Abstract
OBJECTIVE
To estimate Brucella canis seropositivity rates for purebred dogs being bred by noncommercial breeders, describe epidemiological findings in infected commercial dog-production facilities, and characterize B canis infection in pet dogs and the risk to human health.
DESIGN
Retrospective descriptive study.
SAMPLE
2,799 canine specimens submitted to the Michigan State University Veterinary Diagnostic Laboratory for B canis testing and records of B canis reports provided to the Michigan Department of Agriculture and Rural Development from 2007 through 2016.
PROCEDURES
Results of B canis laboratory tests and epidemiological findings for reported cases of B canis were reviewed and summarized. Federal and state public health officials were interviewed regarding human B canis infection. State veterinarians were interviewed regarding canine brucellosis reporting and control procedures.
RESULTS
Estimated B canis seropositivity was 0.4% among purebred Michigan dogs owned by noncommercial breeders. Infection was confirmed in dogs from 17 commercial dog-production facilities, 3 shelters, and I rescue agency. Estimated infection prevalence in production facilities ranged from 2 of 22 (9%) to 5 of 6 (83%). Transfer of infected dogs involved 22 Michigan counties and II states. Seven of 20 privately owned infected dogs had diskospondylitis; I also had uveitis. Fifty-three veterinary hospital or diagnostic laboratory personnel had inadvertent exposure to the pathogen. Brucella canis was isolated from I commercial production facility owner.
CONCLUSIONS AND CLINICAL RELEVANCE
B canis was uncommon in purebred dogs being bred by noncommercial breeders but endemic in Michigan commercial facilities producing dogs destined to become household pets. Infected pet dogs caused human B canis exposure, and several pet dogs had debilitating disease not associated with the reproductive system.
In Michigan, canine brucellosis is reportable to the MDARD. In 2007, the MDARD began receiving an unprecedented number of reports of Brucella canis infection, many originating from 1 commercial dog-production facility (P1). Disease investigation by MDARD field veterinarians found approximately 90 dogs residing in the facility, where the first sign of disease recounted by the owner was conception failure among dogs obtained in 2006 from facilities in Indiana, Ohio, West Virginia, and New York. Abortions and stillbirths had then occurred for 10 months; during this time, no pups survived to weaning age. After initial screening of 70 dogs for B canis infection (by RSATa) revealed that 100% were seropositive, blood samples from 25 dogs were submitted to a Tennessee veterinary practice for IFA testing, and 24 (96%) had positive results. Intermittent testing and culling of seropositive dogs (approx 62) continued until December 2007, when another stillbirth occurred. Blood cultures from 3 of the remaining 28 (11%) dogs tested positiveb for B canis. The MDARD then placed the facility under quarantine with stipulations for cleaning and disinfection, which were not accomplished. Eventually all of the remaining dogs were euthanized. Disease investigation revealed that prior to the quarantine, dogs had been sold, bred, or moved among this and 2 other dog-production facilities in the state, and puppies and adult dogs had been sold to the public through pet shops and dog brokers. Dogs from 1 of the 2 recipient facilities were directly or indirectly exchanged with those of 5 other Michigan dog-production facilities. By March 2009, 11 commercial dog-production facilities had been identified and inspected by MDARD field veterinarians, and placed under quarantine. Infection in the facilities was confirmed by B canis-positive culturea,b or AGIDcpac results.
As a result of the frequent interchange of dogs and inadequate recordkeeping, the numbers of adult dogs in the infected commercial facilities could not be accurately determined. However, on the basis of laboratory results and counts obtained during veterinary inspections, these facilities collectively had > 300 adult dogs, and an unknown number of puppies had been sold from these locations. The producers were located in 5 different counties, and trace-forward investigations found infected pet dogs in 3 additional counties. Although the clinical signs and the high prevalence of infection within the breeding colonies were typical of canine brucellosis, the magnitude of the intrastate dog trade revealed during the disease investigation was unexpected.
In early 2010, diskospondylitis was found in 2 unrelated, B canis-infected puppies imported from Kentucky by 2 Michigan animal shelters. The shelters were located in 2 counties, neither of which included the commercial production facilities under quarantine at the time. Finding debilitating clinical disease related to B canis infection in such young dogs was unexpected.
These experiences greatly increased the authors’ appreciation of the interstate and intrastate trade in dogs destined to become household pets and raised concerns about the risk of B canis exposure to people. During this time, the MDARD continued to receive and investigate new reports of canine brucellosis, and the authors began to look at B canis from a different perspective. In our experience, veterinarians’ index of suspicion for B canis infection was largely limited to sexually intact, purebred dogs used for breeding. Whereas the reproductive effects within the infected colonies were as expected, the clinical effects on systems other than the reproductive tract were not; further, the type of dog-breeding facilities involved, the pet quality of the dogs being produced, and the intended markets for those dogs were not considered typical.
To the authors’ knowledge, the risk of human B canis infection from pet dogs that are not breeding animals is presently unknown. The purposes of the study reported here were to retrospectively estimate seropositivity rates for B canis in sexually intact, adult, purebred dogs owned by noncommercial breeders and describe the epidemiological findings in infected commercial dog-production facilities in Michigan during the outbreak. We also sought to characterize cases of B canis infection in pet dogs and reported cases of human B canis infection during this period. To further understand associations with interstate commercial dog trade, the canine brucellosis reporting and control measures of the states associated with the outbreak were investigated.
Materials and Methods
Hard-copy records for cases of canine brucellosis reported to the MDARD as well as electronic files for results of serologic tests, PCR assays, and cultures of samples for B canis submitted to the MSUVDLa from January 1, 2007, through December 31, 2016, were reviewed by 4 of the authors (TDC, JRD, CAJ, and MMS). Keywords for the electronic search included Brucella canis, RSAT, IFA, PCR, canine, culture, and Brucella. Data collected from records of both institutions included owner, county, and signalment. Data collected from the MDARD also included history, clinical signs, diagnostic laboratory names and test results, patient outcome, and findings of MDARD field veterinarians’ inspections of facilities and animals. The MSUVDL and the MDARD are separate entities independent from each other; MSUVDL files contained the results of tests performed as requested by the submitting veterinarian. The MSUVDL forwarded positive B canis test results and results of IFAs indicating antibody titers ≥ 1:160 for Michigan dogs to the MDARD. The choice of veterinary diagnostic laboratory and the type of test requested (eg, serology vs culture) was the purview of the veterinary practitioner who tested the dog and reported results to the MDARD. Therefore, records from the MDARD included hard-copy laboratory results from veterinary diagnostic laboratories other than or in addition to the MSUVDL. Animals were classified as infected when B canis organisms were cultured from blood, tissue, or fluid samples by the veterinary diagnostic laboratories, or when AGIDcpac results were positive. Serologic results from AGID assays using other antigens were not considered to be confirmatory. Seropositive animals that had clinical signs consistent with canine brucellosis and those from facilities that had culture-confirmed or AGIDcpac-confirmed B canis-infected dogs found on the premises were also considered infected. Regardless of which diagnostic laboratory performed the test or which technique was used, a dog was identified as seropositive if results of RSAT or 2ME-RSAT were positive or a serum antibody titer >1:320 was found by IFA. Antibody titers < 1:40 were considered a negative result, and titers between these 2 values or RSAT-positive with 2ME-RSAT-negative results were classified as suspect.
Information was collected from MSUVDL and MDARD records and collated by 3 investigators (TDC, JRD, and CAJ). When dogs with positive results in the MSUVDL records were not found in MDARD files, the submitting veterinarian was interviewed by 1 individual (CAJ) to determine whether the dog was a breeding animal or pet and to record clinical signs and patient outcome. Information from the MDARD records was used to estimate the prevalence of B canis infection in commercial Michigan dog-production facilities. Information from the MSUVDL was used to estimate the seroprevalence of B canis in purebred dogs of breeding age owned by noncommercial Michigan breeders.
