Abstract
OBJECTIVES:
To evaluate the feasibility of using a biobehavioral approach to examine symptom burden in rural residents with advanced cancer.
SAMPLE & SETTING:
21 patients with advanced lung, colorectal, or pancreatic cancer were enrolled at the University of Iowa in Iowa City.
METHODS & VARIABLES:
Using Cleeland’s cytokine-immunologic model of symptom expression, symptom burden (i.e., severity, count, and interference) and inflammatory cytokines were measured for 24 weeks. Potential predictors included demographics, clinical characteristics, optimism, social support, and cancer-related stress. Descriptive statistics, Wilcoxon rank-sum, and Fisher’s exact test were used for analysis.
RESULTS:
Recruitment and retention rates were similar for rural and nonrural patients. Demographics, optimism, and social support were no different between groups. The cancer-related stress total score for rural patients was nearly half of the score of nonrural patients, with rural patients reporting significantly less avoidance. Symptom severity for the five worst symptoms remained moderate during the 24 weeks, whereas nonrural residents reported steady declines in severity of their five worst symptoms. Significant differences in inflammatory cytokines between groups were only found at one time point.
IMPLICATIONS FOR NURSING:
Rural residents who seek care at a cancer center may be clinically and demographically more similar to their nonrural counterparts than to rural residents seeking local care.
Keywords: symptom burden, rural, advanced cancer, isolation, symptom management
Approximately 59 million people live in rural areas in the United States (Blake, Moss, Gaysynsky, Srinivasan, & Croyle, 2017). Rural-dwelling individuals have an increased frequency of late-stage cancer at the time of diagnosis (Singh, Williams, Siahpush, & Mulhollen, 2011) and an increased incidence of cancer mortality compared to individuals living in nonrural areas (Blake et al., 2017). These disparities are thought to be related to restricted access to health care, lower median household income, and fewer years of formal education (Weaver, Geiger, Lu, & Case, 2013). In addition, rural residents experience isolation, report lack of information, and have limited accessibility to services (Duggleby et al., 2010). The challenge of including rural residents who live geographically far from academic centers in research contributes to gaps in understanding the symptom management needs of rural residents with advanced cancer (Gilbertson-White, Saeidzadeh, Yeung, Tykol, & Vikas, 2017).
People with advanced cancer face multiple distressing physical and psychosocial symptoms, such as pain, fatigue, sleep disturbance, and depressed mood (Rhondali et al., 2013) across various cancer diagnoses. Symptoms can affect a person’s function and interfere with cancer treatment outcomes (Wang et al., 2010). Symptom severity is a known predictor of physical function in people with advanced cancer (Salanitro et al., 2012). Symptom distress has been associated with lower quality of life (Kirkova et al., 2010). In addition, rural residents report increased rates of depression (Andrykowski, Steffens, Bush, & Tucker, 2014; Burris & Andrykowski, 2010) and stress related to accessing healthcare services during major illnesses (Hendren et al., 2011). This suggests that symptom occurrence, severity, and interference may be higher in people with advanced cancer living in rural areas.
A growing body of research has evaluated various predictors of cancer symptoms to determine who will experience worse symptom burden, including social factors, such as socioeconomic status (Sullivan et al., 2016) and social support (Kroenke et al., 2013); psychosocial factors such as optimism (Ha & Cho, 2014) and perceived stress (Ho, Fong, & Cheung, 2014); biological factors, such as genetic and genomic markers (Aouizerat et al., 2009; Koleck & Conley, 2016; Miaskowski et al., 2017; Reyes-Gibby et al., 2008) and inflammatory cytokines (Fogelman et al., 2017; Oliveira et al., 2014; Wang et al., 2010). The evidence supporting the relationships among proinflammatory cytokines, including interleukin-1 beta (IL-1b), interleukin-1 receptor antagonist (IL-1RA), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-a), and symptom burden in individuals with cancer is very promising (Wood, Nail, Gilster, Winters, & Elsea, 2006; Wood & Weymann, 2013). Based on this research, it is believed that cancer and treatment of cancer (radiation therapy/chemotherapy) triggers the release of these cytokines, which then lead to the development of symptoms such as pain, fatigue, sleep disturbance, and mood changes (Bower & Lamkin, 2013; Carnio, Di Stefano, & Novello, 2016; Koch, Love-Homan, Espinosa-Cotton, Stanam, & Simons, 2015; Paulsen et al., 2017; Shi et al., 2015; Wang et al., 2008). In addition, elevated perceived stress is known to be associated with increased inflammatory response in both acute and chronic stress models (Elenkov & Chrousos, 2002). Given the challenges of accessing health care and higher rates of advanced cancer diagnoses, rural residents may be experiencing an elevated stress response to these stressors compared to their nonrural peers (Moreno-Smith, Lutgendorf, & Sood, 2010).
Therefore, it is hypothesized that people with advanced cancer living in rural areas will experience elevated inflammatory response and increased symptom burden compared to their nonrural peers. Supporting this hypothesis, in a study of 117 individuals with cancer living in China, the subgroup of participants with moderate to severe ratings on the symptom cluster of pain, fatigue, depression, and sleep disturbance had higher levels of IL-6, had poorer functional status, and were more likely to be rural residents than participants reporting lower severity scores for this symptom cluster (Ji et al., 2017).
Although there is a large body of research on symptoms and cytokines, few studies have evaluated differences between rural and nonrural populations, particularly over time. One explanation for this gap is that rural residents often do not receive all the cancer care from a single healthcare system, making data collection challenging (Gilbertson-White, Aouizerat, & Miaskowski, 2011). In addition, the majority of the symptom and cytokine research focuses on individuals with all stages of disease. Individuals with advanced cancer generally do not receive cure-focused cancer treatment and experience a unique set of stressors. Therefore, the purpose of this study is to describe the recruitment and retention rates for this challenging cancer population. A secondary purpose is to describe preliminary results describing differences and similarities between rural and nonrural residents in terms of inflammatory response and symptom trajectories over the course of treatment for advanced cancer. The cytokine-immunologic model of symptom expression (Cleeland et al., 2003) guided the current study.
Methods
Design, Sample, and Setting
This exploratory pilot study used a longitudinal, observational design of rural and nonrural newly diagnosed individuals with advanced cancer (N = 21). The study was approved by the institutional review board at the University of Iowa. Participants were recruited from a National Cancer Institute (NCI)–designated comprehensive cancer center in eastern Iowa. Individuals were included if they were aged 18 years or older; had advanced non-small cell lung cancer (NSCLC), pancreatic cancer, or colorectal cancer (i.e., stage IIIb or IV); started primary treatment; and were able to read and write in English. The rationale for including these cancer types was that people with these cancers are similar in terms of their reported symptom experiences, prognoses, and treatment protocols when diagnosed at an advanced stage. Individuals were excluded for the following reasons: actively dying, no planned anticancer treatments, or an identified change in cognitive function by the oncologist that would interfere with the patient’s ability to recall his or her health-related experiences from the past week.
