Abstract
Thrombospondins are a family of five secreted proteins that have diverse roles in modulating cellular function. Thrombospondins-1 and 2 were identified as matricellular proteins based on their functional roles combined with their transient appearance or accumulation in extracellular matrix at specific times during development and in response to injury or stress in mature tissues. Thrombospondin-1 is a major component of platelet α-granules, which provides a convenient source for purification of the protein. Methods are described to prepare thrombospondin-1 from human platelets in a biologically active form with minimal degradation or contamination with other platelet proteins. A nondenaturing method is described for removing bound transforming growth factor-β1.
Keywords: thrombospondins, thrombospondin-1, transforming growth factor-β, affinity chromatography, heparin, fibronectin, platelets
INTRODUCTION
The thrombospondin (TSP) family in vertebrates consists of five members (Carlson, Lawler, & Mosher, 2008). One subfamily contains the trimeric proteins TSP1 and TSP2, and the second contains the pentameric TSP3, TSP4, and cartilage oligomeric matrix protein (COMP/TSP5). COMP is a constitutive component of bone and cartilage extracellular matrix, whereas TSP1 and TSP2 are generally present in extracellular matrix only at specific stages of development and during tissue remodeling and responses to injury or inflammation. Many cell types synthesize and secrete TSP1 in response to specific growth factors, but TSP1 that accumulates in extracellular matrix at sites of injury also derives from platelets, where TSP1 is a major component of the α-granule proteins. Consequently, platelets are a convenient source for purification of the protein.
Other members of the thrombospondin family have been isolated from natural sources or prepared as recombinant proteins using plasmid or viral constructs expressed in mammalian or insect cells. Full length and truncated forms of TSP2 have been expressed and purified as recombinant proteins (Annis, Murphy-Ullrich, & Mosher, 2006; Bujak, Pretto, Ritz, Gualandi, Wulhfard, & Neri, 2014; Koch, Hussein, Woeste, Grundker, Frontzek, Emons et al., 2011; Mosher, Huwiler, Misenheimer, & Annis, 2002; Oganesian, Armstrong, Migliorini, Strickland, & Bornstein, 2008). TSP3 has been prepared as a recombinant protein (Chen, Aeschlimann, Nowlen, & Mosher, 1996; Qabar, Derick, Lawler, & Dixit, 1995). Full length and truncated forms of TSP4 have been studied using expression plasmids and baculovirus and adenovirus vectors (Brody, Schips, Vanhoutte, Kanisicak, Karch, Maliken et al., 2016; Misenheimer & Mosher, 2005; Narouz-Ott, Maurer, Nitsche, Smyth, & Paulsson, 2000; Yang, Ma, Ju, Zhang, Li, Zhang et al., 2016). Published methods are available for isolation of COMP from cartilage and preparation of purified recombinant COMP (Happonen, Saxne, Aspberg, Morgelin, Heinegard, & Blom, 2010; Haudenschild, Hong, Yik, Chromy, Morgelin, Snow et al., 2011; Holden, Keene, Lunstrum, Bachinger, & Horton, 2005; Rosenberg, Olsson, Morgelin, & Heinegard, 1998).
Although some fragments of TSP1 have been expressed in E. coli (Klenotic, Page, Misra, & Silverstein, 2011; McDonald, Dimitry, & Frazier, 2003; Miao, Seng, Duquette, Lawler, Laus, & Lawler, 2001), these proteins lack important posttranslational modifications of platelet TSP1 including Asn-linked complex carbohydrates (Furukawa, Roberts, Endo, & Kobata, 1989), O-GlcNAc modification of the EGF-like repeats (Hoffmann, Liu, & Mosher, 2012), and C-mannosylation of Trp and O-glucosyl-β1–3fucosylation of Ser/Thr residues in the type 1 thrombospondin repeats (TSRs) (Heinonen & Maki, 2009; Hess, Keusch, Oberstein, Hennekam, & Hofsteenge, 2008; Hofsteenge, Huwiler, Macek, Hess, Lawler, Mosher et al., 2001; Leonhard-Melief & Haltiwanger, 2010). These posttranslational modifications can alter specific biological functions of TSP1 (Ihara, Manabe, Ikezaki, Inai, Matsui, Ohta et al., 2010; Muroi, Manabe, Ikezaki, Urata, Sato, Kondo et al., 2007). These native glycosylations of TSP1 can only be achieved in eukaryotic cells. Recombinant TSP1 can be expressed in mammalian cells, but purification from this source can be complicated by the strong interaction between TSP1 and fibronectin, and in our experience denaturing conditions are required to remove the major fibronectin contaminant. Others have succeeded to prepare full length and truncated forms of recombinant TSP1 using baculovirus constructs in insect cells (Mosher et al., 2002). These preparations have been valuable for structural analysis and mapping functional domains and antibody recognition sites (Annis et al., 2006; Carlson et al., 2008).
In addition to its specific interaction with fibronectin, other platelet-associated proteins often co-purify with TSP1, including fibrinogen, histidine-rich glycoprotein (Silverstein, Leung, Harpel, & Nachman, 1985), plasminogen (Silverstein et al., 1985), vitronectin (Sun & Mosher, 1990), platelet-derived growth factor (Hogg, Hotchkiss, Jimenez, Stathakis, & Chesterman, 1997), and TGFβ1 and its latency-associated peptide (Murphy-Ullrich & Poczatek, 2000).
Procedures for purification of TSP1 from platelets typically include affinity chromatography utilizing its binding to ligands such as heparin, fibrinogen or polyhistidine (Lawler, Slayter, & Coligan, 1978; Santoro & Frazier, 1987; Tuszynski, Srivastava, Switalska, Holt, Cierniewski, & Niewiarowski, 1985; Vanguri, Wang, Godyna, Ranganathan, & Liau, 2000). Combining affinity chromatography with gel filtration, ultracentrifugation, or ion exchange chromatography, yields TSP1 in a relatively pure form (Clezardin, McGregor, Manach, Robert, Dechavanne, & Clemetson, 1984; Lawler et al., 1978; Roberts, Cashel, & Guo, 1994; Santoro & Frazier, 1987).
For some functional studies trace amounts of specific contaminants in TSP1 purified using these methods may pose problems. Choice of an appropriate method to purify TSP, therefore, must include consideration of which functional activities of TSP1 are critical for the planned studies and which potential contaminants are likely to interfere with assessing the activity of TSP1 in a specific context.
Contamination with adhesive proteins such as fibronectin or vitronectin is easily detected and can be avoided (Sun & Mosher, 1990). Contamination with bioactive levels of TGFβ, however, is more difficult to detect. One method to remove the associated TGFβ from TSP1 of utilizes gel filtration at high pH (Murphy-Ullrich, Schultz-Cherry, & Höök, 1992). In this method, the gel filtration column is equilibrated in Tris-HCl, 150 mM NaCl with the pH raised to 11, well outside the buffering capacity of Tris. There is likely a transient rise in pH exposure as TSP1 elutes from this column at ~ pH 8–9. However, such alkaline conditions rapidly degrade disulfide bonds of proteins (Florence, 1980). Because several biological activities of TSP1 depend on the state of its cysteine disulfides (Roberts, Haverstick, Dixit, Frazier, Santoro, & Ginsburg, 1985; Speziale & Detwiler, 1990; Sun, Skorstengaard, & Mosher, 1992), alkaline-stripped TSP1 may lack some relevant biological activities of the native protein. However, a less harsh gel filtration method for removing TGF-β from platelet TSP1 was recently reported (Lu, Pallero, Lei, Hong, Yang, Suto et al., 2016). This method performs the gel filtration step in Tris-HCl buffer at pH 7.4 with 800 mM NaCl. The use of high salt had been shown to effectively dissociate TGFβ from fibrillin-1 fragments (Kaur & Reinhardt, 2012). The high salt purification results in less aggregation of TSP1 (as measured by light scattering at 320 nm absorbance) than with high pH purification.
