Abstract
The saturated hydrocarbon bisabolane is a diesel fuel substitute that can be derived from sesquiterpene precursors bisabolene or curcumene. These sesquiterpenes are generated from farnesyl diphosphate in reactions catalyzed by eponymous terpenoid cyclases, but they can also be generated by engineered terpenoid cyclases in which cyclization cascades have been reprogrammed by mutagenesis. Here, we describe the X-ray crystal structure determination of F95Q epi-isozizaene synthase (EIZS), in which the new activity of curcumene biosynthesis has been introduced and the native activity of epi-isozizaene biosynthesis has been suppressed. F95Q EIZS generates β- and γ-curcumene regioisomers with greater than 50% yield. Structural analysis of the closed active site conformation, stabilized by the binding of 3 Mg2+ ions, inorganic pyrophosphate, and the benzyltriethylammonium cation, reveals a product-like active site contour that serves as the cyclization template. Remolding the active site contour to resemble curcumene instead of epi-isozizaene is the principal determinant of the reprogrammed cyclization cascade. Intriguingly, an ordered water molecule comprises part of the active site contour. This water molecule may also serve as a final proton acceptor, along with inorganic pyrophosphate, in the generation of curcumene regioisomers; it may also contribute to the formation of sesquiterpene alcohols identified as minor side products. Thus, the substitution of polar side chains for nonpolar side chains in terpenoid cyclase active sites can result in the stabilization of bound water molecules that, in turn, can serve template functions in isoprenoid cyclization reactions.
Keywords: X-ray crystallography, enzyme, protein engineering, energy biotechnology, diesel fuel
Graphical Abstract

1. Introduction
Ubiquitous in all forms of life, terpene cyclases are enzymes that catalyze the conversion of linear isoprenoid substrates such as geranyl diphosphate, farnesyl diphosphate, or geranylgeranyl diphosphate into a wide variety of polycyclic natural products (Cane,1985, 1990; Croteau et al. 1987; Wendt et al., 2000; Tholl, 2006; Austin et al., 2008; Miller & Allemann, 2012; Gao et al., 2012). There are two cyclase classes characterized by distinct α-helical folds and distinct chemical mechanisms for substrate activation (Christianson, 2006, 2008, 2017). Class I enzymes initiate catalysis by metal ion-dependent substrate ionization to form coproduct inorganic pyrophosphate (PPi) and a reactive allylic cation, while class II enzymes initiate catalysis by protonation of a carbon-carbon double bond to generate a tertiary carbocation intermediate. At the level of primary structure, most class I cyclases contain conserved “aspartate-rich” DDXX[D/E] and “NSE” [N/D]DXX[S/T]XX[K/R]E metal ion-binding motifs, in which boldface residues typically coordinate to three catalytic metal ions (usually Mg2+, but sometimes Mn2+) (Aaron & Christianson, 2010). Rarely, a class I cyclase contains two aspartate-rich metal-binding motifs (Gennadios et al., 2009). Class II cyclases contain a DXDD motif in which the central aspartic acid serves as a catalytic general acid (Wendt et al., 2000).
The sesquiterpene cyclase epi-isozizaene synthase (EIZS) is a class I enzyme that converts farnesyl diphosphate (FPP) into epi-isozizaene as the major hydrocarbon product, approximately 80% at 30 °C (Lin et al., 2006, 2009; Aaron et al., 2010). Cyclization fidelity improves markedly at lower temperature, with epi-isozizaene formation approaching 100% at 4 °C (Li et al., 2014). The crystal structure of wild-type EIZS from Streptomyces coelicolor has been solved in complex with three Mg2+ ions, PPi, and the benzyltriethylammonium cation (BTAC), revealing an active site contour largely defined by aromatic residues F95, F96, F198, and W203 (Aaron et al., 2010). In addition to providing a three-dimensional template for catalysis that ensures the proper conformation of FPP prior to the initiation of catalysis, these residues help stabilize cationic intermediates through cation–π interactions.