There is no official definition of rural, but the U.S. Census Bureau (USCB) and the Office of Management and Budget (OMB) have developed definitions that inform how rurality is operationalized. The USCB defines urbanized areas as 50,000 or more people and urban clusters as at least 2,500 and less than 50,000 people (Ratcliffe, Burd, Holder, & Fields, 2016). The OMB designates counties as metropolitan (core urban area of 50,000 or more population), micropolitan (urban core of at least 10,000 but less than 50,000 population), or neither. The remaining counties that are not part of a metropolitan statistical area are considered rural. These definitions have inaccuracies that lead to over- or under-representation of rural areas. The Rural-Urban Commuting Area (RUCA) codes created by the Federal Office of Rural Health Policy (FORHP) incorporate elements from both definitions. The FORHP accepts all non-metropolitan counties as rural and uses an additional method of determining rurality (U.S. Department of Agriculture, 2005). Based on U.S. census data, a RUCA code is assigned to each census tract to account for distance to services. Tracts inside metropolitan counties with the codes 4–10 are considered rural. Because of the small sample size, participants were dichotomized into rural and nonrural based on the RUCA code of their primary residence.
Procedures
Baseline data were collected prior to infusion on the first day of chemotherapy. Subsequent data were gathered in conjunction with the chemotherapy infusion schedule (i.e., every three to four weeks) for as many as four cycles of treatment. The baseline survey consisted of a sociodemographic questionnaire and a battery of measures evaluating psychosocial factors (i.e., optimism, social support, and cancer-related stress). In addition, at all six time points, participants completed the MD Anderson Symptom Assessment Inventory–core (MDASI-core) symptom burden questionnaire, provided blood to test for inflammatory cytokines, and had their medical record reviewed for changes in their treatment or disease. Surveys were completed on the day of the visit in clinic waiting rooms prior to the scheduled infusion. Blood samples for cytokine analysis were collected in conjunction with other scheduled clinical blood draws on the day of the study visit.
Measures
Sociodemographic questionnaire:
The Center for Research in Chronic Disease sociodemographic form was used to collect information about age, race, gender, education, income, and zip code (Sereika & Engberg, 2006). This questionnaire has been used in various chronic disease populations, including cancer (Donovan et al., 2014; Henderson et al., 2010).
Optimism:
The Revised Life Orientation Test is a measure of trait optimism consisting of 10 items. Response options range from 0 (strongly disagree) to 4 (strongly agree). After reverse scoring appropriate items, the six items are summed to create an optimism score with a range of 0–24 (α = 0.68, r = −20).
Validity as a trait measure is supported given no significant differences in scores among long-term survivors, short-term survivors, and those with active, recurrent disease (Donovan, 2003). Internal reliability in cross-sectional studies of individuals with breast cancer had a Cronbach alpha of 0.85 (Carver, Smith, Petronis, & Antoni, 2006; Scheier et al., 2007). The scale also shows good discriminant validity with respect to related concepts such as locus of control and helplessness (r = 0.24, r = −0.35, respectively) (Scheier & Carver, 1985).
Social support:
The Interpersonal Support Evaluation List–Revised (ISEL-R) is a measure of social support (Cohen, 2015; Cohen & Hoberman, 1983; Cohen, Mermelstein, Kamarck, & Hoberman, 1985). The ISEL-R is a 12-item scale assessing three types of social support (appraisal, belonging, and tangible). The long form (40 items) of this scale has shown to have good validity and reliability (α = 0.7) (Merz et al., 2014). The responses for ISEL are based on a four-point scale ranging from 1 (definitely false) to 4 (definitely true) (Fagundes, Lindgren, Shapiro, & Kiecolt-Glaser, 2012). A higher score indicates higher perception of social support (Rogers, Brotherton, Plaza, Durán, & Altamar, 2015).
Cancer-related stress:
The Impact of Events Scale– Revised (IES-R) is a measure of perceived stress related to cancer and its treatment that consists of a 22-item scale comprised of three subscales. Subscales (i.e., avoidance, intrusion, and hyperarousal) are summed to create a total score. Thresholds have been identified for low (8.5 or less), medium (9–19), and high (greater than 19) stress responses with well-established reliability and validity (avoidance, α = 0.82; intrusion, α = 0.78) across cancer populations (Horowitz, Wilner, & Alvarez, 1979).
Symptom burden:
Symptom burden was measured using the symptom severity and symptom interference subscales on the MDASI-core. The symptom severity scale consists of 13 common cancer-related symptoms that are rated on a 0–10 numeric rating scale (NRS) (Sailors et al., 2013), ranging from 0 (do not have the symptom) to 10 (worst severity imaginable). An additional symptom (constipation), which is not part of the MDASI-core but a common symptom in NSCLC, pancreatic, and colorectal cancers, was added for a total of 14 symptoms. The MDASI-core has well-established validity and reliability (core symptoms, α = 0.88; interference, α = 0.92; area under the curve = 0.77) across various cancer diagnoses (Jones et al., 2014). The symptom interference subscale on the MDASI-core measures level of interference from symptoms on general activity, mood, enjoyment of life, walking, relationships with others, and work. The response options use an NRS from 0 (did not interfere) to 10 (interfered completely) (Williams et al., 2013).
The concept of symptom burden was evaluated in four ways: (a) overall symptom severity, (b) severity for the five highest symptoms, (c) total number of moderate and severe symptoms, and (d) symptom interference. Overall symptom severity was defined by the mean NRS severity score for all 14 symptom items. In an effort to better capture the upper range of symptom severity experiences for each participant, the mean severity score was also calculated for the top five most severe symptoms reported at each time point (i.e., severity for the five highest symptoms). Total number of moderate and severe symptoms was defined as a tally of the total number of symptoms rated 4 or greater on the NRS. The symptom interference score was defined by the mean score of the six items that make up the MDASI-interference subscale.
Inflammatory cytokines:
A customized pro-inflammatory cytokine panel was selected that included five cytokines (IL-1β, IL-1RA, IL-6, tumor necrosis factor alpha [TNF-α], and C-reactive protein [CRP]) in the inflammatory pathway that are commonly reported to be associated with cancer-related symptoms (Cleeland et al., 2003; Seruga, Zhang, Bernstein, & Tannock, 2008). Four milliliters of blood were collected from a peripheral blood draw or from a central venous access device using standard techniques with EDTA (ethylenediaminetetraacetic acid) tubes. Samples were kept on ice during transport prior to processing and storage. The time from collection to processing and storage was no longer than two hours. When collected in EDTA tubes and stored at 4°C–8°C, these markers are known to be stable for as long as 24 hours (Aziz et al., 2016). Samples were centrifuged for 10 minutes at 4°C to remove particulates, and plasma was drawn off and stored in a −80°C freezer to prevent contamination and loss of bioactivity.
After being thawed to room temperature, plasma samples were analyzed for quantitative levels of the cytokines using high-sensitivity ELISA kits, as per the manufacturer’s protocol. The ratio of IL-1β/IL-RA x 1,000 was calculated as an additional measure of inflammatory response since this ratio has been shown in some cases to be indicative of an inflammatory environment (Arondel, Singer, Matsukawa, Zychlinsky, & Sansonetti, 1999; Girard et al., 2008; Richette et al., 2008).
Clinical characteristics:
The electronic health record was reviewed at each visit for information about cancer type, stage, and current treatments.