Maintenance of its bound divalent cations is also critical in purification of fully bioactive TSP1. The Ca-repeats of TSP1 cooperatively bind 26 calcium ions, and binding of Ca2+ induces profound conformation changes in the TSP1 molecule (Kvansakul, Adams, & Hohenester, 2004; Lawler & Simons, 1983; Misenheimer & Mosher, 1995; Vuillard, Clezardin, & Miller, 1991). These conformation changes strongly alter the functional activities of TSP in several assays and alter the sensitivity of TSP1 to degradation by certain proteases (Calzada, Kuznetsova, Sipes, Rodrigues, Cashel, Annis et al., 2008; Lawler & Simons, 1983; Misenheimer & Mosher, 1995). Chelation of divalent cations could potentially inhibit proteolysis of TSP1 by calcium-dependent proteases, but the changes in TSP1 conformation induced by chelation of divalent cations may not be completely reversible by their re-addition (Vuillard et al., 1991). Calcium binding is also critical for preventing isomerization of disulfide bonds by protein disulfide isomerase, which is also released during platelet activation (Chen, Lin, & Detwiler, 1992; Hotchkiss, Chesterman, & Hogg, 1996; Hotchkiss, Matthias, & Hogg, 1998; Huang, Detwiler, Milev, & Essex, 1997). Several studies have documented that disulfide bond isomerization catalyzed by this enzyme can alter specific biological functions of TSP1, including exposure of its RGD to integrin binding (Hotchkiss et al., 1998), and covalent or noncovalent interactions of TSP1 with other bioactive proteins released following degranulation of platelets (Hogg et al., 1997; Milev & Essex, 1999). Therefore, achieving optimal biological activity and minimal proteolysis requires maintaining TSP1 in a calcium-replete state throughout its purification.
The following methods for preparation of calcium replete TSP1 employ a combination of positive and negative affinity chromatography. The heparin-binding activity of TSP1 enables positive affinity purification, and negative affinity chromatography on immobilized gelatin removes contaminating fibronectin (Roberts et al., 1994; Santoro & Frazier, 1987). Subsequent size exclusion chromatography removes low molecular weight contaminants and yields electrophoretically homogeneous TSP1.
1. PREPARATION OF THROMBOSPONDIN-1 FROM HUMAN PLATELETS
1.1. Overview
Freshly isolated washed human platelets from healthy donors are activated using thrombin to induce secretion of their α-granule contents, of which TSP1 is a major component. Low speed centrifugation is used to remove aggregated platelets and high speed centrifugation to remove insoluble particulates. The clarified releasate is subjected to negative affinity column chromatography on gelatin-agarose to remove fibronectin followed by positive affinity chromatography on heparin-agarose with stepwise salt elution to resolve TSP1 from other heparin-binding proteins and concentrate the crude TSP1 preparation prior to size exclusion column chromatography (Roberts et al., 1994; Santoro & Frazier, 1987). TSP1 isolation in this manner generally results in yields of 3 to 5 mg from a typical donor, corresponding to approximately 1 mg per unit of platelets (3 × 1011 platelets/unit (Anstall & Urie, 1986)). Donor platelet counts vary, and occasionally platelet rich donors have resulted in yields upwards of 9 mg of TSP1. The same procedure has been used to isolate TSP1 from bovine platelets with yields exceeding 10 mg, and the method can be scaled down to isolate microgram amounts of TSP1 from rodent platelets.
For investigators without access to onsite platelet pheresis facilities, TSP1 can also be purified from pooled, random donor platelet units purchased from the American Red Cross. Typically, fresh units are purchased, prior to outdate, to minimize loss of platelet alpha granule contents. However, because of cost and availability considerations in recent years, we have successfully isolated 5–6 mg TSP1 from releasates from 20 pooled donor units obtained within 1–2 days following outdate. The cost is considerably less than for fresh platelets, and availability is also increased. It is very important to maintain platelet units at room temperature prior to thrombin-stimulated platelet degranulation to avoid premature degranulation.
TSP1 is sensitive to protease cleavage that preferentially removes the N-terminal heparin-binding domain, which can compromise the affinity purification on heparin. Limited cleavage of this domain from one subunit leaves a trimer with two intact heparin-binding domains that will bind to the heparin column and show a minor 120 kDa band on SDS gels after purification (Fig. 1). Inclusion of phenylmethylsulfonyl fluoride effectively inhibits serine proteases, but platelets also contain calcium-dependent proteases that degrade TSP1. Because calcium is used at all steps to maintain TSP1 in its native conformation, performing all steps at 4° C and minimizing processing time is critical to limit Ca-dependent proteolysis. Although this procedural method is straightforward, minimizing the purification time requires careful planning. To ensure optimum TSP1 quality, isolation is preferably completed from obtaining fresh platelets to storage of the purified TSP1 aliquots within one 12–14 hour working day.
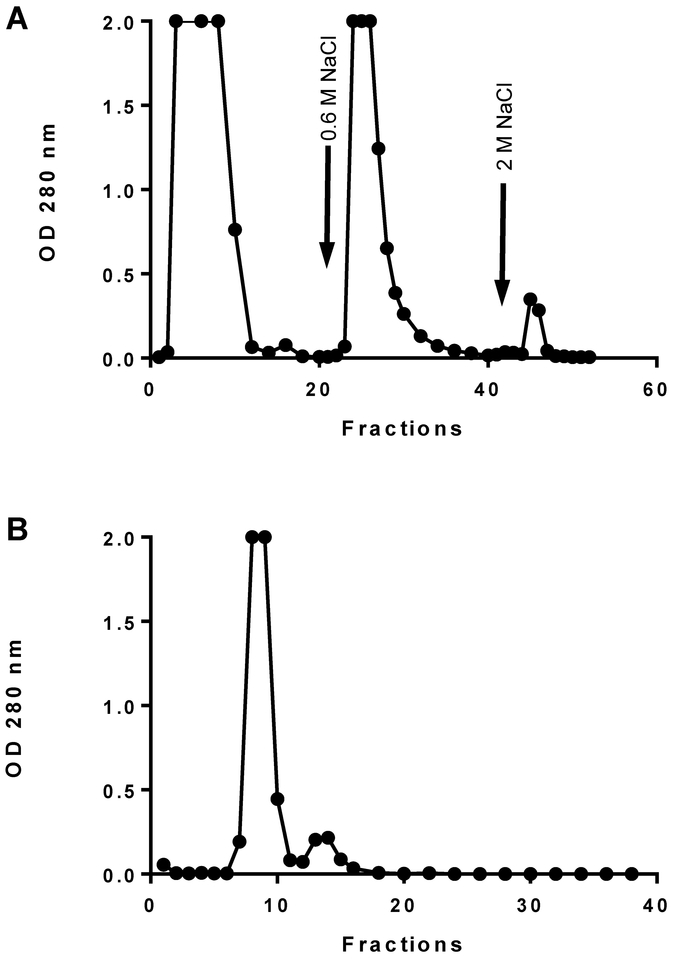
Figure 1.
A, Heparin affinity chromatography of platelet releasate fraction unbound on gelatin agarose. Thrombospondin elutes in the 0.6 M NaCl fraction. B, Gel permeation chromatography of 0.6 M NaCl, heparin-bound fraction on Sephacryl S-300. Thrombospondin elutes in the first peak.
