Abstract
Background
MicroRNA-381 (miR-381) is proven to be involved in many human tumors. Bioinformatics prediction suggests that miR-381 is decreased in renal cell carcinoma. However, its biological functions in clear-cell renal cell carcinoma (ccRCC) remain largely unknown. The present research aimed to evaluate miR-381 expression in renal cancer tissues and its effects on cell proliferation, growth, migration, and chemoresistance.
Material/Methods
Sixty pairs of ccRCC and the adjacent non-tumor specimens were collected during routine therapeutic surgery. Quantitative real-time PCR (qRT-PCR) assay was employed to examine miR-381 expression in the ccRCC tissues and the associated adjacent tissues (the normal tissues adjacent to tumor tissues). Cell transfection assay and Thiazolyl Blue Tetrazolium Bromide (MTT) assay were utilized to observe effects of miR-381 on the cell proliferation, growth, invasion, and chemoresistance in the Caki-1 cell line and 786-O cell line. Flow cytometry was used to assess cell apoptosis. Caki-1 cell and 786-O cell Xenograft BALB/c mouse models were established.
Results
miR-381 expression was downregulated in ccRCC tissues in vivo and in cell lines in vitro. Downregulation of miR-381 promoted growth of cells and restrained the ccRCC cell apoptosis. Increased miR-381 combined with Ci and Pa suppressed the proliferation and enhanced the anti-tumor effects of Ci and Pa at tolerated concentrations in vitro. miR-381 inhibition promoted chemoresistance in vitro.
Conclusions
miR-381 levels were significantly downregulated in renal cancer tissues and miR-381 inhibition promoted tumor cell growth, migration, and chemoresistance.
MeSH Keywords: Carcinoma, Renal Cell; Chemotherapy, Adjuvant; Drug Resistance; MicroRNAs
Background
Renal cell carcinoma (RCC) is the second leading cause of death due to urological malignant tumors, and accounts for about 3% of all adult malignancies. Clear-cell RCC (ccRCC) is considered to be the most frequently occurring RCC histological category. ccRCC is characterized by inherent chemoresistance and radio-resistance [1]. Clinically, ccRCC is treated by radical nephrectomy. However, the recurrence rates and the metastases of RCC are higher after surgery, and the overall survival rate of metastatic ccRCC is about 20% at 5 years [2]. Currently, ccRCC, which rarely responds to chemotherapy and radiotherapy, develops rapidly with application of targeted therapies [3]. The targeting treatments for ccRCC in clinical practice mainly apply the mammalian target of rapamycin (mTOR) inhibition method, but with lower efficacies [4]. Thus, in order to better diagnose, treat, and predict RCC, reliable and specific biomarkers are urgently needed. In recent years, multi-chemoresistance has become a problem in cancer chemotherapy [5].
Although chemoresistance has been studied for many years, the associated knowledge is limited [6]. As a life-threatening disorder, cancer behaviors always cause genetic abnormalities of tumor cells [7]. MicroRNAs (miRNAs) are composed of 21- to 25-nucleotide (nt) non-coding and single-stranded RNAs, which involve many biological processes or effects, including cell growth, cell-fate specification, cell apoptosis, and cell differentiation [8]. At post-transcriptional levels, microRNAs play negative roles in modulating gene-coded protein expression, which is thus extensively applied in the enhancement of clinical diagnosis and treatment. Previous studies have proved that microRNAs participate in multi-chemoresistance in cancers such as lung adenocarcinoma and oral squamous cell carcinoma [9–11].
MicroRNAs (miRNAs) have recently been proven to be useful biomarkers for diagnosis, prediction, and prognosis of cancers and associated treatments [12]. Tang et al. originally proved the existence of miR-381 in glioma cells and clarified their effects. miR-381 expression is mainly distributed in the cells or tissues of brain cancers, such as gliomas, while the expression of miR-381 is increased according to tumor grade [6]. miR-381 is increased glioma cell proliferation in vivo and in vitro, the actions of which were also related to reduced suppression of the AKT signaling pathway and the MEK/ERK signaling pathway [13,14].
In this research, our findings illustrated that miR-381 levels were remarkably decreased in RCC tissues, and further demonstrated that the reduction of miR-381 enhanced tumor cell growth, migration, and chemoresistance.
Material and Methods
Patient sample collection
A total of 60 pairs of ccRCC and adjacent specimen tissues (non-tumor tissues) were collected from routine therapeutic operations at the 2nd Affiliated Hospital of Harbin Medical University, Harbin, China. All the ccRCC cancer tissue samples and the normal tissue samples were then flash-frozen in liquid nitrogen immediately after surgery and kept at −80°C. None of the patients had undergone radiotherapy or chemotherapy prior to the surgery. All patients provided informed consent and the study was for usage of materials or tissue samples. This study was also approved by the Ethics Committee of the 2nd Affiliated Hospital of Harbin Medical University, Harbin, China.
