Abstract
Aims
To investigate if patients with atrial fibrillation (AF) without clear indication for oral anticoagulant (OAC) due to perceived low stroke risk may benefit from OAC treatment when also dementia and intracerebral bleeding risks are considered.
Methods and results
Retrospective study of cross-matched national registries of all individuals in Sweden with a hospital diagnosis of AF between 2006 and 2014 (n = 456 960). Exclusion was made of patients with a baseline CHA2DS2-VASc score >1, not counting female sex, and of patients with previous diagnosis of dementia or intracranial bleeding. After exclusions, 91 254 patients remained in the study of whom 43% used OAC at baseline. Propensity score matching and falsification endpoints were used. Treatment with OAC was associated with lower risk of dementia after adjustment for death as a competing risk [subhazard ratio (sHR) 0.62 with 95% confidence interval (CI) 0.48–0.81]. Regarding the composite brain protection endpoint, OAC treatment was associated with an overall 12% lower risk (sHR 0.88, CI 0.72–1.00). This apparent benefit was restricted to patients aged >65 years, whereas OAC treatment of patients <60 years of age without risk factors appeared to do more harm than good.
Conclusion
Low-risk AF patients who take OAC have lower risk of dementia than those who do not use OAC. Patients age >65 years appear to benefit from OAC treatment irrespective of stroke risk score.
Keywords: Atrial fibrillation, Dementia, Stroke, Intracerebral bleeding, Oral anticoagulation
Introduction
Observational studies have shown that atrial fibrillation (AF) patients are at increased risk of dementia.1,2 Repeated embolization of microscopic clots wears down the brain and causes cognitive decline and dementia much in the same way as embolization of macroscopic clots causes stroke.
However, it is not only vascular dementia, which is increased in AF, Alzheimer’s, and other types of dementia are also more common with AF.3–5 Partly this may be because AF and dementia share many risk factors, e.g. age, hypertension, diabetes, and cardiovascular disease.6–8 An autopsy study of 6205 individuals with neurodegenerative diseases showed that all major dementias have a vascular component, ranging from 61% in frontotemporal dementia to 80% in Alzheimer disease.9 Although dementia has a multifactorial aetiology, the cardiovascular part is the only one which is readily treatable and preventable.10
Recent observational studies have shown that AF patients using oral anticoagulant (OAC) drugs have almost half as high risk of dementia as AF patients not using OAC.11,12 Although non-randomized studies cannot prove causal treatment effects, these observations are in agreement with the hypothesis that OAC prevents embolization of both large and small clots.
Other studies have suggested participation of other mechanisms as well, e.g. interaction between OAC and factors XII to VII in the coagulation cascade, which are involved in the metabolism of beta amyloid 40 and 42, central in Alzheimer’s disease.13,14
Most AF patients are recommended OAC treatment due to high stroke risk associated with advanced age and comorbidity, but those who are young and healthy are not. In Sweden, approximately 15% of the AF population have CHA2DS2-VASc score ≤1 point (not counting female sex).15 According to current guideline recommendations, a 50-year-old patient with AF and no other risk factors could wait 15, or even 25 years, before age makes OAC treatment advisable.16 From a global brain protection perspective, i.e. with the objective to shield the brain from dementia as well as from embolic stroke and intracerebral bleedings (ICHs), it is possible that low-risk patients would be better off with OAC than without.
A randomized placebo controlled (RCT) trial would be ideal for determining net benefit of OAC treatment. It would not be ethically acceptable to randomize patients at risk of stroke to placebo instead of OAC, but it can be done with low-risk patients without clear indication for OAC. However, there are a few obstacles: (i) the observation period cannot be very long because the participants will grow older during the study and the stroke risk will increase to a level where it is no longer safe or ethical to continue without offering OAC treatment and (ii) the incidence of dementia and stroke is low among relatively young low-risk patients. Therefore, an RCT would have to include very large number of patients, especially of the study period has to be short, in order to obtain sufficient statistical power (iii) dementia develops gradually over many years. It is therefore not certain that a study with a short observation period of a couple of years will be able to detect an effect related to OAC treatment.
We decided to use the population wide Swedish health registries to explore this issue, while we are waiting for definite proof from an RCT.
Aims
To determine if AF patients with low stroke risk who use OAC are better protected from brain damage, whether it is dementia, ischaemic stroke, or ICH, than patients not using OAC.
Methods
Study population
All individuals in Sweden with a diagnosis of AF between 2006 and 2014 were identified by civic registration numbers in the nationwide Swedish Patient register and cross matched with the national Dispensed Drug register (n = 456 960). These registers have national coverage and carry information about all residents in Sweden irrespective of citizenship and without practically no individuals lost to follow-up. Validation studies have shown that these registers are well suited for research purposes.17–22 The lookback period for identification of past and current disease was from 1997, when the current version of the International Classification of Diseases (ICD-10) was adopted in Sweden.
Baseline information
Information about current and previous disease was extracted from the Patient register with the use of codes listed in Supplementary material online, Table S1. Individual risk scores for stroke according to CHA2DS2-VASc was obtained from register information about previous stroke/transient ischaemic attack (TIA), hypertension, heart failure, diabetes, vascular disease, age, and sex.23 Exclusion was made of patients with a baseline CHA2DS2-VASc score >1, not counting points for female sex, and of patients with previous diagnosis of dementia or intracranial bleeding.
Follow-up
The first contact with an AF diagnosis after 1 January 2006 was considered as index date. A 30-day blanking period was used in order to avoid double counting events that had caused the index contact. Therefore, follow-up started at index date + 30 days and diagnoses given during these first 30 days considered as belonging to baseline.