1.1.1. Platelet handling and washing
IRB approval must first be obtained to acquire platelets from deidentified healthy donors. Fresh 7 pass platelet apheresis product is obtained from the Blood Bank immediately following donation. The collection bag containing the platelet suspension in plasma must be gently rocked at room temperature before processing to avoid platelet clumping. Addition of anticoagulant acidified citrate-dextrose (ACD) buffer prevents inadvertent platelet activation. The ACD diluted platelet plasma supernatant from the first cell washing step can be retained and used for plasma fibronectin isolation if desired (Akiyama & Yamada, 1985). For purchase of platelets from the American Red Cross, a yearly IRB approval is needed.
Centrifugation and washing of the platelets is done gently to minimize shear that will damage or activate the platelets. However, we have never observed platelet activation in the washing stages. After dilution of the platelets in one sixth volume of ACD, an aliquot of the platelet rich plasma is counted and to assess the required size for the heparin column.
After suspension in the activation buffer platelets become much more susceptible to premature α-granule release, and proper care to avoid stress must be taken. Problems in the past have included premature platelet activation during addition of concentrated Tris to adjust the pH, and resistance to activation after addition of thrombin. Accurate counting of the platelets units helps to ensure proper dilution into Platelet Activation Buffer, which will minimize the platelet releasate gelling following thrombin activation and is useful to prepare the proper size affinity columns.
Activation of platelets at 0.5 units of thrombin per ml will result in a rapid and massive platelet granular release that will be clearly visible as the platelets form a dense aggregate. In the last 20 years, the platelets were non-responsive to thrombin only once, and ionomycin at ~ 100 nM was used successfully to induce their aggregation.
Following platelet activation care must be taken to keep the platelet α-granule releasate on ice and to ensure that all columns, centrifuges and buffers are at 4°C prior to use. The activated platelets are centrifuged at 18,000 x g to remove platelet aggregate debris. A high platelet yield combined with use of an insufficient volume of activation buffer can result in gelling of the TSP1-containing platelet supernatant during high speed centrifugation. This will not affect the final yield provided that the gel is solublized by addition of buffer and gently swirling the tube in warm water.
1.1.2. Preparation for sequential negative and positive affinity column
The tandem gelatin/heparin column setup is crucial for preparing the crude TSP1 sample for the size-exchange column. Column resin volumes have been optimized at 5 ml of gelatin-agarose and 4 ml of heparin-agarose for 8 units of platelets, and can be scaled proportionally for smaller or larger platelet quantities. Note that our experience has shown batch to batch variation in the TSP1 binding capacity of the heparin affinity matrix. Examination of the flow through to detect unbound TSP1 should be performed when first using a new lot.
The upper gelatin-agarose and lower heparin-agarose are connected by ~ 30 cm of 1.19 mm I.D. polyethylene tubing. Flow rates between the two columns can vary, and the excess tubing enables control of the relative hydrostatic pressure to account for any flow differences.
The cell-free platelet release is applied to the tandem columns so that the sample is loaded into the gelatin-agarose column within one to two hours (~24 ml/hour = 0.4 ml/min). After the sample has passed into the gelatin column, apply 3–4 column volumes of Column Buffer to ensure all the TSP1 has entered into the heparin column. The gelatin column can then be disconnected and capped for cleaning and reuse.
The heparin column is washed with the column buffer until the absorbance at 280 nm of the flow-through drops to background (Fig. 1A). Our original protocol called for the use of a Column Buffer containing an intermediate 0.3 M NaCl concentration (Roberts et al., 1994). However, using inspection of the 0.3 M NaCl fractions from recent lots of heparin-agarose showed elution of some TSP1 at this salt concentration. Currently we are using only 0.6 M NaCl Column Buffer to directly elute the TSP1 containing fractions. Care should be used to elute the protein peak from the heparin column in the minimum volume possible to apply to the size exclusion column. The pooled fractions are typically between 4 to 5 ml and ~1.0 to 1.6 absorbance at 280 nm. Further step or gradient elution up to 2 M NaCl can be used to recover platelet factor-4 from the heparin column (Fig. 1A).
Size exclusion chromatography
The post heparin-agarose pooled fractions are careful applied to the size exclusion column after removing any Column Buffer from the head of the 1.9 I.D. x 90 cm Sephacryl S300 column. Care must be employed so that the resin does not dry and crack while also ensuring enough Column Buffer has been removed to keep the sample from being diluted. Apply the sample gently so not to disturb the column packing. The moment the entire sample completely enters the resin, use a small volume (~1 ml) of Column Buffer to rinse the head space of the column. At this point slowly add Column Buffer to create a head volume large enough not disturb the resin packing, and connect a reservoir to the column with one liter of cold Column Buffer. Sample fractions are collected at 2 ml per tube for the first 20 tubes, followed by 0.5 ml per tube until the first major peak is detected by absorbance at 280 nm (Fig. 1B).
Typical peak elution volumes will be expected between 42–57 ml with a flow rate of ~16.8ml/hour. During the 2–3 hours awaiting elution from the Sephacryl column, clean up and tube labeling is recommended. Column fractions will be pooled based on absorbance at 280 nm, recording the total volume and pooled OD280 nm. Currently our lab practice is to separate the fractions into high or low concentration pools. Addition of 20% w/v sucrose to the TSP1 pools must be done slowly to prevent precipitation of the purified TSP1. After the addition of sucrose, the sample will be remeasured for absorbance at 280 nm and volume and labeled as the final TSP1 concentration. Note the extinction coefficient for TSP1 is 0.9 absorbance at 280 nm per 1 mg/ml (Margossian, Lawler, & Slayter, 1981).
Purified TSP1 yields are typically around 0.25 and 0.55 mg/ml at 4 to 5 ml for the low and high pooled fractions. Typical expected yields are 0.5 to 1 mg of TSP1 per unit of platelets. TSP1 is aliquoted in small volumes to minimize freezing-thawing of samples, typically at 10 and 20 μl per tube. Tubes are flash frozen on a dry ice/ethanol bath and stored at −70°C.
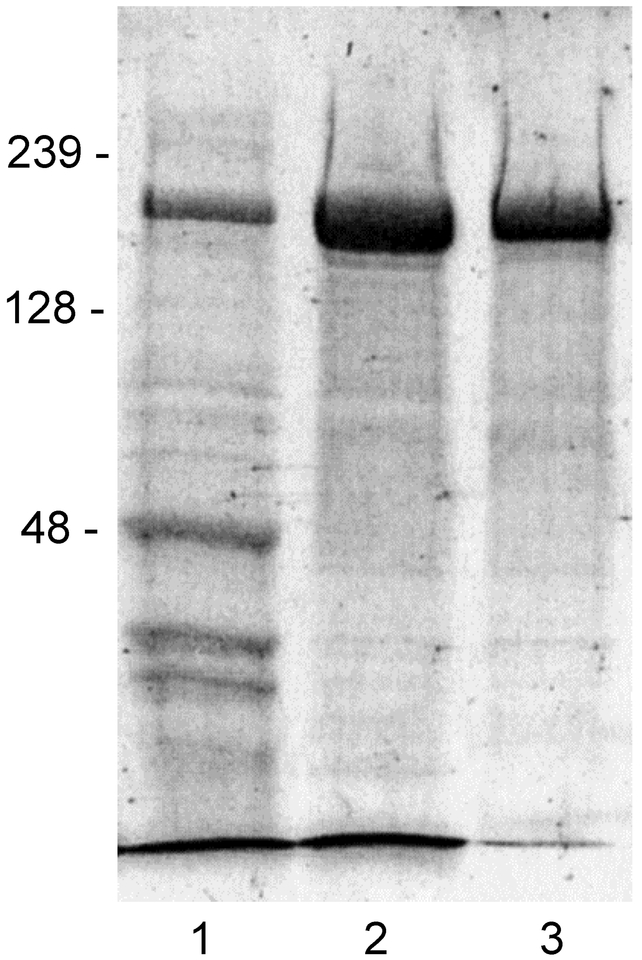
To monitor the quality of this protein isolation, 40 μl samples will be taken at the following steps and evaluated by SDS-PAGE; crude cell-free platelet releasate (pre-gelatin/ heparin columns), heparin flow thru, heparin 0.6 M NaCl pooled fraction (post heparin) and size exclusion column pools. Proteolysis is indicated by appearance of a second band at 140 kDa and free heparin-binding domain which runs at the dye front in 7% gels but can be visualized on a 12% gel (Fig. 2).