States and counties were considered to be involved in the Michigan outbreak when dogs from those locations were moved to Michigan facilities where infection was confirmed and vice versa. State veterinarians of states involved were interviewed by 1 investigator (CAJ) to determine whether B canis was a reportable disease in their state, and if so, what control measures were in place. Human brucellosis is a nationally reportable disease, and officials at the Special Pathogens Branch of the US CDC and public health officials of the states involved with the Michigan outbreak were interviewed by the same investigator regarding the number of reported cases of human brucellosis due to B canis or Brucella isolates that had not been identified as to species during the study years.
Definitions
For the purposes of this investigation, a commercial dog producer was defined as a person operating a commercial dog-breeding facility that emphasized quantity of sales over quality of dogs being bred or sold, as assessed by MDARD field veterinarians’ inspection of the dogs and the facilities. A noncommercial dog breeder was defined as a person operating a purebred dog-breeding facility that emphasized quality of dogs over quantity of dogs being bred or sold. A pet-quality dog was defined as one of undetermined or mixed breeding or a purebred dog that lacked qualities established by a respective US breed registry. Additionally, dogs that were pets or gonadectomized and those from a facility that transferred to or received dogs from brokers, pet shops, humane societies, shelters, or rescue agencies were considered to be pet-quality dogs, regardless of ancestry. A kennel was defined as a structure housing dogs under appropriate husbandry conditions. The term colony referred to a group of dogs, regardless of the facility in which they were kept. An animal shelter was defined as a facility operated by one or more individuals, a municipality, a humane society, or another organization for the care of impounded, stray, or surrendered animals. Networks of animal rescue groups in which such animals were solely placed in foster homes were referred to as rescue agencies.
Results
MSUVDL records
Veterinarians from across the United States and Canada submitted samples for B canis testing, and 14 other diagnostic laboratories forwarded specimens for B canis analysis to the MSUVDL during the study period. Of 2,799 canine specimens, 67 were submitted for B canis culture; 17 (25%) had positive results. Fifteen of the samples with positive culture results were from 36 dogs owned by 1 commercial producer in Michigan. Excluding that facility, 2 of 31 (6%) samples submitted to MSUVDL for culture tested positive for B canis. During the study period, the commercially-available 2ME-RSATd test kit and IFAe reagents were used for B canis serologic testing. When 2ME-RSAT kits were sporadically unavailable, the IFA was used exclusively. The 2ME-RSAT performed was a 2-step procedure. When the first step, RSAT, had a positive result, 2-mercaptoethanol was added to another aliquot of patient serum to decrease nonspecific agglutination, and the RSAT step was repeated.
Of 2,732 submissions for B canis serologic testing during the study period, 2,405 (88%) were from Michigan; 327 were from 33 other states and 3 Canadian provinces. There were multiple submissions from some individual dogs, which contributed to the total number of submissions. Of submissions from Michigan, 15 were from canine blood banks, research laboratories, or shelter or rescue agencies. Serologic results were positive for 60 of 2,732 (2.2%) samples, 50 of which were from Michigan dogs; 38 of the 50 (76%) were from quarantined Michigan commercial facilities, and individual submissions from 12 dogs (8 sexually intact females, 1 sexually intact male, 1 spayed female, and 2 castrated males) were not. Five other Michigan submissions (3 females [2 sexually intact and 1 spayed] and 2 males [both sexually intact]) had suspect serologic results. In addition to Michigan, positive serologic or culture results were found from Oklahoma (n = 3), North Carolina (1), Illinois (1), and Ohio (5). Test results were reported by MSUVDL to the submitting veterinarian. Positive test results were also forwarded to the state veterinarians of states in which canine brucellosis was reportable. Recommendations for additional testing (discussed below) were included in MSUVDL reports for dogs with positive serologic results and those with anti-body titers ≥ 1:160 by IFA.
Of the 2,405 serologic specimens from Michigan dogs, 39 were from dogs < 6 months old, 5 were from dogs ≥ 12 years old, and 126 were from dogs of unspecified age. Breed was not indicated for 131 submissions, 178 were from dogs classified as mixed breed or other breed, and the remaining 2,096 (87%) were from dogs of specific breeds. Specimens were submitted for spayed females (n = 46), castrated males (53), and dogs for which sex was not specified (208) as well as sexually intact dogs (2,098 [1,437 females and 661 males]). Three hundred and seventy-one of 2,098 (18%) samples from sexually intact dogs were from commercial production facilities under quarantine for brucellosis in Michigan.
Given the typical canine interestrous interval of 6 to 8 months, submissions from an individual animal or a breeding pair on 1 occasion during a single year was reflective of responsible breeders’ routine, prebreeding, B canis screening of dogs having no clinical signs suggestive of B canis infection. On the other hand, submissions for > 3 dogs from 1 owner on a single occasion or repeated submissions from the same dog during a single year were typical of screening or monitoring a facility (or a specific dog) when infection was suspected or known. To estimate a seropositivity rate in apparently healthy (ie, without clinical signs of brucellosis) purebred sexually intact Michigan dogs of breeding age owned by noncommercial breeders, the following samples were excluded from evaluation: specimens from Canada or from states other than Michigan; those from dogs of mixed or unspecified breeds; those from dogs with no recorded age, age < 6 months, or age > 12 years; those from dogs that were spayed or castrated; > 3 submissions from the same owner on the same day; and > 1 submission from the same dog during the same year. Segregating the data in this way identified only 1 sexually intact purebred Michigan dog of breeding age that was not associated with a commercial facility and was tested twice during the same year. Because this dog had equivocal 2ME-RSAT results 2 weeks apart, the suspect result was included with the data of those tested once during a year. There were 1,397 purebred sexually intact Michigan dogs of breeding age that fit the predefined criteria for seropositivity analysis. Five (0.4%) dogs, including the 1 dog tested twice, had results classified as suspect (IFA results of 1:160 or equivocal 2ME-RSAT). Five (0.4%) had positive results, and this value was taken as the apparent rate of seropositivity for Michigan purebred dogs owned by noncommercial breeders.
Four of the Michigan dogs that were seropositive for B canis had no record found in MDARD files. One of the 4 dogs was eventually traced back to a quarantined commercial production facility to which it had been returned. For 2 other dogs, the veterinarians who submitted the samples considered the initial serologic results (2ME-RSATa) to have been false positive on the basis of their subsequent testing. The fourth dog was a Labrador Retriever with orchitis that had positive 2ME-RSAT results. This dog had been treated with tetracycline and was subsequently lost to follow-up.
MDARD records
Seventeen commercial dog-production facilities, 3 shelters, 1 rescue agency, and 1 noncommercial breeding facility as well as 30 individually owned dogs were identified in MDARD investigations for reported B canis infection during the study period. The commercial production facilities (P1 through P17), shelters (S1 through S3), and individually owned dogs (C1 through C30) were numbered chronologically as they were detected in Michigan (Figure 1). The previously described facility in which 15 of 36 dogs tested positive by culture was identified as P2. Movement of dogs among states is depicted (Figure 2). There were 11 states (California, Indiana, Iowa, Kentucky, Michigan, Missouri, New York, North Carolina, Ohio, West Virginia, and Wisconsin) involved in the out-break; in Michigan, infected dogs were found in 22 counties. In addition to the MSUVDL, 5 veterinary diagnostic laboratoriesb,c,f–h had been used by Michigan veterinarians for B canis screening or confirmation. Tests included RSAT, 2ME-RSAT, IFA, microagglutination, AGIDcpac, PCR assay, and culture.
