Abstract
Background:
Fear-based disorders, like social anxiety disorder (SAD) and posttraumatic stress disorder (PTSD), are characterized by an exaggerated fear response and avoidance to trigger cues, suggesting a transdiagnostic mechanism of psychopathology. Current theories suggest that abnormalities in conditioned fear is a primary contributor to the pathophysiology of these disorders. The primary goal of this study was to compare acquisition of conditioned stimulus (CS) and aversive unconditioned stimulus (US) contingencies during fear learning and extinction in individuals with SAD and PTSD.
Methods:
In a standard Pavlovian fear conditioning-extinction paradigm we measured subjective US expectancy ratings to different CSs in patients with SAD (n=16) compared to patients with PTSD (n=13) and healthy controls (n=15)
Results:
Both patient groups (SAD, PTSD) acquired differential conditioning between a CS that predicted US (CS+) and a CS that never predicted the US (CS-), however, both groups reported an increased expectancy that the US would occur following the CS-. Additionally, the PTSD group overestimated that the US would occur in general. Neither patient group showed evidence of successful extinction of the CS+-US contingency nor differentiated their expectation of US occurrence between the CS+ and CS- during extinction learning.
Limitations:
Group sample sizes were small and we did not include a trauma-exposed group without PTSD
Conclusions:
Both SAD and PTSD generalize expectations of an aversive outcome across CSs, even when a CS never signals an aversive outcome and PTSD may tend to over-expect threat. Fear learning and extinction abnormalities may be a core feature underlying shared symptoms across fear-based disorders.
Keywords: Social phobia, PTSD, Aversive, Classical conditioning, Threat expectancy
1. Introduction
Experiences involving threat result in strong fear learning, allowing rapid detection of associations between cues in the environment and prediction of imminent threat. Importantly, ever-changing environments require that an individual flexibly re-adjust learned fear such that it would appropriately track the ongoing change in circumstances (e.g., stimulus might cease to signal danger; Schiller and Delgado, 2010). Current theories of anxiety and posttraumatic stress disorder (PTSD) suggest that abnormalities in conditioned fear are a primary contributor to the pathophysiology of these disorders (Briscione et al., 2014; Duits et al., 2015; Lissek et al., 2005). Maladaptive cognitions play a crucial role in the development and maintenance of anxiety and fear responses and while some cognitions may be disorder-specific there are commonalities in cognition across disorders, such as future-oriented perceptions of danger or threat (Hofmann, 2008; Newby et al., 2015; Norton and Paulus, 2015).
In the laboratory, these associative learning processes can be modeled using Pavlovian fear conditioning and extinction models in which fear is first linked to a previously innocuous cue (conditioned stimulus; CS) and is then decreased by presenting the CS alone (producing extinction). To date, findings from patient studies suggest that fear acquisition and/or extinction abnormalities may be a core feature underlying shared symptoms across fear-based disorders (Milad et al., 2005). For instance, patients with PTSD show deficits in the ability to discriminate between a CS that was paired with an aversive outcome (CS+; danger cue) and a CS that was never paired with an aversive outcome (CS-; safety cue), as well as, a general inability to acquire safety signals, and/or increased fear conditioning (Blechert et al., 2007; Grillon and Morgan, 1999; Jovanovic et al., 2010, 2009; Norrholm and Jovanovic, 2011; Norrholm et al., 2011; Orr et al., 2000; Peri et al., 2000).
While the vast majority of research efforts have focused on fear extinction in patients with PTSD there are emerging studies that have examined fear conditioning and extinction in other fear-based disorders and consistently demonstrate evidence of impaired fear extinction (Duits et al., 2015). For example, patients with panic disorder exhibit larger physiological responses during extinction training and rate the extinguished CS as more unpleasant than healthy controls (Michael et al., 2007). Enhanced fear learning has been reported in patients with spider phobia (Schweckendiek et al., 2011) and extinction retention deficits have been reported in patients with obsessive-compulsive disorder (Milad et al., 2013). Two behavioral studies in patients with social anxiety disorder (SAD) have shown increased conditioned fear responses, suggesting increased fear conditionability (Lissek et al., 2008) and increased tendency to not only associate threat to safety cues, but an inability to extinguish conditioned fear responses (Hermann et al., 2002). Together, these studies suggest that fear acquisition and/or extinction abnormalities may be a core feature underlying shared symptoms across fear-based disorders as well as their high degree of comorbidity (Goldstein et al., 2016). However, in the aforementioned fear extinction studies, the experiments conducted in patients with SAD, both used neutral facial expressions as the CS, which may have confounded the overall results.
Previous studies have reported that patients with SAD are likely to interpret neutral and other emotionally ambiguous facial expressions negatively (Cooney et al., 2006; Winton et al., 1995; Yoon and Zinbarg, 2007, 2008), making it possible that neutral faces may not be regarded as neutral stimuli that become a threat cue as a result of associative learning. The baseline difference in CS and/or the unconditioned stimulus (US) meaning in patients with SAD compared to healthy controls may obscure differences in the acquisition and extinction of conditioned fear responding to socially relevant cues. To control for this potential confound and to determine whether patients with SAD, like patients with PTSD, have deficits in general acquisition of fear-related CS-US contingencies and subsequent extinction of these contingencies we used a standard Pavlovian fear conditioning and extinction paradigm with non-social cues as the CSs (colored lights) in patients with SAD, PTSD, and healthy controls (Milad et al., 2005, 2007a). We hypothesized that patients with SAD and patients with PTSD would show deficits in extinction learning as evidence by greater US expectancy ratings during extinction learning and recall compared to healthy volunteers.
2. Materials and methods
2.1. Participants
Forty-four volunteers participated in this study (SAD=16; PTSD=13; Healthy Controls [HC]=15; see Supplemental Table 1 for sample demographics and clinical characteristics) and were recruited from the University of Michigan Anxiety Disorders Clinic, the University of Michigan campus, and surrounding Metro Detroit communities via online advertisements and flyers.
Participants with SAD or PTSD were required to have a primary diagnosis of SAD or PTSD, respectively, while participants in the HC group could not meet criteria for any current or past Axis I disorder. Psychiatric diagnoses based on the DSM-IV criteria (Association, 2000), were established via the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Of note, all clinical assessments were conducted by Dr. Rabinak, who was trained to administer and score these assessments for research-related purposes (See Supplemental Materials and methods for additional inclusion and exlusion critiera and comorbities).
There was no significant difference in age between the HC and SAD groups [t(29)=−1.53, p=.14] nor the SAD and PTSD groups [t(27) =−1.21, p=.24], however the HC group was significantly younger than the PTSD group [t(26)=−2.46, p=.03; Supplemental Table 1], therefore age was included as a covariate in the analyses. Between-group comparisons on sex of the participants, ethnicity, and racial composition are included in the Supplemental Results.