Figure 2.
SDS gel electrophoresis analysis of crude and purified TSP1. 7.5% SDS-PAGE gel with 10 μl of β-mercaptoethanol treated sample/lane: 1) crude releasate, 2) eluate from heparin column, 3) Sephacryl column eluate. Gel was stained using Coomassie brilliant blue, and protein marker migrations are indicated in kDa.
1.2. Protocol
1.2.1. Materials and Reagents
1.2.1.1. Equipment
UV/VIS spectrophotometer (Beckman DU640 or equivalent)
37 °C water bath
pH meter
High speed centrifuge (Sorvall RC5)
Fixed angle rotor (Sorvall SS34, No. 280204)
Swinging Bucket centrifuge (Sorvall Legend XTR or equivalent)
Fraction collector (Gilson micro fractionator FC-80K5)
Pipetmen, P-10, P-200, P-1000
Two chromatography columns: = 1 × 10 cm (Bio-Rad)
One chromatography column = 1.5 × 90 cm (Amersham)
Cold room or refrigerated chromatography cabinet
Pipet aid (Drummond)
1.2.1.2. Supplies
Borosilicate glass tubes, 13 × 100 mm
Polycarbonate centrifuge tubes (Nalgene Oak Ridge High-Speed Polycarbonate Centrifuge Tubes w/Sealing Cap No. 031464)
Disposable pipets, 10 ml
Graduated cylinder, 500 ml
0.65 ml slick tubes: PGC # 505–195
Intramedic polyethylene tubing 1.19 mm ID
7.5% acrylamide SDS gels
1.2.1.3. Column matrix
Heparin-agarose (Sigma H-6508)
Gelatin-agarose (Sigma G-5384)
Sephacryl S 300 high resolution (GE Healthcare Life Sciences)
1.2.1.4. Chemicals
1 M Tris base: (12.1 g/100 ml distilled deionized water)
Hydrochloric acid (12 N HCl)
Leupeptin
Phenylmethylsulphonyl fluoride (PSMF, 100 mM stock in isopropanol)
Potassium hydrogen phosphate trihydrate (K2HPO4· 3H2O
Sodium phosphate dibasic heptahydrate (Na2HPO4 ·7H2O)
Sodium phosphate monobasic monohydrate (NaH2PO4·H2O)
Sodium chloride
Sucrose, ultrapure
Calcium chloride
6-aminohexanoic acid
Benzamidine hydrochloride
Urea, ultrapure
Glucose
Thrombin
Hirudin
Citric acid
Sodium citrate
Isopropanol
Sodium azide (NaN3)
1.2.1.5. Solutions to be prepared
Buffers:
ACD (acid-citrate-dextrose): 40 mM citric acid, 85 mM sodium citrate, 136 mM glucose, pH 7.4
8 g of Citric acid (MW 192.12)
22g of Sodium citrate (MW 258.06)
24.5 g of Glucose (MW 180.16)
pH and make to 1 liter
PWB (Platelet Washing Buffer): 4.3 mM K2HPO4-3H2O, 4.3 mM K2HPO4-3H2O, 24.4 mM NaH2PO4-H2O, 113mM NaCl, pH 6.5
0.98 g of K2HPO4· 3H2O (MW 228.22)
1.15 g of Na2HPO4· 7H2O (MW 268.07)
3.37 g of NaH2PO4· H2O (MW 137.99)
6.6 g of NaCl (MW 58.44)
1 g of 5.5 mM Glucose (MW 180.16)
pH and make to 1 liter
PAB (Platelet Activation Buffer) – 20 mM Tris base, 150 mM NaCl, 0.1mM CaCl2-2H2O, 5.5 mM glucose, pH 7.6
2.43 g of Tris base (MW 121.14)
8.76 g of NaCl (MW 58.44)
14.7 mg of CaCl2· 2H2O (MW 147.0)
1 g of M Glucose (MW 180.16)
pH and make to 1 liter
CB (Chromatography Buffer) – 20 mM Tris base, 150 mM NaCl, 0.1 mM CaCl2-2H2O, pH 7.6
2.43 g of Tris base (MW 121.14)
8.76 g NaCl (MW 58.44)
14.7 mg CaCl2· 2H2O (MW 147.0)
pH and make to 1 liter
Elution Buffer- Chromatography Buffer with 600 mM NaCl– 20 mM Tris base, 600mM NaCl, 0.1 mM CaCl2-2H2O, pH 7.6
Add 2.63 g NaCl to 100ml of Chromatography Buffer as prepared above
Column storage and conditioning buffer – To 1 liter of Chromatography Buffer add 0.02% w/v NaN3 (0.2 g), 1 mM Benzamidine hydrochloride (157 mg), 10 mM 6-aminohexanoic acid (1.31 g), and 0.1 mM PMSF (1 ml of 100 mM stock in isopropanol).
For conditioning a new column, the same buffer is prepared and made to 1 % w/v with bovine serum albumin.
Note: 0.1 mM CaCl2 is the optimal concentration since higher calcium concentrations can result in TSP-1 retention on the gelatin column
Heparin-Agarose column regeneration buffer:
2 M NaCl: Chromatography Buffer supplemented with 1.85 M NaCl (add 10.8 g NaCl to a final volume of 100 ml)
Gelatin-agarose regeneration buffer:
4 M urea: Chromatography Buffer supplemented with 4 M urea (add 24 g urea to 50 ml buffer and bring to final volume of 100 ml)
1.2.2. Methods
1.2.2.1. Preparation and Washing of Fresh Human Platelets
Prepare and have ready the following items at room temperature:
Swinging Bucket centrifuge (Sorvall Legend XTR)
40 ml tubes and caps
500 ml graduated cylinder
ACD buffer
PWB
Biological waste trash
Pipets
Platelet counting hemocytometer (Petroff-Hausser Bacteria Counter, 1/400 sq mm, 1/50 mm deep made by C.A. Hausser & Son, Philadelphia, PA, USA)
Microscope
Measure the volume of the platelet suspension in a 500 ml graduated cylinder.
Add one sixth volume of room temperature ACD and record the final volume.
Mix by gentle inversion of the ACD platelet suspension.
Remove a small aliquot, dilute ~100x and count the units of platelets to calculate the total units of platelets. (One unit = 3 × 1011 platelets)
Divide equally into ~ eight 40 ml capped plastic tubes centrifuge tubes
Centrifuge at 1200 x g for 20 minutes at 20°C.
The plasma supernatant is removed and can be saved for fibronectin isolation.
Any discarded platelet supernatant should be treated as biological waste.