Figure I—
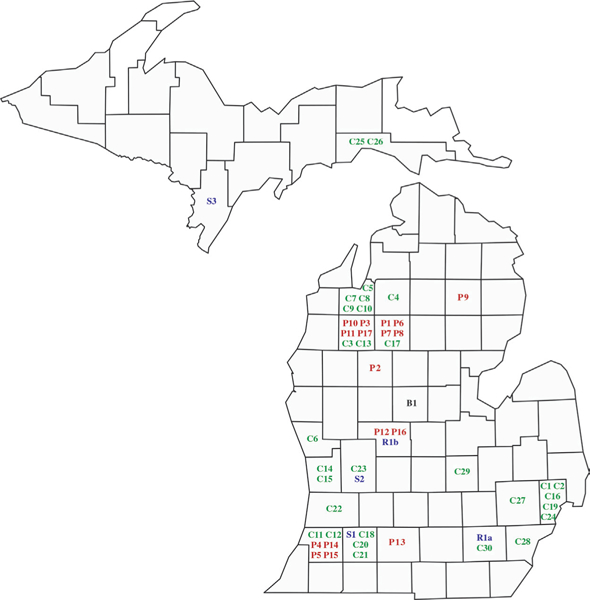
Distribution of Michigan dogs by county during a Brucella canis outbreak from January I, 12007, through December 31, 2016. Numbers for entities in each category were assigned chronologically as dogs potentially infected with B canis were identified; in some instances, specific information such as the location of a home or facility to which a dog had been moved or sold could not be recalled or was not provided, and numbers could not be assigned. B = Noncommercial dog breeder. C = Individually owned dogs (case numbers). P = Commercial dog-production facility. R = Rescue agency (a and b represent 2 different dogs that were placed in foster care homes by the group). S = Shelter.
Figure 2—
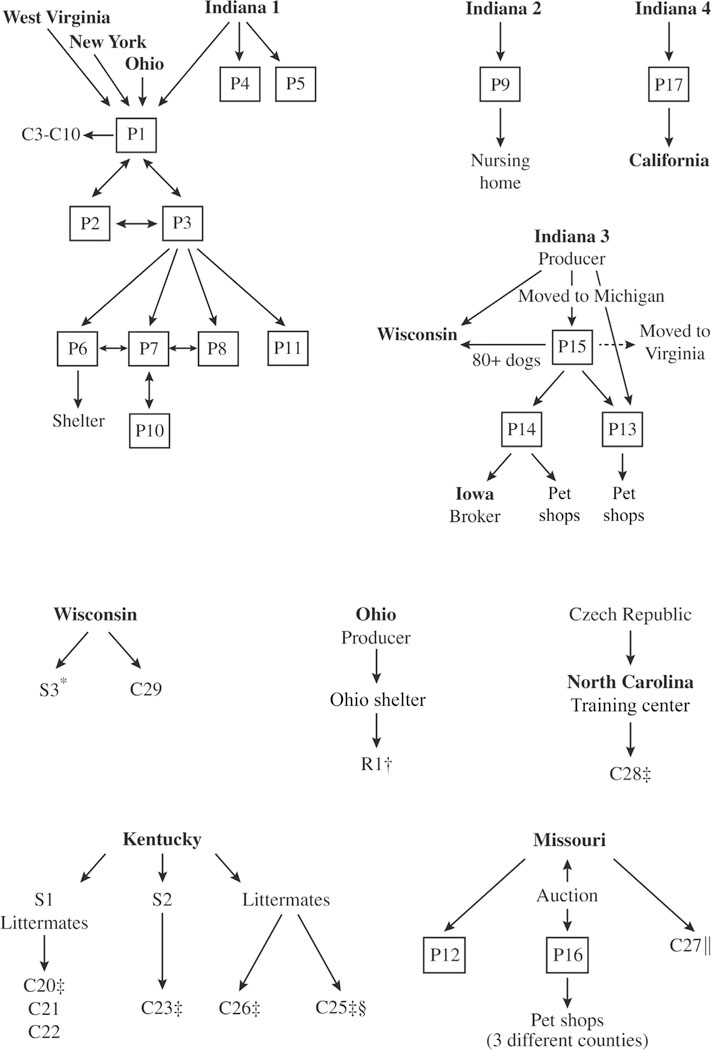
Flow diagrams showing intrastate and interstate movement of dogs to and from Michigan facilities where B canis infection was confirmed, and interstate movement of infected individual dogs. One-way and 2-way movements are indicated by single- and double-headed arrows, respectively. *Brucella canis infection in the owner of a Wisconsin commercial production facility from which infected dogs moved to S3 in Michigan. †Influenza-like symptoms in 3 veterinary hospital staff members who worked with the dog. ‡Infected dogs with diskospondylitis. §Needle stick incurred by a veterinary technician during blood sample collection. ‖Infected dog with diskospondylitis and uveitis. See Figure I for remainder of key.
All facilities, shelters, and rescue agency foster homes were placed under quarantine during disease investigation to confirm the diagnosis and implement control measures. Individually owned dogs were quarantined at home. Zoonotic potential was discussed with the owners in all situations. Quarantines prohibited movement of dogs in and out of the property as well as continued breeding of dogs. Cleaning and sanitizing of facilities were required. The specific requirements for cleaning, sanitation, and addressing animal welfare issues varied according to the conditions found by MDARD veterinarians. Release from quarantine required that these conditions were met and that all remaining dogs on the premises, including those with previously negative results, had 2 consecutive negative (typically AGIDcpac) test results 8 to 12 weeks apart. These conditions were made in writing and the owner’s signature indicated acceptance of them. Compliance was monitored by on-site inspection and review of official laboratory test results.
Commercial dog-production facilities—
During disease investigation, infection was confirmed by culture, AGIDcpa,c or both in ≥ 1 dog in each affected commercial production facility. All 17 commercial production facilities had sold or transferred dogs to other producers, at auction in Missouri, to brokers in Iowa and elsewhere, to shelters or rescue agencies, pet shops, individual owners, or some combination of these (Figure 2). Although producers volunteered information regarding these intended markets, they often indicated they did not recall which specific brokers, shelters, or pet shops were involved. Unknown entities were not assigned a chronological number. Nine of the 17 commercial producers identified themselves by their attire and lifestyle as members of close-knit, faith-based communities that restrict use of technology. Accurate census data and unique, permanent, individual animal identification methods were uncommon, and field veterinarians could not be certain that every dog on these premises was made available to them. Thirteen commercial production facilities were estimated to have 20 to > 100 adult dogs. Four commercial facilities were estimated to have < 20 adult dogs (counts ranged from 6 to 13). Some commercial production facilities also had puppies. Affected dogs were of 35 breeds and various mixed or so-called designer breeds (Supplementary Table SI, available at avmajournals.avma.org/doi/suppl/10.2460/javma.253.3.322). Clinical signs of infected dogs reported by all commercial producers included ≥ 1 of the following: abortion, stillbirth, conception failure, male infertility, and no pups surviving to weaning age.
As previously described and shown (Figure 2), epidemiological investigation of the early brucellosis reports revealed direct or indirect interchange of dogs among commercial facilities P1, P2, P3, P6, P7, P8, and P10. Two other commercial production facilities (P4 and P5) had not interacted with those facilities but had obtained dogs in 2008 from the same commercial facility in Indiana that had supplied dogs to P1 in 2006. The owners of P3 and P11 had facilities on property they shared. A year after P3 was placed under quarantine, MDARD veterinarians found it vacated, and the dogs had been moved to P11. The property was later sold, and no dogs remained. Facility P9 had obtained dogs from a different Indiana production facility than had the others, and prior to quarantine had sold a dog to an undisclosed nursing home. On follow-up inspection by MDARD veterinarians, the P9 property was found vacant.