Cell cultures and treatments
The human RCC cells (Caki-1 cells and 786-O cells) were obtained from the Chinese Academy Cell Resource Center (Shanghai, China). The 786-O cells were cultured in Roswell park memorial institute-1640 (RPMI 1640) (Gibco BRL. Co., Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL. Co.) at 37°C with 5% CO2. The Caki-1 cells were cultured in McCoy’s 5A Media (modified with tricine) supplemented with 10% FBS (Gibco BRL. Co.) at 37°C with 5% CO2. The miR-381 mimics, anti-sense miR, and negative control were synthesized by Genepharma Co. (Shanghai, China). The Caki-1 cells and 786-O cells were seeded onto 6-well plates (Corning-Costar, NY) 24 h before the experiment. When the cell density reached 70–80%, the miR-381 mimics, anti-sense miR, and negative control were transiently transfected into the cells using Lipofectamine™ 2000 reagent (Cat. No. 11668-027, Invitrogen, CA) according to instructions of the manufacturer.
Quantitative real-time PCR assay (qRT-PCR)
Total RNAs were extracted from the cancer tissue samples and the cancer cell lines by using a total RNA Extraction Kit (Cat. No. 1560, Ambion, Inc., Austin, TX). All the primers (Table 1) were synthesized by Shanghai Sangon Biotech Co. (Shanghai, China). Small nuclear U6 small nuclear RNA (snRNA, Sangon Biotech, Shanghai, China) was employed as the internal control. qRT-PCR assay was conducted on complementary DNA (cDNA) with specific primers for miR-381. qRT-PCR assay was conducted with Applied Biosystems PCR equipment (Mode: 7500, PE Gene Applied Biosystems, Foster, CA) and with SYBR Green PCR Master Mix (PE Gene Applied Biosystems., Foster, CA). The differences in miR-381 expression, represented as fold-changes, were calculated with comparative cycle threshold (2−ΔΔCT) method.
Table 1.
Real-time qRT-PCR primers used for quantification of renal cancer cell gene expression.
| Forwards | Reverse | |
|---|---|---|
| Hsa-miR-381-3p | 5′-TATACAAGGGCAAGCTCTCTGT-3′ | – |
| NC mimic | 5′-UUCUCCGAACGUGUCACGUTT-3′ | 5′-ACGUGACACGUUCGGAGAATT-3′ |
| Mimic | 5′-UAUACAAGGGCAAGCUCUCUGU-3′ | 5′-AGAGAGCUUGCCCUUGUAUAUU-3′ |
| NC inhibitor | 5′-CAGUACUUUUGUGUAGUACAA-3′ | – |
| Inhibitor | 5′-ACAGAGAGCUUGCCCUUGUAUA-3′ | – |
Thiazolyl blue tetrazolium bromide (MTT)
The cell activities or growth were assessed with MTT approach. In brief, ccRCC cell lines were seeded onto the 96-well plates (Corning-Costar, NY) at a density of 1×104 cells/well and were cultured at 37°C overnight. Then, the miRNA-381 NC, mimics, and inhibitors were transfected into the cells. After incubation for 24 h, Paclitaxel (Pa) or Cis-platinum (Ci) were added, then continuously incubated for 48 h. Subsequently, the retained old medium was discarded and fresh medium containing MTT was added (MTT at a final concentration of 5 mg/ml, Sigma-Aldrich, St. Louis, MO). After MTT incubation at 37°C for 4 h, dimethyl sulfoxide (DMSO, final concentration of 200 μl/well) was added to dissolve the formed formazan products. Optical density (OD) values were assessed with a spectrophotometer (Mode: EL-800, Bio-Tek Instruments, Winooski, VT) at 490 nm.
Flow cytometry
Determination of cell apoptosis was conducted using flow cytometry assay. Briefly, the apoptosis of cells was evaluated by staining with Annexin V and PE/7-AAD using an Apoptosis Detection Kit (Cat. No. 559763, BD Biosciences, San Jose, CA) following the instructions of the manufacturer. The cells were washed using phosphate-buffered saline (PBS, Beyotime Biotech, Shanghai, China). Then, 100 μl cell suspensions containing 1×105 cells were stained using Annexin V-PE (5 μl) and 7-AAD (5 μl) at room temperature for 15 min. Subsequently, the cells in each well were incubated with 1×binding buffer (400 μl). The apoptosis of cells was analyzed using a FACScan flow cytometer (BD, Mountain View, CA).