Gently suspend the platelet pellet in 25 ml of platelet washing buffer “PWB” by trituration
Resuspend platelets in six centrifuge tubes.
Centrifuge the suspensions at 1200 x g for 15 minutes at room temperature.
Remove and discard the supernatant buffer.
Gently resuspend the pellets in 10 ml of PWB by trituration and place into 6 tubes.
Add additional PWB buffer to fill each tube and mix by trituration.
Centrifuge the tubes as above and discard the supernatant.
1.2.2.2. Activation of Platelets
The method that follows is based on 1.5 units of platelets per 12 ml tube and should be adjusted to suit the available units of platelets.
Prepare and have ready the following items:
High speed centrifuge (Sorvall RC5) and SS-34 rotor at 4°C
pH meter
Ice bucket
1M Tris base
100 U/ml Thrombin
500 Units/ ml Hirudin
500 μg/ml Leupeptin
100 mM PMSF
37 °C water bath
PAB
Suspension of washed platelets for thrombin activation:
Gently resuspend the washed platelet pellets at 1.5 unit per 12 ml in PAB.
Slowly adjust suspensions pH to 7.6–7.9 with addition of small volumes of 1 M Tris base
Use ~50 μl tris to begin with, followed by 10 μl aliquots
Immediately transfer the suspension to the 37° water bath and gently swirl for 1 to 2 minutes to warm.
Platelet activation:
Add thrombin to a final concentration of 0.5 U/ml
(60 μl of 100 U/ml thrombin per tube)
Continue gentle agitation for two minutes
At this step, you will see a massive platelet aggregation
Transfer each activated tube to ice and stop reaction by addition of final concentrations:
2 mM PMSF = 240 μl
10 μg/ml of leupeptin = 240 μl
2 U/ml of hirudin = 48 μl
Mix and chill on ice without agitation.
From this point on the sample needs to be kept at cold
1.2.2.3. Preparation of Cell-Free Supernatant
Centrifuge the suspensions at 3640 x g for 5 min at 4° C
Transfer the supernatant to clean centrifuge tubes and centrifuge at 40,000 x g for 55 minutes at 4° C.
Combine and place the supernatant into a 50 ml tube and place on ice.
If the platelet concentration is unusually high, the solution sometimes gels during this centrifugation. This can be reversed by warming the tube to 15–20 °C and diluting if needed.
Save a 40 μl aliquot to analyze by SDS-PAGE gel
Apply to the coupled gelatin and heparin affinity columns.
1.2.2.4. Tandem Affinity Gelatin and Heparin Columns
The gelatin and heparin columns are connected in series in a cold room so that the sample passes first through the gelatin-agarose column then through the heparin-agarose column. Pre-equilibrate the columns with Column Buffer during centrifugation of the platelet supernatant. The two columns are connected so that fractions are collected in 13 × 100 mm tubes in the fraction collector. It is important to get the sample out of these volumes in as small a volume (4 ml) as possible.
Prepare and have ready the following items:
Tandem affinity columns
UV/VIS spectrophotometer
Fraction collector
CB
Elution Buffer
50 ml
15 ml capped tubes
Clamp the pooled supernatant in 50 ml tube above the tandem columns and connect by syphon through 1.9 mm tubing
Adjust the hydrostatic pressure so the solution loads within 1–2 hours.
To test for protein not retained by gelatin-heparin columns, collect and save a 40 μl aliquot of the sample flow-through.
After the supernatant reservoir is empty, elute the tandem columns with ~15 to 20 ml of CB to clear all the TSP into the lower column.
Remove the gelatin-agarose column, capping and storing to recondition and reuse.
Continue eluting the heparin-agarose column with CB until the OD280 nm falls below 0.04.
Remove the reservoir and allow the remaining buffer in the heparin-agarose column to drain into the column.
Immediately add 1 ml of Elution Buffer and allow it to drain into the column bed.
Apply an additional 1 ml of Elution Buffer to the column and connect a reservoir using the same Elution buffer, collecting 0.5 ml fractions.
Continue eluting until the TSP1 peak is eluted.
Measure and pool the peak containing fractions recording combined volume and OD280 nm
Cap and store the heparin-agarose column to recondition and reuse.
Remove and save a 40 μl aliquot to analyze by SDS-PAGE gel
1.2.2.5. Size Exclusion Chromatography
The 1.9 I.D. x 90 cm Sephacryl S300 column should be pre-equilibrated overnight in cold CB and be connected by ~75 cm of 1.19 mm ID polyethylene tubing to a one liter CB reservoir.
Prepare and have ready the following items:
CB
Fraction collector
13×100 mm Borosilicate glass tubes
15 ml capped tubes
Sucrose
0.65 ml Slick tubes (~200–400 labeled as TSP1)
Disconnect the reservoir at the column head and remove almost all CB.
At the point the column begins to just absorb the last of the CB, carefully apply the sample gently so not to disturb the column packing.
The moment the sample completely enters the column resin, add a small volume (~1ml) of CB to ease any remaining sample into the column.
Slowly add CB to create a head volume large enough not disturb the resin packing with dripping and reconnect the CB reservoir.
Elute the column, collecting 2 to 0.5 ml fractions. The first major peak detected by UV absorbance, which should elute in the first 40–60 ml, contains the TSP1 and will be pooled.
1.2.2.6. Pooling and Storage of Thrombospondin-1
Combine the peak fractions into 15 ml capped tubes and measure the total volume and OD280.
Typically, we divide the peak fractions into two pools based on their OD280 nm for a “high” and “low” pools.
Slowly add ultrapure sucrose to a final concentration of 20% w/v.
Dissolve the sucrose by slowly rolling of the tubes to avoid precipitation of the TSP1
Remeasure the total volume and OD280 nm now that sucrose has been added.
Typical yields are 0.5 to 1 mg of TSP1 per unit of platelets.
Remove and save a 40 μl aliquot for SDS-PAGE gel
Divide the TSP1 pool into small aliquots, flash freeze and store at – 70°C.
1.2.2.7. Analysis and Clean-Up
Aliquots collected for purification should be analyzed on a 7.5% polyacrylamide SDS gel for purity and proteolysis. Use SDS-loading buffer with β-mercaptoethanol. Proteolysis is indicated by appearance of a second band at 140 kDa and free heparin-binding domain which runs at the dye front in 7% gels but can be resolved on a 12% gel.
All columns can and should be saved and reused. The gelatin-agarose column is regenerated with CB containing 4 M urea to elute bound fibronectin. The heparin-agarose column is regenerated with CB containing 2 M NaCl. The gelatin column will exhibit reduced flow after repeated use and should be replaced as required. Most critical is to retain and reuse the Sephacryl column because a good working size-exclusion column will last years and produce higher yields than a newly poured column. This column is washed until OD280 nm is at background. All three columns are stored long term in column storage buffer at 4°C
2. NONDENATURING REMOVAL OF TGFβ
2.1. Purification of TGFβ-depleted Thrombospondin-1.
This protocol is a modification of a method developed in the Deane Mosher lab and recently described as an effective method to dissociate TSP1 and TGFβ (Bale, Westrick, & Mosher, 1985; Lu et al., 2016). Initially, we used a high pH method as described above; however the current high salt method yields TSP1 preparations with reduced aggregation and associated TGFβ (Murphy-Ullrich et al., 1992). Typically, we use either 10 units of fresh, pooled random donor platelets or 20–30 units of recently expired platelets. Yields from random donor platelet units processed within 1–2 days of expiration can vary, but we typically obtain 6–8 mg TSP1 from 30 units of outdated platelets. Purification of TSP1 from platelet releasates involves a two-step process utilizing heparin affinity chromatography and gel permeation chromatography under high salt (0.8 M NaCl) conditions.
