Abstract
Background.
Although adverse effects of severe chronic stress on immunocompetence and physical well-being in older adults have been reported, the immune response to less severe life stress among healthy older adults, particularly among women, is not well understood. Interleukin-6 (IL-6) has been considered a good overall indicator of immune functioning in older adults because of its contribution to the pathogenesis of several age-related conditions such as osteoporosis. Regulation of IL-6 is impaired in elderly adults, and levels of IL-6 increase with stress and depression. This research cross-sectionally examined levels of IL-6 in three groups of healthy older women with varying levels of life stress and mood disturbance and a healthy group of young women.
Methods.
Subjects included 18 caregivers of Alzheimer’s patients, 17 older women assessed one month before relocation of their residence, 15 nonmoving and noncaregiving older women, and 20 younger women. Subjects completed the Profile of Mood States (POMS) and had early morning blood draws.
Results.
Alzheimer’s caregivers reported significantly greater distress than women of all other groups. IL-6 levels in care-givers were significantly higher than those of all other women. The older women had significantly higher IL-6 than young controls, but there were no significant differences in IL-6 between movers and older controls. Among all women, greater depression and distress were related to higher levels of IL-6.
Conclusions.
These findings suggest that in older women, chronic stressors are associated with significant elevations in IL-6 over and above the elevations associated with normal aging, but that moderate stressors may not be related to appreciable elevations in IL-6.
PREVIOUS research has documented adverse effects of severe chronic stress, such as caregiving for Alzheimer’s patients, on immunocompetence, mood, and physical well-being in older adults (1–3). Because of age-related alterations in immunocompetence (4), older adults are thought to be particularly vulnerable to immune effects of life stressors. However, the immune response to more temporary life stress among healthy older adults, particularly among women, is not well understood. This investigation compares three groups of healthy older women with varying levels of life stress and a control group of healthy younger women.
Interleukin-6 (IL-6) is a multifunctional cytokine strongly implicated in processes involved in normal aging as well as in the pathogenesis of a number of age-related conditions (5,6). These include certain types of autoimmune conditions (7), osteoporosis (8), lymphoma (9), multiple myeloma (10), and some of the pathological changes involved in Alzheimer’s disease (11). IL-6 is produced by monocytes, macrophages, mast cells, and endothelial cells, and has a critical role in the regulation of cellular processes such as the acute phase response, osteoclastogenesis, and hematopoiesis (12). It is also responsible for many aspects of immune regulation (6,12). Increased levels of IL-6 commonly occur with disturbances of homeostasis, such as those accompanying inflammation or trauma. The regulation of IL-6 synthesis is normally tightly controlled, but this regulation is impaired in elderly people, and levels ofIL-6 thus tend to increase with age (5,6). Postmenopausal women also lose the inhibiting effect of estrogen on IL-6 gene expression (13–15). Older women are thought to be particularly at risk from increased concentrations of circulating IL-6 because of its contribution to osteoporosis via stimulation of production of osteoclasts, the cells that resorb bone. IL-6 also plays a role in regulation of osteoblast development and function (13–15).
In addition to its effects on the skeletal and immune systems, IL-6 is also implicated in the stress response (16,17). Plasma IL-6 concentrations increase following physical and psychological stressors not necessarily associated with infection or tissue inflammation, such as immobilization, footshock, and conditioned aversive stimuli (16). It is thought that increases in plasma IL-6 during stress come from a nonimmune origin, such as the adrenal gland, and form part of the hormonal response to arousal (16,17), although mediation by central and peripheral catecholaminergic systems also may be involved (18). IL-6 levels are elevated during depression and decrease following successful treatment of major depression with fluoxetene (19).
Because of the critical role of IL-6 in both stress and aging, we examined possible interactive effects of life stress and aging on IL-6 in healthy older women with varying levels of life stress and mood disturbance. Caregiving of Alzheimer’s patients has been implicated as a severe chronic stressor in numerous studies, in part due to the unpredictable, emotionally draining, and socially isolating characteristics of the caregiving role (20). Caregivers tend to have higher levels of depression, greater immune compromise, and poorer health status than age-matched noncaregivers (1,2). Another common life stressor encountered by older adults is housing relocation, particularly as it may be accompanied by uncertainty, physical exertion, and losses such as changes in possessions, social support systems, sense of autonomy, and mobility (21,22). Early studies of involuntary housing relocation among older adults reported marked increases in risk for morbidity and mortality post-relocation (23,24). However, more recent studies have reported positive or neutral effects on health and psychosocial functioning of older adults (e.g., 25,26), with factors such as controllability, predictability, and quality of the new environment serving as important predictors of outcome (e.g., 27,28).