2.2. Additional assessment measures
2.2.1. Liebowitz Social Anxiety Scale (LSAS)
The LSAS is a 24-item, clinician-administered questionnaire used to assess the range of social interactions and performance situations that patients with SAD may fear and/or avoid (Heimberg et al., 1999).
2.2.2. Social Interaction Anxiety Scale (SIAS)
The SIAS is a 20-item, self-report scale that assesses fears of more general social interaction (Mattick and Clarke, 1998).
2.2.3. Clinician-Administered PTSD Scale for DSM-IV (CAPS)
The CAPS the “gold standard” for PTSD assessment and diagnosis and is clinician-administered. Severity ratings are based on symptom frequency and intensity (Blake et al., 1995).
2.2.4. Life Stressor Checklist-Revised (LSC-R)
The LSC-R is a self-report measure designed to screen for potentially traumatic events in a person’s lifetime (Wolfe and Kimerling, 1997).
2.2.5. PTSD Checklist-Civilian (PCL-C)
The PCL-C is a 17-item, self-report measure reflecting DSM-IV symptoms of PTSD. The PCL-C asks about symptoms in relation to generic “stressful experiences” and can be used in aiding diagnostic assessment of PTSD (Blanchard et al., 1996; Weathers et al., 1993).
2.2.6. Hamilton Anxiety Scale (HAM-A)
The HAM-A is a 14-item, clinician-administered questionnaire that measures severity of a patient’s anxiety (Hamilton, 1959).
2.2.7. Hamilton Depression Scale (HAM-D)
The HAM-D is a 21-item, clinician-administered questionnaire used to determine a patient’s level of depression (Hamilton, 1960).
2.2.8. Beck Depression Inventory (BDI-II)
The BDI-II is a 21-item, self-report questionnaire used to measure severity of depression (Beck et al., 1974; Beck and Steer, 1984; Beck et al., 1996; Beck et al., 1961).
2.2.9. State-Trait Anxiety Inventory (STAI)
The STAI is a 40-item, self-report measure of trait and state anxiety (Spielberger et al., 1983).
Between-group differences on the additional assessment measures are presented in the Supplemental Results and Supplemental Table 1. All participants gave written informed consent after explanation of the experimental protocol with research staff and were monetarily compensated for their time, as approved by the University of Michigan Institutional Review Board.
2.3. Fear conditioning, extinction, and testing procedures
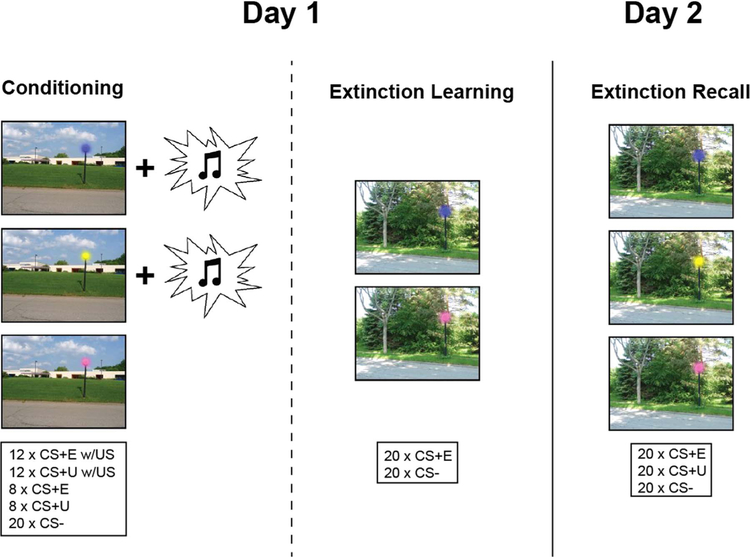
Participants were studied over 2 consecutive days (see Fig. 1) using a similar design developed and validated by Milad and colleagues (Milad et al., 2005, 2007a), which manipulates context using an ABB design (i.e., in which the context is the same during extinction learning and testing, but different from the context in which fear is first acquired). Digital photographs of two different outdoor scenes (school building and forest) constituted the visual contexts. Each scene contained a street light and different colors (blue, pink, yellow) of the lit street light constituted the conditioned stimuli (CSs).
Fig. 1.
Schematic of the experimental protocol.
On Day 1, all participants underwent partial discrimination fear conditioning, in which they were presented with two CSs on a computer screen (CS+s; e.g., blue and yellow lights) that co-terminated with an aversive white noise burst US through a pair of headphones (500 ms, 100 dB) at a partial reinforcement rate of 60% (i.e., partial reinforcement extinction effects; cf. (i.e., partial reinforcement extinction effects; cf. Milad et al., 2009; Milad et al., 2007b)). A third CS (e.g. pink light) was presented during fear conditioning but never paired with the US (CS-). Fear acquisition consisted of 8 non-reinforced presentations of each of the CS+s, intermixed with an additional 12 presentations of each CS+ that co-terminated with the US, and 20 presentations of the CS-, all presented within the conditioning context (Context A). After approximately a 15 min break all participants underwent an extinction session in which one of the CS+s (e.g., blue light) was extinguished (CS+E) whereas the other CS+(e.g., yellow light) was not (CS+U; i.e., this CS+ was not presented during extinction learning). During extinction learning the CS+E was presented in the absence of the US. There were 20 CS+E and 20 CS- trials presented in the extinction context (Context B).
To assess extinction retention, we conducted an extinction memory recall test approximately 24 h after the extinction learning session (Day 2). Of note, all participants returned and completed the sessions on Day 2. Extinction recall consisted of 20 non-reinforced presentations of each of the CSs (CS+E, CS+U, and CS-) in Context B. In each experimental session (fear acquisition, extinction learning, extinction recall) the context picture was presented for 7–12 s: 3–8 s alone followed by 4 s in combination with the CS+ or CS−. The mean intertrial interval was 6 s (range: 4–9 s) determined from the offset of the CS to the onset of the context. The designation of colored lights (blue, yellow, or pink) as CS+E, CS+U, or CS-, and the context (building or forest) as Context A or Context B was counterbalanced across the participants. In addition, the order of trials was pseudo-randomized, such that no more than 2 presentations of the same colored light (pink, yellow, or blue) occurred in a row.
