Abstract
Inhibition and genetic deletion of fatty acid-binding proteins (FABPs) 5 and 7 have been shown to increase the levels of the endocannabinoid anandamide as well as the related N-acylethanolamine’s palmitoylethanolamide and oleoylethanolamide. This study examined the role of these FABPs on forced-swim (FS) behavior and on sucrose consumption in two experiments: (experiment 1) using wild-type (WT) mice treated with the FABP inhibitor SBFI26 or vehicle and (experiment 2) using WT and FABP5/7 deficient mice. Results from experiment 1 showed that acute treatment with SBFI26 did not have any effect on sucrose intake or FS behavior in mice. In experiment 2, male and female FABP5/7 deficient mice showed significant increases in sucrose consumption (25 and 21%, respectively) compared with their WT counterparts. In addition, immobility time during the FS was decreased by 27% in both male and female FABP5/7 knockout mice compared with their WT counterparts. The fact that such differences were seen between the acute pharmacological approach and the genetic approach (gene deletion) of FABP needs to be further investigated. The function of FABPs and their specific effects on endocannabinoid anandamide, oleoylethanolamide, and palmitoylethanolamide may play an important role in the development of reward and mood behaviors and could provide opportunities for potential therapeutic targets.
Keywords: anandamide, endocannabinoid, FABP, fatty acid-binding proteins, forced swim, SBFI26
Introduction
Endocannabinoid (eCB) signaling plays an important role in the regulation of a plethora of physiological functions. Dysregulation of eCB signaling has been implicated in various psychiatric disorders including depression (Rutkowska and Jachimczuk, 2004; Bambico et al., 2010; Umathe et al., 2011; Kruk-Slomka et al., 2015); drug abuse (Moreira et al., 2015; Parsons and Hurd, 2015), alcoholism (Basavarajappa and Hungund, 2002; Thanos et al., 2005; Parolaro et al., 2007), obesity (Mazier et al., 2015), reward deficiency syndrome (Comings and Blum, 2000); anxiety (Batista et al., 2014), stress (Hill and McEwen, 2010; Hillard, 2014; Gray et al., 2015), and pain sensation (Hohmann and Suplita, 2006; Kaczocha et al., 2015). eCB signaling is primarily mediated through N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol that act through the cannabinoid 1 and 2 receptors (CB1R and CB2R, respectively) (Hill and McEwen, 2010; Pertwee et al., 2010).
Recent work has largely focused on the role of eCB signaling in reward-related behaviors, and on its interaction with the neurotransmitter dopamine (DA) (or a review see Panagis et al., 2014). DA is intricately tied to our understanding of hedonic behavior (Wise, 2008), and the ability of the eCBs to impact DA signaling has many implications for addiction. CB1Rs are ubiquitous expressed throughout the brain, including the mesolimbic DAergic system, which is a key neuronal circuit in regulating motivational and emotional processing (Laviolette and Grace, 2006; Melis et al., 2014; Wang and Lupica, 2014). Previous research has shown that eCBs alter DA release in the nucleus accumbens (NAc) by influencing the strength of synaptic inputs onto ventral tegmental area (VTA) DA neurons (Szabo et al., 2002; Riegel and Lupica, 2004; Lupica and Riegel, 2005; Haj-Dahmane and Shen, 2010). Consequently, eCB signaling within the VTA is thought to be involved in the regulation of reward-related behaviors.
Because of the lipophilic properties of eCBs, they require assistance from carriers to move in and around the cell. There are several known eCB carriers including albumin, Hsp70, and fatty acid-binding proteins (FABPs) (Nicolussi and Gertsch, 2015). Only recently have FABPs been shown to shuttle AEA through the cell and to intracellular targets (Kaczocha et al., 2009). There are three FABPs that are highly expressed in the central nervous system: FABP3, FABP5, and FABP7. A recently developed inhibitor, SBFI26, inhibits two of these FABPs (FABP5 and FABP7), and has been deemed an effective method of indirectly raising AEA and producing antinociceptive and anti-inflammatory properties (Berger et al., 2012). Following intraperitoneal injection, plasma and brain SBFI26 levels have been shown to peak within 1 h, with an approximate half-life of 3 h (Kaczocha et al., 2014). In addition to AEA, it has also been shown that both SBFI26-treated mice and mice with genetic ablation of FABP5 and FABP7 genes display elevations in related N-acylethanolamine’s palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) (Kaczocha et al., 2015; Peng et al., 2017). Furthermore, SBFI26 shows therapeutic potential for pain management, without the risk of abuse (Thanos et al., 2016). The effects of FABP inhibition on sucrose intake and its effects on forced-swim (FS) behavior are poorly characterized in the literature. Therefore, the current study sought to expand on the potential regulatory actions of FABPs on sucrose intake and FS behavior, which has been widely used in screening for antidepressant-like behavior in animal models (Cryan et al., 2005; Pollak et al., 2010; Bogdanova et al., 2013).