Xenograft study
The athymic BALB/c mice (males, 4 weeks old) were obtained from Shanghai Laboratory Animal Company (SLAC, Shanghai, China). All mice were fed kept in specific pathogen-free (SPF) conditions based on the protocols, and the conducted experiments were approved by Institutional Animal Care and Use Committee (IACUC) of Harbin Medical University, Harbin, China. Caki-1 cells were harvested (1×107 cells/ml) and we injected 0.1 ml of cells into the skin of the front legs. Intraperitoneal injection for the Pa or PBS, Ci was firstly started into 2 mice each in either Caki-1 group on the 8th day, and subsequently repeated at least 5 times by once in 2 days. The tumor weight and sizes were measured in miR-381 mimics (6 mice on right side, including 3 mice for cisplatin treatment and 3 mice for paclitaxel treatment) and negative controls (NC, 6 mice on left side, including 3 mice for cisplatin treatment and 3 mice for paclitaxel treatment) every 2 days. At 25 days after injection of cells, mice were sacrificed and the tumor sizes/weights were evaluated and tissues were stored at −80°C for the analysis.
Chemotherapeutics
All of the chemotherapeutic drugs used, including paclitaxel (Pa, Taiji, Sichuan, China), and cisplatin (Ci, Haosen, Jiangsu, China), were of clinical grade. Chemoresistance experiments (the IC50 evaluation) were performed according to standard protocol.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 computer software (SPSS Inc., Chicago, IL, USA). All experiments were carried out at least 3 times, and all samples were analyzed in triplicate. Tukey’s post-hoc test validated analysis of variance (ANOVA) was used to compare the differences in miR-381 expression between cancer tissues and the normal tissues. The relationships between miR-381 expression and clinico-pathological parameters were analyzed with the Pearson χ2 test. Kaplan-Meier analysis was utilized to analyze overall survival. p<0.05 represented a statistically significant difference.
Results
miR-381 expressions were downregulated in ccRCC tissues and cancer cell lines
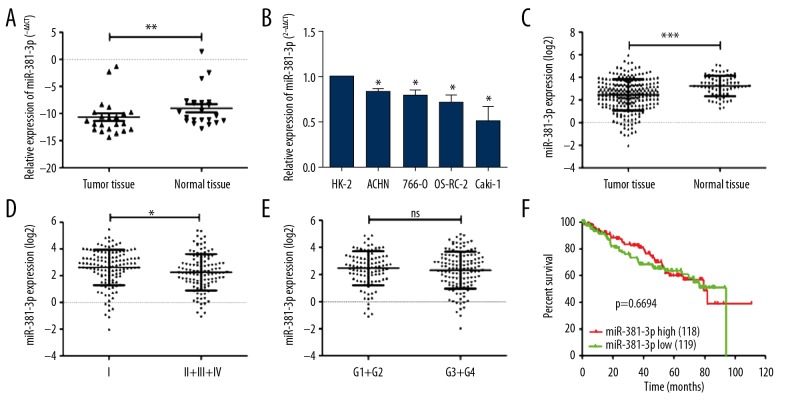
To evaluate functions of miR-381, the expressions of miR-381 were analyzed in ccRCC cancer tissues and adjacent normal tissues (Table 2). qRT-PCR analysis results indicated that miR-381 expression was significantly decreased in the ccRCC tissues compared to that in adjacent normal tissues (Figure 1A, p<0.05). Similarly, miR-381 expression was reduced in ccRCC cell lines (Figure 1B), which suggests that the downregulated miR-381 expression may be related to ccRCC, in agreement with the Cancer Genome Atlas (TCGA) database (Figure 1C). miR-381 expression was also significantly decreased in tissues at stages II/III/IV compared to tissues at stage I (Figure 1D), but there was no significant difference between grade 1/2 and grade 3/4 (Figure 1E). However, survival analysis findings showed that high expression of miR-381 did not affect overall survival in ccRCC patients (Figure 1F).
Table 2.
Correlation between clinic pathological characteristics and miR-381-3p expression levels in ccRCC patients.
| Characteristics | Number of patients | miR-381-3p | χ2 | p Value | |
|---|---|---|---|---|---|
| High expression (%) | Low expression (%) | ||||
| Sex | 118 | 119 | 0.362 | 0.548 | |
| Male | 161 | 78 (66.11) | 83 (69.75) | ||
| Female | 76 | 40 (33.89) | 36 (30.25) | ||
| Age | 0.844 | 0.358 | |||
| ≤65 | 158 | 82 (69.50) | 76 (63.87) | ||
| >65 | 79 | 36 (30.50) | 43 (36.13) | ||
| TNM stage | 7.813 | 0.005 | |||
| I | 121 | 71 (60.17) | 50 (42.02) | ||
| II+III+IV | 116 | 47 (39.83) | 69 (57.98) | ||
| Grade | 0.035 | 0.851 | |||
| Grade 1 + grade 2 | 101 | 51 (43.22) | 50 (42.02) | ||
| Grade 3 + grade 4 | 136 | 67 (56.78) | 69 (57.98) | ||
| Lymph node metastasis | 0.585 | 0.444 | |||
| No | 95 | 46 (38.99) | 49 (41.18) | ||
| Yes | 8 | 5 (4.24) | 3 (2.53) | ||
Figure 1.