The substitution of other amino acids for these aromatic residues can dramatically reprogram the cyclization cascade to yield different cyclization products (Aaron et al., 2010; Li et al., 2014; Blank et al., 2017). Some amino acid substitutions yield cyclases that have completely lost the capacity for epi-isozizaene generation, but instead generate brand new sesquiterpene products (Li et al., 2014; Blank et al., 2017). The engineering of a terpene cyclase to generate completely new cyclization products represents a significant advance, especially if such products are commercially useful. For example, F95Q EIZS catalyzes the cyclization of FPP to form the β and γ regioisomers of curcumene as major cyclization products (54% total at 30°C) with a specific activity 33% of that measured for the wild-type enzyme (Figure 1) (Blank et al., 2017). As noted for F95H EIZS, which generates 50% β-curcumene at 30 °C, curcumene regioisomers can be chemically hydrogenated to yield bisabolane (Li et al., 2014), a diesel fuel substitute (Peralta-Yahya et al., 2011) (Figure 1). Engineered bacterial cyclases can be used as robust components in metabolic engineering experiments to expand biosynthetic capabilities beyond the limitations of naturally occurring cyclases (Li et al., 2014; McAndrew et al., 2011).
Figure 1.
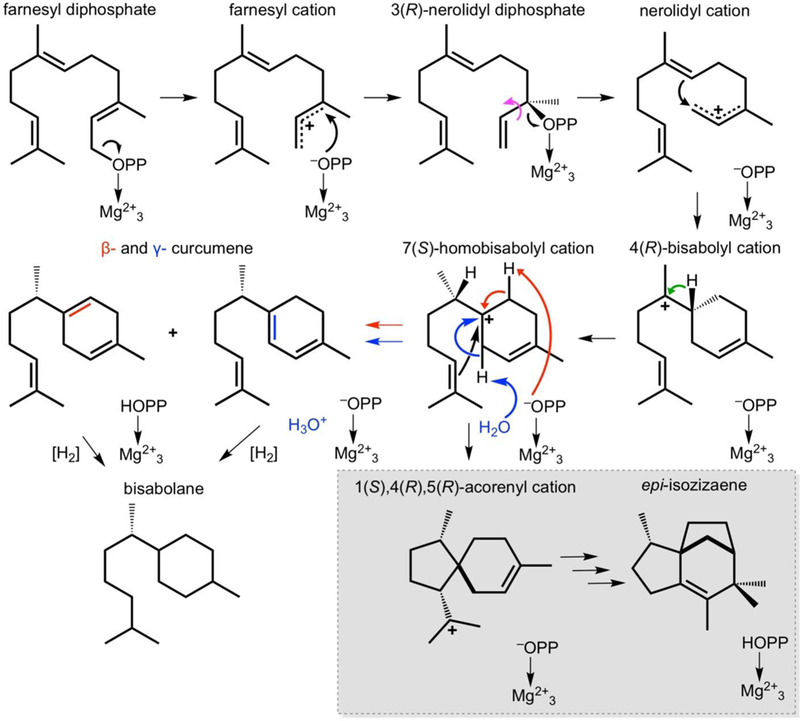
Proposed mechanism for F95Q EIZS-catalyzed cyclization of farnesyl diphosphate to form β- and γ-curcumene regioisomers. C3-C2 bond rotation indicated by magenta arrow, 1,2-hydride shift indicated by green arrow. Red and blue arrows correspond to proton elimination reactions yielding β- and γ-curcumene, respectively. The deprotonation reaction leading to γ-curcumene could be mediated by water #372 trapped in the active site as indicated, or it could be mediated by metal-bound inorganic pyrophosphate (–OPP) as discussed in the text. Although the stereochemistry of β- and γ-curcumene has not been established, it is presumed to derive from the 7(S)-homobisabolyl cation definitively established in the biosynthesis of epi-isozizaene. Even if the 7(R)-homobisabolyl cation is formed, either enantiomer of β- and γ-curcumene could be chemically hydrogenated to yield diesel fuel substitute bisabolane.
Here, we report the crystal structure of F95Q EIZS complexed with Mg2+3-PPi and BTAC, which partially mimics the bisabolyl cation intermediate in the FPP cyclization cascade leading to β- and γ-curcumene. Structural comparisons with F95N, F95H, and F95C EIZS, which also generate curcumene regioisomers (Li et al., 2014; Blank et al., 2017), are also discussed. These studies comprise the first step in evaluating F95 mutants of EIZS as potential “plug and play” components for synthetic biology approaches toward the generation of hydrocarbon fuels.