Methods
Subjects
Male and female C57BL/6J wild-type (WT) and FABP5/7 knockout (KO) mice (22–30 g, 20–25 weeks old) were used as previously described (Matsumata et al., 2012). The animals were single housed at room temperature (22°C) and in controlled humidity conditions and kept on a 12 h inverted light cycle beginning at 0900 h with free access to water and food. The animals were habituated to the experimental room for 1 week before testing. The procedures for this study conform to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the protocol was approved by the University Institutional Animal Care and Use Committee at the University of Buffalo (Buffalo, New York, USA).
Sucrose preference
The sucrose preference test procedure was adopted from the Brown Institute for Brain Science – Rodent Neurodevelopmental Behavioral Testing Facility (Providence, Rhode, USA) and performed as previously described with minor modifications (Bitanihirwe et al., 2010). Briefly, mice were given 3 days to acclimate to the setup with access to bottles, containing water and 2% sucrose (Sigma Aldrich, St. Louis, Missouri, USA). This was followed by 4 days during which consumption was recorded by measuring the weight of the water and sucrose bottles every 24 h, at15.00 h. Mice in the SBFI26 experiment were injected with their respective dose of SBFI26 4 h into their dark cycle each day during the last 4 days of the experiment. The position of the two bottles (right or left side) was switched daily throughout the duration of the procedure. A ‘dummy’ cage containing the same bottles given to each mouse was also measured. Dummy bottles were flipped along with all others to measure accidental disturbance of the cage rack or other variables.
Forced-swim test
The forced-swim test (FST) was conducted as previously described (Delis et al., 2013). For the SBFI26 experiment, mice were injected with drug 90 min before testing. Mice were placed in a 25 cm glass cylinder (14 cm in diameter) with 20 cm of water at 24± 2°C for a total of 6 min. The first 2 min were counted as habituation, whereas the last 4 min were counted as the testing period. A mounted camera (Canon Inc., New York, USA) (~2–3 feet above) was used to capture the runs; the video footage was subsequently saved for future analysis using the TopScan program (Clever Sys Inc., Reston, Virginia, USA), which characterized behavior by the amount of movement exerted.
Open-field test
Mice were habituated to the room 30 min before testing. Then, mice were run in an open-field arena (16′ × 16′) photo beam activity monitoring system (Coulbourn Instruments, Allentown, Pennsylvania, USA) for a period of 60 min. Distance traveled was examined to identify changes in overall locomotor activity.
Drug
The FABP inhibitor SBFI26 was synthesized as described by Berger et al. (2012). The drug was dissolved in DMSO : cremophor-EL : 0.9% saline (4% DMSO : 10% Cremophor-EL). Mice received intraperitoneal injections at a volume of 10 μl/g body weight.
Statistical analysis
All data are represented as group mean + standard error of the mean. For the sucrose testing, the last 4 days of sucrose and water consumption were averaged into 1 value for each animal. All follow-up post-hoc interactions were assessed using Tukey’s honest significant difference test. Statistical significance was set at α of 0.05. All statistics and graphing were performed using SigmaPlot 11.0 (Systat Software Inc., San Jose, California, USA).