Evaluation of miR-381 expression in ccRCC cancer tissues and cancer cells. (A) Expression of miR-381 was examined by using real-time PCR assay in human ccRCC cancer tissues and the associated adjacent non-cancer tissues (normal tissues adjacent to cancer tissues). ** p<0.01. (B) Different expression of miR-381 between normal kidney cells (HK-2 cells were used as control) and the ccRCC cancer cells. * p<0.05. (C) Expression of miR-381 was analyzed using the TCGA database. *** p<0.001. (D) Different expression of miR-381 at stage I and stage II/III/IV cancers. * p<0.05. (E) Differences in miR-381 expression in grade I/II cancer tissues and grade III/IV cancer tissues. (F) The overall survival analysis of miR-381-3p by Kaplan-Meier curve. * p<0.05, ** p<0.01, *** p<0.001 represents the differences between 2 groups.
Downregulation of miR-381 restrained apoptosis and promoted cell proliferation of ccRCC cancer cells
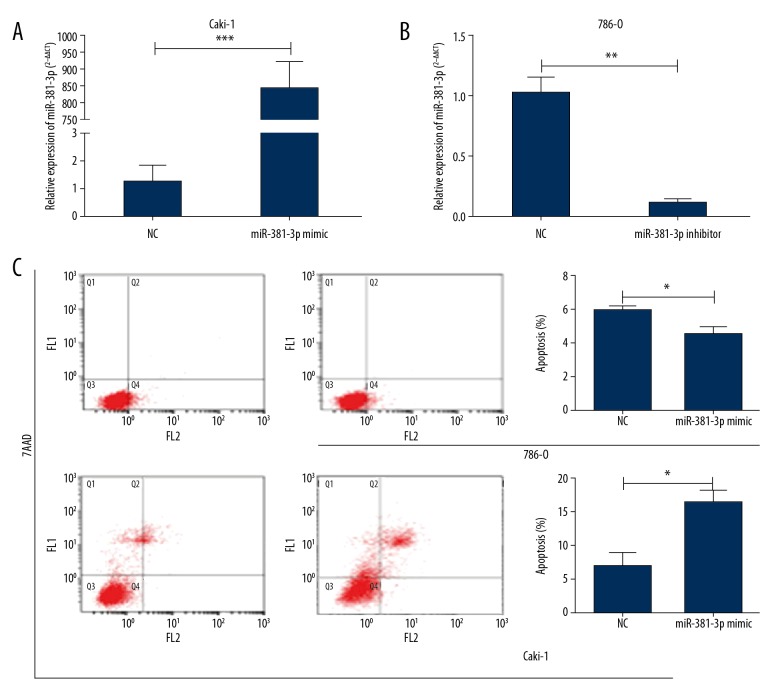
To detect functions of miR-381 in progression of ccRCC, the loss-of-function assay and the gain-of-function assay were conducted. According to endogenous expression of miR-381 in ccRCC cell lines (Figure 1B), we overexpressed miR-381 in Caki-1 cells (Figure 2A) and silenced miR-381 expression in 786-O cells (Figure 2B). To clarify effects of miR-381 expression on the proliferation of ccRCC cells, apoptosis was evaluated in ccRCC cells using flow cytometry assay. Our findings demonstrated that miR-381 overexpression remarkably promoted apoptosis in Caki-1 cells (Figure 2C).
Figure 2.
Downregulation of miR-381 repressed the proliferation and induced apoptosis of ccRCC. (A) In Caki-1, the expressions were significantly enhanced after 48-h transfection with miR-381, as shown by qPCR analysis, *** p<0.001. (B) In 786-O, the expression level was significantly reduced after 48-h transfection with miR-381 inhibitor, as shown by qPCR analysis, ** p<0.01. (C) The effect on cell apoptosis after 48-h transfection with miR-381 mimics (Caki-1) or inhibitor (786-O), * p<0.05.