Commercial production facilities continued to be identified during each year of the 10-year study period except 2011, and this revealed additional inter-state or intrastate movement (or both) of dogs (Figure 2). For example, a commercial producer from Indiana (different from the 2 previously identified Indiana facilities) had moved to Michigan along with hundreds of dogs and established a facility (P15) on the property of another Michigan producer (P14). At the same time, additional dogs had been moved from the Indiana facility to a second Michigan facility (P13) and to a facility in Wisconsin. Dogs from P14 were sold to a broker in Iowa and pet shops in Michigan. The P15 facility was under quarantine for 2 years (from September 2014 through October 2016) until the conditions to lift quarantine were met, after which time the producer announced the intention to leave Michigan and transfer all remaining dogs (approx 88) to the Wisconsin facility. The producer then moved to Virginia. The owner of P17 acquired a dog from a fourth commercial facility in Indiana and had sold 1 dog to a person in California and puppies to people in 8 different Michigan counties.
As a consequence of inadequate recordkeeping or failure of disclosure by all 17 producers, trace-forward investigation was often difficult. For example, of the many dogs transferred to private ownership from facility P1 prior to quarantine, trace-forward for only 8 dogs was possible. These 8 dogs (C3 through C10) were pet male and female dogs of 3 breeds that were residing in 4 different counties (Figure 1). All but 2 (both females) had been gonadectomized. Five of the 8 dogs tested were positive for anti-B canis antibody by RSAT,g 2ME-RSAT,a IFA,g or a combination of these methods; 3 also had suspect AGIDcpac results, and 1 also had a B canis-positive blood culture.a On the basis of these test results and their known source from an infected colony, all 8 were considered to be infected. The owners were advised of zoonotic risk, and they all opted to keep their pets. One neutered male and 1 spayed female were treated with antimicrobials. Owners of the 2 sexually intact females indicated intention to spay. All 8 were lost to further follow-up. Facility P16 obtained dogs from auction in Missouri and had hundreds of dogs and puppies. Abortion and conception failure were noted for 6 months before serum from 4 dogs was submitted to a Tennessee veterinary practice and positive IFA results were reported for each. On that basis, culling began and 66 adult dogs and 89 puppies were documented as having been euthanized by a veterinarian. At the time MDARD was notified and placed the facility under quarantine, there were 31 dogs remaining and all had negative AGIDcpac results. Disease investigation found that all of the puppies or dogs previously sold from P16 to pet shops in 3 different Michigan counties had been adopted by private owners and were lost to follow-up.
Commercial producers rarely tested all dogs at the same time or by the same method. For example, the owners of P4, P6, and P10 initially tested only 1 to 3 of many dogs, and each dog tested was seropositive (2ME-RSATa,b). Another commercial production facility (P2) had > 50 dogs, 32 of which were identified as seropositive for B canis by 2ME-RSATa on 1 occasion, and on 1 occasion 32 dogs from that facility had B canis-positive culturea results, but they were not necessarily the same 32 animals. However, on 1 occasion, blood or vaginal fluid was submitted for culture from 29 dogs from P2, and 6 (21%) were positive for B canis. There were 6 dogs in P17, and 2 of the 4 dogs tested were infected (AGIDcpac-positive). Nine of 32 (28%) dogs tested on 1 occasion at P3 had positive B canis blood cultureb results. Another commercial facility (P12) had only 6 dogs, 5 of which (83%) were found to be infected by positive AGIDcpab results. As testing and culling continued within colonies, the number of animals tested, the types of tests performed (serology versus culture or AGIDcpac), and the proportion of positive test results varied. From 2007 through 2016, for individual colonies with > 3 dogs tested on 1 occasion, the proportions of dogs known to have had positive AGIDcpac or B canis-positive culture results among dogs known to have been tested simultaneously ranged from 2 of 22 (9%; P7) to 5 of 6 (83%; P12). This range was taken to represent the prevalence of infection in Michigan commercial dog-production facilities.
On some occasions, some commercial producers refused to sign the quarantine agreement or refused to allow MDARD inspection of their property. Law enforcement personnel were involved with 6 of the quarantines, sometimes for reasons in addition to presence of B canis. Commercial producers often refused to provide information about the source or disposition of their dogs, and at least 1 continued acquiring new dogs while the facility was under quarantine. Three producers were known to have kept and retested some seropositive dogs while culling others. Despite having a quarantine imposed, 7 commercial producers in Michigan were known to have moved dogs to other facilities, including ≥ 2 animal shelters and 5 pet shops; 3 producers claimed to have killed many of their dogs themselves, but this was verifiable at only 1 facility. In 3 instances, producers sold or vacated their property, and no dogs remained. Some facilities were adequately cleaned and sanitized. Some producers remodeled or razed existing buildings or built new kennels.
Shelters—
Three Michigan shelters were known to have been involved in the outbreak; 2 had imported dogs from Kentucky to increase adoption availability. One Michigan shelter (S1) had imported a bitch with her 5 puppies and 2 other adult dogs from a Kentucky shelter. Clinical signs consistent with diskospondylitis were recognized in 1 of the puppies (C20; Figures 1 and 2). Results of a 2ME-RSATa and AGIDcpac were both positive, and the puppy was euthanized. Two of the remaining 4 puppies (C21 and C22) also had positive 2ME-RSATa and AGIDcpac results but had been adopted as pets before results were known, and 1 was living in another county. Both owners were advised of the zoonotic potential, and both elected to keep their pets under permanent quarantine. The dam had positive 2ME-RSAT but negative AGIDcpac results; after being spayed, this dog was lost to follow-up. Test results for the other 2 puppies and 2 adult dogs from Kentucky were negative. The second shelter (S2) had imported an 8-week-old puppy (C23) from Kentucky that was adopted by a new owner. The puppy was found to have diskospondylitis 7 months later. Results of IFA and AGIDcpac were positive for B canis, and the dog was euthanized.
A third shelter (S3) was associated with a Wisconsin commercial producer from whom B canis had been isolated during a hospital visit. Brucella canis subsequently was isolated from one of this producer’s dogs, and the dog was euthanized in Wisconsin. Later, Wisconsin law enforcement officials impounded the producer’s remaining 21 dogs for reasons unrelated to brucellosis, and moved them to Michigan shelter S3 (Figures 1 and 2). Ten of the 21 (48%) Wisconsin dogs tested positive for B canis by microagglutinationc and AGIDcpac and were euthanized; the remaining 11 dogs had negative results at that time and again 8 weeks later.
Rescue agency—
The rescue agency known to be involved in the outbreak (R1) obtained Cavalier King Charles Spaniels from various sites throughout the United States, including an Ohio shelter that received dogs from a nearby Ohio commercial dog producer. Two dogs from the Ohio shelter were placed with a Michigan foster care provider (Figures 1 and 2). One of these dogs was castrated and tested for B canis. A positive IFAf result was confirmed by AGIDcpac 2 weeks later; by this time, 3 people from the veterinary hospital had reported influenza-like symptoms to the MDHHS. The dog was euthanized. Five other dogs of various breeds in the same foster home tested negative by 2ME-RSATa and IFA.a The other dog imported from Ohio had already been adopted from the foster provider by new owners and moved to another Michigan county. This dog and 2 others in the same household tested negative by 2ME-RSATa and IFA.a
Noncommercial breeder—
The 1 noncommercial breeding facility (B1; Figure 1) identified in MDARD records during the study period had 10 adult dogs of 2 breeds housed in 2 kennels. One bitch inseminated with semen imported from Mississippi aborted. Neither the stud nor the semen had been tested for B canis. The bitch’s results of 2ME-RSAT,a IFAa (1:160), and microagglutination testingc were positive; culturea and AGIDcpac results were negative. The other 9 dogs in the facility tested negative by AGIDcpa.c The bitch that aborted had negative microagglutinationc and AGIDcpac results 2 months later, and the initial serologic results were considered to be false positive.