The Patient register and the Cause of Death register were used to detect events during follow-up. Censoring was made on the date of the event, death, emigration, or end of follow-up on 31 December 2014. Endpoints were a new diagnosis of dementia, ischaemic stroke, ICH, and a composite of these in an attempt to assess the net clinical benefit of OAC use.
Drug use
Drugs dispensed within 6 months before start of follow-up were considered as reflecting current drug use. Exposure to OACs during follow-up was assessed through dispensed quantities and dispensing intervals. For warfarin, which has no fixed dose, a modification to a previously validated method was used which assumes that all days between two subsequent dispensations are days on treatment if the interval does not exceed 6 months.24 If it exceeded 6 months, or if there were no more dispensations, treatment was assumed to have ended after 3 months. For determination of the number of days non-vitamin K oral anticoagulants (NOACs) would last, information about dispensed quantities and the standard dose for the strength of the drug was used. Outcome analyses were made according to OAC exposure during follow-up, where the analysis was restricted to patients who either never used OAC, and to patients who had access to OAC lasting at least 80% of the days at risk. Outcomes analysed according to treatment at baseline without consideration of later changes are also presented in analogy with the intention to treat principle.
Statistical methods
Individual propensity scores for the likelihood of OAC treatment at baseline were obtained through logistic regression. The propensity scores were matched 1:1 without replacement between patients with and without OAC treatment at baseline. A caliper of 0.001 on logit transformed scores was used.
For the comparison of outcomes with NOACs and vitamin K antagonist (VKA), another propensity score matching was performed in the same way.
Baseline characteristics before and after propensity score matching are presented descriptively with differences between groups assessed with standardized differences. Absolute standardized differences of ≥0.10 are considered as indicative of a significant imbalance between the groups.
Incidence rates are reported as number of events per 100 patient years at risk, with 95% confidence intervals (CIs). Competing risk regression of subhazard ratios (sHRs) accounting for the risk of dying before reaching specified outcomes was done according to the method of Fine and Gray25 to evaluate the association between outcomes and treatment.
For analyses according to treatment during follow-up, patients who did not have OAC >80% of the time or belonged to those who never used it, were excluded from the propensity-score matched cohorts since matching on future behaviour is not allowed and would have biased the results. Analyses made according to treatment at baseline, without consideration of later changes are also shown.
In order to assess the likelihood of hidden confounding regarding which patients that were given OAC treatment, a composite falsification endpoint was used.26 Conditions that influences decisions about treatment which is not documented by code but may affect outcome, e.g. if a patient looks frail or fit, may constitute hidden bias. A falsification endpoint consisting of events that occur more often in frail patients, but has no causal relationship with the treatment, can detect presence, direction and magnitude of such unknown bias. If the falsification endpoint, after adjustments for known cofactors, occurs significantly more often in one group than in the other, this indicates the presence of hidden confounding and that the groups were not balanced at baseline. For this study, a composite falsification endpoint consisting of hospitalization for a fall accident, pneumonia or a first-time diagnosis of osteoarthritis, diabetes, or chronic obstructive pulmonary disease was used. P-values <0.05 were considered significant. A composite falsification endpoint was considered as more robust and less likely to be affected by chance.
Data management and analyses were performed in Stata 14.2 (Stata Corp., College Station, TX, USA).
The study was approved by the local ethics committee (approvals #2014/894-31, #2014/876-31/4, #2014/1065-31) and conformed to the Declaration of Helsinki.
Results
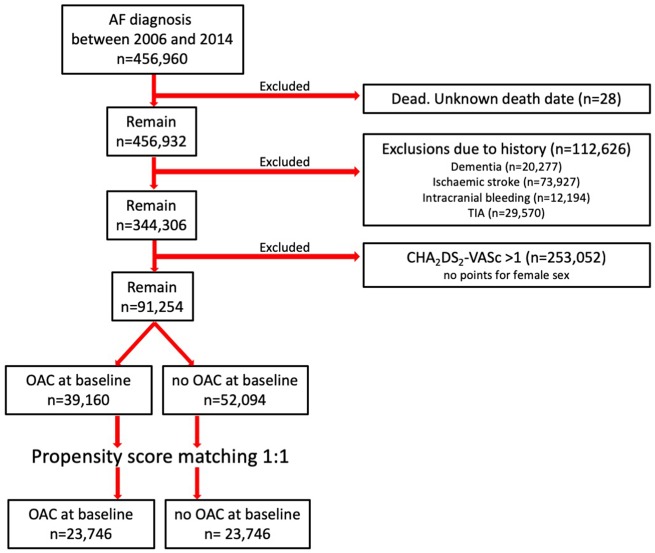
Of the total AF population, approximately 20% (91 254 out of 456 960) satisfied the inclusion and exclusion criteria (Figure 1). Despite low risk of ischaemic stroke, 43% were using OAC at baseline (39 160 out of 91 254). One-third of those with CHA2DS2-VASc score 0 points used OAC (13 332 out of 40 545).
Figure 1.
Inclusion and exclusions of study patients.
Patients using OAC were older (mean age 61.4 vs. 57.1 years), had more often heart failure (8.8% vs. 2.6%) and a history of venous thromboembolism (4.7% vs. 1.7%) (Table 1). They also had valvular disease and used cardiovascular drugs more often. Patients without OAC had alcohol related diagnoses and cancer more often than OAC treated patients. Propensity score matching produced two cohorts with and without OAC treatment of equal size (n = 23 746) who were similar on all tested covariates (Table 1).
Table 1.