Increased miR-381 combined with Ci and Pa suppressed cancer cell proliferation and potentiated anti-tumor effects of Ci and Pa at tolerated concentrations in vitro
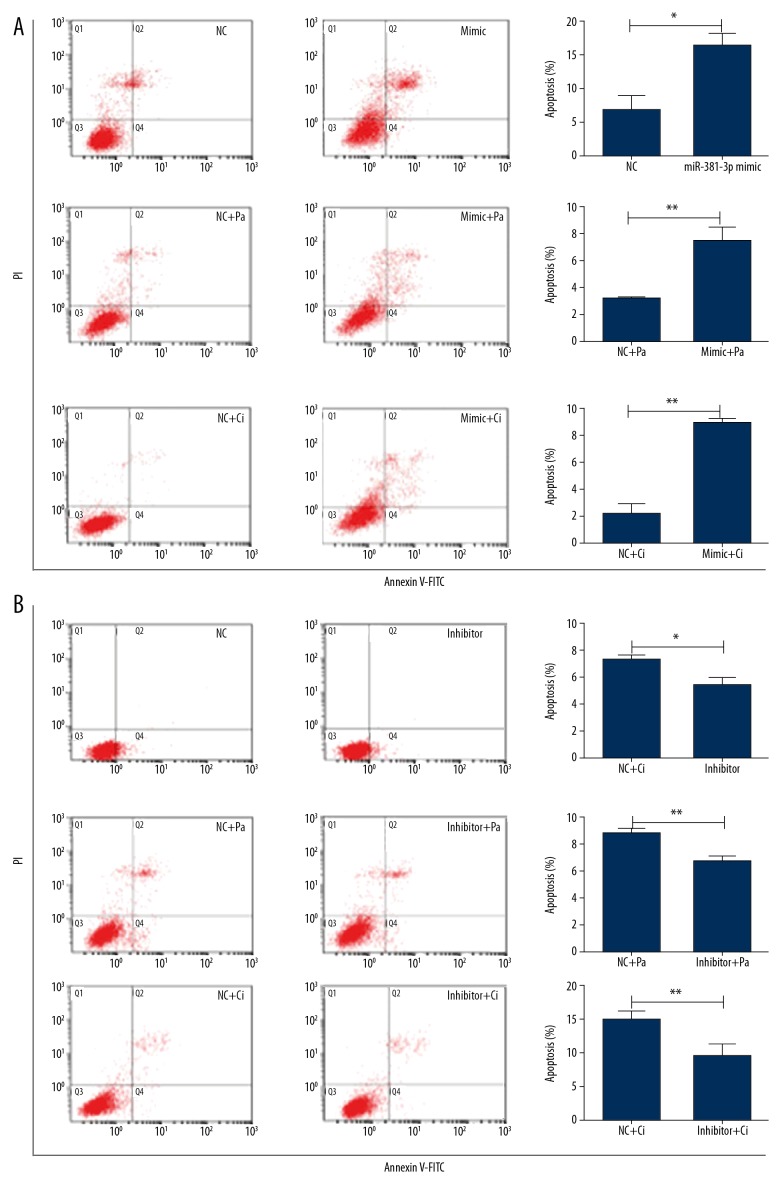
To investigate effects of miR-381 on proliferation of 786-O cells and Caki-1 cells, we evaluated the abilities of miR-381 to sensitize cells treated with Ci or Pa by using flow cytometry. We detected relative cell apoptosis after Ci and Pa treatment with miR-381 (or miR-381 expression) or not for 48 h. The 786-O cells and Caki-1 cells were treated with Ci and Pa at final concentrations of 0 μg/ml or 20 μg/ml, respectively. Transfection of miR-381 mimics into the Caki-1 cell lines significantly enhanced cell apoptosis by 3-fold after combined treatment compared to that with Ci or Pa treatment alone (Figure 3A). In contrast, after transfection with miR-381 inhibitor in 786-O cell lines, cell apoptosis was significantly decreased compared with the control, and the anti-tumor effects were obviously attenuated following Ci or Pa monotherapy (Figure 3B).
Figure 3.
miR-381 combined with Ci and Pa suppressed cancer cell proliferation and enhanced anti-tumor effects of the Ci and Pa on cancer cells at the tolerated concentration. (A) Effect on cell apoptosis after transfected with miR-381 mimics (Caki-1) after treatment with Pa or Ci. ** p<0.01 represents differences between 2 groups. (B) The effect on cell apoptosis after transfected with miR-381 inhibitors (786-O) after treatment with Pa or Ci. * p<0.05 and ** p<0.01 represents differences between 2 groups.