Analyses
Feasibility was established by evaluating recruitment and retention rates. SAS, version 9.4, was used for statistical analysis. Descriptive statistics (means and standard deviation or percentages) were calculated for demographic and psychosocial variables at baseline and compared between rural and nonrural participants using Wilcoxon rank-sum exact test for continuous variables and Fisher’s exact test for categorical variables. Means and standard deviations were calculated for symptom burden variables and cytokines at all six time points and compared for rural and nonrural participants using Wilcoxon rank-sum exact test. Because of the exploratory nature of this feasibility study and a small sample size, a p value of less than 0.1 was used to indicate significant differences, and no adjustment for multiple comparisons were made (Kang, Hong, Esie, Bernstein, & Aral, 2017).
Results
Recruitment and Retention
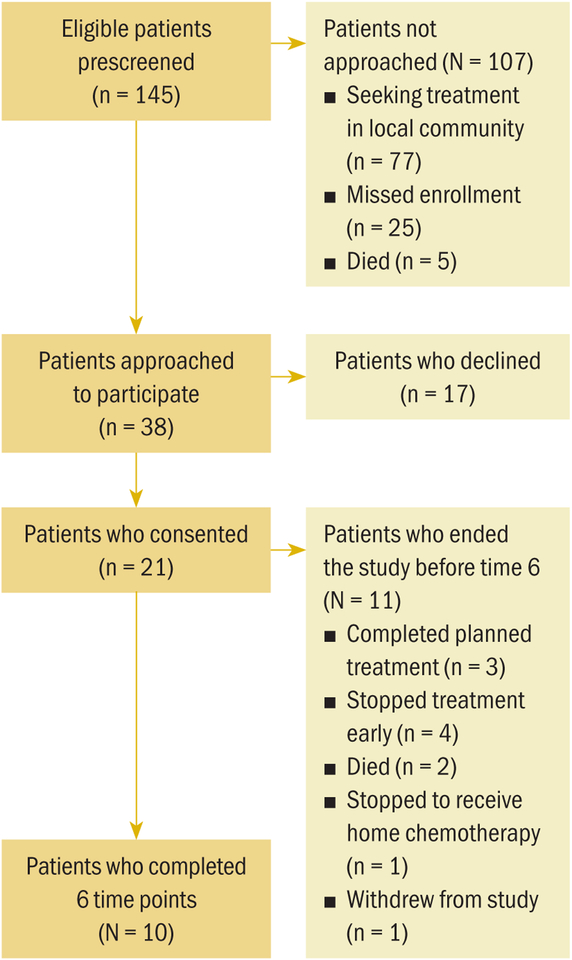
One hundred and forty-five potential participants were prescreened and found to be eligible; however, 73% of those individuals were not approached because they (a) planned to seek treatment in their local community and would not be able to participate in data collection for the length of entire planned treatment, (b) started their cancer treatment between the time of screening and scheduled enrolled (e.g., emergently hospitalized and started chemotherapy on the weekend), or (c) died between the time of screening and scheduled enrolled. Twenty-one consented to participate and provided baseline data. Figure 1 depicts the number of individuals screened and approached who consented (n = 21, 8 rural and 13 nonrural) and completed the study (n = 10, 4 rural and 6 nonrural). From the time of consent, the dropout rate by time 6 (T6) was approximately 50% in the rural and nonrural groups.
FIGURE 1.
Flow Diagram for Sample
Sample
The participants had a mean age of 60 years (SD = 9.3). The majority of participants were Caucasian (n = 20), male (n = 12), and had at least some college education (n = 16) (see Table 1). Rural participants were not statistically significantly different from their nonrural counterparts in terms of race, gender, education, and income levels.
TABLE 1.
Sample Characteristics
| Total (N = 21) | Rural (N = 8) | Nonrural (N = 13) | |||||
|---|---|---|---|---|---|---|---|
| Variable | SD | SD | SD | pb | |||
| Age (years) | 59.95 | 9.27 | 59.75 | 9.54 | 60.08 | 9.48 | 0.632 |
| Optimisma | 4.29 | 4.11 | 14 | 2.88 | 14.46 | 4.82 | 0.736 |
| Variable | n | n | n | pc | |||
| Gender | |||||||
| Male | 12 | 4 | 8 | 0.673 | |||
| Female | 9 | 4 | 5 | - | |||
| Race | |||||||
| Caucasian | 20 | 8 | 12 | 1 | |||
| African American | 1 | - | 1 | - | |||
| Education | |||||||
| High school/GED | 5 | 2 | 3 | 1 | |||
| Some college/degree | 16 | 6 | 10 | - | |||
| Household income ($) | |||||||
| 50,000 or less | 8 | 2 | 6 | 0.642 | |||
| More than 50,000 | 12 | 5 | 7 | - | |||
| Not reported | 1 | 1 | - | - | |||
| Employment | |||||||
| Employed | 11 | 5 | 6 | 0.659 | |||
| Not employed | 10 | 3 | 7 | - | |||
| Cancer type | |||||||
| Lung | 9 | 2 | 7 | 0.506 | |||
| Pancreatic | 3 | 2 | 1 | - | |||
| Colorectal | 9 | 4 | 5 | - | |||
| Completion of all visits | |||||||
| Yes | 10 | 4 | 6 | 1 | |||
| No | 11 | 4 | 7 | - | |||
Optimism was assessed with the Revised Life Orientation Test, with responses ranging from 0 (strongly disagree) to 4 (strongly agree). After reverse scoring, six items were summed to create an optimism score with a range of 0–24.
Wilcoxon rank-sum exact test
Fisher’s exact test
Psychosocial Characteristics
At baseline, rural participants reported lower scores than nonrural participants on all three IES subscales (intrusion, p = 0.057; avoidance, p = 0.023; hyper-arousal, p = 0.229) (see Table 2). The total IES score for rural participants was almost half the score for nonrural participants ( = 12.5 [SD = 11.8] versus = 24.6 [SD = 14], p = 0.06). Optimism scores were similar for rural and nonrural residents, while they were less variable for rural residents ( = 14 [SD = 2.9] versus = 14.5 [SD = 4.8], p = 0.74). Perceived stress was also similar for rural and nonrural residents, but both populations showed high deviation in terms of perceived stress scores (coefficient of variant = 68.5% and 65.6%, respectively). Regarding interpersonal support, there were no statistically significant differences between the groups: both rural and nonrural groups reported, on average, moderate to high feelings of belonging, appraisal, and tangibility of support system.
TABLE 2.