Individually owned dogs—
Reasons for testing individually owned dogs (C1 through C30) included routine prebreeding screening, requirements for international movement, inclusion in trace-forward epidemiological investigations, other possible exposure to known infected dogs, and clinical signs suggestive of brucellosis. Distribution of these dogs and their sources (where known) is shown (Figures 1 and 2). Eighteen of the 30 dogs had epidemiological links to probable or confirmed endemic sources of B canis (8 to commercial producers in Michigan, 4 to Michigan shelters that acquired dogs from Kentucky, 2 litter-mates illegally [no certificate of veterinary inspection or rabies vaccination] imported from Kentucky, 1 dog illegally imported from Wisconsin, 1 legally imported from North Carolina, 1 legally imported from Missouri, and 1 transferred to a rescue agency from a shelter in Ohio). The other 12 dogs had no apparent connection to endemic areas; however, several were described as having been rescued. In all, 20 of the 30 dogs were confirmed to be infected with B canis.
Eleven of 12 dogs tested for reasons other than trace-forward investigations had no clinical signs but had initial positive 2ME-RSAT or IFA results. One dog (C30) had positive PCR assayf results from a sample obtained by vaginal swabbing. Seven of the 12 dogs (C11, C12, C14, C18, C19, C29, and C30) were tested as part of routine prebreeding evaluations. One of these (C29) was a Bull Mastiff bitch that was shipped sight unseen from Wisconsin and had positive 2ME-RSATa and IFAa (1:320) results. Despite a recommendation for confirmatory testing, the dog was euthanized without further testing. The initial results from the other 6 dogs evaluated during prebreeding screening were classified as false positive on the basis of additional testing that included negative AGIDcpac results on ≥ 1 occasion or negative vaginal fluid or blood culture results. Results from 3 other dogs without clinical signs were also classified as false positive. Two of these 3 dogs (C13 and C17) were tested because they had freely roamed onto neighboring property that was under quarantine because of dogs infected with B canis. The third (C15) was tested as a requirement for importation into South Africa. Although no treatment was disclosed, there was concern that dog C18, tested as a prebreeding precaution, might have received antimicrobials during the time of confirmatory testing, which could have caused false-negative results; this dog was a therapy dog that visited a local hospital. The remaining 2 of 12 dogs with no clinical signs were the previously described littermates that were adopted from shelter S1 (C21 and C22) before the positive serologic test results were known. Another 8 dogs without clinical signs (C3 through C10) had been identified and tested during the previously described trace-forward investigations for a commercial production facility (P1).
Ten individually owned dogs were tested for B canis because of clinical signs suggestive of brucellosis. One dog, a Boxer described as having been rescued (C1), aborted a litter and subsequently had a positive IFAg result. The owner chose to keep the dog under home quarantine. A pit bull-type mixed-breed dog (C2) that was adopted from an unknown source had a bleeding vaginal tumor and tested positive by IFA,f microagglutination,c and AGIDcpa.c The tumor was excised, and the dog was spayed and treated for 30 days with doxycycline and then quarantined at home. Dogs C1 and C2 were subsequently lost to follow-up. A Springer Spaniel (C16) that was febrile and had joint swelling after delivering a litter tested positive by 2ME-RSATa but was also seropositive for Borrelia spp; after additional testing with negative 2ME-RSATa and IFAa results over 2 months and resolution of the joint swelling, the initial result was considered false positive. The other 7 dogs with clinical signs (C20 and C23 through C28) were tested because they had evidence of back pain; all 7 were determined to have diskospondylitis, and 1 also had uveitis. These 7 dogs were ≤ 3 years old and only one was sexually intact. Two were the previously described puppies (C20 and C23) imported from Kentucky by shelters S1 and S2.
One of the dogs tested because of clinical signs, a 3-year-old, neutered male pit bull-type crossbred dog (C24) had been received as a puppy in 2009 from a neighbor who adopted the dam (described as a rescue) along with her litter of 10 puppies. This dog was neutered as a puppy; after developing thoracolumbar diskospondylitis that was unresponsive to treatment, it was referred to the MSU Veterinary Medical Center for further evaluation in 2012. A serologic test (RSATd) for B canis at the time of admission had negative results, and a specimen of intervertebral disk material (collected by fine-needle aspiration) was submitted for culture but was not labeled as potentially containing a Brucella sp. After incubation for 7 days, B canis was isolated; by this time, 21 hospital staff members and 5 laboratory employees had been exposed to the dog’s bodily fluids, disk material, or both. In all, 49 people were classified by MSU Occupational Health officials and the MDHHS according to CDC guidelines as having been exposed to B canis (including 28 who had worked in the intensive care ward where the dog was hospitalized for 3 days) during this event. Daily monitoring for fever was ordered for all exposed individuals, and prophylactic antimicrobial treatment was recommended for some, depending on the degree of exposure. One veterinary technician who had direct contact with the dog reported influenza-like symptoms. Despite being informed of the grave prognosis and the zoonotic risk, the owners elected to take the dog home, and it was euthanized shortly afterward. The source of infection remained unknown. The patient had been a house pet since its adoption and was the only dog in the household; it reportedly never roamed freely, although it was taken to dog parks. The dam had been spayed in 2009 after being taken in by the neighbor and had negative RSATc and AGIDcpac results for B canis in 2012. As of 2015, the dam continued to be healthy.
A female Labrador Retriever-cross puppy (C25) and 2 littermates had been acquired by an individual from another person in Kentucky. One of the littermates (C26) was given to a friend, and the other was given away through an internet placement. No records were kept. The 2 remaining dogs (C25 and C26) had been gonadectomized, and had reportedly always shown signs of back pain. Thoracolumbar diskospondylitis was diagnosed in 1 dog (C25) at 3 years of age; an IFAa for B canis had positive results (> 1:20,480), whereas the result of AGIDcpac was deemed suspect, and blood culturec results were negative. A veterinary technician sustained a needle stick while collecting a venous blood sample from the dog but did not become ill. The MDHHS and CDC recommended that this individual undergo prophylactic antimicrobial treatment. Because of similar clinical signs, the other dog (C26) was then tested by microagglutinationc and AGIDcpac, both with positive results. Both dogs were euthanized. Other dogs (n = 9) that were known to have contact with the 2 affected dogs tested negative by AGIDcpa.c
An 18-week-old female Labrador Retriever-Poodle cross (C27) was imported to Michigan from Missouri with a certificate of veterinary inspection and information that both the sire and dam were brucellosis-negative. At the time the dog was spayed, uveitis was detected; signs of back pain subsequently developed, and lumbar diskospondylitis was diagnosed. Results of IFAf and AGIDcpac were positive for B canis, and the dog was euthanized. The owner reported that the dog had attended a local canine daycare facility where approximately 50 dogs were typically present on any given day. Information about B canis was posted at the facility, and informational fliers were distributed to patrons. After 6 months, there had been no reports of illness in other dogs.
A sexually intact male German Shepherd Dog (C28) was imported at 10 months of age from the Czech Republic with requisite paperwork by a dog-training facility in North Carolina. After approximately 4 months of explosives detection training, the dog was purchased by a federal agency. The dog resided at the home of its handler in Michigan but was worked in several states by 3 federal agencies and by the police departments of 3 Michigan universities. Signs of lumbar pain had been noticed during the first month, and hind limb weakness was evident within 6 months after the dog was purchased. After 18 months, a diagnosis of diskospondylitis was confirmed. Results of an IFAg and RSAT were positive, an AGIDcpac result was considered suspect, and contamination was found on culturec of a blood sample. The agency responsible for the dog declined further testing, and the dog was euthanized. All of the entities involved were notified by MDARD of potential B canis exposure. Of the individually owned dogs with diskospondylitis, only C28 was sexually intact, and this dog had never been bred.