Baseline characteristics, before and after propensity score matching
| Before matching |
Propensity-score matched |
|||||
|---|---|---|---|---|---|---|
| No OAC (n = 52 094) | OAC (n = 39 160) | Standardized differences | No OAC (n = 23 746) | OAC (n = 23 746) | Standardized differences | |
| Age, mean | 57.1 | 61.4 | −0.411 | 60.8 | 61.5 | −0.079 |
| Female sex | 35.1% | 29.1% | 0.129 | 29.9% | 30.7% | −0.017 |
| CHA2DS2-VASc, mean | 0.83 | 0.95 | −0.174 | 0.88 | 0.91 | −0.042 |
| >1 year since first AF diagnosis | 20.5% | 20.7% | −0.004 | 18.7% | 19.7% | −0.024 |
| Myocardial infarction | 1.7% | 1.2% | 0.041 | 1.2% | 1.3% | −0.001 |
| Peripheral artery disease | 0.6% | 0.9% | −0.027 | 0.4% | 0.5% | −0.014 |
| Heart failure | 2.6% | 8.8% | −0.267 | 2.5% | 2.8% | −0.016 |
| Any valvular disease | 3.7% | 10.6% | −0.269 | 3.7% | 4.4% | −0.035 |
| Pacemaker/ICD | 3.2% | 4.7% | −0.076 | 2.8% | 3.2% | −0.023 |
| Hypertension | 13.5% | 15.1% | −0.046 | 15.3% | 15.3% | 0.002 |
| Diabetes | 2.0% | 2.3% | −0.016 | 1.7% | 1.9% | −0.016 |
| Renal disease | 0.9% | 0.6% | 0.038 | 0.5% | 0.4% | 0.005 |
| Liver disease | 1.8% | 0.8% | 0.090 | 0.5% | 0.6% | −0.006 |
| Hospitalization for bleeding | 5.7% | 4.4% | 0.058 | 3.2% | 3.4% | −0.012 |
| Venous thromboembolism | 1.7% | 4.7% | −0.174 | 2.0% | 2.1% | −0.010 |
| Hypothyroidism | 2.8% | 2.4% | 0.020 | 1.9% | 2.1% | −0.010 |
| Thyrotoxicosis | 1.5% | 1.8% | −0.023 | 1.2% | 1.4% | −0.019 |
| Osteoarthritis | 10.5% | 12.4% | −0.059 | 12.1% | 12.6% | −0.016 |
| Parkinson’s disease | 0.4% | 0.3% | 0.010 | 0.3% | 0.3% | −0.012 |
| COPD | 3.3% | 3.0% | 0.013 | 2.0% | 2.4% | −0.028 |
| Alcohol index | 6.0% | 2.6% | 0.169 | 2.2% | 2.5% | −0.018 |
| Cancer ≤3 years | 7.0% | 4.0% | 0.131 | 4.2% | 4.4% | −0.007 |
| Frequent falls | 1.3% | 0.9% | 0.044 | 0.6% | 0.7% | −0.013 |
| Drugs used at baseline | ||||||
| ACE inhibitor or ARB | 17.6% | 34.5% | −0.394 | 23.1% | 23.2% | −0.003 |
| Diuretic | 11.3% | 22.5% | −0.303 | 12.4% | 13.1% | −0.020 |
| Statin | 14.0% | 18.1% | −0.114 | 15.7% | 16.5% | −0.023 |
| Nitrates | 5.0% | 4.6% | 0.006 | 4.1% | 4.4% | −0.016 |
| Beta blocker | 59.8% | 82.5% | −0.516 | 78.2% | 78.6% | −0.010 |
| Antiarrhythmic drug Class 1 or 3 | 5.5% | 6.3% | −0.035 | 5.6% | 6.7% | −0.047 |
| Digoxin | 4.1% | 16.9% | −0.428 | 4.8% | 5.3% | −0.026 |
The mean follow-up for the composite brain protection endpoint was 4.7 ± 2.8 years (median 4.7 years, interquartile range 2.2–7.2 years). Mean follow-up was between 4.8 and 4.9 years for all other endpoints.
Dementia
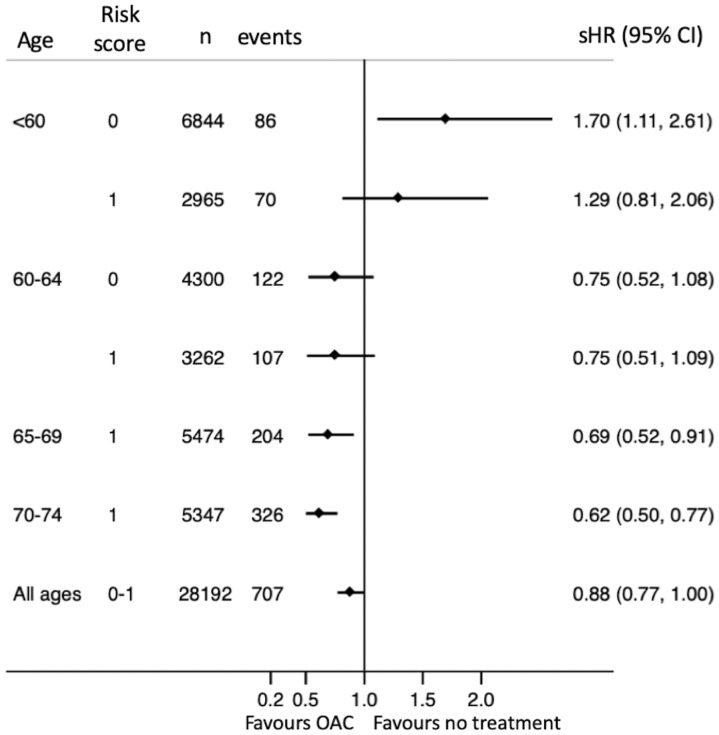
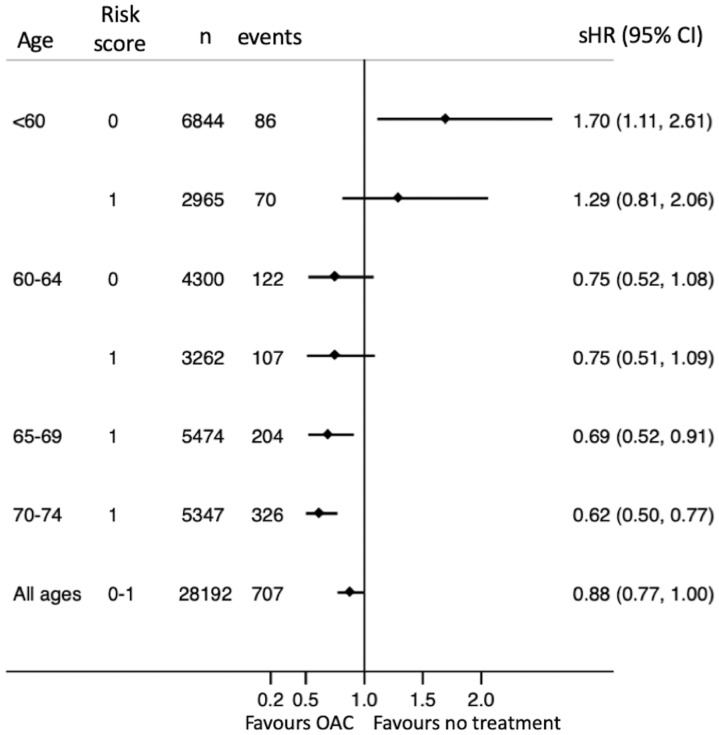