At the beginning of each session participants were told that they may or may not hear a loud noise burst and were instructed to pay attention to the computer screen and try to figure out the relationship between the color of the light and the noise burst. During each presentation of the colored light stimuli, participants were asked to rate their expectancy that the US would occur on a three-button response pad (“Will you hear a loud noise burst?”: 0=Definitely not; 50=Unsure; 100=Definitely)(Dunsmoor et al., 2008; Knight et al., 2004; Norrholm et al., 2006). US expectancy was scored as the first response within 3 s of CS onset and was used to assess learning of the stimulus contingencies during each session (e.g. CS-US, CS-noUS). Of note, we used US expectancy ratings as our primary measure of conditioned fear, rather than psychophysiological responding (e.g. skin conductance; Jovanovic et al., 2006; Soeter and Kindt, 2010; Warren et al., 2014). The main drawback of using psychophysiological measures as indicators of fear is the large inter-individual variability of autonomic responses, which limits the direct comparability between subjects (Picard et al., 2016). Additionally, some participants do not show measurable levels of SCR during fear conditioning, which precludes assessment of fear conditioning (Bjorkstrand, 1990; Schell et al., 1991). The US expectancy measure gives an indication about the extent to which participants expect the aversive outcome and there is sufficient evidence for the face validity, diagnostic validity, predictive validity, and construct validity of the US-expectancy measure (Boddez et al., 2013). As such, US expectancy mimics threat expectancies, which are a clear-cut symptom of pathological fear.
In addition, participants were asked to provide a rating of subjective anxiety using the Subjective Units of Discomfort Scale (SUDS; 0=no anxiety; 25=mild anxiety, alert, able to cope; 50=moderate anxiety, some trouble concentrating; 75=severe anxiety, thoughts of leaving; 100=very severe anxiety, worst ever experienced; Hope et al., 2000; Wolpe and Lazarus, 1966) at three time points during the extinction learning session: before the start of the session, in the middle of the session, and at the end of the session; and before the beginning of the extinction recall session. The SUDS ratings were collected as a secondary measure of fear reactivity. Headphones remained in place during all sessions (removed only during breaks).
Of note, all female subjects completed study sessions about 1 week prior to menses onset (based on self-reports of last period and cycle length), to ensure that they were studied while estrogen levels were low. This restriction was based on evidence that high estradiol levels can facilitate fear extinction (Milad et al., 2010; Zeidan et al., 2011).
2.4. Data analysis
US expectancy and SUDS ratings were analyzed using analysis of variance (ANOVA) controlling for age. Post-hoc comparisons between and within groups, using independent and paired t-tests, respectively, were performed after a significant main effect or interaction. We used a significance threshold of p < .05 (two-tailed), corrected for multiple comparisons using Bonferroni correction. We also calculated and report Cohen’s d, an index of effect size, for between-group effects (Cohen, 1988) and d for within-group/repeated measures effects (dRM; Equation 8 from Morris and DeShon, 2002), as well as confidence intervals.
3. Results
3.1. Fear acquisition
An ANOVA of US expectancy ratings with one, three-level factor – group (HC, SAD, PTSD), and two, two-level factors—stimulus (CS +[collapsed across CS+E and CS+U], CS-) and time (early acquisition, late acquisition), controlling for age–revealed a significant main effect of group (F(2,39)=5.80, p=.006), main effect of stimulus (F(1,39) =13.03, p=.001), and group by stimulus interaction (F(2,39)=7.54, p=.002).
All groups acquired differential US expectancies between the CS+ and CS-, however at different rates. Specifically, participants in the HC and SAD groups expected the US to occur during the CS+ more than during the CS- during early and late acquisition, whereas the PTSD group didn’t differentiate US expectancy to the CS+ relative to the CS-until late acquisition (Table 1; Fig. 2A). Of note, both patient groups had a significantly greater expectations that the US would occur to the CS- during early acquisition compared to the HC group and this difference between the PTSD and HC group was significant during late acquisition as well (Table 2; Fig. 2A). The PTSD group also expected the US to occur more during the CS- compared to the SAD group.
Table 1.
Significant within-group comparisons of US expectancy ratings during fear acquisition, extinction learning, and extinction recall.
| t-score | df | dRM | Lower | Upper | ||
|---|---|---|---|---|---|---|
| Fear acquisition | ||||||
| CS+ vs. CS− Early | 8.92 | 14 | 3.95 | 48.35 | 78.98 | HC*** |
| 2.75 | 15 | .69 | 7.33 | 57.64 | SAD* | |
| CS+ vs. CS− Late | 10.40 | 14 | 4.17 | −.34 | 10.34 | HC*** |
| 11.43 | 14 | .88 | 4.26 | 36.93 | SAD*** | |
| 2.30 | 12 | .68 | .87 | 19.90 | PTSD* | |
| Extinction learning | ||||||
| CS+E vs. CS− Early | 3.77 | 13 | 1.29 | 11.51 | 42.47 | HC** |
| CS+E Early vs. Late | 3.98 | 13 | 1.79 | 11.98 | 40.41 | HC** |
| CS− Early vs. Late | 2.53 | 12 | .71 | 2.06 | 27.94 | PTSD* |
| Extinction recall test | ||||||
| CS+ E vs. CS− | 6.61 | 14 | 1.71 | 7.38 | 14.47 | HC*** |
| 2.25 | 14 | .59 | .32 | 13.68 | SAD* | |
| 2.40 | 12 | .68 | 1.21 | 24.73 | PTSD* | |
| CS+U vs. CS− | 2.60 | 14 | .76 | 2.43 | 25.42 | HC* |
HC, healthy controls; SAD, social anxiety disorder; PTSD, posttraumatic stress disorder; Within-group comparisons were performed using paired t-tests, corrected for multiple comparisons using Bonferroni correction;
p≤.05.
p≤.01.
p≤.001.
Fig. 2. Mean US expectancy ratings during fear acquisition, extinction learning, and extinction recall.
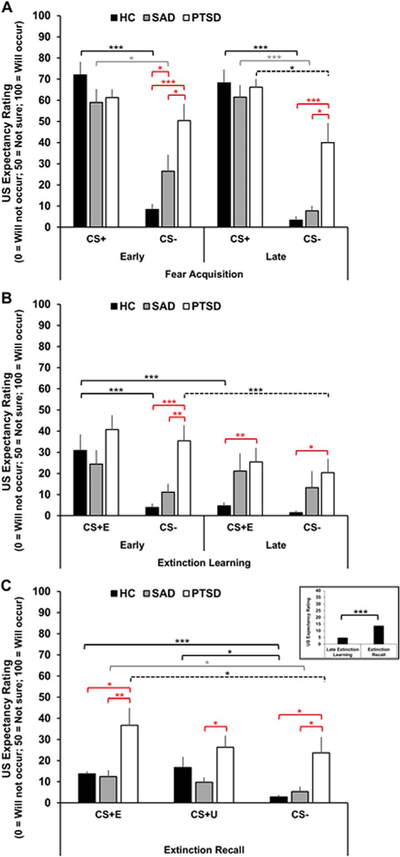
A, Mean US expectancy ratings to the CS+ and CS- during early and late fear acquisition. B, Mean US expectancy ratings to the CS+E and CS- during early and late extinction learning. C, Mean US expectancy ratings to the CS+E (left), CS+U (middle) and CS- (right) during the extinction recall test. HC (black bars), SAD (gray bars), and PTSD (white bars). Inset shows a bar graph representing the mean US expectancy ratings to the CS+E during late extinction learning and extinction recall in the HC group. Error bars are standard error of the mean (SEM). Brackets indicate significant differences within groups (HC, black; SAD, gray; PTSD, dashed) and between groups (red). *p≤.05**p≤.01***p≤.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Table 2.