Psychosocial Characteristics of Sample
| Total (N = 21) | Rural (N = 8) | Nonrural (N = 13) | |||||
|---|---|---|---|---|---|---|---|
| Evaluation | SD | SD | SD | p | |||
| Interpersonal Support Evaluationa | |||||||
| Appraisal subscale | 12.71 | 2.7 | 12.75 | 2.71 | 12.69 | 2.81 | 0.928 |
| Belonging subscale | 13.14 | 2.63 | 12.63 | 2.93 | 13.46 | 2.5 | 0.604 |
| Tangible subscale | 13.67 | 2.03 | 13 | 2.51 | 14.07 | 1.66 | 0.396 |
| Perceived Stress Scoreb | |||||||
| Overall | 6.62 | 4.3 | 6.38 | 4.37 | 6.77 | 4.44 | 0.986 |
| Impact of Events Scale-Revised | |||||||
| Overallc | 19.75 | 14.17 | 12.5 | 11.78 | 24.58 | 13.96 | 0.06 |
| Intrusion subscaled | 1 | 0.92 | 0.5 | 0.54 | 1.33 | 0.99 | 0.057 |
| Avoidance subscaled | 1.05 | 0.83 | 0.5 | 0.93 | 1.42 | 0.52 | 0.023 |
| Hyperarousal subscaled | 0.8 | 0.77 | 0.5 | 0.54 | 1 | 0.85 | 0.229 |
Scores range from 4 (definitely true) to 16 (definitely false).
Scores range from 0 (never) to 16 (very often).
Scores range from 1 (not at all) to 88 (often).
Scores range from 1 (not at all) to 4 (often).
Symptom Burden
Mean overall symptom severity, mean severity for the five highest symptoms, total number of moderate or severe symptoms, and mean symptom interference are shown for all six time points in Tables 3 and 4. At the beginning of the study, rural participants tended to report lower mean overall symptom severity scores than nonrural participants (1.4 [SD = 1.5] for rural versus 2.4 [SD = 2.6]) for nonrural participants (d = 0.46, p = 0.33).
TABLE 3.
Symptom Burden, Interference, and Cytokines for Rural and Nonrural Participants at Time Points 1–3
| Time 1 (N = 21) | Time 2 (N = 19) | Time 3 (N = 18) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | SD | d/p | n | SD | d/p | n | SD | d/p | |||
| Symptom severity | ||||||||||||
| Rural | 8 | 1.4 | 1.51 | 0.46 | 7 | 1.9 | 1.04 | 0.08 | 7 | 1.39 | 1.26 | 0.11 |
| Nonrural | 12 | 2.38 | 2.59 | 0.33 | 12 | 2.08 | 2.6 | 0.31 | 10 | 1.54 | 1.6 | 0.98 |
| Top 5 symptoms | ||||||||||||
| Rural | 8 | 3.05 | 2.96 | 0.4 | 7 | 4.37 | 2.46 | −0.29 | 7 | 3.23 | 2.77 | −0.11 |
| Nonrural | 12 | 4.23 | 3.21 | 0.31 | 12 | 3.55 | 3.3 | 0.25 | 10 | 2.96 | 2.42 | 1 |
| Symptoms > 3a | ||||||||||||
| Rural | 8 | 2.38 | 3.2 | 0.42 | 7 | 3.57 | 2.07 | −0.15 | 7 | 2.14 | 2.34 | 0.09 |
| Nonrural | 12 | 4 | 4.57 | 0.38 | 12 | 3 | 4.73 | 0.24 | 10 | 2.4 | 3.63 | 0.89 |
| Symptom interference | ||||||||||||
| Rural | 8 | 1.63 | 2.45 | 0.7 | 7 | 1.86 | 1.35 | 0.23 | 7 | 2.14 | 1.86 | −0.12 |
| Nonrural | 13 | 3.69 | 3.43 | 0.15 | 12 | 2.33 | 2.57 | 0.94 | 10 | 1.9 | 2.38 | 0.65 |
| IL-1RA | ||||||||||||
| Rural | 8 | 524.6 | 266.6 | −0.11 | 8 | 403.5 | 196.1 | 0.07 | 7 | 723.8 | 244.1 | 0.41 |
| Nonrural | 13 | 481.7 | 462.1 | 0.3 | 10 | 421.1 | 321.5 | 0.76 | 10 | 993.4 | 867.7 | 0.66 |
| IL-1β | ||||||||||||
| Rural | 8 | 0.31 | 0.58 | −0.21 | 8 | 0.03 | 0.08 | 0.52 | 7 | 0.04 | 0.1 | 0.74 |
| Nonrural | 13 | 0.23 | 0.31 | 0.82 | 10 | 0.21 | 0.49 | 0.35 | 10 | 0.18 | 0.25 | 0.08 |
| IL-6 | ||||||||||||
| Rural | 8 | 9.71 | 13.65 | −0.25 | 8 | 4.14 | 3.23 | 0.4 | 7 | 8.15 | 6.56 | −0.62 |
| Nonrural | 13 | 6.97 | 10.05 | 0.4 | 10 | 9.89 | 20.27 | 0.9 | 10 | 5.14 | 4 | 0.38 |
| TNFα | ||||||||||||
| Rural | 8 | 1.85 | 0.94 | −0.31 | 8 | 2.05 | 1.02 | −0.76 | 7 | 2.05 | 1.07 | −0.31 |
| Nonrural | 13 | 1.47 | 1.44 | 0.09 | 10 | 1.41 | 0.77 | 0.15 | 10 | 1.77 | 0.89 | 0.66 |
| CRPb | ||||||||||||
| Rural | 8 | 12.83 | 19.54 | −0.11 | 8 | 10.23 | 60.71 | 0.17 | 8 | 2.95 | 3.44 | −1.02 |
| Nonrural | 13 | 10.96 | 16.27 | 0.96 | 10 | 12.21 | 16.02 | 0.52 | 10 | 7.44 | 11.21 | 0.07 |
| IL-1β/IL-1RA ratio | ||||||||||||
| Rural | 8 | 0.6 | 1.2 | −0.06 | 8 | 0.12 | 0.35 | 0.4 | 7 | 0.06 | 0.14 | 0.56 |
| Nonrural | 13 | 0.55 | 0.57 | 0.54 | 10 | 0.32 | 0.61 | 0.43 | 10 | 0.27 | 0.48 | 0.06 |
Symptom severity is rated on a 0–10 numeric rating scale ranging from 0 (not present) to 10 (worst imaginable). Scores greater than 3 represent moderate or severe symptom intensity.
CRP values were expressed in mg/dl; all other cytokines values in pg/dl CRP—C-reactive protein; d/p—Cohen’s d for comparisons of rural to nonrural participants at each time point; p value for the Wilcoxon two-sample exact test comparing rural to nonrural participants at each time point; IL—interleukin; TNF—tumor necrosis factor
Note. Because of missing data, some values in the n columns may not equal total N.
TABLE 4.
