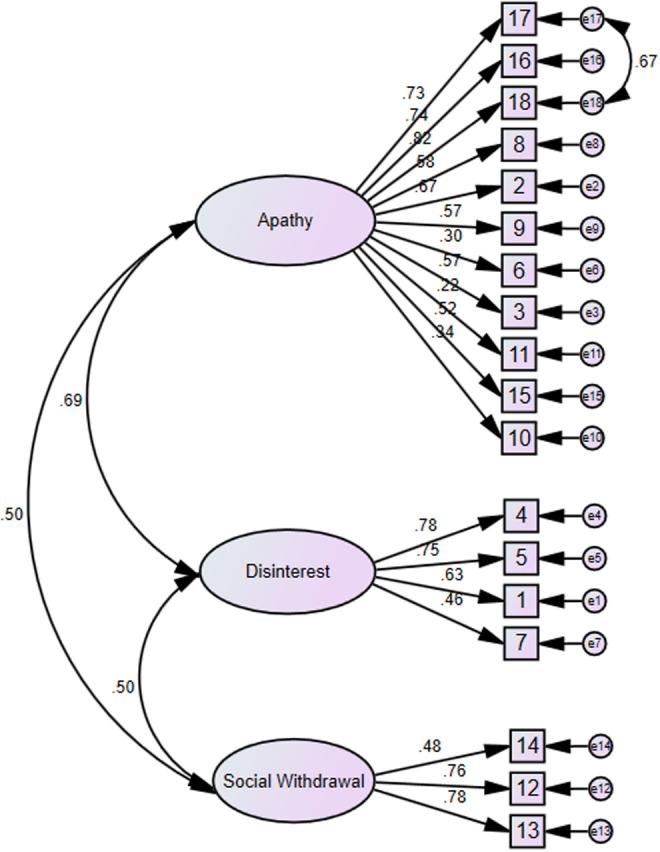
Confirmatory factor analysis results of Apathy Evaluation Scale, self-rated version (AES-S).
Reliability
Reliability coefficients (Cronbach α) were .82 for the “apathy” subscale, .71 for the “disinterest” subscale, and .70 for the “social withdrawal” subscale, demonstrating acceptable internal consistency for each factor.
Convergent and Discriminant Validity
Table 3 presents the results of the correlational analyses. As expected, there were modest to high correlations among the 3 subscales of the AES-S. Likewise, the AES-I total score was significantly correlated with the apathy, disinterest, and social withdrawal subscales of the AES-S. Suggesting convergent validity, the apathy subscale was significantly associated with the apathy item of the NPI-Q, suggesting good convergent validity. As expected, apathy, disinterest, and social withdrawal subscales were modestly correlated with the depression score of the NPI-Q and with the CES-D total score. We used the CES-D total score for validation purposes. However, as an additional analysis, we calculated the anhedonia score from the CES-D to see how disinterest factor of the AES-S correlates with anhedonia score of the CES-D. Based on this analysis, anhedonia was significantly correlated with disinterest (r = 0.23, P < .001). Discriminant validity was assessed by examining the association between the subscales of the AES-S and the report of delusions on the NPI-Q. There was no association between these measures. We tested whether subscales of AES-S correlate with cognitive functioning and found only the apathy subscale was significantly associated with MMSE total score. No significant correlations were found between MMSE and disinterest and social withdrawal subscales.
Table 3.
Pearson Product–Moment Correlation Between AES-S Subscales and AES-I, NPI-Q Apathy, NPI-Q Depression, NPI-Q Delusion, MMSE, and CES-D.
| Measures | Apathy | Disinterest | Social Withdrawal |
|---|---|---|---|
| Apathy | - | ||
| Disinterest | 0.56a | - | |
| Social withdrawal | 0.43a | 0.42a | - |
| AES-I | 0.27a | 0.25a | 0.17b |
| NPI-Q apathy | 0.13c | 0.01 | −0.04 |
| NPI-Q depression | 0.27a | 0.25a | 0.20a |
| NPI-Q delusions | 0.04 | 0.01 | −0.06 |
| MMSE | −0.14b | −0.06 | −0.04 |
| CES-D | 0.46a | 0.32a | 0.27a |
Abbreviations: AES-S, apathy Evaluation Scale, self-rated; AES-I, Apathy Evaluation Scale, Informant-rated; CES-D, Center of Epidemiological Studies Depression Scale, MMSE, Mini-Mental State Examination; NPI-Q, Neuropsychiatric Inventory Questionnaire.
a P <.001.
b P < .01.
c P < .05.