miR-381 inhibition promoted the in vitro chemoresistance levels
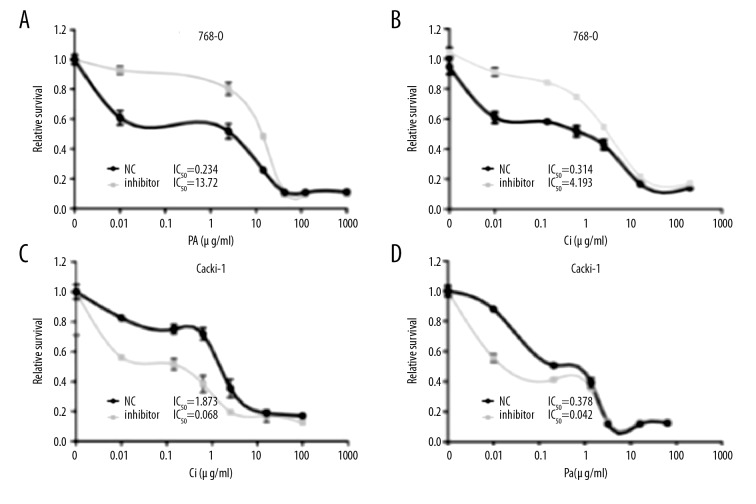
We assessed the effects of miR-381 expression on multi-drug resistance, showing that inhibition of miR-381 expression remarkably increased cell growth and chemoresistance as shown by MTT assay (Figure 4A, 4B). Conversely, overexpression of miR-381 enhanced chemo-sensitive as shown by MTT assay (Figure 4C, 4D). These findings suggest that reduction of miR-381 promoted proliferation of ccRCC cells in vitro, and the increased miR-381 levels enhanced the chemo-sensitivity in caki-1-derived tumor xenografts of nude mice.
Figure 4.
miR-381 suppression promoted cell growth and chemoresistance in vitro. A and B. The IC50 of Pa (A) and Ci (B) post-transfection with miR-381 inhibitors in the 786-O cells. (C, D) IC50 changes after transfection with miR-381mimics in Caki-1 cells.
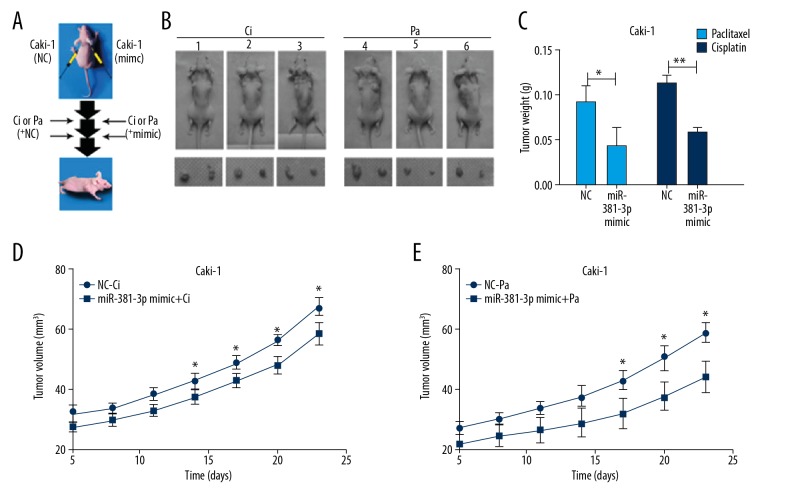
To explore the promising tumor-suppressing effects of miR-381 on cells, the Caki-1 cells were subcutaneously injected into back sides of the front legs of nude mice. To minimize inter-mouse bias, we subcutaneously injected the Caki-1 (at density of 1.5×107 cells/site) cells into 2 back sides of front legs of the other 6 mice. Intra-tumor miR-381 injections or the negative control (NC) into tumors derived from caki-1 were initiated from the 5th day, then repeated 5 times and conducted once for 2 days. PBS, Pa, or Ci intraperitoneal injections were initiated on the 8th day into the 2 mice (Figure 5A, 5B). Tumor weights were evaluated on the 25th day. Mice injected with miR-381 had tumors that were significantly lighter than in mice injected with NC (Figure 5C). We also drew growth curves of xenograft tumors (Figure 5D, 5E). All these results suggest that miR-381 promotes chemotherapy sensitivity.
Figure 5.
miR-381 restrained both cell proliferation and chemoresistance for the Caki-1 cell-derived tumor xenografts (n=6 mice). (A) Experimental scheme. (B) Graphs for representative tumor-established mice at day 25. (C) Tumor weight from different tumors isolated from different mice were calculated and the images were plotted, * p<0.05, ** p<0.01 represents differences between 2 groups. (D, E) Tumor volumes of different mice were calculated and the images were plotted, * p<0.05, ** p<0.01 represents differences between 2 groups.