2.2. Buffers and reagents:
2.2.1. Platelet wash buffers and reagents for α-granule release
Acid Citrate-dextrose (ACD), pH 4.6
Make it no more than 1 day before use.
To make 500 ml;
| Dextrose | 9.9 g | 0.1 M |
| Sodium Citrate (MW 258.1) | 12.45 g | 0.1 M |
| Citric Acid (MW 192.1) | 6.7 g | 0.07 M |
pH 4.6 with 1 N NaOH
Hepes wash buffer, pH 7.6
To make 2 L;
| ACD | 200 ml of stock from above | 10% |
| NaCl | 17.52 g | 0.15 M |
| Hepes | 23.82 g | 0.05 M |
| Dextrose | 1.8 g | 5 mM |
pH 7.6 with 10 N NaOH
Hepes resuspension buffer, pH 7.6
To make 100 ml;
| Hepes | 2.38 g | 0.1 M |
| NaCl | 0.86 g | 0.15 M |
| Dextrose | 0.08 g | 5 mM |
pH 7.6 with 4 N NaOH.
Thrombin(Sigma, cat# T7009)
Reconstitute with PBS to final concentration of 100 Units/ml. Thrombin can be made fresh or stored frozen in 500 μl aliquots, thawed just prior to use.
Nitrophenyl p-guanidine Benzoate (NPGB, Sigma cat# N8010)
Prepare fresh just prior to thrombin stimulation. Use glass tubes only.
| NPGB | 0.033 g | 100 mM |
| Dimethyl Formamide | 1 ml |
Use at 1 mM final concentration (500 μl in 50 ml releasate).
2.2.2. Buffers for Chromatography
For heparin affinity chromatography
Tris buffered saline with Calcium pH 7.4 (TBS-C)
| Tris HCl | 0.01 M |
| NaCl | 0.15 M |
| CaCl2 (make from stock of 100 mM CaCl2, 50 mM MgCl2: use 1 ml for 1 liter of buffer) | 0.1 mM |
Strip buffer (0.55 M NaCl) , pH 7.4
| Tris HCl | 0.01 M |
| NaCl | 0.55 M |
| CaCl2 (make from stock of CaCl2, MgCl2 as above) | 1 mM |
Platelet Factor 4 strip buffer (2.0 M NaCl), pH 7.4
| Tris HCl | 0.01 M |
| NaCl | 2 M |
Gel filtration buffer (0.8 M NaCl) , pH 7.4
| Tris HCl | 0.01 M |
| NaCl | 0.8 M |
| CaCl2 (make from stock of CaCl2, MgCl2 as above) | 1 mM |
Note: We typically filter buffers through a 0.22 μm filter to remove any possible particulates or bacterial contamination. Although TSP1 is purified in an open system and the buffers are not considered sterile, we find that “cleaning” the buffers by filtering reduces chances of contamination of cell cultures with short-term TSP1 use.
2.3. Platelet Preparation and Release Reaction
It is very important to use only plastic utensils when dealing with platelets and thrombospondin 1.
Pool platelets from 10–20 random donor units.
Add ACD to ¼ final volume.
Transfer to 50 ml conical centrifuge tubes and centrifuge at 300 xg for 10 minutes to pellet red blood cells. Discard pellet.
Centrifuge supernatants at 2,700 xg at room temperature for 15 minutes to pellet platelets.
Wash pelleted platelets three times with Hepes wash buffer (~ 50 ml) as above (2700 xg, 15 minutes),
After last wash, decant tubes, and gently resuspend platelets in a small volume of Hepes resuspension buffer. Pool washed platelets from all tubes and resuspend to a total volume of 50 ml with Hepes resuspension buffer.
During the last wash, prepare NPGB and thaw thrombin stock.
Place resuspended, washed platelets in a plastic beaker in a 37°C water bath to warm.
Add thrombin to platelet prep with constant gentle stirring and time reaction with a stopwatch. At ~1 min 45 sec, you will see the platelets start to aggregate.
Terminate the reaction at 2 minutes with NPGB and stir for an additional minute at room temperature. Place platelets on ice.
Centrifuge for 20 min at 27,000 x g at 4°C.
Decant supernatant (releasate) into two 50 ml centrifuge tubes. Add 10 ml of resuspension buffer to each tube. Discard aggregated platelet pellets.
Snap freeze releasate using a dry ice/methanol bath or with immersing in liquid nitrogen. Store overnight at −70°C. Thrombospondin can be purified from releasates stored for several months.
Just prior to chromatographic purification, thaw releasate in 37° water bath. Centrifuge for 10 min at 4°C at 2700 xg. Use a wooden applicator stick (like a cotton swab stick) to trap and remove the fibrin clot. Avoid repeated freezing and thawing of the releasate.
2.4. Chromatography
2.4.1. Heparin Affinity purification
This step isolates heparin-binding proteins from the platelet releasate. These proteins include TSP1, β-thromboglobulin, and platelet factor 4 among others.
Prepare either heparin-Sepharose or heparin-agarose columns with an approximate bed volume of 6 ml. Wash and equilibrate the column with 200 ml TBS-C to remove azide containing storage buffer. Block non-specific binding sites with 1 ml 10% BSA. Wash unbound BSA with at least 150 ml TBS-C, monitoring absorbance at 280 nm.
Add releasate to column. Collect small aliquots of releasate and flow through for later analyses by SDS-PAGE.
Wash column with TBS-C until absorbance 280 nm is near baseline.
Attach column to fraction collector and collect ~ 1ml fractions.
Elute heparin binding proteins (TSP1 fraction) with 0.55M NaCl Strip buffer in 1ml fractions.
Read absorbance of fractions at 280 nm. Pool fractions with absorbance >1.0 and label as “Pool 1”. Trailing fractions with absorbance of 0.2–0.9 can be pooled as “Pool 2.”
Platelet Factor 4 will still be bound to the heparin-affinity resin and it is necessary to elute Platelet Factor 4 with 2 M NaCl so that the heparin column can be re-used.
Wash the column with 200 ml TBS-C and store at 4°C with 0.5 ml 20% azide.
2.4.2. Gel Filtration equilibrated in Tris buffered 0.8 M NaCl, pH 7.4 to dissociate TGFβ
Column preparation:
We have long used Bio-Rad A 0.5M Gel or BioGel P-300 as the resin for the gel permeation step. These resins are no longer available, however, Sephacryl S300 would be a suitable replacement. The column can be poured, packed, and blocked with TBS-C, pH 7.4 prior to use. To avoid sample dilution, the sample size should be no more than 2% of the bed volume. Blue dextran, found in many commercially available gel filtration calibration kits for calibrating of void and total bed volumes, can be used to both determine the void volume and determine the quality of the bed packing: you can visually inspect the column to make sure that blue dextran moves evenly through the column. This column can be reused multiple times if stored properly at 4°C with azide. If the flow rate starts to slow or if there is evidence of proteolysis in samples, the resin should unpacked and cleaned with 1% Triton-X 100 in TBS-C. The typical bed volume we use is ~100–120 ml.
On the day of use, the column should be washed to remove any azide and equilibrated with 2–3 column volumes of Tris buffered 0.8 M NaCl, pH 7.4. Column is now ready to be loaded with sample (Pool #1). Once sample has entered bed, gently layer on Tris buffered 0.8M NaCl on top of column bed. Attached column to fraction collector and pump to regulate flow. Try to keep a flow rate of 0.35 ml/min. TSP1 will elute near the void volume and fraction volume can be reduced at this time to ~ 2 min (0.7 ml) per tube. Be sure to use silanized plastic tubes for collection to minimize non-specific absorption to tube surface. TGFβ will elute near the tail of the TSP1 peak and also near the total bed volume.