Different types of control are thought to influence the appraisal of stress in any particular life situation (29). These include behavioral control (ability to take concrete action to reduce the impact of a stressor), cognitive control (ability to think differently about an event to modify its impact), and decisional control (the opportunity to choose between alternative courses of action). The situation of caregiving is often undertaken with little or no decisional control, may present limited opportunities for behavioral control, may severely test a caregiver’s capacity for cognitive control, and may present the possibility of long-term exposure for an indefinite period in the future (20). In contrast, even though it may present challenges along the way, the process of voluntary relocation offers many opportunities for decisional and behavioral control, and exposure to a relatively finite event. These factors, taken together with empirical findings noted above regarding the sequelae of voluntary relocation, underlie our conceptualization of voluntary housing relocation as a moderately challenging but temporary life stressor.
This study cross-sectionally examined three groups of older women and a comparison group of young controls: Alzheimer’s caregivers were selected as a high chronic stress group; women anticipating voluntary housing relocation were selected as a moderate stress group; noncaregiving and nonmoving older women served as older controls, and healthy younger women composed the young control group. It was predicted that the greatest distress would be seen in Alzheimer’s caregivers, followed by older movers, and that distress levels in the two control groups would be approximately equivalent. It was also hypothesized that IL-6 would be positively related to both distress and age, and that older women with the greatest distress would have the highest IL-6 levels. Specifically, it was predicted that Alzheimer’s caregivers would have the highest IL-6 levels, followed by older movers, then by the older controls, and that the lowest levels would be seen in young controls.
Method
Sample Characteristics
Subjects included 71 women: 18 Alzheimer’s caregivers, 17 older women assessed one month ‘before moving their residence, 15 healthy nonmoving and noncaregiving older women, and 21 healthy younger women. The older subjects were participants in a larger study of Alzheimer’s caregivers or in a study of older adults undergoing community housing relocation. These studies were conducted concurrently, and blood samples were processed in the same laboratory by the same laboratory personnel. Participants were drawn from the same geographical region in a semirural midwestern state. Caregivers were recruited from advertisements in church bulletins, Alzheimer support groups, local newspapers, church groups, and community centers. Movers and older controls were recruited from senior centers, church groups, and newspaper advertisements; movers were also recruited from waiting lists of community-based congregate living facilities. All movers had chosen to relocate voluntarily and were moving to community congregate living facilities where they would be living independently. Young volunteers were recruited from community advertising.
Exclusion criteria.—
Caregivers, movers, and older controls had similar exclusion criteria. These included the following: use of systemic corticosteroids within last 3 months; history of chemotherapy or radiation in the past 5 years; active diseases that could affect immune function, such as cancer, rheumatoid arthritis, AIDS, lupus, and multiple sclerosis; presence of an infectious disease such as a cold or flu within the last 2 weeks; serious illness requiring hospitalization or bedrest of more than 7 days within the last 3 months; cognitive impairments precluding ability to answer questionnaires; bereavement within 6 months of study entry; age under 60 (caregivers); age under 65 (movers and elderly controls). Young controls had the same exclusion criteria, except that they were required to be younger than age 55 and not taking birth control pills or beta blockers.
Assessments
Demographic and control variables.—
Demographic information such as age, ethnicity, income, education, and marital status was assessed. Subjects reported average alcohol and cigarette intake and sleep over the last 7 days, and use of hormone replacement therapy (HRT) or beta blockers to assess for factors that could potentially affect outcome measures (30).
Distress.—
The Profile of Mood States [POMS; (31)] is a self-report scale comprising 65 mood adjectives such as “friendly,” “tense,” “angry.” These are rated on a 5-point scale from (0) not at all to (4) extremely, according to mood over the last week. Six factors (anxiety, depression, anger, vigor, fatigue, and confusion) have been identified by factor analyses to describe the items in this scale (32). A total mood disturbance (TMD) score is obtained by summing all scores except vigor and subtracting vigor. Thus, TMD scores can be negative. A 37-item POMS Short Form (POMS-SF: 32) has been developed to reduce response burden in populations such as the elderly. POMS-SF subscale scores have high correlations with the original sub-scales (rs = 0.80s to 0.90s). Internal consistencies for the POMS-SF, are equivalent to those for the original POMS, with Cronbach’s alpha generally ranging between 0.80 and 0.96(32). Moving women and older controls were administered the POMS-SF, whereas caregivers and young adult women completed the original POMS scale (see Note 1). To standardize POMS responses for all groups, the 37 POMS-SF items were extracted from the responses of all groups, and these items were used in all POMS analyses.