Mean US expectancy ratings and significant between-group comparisons during fear acquisition, extinction learning, and extinction recall.
| HC |
SAD |
PTSD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M % | SD | M % | SD | M % | SD | t-score | Df | d | 95% CI |
|||
| Lower | Upper | |||||||||||
| Fear acquisition | ||||||||||||
| Early | ||||||||||||
| CS+ | 72 | 24.06 | 58.98 | 25.37 | 61.24 | 14.16 | ||||||
| CS− | 8.33 | 10.29 | 26.49 | 31.03 | 50.38 | 28.68 | −2.21 | 18.46 | .80 | −35.36 | −.96 | HC < SAD* |
| −5.01 | 14.67 | 1.95 | −59.96 | −24.14 | HC < PTSD*** | |||||||
| −2.13 | 27 | .80 | −46.88 | −.90 | SAD < PTSD* | |||||||
| Late | ||||||||||||
| CS+ | 68.17 | 24.43 | 61.46 | 23.05 | 66.15 | 14.38 | ||||||
| CS− | 3.33 | 6.99 | 7.67 | 9.23 | 40.00 | 33.22 | −3.90 | 12.92 | 1.53 | −56.97 | −16.37 | HC < PTSD** |
| −3.40 | 13.61 | 1.33 | −52.81 | −11.86 | SAD < PTSD* | |||||||
| Extinction learning | ||||||||||||
| Early | ||||||||||||
| CS+E | 30.92 | 28.00 | 24.41 | 25.67 | 40.77 | 24.65 | ||||||
| CS− | 3.93 | 7.12 | 11.15 | 15.14 | 35.38 | 26.57 | 4.13 | 13.60 | 1.87 | −47.83 | −12.09 | HC < PTSD*** |
| 2.91 | 18.44 | 1.16 | −41.73 | −6.74 | SAD < PTSD** | |||||||
| Late | ||||||||||||
| CS+E | 4.72 | 6.12 | 21.17 | 31.92 | 25.38 | 24.28 | 2.98 | 13.41 | 1.36 | −35.59 | −5.74 | HC < PTSD** |
| CS− | 1.43 | 3.63 | 13.33 | 29.98 | 20.38 | 23.40 | 2.89 | 12.54 | 1.40 | −33.19 | −4.72 | HC < PTSD* |
| Extinction recall test | ||||||||||||
| CS+E | 13.67 | 4.81 | 12.33 | 11.78 | 36.67 | 29.53 | 2.78 | 12.55 | 1.34 | −40.96 | −5.04 | HC < PTSD* |
| 2.79 | 15.29 | 1.18 | −42.93 | −5.74 | SAD < PTSD** | |||||||
| CS+U | 16.67 | 19.52 | 9.67 | 8.96 | 26.37 | 20.43 | 2.73 | 15.95 | 1.14 | −29.68 | −3.72 | SAD < PTSD* |
| CS− | 2.74 | 3.89 | 5.33 | 8.96 | 23.70 | 26.72 | 2.80 | 12.44 | 1.37 | −37.19 | −4.73 | HC < PTSD* |
| 2.37 | 14.34 | 1.03 | −34.98 | −1.75 | SAD < PTSD* | |||||||
HC, healthy controls; SAD, social anxiety disorder; PTSD, posttraumatic stress disorder; Group comparisons were performed using independent t-tests, corrected for multiple comparisons using Bonferroni correction;
p≤.05.
p≤.01.
p≤.001.
3.2. Extinction learning
An ANOVA of US expectancy ratings with one, three-level factor– group (HC, SAD, PTSD), and two, two-level factors—stimulus (CS+E, CS-) and time (early extinction, late extinction), controlling for age– revealed a significant main effect of time (F(1,38)=18.86, p < .001), group by time interaction (F(2,38)=3.68, p=.03), and stimulus by time interaction (F(1,38)=6.06, p=.02).
Not surprisingly, during early extinction the HC group expected the US to occur when presented with the CS+E, but by the end of the extinction session the HC group no longer expected the US during the CS+E, suggesting they were able to learn the extinction contingency (CS-noUS; Table 1; Fig. 2B). US expectancy ratings were similarly low during the CS- across the extinction learning session in the HC group. Additionally, during early extinction the HC group expected the US to occur during the CS+E and did not expect the US to occur during the CS-. This is further evidence that the HC group successfully acquired differential stimulus contingencies between the CSs and US during the previous fear acquisition phase. During late extinction there was no difference between US expectancy ratings to the CS+E and CS-. Interestingly, US expectancy rating during the CS+E did not change over the course of extinction (early vs. late) in either the SAD or PTSD groups (Table 1; Fig. 2B). Additionally, during early extinction US expectancy ratings during the CS+E did not differ from CS- ratings in either patient group. US expectancy ratings to the CS- were significantly higher during early compared to late extinction in the PTSD group; there was no difference in the SAD group (Table 1; Fig. 2B).
Between group analyses revealed no significant differences in US expectancy ratings to the CS+E or CS- during early or late extinction between the HC and SAD groups (ps > .10), but compared to the HC group, the PTSD group expected that the US would occur during the CS +E during late extinction and during the CS- during both early and late extinction. The PTSD group also indicated that they expected the US to occur during the CS- during early extinction to a greater extent than the SAD group (Table 2; Fig. 2B).
An ANOVA of SUDS ratings with two, three-level factor–group (HC, SAD, PTSD), and time (pre-extinction, mid-extinction, post-extinction), controlling for age–revealed a significant main effect of group [F(2,40)=8.45, p=.001]. There was no significant difference in SUDS ratings during pre- and mid-extinction in the HC group, however the HC group did report significantly lower SUDS ratings post-compared to pre-extinction (Table 3; Fig. 3). Both the SAD and PTSD groups reported significantly higher SUDS ratings than the HC group pre-, mid-, and post-extinction (Table 3; Fig. 3). Of note, SUDS ratings were not significantly different between the SAD and PTSD group and did not significantly change over the course of extinction in either group (ps > .50).
Table 3.