Results
Sucrose consumption
FABP5/7 KO mice
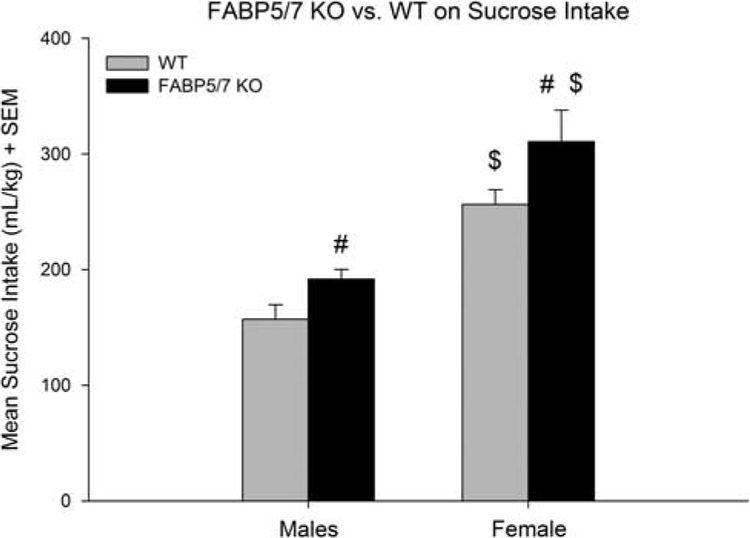
A two-way analysis of variance (ANOVA) revealed significant main effects on sucrose consumption of genotype [F(1,48) =12.12; P < 0.001] and sex [F(1,48) = 54.88; P < 0.001], but not the genotype×sex interaction [F(1,48) = 0.19; NS], suggesting that the increased sucrose consumption seen in FABP5/7 KOs was independent of sex (Fig. 1). Follow-up comparisons showed that FABP5/7 KO females consumed 21% more than their WT counterparts (P < 0.05) and 52% more than the FABP5/7 KO males (P < 0.001); FABP5/7 KO males consumed 25% more than their WT counterparts (P < 0.05); WT females consumed 58% more than the WT males (P < 0.001). A separate two-way ANOVA for water consumption revealed no significant effect of genotype [F(1,48) = 0.81; NS] or sex [F(1,48) = 1.56; NS]. However, nonsignificant increases in water consumption were seen in the FABP5/7 KO mice. Regarding sucrose preference, a two-way ANOVA revealed a significant main effect of sex [F(1,48) = 4.53; P < 0.05], but not of genotype [F(1,48) =0.67; NS]. Female WTs were shown to have a higher preference compared with male WT (P <0.05). It is likely that the small increase in water consumption in the FABP5/7 KO mice was responsible for the lack of difference in sucrose preference between genotypes.
Fig. 1.
Sucrose consumption (mean daily intake over 4 days). #Difference within sex, between genotype; $Difference within genotype, between sex. FABP5/7 knockout (KO) females (n = 7) consumed 21% more than their wild type (WT) (n = 15) counterparts (P < 0.001) and 52% more than the FABP5/7 KO males (P < 0.001). FABP5/7 KO males (n = 16) consumed 25% more than their WT counterparts (n = 14; P < 0.01). WT females consumed 58% more than the WT males (P < 0.001).
SBFI26-treated mice
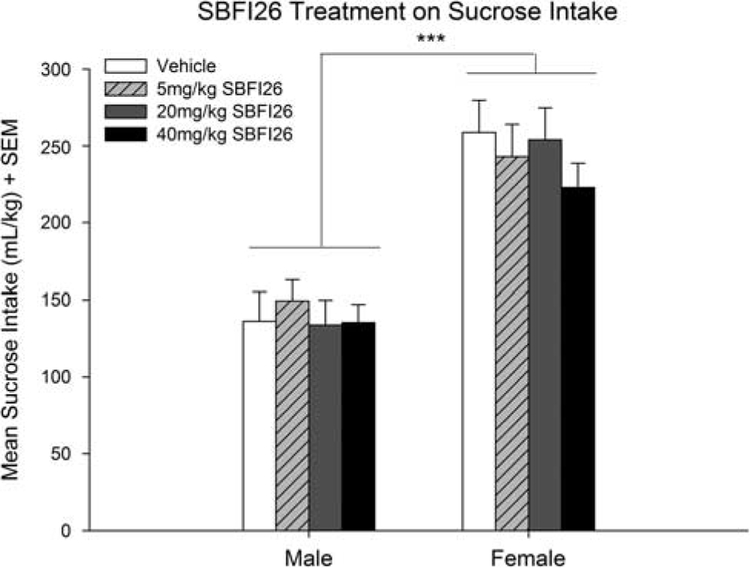
A two-way ANOVA found a significant effect of sex [F(1,71) = 74.24; P < 0.001], but no significant main effect of SBFI26 treatment [F(3,71) = 0.48; NS] on sucrose consumption (Fig. 2). A separate two-way ANOVA for water consumption revealed no significant effect on consumption of treatment [F(3,71) = 2.26; NS] or sex [F(1,71) = 0.97; NS]. Finally, a two-way ANOVA showed no significant differences in preference for sucrose for SBFI26 treatment [F(3,71) = 2.08; NS] or sex [F(1,71) = 1.41; NS].