Interleukin-6 (IL-6).—
Detection of interleukin-6 (IL-6) present in plasma was performed by an ELISA using a standard kit (R&D Diagnostics, Minneapolis, MN; High Sensitivity IL-6 Quantikine Kit), with measurements performed according to manufacturer’s instructions and results interpolated from the standard reference curve provided with the kit.
Plasma albumin.—
Plasma albumins were assessed to control for nutritional status. Assays were performed using an endpoint method based on a modification of the Doumas bromcresol green (BCG) dye-binding reaction in which BCG binds selectively with albumin (33).
Procedures
Psychosocial and health assessments for older women were administered by a trained research assistant and a project nurse in the subject’s home. Relocating women participated in assessments one month prior to moving. Caregivers received assessments prior to commencement of an intervention. Peripheral venous blood samples were collected by a study nurse prior to administration of questionnaires. Blood samples were collected in 10 ml heparin vacutainer tubes (Becton Dickinson; Rutherford, NJ) between 7:30 and 11 am to minimize potential circadian changes. Younger comparison subjects received early morning blood draws and completed questionnaires in the University Clinical Research Center. Blood samples were kept at room temperature until processing. Plasma samples were separated, aliquoted, and frozen at −40°C immediately upon arrival at the laboratory. Samples from all groups were coded and assayed in batches by a laboratory technician blind to the hypotheses of the study.
Statistical analyses.—
Before proceeding with formal data analysis, distributions of all variables were examined for outliers and non-normality. A logarithmic transformation was used to normalize IL-6 data, which were positively skewed. Chi-square tests and one-way analyses of variance (ANOVAs) were used to establish equivalence at baseline between groups on demographic and control variables. A multivariate analysis of variance (MANOVA) and follow-up univariate and post hoc tests were used to test for group differences in mood. A one-way ANOVA and follow-up post hoc tests were used to compare levels of IL-6 between groups. Pearson correlations were performed to assess relationships among distress and immune variables. The Statistical Package for the Social Sciences (SPSS, Inc.; Chicago, IL) version 7.5 was used for data analyses.
Results
Sample Characteristics
All subjects in this study were Caucasian. The mean age of young controls was approximately 40 (±9.20years), whereas older women ranged from age 60–89. Caregivers were significantly younger than the other older women, with a mean age of 70.94 (±8.32) in contrast to movers (M =79.59± 5.53) and controls (M =76.07 ± 6.17), F(2,47) = 7.07, p <.002. The women in this study were largely married or living with a partner, although 47% of movers and 20% of controls were widowed. Chi-square tests indicated significant differences between the groups in relationship status [χ2 (12) = 37.85, p <.001]. The income distribution of the sample ranged from households reporting annual incomes of less than $10,000 to those reporting over $50,000. A Mantel Haenszel test of linear association revealed no significant differences among groups with respect to income (p> .50). Education of women in the sample ranged from some high school education to postgraduate education. A Mantel Haenszel test indicated that there were significant differences between groups in education (p < .05). (See Table 1.)
Table 1.
Demographics of the Study Population
| Measure | Alzheimer’s Caregivers | Movers | Older Controls | Younger Controls |
|---|---|---|---|---|
| Age (yrs; M ± SD) | 70.9 ± 8.32 | 79.6 ± 5.53 | 76.1 ± 6.17 | 39.9 ± 9.20 |
| Sleep (hrs; M ± SD) | 6.17 ± .86 | 7.09 ± 1.64 | 7.17 ± .90 | 7.36 ± 1.40 |
| Income | ||||
| < $10,000 | 5.5% | 0.0% | 28.6% | 0.0% |
| $10,000–$30,000 | 66.7 | 37.5 | 21.4 | 23.8 |
| $20,000–$50,000 | 22.3 | 56.2 | 21.4 | 42.9 |
| >$50,000 | 5.5 | 6.3 | 28.6 | 33.3 |
| Education | ||||
| Some high school | 5.6% | 0.0% | 14.3% | 0.0% |
| High school graduate | 55.6 | 11.7 | 14.3 | 23.8 |
| Trade school/some college | 22.2 | 23.5 | 28.6 | 38.2 |
| College graduate | 16.7 | 47.1 | 21.4 | 19.0 |
| Postgraduate | 0.0 | 17.6 | 21.4 | 19.0 |
| Marital Status | ||||
| Single | 0.0% | 0.0% | 0.0% | 19.0% |
| Divorced | 0.0 | 0.0 | 0.0 | 9.5 |
| Married/living with partner | 100.0 | 52.0 | 80.0 | 71.5 |
| Widowed | 0.0 | 47.0 | 20.0 | 0.0 |
Note: Percentages shown for Income, Education, and Marital Status.