Mean SUDS ratings and significant between-group comparisons during extinction learning and extinction recall.
| HC |
SAD |
PTSD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | t-score | df | d | 95% CI |
|||
| Lower | Upper | |||||||||||
| Extinction Learning | ||||||||||||
| Pre | 15.33 | 18.46 | 33.13 | 17.02 | 36.15 | 18.95 | 2.79 | 29 | 1.00 | −30.82 | −4.76 | HC < SAD** |
| 2.94 | 26 | 1.11 | −35.38 | −6.26 | HC < PTSD** | |||||||
| Mid | 8.67 | 14.07 | 31.85 | 18.70 | 30.00 | 17.80 | 3.88 | 29 | 1.42 | −35.43 | −10.99 | HC < SAD*** |
| 3.54 | 26 | 1.34 | −33.72 | −8.95 | HC < PTSD** | |||||||
| Post | 5.33 | 10.60 | 28.13 | 20.40 | 32.31 | 19.64 | 3.86 | 29 | 1.47 | −34.86 | −10.72 | HC < SAD*** |
| 4.61 | 26 | 1.78 | −39.01 | −14.94 | HC < PTSD*** | |||||||
| Extinction recall test | ||||||||||||
| Pre | 4.00 | 7.37 | 14.38 | 17.11 | 19.23 | 17.54 | 2.17 | 29 | .85. | −20.17 | −.58 | HC < SAD* |
| 2.92 | 15.64 | 1.22 | −26.32 | −4.14 | HC < PTSD** | |||||||
HC, healthy controls; SAD, social anxiety disorder; PTSD, posttraumatic stress disorder; Group comparisons were performed using independent t-tests, corrected for multiple comparisons using Bonferroni correction;
p≤.05.
p≤.01.
p≤.001.
Fig. 3. Mean SUDS ratings before, during, and after the extinction learning session and before the extinction recall test.
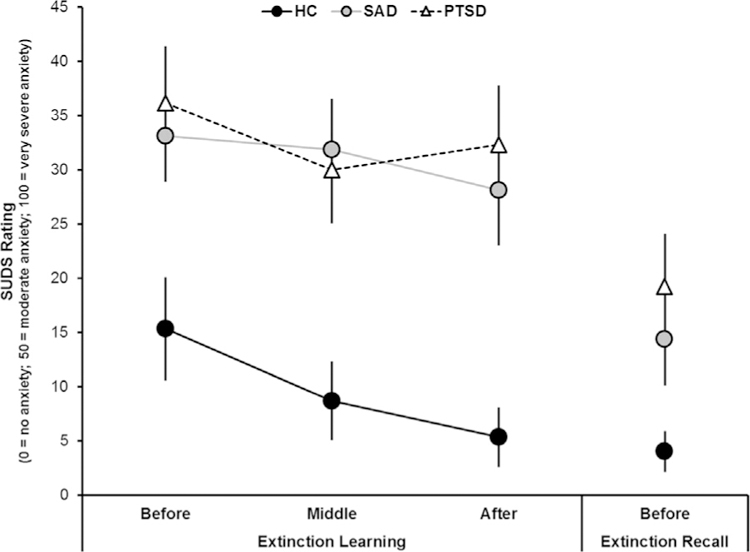
HC (black circles), SAD (gray circles), and PTSD (white triangles). Error bars are standard error of the mean (SEM).
To explore whether SUDS ratings during mid- and post-extinction learning were associated with US expectancy ratings to the CS+E during late extinction we conducted a Pearson’s correlation analysis across all groups. We found that SUDS ratings at mid-extinction and post-extinction were positively correlated with US expectancy ratings to the CS+E during late extinction (SUDS mid-extinction: r(42)=.41, p=.007; SUDS post-extinction: r(42)=.54, p < .001).
Next, we conducted a mediation analysis using PROCESS software (2.16.2; Hayes, 2013) implemented in SPSS to test for the mediating effects of SUDS ratings mid- and post-extinction on the association between group and US expectancy ratings to the CS+E during late extinction. We tested mediation with 2 dummy coded variables to represent the 3 groups, using the HC group as the reference group. Using ordinary least squares regression, 2 separate analyses were conducted to estimate the linear model. As recommended for small samples, we used nonparametric bootstrapping analyses (based on 50,000 bootstrapped samples; see Preacher and Hayes, 2004; Preacher et al., 2007) to test the relative indirect effect. In these 2 analyses, mediation is significant at p < .05 if the 95% bias-corrected and accelerated confidence intervals for the indirect effect do not include “0” (Hayes, 2013; Preacher and Hayes, 2004; Preacher et al., 2007). Thus, the indirect effect (ab) through SUDS ratings during mid- and post-extinction and the direct effect (c′) of the group on US expectancy ratings to the CSs during late extinction had to be interpreted in light of the coding of the dummy variables (which compared SAD vs. PTSD).
Mediation analyses indicated that SUDS ratings during post-extinction, but not mid-extinction, significantly mediated the relationship between the patient (SAD, PTSD) groups (compared to the HC group) and US expectancy ratings to the CS+E during late extinction (abSAD=16.32, 95% CI [3.46, 43.21]; abPTSD=20.08, 95% CI [4.90, 50.00]). Relative direct effects of group on US expectancy ratings to the CS+E during late extinction were not significant, suggesting that SUDS ratings post-extinction fully mediated the pathway between group and US expectancy to the CS+E during late extinction.
3.3. Extinction recall test
An ANOVA of US expectancy ratings averaged during the first four trials of the extinction recall test with two, three-level factors–group (HC, SAD, PTSD) and stimulus (CS+E, CS+U, CS-), controlling for age–revealed a significant main effect of group (F(2,39)=7.00, p=.003) and main effect of stimulus (F (2,78)=9.94, p < .001).
Surprisingly, there was no significant differences in US expectancy ratings during the CS+E and CS+U within any of the groups (ps > .08; Table 1; Fig. 2C). However, all groups expected the US to occur during the CS+E to a greater extent than during the CS- (Table 1; Fig. 2C). The HC group also expected the US to occur during the CS+U to a greater extent than during the CS-; there were no significant differences in either of the patient groups. Contrary to our hypothesis, the HC group expected the US to occur to a greater extent during the CS+E during extinction recall compared to their expectations reported during late extinction learning (t(13)=5.06, p < .001, dRM=1.40, 95% CI [5.07, 12.63]), suggesting some spontaneous recovery of the initial CS-US contingency even after successful learning of the CS-noUS contingency during extinction learning (see inset of Fig. 2C).
Between group analyses revealed no significant differences in US expectancy ratings to the CS+E, CS+U, or CS- during extinction recall between the HC and SAD groups (ps > .10). However, compared to the HC and SAD groups, the PTSD group expected the US to occur to a greater extent during both the CS+E and the CS- (Table 3; Fig. 2C). The PTSD group also expected the US to occur during the CS+U to a greater extent compared to the SAD group.