During follow-up, 956 patients received a first-time diagnosis of dementia. The incidence was 0.27 compared with 0.16 per 100 years at risk among patients who never used OAC and patients who used OAC at least 80% of the time, respectively (Table 2). The incidence increased as patients got older (Figure 2). Treatment with OAC was associated with lower risk of dementia (sHR0.62 with 95% CI 0.48–0.81). This apparent benefit was primarily seen in patients with CHA2DS2-VASc score 1 [hazard ratio (HR) 0.57, CI 0.43–0.74]. Few young patients with CHA2DS2-VASc score 0 got a dementia diagnosis (17 aged <60 and 31 aged 60–64) and hence the CIs were wide and the benefit of treatment uncertain (sHR 0.44, CI 0.19–1.02). There was a trend towards more benefit with increasing age (Figures 3 and 4).
Table 2.
Incidence rates (IR) and subhazard ratios (sHR) for outcomes in relation to OAC treatment
| As treated during follow-upa |
As treated at baselineb |
||||||
|---|---|---|---|---|---|---|---|
| Events | IR per 100 risk years (95% CI) | sHR (95% CI) | Events | IR per 100 risk years (95% CI) | sHR (95% CI) | ||
| Composite brain endpoint | No OAC | 535 | 0.84 (0.77–0.92) | Reference | 1279 | 1.14 (1.08–1.20) | Reference |
| OAC | 380 | 0.72 (0.81–0.92) | 0.88 (0.77–1.00) | 938 | 0.86 (0.81–0.92) | 0.77 (0.70–0.83) | |
| Dementia | No OAC | 174 | 0.27 (0.23–0.31) | Reference | 288 | 0.25 (0.22–0.28) | Reference |
| OAC | 88 | 0.16 (0.13–0.20) | 0.62 (0.48–0.81) | 215 | 0.19 (0.17–0.22) | 0.79 (0.66–0.94) | |
| Ischaemic stroke | No OAC | 331 | 0.52 (0.46–0.58) | Reference | 955 | 0.84 (0.79–0.90) | Reference |
| OAC | 246 | 0.46 (0.41–0.53) | 0.92 (0.78–1.09) | 618 | 0.56 (0.52–0.61) | 0.68 (0.61–0.75) | |
| ICH | No OAC | 63 | 0.10 (0.08–0.12) | Reference | 116 | 0.10 (0.08–0.12) | Reference |
| OAC | 60 | 0.11 (0.09–0.14) | 1.19 (0.84–1.70) | 148 | 0.13 (0.11–0.16) | 1.35 (1.06–1.72) | |
| Death from any cause | No OAC | 1461 | 2.25 (2.14–2.37) | Reference | 1885 | 1.63 (1.56–1.70) | Reference |
| OAC | 659 | 1.23 (1.14–1.33) | 0.54 (0.50–0.60) | 1465 | 1.32 (1.25–1.39) | 0.81 (0.75–0.87) | |
| Falsification endpointc | No OAC | 2652 | 4.61 (4.44–4.79) | Reference | 5084 | 5.05 (4.92–5.20) | Reference |
| OAC | 2381 | 5.14 (4.94–5.35) | 1.14 (1.08–1.20) | 4964 | 5.19 (5.04–5.33) | 1.03 (1.00–1.08) | |
Comparison of outcomes for 17 092 patients never exposed to OAC and 20 329 patients who used OAC >80% of time at risk, all within the propensity-score matched cohorts.
Comparison of outcomes for patients propensity score matched for the likelihood of OAC treatment at baseline, with 23 746 patients in each group.
Composite endpoint consisting of fall accident, pneumonia, newly diagnosed osteoarthritis, newly diagnosed diabetes, and newly diagnosed chronic obstructive pulmonary disease.
Figure 2.