Control variables.—
Alzheimer’s caregivers reported significantly fewer hours of sleep than other women, F(3,67) =3.28, p < .05. Caregivers averaged 6.2 ± 8.6 hours of sleep per night, whereas all other women averaged at least 7.1 ± 1.64 hours per night. There were no significant differences between groups in use of cigarettes or alcohol (both ps > .12). One mover, two older controls, two caregivers, and no younger controls used beta blockers. The differences between groups in use of beta blockers were not significant, χ2 (3) = 2.91, p > .39. Four movers, four older controls, five caregivers, and no young controls used HRT. These differences were marginally significant, χ2 (3) = 6.80, p < .09. There were no significant differences between the groups in albumin levels [F(3,64) = 1.50, p =.22], and all albumin levels were within the normal range (34).
As predicted, age was significantly associated with higher IL-6 levels (r = .54, p < .001). There was a trend toward an inverse relationship between use of HRT and IL-6levels (r = −.20, p < .09). Otherwise, there were no significant relationships between control or demographic variables and IL-6 (all ps > .15).
Distress.—
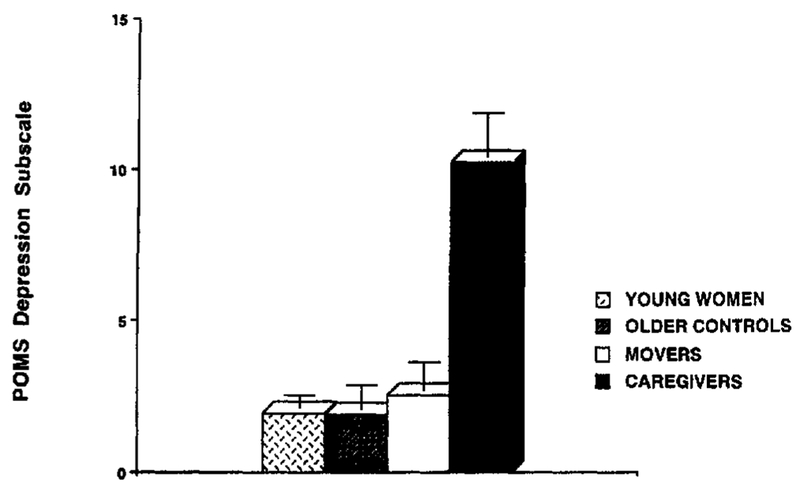
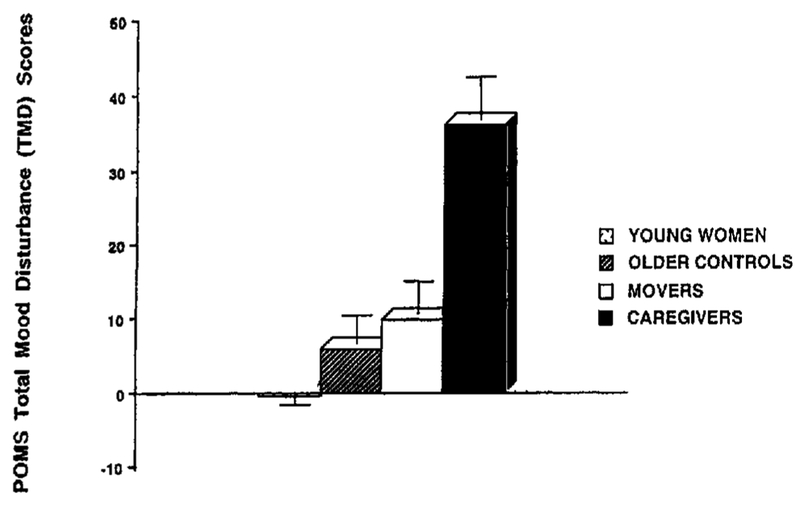
A MANOVA was performed on all POMS sub-scales to determine between-group differences. The entire model was significant, F(18, 176) = 3.82, p < .001. Univariate tests showed significant differences between groups for all POMS subscales (all ps <.005). (See Note 2 and Table 2.) Follow-up Tukey’s post hoc comparisons were conducted to evaluate pairwise differences among these means. Caregivers showed significantly greater distress than women of all other groups on the POMS TMD and on all POMS subscales except vigor (all ps <.001). (See Figures 1 and 2.) Older women, regardless of group, reported significantly less vigor than the younger controls (all ps < .01), but there were no significant differences in vigor reported between groups of older women. In addition, movers showed a trend toward higher total mood disturbance as compared to young controls (p = .06), suggesting mildly elevated distress among relocating women. Otherwise, distress levels did not differ between the three noncaregiving groups.