Symptom Burden, Interference, and Cytokines for Rural and Nonrural Participants at Time Points 4–6
| Variable | Time 4 (N = 15) | Time 5 (N = 12) | Time 6 (N =10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | SD | d/p | n | SD | d/p | n | SD | d/p | |||
| Symptom severity | ||||||||||||
| Rural | 7 | 1.37 | 1.23 | −0.03 | 5 | 1.98 | 1.28 | −0.91 | 4 | 1.73 | 1.65 | −0.8 |
| Nonrural | 8 | 1.33 | 1.79 | 0.44 | 7 | 0.91 | 1.28 | 0.11 | 6 | 0.9 | 0.71 | 0.71 |
| Top 5 symptoms | ||||||||||||
| Rural | 7 | 3.09 | 2.49 | −0.32 | 5 | 4.88 | 3.22 | −1.36 | 4 | 4 | 3.7 | −0.88 |
| Nonrural | 8 | 2.35 | 2.49 | 0.41 | 7 | 1.83 | 1.79 | 0.05 | 6 | 2.03 | 1.3 | 0.71 |
| Symptoms > 3a | ||||||||||||
| Rural | 7 | 1.71 | 2.06 | 0.18 | 5 | 3 | 2.74 | −0.66 | 4 | 2.75 | 3.2 | −0.85 |
| Nonrural | 8 | 2.25 | 3.88 | 0.74 | 7 | 1.43 | 2.51 | 0.56 | 6 | 1 | 1.55 | 0.6 |
| Symptom interference | ||||||||||||
| Rural | 7 | 2.71 | 2.69 | −0.71 | 5 | 3 | 2.74 | −1.58 | 4 | 2.25 | 2.63 | −0.92 |
| Nonrural | 8 | 1.25 | 1.67 | 0.34 | 7 | 0.43 | 0.53 | 0.24 | 6 | 0.83 | 0.75 | 0.72 |
| IL-1RA | ||||||||||||
| Rural | 7 | 627.8 | 417.5 | 0.08 | 5 | 449.7 | 166.1 | 0.26 | 4 | 368.5 | 225.3 | −0.5 |
| Nonrural | 8 | 658.6 | 449.1 | 0.84 | 7 | 523.8 | 381.8 | 0.88 | 6 | 288.6 | 140.32 | 0.76 |
| IL-1β | ||||||||||||
| Rural | 7 | 0.14 | 0.22 | −0.48 | 5 | 0.01 | 0.02 | 0.55 | 4 | 0.44 | 0.49 | 0.83 |
| Nonrural | 8 | 0.07 | 0.1 | 1 | 7 | 0.04 | 0.08 | 0.68 | 6 | 0.89 | 0.67 | 0.38 |
| IL-6 | ||||||||||||
| Rural | 7 | 8.92 | 9.16 | −0.6 | 5 | 15.82 | 24.94 | −0.66 | 4 | 6.86 | 5.8 | −0.48 |
| Nonrural | 8 | 5.19 | 3.76 | 0.84 | 7 | 5.91 | 6.06 | 0.76 | 6 | 4.45 | 5.51 | 0.26 |
| TNFα | ||||||||||||
| Rural | 7 | 4.71 | 1.54 | −0.9 | 5 | 5.3 | 2.8 | −0.84 | 4 | 7.31 | 3.6 | −1.26 |
| Nonrural | 8 | 3.42 | 1.54 | 0.17 | 7 | 3.5 | 2.01 | 0.27 | 6 | 4.55 | 1.36 | 0.48 |
| CRPb | ||||||||||||
| Rural | 7 | 2.56 | 1.79 | 0.29 | 5 | 46.4 | 97.6 | −0.72 | 4 | 2.04 | 1.38 | −0.97 |
| Nonrural | 8 | 5.22 | 12.7 | 0.35 | 7 | 5.7 | 45.44 | 0.88 | 6 | 0.87 | 1.31 | 0.17 |
| IL-1β/IL-1RA ratio | ||||||||||||
| Rural | 7 | 0.13 | 0.19 | −0.35 | 5 | 0.01 | 0.03 | 0.43 | 4 | 0.94 | 1.01 | 1.29 |
| Nonrural | 8 | 0.08 | 0.12 | 0.83 | 7 | 0.04 | 0.09 | 0.84 | 6 | 4.04 | 3.32 | 0.19 |
Symptom severity is rated on a 0–10 numeric rating scale ranging from 0 (not present) to 10 (worst imaginable). Scores greater than 3 represent moderate or severe symptom intensity.
CRP values were expressed in mg/dl; all other cytokines values in pg/dl CRP—C-reactive protein; d/p—Cohen’s d for comparisons of rural to nonrural participants at each time point; p value for the Wilcoxon two-sample exact test comparing rural to nonrural participants at each time point; IL—interleukin; TNF—tumor necrosis factor
Examination of mean severity for the five highest symptoms revealed a distinct pattern for rural and nonrural participants. The mean severity score at baseline for the five highest symptoms averaged 3 (SD = 2.9) for rural and 4.2 (SD = 3.2) for nonrural participants. Starting at T2, mean severity scores for the top five symptoms were increasing for rural participants (to 4 [SD = 3.7], on average, at T6) and declining for nonrural participants (to 2 [SD = 1.3], on average, at T6).
Total number of moderate or severe symptoms at T1 averaged 2.4 (SD = 3.2) for rural participants and 4 (SD = 4.6) for nonrural participants (p = 0.38). Mean symptom interference with life averaged 1.6 (SD = 2.5) for rural participants and 3.7 (SD = 3.4) for nonrural participants (p = 0.15). Toward the end of the study, means for nonrural participants decreased to 0.8 (SD = 0.7). None of the differences between the groups’ symptom burden measures at any time point were statistically significant.
Circulating Proinflammatory Cytokines
The greatest differences between rural and nonrural groups were observed at T3 for CRP, IL-β, and IL-1β/IL-1RA, with rural residents having lower levels than nonrural residents. The mean for CRP was 2.95 mg/dl (SD = 3.44) for rural participants compared to 7.45 mg/dl (SD = 11.22) for nonrural participants (p = 0.07). The mean for IL-1β was 0.04 pg/dl (SD = 0.1) for rural residents compared to 0.18 pg/dl (SD = 0.25) for nonrural residents (p = 0.08). The mean for IL-1β/IL-1RA was 0.27 pg/dl (SD = 0.48) for rural residents compared to 0.06 pg/dl (SD = 0.14) pg/dl for nonrural residents. In addition, TNF-α was higher in rural residents at baseline, with a mean of 1.85 pg/dl (SD = 0.94) compared to 1.47 pg/dl (SD = 1.44) for nonrural residents (p = 0.09). Statistically significant differences in cytokine values were not observed at any other time point.
Discussion
This feasibility study assessed the symptom burden and cytokine relationship in rural and nonrural individuals with advanced cancer during a period of 24 weeks. This study may be the first to explore the relationships among cancer symptoms and cytokines longitudinally in rural residents. It is notable that the majority of research exploring relationships among cancer symptoms and cytokines is conducted in urban, tertiary care centers with a study population that is primarily urban and suburban. The unique experiences and challenges of rural individuals with advanced cancer are not reflected in the symptom and cytokine literature. This study reflects the challenges inherent in including this population in biobehavioral research (Gilbertson-White, Bohr, & Wickersham, 2017). Given how quickly treatment decisions are made and implemented, it can be extremely difficult to obtain baseline data prior to starting cancer treatment. One hundred and forty-five individuals were prescreened and found to be eligible; however, 73% of those individuals were not approached for various circumstances that would affect their ability to participate in all planned study visits. Prescreening occurred prior to obtaining informed consent and no personal health information was collected; therefore, the authors are unable to determine if rural residents were more likely to be in the group of individuals screened but not recruited. It is a reasonable assumption that some individuals in rural areas, particularly those at a greater distance from the tertiary care cancer center, would elect to receive treatment at a local cancer center. More research is needed to determine what factors influence rural residents’ choices to receive cancer treatment at a tertiary care center versus a local cancer center. It is likely that the financial and time commitment of traveling a long distance from rural areas for people with cancer as well as family members can influence decisions about where to receive care. In addition, factors such as insurance coverage, availability of work medical leave, education level, and endorsement of care facility from local providers may affect rural residents’ choices in where they seek care for their cancer.