Read absorbance of the eluted peak at 280 nm and at 320 nm to determine protein concentration. We use the following formula: (Absorbance at 280 nm) – 1.7(Absorbance at 320 nm) divided by the extinction coefficient of TSP1 (0.9) = mg/ml. The absorbance at 320 nm detects light scattering due to aggregation (Bale et al., 1985; Margossian et al., 1981). This measurement is a good assessment of protein quality and can be expected to be elevated if TSP1 is subjected to denaturing conditions or upon freeze/thawing.
Desalting TSP1 purified with 0.8 M NaCl buffer: It will be necessary to desalt the purified protein. To achieve this, use desalting columns or Amicon concentrators with MWCO below 30K daltons. Spin down at 3000 rpm/10–15 minutes, compare volumes (starting/final), and calculate how much TBS-Ca/Mg pH 7.4 is necessary to add to achieve a final NaCl concentration of 0.15 M. Alternately, we have had success by dialyzing TSP1 against several changes of 1–2 liters of TBS-C at 4°C.
To minimize aggregation, freeze and thaw TSP1 rapidly in small volumes (minimal 0.25 ml). Use a 37°C water bath to thaw, and either a dry ice/methanol bath or immersion in liquid nitrogen to snap freeze the protein. Store at −70–80°C and avoid repeated freeze/thawing. Remeasure concentration after thawing. Do not store at 4°C for more than 1–2 days to avoid possible proteolysis.
Evaluate protein quality (proteolysis, purity, aggregation) by running releasate, heparin-pass through, 0.55M NaCl peak, and gel filtration peaks on SDS-PAGE with Coomassie blue staining.
2.5. Evaluating purified TSP1 for contaminating TGF-β activity
We evaluate our TSP1 preparations for associated TGF-β activity by measuring active TGFβ in TSP1 samples using the R &D Systems Quantikine ELISA for human TGFβ1 (Catalogue # DB100B). Typically, we detect about 4–8 fM TGFβ activity per nM TSP1 trimer (0.3–0.6 pM TGFβ in 20–28 μg/ml TSP1). This a sufficiently low background level of TGF-β activity to allow us to detect TSP1-mediated activation and to avoid eliciting cellular responses to TGFβ. If it is necessary to achieve lower levels of TGFβ, then TSP1 can be further purified by passing through an anti-LAP or anti-TGFβ affinity column (Ribeiro, Poczatek, Schultz-Cherry, Villain, & Murphy-Ullrich, 1999). These columns can be costly to generate and one also risks losing TSP1 through this additional chromatography step: we have not found it necessary to use this step for most of our assays.
REFERENCES
- Akiyama SK, & Yamada KM (1985). The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem, 260, 4492–4500. [PubMed] [Google Scholar]
- Annis DS, Murphy-Ullrich JE, & Mosher DF (2006). Function-blocking anti-thrombospondin-1 monoclonal antibodies. Thromb. Haem, 4, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstall HS, & Urie PM (1986) A Manual of Hemotherapy (pp. 82–89). New York: John Wiley and Sons. [Google Scholar]
- Bale MD, Westrick LG, & Mosher DF (1985). Incorporation of thrombospondin into fibrin clots. J Biol Chem, 260, 7502–7508. [PubMed] [Google Scholar]
- Brody MJ, Schips TG, Vanhoutte D, Kanisicak O, Karch J, Maliken BD, et al. (2016). Dissection of Thrombospondin-4 Domains Involved in Intracellular Adaptive Endoplasmic Reticulum Stress-Responsive Signaling. Mol Cell Biol, 36, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak E, Pretto F, Ritz D, Gualandi L, Wulhfard S, & Neri D (2014). Monoclonal antibodies to murine thrombospondin-1 and thrombospondin-2 reveal differential expression patterns in cancer and low antigen expression in normal tissues. Exp Cell Res, 327, 135–145. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Kuznetsova SA, Sipes JM, Rodrigues RG, Cashel JA, Annis DS, et al. (2008). Calcium indirectly regulates immunochemical reactivity and functional activities of the N-domain of thrombospondin-1. Matrix Biol, 27, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CB, Lawler J, & Mosher DF (2008). Structures of thrombospondins. Cell Mol Life Sci, 65, 672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Aeschlimann D, Nowlen J, & Mosher DF (1996). Expression and initial characterization of recombinant mouse thrombospondin 1 and thrombospondin 3. FEBS Lett, 387, 36–41. [DOI] [PubMed] [Google Scholar]
- Chen K, Lin Y, & Detwiler TC (1992). Protein disulfide isomerase activity is released by activated platelets. Blood, 79, 2226–2228. [PubMed] [Google Scholar]
- Clezardin P, McGregor JL, Manach M, Robert F, Dechavanne M, & Clemetson KJ (1984). Isolation of thrombospondin released from thrombin-stimulated human platelets by fast protein liquid chromatography on an anion-exchange Mono-Q column. J Chromatogr, 296, 249–256. [DOI] [PubMed] [Google Scholar]
- Florence TM (1980). Degradation of protein disulphide bonds in dilute alkali. Biochem J, 189, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Roberts DD, Endo T, & Kobata A (1989). Structural study of the sugar chains of human platelet thrombospondin. Arch Biochem Biophys, 270, 302–312. [DOI] [PubMed] [Google Scholar]
- Happonen KE, Saxne T, Aspberg A, Morgelin M, Heinegard D, & Blom AM (2010). Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum, 62, 3574–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Hong E, Yik JH, Chromy B, Morgelin M, Snow KD, et al. (2011). Enhanced activity of transforming growth factor beta1 (TGF-beta1) bound to cartilage oligomeric matrix protein. J Biol Chem, 286, 43250–43258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen TY, & Maki M (2009). Peters’-plus syndrome is a congenital disorder of glycosylation caused by a defect in the beta1,3-glucosyltransferase that modifies thrombospondin type 1 repeats. Ann Med, 41, 2–10. [DOI] [PubMed] [Google Scholar]
- Hess D, Keusch JJ, Oberstein SA, Hennekam RC, & Hofsteenge J (2008). Peters Plus syndrome is a new congenital disorder of glycosylation and involves defective Omicron-glycosylation of thrombospondin type 1 repeats. J Biol Chem, 283, 7354–7360. [DOI] [PubMed] [Google Scholar]
- Hoffmann BR, Liu Y, & Mosher DF (2012). Modification of EGF-like module 1 of thrombospondin-1, an animal extracellular protein, by O-linked N-acetylglucosamine. PLoS One, 7, e32762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, et al. (2001). C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem, 276, 6485–6498. [DOI] [PubMed] [Google Scholar]
- Hogg PJ, Hotchkiss KA, Jimenez BM, Stathakis P, & Chesterman CN (1997). Interaction of platelet-derived growth factor with thrombospondin 1. Biochem J, 326, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden P, Keene DR, Lunstrum GP, Bachinger HP, & Horton WA (2005). Secretion of cartilage oligomeric matrix protein is affected by the signal peptide. J Biol Chem, 280, 17172–17179. [DOI] [PubMed] [Google Scholar]
- Hotchkiss KA, Chesterman CN, & Hogg PJ (1996). Catalysis of disulfide isomerization in thrombospondin 1 by protein disulfide isomerase. Biochemistry, 35, 9761–9767. [DOI] [PubMed] [Google Scholar]
- Hotchkiss KA, Matthias LJ, & Hogg PJ (1998). Exposure of the cryptic Arg-Gly-Asp sequence in thrombospondin-1 by protein disulfide isomerase. Biochim Biophys Acta, 1388, 478–488. [DOI] [PubMed] [Google Scholar]