Table 2.
Means and (Standard Deviations) for Profile of Mood State (POMS) Scores
| Measure | Alzheimer’s Caregivers | Movers | Older Controls | Younger Controls | F(3,67) |
|---|---|---|---|---|---|
| Anxiety | 10.83 (5.16) | 5.24 (5.04) | 4.60 (2.87) | 3.71 (2.63) | 11.42*** |
| Depression | 10.28 (6.21) | 2.53 (3.61) | 1.87 (3.09) | 1.95 (1.75) | 18.99*** |
| Anger | 9.22 (4.56) | 3.00 (3.92) | 3.67 (5.56) | 3.48 (1.72) | 9.47*** |
| Vigor | 10.67 (4.10) | 9.53 (5.44) | 11.87 (5.37) | 15.71 (3.33) | 6.84*** |
| Fatigue | 11.11 (4.98) | 5.82 (4.46) | 5.20 (5.49) | 3.90 (3.34) | 8.98*** |
| Confusion | 5.44 (3.55) | 2.94 (3.19) | 2.40 (2.35) | 2.24 (1.70) | 5.25** |
| POMS TMD | 36.22 (23.60) | 5.87 (14.94) | 10.00 (17.35) | −.43 (9.23) | 16.83*** |
p< .01;
p< .001.
Figure 1.
Means (and SEM) for POMS Depression in four groups of healthy women. Tukey’s post hoc comparisons for caregivers versus all other groups, p< .001.
Figure 2.
Means (and SEM) for POMS Total Mood Disturbance in four groups of healthy women. Tukey’s post hoc comparisons for caregivers versus all other groups, p < .001; movers versus young controls, p =.06.
Interleukin-6.—
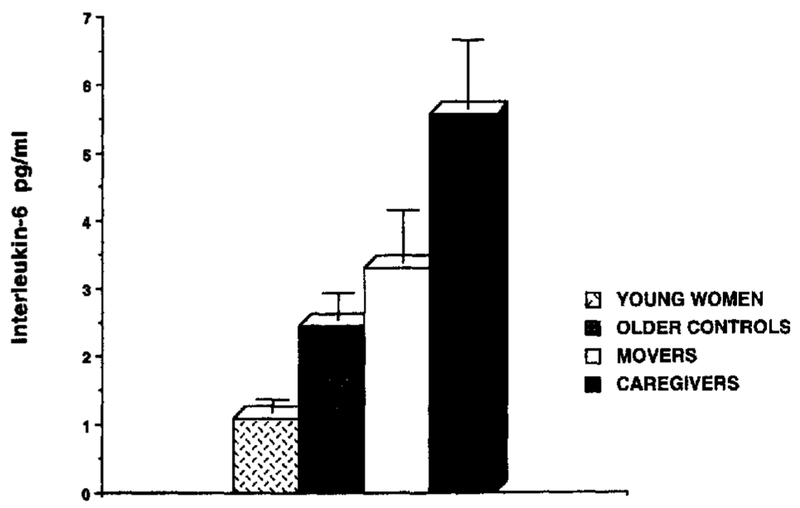
There was a significant difference in IL-6 levels between groups, F(3,67) =19.25, P < .001 (see Note 2). The means of IL-6 levels were ordered as expected across the four groups. Alzheimer’s caregivers had the highest IL-6 levels (M =5.70 pg/ml ± 4.30), followed by movers (M = 3.32 pg/ml ± 3.12), then by older controls (M = 2.44 pg/ml ± 1.60), then by young controls (M =1.11 pg/ml ± .76). Follow-up Tukey’s post hoc comparisons indicated that caregivers demonstrated significantly higher levels of IL-6 than all three groups of noncaregivers (caregivers vs movers, p <.03; caregivers vs older controls, p < .005; caregivers vs young controls, p < .001).In addition, both groups of older noncaregivers had significantly higher levels of IL-6 than young controls (movers vs young controls, p < .001; older controls vs young controls, p < .001). (See Figure 3.)
Figure 3.
Means (and SEM) for Interleukin-6 (pg/ml) in four groups of healthy women. Tukey’s post hoc comparison for caregivers versus movers, p < .03; caregivers versus older controls, p < .005; caregivers versus young controls, p < .001; movers versus young controls, p < .001; older controls versus young controls, p < .001.