Discussion
miRNAs were first discovered in C. elegans, and are now known to exist in many animals, plants, and viruses [15]. miRNAs are derived from endogenous transcription, which is initiated by slicing of RNase III-associated enzymes. miRNAs play critical roles in regulating gene overexpression or gene silencing by binding or attaching to 3-un-translated regions (3′-UTR) of the targeted mRNAs. miRNAs also participate in many biological processes and functions such as cell differentiation, apoptosis, cell growth, embryonic development, cell migration, and invasion [16].
A previous study also reported that miRNAs can either suppress the proliferation of tumor cells or act as a tumor oncogene to initiate tumorigenesis [17]. miRNAs can modulate the differentially expressed genes in tumor cells, which has been used to discover clinical therapeutic approaches. Recent research has also demonstrated that miRNAs can inhibit the TRAIL signal pathway to promote chemotherapy resistance of cancer or enhance the STAT signal pathway to promote the sensitivity to TAX Taxol of cancer [18]. Aberrant expression of miR-381 has been discovered in many cancers, such as liver cancer, breast cancer, lung cancer, and colon cancer [19–21]. Previous functional investigations have also reported that miR-381 has controversial effects of tumor-suppressive and oncogenic effects in the distinguish human tumor tissues [7–10]. Therefore, we speculated that the precise functions of the miR-381 may be tissue-specific or cell-specific in these cancers. Also, discover and screening for the specific biomarkers for the RCC treatment is urgent for overcoming the drug resistance in clinical. Recent studies reported that dysregulated miRNAs are common in many cancers. Our own research and that of others shows that global downregulated expression of miRNA is a remarkable characteristic of ccRCC [6,12,15,16,19]. However, the functions of miR-381 in the pathogenesis of ccRCC have not been fully clarified until now. Previous studies showed that MiR-381 is the most remarkably decreased molecule in ccRCC cancer tissues and cells [6 12,15,16,19,22]. There has been no previous report on miR-381 regulating proliferation and chemoresistance of Pa and Ci. Therefore, we selected miR-381 as a specific molecule to investigate chemoresistance and proliferation in ccRCC. Pa and Ci and their associated compounds are extensively applied in treating RCC in clinical practice, but the sensitivity of RCC cells to these compounds is relatively low. Therefore, chemoresistance induces poor prognosis and chemo-agents cause many adverse effects in cancer patients.
Although we found some interesting results, the present study has certain limitations. First, the tumor volume and size in the xenograft BALB/c mice were relatively small, which might be caused by the lower dosage of Caki-1 cells injected into the mice. Second, this study only observed the effects of microRNA-381 on tumor growth and chemoresistance of clear-cell renal cell carcinoma; therefore, the mechanism for these effects was not explored. In the future studies, we intend to explore the expression of proliferation- and apoptosis-associated biomarkers to confirm the potential therapeutic effects.
Conclusions
We found that miR-381 is a potential biomarker for differentiating ccRCC tissues from normal tissues, and it plays critical roles in suppressing ccRCC cancer cell metastasis and cell proliferation. In the present study we explored the functional characteristics and chemo-drug resistance of miR-381 in ccRCC in vivo. We found that the upregulation of miR-381 significantly inhibited the cell proliferation and enhance the chemo-sensitivity of ccRCC cells. The effect of miR-381 expression on ccRCC cells was also evaluated both in vitro and in vivo, and the data showed that miR-381 enhanced the sensitivity of cells to Pa and Ci treatment. Moreover, the suppression of miR-381 expression also enhanced the chemoresistance and the cell proliferation both in vitro and in vivo. Our results show the tumor-inhibitive roles of miR-381 in ccRCC. Therefore, miR-381 might be a useful biomarker for chemotherapy of ccRCC.
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China (Grant No. 81171996; 81272289) and the Scientific Research Projects of Health Commission of Heilongjiang Province (Grant No. 2018362)
Conflict of interest
The authors declare no competing financial or commercial interests in this manuscript.