2. Results
2.1. Crystal structure determination
The 1.91 Å resolution crystal structure of the F95Q EIZS-Mg2+3-PPi-BTAC complex reveals the characteristic class I terpene cyclase fold identical to that of the wild-type EIZS-Mg2+3-PPi-BTAC complex (PDB 3KB9), with a root-mean-square (rms) deviation of 0.11 Å for 300 Cα atoms (Figure 2). The active site of F95Q EIZS is in the fully closed conformation, stabilized by full-occupancy binding of 3 Mg2+ ions and the PPi anion. In the fully closed conformation, the active site is completely encapsulated, thereby preventing premature quenching of carbocation intermediates by bulk solvent during catalysis. The overall polypeptide chain is well ordered, with no major gaps in the electron density corresponding to main chain atoms. However, the chain termini are disordered: the N-terminal hexahistidine tag and M0-I15 at the N-terminus, and E356-K361 at the C-terminus, lack well-ordered electron density and are omitted from the final model. Polypeptide chain termini are similarly disordered in the wild-type EIZS-Mg2+3-PPi-BTAC complex.
Figure 2.

(Left) Ribbonplot of F95Q EIZS color-coded as follows: backbone atoms, blue; DDXXD metal-binding motif, red; NSE metal-binding motif, orange; BTAC, stick figure with pink C atoms and blue N atom; magnesium ions, green spheres; PPi, stick figure with orange P atoms and red O atoms. (Right) Superposition of the F95Q EIZS-Mg2+3-PPi-BTAC complex with the wild-type EIZS-Mg2+3-PPi-BTAC complex (all atoms yellow; PDB 3KB9).
Electron density for the Q95 side chain is well defined (Figure 3a). Although the carboxamide group rotamer cannot be unambiguously established, the rotamer was selected that oriented the side chain carbonyl group into the active site cavity. As refined in this conformation, the carboxamide NH2 group donates a hydrogen bond to Y91 (2.8 Å), and the carboxamide C=O group accepts a hydrogen bond from water #372 (3.1 Å) and also makes a weakly polar interaction with an indole C-H of W203 (3.2 Å) (Figure 3b). The conformation of Q95 is such that the side chain packs against residues defining the active site surface, such that the enclosed active site volume of 331 Å3 is greater than that of 303 Å3 measured for the wild-type enzyme. The volume of the hydrocarbon moiety of FPP is 245 Å3; there is a relatively snug fit when substrate binds to wild-type EIZS, but this fit loosens when the substrate binds to F95Q EIZS. Increased active site volume results in a more permissive template that is not as strict in exerting conformational control over the substrate and reactive carbocation intermediates, thereby accounting for the generation of only 6% epi-isozizaene by this mutant. Since the majority of products generated by F95Q EIZS derive from the bisabolyl or homobisabolyl cations, the additional active site volume introduced at the F95 position must compromise the homobisabolyl cation conformation required to form the acorenyl cation enroute to epiisozizaene generation (Figure 1).
Figure 3.
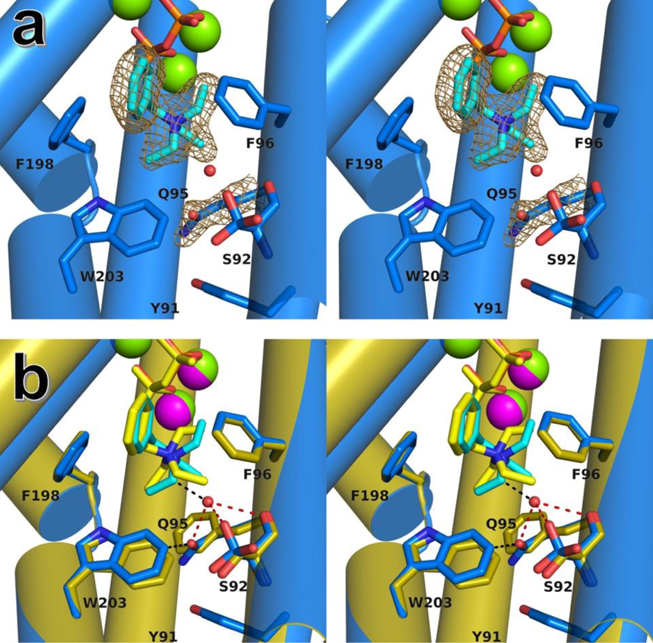
(a) Stereoview of F95Q EIZS-Mg2+3-PPi-BTAC complex, showing Polder omit maps for BTAC and Q95 (contoured at 6σ and 4σ, respectively). Color codes are as follows: C (protein) = blue, C (BTAC) = cyan, N = dark blue, O = red, P = orange, Mg2+ ions = green spheres. Water #372 is shown as a small red sphere. (b) Complex in (a) superimposed on the wild-type EIZS-Mg2+3-PPi-BTAC complex (gold; Mg2+ ions = magenta spheres). Hydrogen bond interactions are indicated by red dashed lines. Other interactions are shown as black dashed lines.