The dropout rate in this study (50%) reflects the rapidly changing health status of people with advanced cancer. Notably, there was no difference in the rate of dropout for rural and nonrural residents. The number of participants in each category for early dropout were too small to detect a difference between rural and nonrural residents. One participant actively chose to end participation in the study; the remaining 10 all ended participation due to changes in their clinic situation. This suggests that the dropout may be related to the cancer progression rather than rurality. In this sample, it appears that the dropout rate may also be related to the type of cancer (i.e., lung cancer progressing faster than colorectal cancer). More research is needed in a larger sample size with multiple cancer types to determine if rurality interacts with treatment decisions (i.e., are rural residents receiving care at an academic medical center more likely to end treatment sooner than their nonrural peers?). In addition, to more fully understand the experience of rural residents, research needs to move beyond the academic medical center and include individuals who are seeking care at their local cancer clinic.
Rural and nonrural residents were similar at baseline for demographic and clinical characteristics as well as on measures of social support and optimism. Of note, rural residents had lower scores on the measure specific to cancer-related stress (IES-R) and were statistically lower in their avoidance scores. The authors expected to find scores on cancer-related stress to be higher for rural residents given the greater number of stressors they encounter in receiving their cancer care (Hendren et al., 2011). One explanation is that the rural residents in this study are proactive individuals as evidenced by their choice to travel great distances to receive care at an academic medical center. Additional research is needed with individuals receiving care at both academic medical centers as well as community cancer centers to determine if there are differences in cancer-related stress associated between rural and nonrural residents with advanced cancer.
In the initial analysis of symptom burden compared to nonrural participants, rural participants did not have statistically higher overall symptom severity at any time point. However, when the authors evaluated symptom severity more closely by focusing on the five most severe symptoms at each time point, they found that, from T2 onward, rural residents had higher severity scores that did not improve over time. Nonrural residents saw a gradual decline in the mean severity of their five most severe symptoms over time. In addition, an interesting pattern was noted on symptom interference. Although rural participants reported lower symptom interference initially (T1 and T2), the pattern switched at T3, with rural participants reporting higher symptom interference for the remainder of the study. One interpretation is that, for rural participants, the influence symptoms have on their life may become greater over time given the context of cancer treatment for those living in rural areas (e.g., greater travel distance to healthcare providers) (Duggleby et al., 2010) and smaller or more spread out support network (Cacioppo & Cacioppo, 2014; Steptoe, Shankar, Demakakos, & Wardle, 2013; Tittman, Harteau, & Beyer, 2016). Although symptoms seem manageable at first, their interference with life becomes more apparent over time. As a feasibility study with a small sample, the analyses were not adequately powered to detect statistical significance. However, the effect size (e.g., Cohen’s d) for top five severity symptoms increased in magnitude at each time point, suggesting the difference in this sample may represent a clinically meaningful difference between rural and nonrural residents. More research is needed to determine if this is simply an effect of a small sample size or if severity of the worst symptoms for nonrural residents does not improve over time.
Of note, three markers of inflammation (CRP, IL-1β, and IL-1β/IL-1RA) were found to be statistically significantly lower for rural participants compared to nonrural participants at T3. At all other time points, there was no detectable pattern in the cytokine levels over time or when comparing rural to nonrural participants. This result does not support the authors’ hypothesis that rural residents experience more stressors and, subsequently, will have higher levels of circulating inflammatory cytokines. However, given that significant differences in avoidance scores were present in this sample, with rural residents being less avoidant, one explanation is that this group of rural residents manages stress particularly well, which is reflected in their cytokine levels. This finding merits further exploration in a larger sample size, including residents in rural areas not receiving care at an academic medical center.
Limitations
Limitations of this research include the small sample size and recruitment of participants exclusively from an academic medical center rather than clinics located in rural communities. Also, other factors that may have contributed to stress, including care-giving responsibilities and sensory impairments, were not included. This study was not powered to detect statistical differences in symptom burden and cytokines; therefore, the patterns noted can only be viewed as exploratory and in need of additional study. Participation rates were similar across rural and nonrural participants, demonstrating that rural participants are willing to take part in symptom research despite the added burdens they face related to receiving cancer care. In addition, racial and ethnic diversity was limited in this study. Future research is needed that includes various racial and ethnic groups as well as variation in geographical rural areas
Implications for Nursing
For nurses providing care to individuals with advanced cancer living in rural areas, it is important to consider how rurality can influence their cancer care. A thorough assessment of their symptoms, including symptom interference, is needed. In addition, an awareness of how living in a rural area can be an added burden that may contribute to chronic stress can help nurses be mindful of these challenges while providing care. Rural residents who choose to travel great distances to seek care at an academic medical center may represent a unique type of individual with access to supportive resources making that choice possible. Additional research is needed in a larger sample size where factors, such as optimism, perceived stress, and social support, can be included in a multiregression model to better understand inflammatory stress response and symptom development in rural residents with advanced cancer. For researchers conducting studies in a rural setting, this study draws attention to how recruitment is challenging and longevity of participation is dependent on clinical factors, which can change rapidly in advanced disease.
Conclusion
This study is the first to explore differences in symptom burden and cytokine response in rural residents with advanced cancer. Surprising findings include lower scores in rural participants on the avoidance subscale on the measure of the cancer-related stress as well as the similar symptom burden scores and cytokine responses between rural and nonrural participants. These differences merit further research via large-scale longitudinal studies. Research including people with advanced cancer (rural and nonrural) receiving care in community cancer centers as well as at tertiary care centers will provide better insights into the unique experiences of rural individuals with advanced cancer. In terms of feasibility, the major lesson learned in this study is that rural residents who seek care at an academic medical center may not be representative of all rural residents with advanced cancer. In fact, it is likely that they are very different from rural residents who receive care in their local communities. In addition, participation in longitudinal biobehavioral research is highly dependent on complex, ever-changing clinical factors, and research protocols need to take these events into account when designing future studies.
KNOWLEDGE TRANSLATION.
Personality factors, such as avoidance, may play an important role in the development of symptoms in people with advanced cancer.
Differences in inflammatory response between rural and nonrural people with advanced cancer may represent an interaction of biological and psychosocial factors in these populations.
More biobehavioral research is needed to better understand how rurality interacts with stress response and symptom burden in patients with advanced cancer.
Acknowledgments
Funding was provided through the University of Iowa’s Barbara and Richard Csomay Center for Gerontological Excellence (PI: Gilbertson-White) and the University of Iowa Holden Comprehensive Cancer Center (P30 CA086862, PI: G.J. Weiner). No other financial relationships to disclose.