Both the SAD and PTSD groups reported significantly higher SUDS ratings than the HC group right before the extinction recall test (Table 3; Fig. 3). Pre-extinction recall SUDS ratings were not significantly different between the SAD and PTSD groups. To explore whether SUDS ratings right before the extinction recall test were associated with US expectancy ratings to the CSs during extinction recall we conducted a Pearson’s correlation analysis across all groups. We found that pre-extinction recall SUDS ratings were positively correlated with US expectancy ratings to the CS+E (r(39)=.37, p=.02) and CS- (r(39)=.32, p=.05) during extinction recall., however follow-up analyses to test the mediating effects of SUDS prior to extinction recall on the association between group and CSs were not significant.
Of note, none of the US expectancy ratings during any of the session (fear acquisition, extinction learning, and extinction memory recall) were significantly correlated with SAD or PTSD symptom severity (LSAS; CAPS and PCL) in the SAD or PTSD groups, respectively (ps >50).
4. Discussion
In the present study we found that both patient groups (SAD, PTSD) could acquire differential conditioning between a danger cue (CS+) and safe cue (CS-). However, both groups associated the US to safe cues and the PTSD group also tended to overestimate US occurrence across all cues. Neither patient group showed evidence of successful extinction learning nor did they differentiate US expectancy between the CS+E and the CS- during extinction learning. Moreover, neither patient group differentiated CS+E from CS+U in terms of US expectancy during extinction recall and the PTSD group continued to overestimate US expectancy. Together, these preliminary findings suggest that, in SAD and PTSD, expectancy of an aversive event (US) is generalized across neutral stimuli even if the stimuli never signaled aversive outcomes. Moreover, individuals with PTSD, specifically, may tend to inflate the aversive associations of stimuli.
During fear acquisition the SAD group displayed normal fear acquisition (no different than HC group), such that they rated the US as more likely to occur during the CS+ than during the CS-. However, during early acquisition the SAD group reported that they expected the US to occur during the CS- compared to the HC group, suggesting difficulty acquiring contingencies that are safe (CS-). This finding is consistent with a prior fear conditioning study, in which patients with SAD also reported an increased US expectancy, specifically to the CS-, compared to HCs (Hermann et al., 2002). Our preliminary results suggest that individuals with SAD may initially default towards associating the US across stimuli, but are able to quickly acquire the appropriate CS-US contingencies with repeated experience with the safe cue in the absence of an aversive outcome.
The opposite appears to be true for PTSD. Patients with PTSD did not discriminate US occurrence between the CSs until late acquisition and even then still maintained the expectation that the US would occur during the CS-. This finding is consistent with previous reports of failure to discriminate between the CS+ and CS-, a general inability to acquire safety signals, and increased fear conditioning in patients with PTSD (Blechert et al., 2007; Duits et al., 2015; Grillon and Morgan, 1999; Jovanovic et al., 2010, 2009; Lissek et al., 2005; Norrholm and Jovanovic, 2011; Norrholm et al., 2011; Orr et al., 2000; Peri et al., 2000). Several prior studies have shown that individuals with PTSD have difficultly discriminating between safety and danger cues as evidenced by no difference in fear potentiated startle magnitude to both cues, which may suggest an impaired ability to translate and inhibit fear responses in the presence of a safety cue and/or tend to generalize fear to stimuli that resemble the CS+(Duits et al., 2015; Jovanovic et al., 2012, 2010; Lissek et al., 2005). Increased expectancy of aversive events (US) to safety cues may also be a reflection of hyperarousal symptoms in PTSD. For instance, increased fear potentiated startle responses to a safety cue (CS-) have been reported to predict hyperarousal symptoms in PTSD (Glover et al., 2011) and suppression of cortisol in patients with PTSD has been shown to reduce exaggerated fear responses to a CS+(Jovanovic et al., 2011). Of note, unlike previous studies, individuals with PTSD in our study were eventually able to acquire differential CS-US contingencies between the CS+ and CS-.
During extinction learning there were no significant differences in US expectancy ratings to the CS+E or CS- during early or late extinction between the HC and SAD groups. However, whereas the HC group’s expectations of the US occurring during the CS+E decreased over time, the SAD group’s expectations that the US would occur during the CS+E remained high and did not change. Likewise, the PTSD group’s expectations that the US would occur during the CS+E did not change over the course of the extinction session. Additionally, at the beginning of extinction, US expectancy ratings to the CS+E did not differ from the CS- in either patient group, suggesting that both SAD and PTSD groups did not differentiate CS-US contingencies between the two stimuli. Moreover, while US expectancy ratings to the CS+E and the CS- did not differ between the HC and SAD groups, the PTSD group had increased expectations that the US would occur to the CS+E during late extinction and to the CS- during early and late extinction compared to the HC group. The PTSD group also reported greater expectations that the US would occur to the CS- during early extinction compared to the SAD group. Similar to what we observed during fear acquisition, these preliminary findings suggest that the PTSD group had difficulty acquiring the appropriate CS-US contingencies to the CSs during extinction learning. Importantly, the failure to extinguish US expectancy to the CSs in the SAD and PTSD groups may be secondary to the lack of discrimination and over-expectation of the US occurring during fear acquisition. In addition, a general heightened anxiety level (SUDS ratings) in the SAD and PTSD groups may have interfered with their ability to change (decrease) US expectancy to the CSs during extinction learning. The results of the mediation analysis support this interpretation. We found that SUDS ratings collected at the end of extinction learning mediated the association between group and US expectancy ratings to the CS+E during late extinction.
Another possible interpretation for the lack of extinction learning in the patient groups may be related to low estrogen levels in female participants during extinction. In the present study all female subjects completed study sessions about 1 week prior to menses onset when estrogen levels were low, based on evidence that high estradiol levels can facilitate fear extinction (Milad et al., 2010; Zeidan et al., 2011). However, other studies support the opposite effect—among women with PTSD and low estrogen levels, fear responding is higher during extinction learning (Glover et al., 2012, 2015). Unfortunately, the majority of our participant sample was female, thus limiting our ability to compare the effects of gender within our patient groups.
Since we did not observe extinction of the CS-US contingency in either patient group during the extinction learning session it is difficult to interpret their results during extinction recall. Neither patient group rated the CS+E and the CS+U differently, which could be that they never learned the extinction association, as may be suggested by the extinction learning session. Consistent with the previous sessions, the PTSD group continued to overestimate threat to all CSs (CS+E, CS+U, CS-). In addition, patients with SAD and patients with PTSD reported higher levels of anxiety at the beginning of the extinction recall test compared to the HC group. However, what is most interesting is that while neither patient group displayed within-session habituation of subjective anxiety during extinction learning, their self-reported anxiety levels were significantly lower on the following day prior to the extinction recall session, suggesting some between-session habituation of anxiety. Contrary to what we would expect, US expectancy ratings to the CS+E during extinction recall were significantly higher than those reported during late extinction learning in the HC group, suggesting some spontaneous recovery of fear following successful within-session extinction learning.