The F95Q substitution stabilizes the binding of water #372 through hydrogen bonding. In addition, water #372 forms hydrogen bonds with rotamer C of the side chain hydroxyl group of S92 and the backbone carbonyl oxygen of S92. Since water #372 is tethered in place by multiple hydrogen bonds and is not sterically required to be displaced by FPP binding, it likely remains firmly bound in the active site throughout catalysis. While the aromatic ring of BTAC remains sandwiched in place by F96 and F198 in wild-type EIZS and F95Q EIZS complexes, the triethylamino moiety of BTAC undergoes a conformational change in the active site of F95Q EIZS to accommodate the binding of water #372. This water molecule may be responsible for the generation of sesquiterpene alcohol side products α-bisabolol (13%), (E)-nerolidol (3%), and acorenol (2%), by quenching the corresponding carbocation intermediates (Blank et al., 2017). Notably, if water #372 does not react with carbocation intermediates, then it serves as part of the active site contour that governs the conformation of the substrate as well as carbocation intermediates. Water #372 is unique to F95Q EIZS and is not observed in the active site of any other F95 mutant studied by X-ray crystallography (Li et al., 2014; Blank et al., 2017).
2.1. Modeling enzyme-intermediate and enzyme-product complexes
Analysis of the three-dimensional contour of the enclosed active site of wild-type EIZS reveals a product-like shape that is highly complementary to the molecular structure of epi-isozizaene (Figure 4a). Thus, the active site contour serves as a template for cyclization and enforces the proper sequence of carbon-carbon bond-forming reactions, including the compressed conformation of the homobisabolyl cation required to form the acorenyl cation (Figure 1).
Figure 4.

(a) Stereoview of the EIZS-Mg2+3-PPi-BTAC complex, less the BTAC molecule. Color coding is as follows: Mg2+ ions = large magenta spheres; F95 and PPi are shown with C = aqua, O = red, P = orange. The active site contour is represented by firebrick red meshwork and is fit by a model of epi-isozizaene (gold). (b) Stereoview of the F95Q EIZS-Mg2+3-PPi-BTAC complex, less the BTAC molecule. Color coding is as follows: Mg2+ ions = large green spheres, water #372 = small red sphere; Q95 and PPi are shown with C = green, N = dark blue, O = red, P = orange. The Q95---water #372 hydrogen bond is represented by a blue dashed line. The active site contour is represented by purple meshwork and is fit by models of β- and γ-curcumene and the 7(S)-homobisabolyl cation (red, blue, and gold, respectively).
In contrast, analysis of the three-dimensional contour of the enclosed active site of F95Q EIZS reveals a more extended contour that is highly complementary to the extended conformation of β- and γ-curcumene, as well as the 7(R)-homobisabolyl cation intermediate preceding the formation of these products (Figure 4b). Thus, the active site contour is still quite product-like in shape, but since this contour has been remolded by mutagenesis it serves as a template for the generation of new products. Binding modes calculated for β-curcumene, γ-curcumene, and the homobisabolyl cation with the docking software NRGsuite FlexAID (Gaudreault et al. 2015) reveal a consistent binding orientation in which the pendant isoprenoid tail occupies the newly introduced volume resulting from the F95Q substitution. The conformation of the homobisabolyl cation is such that it would easily support the 1,2-hydride transfer leading from the bisabolyl cation precursor.