Among all subjects, women reporting greater depression, anger, fatigue, or total mood disturbance had significantly higher levels of IL-6, (rs from .24-.35, all ps < .05), and women with greater vigor had lower IL-6 levels (r = −.30, p = .01). Levels of IL-6 were not related to anxiety or confusion (ps>,.11). (See Note 3.)
Discussion
This is one of the first investigations examining levels of IL-6 in women as related to both stress and age. Alzheimer’s caregivers demonstrated higher levels of mood disturbance than women in any of the other groups, who did not differ from each other except for the trend for movers to show elevations in total distress. All groups of older women reported less vigor than younger women. All older women showed significant elevations of IL-6 compared to younger women. In addition, Alzheimer’s caregivers demonstrated significant elevations in IL-6 compared to older women anticipating housing relocation and older controls. Although women planning to move had slightly higher IL-6levels than the elderly controls, these differences were not significant, suggesting that such temporary stressors among older women produce less marked changes in IL-6 than do severe chronic stressors.
These findings suggest that in older women, chronic stressors such as those accompanying Alzheimer’s caregiving are related to elevations in IL-6, over and above the elevations associated with normal aging or the combination of aging and temporary life stress. These findings are particularly striking in light of the fact that the mean age of the caregivers was approximately 6–9 years younger than the other two groups of older women. This, if anything, would have contributed to a tendency to lower, rather than higher levels of IL-6 in the caregivers. In all subjects, IL-6 elevations were significantly associated with higher levels of depression, anger, fatigue, and total distress, and with less vigor.
These findings are consistent with previous reports of elevated IL-6 with aging (5,6) and also with findings of IL-6 increases accompanying chronic depression (19). One previous investigation (35) reported no differences in levels of LPS- or fluzone-stimulated IL-6 between Alzheimer’s caregivers and age-matched noncaregiving controls, although lower levels of stimulated interleukin-2 and interleukin-1β were found in caregivers. The use of stimulated IL-6 and a sample including both men and women may have contributed to differences in findings between that study and the present investigation and point to the need for further research in this area.
Mechanisms underlying IL-6 elevations in response to chronic stress are not well defined. Prolonged stress is thought to interfere with an older organism’s ability to terminate glucocorticoid secretion at the end of a stressor, resulting in chronic glucocorticoid elevations (36) and concomitant alterations in aspects of immune functioning (37). In situations of acute stress, previous work has implicated involvement of the hypothalamic-pituitary-adrenocortical (HPA) axis in the enhancement of IL-6 production (16), although some findings favor a role of central and peripheral catecholaminergic systems independent of the HPA axis (18). The possibility of adrenal production of IL-6 as part of the organismic response to arousal (16, 38) is consistent with the associations of chronic stress with IL-6 elevations observed in the present investigation. Although pharmacologic doses (i.e., concentrations used for external administration) of glucocorticoids have been shown to inhibit production of IL-6(39), physiological (i.e., levels similar to those found in the body) doses of glucocorticoids may either not suppress (40) or may even stimulate IL-6 (41). This suggests a possible physiological basis whereby chronic stress in elderly people might coexist with elevated IL-6.
Elevated levels of IL-6 have been noted in chronic fatigue patients exposed to a severe life stressor such as Hurricane Andrew, particularly in those patients using cognitive avoidance or high levels of negative emotional expression to deal with the trauma (42). These findings suggest that styles of cognitive and emotional processing of life stressors may be related to elevations in IL-6. Thus, it is possible that behavioral interventions enabling chronically stressed older women such as Alzheimer’s care-givers to adapt to chronic life stressors and decrease negative affectivity may also have immune and health implications.
Limitations of this study include the absence of direct measurement of perceived life stress or use of a more sensitive measure of depression. As screening for the older controls excluded only major confounding life stressors such as those associated with Alzheimer’s caregiving, moving, bereavement, or presence of a chronic immunomodulatory illness, it is possible that these women may have had life stressors not assessed by the current study. This would have resulted in a bias against detection of group differences. Because this study lacked control groups composed of young caregivers and young movers, it is difficult to totally dissociate the effects of these stressors from the interactive effect of these stressors with the aging process. Future work in this area would benefit from use of young control groups with stressors equivalent to those experienced by the older women.
We do not have data indicating whether the IL-6 elevations observed in this study actually have clinical significance for health outcomes in the populations studied. As there is evidence that IL-6 is related to osteoporosis (8), functional disability (43), and tumor progression in cancers [e.g., ovarian cancer which predominantly affects older women (44)], chronic elevations in IL-6 among elderly adults could have profound health ramifications. Future work (e.g., examining IL-6 and incidence of osteoporosis in a sample including older caregivers) would be extremely useful in studying the clinical significance of IL-6 elevations.