- Huang EM, Detwiler TC, Milev Y, & Essex DW (1997). Thiol-disulfide isomerization in thrombospondin: effects of conformation and protein disulfide isomerase. Blood, 89, 3205–3212. [PubMed] [Google Scholar]
- Ihara Y, Manabe S, Ikezaki M, Inai Y, Matsui IS, Ohta Y, et al. (2010). C-Mannosylated peptides derived from the thrombospondin type 1 repeat interact with Hsc70 to modulate its signaling in RAW264.7 cells. Glycobiology, 20, 1298–1310. [DOI] [PubMed] [Google Scholar]
- Kaur J, & Reinhardt DP (2012). Immobilized metal affinity chromatography co-purifies TGF-beta1 with histidine-tagged recombinant extracellular proteins. PLoS One, 7, e48629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenotic PA, Page RC, Misra S, & Silverstein RL (2011). Expression, purification and structural characterization of functionally replete thrombospondin-1 type 1 repeats in a bacterial expression system. Protein Expr Purif, 80, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Hussein F, Woeste A, Grundker C, Frontzek K, Emons G, et al. (2011). CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat, 128, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvansakul M, Adams JC, & Hohenester E (2004). Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. Embo J, 23, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, & Simons ER (1983). Cooperative binding of calcium to thrombospondin. The effect of calcium on the circular dichroism and limited tryptic digestion of thrombospondin. J Biol Chem, 258, 12098–12101. [PubMed] [Google Scholar]
- Lawler JW, Slayter HS, & Coligan JE (1978). Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem, 253, 8609–8616. [PubMed] [Google Scholar]
- Leonhard-Melief C, & Haltiwanger RS (2010). O-fucosylation of thrombospondin type 1 repeats. Methods Enzymol, 480, 401–416. [DOI] [PubMed] [Google Scholar]
- Lu A, Pallero MA, Lei W, Hong H, Yang Y, Suto MJ, et al. (2016). Inhibition of Transforming Growth Factor-beta Activation Diminishes Tumor Progression and Osteolytic Bone Disease in Mouse Models of Multiple Myeloma. Am J Pathol, 186, 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian SS, Lawler JW, & Slayter HS (1981). Physical characterization of platelet thrombospondin. J Biol Chem, 256, 7495–7500. [PubMed] [Google Scholar]
- McDonald JF, Dimitry JM, & Frazier WA (2003). An amyloid-like C-terminal domain of thrombospondin-1 displays CD47 agonist activity requiring both VVM motifs. Biochemistry, 42, 10001–10011. [DOI] [PubMed] [Google Scholar]
- Miao WM, Seng WL, Duquette M, Lawler P, Laus C, & Lawler J (2001). Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and -independent mechanisms. Cancer Res, 61, 7830–7839. [PubMed] [Google Scholar]
- Milev Y, & Essex DW (1999). Protein disulfide isomerase catalyzes the formation of disulfide-linked complexes of thrombospondin-1 with thrombin-antithrombin III. Arch Biochem Biophys, 361, 120–126. [DOI] [PubMed] [Google Scholar]
- Misenheimer TM, & Mosher DF (1995). Calcium ion binding to thrombospondin 1. J Biol Chem, 270, 1729–1733. [DOI] [PubMed] [Google Scholar]
- Misenheimer TM, & Mosher DF (2005). Biophysical characterization of the signature domains of thrombospondin-4 and thrombospondin-2. J Biol Chem, 280, 41229–41235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, Huwiler KG, Misenheimer TM, & Annis DS (2002). Expression of recombinant matrix components using baculoviruses. Methods Cell Biol, 69, 69–81. [DOI] [PubMed] [Google Scholar]
- Muroi E, Manabe S, Ikezaki M, Urata Y, Sato S, Kondo T, et al. (2007). C-Mannosylated peptides derived from the thrombospondin type 1 repeat enhance lipopolysaccharide-induced signaling in macrophage-like RAW264.7 cells. Glycobiology, 17, 1015–1028. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, & Poczatek M (2000). Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev, 11, 59–69. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Schultz-Cherry S, & Höök M (1992). Transforming growth factor-b complexes with thrombospondin. Mol Biol Cell, 3, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narouz-Ott L, Maurer P, Nitsche DP, Smyth N, & Paulsson M (2000). Thrombospondin-4 binds specifically to both collagenous and non-collagenous extracellular matrix proteins via its C-terminal domains. J Biol Chem, 275, 37110–37117. [DOI] [PubMed] [Google Scholar]
- Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, & Bornstein P (2008). Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol Biol Cell, 19, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qabar A, Derick L, Lawler J, & Dixit V (1995). Thrombospondin 3 is a pentameric molecule held together by interchain disulfide linkage involving two cysteine residues. J Biol Chem, 270, 12725–12729. [DOI] [PubMed] [Google Scholar]
- Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, & Murphy-Ullrich JE (1999). The activation sequence of thrombospondin-1 interacts with the latency- associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem, 274, 13586–13593. [DOI] [PubMed] [Google Scholar]
- Roberts DD, Cashel J, & Guo N (1994). Purification of thrombospondin from human platelets. J Tissue Cult Methods, 16, 217–222. [Google Scholar]
- Roberts DD, Haverstick DM, Dixit VM, Frazier WA, Santoro SA, & Ginsburg V (1985). The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J Biol Chem, 260, 9405–9411. [PubMed] [Google Scholar]
- Rosenberg K, Olsson H, Morgelin M, & Heinegard D (1998). Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem, 273, 20397–20403. [DOI] [PubMed] [Google Scholar]
- Santoro SA, & Frazier WA (1987). Isolation and characterization of thrombospondin. Methods Enzymol, 144, 438–446. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Leung LL, Harpel PC, & Nachman RL (1985). Platelet thrombospondin forms a trimolecular complex with plasminogen and histidine-rich glycoprotein. J Clin Invest, 75, 2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale MV, & Detwiler TC (1990). Free thiols of platelet thrombospondin. Evidence for disulfide isomerization. J Biol Chem, 265, 17859–17867. [PubMed] [Google Scholar]
- Sun X, & Mosher DF (1990). Contamination of thrombospondin with vitronectin. Blood, 76, 1666–1668. [PubMed] [Google Scholar]
- Sun X, Skorstengaard K, & Mosher DF (1992). Disulfides modulate RGD-inhibitable cell adhesive activity of thrombospondin. J Cell Biol, 118, 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski GP, Srivastava S, Switalska HI, Holt JC, Cierniewski CS, & Niewiarowski S (1985). The interaction of human platelet thrombospondin with fibrinogen. Thrombospondin purification and specificity of interaction. J Biol Chem, 260, 12240–12245. [PubMed] [Google Scholar]
- Vanguri VK, Wang S, Godyna S, Ranganathan S, & Liau G (2000). Thrombospondin-1 binds to polyhistidine with high affinity and specificity. Biochem J, 347, 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillard L, Clezardin P, & Miller A (1991). Models of human platelet thrombospondin in solution. A dynamic light-scattering study. Biochem J, 275, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Ma SP, Ju F, Zhang YP, Li ZC, Zhang BB, et al. (2016). Thrombospondin-4 Promotes Neuronal Differentiation of NG2 Cells via the ERK/MAPK Pathway. J Mol Neurosci, 60, 517–524. [DOI] [PubMed] [Google Scholar]