Group Differences in Apathy Levels
A series of independent-samples t tests were conducted to compare AES-S scores in carriers and noncarriers of a genetic risk factor, APOEe4, and between parental history groups. No differences were found in the AES-S scores for e4 allele noncarriers (mean = 24.57, standard deviation [SD] = 5.48) versus e4 allele positive status (mean = 25.35, SD = 6.62), t 312 = −1.13, P = .26. Likewise, no differences were found in the AES-S scores for positive parental history (mean = 24.75, SD = 5.52) versus negative parental history (mean = 24.40, SD = 5.81); t 286 = −0.43, P = .67.
Discussion
Our results suggest the AES-S is a useful and reliable instrument to quantify and measure the severity of apathy symptoms in cognitively healthy middle-aged individuals at risk for AD. On average, these cognitively healthy, middle-aged participants report a minimal level of apathy symptoms. Nonetheless, exploratory and confirmatory factor analyses results suggest the AES-S exhibits a 3-factor structure in this population: (1) apathy, (2) disinterest, and (3) social withdrawal. The 3-factor structure found in the current study is largely similar to previous factor analysis findings obtained from differing clinical cohorts using either the AES self-rated version 5,23 or clinician version. 5,12,13 In contrast, a 2-factor structure in the AES-S was found in a sample of individuals with cognitive deficits or dementia. 14 Our data were collected from unique population, differing from participants of Marin and colleagues’ original study. 5 Understandably, our participants may experience, evaluate, or perform quite differently with respect to measures of apathy. Therefore, the heterogeneity of research participants in each study can change items of specific factors of the AES-S.
All the AES-S subscales were found to have a significant correlation with the AES-I total score, and yet only the apathy subscale of AES-S was correlated with the apathy score of the NPI-Q. As expected, the subscales of the AES-S were associated with CES-D total score and the depression item of the NPI-Q; however, the levels of associations were small to moderate. Interestingly, the association between the depression item of NPI-Q and apathy factor was higher than the association between the apathy item of NPI-Q and the apathy factor. This finding is consistent with previous findings. 14 Although apathy and depression are distinct neuropsychiatric syndromes, it is important to note that there is considerable overlap between apathy and depression measurement, perhaps because depression questionnaires assess apathy secondary to depression. 24,25 The subscales of the AES-S were not significantly associated with the delusions item of the NPI-Q. Overall, the correlation coefficients indicated the AES-S has acceptable convergent and discriminate validity for cognitively healthy middle-aged individuals at risk for AD.
Research exploring the relationship between apathy and cognitive functioning is common in cognitively impaired populations 12 ; the relationship between apathy and cognitive functioning is not well explored in cognitively healthy middle-aged cohort nonetheless. Our analyses suggest an exclusive association between cognitive performance and apathy, which only the apathy subscale of AES-S had a significant inverse correlation with MMSE scores. Such finding denotes a higher level of apathy was associated with worse cognitive performance. The mechanism underlying this association cannot be determined in these analyses. It is possible that subtle cognitive defic?its underlying apathy or the reverse or that both are reflective of underlying preclinical pathology.