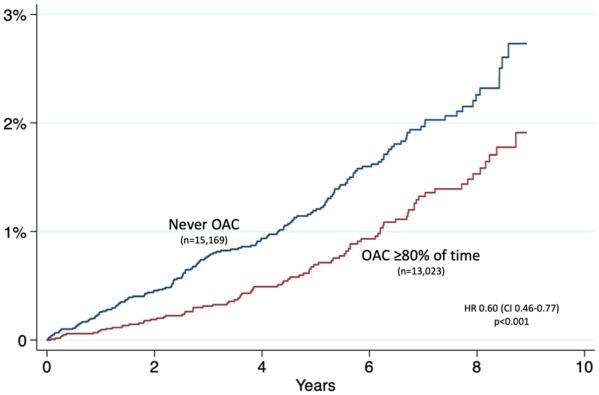
Unadjusted incidence of dementia in relation to oral anticoagulant treatment.
Figure 3.
Subhazard ratios for dementia in relation to oral anticoagulant use.
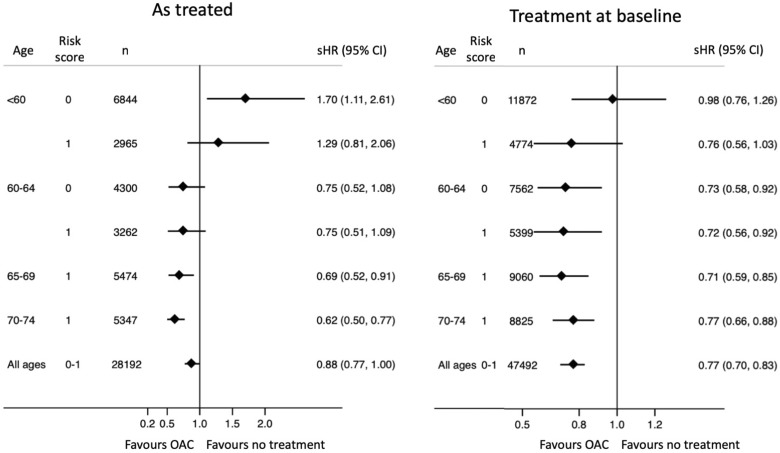
Figure 4.
Subhazard ratios for the composite brain endpoint in relation to oral anticoagulant use.
Take home figure.
Patients with atrial fibrillation aged 65–74 years without other stroke risk factors who take oral anticoagulants have lower risk of the composite of dementia, ischaemic stroke, or intracerebral bleeding than patients not taking oral anticoagulants.
Among patients without OAC at baseline, the strongest predictor for dementia was age followed by Parkinson’s disease, alcohol and repeated hospitalizations for fall accidents (Table 3). Predictors for ischaemic stroke were the constituents of the CHA2DS2-VASc score; age, hypertension, vascular disease, and heart failure but not diabetes. Instead, alcohol and frequent falls showed association with stroke risk in this low-risk population. Predictors for ICH were age, diabetes, heart failure, and alcohol. Women had lower risk of ICH than men (sHR 0.66, CI 0.49–0.90).
Table 3.
Factors associated with outcomes among 52 094 AF patients without OAC at baseline, adjusted for the competing risk of dying from other causes
| Composite brain endpoint sHR (95% CI) | Dementia sHR (95% CI) | Ischaemic stroke sHR (95% CI) | ICH sHR (95% CI) | |
|---|---|---|---|---|
| Age | ||||
| <60 | Reference | Reference | Reference | Reference |
| 60–64 | 2.04 (1.81–2.30) | 4.37 (3.02–6.34) | 1.83 (1.60–2.08) | 2.49 (1.73–3.59) |
| 65–69 | 3.25 (2.86–3.70) | 9.32 (6.53–13.31) | 2.66 (2.30–3.09) | 3.50 (2.28–5.37) |
| 70–74 | 4.86 (4.30–5.49) | 21.71 (15.51–30.39) | 3.56 (3.08–4.10) | 3.37 (2.16–5.24) |
| Female sex | 1.05 (0.96–1.14) | 1.08 (0.90–1.29) | 1.10 (0.99–1.21) | 0.66 (0.49–0.90) |
| >1 year since first AF diagnosis | 1.12 (1.02–1.23) | 1.14 (0.94–1.39) | 1.10 (0.99–1.23) | 1.06 (0.77–1.47) |
| Myocardial infarction | 1.22 (0.87–1.71) | 0.17 (0.02–1.23) | 1.58 (1.11–2.24) | 0.26 (0.04–1.82) |
| Peripheral artery disease | 1.74 (1.09–2.79) | 1.30 (0.32–5.27) | 1.72 (1.01–2.93) | 1.78 (0.43–7.34) |
| Heart failure | 1.31 (1.01–1.70) | 0.25 (0.06–1.05) | 1.39 (1.04–1.85) | 1.96 (1.01–3.79) |
| Any valvular disease | 1.22 (1.01–1.48) | 1.43 (0.98–2.10) | 1.20 (0.96–1.50) | 0.99 (0.52–1.90) |
| Pacemaker/ICD | 1.03 (0.84–1.27) | 0.94 (0.61–1.45) | 1.10 (0.87–1.39) | 0.87 (0.43–1.76) |
| Hypertension | 1.41 (1.22–1.64) | 1.20 (0.80–1.79) | 1.49 (1.27–1.76) | 1.05 (0.66–1.69) |
| Diabetes | 1.34 (0.99–1.81) | 1.25 (0.60–2.74) | 1.25 (0.88–1.78) | 2.40 (1.19–4.83) |
| Renal disease | 1.40 (0.97–2.01) | 1.46 (0.67–3.17) | 1.24 (0.80–1.93) | 1.57 (0.55–4.45) |
| Liver disease | 1.05 (0.79–1.39) | 1.40 (0.82–2.40) | 0.94 (0.66–1.32) | 0.94 (0.41–2.17) |
| Hospitalization for bleeding | 1.02 (0.86–1.20) | 1.27 (0.93–1.73) | 0.98 (0.80–1.19) | 1.03 (0.61–1.74) |
| Venous thromboembolism | 1.25 (0.95–1.64) | 0.83 (0.42–1.62) | 1.35 (0.99–1.83) | 1.13 (0.46–2.75) |
| Hypothyroidism | 1.11 (0.89–1.39) | 1.11 (0.70–1.74) | 1.09 (0.84–1.41) | 1.12 (0.48–2.60) |