Two additional Michigan dogs with testicular enlargement were reported to MDARD as suspected of having canine brucellosis. Both owners refused testing. These dogs were not numbered among the individually owned dogs involved in the outbreak.
Human exposure and cases of brucellosis
Staff members at 3 veterinary hospitals and the MSUVDL, totaling 53 people, were classified by MDHHS according to CDC guidelines as having been exposed to B canis, with 4 individuals reporting influenza-like symptoms as described in previous results. The MDHHS together with the MSU Occupational Health office or the CDC, were involved in investigation of these human exposures. One Michigan dog producer was reputed to have brucellosis, but this was not reported to the MDHHS. From 2007 through 2016, there were 21 human brucellosis cases in Michigan attributed to Brucella abortus, Brucella melitensis, or Brucella suis on the basis of culture results. Two other people had positive serologic results for Brucella spp that were not identified more specifically. No human cases of B canis infection were identified in Michigan.
From 2008 through 2015, 14 probable or confirmed human cases of B canis in various states were reported to the CDC Bacterial Special Pathogens Branch.i Five of those were from states associated with the Michigan outbreak, including California (n = 1), New York (1), Wisconsin (1), and West Virginia (2). Four infected individuals had reported contact with dogs, and 1 had a presumptive link to laboratory exposure identified on epidemiological investigation. The infected individual in California was hospitalized because of severity of the illness. In addition to the previously described Wisconsin dog producer, at least 1 other human case of the disease during this 8-year time span was directly associated with unregulated interstate commercial dog trade. A 3-year-old child in New York acquired brucellosis and required hospital care after being exposed by contact with an infected 8-week-old Yorkshire Terrier puppy in the same household. The puppy had been obtained from a New York pet shop, which had received it from a commercial dog-production facility in Iowa.1
As of April 2018, the CDC’s 2016 human brucellosis surveillance data were incomplete, and only 6 of the 11 states associated with the outbreak had submitted their data to the agency.i Officials from Michigan, North Carolina, New York, Wisconsin, and Ohio reported no human cases of B canis infection in 2016. A veterinary practitioner in Missouri became infected following exposure during necropsy of a dog that had tested positive for B canis by culture. The dog was owned by a breeder, but whether the dog was part of the breeding colony could not be determined. There was no known connection to interstate transport of dogs, and the veterinarian was not hospitalized.
Other findings
There were 2 apparent false-negative RSAT results. One was for a dog with diskospondylitis (C24). Long-term antimicrobial treatment could have caused the negative serologic test result, whereas subsequent culture of disk material yielded B canis. The other was for a sample taken at the time of abortion from a bitch at a commercial production facility (P9). The same dog subsequently had a positive 2ME-RSATa result during screening of the entire colony and was euthanized. There was no information about antimicrobial administration in the files for the commercial facility (P9), and a follow-up visit found the property vacated as previously described.
On the basis of comments made by some commercial producers and some of their veterinarians, as well as some shelter veterinarians and rescue agency members, it became apparent that some commercial producers, brokers, pet shops, shelters, and rescue agencies knowingly accepted dogs from facilities in which B canis was endemic. Despite this knowledge, none followed appropriate biosecurity measures for protection against introducing B canis into their facilities. Some organizations knowingly accepted dogs from infected commercial facilities and shelters to prevent their euthanasia, and at least 1 advertised online that rescued animals were available for adoption because they could no longer have puppies. With the exception of the previously described canine daycare facility, the study found no evidence that the presence of B canis infection in commercial production facilities, shelters, or rescue agencies had been disclosed to patrons. When adequate records for the disposition of dogs existed, the MDARD notified new owners of the potential zoonotic disease exposure and recommended brucellosis testing.
Consultation with veterinarians in Michigan revealed that not all realized B canis infection is a reportable disease. Of the 11 states involved in the Michigan outbreak, it was and is currently reportable in all but 4 (California, Kentucky, New York, and North Carolina). With the exception of Wisconsin, none of the 11 states, including Michigan, had or currently has published regulations for B canis testing prior to breeding, sale, acquisition, intrastate transport, or importation of dogs. For dogs obtained at auction, Wisconsin does require documentation of a negative result for brucellosis testing conducted ≤ 30 days prior to importation. State animal health officials from states in which canine brucellosis is not a reportable disease had no guidelines for control and prevention. Officials from states in which canine brucellosis is a reportable disease (including the study authors), indicated that the absence of universally accepted guidelines for control and prevention of B canis infection delayed their responses during the outbreak because of the time and effort needed to develop and implement their own protocols. Direct comparisons among states’ protocols could not be made because they were still evolving, a situation that also was found in a recent national survey by the National Association of State Public Health Veterinarians.j The state of Missouri had published a laypersons’ form including guidelines for certification of a canine brucellosis-free breeding facility,k and many of those procedures could be applicable in cases of newly recognized infection.
It also was found that state animal health officials did not routinely share infectious disease data with each other. Animal health and public health officials of some states readily shared reports of B canis infection in dogs and people with each other, whereas others did not.
Discussion
Our findings indicated that Michigan’s practicing veterinarians’ index of suspicion for B canis infection was largely limited to sexually intact, adult purebred dogs that were being bred. The large sample size of serologic test results from the MSUVDL provided the opportunity to estimate a seroprevalence of B canis in such dogs. The RSAT is known to be highly sensitive, with few false-negative results; however, the RSAT is also known to lack specificity, so that false-positive results are common.2 The IFA might be somewhat less sensitive than the RSAT.2 Given the sensitivity but the possibility of false-positive results, reliance on these test results could yield an overestimation of prevalence. Contrary to commonly held expectations, we found a low seroprevalence (0.4% of 1,397 laboratory submissions) among sexually intact, purebred Michigan dogs of breeding age owned and tested by noncommercial breeders. Instead, our findings indicated that pet-quality purebred or so-called designer mixed-breed puppies and dogs specifically intended for sale to brokers or pet shops as well as dogs transferred to shelters and rescue agencies from commercial production facilities or from other states were of greater concern to the public health1,3,4 and the general dog population in this outbreak.
In one of the few commercial facilities (P12) in which all the dogs were known to have been tested at the same time, the prevalence of confirmed infection was 5 of 6 (83%). When all the dogs in a facility were not tested at the same time, the prevalence of infection was estimated on the basis of the number of dogs with confirmed infection compared with the number of dogs tested simultaneously. Given the high estimated and actual prevalence (range, 9% to 83%) of B canis infection in dogs within the 17 infected commercial dog-production facilities, and with as many as 5 of 8 dogs (62%) in 1 trace-forward investigation found to be infected, an alarming but unknown number of infected pet dogs exposed unsuspecting dog owners to B canis. Staff members in 3 veterinary hospitals and the MSUVDL were inadvertently exposed, and 1 dog from an infected commercial production facility was sold to a nursing home. One infected dog had attended a busy canine daycare facility on 2 occasions before the diagnosis was made. Neighbors had filed a complaint that 1 privately owned dog with confirmed B canis infection was allowed to play with neighborhood children and had access through a fence to a dog living on adjacent property after the owner had agreed to conditions of quarantine. Some neighborhood dogs freely roamed onto property that had been quarantined. These results, taken with the continued movement of dogs from quarantined commercial facilities previously described, showed that even with quarantines imposed, it was not feasible to prevent infected dogs from exposing other dogs and people to B canis.