In group comparisons of levels of apathy, AES-S scores did not statistically differ when risk factors (ie, APOEe4 and parental history) were examined. Previous findings regarding the relationship between APOEe4 status and apathy using clinical cohorts have been mixed. One study found higher rates of apathy in APOEe4 carriers with AD. 26 However, no significant difference was found between APOEe4 carriers and noncarriers with AD on the NPI depression–apathy score. 27
More than 70% of persons in the early stage of AD experience apathy, which often interferes with their social, physical, and mental functioning. 28 Additionally, apathy is associated with mortality risk in cognitively impaired older adults. 9,29 The development of AD neuropathology likely begins long before clinical signs become evident. 30 In these preclinical stages, behavioral and psychological symptoms, including apathy, may be early disease indicators of AD pathology in cognitively healthy older individuals. For example, mood changes in cognitively healthy participants are associated with increased levels of traditional AD biomarkers, including CSF and PET Pittsburg Compound B. 31 Some have suggested behavioral, mood, and personality changes are among the earliest signs of underlying neuropathological processes, 32 supporting the necessity for valid examinations for behavioral symptoms such as apathy in the preclinical stages.
Examining the preclinical phase of AD with less invasive ways may also be more acceptable among patients, which may increase treatment adherence and ultimately help health-care professionals control the progression of AD. This study demonstrates the AES-S has acceptable psychometric properties when used in a population of cognitively healthy middle-aged individuals at risk for AD. Given apathy is a common and easily measurable feature in AD and is a candidate behavioral marker in the preclinical phase, identifying a valid, cost-effective, and noninvasive assessment tool for apathy is imperative. Health-care professional should educate their patients and families to pay attention to such behavioral symptoms given the general public may only pay attention to memory loss and see it as the sole symptom of AD.
The current study presents some limitations. First, our sample comprises a nonclinical, cognitively healthy middle-aged group whose levels of apathy are generally low and whose range of AES-S scores is restricted. Thus, caution should be used in the interpretation of the correlation coefficients used to assess validity of the measure as some correlations may be underestimates of associations. Second, most participants were Caucasian and well educated; thus, the generalizability to other populations is limited. Third, the 3-factor structure solution we identified in the AES-S has not been tested for stability across samples or time, that is, longitudinally. Future studies should use CFA in a different sample. Fourth, we did not use receiver operating characteristic analysis to calculate cutoff values. However, researchers reported cutoff values on the AES for healthy participants and patients. For example, a score of 34 or higher indicates apathy for healthy participants (2 SD above mean). 33 Finally, this is a cross-sectional study and thus cannot assess the usefulness of the AES-S as a marker of AD risk; therefore, the predictive value of the AES-S still needs to be evaluated through longitudinal studies. Despite these limitations, the current study provides evidence of the validity of a simple, noninvasive measure of apathy in a nonclinical population.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data collection and contributions by author Carey E. Gleason is supported by funding from NIH-NIA for African Americans Fighting Alzheimer’s at Midlife (AA-FAIM, R01 AG054059) and the Wisconsin Alzheimer’s Disease Center (P50 AG033514). Mary F. Wyman was also supported by funding from VA Advanced Fellowship in Women’s Health. This is GRECC Manuscript number 001-2018.
ORCID iD: Emre Umucu  http://orcid.org/0000-0002-3945-6975
http://orcid.org/0000-0002-3945-6975
Mary Wyman  http://orcid.org/0000-0001-5371-9105
http://orcid.org/0000-0001-5371-9105
References
- 1. Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19(3):163–165. [DOI] [PubMed] [Google Scholar]
- 2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delrieu J, Desmidt T, Camus V, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Apathy as a feature of prodromal Alzheimer’s disease: an FDG-PET ADNI study. Int J Geriatr Psychiatry. 2015;30(5):470–477. [DOI] [PubMed] [Google Scholar]
- 5. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–162. [DOI] [PubMed] [Google Scholar]