References
- 1.Huang T, Wang X, Yang X, et al. Long non-coding RNA DUXAP8 enhances renal cell carcinoma progression via downregulating miR-126. Med Sci Monit. 2018;24:7340–47. doi: 10.12659/MSM.910054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viers BR, Houston Thompson R, et al. Preoperative neutrophil-lymphocyte ratio predicts death among patients with localized clear-cell renal carcinoma undergoing nephrectomy. Urol Oncol. 2014;32:1277–84. doi: 10.1016/j.urolonc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 3.von Roemeling CA, Radisky DC, Marlow LA, et al. Neuronal pentraxin 2 supports clear-cell renal cell carcinoma by activating the AMPA-selective glutamate receptor-4. Cancer Res. 2014;74:4796–810. doi: 10.1158/0008-5472.CAN-14-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YC, Chien LH, Huang BM, et al. Aqueous extracts of toona sinensis leaves inhibit renal carcinoma cell growth and migration through JAK2/stat3, Akt, MEK/ERK, and mTOR/HIF-2alpha pathways. Nutr Cancer. 2016;68:654–66. doi: 10.1080/01635581.2016.1158292. [DOI] [PubMed] [Google Scholar]
- 5.Bahrami A, Aledavood A, Anvari K, et al. The prognostic and therapeutic application of microRNAs in breast cancer: Tissue and circulating microRNAs. J Cell Physiol. 2018;233:774–86. doi: 10.1002/jcp.25813. [DOI] [PubMed] [Google Scholar]
- 6.Tang H, Liu X, Wang Z, et al. Interaction of hsa-miR-381 and glioma suppressor LRRC4 is involved in glioma growth. Brain Res. 2011;1390:21–32. doi: 10.1016/j.brainres.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Rothschild SI, Tschan MP, Jaggi R, et al. MicroRNA-381 represses ID1 and is deregulated in lung adenocarcinoma. J Thorac Oncol. 2012;7:1069–77. doi: 10.1097/JTO.0b013e31824fe976. [DOI] [PubMed] [Google Scholar]
- 8.Juan D, Alexe G, Antes T, et al. Identification of a microRNA panel for clear-cell kidney cancer. Urology. 2010;75:835–41. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Hill S, Lichenstein S, Wang S, et al. Caudate volume in offspring at ultra high risk for alcohol dependence: COMT Val158Met, DRD2, externalizing disorders, and working memory. Adv Mol Imaging. 2013;3:43–54. doi: 10.4236/ami.2013.34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng B, Wang R, Song HZ, et al. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–76. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- 11.Yu ZW, Zhong LP, Ji T, et al. MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral Oncol. 2010;46:317–22. doi: 10.1016/j.oraloncology.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Yi Z, Fu Y, Zhao S, et al. Differential expression of miRNA patterns in renal cell carcinoma and nontumorous tissues. J Cancer Res Clin Oncol. 2010;136:855–62. doi: 10.1007/s00432-009-0726-x. [DOI] [PubMed] [Google Scholar]
- 13.Weng L, Wu X, Gao H, et al. MicroRNA profiling of clear-cell renal cell carcinoma by whole-genome small RNA deep sequencing of paired frozen and formalin-fixed, paraffin-embedded tissue specimens. J Pathol. 2010;222:41–51. doi: 10.1002/path.2736. [DOI] [PubMed] [Google Scholar]
- 14.Jung M, Mollenkopf HJ, Grimm C, et al. MicroRNA profiling of clear-cell renal cell cancer identifies a robust signature to define renal malignancy. J Cell Mol Med. 2009;13:3918–28. doi: 10.1111/j.1582-4934.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakada C, Matsuura K, Tsukamoto Y, et al. Genome-wide microRNA expression profiling in renal cell carcinoma: Significant downregulation of miR-141 and miR-200c. J Pathol. 2008;216:418–27. doi: 10.1002/path.2437. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Ohms SJ, Li Z, et al. Changes in the expression of miR-381 and miR-495 are inversely associated with the expression of the MDR1 gene and development of multi-drug resistance. PLoS One. 2013;8:e82062. doi: 10.1371/journal.pone.0082062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierinckx A, Roche M, Legras-Lachuer C, et al. MicroRNAs in pituitary tumors. Mol Cell Endocrinol. 2017;456:51–61. doi: 10.1016/j.mce.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Garofalo M, Quintavalle C, Di Leva G, et al. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845–55. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Zhao Q, Fan L, et al. Downregulation of MicroRNA-381 promotes cell proliferation and invasion in colon cancer through up-regulation of LRH-1. Biomed Pharmacother. 2015;75:137–41. doi: 10.1016/j.biopha.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Ming J, Zhou Y, Du J, et al. miR-381 suppresses C/EBP alpha-dependent Cx43 expression in breast cancer cells. Biosci Rep. 2015;35:e00266. doi: 10.1042/BSR20150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Zhao S, Pang X, et al. MicroRNA-381 suppresses cell growth and invasion by targeting the liver receptor homolog-1 in hepatocellular carcinoma. Oncol Rep. 2016;35:1831–40. doi: 10.3892/or.2015.4491. [DOI] [PubMed] [Google Scholar]
- 22.Dai J, Xu LJ, Han GD, et al. MicroRNA-125b promotes the regeneration and repair of spinal cord injury through regulation of JAK/STAT pathway. Eur Rev Med Pharacol Sci. 2018;22:582–89. doi: 10.26355/eurrev_201802_14271. [DOI] [PubMed] [Google Scholar]