REFERENCES
- Andrykowski MA, Steffens RF, Bush HM, & Tucker TC (2014). Disparities in mental health outcomes among lung cancer survivors associated with ruralness of residence. Psycho-Oncology, 23, 428–436. 10.1002/pon.3440 [DOI] [PubMed] [Google Scholar]
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, … Miaskowski C (2009). Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing, 11, 27–41. 10.1177/1099800409333871 [DOI] [PubMed] [Google Scholar]
- Arondel J, Singer M, Matsukawa A, Zychlinsky A, & Sansonetti PJ (1999). Increased interleukin-1 (IL-1) and imbalance between IL-1 and IL-1 receptor antagonist during acute inflammation in experimental Shigellosis. Infection and Immunity, 67, 6056–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N, Detels R, Quint JJ, Li Q, Gjertson D, & Butch AW (2016). Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine, 84, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, & Croyle RT (2017). Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiology, Biomarkers and Prevention, 26, 992–997. 10.1158/1055-9965.epi-17-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, & Lamkin DM (2013). Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain, Behavior, and Immunity, 30, S48–S57. 10.1016/j.bbi.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris JL, & Andrykowski M (2010). Disparities in mental health between rural and nonrural cancer survivors: A preliminary study. Psycho-Oncology, 19, 637–645. 10.1002/pon.1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, & Cacioppo S (2014). Social relationships and health: The toxic effects of perceived social isolation. Social and Personal Psychology Compass, 8, 58–72. 10.1111/spc3.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnio S, Di Stefano RF, & Novello S (2016). Fatigue in lung cancer patients: Symptom burden and management of challenges. Lung Cancer, 7, 73–82. 10.2147/lctt.s85334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Smith RG, Petronis VM, & Antoni MH (2006). Quality of life among long-term survivors of breast cancer: Different types of antecedents predict different classes of outcomes. Psycho-Oncology, 15, 749–758. 10.1002/pon.1006 [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, … Lee BN (2003). Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer, 97, 2919–2925. 10.1002/cncr.11382 [DOI] [PubMed] [Google Scholar]
- Cohen S (2015). ISEL-12: Basic psychometric information. Retrieved from http://www.psy.cmu.edu/~scohen/scales.html
- Cohen S, & Hoberman HM (1983). Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology, 13, 99–125. 10.1111/j.1559-1816.1983.tb02325.x [DOI] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, & Hoberman HM (1985). Measuring the functional components of social support In Sarason IG & Sarason BR (Eds.), Social support: Theory, research, and applications (pp. 73–94). Dordrecht, Holland: Springer. [Google Scholar]
- Donovan HS (2003). The role of cognitive and emotional representations in cancer symptom management (Doctoral dissertation). University of Wisconsin-Madison, Madison, WI. [Google Scholar]
- Donovan HS, Ward SE, Sereika SM, Knapp JE, Sherwood PR, Bender CM, … Ingel R (2014). Web-based symptom management for women with recurrent ovarian cancer: A pilot randomized controlled trial of the WRITE Symptoms intervention. Journal of Pain and Symptom Management, 47, 218–230. 10.1016/j.jpainsymman.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby WD, Penz KL, Goodridge DM, Wilson DM, Leipert BD, Berry PH, … Justice CJ (2010). The transition experience of rural older persons with advanced cancer and their families: A grounded theory study. BMC Palliative Care, 9, 5 10.1186/1472-684X-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, & Chrousos GP (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences, 966, 290–303. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Lindgren ME, Shapiro CL, & Kiecolt-Glaser JK (2012). Child maltreatment and breast cancer survivors: Social support makes a difference for quality of life, fatigue and cancer stress. European Journal of Cancer, 48, 728–736. 10.1016/j.ejca.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman DR, Morris J, Xiao L, Hassan M, Vadhan S, Overman M, … Wang XS (2017). A predictive model of inflammatory markers and patient-reported symptoms for cachexia in newly diagnosed pancreatic cancer patients. Supportive Care in Cancer, 25, 1809–1817. 10.1007/s00520-016-3553-z [DOI] [PubMed] [Google Scholar]
- Gilbertson-White S, Aouizerat BE, & Miaskowski C (2011). Methodologic issues in the measurement of cytokines to elucidate the biological basis for cancer symptoms. Biological Research for Nursing, 13, 15–24. 10.1177/1099800410379497 [DOI] [PubMed] [Google Scholar]
- Gilbertson-White S, Bohr N, & Wickersham KE (2017). Conducting biobehavioral research in patients with advanced cancer: Recruitment challenges and solutions. Biological Research for Nursing, 19, 481–490. 10.1177/1099800417709529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson-White S, Saeidzadeh S, Yeung CW, Tykol H, & Vikas P (2017). Palliative and supportive interventions to improve patient-reported outcomes in rural residents with cancer Journal of Community and Supportive Oncology, 15, e248–e255. 10.12788/jcso.0348 [DOI] [Google Scholar]
- Girard S, Kadhim H, Larouche A, Roy M, Gobeil F, & Sébire G (2008). Pro-inflammatory disequilibrium of the IL-1 beta/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine, 43, 54–62. 10.1016/j.cyto.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Ha EH, & Cho YK (2014). The mediating effects of self-esteem and optimism on the relationship between quality of life and depressive symptoms of breast cancer patients. Psychiatry Investigation, 11, 437–445. 10.4306/pi.2014.11.4.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WA, Schlenk EA, Kim KH, Hadigan CM, Martino AC, Sereika SM, & Erlen J (2010). Validation of the MOS-HIV as a measure of health-related quality of life in persons living with HIV and liver disease. AIDS Care, 22, 483–490. 10.1080/09540120903207292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren S, Chin N, Fisher S, Winters P, Griggs J, Mohile S, & Fiscella K (2011). Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. Journal of the National Medical Association, 103, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RT, Fong TC, & Cheung IK (2014). Cancer-related fatigue in breast cancer patients: Factor mixture models with continuous non-normal distributions. Quality of Life Research, 23, 2909–2916. 10.1007/s11136-014-0731-7 [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, & Alvarez W (1979). Impact of event scale: A measure of subjective stress. Psychosomatic Medicine, 41, 209–218. [DOI] [PubMed] [Google Scholar]
- Ji YB, Bo CL, Xue XJ, Weng EM, Gao GC, Dai BB, … Xu CP (2017). Association of inflammatory cytokines with the symptom cluster of pain, fatigue, depression, and sleep disturbance in Chinese patients with cancer. Journal of Pain and Symptom Management, 54, 843–852. 10.1016/j.jpainsymman.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Jones D, Zhao F, Fisch MJ, Wagner LI, Patrick-Miller LJ, Cleeland CS, & Mendoza TR (2014). The validity and utility of the MD Anderson Symptom Inventory in patients with prostate cancer: Evidence from the Symptom Outcomes and Practice Patterns (SOAPP) data from the Eastern Cooperative Oncology Group. Clinical Genitourinary Cancer, 12, 41–49. 10.1016/j.clgc.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Hong J, Esie P, Bernstein KT, & Aral S (2017). An illustration of errors in using the P value to indicate clinical significance or epidemiological importance of a study finding. Sexually Transmitted Diseases, 44, 495–497. 10.1097/OLQ.0000000000000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkova J, Walsh D, Rybicki L, Davis MP, Aktas A, Tao J, & Homsi J (2010). Symptom severity and distress in advanced cancer. Palliative Medicine, 24, 330–339. 10.1177/0269216309356380 [DOI] [PubMed] [Google Scholar]