| Thyrotoxicosis | 0.86 (0.61–1.22) | 0.92 (0.44–1.89) | 0.87 (0.59–1.30) | 0.32 (0.04–2.31) |
| Osteoarthritis | 1.10 (0.97–1.24) | 1.17 (0.93–1.48) | 1.06 (0.92–1.22) | 0.93 (0.61–1.43) |
| Parkinson’s disease | 2.36 (1.63–3.41) | 5.66 (3.56–9.02) | 0.84 (0.42–1.70) | — |
| COPD | 0.96 (0.79–1.18) | 1.09 (0.75–1.58) | 0.94 (0.74–1.20) | 0.72 (0.35–1.49) |
| Alcohol index | 1.64 (1.40–1.93) | 2.00 (1.43–2.81) | 1.61 (1.34–1.94) | 1.68 (1.06–2.66) |
| Cancer ≤3 years | 0.82 (0.70–0.96) | 0.60 (0.42–0.84) | 0.84 (0.70–1.01) | 1.34 (0.87–2.08) |
| Frequent falls | 1.70 (1.33–2.17) | 1.85 (1.21–2.83) | 1.65 (1.23–2.21) | 1.55 (0.69–3.51) |
| Drugs dispensed within preceding 6 months | ||||
| ACE inhibitor or ARB | 1.14 (1.02–1.27) | 1.17 (0.93–1.49) | 1.11 (0.98–1.27) | 1.36 (0.96–1.94) |
| Diuretic | 0.96 (0.85–1.08) | 0.83 (0.65–1.07) | 0.98 (0.86–1.13) | 1.23 (0.86–1.76) |
| Statin | 0.96 (0.85–1.08) | 1.16 (0.92–1.46) | 0.87 (0.76–1.01) | 1.12 (0.77–1.61) |
| Nitrates | 1.11 (0.94–1.30) | 1.31 (0.97–1.77) | 1.07 (0.88–1.31) | 0.69 (0.37–1.27) |
| Beta blocker | 1.13 (1.03–1.23) | 0.89 (0.75–1.06) | 1.19 (1.08–1.32) | 1.42 (1.06–1.90) |
| Antiarrhythmic drug Class 1 or 3 | 0.92 (0.77–1.09) | 0.94 (0.65–1.36) | 0.92 (0–75–1.13) | 1.18 (0.69–2.01) |
| Digoxin | 1.30 (1.11–1.51) | 1.38 (1.04–1.85) | 1.20 (1.00–1.44) | 1.50 (0.93–2.43) |
Ischaemic stroke
The incidence of ischaemic stroke was 0.46 and 0.52 per years of risk with and without OAC, respectively. Oral anticoagulant treatment was not associated with lower risk of ischaemic stroke in this population with CHA2DS2-VASc 0–1 points (sHR 0.92, CI 0.78–1.09). Predictors for stroke were largely the same as the component risk factors of CHA2DS2-VASc, i.e. age, vascular disease, heart failure, and hypertension (Table 3). Female sex and diabetes did not predict stroke, in contrast to alcohol where a previous alcohol related diagnosis was associated with higher stroke risk.
Intracerebral bleeding
The incidence of ICH was similar with and without OAC (0.10 and 0.11 events per 100 years at risk, respectively). The incidence was higher among those who used OAC at baseline but stopped during follow-up (0.8 vs. 0.5%, P < 0.001) as OAC treatment was frequently terminated after an ICH. Risk factors for ICH were age, diabetes, hypertension, heart failure, renal disease, Parkinson’s disease, frequent falls, and alcohol. Female sex was associated with significantly lower adjusted risk of haemorrhagic stroke compared with men (HR 0.65, CI 0.48–0.89) (Table 3).
The composite brain health endpoint
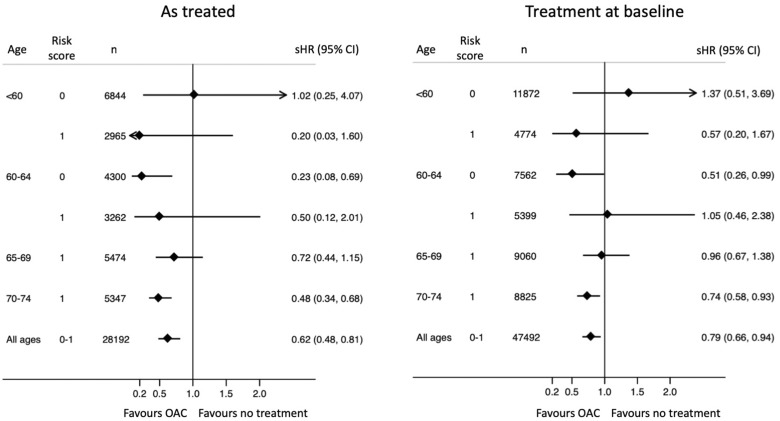
The composite endpoint consisting of ischaemic or haemorrhagic stroke or dementia occurred at 0.84 and 0.72 events per 100 years at risk with and without OAC, respectively. Treatment with OAC was associated with an overall 12% lower risk (HR 0.88, CI 0.77–1.00). When subdivided according to age, however, this benefit seemed to be restricted to patients aged >65 years, whereas OAC treatment of patients <60 years of age with no risk factors appeared to convey more harm rather than benefit when analysed according to OAC exposure during follow-up (Figure 3, left), but not when analysed according to treatment at baseline (Figure 3, right).