Fig. 2.
Effects of SBFI26 administration on sucrose consumption in male and female mice. No differences were found between the doses given in either males or females (n = 10 and 9 for vehicle, n = 10 and 9 for 5 mg/kg, n = 10 and 9 for 20 mg/kg, n = 10 for 40 mg/kg, respectively for males and females). An overall sex effect was found where females consumed more than males; ***P < 0.001.
Forced swim
FABP5/7 KO mice
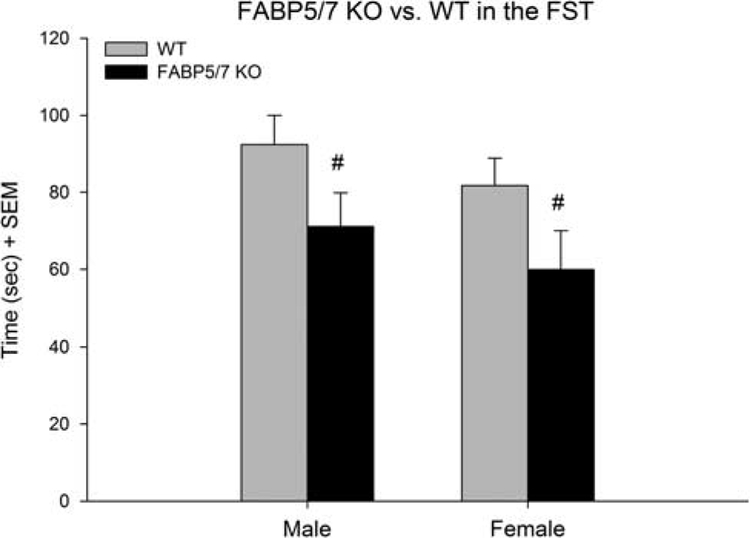
Immobility time in the FS was analyzed using a two-way ANOVA with the factors of genotype (FABP5/7 KO, WT) and sex (male and female) (Fig. 3). There was a significant main effect of genotype [F(1,47) = 10.33; P < 0.01]. Additional post-hoc tests used Tukey’s honest significant difference: FABP5/7 KO males showed a 27.0% decrease in immobility time compared with WT males (P < 0.05). Female FABP5/7 KOs showed a 26.7% decrease in immobility compared with WT females (P < 0.05).
Fig. 3.
Forced-swim behavior in wild type (WT) and FABP5/7 deficient mice. #A significant effect of genotype, within sex differences. Male (n = 16) and female (n = 6) FABP5/7 KOs showed a 27% (P < 0.05) and 27% (P < 0.05) decrease in immobility compared with WT counterparts (n = 14 and 15, respectively). FST, forced-swim test.