The positively charged carbon atoms of the bisabolyl and homobisabolyl carbocation intermediates would be positioned in the same vicinity as the positively charged nitrogen atom of BTAC in the F95Q EIZS-Mg2+3-PPi-BTAC complex. Therefore, these carbocations would similarly be stabilized by cation-π interactions with F96 and F198, as observed for BTAC in Figure 3. Additional electrostatic stabilization of carbocation intermediates and their flanking transition states would be provided by the PPi anion. The break in helix G near F198 (Figure 3) may provide further electrostatic stabilization, since the negative electrostatic potential of the C-terminal end of the helix break is oriented into the active site. This structural feature is conserved in terpenoid synthases as first described in squalene synthase (Pandit et al., 2000) and subsequently in other terpenoid cyclases (Baer et al., 2014a,b).
Insofar that water #372 is chemically inert with respect to carbocation intermediates in the cyclization reactions catalyzed by F95Q EIZS, its molecular surface must contribute to the active site contour that serves as the template for catalysis. The active site contour of F95Q EIZS takes into account the likelihood that water #372 serves as part of the template that governs the binding orientation of the homobisabolyl cation. However, it is possible that water #372 also plays a role as a general base in the final step of the reaction leading to product formation. The 7(S)-homobisabolyl cation undergoes proton elimination at C5 or C1 to yield β-curcumene and γ-curcumene, respectively. Given the modeled binding orientation of the homobisabolyl cation in Figure 4b, water molecule #372 is poised to accept the C1 proton of the homobisabolyl cation to generate γ-curcumene. The other possible general base in the active site, the PPi anion, is properly oriented to accept the C5 proton to generate β-curcumene. The possibility of tandem general base catalysis by water #372 and the PPi anion may account for nearly identical yields of β-curcumene and γ-curcumene, with the slight preference of β-curcumene formation resulting from the PPi anion being a stronger base than a water molecule. Of course, the PPi anion could accept the C1 proton from the homobisabolyl cation if the 6-membered ring were to flip 180°. However, since F95H EIZS generates 50% β-curcumene and cannot fit a trapped water molecule comparable to water #372 in F95Q EIZS, this may imply that the generation of γ-curcumene requires water #372 or its equivalent.
3. Discussion
There is precedent for the binding of water molecules in the hydrophobic active site cavity of EIZS, but none correspond to the position observed for water #372 in F95Q EIZS. For example, the increased active site volume of W203Y EIZS enables the binding of two new water molecules in the active site, and these water molecules may quench carbocation intermediates to yield sesquiterpene alcohol products (Blank et al., 2017). Specifically, quenching of the nerolidyl cation and bisabolyl cation by an active site water molecule results in the formation of (E)-nerolidol (10%) and (Z)-γ-bisabolene (42%).
Other amino acid side chains substituted for F95 similarly result in enzymes that generate major products resulting from the quenching of the bisabolyl cation or homobisabolyl cation intermediate, either by proton elimination or by solvolysis. For example, F95H EIZS generates β-curcumene (50%) (Li et al., 2014), F95N EIZS generates β-curcumene (28%) and α-bisabolol (18%), and F95C EIZS generates γ-curcumene (22%) and (E)-nerolidol (9%). The crystal structures of these mutants each reveal an enlarged active site cleft resulting from the substitution of a smaller side chain for that of F95. Even though the reduction in active site volume is relatively little for F95H EIZS, the substitution of a polar imidazole side chain for the aromatic side chain of F95 appears to influence the derailment of the cyclization cascade to form β-curcumene (Li et al., 2014). It is interesting, too, that F95Y EIZS generates (E)-nerolidol (27%) despite the larger side chain substituted for F95 (Blank et al., 2017). Here, the introduction of a polar side chain in place of F95 enables solvent access to the active site to enable quenching of the nerolidyl cation by a water molecule.
In EIZS mutants containing a smaller, polar side chain substituted for F95 (F95N, F95Q, F95C), it is interesting to note that the generation of epi-isozizaene is substantially reduced to 5–6% of total sesquiterpene products formed. In contrast, F95Y EIZS and F95H EIZS generate 67% and 44% epi-isozizaene, respectively. The substitution of a comparably sized but polar side chain in place of F95 preserves some measure of cyclization fidelity for epi-isozizaene generation, since the net change to the three-dimensional active site contour is minimal. Thus, it appears that the introduction of polarity into the hydrophobic active site is responsible for derailing the cyclization cascade in the F95N, F95Q, and F95C mutants.