NOACs compared with VKA
Among 39 160 patients using OAC at baseline, 2926 (7.5%) used a NOAC. A new propensity score matching procedure generated two cohorts with 2528 patients in each. A comparison of these showed lower incidence of the composite endpoint with NOAC than with VKA (NOAC 0.42, CI 0.19–0.93 vs. VKA 0.74, CI 0.55–1.00 per 100 years at risk). The point estimate for the HR favoured NOACs over VKA but did not reach statistical significance (HR 0.47, CI 0.18–1.22). No ICH events occurred in the NOAC cohort compared with three events in the VKA cohort.
The falsfication endpoint
The composite falsification endpoint had more events in the OAC treated group indicating that there was more undocumented frailty in the OAC group than in the other group (sHR 1.14, CI 1.08–1.20). Hidden bias could therefore led to an underestimation of the benefit of OAC treatment.
Discussion
Our main finding is that the brains of AF patients over 65 years appeared to fare better with OAC than without OAC, irrespective of other risk factors. If this observation holds true and can be confirmed by others, counting of risk scores may not be needed in order to decide whether to offer OAC or not since age alone is enough to tip the balance in favour of treatment (unless there are strong reasons against). Then 65 years could substitute 75 years as cut-off age as in the current guideline recommendations.
Age 60–65 years would then be the new grey zone. Algorithms and rules of thumb are poor companions in grey zones where decisions has to be individualized according to circumstances and preferences.
Risk scores will still be needed for decisions about OAC treatment among the youngest aged <60 years.
Interestingly, the results appeared to favour NOACs more than VKA. The incidence rate and HR for the combined endpoint was about half as high with NOACs as with VKA, and the point estimate for the HR 0.47 favoured NOACs, but the number of patients available for the analysis was low and hence the CIs were too wide for statistical significance. A previous study by Jacobs et al.27 suggested that NOACs were better than VKA for dementia protection, but failed to show a statistically significant difference (HR 0.57, CI 0.17–1.97). In a previous study by our group we found no difference in dementia risk between NOAC and VKA (HR 0.97 CI 0.67–1.40) when analysed according to all ages and all risk strata, but there was an interaction with age where the youngest patients appeared to benefit (age 65–74 years: HR 0.66, CI 0.24–1.79).11
Hypertension, diabetes, smoking, alcohol, obesity, and physical inactivity are important causes for stroke in the general population. They are also risk factors for development of AF and therefore common in AF populations. Ninety percent of all strokes are considered to be due to risk factors which are possible to do something about. A prospective study with a multifactorial approach including not only OAC treatment but optimized treatment of other cardiovascular risk factors including life style interventions is therefore motivated in order to protect the brains of AF patients from dementia as well as from ischaemic stroke and ICH.
It was not possible to perform analyses according to AF was paroxysmal, persistent, or permanent because the codes for AF subtype are too infrequently used to be useful. Furthermore, such analyses are complicated by the fact that AF is a progressive disease and many patients will change type of AF during a long observation period as in this study. Considering that we studied low-risk patients with CHA2DS2-VASc scores of 0 or 1, it is likely that a high proportion had paroxysmal type of AF when they were enrolled.
Few patients in this study received a clinical diagnosis of dementia during follow-up. A clinical diagnosis is a late event in the disease process, which generally does not occur until the cognitive decline is interfering with the activities of daily life. Validation studies of clinical dementia diagnoses in the Swedish Patient register have shown over 98% specificity compared with adjudicated individual cognitive testing, but poor sensitivity ranging between 26% and 43%.20,22 Among patients younger than 75 years the median delay from the first symptoms of AF until a clinical diagnosis was established was 8 years.22
A prospective randomized trial with evaluation of cognitive function at baseline and during follow-up would show higher incidence of dementia than we have done in this study since the sensitivity would be higher. Switching to the endpoint minimal cognitive impairment, which precedes dementia, would also yield higher incidence rates. BRAIN-AF (clinicaltrials.gov: NCT02387229) is a study randomizing low-risk AF patients aged 30–62 years to rivaroxaban or standard care with a composite endpoint of stroke, TIA, and neurocognitive decline. The data collection are planned to be completed in 2022.
Limitations
The most important limitation is the lack of randomization and its vulnerability to hidden confounding regarding decisions whether or not to treat with OAC. Differences between treatment groups which affect treatment decisions can be adjusted for if the information is available in the registreis, otherwise it will cause hidden confounding. Such confounding is particularly common in relation to life style factors like for which diagnostic codes seldom are used. Hidden confounding may also arise from the binary yes/no-nature of registries, e.g. the same code is used for borderline and malignant hypertension although the prognosis is very different.
We used propensity score matching to reduce the risk of confounding, and a falsification endpoint in order to discover the presence of confounding by indication but found no signs of a hidden bias exaggerating the beneficial effect of OAC treatment. On the contrary, the falsification endpoint indicated that there was a modest bias working against, rather than in favour of OAC treatment.
We have presented results made both according to treatment during follow-up and according to treatment at baseline. Although both approaches have their problems, we have chosen the on treatment approach for the main results, because the long observation period inevitably confers cross-over problems when patients stop and start OAC treatment. A weakness with this is that we could not propensity score match for adherence to treatment which lay in the future and was not known at baseline. We therefore used the propensity score matched cohorts from the analyses made according to treatment at baseline, and excluded patients in the untreated cohort when they began with OAC and patients in the treated cohort if they had not used OAC at least 80% of the time at risk. This means that patients who were intentionally treated with OAC for a shorter period, e.g. in conjunction with DC cardioversion, were excluded. These exclusions may have upset the balancing obtained by the matching procedure by excluding relatively healthier patients from the OAC cohort and thus biasing the results against OAC benefit.