As mentioned in the introduction, there have only been two prior behavioral studies that have looked at fear learning and extinction in SAD and they produced conflicting findings (Hermann et al., 2002; Lissek et al., 2008). These conflicting results may be a function of the stimuli that were used. One study used socially relevant audiovisual stimuli as the US (e.g., critical facial expression with negative feedback), whereas the other study used an aversive odor as the US. Both studies also used neutral facial expressions as the CS. Previous studies have reported that patients with SAD are likely to interpret neutral and other emotionally ambiguous facial expressions negatively (Cooney et al., 2006; Winton et al., 1995; Yoon and Zinbarg, 2007, 2008), making it possible that neutral faces may not be regarded as neutral stimuli that become a threat cue as a result of associative learning. The baseline difference in CS and/or US meaning in patients with SAD compared to HCs may obscure differences in the acquisition and extinction of conditioned fear responding to socially relevant cues. In our study we controlled for this potential confound and used non-social cues as the CSs (colored lights) and found that patients with SAD associated threat across the danger and safe cues, similar to findings from the aforementioned studies by Hermann and colleagues (Hermann et al., 2002).
4.1. Limitations
There are some important limitations that should be considered when interpreting the present results. For instance, the sample size is relatively small, especially in patients with PTSD, therefore these findings should be considered preliminary and require replication with larger sample sizes. In addition, we did not include a trauma-exposed group without PTSD, therefore we cannot differentiate whether our findings in the PTSD group are related to trauma exposure broadly or specifically to the development of PTSD following trauma exposure. Although we used validated assessment measures to classify participants into the SAD, PTSD, or HC group and participants with SAD or PTSD endorsed high SAD or PTSD symptoms, respectively, compared to each other and the HC group, we could not confirm accuracy of diagnosis because we only had one rater and thus lacked measures of inter-rater reliability. We also did not measure psychophysiological responding (e.g. skin conductance, heart rate, and startle) as an index of conditioned fear; instead we used subjective US expectancy ratings. The US expectancy measure does not grasp the arousal and behavioral outcomes of fear and anxiety, however a limitation of every outcome measure is that it highlights certain aspects of the phenomenon under study, but ignores other aspects. Although, it is possible there is a dissociation between subjective and physiological responses (Hermann et al., 2002; Norrholm et al., 2006, 2008), several other studies have demonstrated coherence between physiological measures and US expectancy ratings (Blechert et al., 2007; Norrholm et al., 2011; Unger et al., 2003). Various experimental- and/or participant-related factors may contribute to whether this dissociation between physiological measures and subjective ratings is present or absent, including, but not limited to: 1) the arousing quality of the US (e.g., odor, shock, white noise in relation to the physiological response measured Hermann et al., 2002); 2) the timing of the extinction session following acquisition (immediate vs. delayed; Norrholm et al., 2008); and 3) nonspecific effects of symptomology on physiological responsiveness. For instance, patients with SAD who are comorbid for avoidant personality disorder are less physiologically responsive (Hofmann et al., 1995) and patients with depression have lower skin conductance responses (Dawson et al., 1985).
Maladaptive cognitions play a crucial role in the development and maintenance of anxiety and fear responses. While some cognitions may be disorder-specific there are commonalities in cognition across disorders, such as future-oriented perceptions of danger or threat (Hofmann, 2008; Newby et al., 2015; Norton and Paulus, 2015; Watson, 2005). During extinction learning, changes in the CS-US expectancy is a key component of fear reduction and it has been shown, in patients with SAD, that treatment changes during exposure therapy are mediated via changes in cognitions (Hofmann, 2004; Newby et al., 2015; Norton and Paulus, 2015). In the present study both SAD and PTSD groups associated threat to the CS- despite it never being presented with the US, which may be related to their shared symptomology—an exaggerated fear response. A recent neuroimaging meta-analysis suggested that shared symptoms across SAD and PTSD may be associated with a common underlying neurobiology—specifically, amygdala and insula hyperactivity (Etkin and Wager, 2007). In the present study, the PTSD group, specifically, tended to inflate the threat value across the CSs, which may be a related to symptomology that is unique to PTSD, such as symptoms of emotional dysregulation (e.g., hypervigilance, hyperarousal, dissociation, emotional numbing, and re-experiencing; Association, 2013a; Association, 2013b). In fact, only PTSD, not SAD, demonstrate hypoactivation in prefrontal regions associated with the experience or regulation of affect (Etkin and Wager, 2007), which may be related to PTSD-unique symptoms. Indeed, while PTSD shares avoidance and hyperarousal symptomology with other fear-based disorders (SAD, specific phobia, panic), it is also characterized by symptoms that are more consistent with distress-related disorders (major depression), such as significantly diminished interest in activities (Watson, 2005). Therefore, unlike SAD, it is not clear whether PTSD is truly a fear-based disorders, distress disorder, or a combination of both, and its link to both fear and distress may be related to the divergent findings between PTSD and SAD. Thus, while additional research is needed to clarify differences and similarities in fear abnormalities across anxiety and trauma-related conditions and their specific symptom profiles, initial evidence suggests that maladaptive CS-US expectancy may be a core feature underlying their related psychopathology.
Supplementary Material
Acknowledgements
The authors would like to thank Katie Prater for her assistance in designing and programming the experimental task.
Role of funding source
The project described was supported by the National Center for Research Resources, Grant UL1RR024986, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000433. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. These funding sources were not directly involved in the study design; collection, analysis and interpretation of the data; writing of the report; or the decision to submit the article for publication.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jad.2016.09.018.