In conclusion, the crystal structure determination of the F95Q EIZS-Mg2+3-PPi-BTAC complex illuminates the molecular basis of the reprogrammed FPP cyclization cascade leading to the formation of regioisomers β-curcumene and γ-curcumene, both of which can be chemically hydrogenated to yield the diesel fuel substitute bisabolane. While F95Q EIZS is almost identical in overall structure to wild-type EIZS, the increased active site volume and elongation of its three-dimensional contour, along with the introduction of the polar glutamine side chain that stabilizes the binding of water #372, yields a template for catalysis that is highly complementary in shape to the extended conformation of the curcumene products. The PPi co-product as well as water #372 are possible general bases that mediate deprotonation of the homobisabolyl cation intermediate in the final step of catalysis. Additionally, water #372 comprises part of the active site contour that governs substrate and intermediate conformations in catalysis. This water molecule may also account for the formation of sesquiterpene alcohols formed as minor side products.
It is remarkable that a bound water molecule in a terpenoid cyclase active site does not pose a significant risk to a cationic isoprenoid cyclization cascade unless it is precisely positioned to react with an sp2-hybridized carbon atom in a catalytic intermediate. This suggests significant potential for exploring and exploiting carbocation-mediated organic synthesis using enzymes in aqueous solution, which represents an environmentally-friendly option for organic synthesis of terpenoid natural products.
4. Materials and Methods
4.1. Crystallization
Recombinant F95Q EIZS was prepared as previously described (Blank et al., 2017). Crystals were grown using the sitting drop vapor diffusion method at 21 °C. Typically, a 0.5-μL drop of protein solution [4 mg/mL F95Q EIZS, 20 mM Tris (pH = 7.5), 300 mM NaCl, 10 mM MgCl2, 2 mM tris(2-carboxyethyl)phosphine (TCEP), 2 mM sodium pyrophosphate, 2 mM benzyltriethylammonium chloride, 10% (v/v) glycerol] was added to a 0.6-μL drop of precipitant solution [0.2 M magnesium acetate tetrahydrate, 0.1 M sodium cacodylate trihydrate (pH = 6.5), 20% (w/v) PEG 8000]. Immediately afterwards, a 0.1-μL drop of 100X diluted wild-type EIZS crystallization microseeding stock was added, the preparation of which has been reported previously (Blank et al., 2017). The resulting 1.2-μL drop was equilibrated against a 100-μL reservoir of precipitant solution at 21 °C. Crystals appeared after 1 week and were cryoprotected with 30% (v/v) glycerol and flash-frozen in liquid nitrogen.
4.2. Data collection and structure determination
Crystals diffracted X-rays to 1.91 Å resolution at the Advanced Photon Source, Argonne National Laboratory, Northeastern Collaborative Access Team beamline 24-ID-E. Data were collected over 180° with 20% transmission of the 12.68 keV beam and 250 mm crystal-to-detector distance. Crystals belonged to space group P21 with the following unit cell dimensions: a = 53.25 Å, b = 47.09 Å, c = 75.59 Å, β = 95.13° (one monomer in the asymmetric unit). Diffraction data were indexed and integrated in iMOSFILM (Battye et al., 2011) and scaling was performed in AIMLESS (Evans et al., 2013). The crystal structure of the F95Q EIZS-Mg2+3-PPi-BTAC complex was determined by molecular replacement with PHASER (McCoy et al., 2007) using the structure of the wild-type EIZS-Mg2+3-PPi-BTAC complex (PDB 3KB9) as a search probe. Manual model building and refinement were performed with COOT and PHENIX, respectively (Emsley et al., 2010, Adams et al., 2010). Solvent molecules and residues with multiple conformations were modeled during the final stages of refinement; residues with multiple conformations included Q33, S92, V97, T123, S127, R174, E192, R227, K247, S260, R278, C283, V290, R292, and S327. Validation of the structure was performed initially using MOLPROBITY (Chen et al., 2010). All data collection and refinement statistics are detailed in Table 1.
Table 1.