Dogs infected with B canis are typically afebrile and appear healthy, other than having reproductive problems.2 However, we found an unexpectedly high rate5,6 of serious, nonreproductive tract illness among privately owned infected dogs. Of 20 privately owned dogs with confirmed infection, 7 (35%) developed diskospondylitis and were euthanized. One of these 7 dogs also had uveitis. The findings confirmed that B canis can cause clinically important illnesses in puppies and adult dogs, even those that are not used for breeding. The financial and emotional costs to the owners of these dogs were high.
A strong association of canine brucellosis with the unregulated interstate commercial dog trade has been reported,7–9 but to the authors’ knowledge, no such reports have described the extent, frequency, and rapidity of movement of the dogs. On some occasions during the outbreak described in this report, seropositive dogs had already had been transferred to new owners from shelters or the rescue agency before the positive results of B canis tests were known. Brucella canis has been identified in Czechoslovakia,2 and 1 dog (C28) was imported from the Czech Republic to North Carolina where B canis is not a reportable disease.
The high index of suspicion for B canis infection in actively breeding dogs and the emphasis on venereal transmission were understandable, given that the most commonly recognized clinical signs (conception failure, abortion, stillbirth, epididymal enlargement, and infertility)2,10–13 are associated with the reproductive system. However, ingestion, direct contact (through mucous membranes or by inoculation through skin), and inhalation of aerosolized material are more common routes of transmission.2,11,14 Organisms are shed in aborted material and in vaginal discharge after abortion (1 × 107 bacteria/mL to 1 × 1010 bacteria/mL) and readily contaminate the environment,11,12 thus exposing other animals. Organisms are also shed in urine (1 × 103 bacteria/mL to 1 × 106 bacteria/mL), with greater concentrations in samples from males than from females.13,15 Urinary shedding persists for ≥ 3 months beyond initial infection, contaminating the environment.15 Urine exposure is especially important when housing is shared; for example, after 4 to 6 months of close contact or cohabitation of same-sex dogs, infection was transmitted from infected to susceptible males and from infected to susceptible females.15 Brucella canis is also transmitted via contaminated fomites.16 As previously mentioned, venereal as well as transplacental transmission is known to occur; large numbers of organisms are shed in semen, particularly during the first 6 to 8 weeks of infection, and shedding persists for 60 weeks to ≥ 2 years.13 As with other Brucella spp, B canis is shed in milk and other bodily fluids.
Given the same exposure, any dog, regardless of its sex, whether it is neutered or sexually intact, and whether it is used for breeding or not, could become infected with B canis. Nevertheless, approximately twice as many samples submitted for B canis serologic testing to MSUVDL from Michigan dogs for which sex was specified were from sexually intact or spayed females than from sexually intact or castrated males (1,483/2,197 [67.5%] and 714/2,197 [32.5%] submissions from females and males, respectively). This was also true for the subset of sexually intact purebred adult dogs of breeding age (n = 1,397) identified as undergoing routine prebreeding serologic screening; 958 (68.6%) of these dogs were female and 439 (31.4%) were male. Investigators of a study17 on B canis genotypes in samples submitted to the state veterinary diagnostic laboratory in Kansas also identified more samples from female (87/133 [65%]) than male dogs. Yet in the present study, 4 of the 7 dogs with diskospondylitis were males, 2 were females, and the sex of 1 was not reported. Other investigators reported diskospondylitis with B canis infection in 22 dogs, including 18 (82%) sexually intact males, 1 castrated male, and 3 spayed females.18 These findings suggest that B canis infection in male dogs is under-recognized, especially considering the reproductive differences between the sexes. For example, abortion is easily recognized in females, compared with the insidious development of infertility in males. Considering venereal transmission alone, 1 male might expose, or be exposed by, several bitches in any given year, whereas 1 bitch would likely be bred only once.
The number of veterinary hospital and laboratory staff who had inadvertent exposure to B canis from pet dogs was surprising at the time of this investigation. However, others have since reported similar findings,1,19,20 which suggests that awareness of potential exposure to this zoonotic pathogen deserves greater attention. Brucella spp are highly infectious to people and are considered the most common cause of laboratory-acquired infections.21 They are easily aerosolized and easily transmitted through airways, with a dose of 10 to 100 organisms considered sufficient for infection.21 Human B canis infection has been strongly linked with exposure to infected dogs and laboratory specimens.1,4,10,11,19,20,22 There is little difference in the clinical manifestations of B canis and other Brucella spp.23 The most common signs are undulant fever, fatigue, headache, chills, weight loss, malaise, night sweats, emesis, cough, and diarrhea. Brucella canis has also been associated with lymph-adenopathy, hepatosplenomegaly, hepatic dysfunction, bone marrow dysfunction, macular rash, vegetative endocarditis, epidural abscesses, arthralgia, osteomyelitis, pleural effusion, pulmonary nodules, peritonitis, mycotic aneurysms, and flaccid paralysis.1,19,23–35 Illness has been severe enough to require intensive and prolonged antimicrobial treatment, repeated and prolonged hospitalizations, and advanced or invasive treatments such as mechanical ventilation and surgical debridement of B canis lesions, including craniotomy29 and aortic valve replacement.22 Brucella canis infection can also cause death in people.36
The prevalence of B canis infection in people is unknown. Although brucellosis is a disease reportable to the CDC and the organism is classified as an agent of interest for bioterrorism,21 species identification of the organism is not yet mandatory. The commonly used screening test, a Brucella spp microagglutination test, detects antibodies against B abortus, B melitensis, and B suis, without differentiating among these pathogens. At present, there is no serologic assay approved in the United States to screen people for B canis, although veterinary RSATs, tube agglutination tests, and ELISAs have been shown to detect B canis antibodies in human serum.20,22,33,35 Brucella canis is slow-growing and often not detected during the 48- to 72-hour incubation period typically used for routine microbial cultures. For these reasons, and a low index of suspicion, the consensus among experts is that human B canis infection is underrecognized.1,10,29
We also found that the extensive testing, time, and cost to the client needed to confirm, monitor, and eradicate infection was unexpected by the veterinarians who consulted us about testing and control procedures for their clients during the outbreak. The variety of serologic tests used by the same facilities indicated confusion about which tests are most useful under which circumstances. Although isolation of the organism provides a definitive diagnosis,2,18,37–39 it is impractical for routine prebreeding screening. In a circumstance where disease prevalence is low and animals have no clinical signs of brucellosis, a screening test with high sensitivity is ideal. The inhouse RSAT is often used2,10,39 for this reason and because it is expeditious, compared with blood culture. However, the cell wall lipopolysaccharide antigens of B canis or B ovis used in the RSAT and other serologic tests are not unique to these organisms. Therefore, the antibodies detected by these assays are not specific for B canis infection, and false-positive results are common in this and other tests that rely on these antigens, including the 2ME-RSAT, 2-mercaptoethanol tube agglutination test, IFA, agar gel immunodiffusion with cell-wall lipopolysaccharide antigen, and ELISA. Addition of 2-mercaptoethanol eliminates the less specific reactions of IgM antibodies, but false-positive rates can still be as high as 50% to 60%18 owing to cross-reaction with other bacteria or hemolysis in the specimen.2 Reliability of test results and the accuracy of interpretation are extremely variable among laboratories.39 Otherwise, there is no inherent advantage of one cell-wall antigen-based assay over another. Positive results from any such test must be confirmed by a method that has high specificity for B canis. Internal cytoplasmic protein antigens are highly specific for Brucella spp, and the AGIDcpac used in this study is currently considered to be the serologic test with the greatest specificity for Brucella antibodies.2 Isolation of B canis by culture establishes a definitive diagnosis.2,37–39
Molecular methods such as PCR assay have been used for identification and subtyping of Brucella spp in blood and tissue specimens. A minimum number of viable Brucella organisms is necessary for successful isolation of the organism by culture.2 Similarly, the diagnostic sensitivity of the PCR assay depends on the amount and purity of bacterial nucleic acids extracted from the specimen.40 It follows that success of blood culture or PCR assay of whole blood samples would be most likely when samples of adequate size are obtained during times of bacteremia. At other stages of infection, Brucella spp are barely detectable by either method.2,41 For these reasons, repeated sampling or testing of various specimen types is recommended.41 The variety of DNA extraction methods, primers, and amplification techniques used to detect B canis in dogs make direct comparisons among study results difficult.17,40,41 At present, it appears that commonly used commercial kits for DNA extraction from canine blood samples are not, by themselves, sufficiently sensitive to recommend replacing blood culture for diagnosis of B canis in dogs.40,41 Results depend on the quality of the sample submitted as well as the methodology used at the diagnostic laboratory and experience of personnel.41 On the other hand, molecular techniques applied to bacterial isolates have effectively identified genetic markers of the causative strains of B canis in epidemiological investigations.8,17,41
When B canis is endemic (ie, prevalence is high) or when recent exposure (within 1 to 8 weeks) is suspected, blood culture is the preferred test for screening as well as confirmatory diagnosis. Antibody titers may not reach detectable levels until 8 to 12 weeks after inoculation.37,39 Bacteremia persists for 6 months to 5.5 years and subsides as infection becomes chronic.2,13 Titers decline as bacteremia subsides, even though the organism is still present in tissues.13 In certain circumstances, culture of specimens other than or in addition to blood is indicated. Culture of postabortion vaginal discharge is helpful11,12,16; also, shedding of B canis in semen can persist for ≥ 2 years,13 and urine cultures can yield isolates of the organism.4,13,15 Persistence of the organism in the prostate is thought to explain the greater number of organisms recovered from the urine of males than females. Brucella canis also can be recovered from lymph nodes, spleen, liver, bone marrow, prostate, epididymis, and placenta as well as the lumen of a gravid or postabortion uterus of dogs2,13,42; it is rarely recovered from the uterus or the vagina under other conditions.12,43 When B canis causes uveitis or diskospondylitis, it may be recovered from the affected tissues. Antimicrobial treatment sometimes causes negative culture and titer results despite the presence of B canis in tissues,2,12–14 confounding interpretation of results.