SBFI26-treated mice
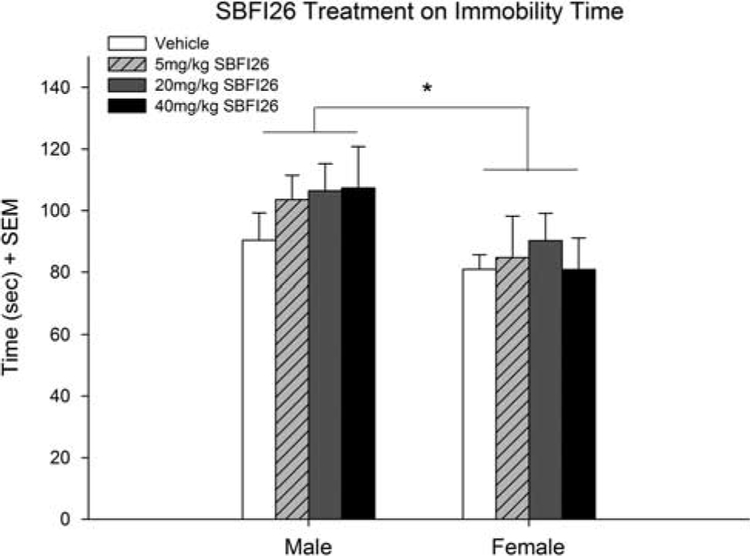
A two-way ANOVA was conducted with the factors of treatment (vehicle, 5, 20, and 40 SBFI26) and sex (male and female) (Fig. 4). No effect of treatment on immobility time was observed [F(3,68) = 0.57; NS], but there was a small effect of sex [F(1,68) = 6.33; P < 0.05], with females overall showing less immobility time.
Fig. 4.
Immobility time in the FS of male and female mice following 90 min pretreatment of SBFI26 (vehicle, 5, 20, and 40 mg/kg). No treatment effect was seen on immobility time, though a small effect was of sex seen (*P < 0.05) (n = 10 for vehicle, n = 10 for 5 mg/kg, n = 10 for 20 mg/kg, n = 9 for 40 mg/kg for both male and female). FS, forced swim.
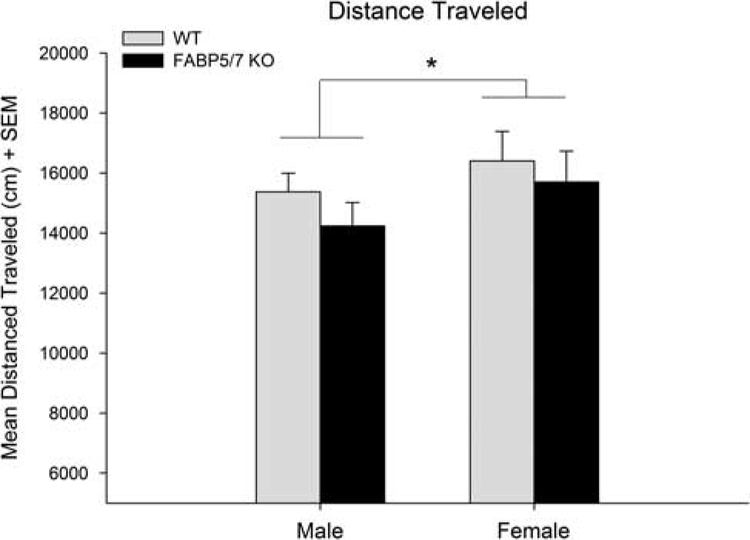
Distance traveled in the open field
A two-way ANOVA did not show any significant effect of genotype [F(1,37) = 3.41; P = 0.073], but did show an overall effect of sex [F(1,37) = 5.14; P < 0.05], where females were more active than males (Fig. 5).
Fig. 5.
Distance traveled in the open-field test. There was no significant effect of genotype. Overall, females were more active than males (*P < 0.05) (male WT: n = 12, female WT: n = 12, male FABP5/7 KO: n = 12, female FABP5/7 KO: n = 7). KO, knockout; WT, wild type.
Discussion
The recent demonstration that FABPs act as intracellular carriers of AEA has introduced new research questions pertaining to the processing of pain and reward signals (Kaczocha et al., 2014; Thanos et al., 2016). Here, we show that both male and female mice lacking the genes for FABP5 and FABP7 consume more sucrose (25 and 21%, respectively) and display reduced immobility time (27% for both) compared with their WT counterparts. In contrast, pharmacological inhibition of FABP5 and FABP7 using the novel inhibitor, SBFI26, did not change sucrose consumption or immobility time. There are several possible mechanisms for the effects of FABP inhibition/deletion.