- 6. Guercio B, Donovan NJ, Munro CE, et al. The Apathy Evaluation Scale: a comparison of subject, informant, and clinician report in cognitively normal elderly and mild cognitive impairment. J Alzheimers Dis. 2015;47(2):421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richard E, Schmand B, Eikelenboom P, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33(2-3):204–209. [DOI] [PubMed] [Google Scholar]
- 8. Lam LC, Tam CW, Chiu HF, Lui VW. Depression and apathy affect functioning in community active subjects with questionable dementia and mild Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(5):431–437. [DOI] [PubMed] [Google Scholar]
- 9. van der Linde RM, Matthews FE, Dening T, Brayne C. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: the importance of apathy. Int J Geriatr Psychiatry. 2017;32(3):306–315. [DOI] [PubMed] [Google Scholar]
- 10. Radakovic R, Abrahams S. Developing a new apathy measurement scale: Dimensional Apathy Scale. Psychiatry Res. 2014;219(3):658–663. [DOI] [PubMed] [Google Scholar]
- 11. Ang YS, Lockwood P, Apps MA, Muhammed K, Husain M. Distinct subtypes of apathy revealed by the Apathy Motivation Index. PLos One. 2017;12(1):e0169938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsieh CJ, Chu H, Cheng JJ, Shen WW, Lin CC. Validation of Apathy Evaluation Scale and assessment of severity of apathy in Alzheimer’s disease. Psychiatry Clin Neurosci. 2012;66(3):227–234. [DOI] [PubMed] [Google Scholar]
- 13. Faerden A, Nesvåg R, Barrett EA, et al. Assessing apathy: the use of the Apathy Evaluation Scale in first episode psychosis. Eur Psychiatry. 2008;23(1):33–39. [DOI] [PubMed] [Google Scholar]
- 14. Clarke DE, Reekum RV, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the Apathy Evaluation Scale. J Neuropsychiatry Clin Neurosci. 2007;19(1):57–64. [DOI] [PubMed] [Google Scholar]
- 15. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 17. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. [DOI] [PubMed] [Google Scholar]
- 18. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 19. Prooijen JWV, Kloot WAVD. Confirmatory analysis of exploratively obtained factor structures. Educ Psychol Meas. 2001;61(5):777–792. [Google Scholar]
- 20. Weston R, Gore PA, Chan F, Catalano D. An introduction to using structural equation models in rehabilitation psychology. Rehabilitation Psychol. 2008;53(3):340–356. [Google Scholar]
- 21. Byrne B. Structural equation modeling with AMOS: basic concepts, applications and programming. Hillsdale, NJ: Lawrence Erlbaum Associates; 2001. [Google Scholar]
- 22. Bollen K, Lennox R. Conventional wisdom on measurement: a structural equation perspective. Psychological Bulletin. 1991;110(2):305–314. [Google Scholar]
- 23. Raimo S, Trojano L, Spitaleri D, Petretta V, Grossi D, Santangelo G. Apathy in multiple sclerosis: a validation study of the apathy evaluation scale. J Neurol Sci. 2014;347(1-2):295–300. [DOI] [PubMed] [Google Scholar]
- 24. Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314–319. [DOI] [PubMed] [Google Scholar]
- 25. Tagariello P, Girardi P, Amore M. Depression and apathy in dementia: same syndrome or different constructs? A critical review. Arch Gerontol Geriatr. 2009;49(2):246–249. [DOI] [PubMed] [Google Scholar]
- 26. Monastero R, Mariani E, Camarda C, et al. Association between apolipoprotein E epsilon4 allele and apathy in probable Alzheimer’s disease. Acta Psychiatr Scand. 2006;113(1):59–63. [DOI] [PubMed] [Google Scholar]
- 27. Chen CS, Ouyang P, Yeh YC, et al. Apolipoprotein E polymorphism and behavioral and psychological symptoms of dementia in patients with Alzheimer Disease. Alzheimer Dis Assoc Disord. 2012;26(2):135–139. [DOI] [PubMed] [Google Scholar]
- 28. Buettner LL, Fitzsimmons S, Atav S, Sink K. Cognitive stimulation for apathy in probable early-stage Alzheimer’s. J Aging Res. 2011;2011:480890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Linde RM, Stephan BC, Savva GM, Dening T, Brayne C. Systematic reviews on behavioural and psychological symptoms in the older or demented population. Alzheimers Res Ther. 2012;4(4):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Babulal GM, Ghoshal N, Head D, et al. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am J Geriatr Psychiatry. 2016;24(11):1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ismail Z, Agüera-Ortiz L, Brodaty H, et al. NPS Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA of ISTAART). The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56(3):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kant R, Duffy J, Pivovarnik A. Prevalence of apathy following head injury. Brain Inj. 1998;12(1):87–92. [DOI] [PubMed] [Google Scholar]