Data collection and refinement statistics for the F95Q EIZS-Mg2+3-PPi-BTAC complex
| Synchrotron | APS |
| Beamline | 24-ID-E |
| Detector | CCD |
| Energy | 12.68 keV |
| Space group | P21 |
| a, b, c (Å) | 53.25, 47.09, 75.59 |
| α, β, γ (°) | 90.00, 95.13, 90.00 |
| Resolution (Å)a | 53.04–1.91 (1.98–1.91) |
| Total/unique no. of reflections | 104897/29257 (7131/1968) |
| Rmergea,b | 0.189 (1.009) |
| Rp.i.m.a,c | 0.116 (0.611) |
| CC1/2a,d | 0.987 (0.551) |
| I/σ(I)a | 8.1 (2.1) |
| Redundancy a | 3.6 (3.6) |
| Completeness (%) a | 100.0 (100.0) |
| Rwork a,e | 0.155 (0.226) |
| Rfree a,e | 0.203 (0.278) |
| No. of non-hydrogen atoms: | |
| protein | 2868 |
| ligands | 49 |
| solvent | 372 |
|
Average B-factors (Å2) |
|
| protein | 16 |
| ligands | 24 |
| solvent | 29 |
|
Rms deviation from ideal geometry |
|
| bonds (Å) | 0.016 |
| angles (°) | 1.3 |
| Ramachandran plot (%) f | |
| favored | 99.11 |
| allowed | 0.89 |
| outliers | 0.00 |
| Rotamer outliers (%) | 0.66 |
| Clashscore | 4.9 |
| PDB accession code | 6OFV |
Values in parentheses correspond to data in highest resolution shell.
mean intensity of Ihkl calculated from replicate measurements. Note that Rmerge can sometimes be inordinately high for highly redundant data sets, in which case CC1/2 can be a better indicator of data quality.
= mean intensity of Ihkl calculated from replicate measurements, N = number of reflections.
Pearson correlation coefficient between random half-datasets.
for reflections contained in the working set. |Fo| = observed structure factor amplitude, |Fc| = calculated structure factor amplitude. Rfree is calculated in the same manner using test set reflections held aside during refinement.
Calculated with MolProbity.
4.3. Modeling
Molecular models of the closed conformations of wild-type EIZS and F95Q EIZS without bound BTAC were generated using COOT (Emsley et al., 2010). The atomic coordinates of epi-isozizaene as well as β- and γ-curcumene were generated in .pdb format by converting a SMILES string generated from the SMILES Translator and Structure File Generator (National Cancer Institute, https://cactus.nci.nih.gov/translate/). Molecular geometries were optimized using Phenix (Adams et al., 2010). Active site pocket meshes were initially generated using the DoGSiteScorer (http://dogsite.zbh.uni-hamburg.de), the results of which guided the cavity function in Pymol. GetCleft and FlexAID as implemented in the NRGsuite plugin were used to generate enclosed active site contours, into which catalytic products and the homobisabolyl cation intermediate could be docked (Gaudreault et al. 2015). The resulting models of enzyme-ligand complexes underwent minor manual adjustments to optimize enzyme-ligand fit, but the final conformations largely followed the autodocking solutions.
5. PDB accession number
Atomic coordinates and structure factor amplitudes of F95Q EIZS have been deposited in the Protein Data Bank (www.rcsb.org) with accession number 6OFV.
Highlights.
The active site contour is a template for catalysis by a terpenoid cyclase
Active site mutagenesis reprograms the reaction sequence of a terpenoid cyclase
Crystal structure of the mutant cyclase reveals a modified active site contour
Modified active site contour fits the molecular shape of the new products generated
Acknowledgements
We thank Alaina Stockhausen for technical assistance. Additionally, we thank the NIH for grant GM56838 in support of this research. Finally, we thank the Northeastern Collaborative Access Team (NE-CAT) beamlines, which are funded by NIH grant P41 GM103403, and Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated by Argonne National Laboratory under contract DE-AC02–06CH11357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron JA and Christianson DW (2010) Trinuclear metal clusters in catalysis by terpenoid synthases. Pure and Applied Chemistry 82, 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaron JA, Lin X, Cane DE, and Christianson DW (2010) Structure of epi-isozizaene synthase from Streptomyces coelicolor A3(2), a platform for new terpenoid cyclization templates. Biochemistry 49, 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ,Richardson DC, Richardson JS, Terwilliger TC, and Zwart PH (2010) PHENIX: a comprehensive python-based system for macromolecular structure solution Acta Cryst D66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MB, O’Maille PE, 2008. Evolving biosynthetic tangos negotiate mechanistic landscapes. Nat. Chem. Biol 4, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer P, Rabe P, Citron CA, de Oliveira Mann CC, Kaufmann N, Groll M, Dickschat JS, 2014a. Hedycaryol synthase in complex with nerolidol reveals terpene cyclase mechanism. ChemBioChem 15, 213–216. [DOI] [PubMed] [Google Scholar]