One potential explanation of these results is through the activation of the CB1 receptor. The role of eCB signaling on reward and FS behavior is known to be mediated through the effects of AEA, 2-arachidonoylglycerol and CB1 receptors. Wang et al. (2015) have shown that eCB signaling is a regulator of DA activity within the VTA, primarily through CB1 activation on GABAergic neurons. Enhancement of eCB signaling has been shown to drive positive reinforcement, whereas attenuating eCB signaling blunts it (Lupica et al., 2004; Fattore et al., 2010). CB1 KO mice have been shown to consume less sucrose than their WT counterparts in a two choice paradigm (Sanchis-Segura et al., 2004). Pharmacologically blocking CB1 receptors also diminishes sucrose feeding and drinking behavior (Arnone et al., 1997; Freedland et al., 2001; Poncelet et al., 2003). In contrast, pharmacological compounds that raise EC levels through inhibition of fatty acid amide hydrolase (FAAH) also impact sucrose consumption. Specifically, treatment with URB597 (FAAH inhibitor) potentiated sucrose intake (Vinod et al., 2012). Enhancing eCB signaling in the NAc through anandamide microinjections was shown to amplify the hedonic impact of natural reward (Mahler et al., 2007). Also, CB1 receptor activation through the administration of Δ9-tetrahydrocannabinol was found to increase the hedonic response to sucrose ingestion. Increases in DA have been observed in the shell of the NAc as a result of Δ9-tetrahydrocannabinol and WIN55212–2 administration (Tanda et al., 1997; De Luca et al., 2012). One possible mechanism for the CB1-induced increases of DA seen within the NAc is thought to be disinhibition of GABA projections (through CB1 activation) to VTA DA neurons from local and outside sources (Szabo et al., 2002; Riegel and Lupica, 2004; Lupica and Riegel, 2005).
Our results also demonstrate that mice with FABP5/7 deletion show reduced FS immobility time, which may be indicative of antidepressant-like behavior. This difference in immobility time is unlikely to be a result of differences in motor activity, given the lack of difference seen in the open-field test (Fig. 5). Differences in immobility time are thought to be associated with differences in a depressive-like phenotype, given the fact that antidepressants have known to decrease immobility time in the FST (Pollak et al., 2010; Bogdanova et al., 2013). However, it needs to be stated that the FSTs were carried out in mice that had not undergone any procedure designed to elicit a depressive-like phenotype. Instead, these results offer a potential screening of depressive-related behavior that needs to be further explored in depression models. The finding of reduced immobility time in mice with pharmacologically or genetically induced increases in eCB signaling has been previously reported. Enhanced AEA signaling has been shown to decrease FS immobility time (Gobbi et al., 2005; Hill and Gorzalka, 2005). It has also been reported that exogenous CB1 agonists also significantly reduces immobility time (Kruk-Slomka et al., 2015). Treatment with other CB1 agonist or AEA-raising compounds have also shown reduced immobility time in the FST (Rutkowska and Jachimczuk, 2004; Umathe et al., 2011). In addition, Bambico et al. (2010) have shown that FAAH KO mice display reduced immobility time, which was normalized by a CB1 blocker (Bambico et al., 2010). In addition, chronic administration of the CB1 blocker, rimonabant, increased immobility time (Hillard, 2014). Thus, the differences in sucrose consumption and FS behavior between WT and FABP5/7 mice may be the result of enhanced CB1 activation.
We recently reported that SBFI26 decreases ethanol consumption in mice (Figueiredo et al., 2017). As decreases in ethanol consumption are known to occur following blockade of the CB1 receptor, this observation indicates that SBFI26 may interfere with the ability of AEA to bind to CB1. Similarly, Seillier and Giuffrida (2018) have demonstrated that administration of OMDM-2, which has been shown to target transporter proteins, including FABP5 (Kaczocha et al., 2012), impairs social interaction in male Wistar rats and is consistent with reduced activation of CB1 receptors. Given these findings, it is suprising that SBFI26 did not change sucrose consumption. It might have to do with the fact that SBFI26’s effects on ethanol consumption were observed in a 6-h paradigm and our sucrose consumption was measured over 24 h.