Analyses made on treatment may also have been affected by events during follow-up, e.g. patients with an ICH, while on OAC and therefore stopped using OAC could be excluded which then would bias results in favour of OAC treatment. Patients with early, but uncoded dementia, may also have been taken off treatment, which also may have led to overestimation of the beneficial effects of OAC in on treatment analyses. It is less likely that ischaemic stroke, while on OAC treatment would lead to cessation of treatment. Thus, there are several problems with the on treatment analyses and the results should preferably be seen together with the analyses made according to treatment at baseline.
The low sensitivity for detection of early stages of dementia through registers means the exclusion of all patients with a diagnosis of dementia did not exclude all patients with dementia. It is conceivable that doctors may have chosen to treat patients with undocumented early dementia in other ways than they treated others, although the falsification endpoint did not indicate that this played a role. It has also to be remembered that this was a highly selected low-risk population without elderly or frail patients and thus considerations about whether patients could manage OAC treatment must have been rare.
Conclusions
Men and women with AF and CHA2DS2-VASc score 0 or 1 who use OACs appear to have lower risk of dementia than those who do not use OACs. In a composite endpoint of dementia, ischaemic stroke, and ICH, there was a benefit of anticoagulants in patients 65 years and older. It may be relevant to consider this when making decisions about oral anticoagulation.
Conflict of interest: L.F. has received consultancy fees from Bayer, Boehringer-Ingelheim, BMS, Pfizer and Sanofi. T.A. has no conflicts of interest to declare. M.R. has received grants/consultancy fees from Abbott, Bayer, BMS, Pfizer and Zenicor.
Supplementary Material
See page 2336 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz414)
References
- 1. Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Horton R, Burkhardt JD, Lakkireddy D, Reddy YM, Casella M, Dello Russo A, Tondo C, Natale A.. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm 2012;9:1761–1768. [DOI] [PubMed] [Google Scholar]
- 2. Singh-Manoux A, Fayosse A, Sabia S, Canonico M, Bobak M, Elbaz A, Kivimäki M, Dugravot A.. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J 2017;38:2612–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD.. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm 2010;7:433–437. [DOI] [PubMed] [Google Scholar]
- 4. Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A.. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke 1997;28:316–321. [DOI] [PubMed] [Google Scholar]
- 5. Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, McCurry SM, Larson EB.. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc 2011;59:1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J.. Pathophysiologic relationship between Alzheimer's disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement (Amst) 2017;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ihara M, Washida K.. Linking atrial fibrillation with Alzheimer's disease: epidemiological, pathological, and mechanistic evidence. J Alzheimer's Dis 2018;62:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Toledo Ferraz Alves TC, Ferreira LK, Wajngarten M, Busatto GF.. Cardiac disorders as risk factors for Alzheimer's disease. J Alzheimer's Dis 2010;20:749–763. [DOI] [PubMed] [Google Scholar]
- 9. Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ.. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013;136:2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V.. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimer's Dement 2018;14:148–156. [DOI] [PubMed] [Google Scholar]
- 11. Friberg L, Rosenqvist M.. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018;39:453–460. [DOI] [PubMed] [Google Scholar]
- 12. Ding M, Fratiglioni L, Johnell K, Santoni G, Fastbom J, Ljungman P, Marengoni A, Qiu C.. Atrial fibrillation, antithrombotic treatment, and cognitive aging: a population-based study. Neurology 2018;91:e1732–e1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang L, Bhattacharya A, Li Y, Zhang Y.. Anticoagulants inhibit proteolytic clearance of plasma amyloid beta. Oncotarget 2018;9:5614–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suidan GL, Singh PK, Patel-Hett S, Chen ZL, Volfson D, Yamamoto-Imoto H, Norris EH, Bell RD, Strickland S.. Abnormal clotting of the intrinsic/contact pathway in Alzheimer disease patients is related to cognitive ability. Blood Adv 2018;2:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friberg L, Rosenqvist M, Lip GY.. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 16. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO.. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O.. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 2010;25:95–102. [DOI] [PubMed] [Google Scholar]
- 19. Appelros P, Terent A.. Validation of the Swedish inpatient and cause-of-death registers in the context of stroke. Acta Neurol Scand 2011;123:289–293. [DOI] [PubMed] [Google Scholar]
- 20. Dahl A, Berg S, Nilsson SE.. Identification of dementia in epidemiological research: a study on the usefulness of various data sources. Aging Clin Exp Res 2007;19:381–389. [DOI] [PubMed] [Google Scholar]
- 21. Friberg L, Skeppholm M.. Usefulness of Health Registers for detection of bleeding events in outcome studies. Thromb Haemost 2016;116:1131–1139. [DOI] [PubMed] [Google Scholar]
- 22. Jin YP, Gatz M, Johansson B, Pedersen NL.. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology 2004;63:739–741. [DOI] [PubMed] [Google Scholar]
- 23. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 24. Skeppholm M, Friberg L.. Adherence to warfarin treatment among patients with atrial fibrillation. Clin Res Cardiol 2014;103:998–1005. [DOI] [PubMed] [Google Scholar]
- 25. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 26. Groenwold RH. Falsification end points for observational studies. JAMA 2013;309:1769–1770. [DOI] [PubMed] [Google Scholar]
- 27. Jacobs V, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD, Mallender C, Osborn JS, Stevens SM, Weiss JP, Woller SC, Bunch TJ.. Long-term population-based cerebral ischemic event and cognitive outcomes of direct oral anticoagulants compared with warfarin among long-term anticoagulated patients for atrial fibrillation. Am J Cardiol 2016;118:210–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.