- Baer P, Rabe P, Fischer K, Citron CA, Klapschinski TA, Groll M, Dickschat JS, 2014b. Induced-fit mechanism in class I terpene cyclases. Angew. Chem. Int. Ed 53, 7652–7656. [DOI] [PubMed] [Google Scholar]
- Battye TGG, Kontogiannis L, Johnson O, Powell HR, Leslie AGW, 2011. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Cryst D67, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank PN, Barrow GH, Chou WKW, Duan L, Cane DE, Christianson DW, 2017. Substitution of aromatic residues with polar residues in the active site pocket of epi-isozizaene synthase leads to the generation of new cyclic sesquiterpenes. Biochemistry 56, 5798–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane DE, 1990. Enzymic formation of sesquiterpenes. Chem. Rev 90, 1089–1103. [Google Scholar]
- Cane DE, 1985. Isoprenoid biosynthesis. Stereochemistry of the cyclization of allylic pyrophosphates. Acc. Chem. Res 18, 220–226. [Google Scholar]
- Chen VB, Arendall WB III, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC, 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Cryst D66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson DW, 2006. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev 106, 3412–3442. [DOI] [PubMed] [Google Scholar]
- Christianson DW, 2008. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol 12, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson DW, 2017. Structural and chemical biology of terpenoid cyclases. Chem. Rev 117, 11570–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, El-Bialy H, Dehal SS, 1987. Metabolism of monoterpenes. Plant Physiol 84, 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K, 2010. Features and development of Coot. Acta Cryst D66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PR, Murshudov GN, 2013. How good are my data and what is the resolution? Acta Cryst D69, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Honzatko RB, Peters RJ, 2012. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat. Prod. Rep 29, 1153–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault F, Morency LP, Najmanovich RJ, 2015. NRGsuite: a PyMOL plugin to perform docking simulations in real time using FlexAID. Bioinformatics 31, 3856–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennadios HA, Gonzalez V, Di Costanzo L, Li A, Yu F, Miller DJ, Allemann RK, Christianson DW, 2009. Crystal structure of (+)-δ-cadinene synthase from Gossypium arboreum and evolutionary divergence of metal binding motifs for catalysis. Biochemistry 48, 6175–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Chou WKW, Himmelberger JA, Litwin KM, Harris GG, Cane DE, Christianson DW, 2014. Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 53, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Cane DE, 2009. Biosynthesis of sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor. Mechanism and stereochemistry of the enzymatic formation of epi-isozizaene. J. Am. Chem. Soc 131, 6332–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Hopson R, Cane DE, 2006. Genome mining in Streptomyces coelicolor: molecular cloning and characterization of a new sesquiterpene synthase. J. Am. Chem. Soc 128, 6022–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, Toub O, Lund ST, Bohlmann J, 2010. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol 10, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew RP, Peralta-Yahya PP, DeGiovanni A, Pereira JH, Hadi MZ, Keasling JD, Adams PD, 2011. Structure of a three-domain sesquiterpene synthase: a prospective target for advanced biofuels production. Structure 19, 1876–1884 [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, 2007. Phaser crystallographic software. J. Appl. Cryst 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Allemann RK, 2012. Sesquiterpene synthases: passive catalysts or active players? Nat. Prod. Rep 29, 60–71. [DOI] [PubMed] [Google Scholar]
- Pandit J, Danley DE, Schulte GK, Mazzalupo S, Pauly TA, Hayward CM, Hamanaka ES, Thompson JF, Harwood HJ Jr., 2000. Crystal structure of human squalene synthase: a key enzyme in cholesterol biosynthesis. J. Biol. Chem 275, 30610–30617. [DOI] [PubMed] [Google Scholar]
- Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, Lee TS, 2011. Identification and microbial production of a terpene-based advanced biofuel. Nat Commun 2, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D, 2006. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant. Biol 9, 297–304. [DOI] [PubMed] [Google Scholar]
- Wendt KU, Schulz GE, Corey EJ, Liu DR, 2000. Enzyme mechanisms for polycyclic triterpene formation. Angew. Chemie Int. Ed 39, 2812–2833. [PubMed] [Google Scholar]