It has been previously shown that both SBFI26 administation and FABP5/7 gene deletion raise OEA and PEA levels (Kaczocha et al., 2015; Peng et al., 2017). It is also possible that both FABP gene deletion and pharmacological inhibition prevent the activation of the CB1 receptor and the effects have more to do with the actions of OEA and PEA, which activate the nuclear peroxisome proliferator-activated receptor-α. Peng et al. (2017) suggested that the effects of FABP5/7 KO on nociception are mediated through the transient receptor potential vanilloid 1 and peroxisome proliferator-activated receptor-α, rather than by CB1 (Peng et al., 2017). Elevation of OEA/PEA may be responsible for the increased sucrose consumption and decreased immobility time seen in the FABP5/7 KOs. This is supported by the fact that both OEA and PEA administration can reduce immobility time in the FST as well as the tail suspension test (Yu et al., 2011, 2015). Moreover, chronic treatment of OEA (1.5, 3, and 6 mg/kg) has been shown to increase sucrose preference following chronic unpredictable mild stress (Jin et al., 2015). Finally, it cannot be ruled out that SBFI26 or FABP5/7 gene deletion alters the functioning or expression of other known eCB carriers, such as Hsp70 or albumin (Nicolussi and Gertsch, 2015). More information is needed to shed light on why differences in behavior are observed between pharmacological inhibition and genetic deletion of FABPs.
Our findings suggest that genetic deletion, but not pharmacological inhibiton, of FABP5 and FABP7, affects sucrose intake and antidepressant-related behaviors. Further investigation will be needed to identify the precise mechanism behind these behavioral changes induced by genetic FABP5/7 deletion. One limitation to this study was the concentation of sucrose used. Because of the fact that WT mice show a high preference for the 2% sucrose solution, it would be difficult to detect increases in preferences within the FABP5/7 KO mice, if any exist. Further work will be needed to examine how FABPs regulate sucrose intake and preference at different sucrose concentrations.
Acknowledgements
This research was funded by the NY Research Foundation (RIAQ0940) and the NIH (DA035923 and DA035949).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G (1997). Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132:104–106. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G (2010). Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 35:2083–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL (2002). Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids 66:287–299. [DOI] [PubMed] [Google Scholar]
- Batista LA, Gobira PH, Viana TG, Aguiar DC, Moreira FA (2014). Inhibition of endocannabinoid neuronal uptake and hydrolysis as strategies for developing anxiolytic drugs. Behav Pharmacol 25:425–433. [DOI] [PubMed] [Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, et al. (2012). Targeting fatty acid binding protein (FABP) anandamide transporters – a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS ONE 7:e50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U (2010). Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 35:2462–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF (2013). Factors influencing behavior in the forced swim test. Physiol Behav 118:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K (2000). Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res 126:325–341. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569. [DOI] [PubMed] [Google Scholar]
- Delis F, Thanos PK, Rombola C, Rosko L, Grandy D, Wang G-J, Volkow ND (2013). Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav Neurosci 127:95–105. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G (2012). Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 63:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Pistis M, Fratta W (2010). The endocannabinoid system and nondrug rewarding behaviours. Exp Neurol 224:23–36. [DOI] [PubMed] [Google Scholar]
- Figueiredo A, Hamilton J, Marion M, Blum K, Kaczocha M, Haj-Dahmane S, et al. (2017). Pharmacological inhibition of brain fatty acid binding protein reduces ethanol consumption in mice. J Reward Defic Syndr Addict Sci 3:21–27. [PMC free article] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ (2001). Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res 25:277–282. [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. (2005). Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 102:18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, et al. (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 35:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY (2010). Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol 588:2589–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB (2005). Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol 15:593–599. [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS (2010). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry 34:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ (2014). Stress regulates endocannabinoid-CB1 receptor signaling.Semin Immunol 26:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL 2nd (2006). Endocannabinoid mechanisms of pain modulation. AAPS J 8:E693–E708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Yu HL, Tian L, Zhang F, Quan ZS (2015). Antidepressant-like effects of oleoylethanolamide in a mouse model of chronic unpredictable mild stress. Pharmacol Biochem Behav 133:146–154. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Deutsch DG (2009). Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci USA 106:6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG (2012). Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem 287:3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Rebecchi MJ, Ralph BP, Teng YH, Berger WT, Galbavy W, et al. (2014). Inhibition of fatty acid binding proteins elevates brain anandamide levels and produces analgesia. PLoS ONE 9:e94200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O’Rourke J, et al. (2015). Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Mol Pain 11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk-Slomka M, Michalak A, Biala G (2015). Antidepressant-like effects of the cannabinoid receptor ligands in the forced swimming test in mice: mechanism of action and possible interactions with cholinergic system. Behav Brain Res 284:24–36. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA (2006). The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci 63:1597–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC (2005). Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48:1105–1116. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF (2004). Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol 143:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC (2007). Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32:2267–2278. [DOI] [PubMed] [Google Scholar]
- Matsumata M, Sakayori N, Maekawa M, Owada Y, Yoshikawa T, Osumi N (2012). The effects of Fabp7 and Fabp5 on postnatal hippocampal neurogenesis in the mouse. Stem Cells 30:1532–1543. [DOI] [PubMed] [Google Scholar]
- Mazier W, Saucisse N, Gatta-Cherifi B, Cota D (2015). The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metab 26:524–537. [DOI] [PubMed] [Google Scholar]
- Melis M, Sagheddu C, De Felice M, Casti A, Madeddu C, Spiga S (2014). Enhanced endocannabinoid-mediated modulation of rostromedial tegmental nucleus drive onto dopamine neurons in Sardinian alcohol-preferring rats. J Neurosci 34:12716–12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Jupp B, Belin D, Dalley JW (2015). Endocannabinoids and striatal function: implications for addiction-related behaviours. Behav Pharmacol 26:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolussi S, Gertsch J (2015). Endocannabinoid transport revisited. Vitam Horm 98:441–485. [DOI] [PubMed] [Google Scholar]
- Panagis G, Mackey B, Vlachou S (2014). Cannabinoid regulation of brain reward processing with an emphasis on the role of CB1 receptors: a step back into the future. Front Psychiatry 5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolaro D, Vigano D, Realini N, Rubino T (2007). Role of endocannabinoids in regulating drug dependence. Neuropsychiatr Dis Treat 3:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL (2015). Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Studholme K, Kanjiya MP, Luk J, Bogdan D, Elmes MW, et al. (2017). Fatty-acid-binding protein inhibition produces analgesic effects through peripheral and central mechanisms. Mol Pain 13:1744806917697007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, Rey CE, Monje FJ (2010). Rodent models in depression research: classical strategies and new directions. Ann Med 42:252–264. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P (2003). Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett 343:216–218. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR (2004). Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24:11070–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska M, Jachimczuk O (2004). Antidepressant-like properties of ACEA (arachidonyl-2-chloroethylamide), the selective agonist of CB1 receptors. Acta Pol Pharm 61:165–167. [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline BH, Marsicano G, Lutz B, Spanagel R (2004). Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology (Berl) 176:223–232. [DOI] [PubMed] [Google Scholar]
- Seillier A, Giuffrida A (2018). The cannabinoid transporter inhibitor OMDM-2 reduces social interaction: further evidence for transporter-mediated endocannabinoid release. Neuropharmacology 130:1–9. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I (2002). Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci 15:2057–2061. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Chiara GD (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science 276:2048–2050. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND (2005). Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res 164:206–213. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Clavin BH, Hamilton J, O’Rourke JR, Maher T, Koumas C, et al. (2016). Examination of the addictive and behavioral properties of fatty acid-binding protein inhibitor SBFI26. Front Psychiatry 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umathe SN, Manna SS, Jain NS (2011). Involvement of endocannabinoids in antidepressant and anti-compulsive effect of fluoxetine in mice. Behav Brain Res 223:125–134. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Xie S, Psychoyos D, Hungund BL, Cooper TB, Tejani-Butt SM (2012). Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS ONE 7:e36743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lupica CR (2014). Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes. Prog Neuropsychopharmacol Biol Psychiatry 52:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Treadway T, Covey DP, Cheer JF, Lupica CR (2015). Cocaine-induced endocannabinoid mobilization in the ventral tegmental area. Cell Rep 12:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2008). Dopamine and reward: the anhedonia hypothesis 30 years on.Neurotox Res 14:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HL, Deng XQ, Li YJ, Li YC, Quan ZS, Sun XY (2011). N-palmitoylethanolamide, an endocannabinoid, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacol Rep 63:834–839. [DOI] [PubMed] [Google Scholar]
- Yu HL, Sun LP, Li MM, Quan ZS (2015). Involvement of norepinephrine and serotonin system in antidepressant-like effects of oleoylethanolamide in the mice models of behavior despair. Neurosci Lett 593:24–28. [DOI] [PubMed] [Google Scholar]