- Koch AT, Love-Homan L, Espinosa-Cotton M, Stanam A, & Simons AL (2015). MyD88-dependent signaling decreases the antitumor efficacy of epidermal growth factor receptor inhibition in head and neck cancer cells. Cancer Research, 75, 1657–1667. 10.1158/0008-5472.CAN-14-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleck TA, & Conley YP (2016). Identification and prioritization of candidate genes for symptom variability in breast cancer survivors based on disease characteristics at the cellular level. Breast Cancer, 8, 29–37. 10.2147/BCTT.S88434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Kwan ML, Neugut AI, Ergas IJ, Wright JD, Caan BJ, … Kushi LH (2013). Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Research and Treatment, 139, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EL, Roesch SC, Malcarne VL, Penedo FJ, Llabre MM, Weitzman OB, … Gallo LC (2014). Validation of interpersonal support evaluation list-12 (ISEL-12) scores among English-and Spanish-speaking Hispanics/Latinos from the HCHS/SOL Sociocultural Ancillary Study. Psychological Assessment, 26, 384–394. 10.1037/a0035248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Conley YP, Mastick J, Paul SM, Cooper BA, Levine JD, … Kober KM (2017). Cytokine gene polymorphisms associated with symptom clusters in oncology patients undergoing radiation therapy. Journal of Pain and Symptom Management, 54, 305–316. 10.1016/j.jpainsymman.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Smith M, Lutgendorf SK, & Sood AK (2010). Impact of stress on cancer metastasis. Future Oncology, 6, 1863–1881. 10.2217/fon.10.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira KG, von Zeidler SV, Lamas AZ, Podestá JR, Sena A, Souza ED, … Bissoli NS (2014). Relationship of inflammatory markers and pain in patients with head and neck cancer prior to anticancer therapy. Brazilian Journal of Medical and Biological Research, 47, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen Ø, Laird B, Aass N, Lea T, Fayers P, Kaasa S, & Klepstad P (2017). The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLOS ONE, 12, e0177620 10.1371/journal.pone.0177620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe M, Burd C, Holder K, & Fields A (2016). Defining rural at the U.S. Census Bureau. Retrieved from https://www.census.gov/library/publications/2016/acs/acsgeo-1.html [Google Scholar]
- Reyes-Gibby CC, Wu X, Spitz M, Kurzrock R, Fisch M, Bruera E, & Shete S (2008). Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncology, 9, 777–785. 10.1016/S1470-2045(08)70197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhondali W, Yennurajalingam S, Chisholm G, Ferrer J, Kim SH, Kang JH, … Bruera E (2013). Predictors of response to palliative care intervention for chronic nausea in advanced cancer outpatients. Supportive Care in Cancer, 21, 2427–2435. 10.1007/s00520-013-1805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richette P, François M, Vicaut E, Fitting C, Bardin T, Corvol M, … Rannou F (2008). A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis. Journal of Rheumatology, 35, 1650–1654. [PubMed] [Google Scholar]
- Rogers HL, Brotherton HT, Plaza SLO, Durán MAS, & Altamar MLP (2015). Depressive and anxiety symptoms and social support are independently associated with disease-specific quality of life in Colombian patients with rheumatoid arthritis. Revista Brasileira de Reumatologia, 55, 406–413. 10.1016/j.rbr.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Sailors MH, Bodurka DC, Gning I, Ramondetta LM, Williams LA, Mendoza TR, … Cleeland CS (2013). Validating the M. D. Anderson Symptom Inventory (MDASI) for use in patients with ovarian cancer. Gynecologic Oncology, 130, 323–328. 10.1016/j.ygyno.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro AH, Hovater M, Hearld KR, Roth DL, Sawyer P, Locher JL, … Ritchie CS (2012). Symptom burden predicts hospitalization independent of comorbidity in community-dwelling older adults. Journal of the American Geriatrics Society, 60, 1632–1637. 10.1111/j.1532-5415.2012.04121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, & Carver CS (1985). Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology, 4, 219–247. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga SL, Knapp J, & Gerszten K (2007). Moderators of interventions designed to enhance physical and psychological functioning among younger women with early-stage breast cancer. Journal of Clinical Oncology, 25, 5710–5714. 10.1200/JCO.2007.11.7093 [DOI] [PubMed] [Google Scholar]
- Sereika SM, & Engberg SJ (2006). Development of standardized sociodemographic and co-morbidity questionnaires. Paper presented at the Sigma Theta Tau International Honor Society of Nursing 17th International Nursing Research Congress, Montreal, Quebec. Retrieved from https://stti.confex.com/stti/congrs06/techprogram/paper_30321.htm [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, & Tannock IF (2008). Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews. Cancer, 8, 887–899. 10.1038/nrc2507 [DOI] [PubMed] [Google Scholar]
- Shi Q, Wang XS, Li G, Shah ND, Orlowski RZ, Williams LA, … Cleeland CS (2015). Racial/ethnic disparities in inflammatory gene single-nucleotide polymorphisms as predictors of a high risk for symptom burden in patients with multiple myeloma 1 year after diagnosis. Cancer, 121, 1138–1146. 10.1002/cncr.29154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Williams SD, Siahpush M, & Mulhollen A (2011). Socioeconomic, Rural-urban, and racial inequalities in US cancer mortality: Part I—all cancers and lung cancer and part II—colorectal, prostate, breast, and cervical cancers. Journal of Cancer Epidemiology, 2011, 107497 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, & Wardle J (2013). Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences of the United States of America, 110, 5797–5801. 10.1073/pnas.1219686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DR, Forsberg CW, Ganzini L, Au DH, Gould MK, Provenzale D, … Slatore CG (2016). Depression symptom trends and health domains among lung cancer patients in the CanCORS study. Lung Cancer, 100, 102–109. 10.1016/j.lungcan.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittman SM, Harteau C, & Beyer KM (2016). The effects of geographic isolation and social support on the health of Wisconsin women. WMJ, 115, 65–69. [PubMed] [Google Scholar]
- U.S. Department of Agriculture. (2005). Rural–urban commuting area codes. Retrieved from https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, … Giralt SA (2008). Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer, 113, 2102–2109. 10.1002/cncr.23820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, … Liao Z (2010). Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, Behavior, and Immunity, 24, 968–974. 10.1016/j.bbi.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Geiger AM, Lu L, & Case LD (2013). Rural-urban disparities in health status among US cancer survivors. Cancer, 119, 1050–1057. 10.1002/cncr.27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LA, Garcia Gonzalez AG, Ault P, Mendoza TR, Sailors ML, Williams JL, … Cortes JE (2013). Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood, 122, 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Gilster A, Winters KA, & Elsea CR (2006). Cancer chemotherapy-related symptoms: Evidence to suggest a role for proinflammatory cytokines. Oncology Nursing Forum, 33, 535–542. 10.1188/06.ONF.535-542 [DOI] [PubMed] [Google Scholar]
- Wood LJ, & Weymann K (2013). Inflammation and neural signaling. Current Opinion in Supportive Palliative Care, 7, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]