The present findings suggest that among older women, the effects of aging, mood, and chronic life stress may be interactive and may affect regulation of interleukin-6, a cytokine that is implicated both in the aging process and in women’s health problems such as osteoporosis. Future research should be directed toward understanding the physiological and psychological mechanisms that may contribute to IL-6 dysregulation in older women.
Acknowledgments
This study was funded in part by the National Institute on Aging Interdisciplinary Research Training Program on Aging (T32 AAG00214), by the National Institute of Nursing Research through a grant entitled Gerontological Nursing Interventions Research Center (P30NR03979), and by the Office of Research on Women’s Health (ROINR034340452).
Appendix
Notes
The use of the full POMS versus the POMS-SF was related to differences in the study protocols in which these subjects were participants.
As a conservative measure, all analyses were also conducted covarying for age, education, relationship status, HRT, and sleep. With the exception of univariate tests for vigor, findings for these analyses remained significant (Multivariate POMS: F(18,156) = 2.69, P =.001; all univariate ps < .01; IL-6 ANOVA with covariates: F(3,62) =4.35, p < .001).
When these analyses were repeated as partial correlations with covariates, all correlations maintained significance except for vigor.
References
- 1.Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer’s disease victims. Psychosom Med. 1987;49:523–535. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. [DOI] [PubMed] [Google Scholar]
- 3.Vitaliano PP, Russo J, Young HM, Ten L, Maiuro RD. Predictors of burden in spouse caregivers of individuals with Alzheimer’s disease. Psychol Aging, 1991;6:393–402. [DOI] [PubMed] [Google Scholar]
- 4.Makinodan T, Hahn TJ, McDougall S, Yamaguchi DT, Fang M, Iida-Klein A. Cellular immunosenescence: an overview. Exp Gerontol. 1991;26:281–288. [DOI] [PubMed] [Google Scholar]
- 5.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. [DOI] [PubMed] [Google Scholar]
- 6.Daynes RA, Araneo BA, Ershler WB, Maloney C, Li G-Z, Ryu S-Y. Altered regulation of IL-6 production with normal aging. J Immunol. 1993;150:5219–5230. [PubMed] [Google Scholar]
- 7.Graeve L, Baumann M, Heinrich PC. IL-6 in autoimmune diseases. Clin Invest. 1993;71:664–671. [DOI] [PubMed] [Google Scholar]
- 8.Roodman GD Interleukin-6: an osteotrophic factor? J Bone Miner Res. 1992;7:475–478. [DOI] [PubMed] [Google Scholar]
- 9.Merz H, Fliedner A, Orscheschek K, et al. Cytokine expression in T-cell lymphomas and Hodgkin’s disease: its possible implication in autocrine or paracrine production as a potential basis for neoplastic growth. Am J Pathol. 1991;139:1173–1180. [PMC free article] [PubMed] [Google Scholar]
- 10.Nachbaur DM, Herold M, Manesch A, Huber H. Serum levels of inter-leukin-6 in multiple myeloma and other hematologic disorders: correlation with disease activity and other prognostic parameters. Ann Hernatol. 1991;62:54–58. [DOI] [PubMed] [Google Scholar]
- 11.Bauer J, Ganter D, Strauss S, Stadtmuller G, Frommberger U, Bauer H, Volk B, Berger M. The participatory interleuken-6 in the pathogenesis of Alzheimer’s Disease. Research in Immunol. 1992;143:650–657. [DOI] [PubMed] [Google Scholar]
- 12.Van Snick JL. Interleukin-6: an overview. Ann Rev Immunol. 1990;8: 253–278. [DOI] [PubMed] [Google Scholar]
- 13.Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by Interleukin-6. Science. 1992;257:88–91. [DOI] [PubMed] [Google Scholar]
- 14.Manolagas SC, Bellido T, Jilka RL. New insights into the cellular, biochemical, and molecular basis of postmenopausal and senile osteoporosis: roles of IL-6 and gp130.Int J Immunopharmacol. 1995;17:109–116. [DOI] [PubMed] [Google Scholar]
- 15.Manolagas SC, Jilka RL. Mechanisms of diseases: bone marrow, cytokines, and bone remodeling-emergency insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. [DOI] [PubMed] [Google Scholar]
- 16.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. [DOI] [PubMed] [Google Scholar]
- 17.Judd AM, McLeod RM. Adrenocorticotropin increases interleukin-6 release from rat adrenal zona glomerulosa cells. Endocrinology. 1992;130: 1245–1254. [DOI] [PubMed] [Google Scholar]