References
- Association, A.P., 2000. Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Author, Washington D.C. [Google Scholar]
- Association, A.P., 2013a. Anxiety Disorders, Diagnostic and Statistical Manual of Mental Disorders 5th ed.. American Psychiatric Publishing, Washington D.C. [Google Scholar]
- Association, A.P., 2013b. Trauma- and Stressor-Related Disorders, Diagnostic and Statistical Manual of Mental Disorders 5th ed.. American Psychiatric Publishing, Washington D.C. [Google Scholar]
- Beck A, Rial W, Rickets K, 1974. Short form of depression inventory: cross-validation. Psychol. Rep 34, 1184–1186. [PubMed] [Google Scholar]
- Beck A, Steer R, 1984. Internal consistencies of the original and revised Beck Depression Inventory. J. Clin. Psychol 40, 1365–1367. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Ball RW, 1996. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Personal. Assess 67, 588–597. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Bjorkstrand PA, 1990. Effects of conditioned stimulus pre-exposure on human electrodermal conditioning to fear-relevant and fear-irrelevant stimuli. Biol. Psychol 30, 35–50. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a Clinician-Administered PTSD Scale. J. Trauma Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA, 1996. Psychometric properties of the PTSD Checklist (PCL). Behav. Res Ther 34, 669–673. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH, 2007. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav. Res Ther 45, 2019–2033. [DOI] [PubMed] [Google Scholar]
- Boddez Y, Baeyens F, Luyten L, Vansteenwegen D, Hermans D, Beckers T, 2013. Rating data are underrated: Validity of US expectancy in human fear conditioning. J. Behav. Ther. Exp. Psychiatry 44, 201–206. [DOI] [PubMed] [Google Scholar]
- Briscione MA, Jovanovic T, Norrholm SD, 2014. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Front. Psychiatry 5, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences 2nd ed.. Lawrence Earlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH, 2006. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res 148, 55–59. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Braaten JR, Catania JJ, 1985. Diagnostic utility of autonomic measures for major depressive disorders. Psychiatry Res 15, 261–270. [DOI] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, Baas JM, 2015. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 32, 239–253. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC, 2008. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage 40, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD, 2012. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol. Psychiatry 72, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Norrholm SD, 2015. Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biol. Psychiatry 78, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T, 2011. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety 28, 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, Grant BF, 2016. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc. Psychiatry Psychiatr. Epidemiol 51, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA 3rd, 1999. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J. Abnorm Psychol 108, 134–142. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. Br. J. Med. Psychol 32, 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach Guilford Publications. [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR, 1999. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol. Med 29, 199–212. [DOI] [PubMed] [Google Scholar]
- Hermann C, Ziegler S, Birbaumer N, Flor H, 2002. Psychophysiological and subjective indicators of aversive pavlovian conditioning in generalized social phobia. Biol. Psychiatry 52, 328–337. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, 2004. Cognitive mediation of treatment change in social phobia. J. Consult Clin. Psychol 72, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, 2008. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin. Psychol. Rev 28, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Newman MG, Ehlers A, Roth WT, 1995. Psychophysiological differences between subgroups of social phobia. J. Abnorm. Psychol 104, 224–231. [DOI] [PubMed] [Google Scholar]
- Hope D, Heimberg R, Juster H, Turk C, 2000. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach Oxford University Press, USA. [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M, 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ, 2010. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress. Anxiety 27, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ, 2009. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res 167, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ, 2006. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav. Neurosci 120, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B, Ressler KJ, 2011. Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology 36, 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ, 2004. Neural substrates mediating human delay and trace fear conditioning. J. Neurosci 24, 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, Grillon C, 2008. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am. J. Psychiatry 165, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS, 2005. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res Ther 43, 1391–1424. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Clarke JC, 1998. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav. Res. Ther 36, 455–470. [DOI] [PubMed] [Google Scholar]
- Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH, 2007. Fear conditioning in panic disorder: Enhanced resistance to extinction. J. Abnorm. Psychol 116, 612–617. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S, 2013. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 70, 608–618, (quiz 554). [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL, 2005. Context modulation of memory for fear extinction in humans. Psychophysiology 42, 456–464. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL, 2007a. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL, 2007b. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM, 2010. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SB, DeShon RP, 2002. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 7, 105–125. [DOI] [PubMed] [Google Scholar]
- Newby JM, McKinnon A, Kuyken W, Gilbody S, Dalgleish T, 2015. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clin. Psychol. Rev 40, 91–110. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, 2011. Translational fear inhibition models as indices of trauma-related psychopathology. Curr. Psychiatry Rev 7, 194–204. [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ, 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ, 2006. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem 13, 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, Rothbaum B, Duncan EJ, 2008. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behav. Neurosci 122, 1016–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton PJ Paulus DJ, 2015. Toward a unified treatment for emotional disorders: update on the science and practice. Behav. Ther [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK, 2000. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J. Abnorm. Psychol 109, 290–298. [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY, 2000. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatry 47, 512–519. [DOI] [PubMed] [Google Scholar]
- Picard RW, Fedor S, Ayzenberg Y, 2016. Multiple arousal theory and daily-life electrodermal activity asymmetry. Emot. Rev 8, 62–75. [Google Scholar]
- Preacher KJ, Hayes AF, 2004. SPSS and SAS procedures for estimating indirect effects in simple mediation models.. Behav. Res. Methods Instrum. Comput.: J. Psychon 36, 717–731. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF, 2007. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivar. Behav. Res 42, 185–227. [DOI] [PubMed] [Google Scholar]
- Schell AM, Dawson ME, Marinkovic K, 1991. Effects of potentially phobic conditioned stimuli on retention, reconditioning, and extinction of the conditioned skin conductance response. Psychophysiology 28, 140–153. [DOI] [PubMed] [Google Scholar]
- Schiller D, Delgado MR, 2010. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci 14, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweckendiek J, Klucken T, Merz CJ, Tabbert K, Walter B, Ambach W, Vaitl D, Stark R, 2011. Weaving the (neuronal) web: fear learning in spider phobia. Neuroimage 54, 681–688. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 (Suppl. 20), 22–33, quiz 34–57. [PubMed] [Google Scholar]
- Soeter M, Kindt M, 2010. Dissociating response systems: erasing fear from memory. Neurobiol. Learn Mem 94, 30–41. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, et al. , 1983. Manual for the State-Trait Anxiety Inventory: STAI (Form Y) Consulting Psychologist Press, Palo Alto, CA. [Google Scholar]
- Unger W, Evans IM, Rourke P, Levis DJ, 2003. The S-S construct of expectancy versus the S-R construct of fear: which motivates the acquisition of avoidance behavior? J. Gen. Psychol 130, 131–147. [DOI] [PubMed] [Google Scholar]
- Warren VT, Anderson KM, Kwon C, Bosshardt L, Jovanovic T, Bradley B, Norrholm SD, 2014. Human fear extinction and return of fear using reconsolidation update mechanisms: the contribution of on-line expectancy ratings. Neurobiol. Learn Mem 113, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, 2005. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J. Abnorm Psychol 114, 522–536. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz B, Herman D, Huska J, Keane T, 1993. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Annual Covention of the International Society for Traumatic Stress Studies, San Antonio, TX. [Google Scholar]
- Winton EC, Clark DM, Edelmann RJ, 1995. Social anxiety, fear of negative evaluation and the detection of negative emotion in others. Behav. Res Ther 33, 193–196. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R, 1997. Gender issues in the assessment of Posttraumatic Stress Disorder. In: Wilson J, Keane T (Eds.), Assessing Psychological Trauma and PTSD Guilford, New York, 192–238. [Google Scholar]
- Wolpe J, Lazarus AA, 1966. Behavior Therapy Techniques Pergamon Press, New York. [Google Scholar]
- Yoon KL, Zinbarg RE, 2007. Threat is in the eye of the beholder: social anxiety and the interpretation of ambiguous facial expressions. Behav. Res Ther 45, 839–847. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Zinbarg RE, 2008. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. J. Abnorm. Psychol 117, 680–685. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR, 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry 70, 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.