- 18.Arimura A, Atsushi T, Komaki G. Interactions between cytokines and the hypothalamic-pituitary-adrenal axis during stress. Ann NY Acad Sci. 1994;739:276–281. [DOI] [PubMed] [Google Scholar]
- 19.Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann NY Acad Sci. 1995;762:474–476. [DOI] [PubMed] [Google Scholar]
- 20.Light E, Liebowitz BD. Alzheimer’s Disease Treatment and Family Stress: Directions for Research. Rockville, MD: National Institute of Mental Health;1989. [Google Scholar]
- 21.Fisher BJ. The stigma of relocation to a retirement facility. J Aging Studies. 1990;4:47–59. [Google Scholar]
- 22.Golant SM Matching congregate housing settings with a diverse elderly population: research and theoretical considerations. J Housing Elderly. 1991;9:21–38. [Google Scholar]
- 23.Aldrich CK, Mendkoff E. Relocation of the aged and disabled: a mortality study. J Am Geriatr Soc. 1963;11:401–408. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman MA. Relationship of mortality rates to entrance to a home for the aged. Geriatrics. 1961;16:515–519., [PubMed] [Google Scholar]
- 25.Carp PM. Short-term and long-term prediction of adjustment to a new environment. J Gerontol. 1974;29:444–453. [DOI] [PubMed] [Google Scholar]
- 26.Armer J Elderly relocation to a congregate setting: factors influencing adjustment. Issues Ment Health Nurs. 1993;14:157–172. [DOI] [PubMed] [Google Scholar]
- 27.Psychological Bourestom N. and physiological manifestations of relocation. Psychiatric Med. 1984;2:57–90. [PubMed] [Google Scholar]
- 28.Ryff CD, Essex MJ. The interpretation of life experience and well-being: the sample case of relocation. Psychol Aging. 1992;7:507–517. [DOI] [PubMed] [Google Scholar]
- 29.Averill JR. Personal control over aversive stimuli and its relationship to stress. Psychol Bull. 1973;80:286–303. [Google Scholar]
- 30.Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. [DOI] [PubMed] [Google Scholar]
- 31.McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 32.Curran SL, Andrykowski MA, Studts JL. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995;7:80–83. [Google Scholar]
- 33.Doumas BT, Biggs HG. Determination of serum albumin. Standard Meth Clin Chem. 1972;7:175–188. [Google Scholar]
- 34.Cooper J, Garnder C. Effect of aging on serum albumin. J Am Geriatr Soc. 1989;37:1039–1042. [DOI] [PubMed] [Google Scholar]
- 35.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci. 1996;93:3043–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986; 7:284–301. [DOI] [PubMed] [Google Scholar]
- 37.Gatti G, Cavallo R, Sartori ML, et al. Inhibition of human natural killer cell activity by cortisol J Steroid Biochem. 1987;265:29–58. [DOI] [PubMed] [Google Scholar]
- 38.Matta SG, Weatherbee J, Sharp BM. A central mechanism is involved in the secretion of ACTH in response to IL-6 in rats: comparison to and interaction with IL-lβ. Neuroendocrinology. 1992;56:516–525. [DOI] [PubMed] [Google Scholar]
- 39.Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL-6 from monocytes, endothelial cells, and fibroblasts. Eur J Immunol.1990;20:2439–2443. [DOI] [PubMed] [Google Scholar]
- 40.DeRijk R, Michelson D, Karp B, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1β (IL-1β, Il-6, and tumor necrosis factor(TNF-a) production in humans: high sensitivity of TNFa and resistance of IL-6. J Clin Endocrinol Metab. 1997;82:1–10. [DOI] [PubMed] [Google Scholar]
- 41.Liao J, Keiser JA, Scales WE, Kunkel SL, Kluger MJ. Role of corticosterone in TNF and IL-6 production in isolated perfused rat liver. Am J Physiol.1995;268:R669–R706. [DOI] [PubMed] [Google Scholar]
- 42.Costello N, Antoni M, Baldewicz T, Lutgendorf S, Klimas N, Schneider-man N. Coping and emotional expression effects upon distress, illness burden, and cytokines in CFS patients after Hurricane Andrew. Psychosom Med. 1998;60:121–122.(Abstract) [Google Scholar]
- 43.Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol Med Sci. 1997;52A:M201–M208. [DOI] [PubMed] [Google Scholar]
- 44.Berek JS, Chung C, Kaldi K, Watson HM, Knox RM, Martinez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991;164:1038–1042. [DOI] [PubMed] [Google Scholar]