We found that veterinarians were torn between implementation of control measures and their allegiance to their clients and patients. Not surprisingly, veterinarians and their clients were reluctant to cull infected animals that appeared otherwise healthy, particularly when these were household pets. Many wanted to pursue treatment, isolation of the dog, or both. To date, the evidence shows that, despite antimicrobial treatment, the organism can still be recovered from urine and is rarely cleared from the mononuclear phagocyte system, the prostate, or other internal organs.2,4,10 Treatment regimens reported to date, including multiple 30-day courses of enrofloxacin for 38 months, have reported failure rates as high as 10 of 12 dogs when animals that died or were removed from the study were included among the treatment failures.44 After confinement, castration, and a 30-day course of enrofloxacin, infected male dogs continued to have positive culture and serologic test results.4 These and other treatment protocols have been reviewed.2 To date, no treatment regimens have 100% efficacy, but those involving single antimicrobial treatment have been least effective. For that reason, if treatment is considered, treatment with multiple antimicrobials is recommended.2,18 Treated dogs are readily susceptible to reinfection.2 Monitoring efficacy of treatment is difficult because bacteremia and serum antibody titers characteristically diminish with chronicity of infection as well as in response to antimicrobial administration.2 Some treated pregnant bitches can carry a litter to term during that time12,42,43; however, they are not cured. Achieving success is so difficult that culture of bone marrow is the gold standard to confirm eradication of brucellosis from a human patient.14 Clearly, treatment efficacy < 100% will not eradicate infection from a colony. Neutering of infected pet dogs eliminates venereal transmission, but shedding in urine and other secretions still occurs. Because successful treatment is unlikely and because infected animals remain a source of infection for other dogs and unsuspecting people, treatment is usually ill-advised.4,10,43
Evidence from studies42–46 of spontaneously occurring B canis infection has shown that the organism is not eliminated from a colony until infected animals are culled, regardless of treatment and even when infected animals are isolated in separate buildings. Because antibody titers or bacteremia may be detectable 3 to 4 weeks after exposure, all colony members should be tested monthly. Failure to test all the remaining dogs or testing less frequently than every 30 days will likely delay recognition of infected animals. Dogs with confirmed infection must be culled promptly if the infection is to be eliminated. Because of continued exposure to infected dogs that have not yet been identified and culled, and because of the time necessary for growth in culture or development of detectable antibody titers, the prevalence of infection in a colony often does not decline until testing and culling have continued for several months.7,42,46 Because antibody titers may take as long as 12 weeks to become detectable, monthly colony-wide testing of all remaining dogs, including those with negative test results during the previous months, must continue until all results are negative for all remaining dogs for ≥ 3 consecutive months2 if infection is to be eradicated. It is important that owners understand this so that expectations are realistic. New animals should be tested shortly before acquisition and then isolated and tested for ≥ 2 months before introduction to the colony.
Limitations of the present study included its retrospective nature, which resulted in identical data not necessarily being present in every record. Complete examination of every dog in a facility often was not practical or economically feasible. Census data, individual animal identification, compliance with quarantine, and records of animal acquisition and disposition were incomplete. The commonly used RSATd was sporadically unavailable from April 2012 through December 2013. Prior to September 2011, the CDC brucellosis case report form did not include contact with dogs among the listed factors potentially associated with human brucellosis. Further, not all Brucella isolates cultured from human samples were identified as to species. These limitations likely resulted in underestimation of the prevalence of B canis infection in dogs and in people. Nevertheless, the results clearly demonstrated that a one-health approach between federal and state agencies is needed to help prevent spread of the disease.
The National Association of State Public Health Veterinarians and the Council of State and Territorial Epidemiologists have recommended improved communication and data sharing among animal health and public health officials and for inclusion of the Brucella species in CDC notifications.47 The AVMA has recommended commitment by state and federal agencies to eradicate brucellosis in all species, including dogs.48 Ultimately, it is in the best interest of those engaged in the breeding, sale, transfer, and adoption of dogs to help stop the spread of canine brucellosis. Veterinarians can help to achieve this goal by promoting voluntary client implementation of a stringent program to prevent, control, and eradicate canine brucellosis. Components of such a program include keeping complete records of animal breeding, acquisition and disposition; ensuring individual animals are identifiable; verifying that dogs test negative for B canis prior to breeding, import, export, acquisition, and sale; conducting disease surveillance within breeding colonies and eradicating the disease when it is found; and following strict biosecurity protocols.
Michigan’s B canis outbreak was associated with the commercial production and trade of pet-quality dogs that were destined primarily to be purchased or adopted as pets. It seems prudent for veterinarians to include B canis screening in their recommendations to clients with newly acquired dogs of any age or breed, whether neutered or sexually intact, especially those obtained from commercial dog producers, pet shops, shelters, or rescue agencies. Dog owners, veterinary staff, and laboratory staff should adhere to strict personal protective protocols when handling potentially exposed dogs and their specimens.
Acknowledgments
No third-party funding or support was received in connection with this project or the writing or publication of the manuscript. The authors declare that there were no conflicts of interest.
Preliminary data from 2007 through 2010 were presented orally at the Michigan Veterinary Conference, Lansing, Mich, January 2011; and the International Symposium on Canine and Feline Reproduction, Whistler, BC, Canada, July 2012.
Use of trade names and commercial sources is for identification only and does not imply endorsement by agencies involved with the study. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or other represented agencies.
The authors thank Nicole Grosjean of MSUVDL for technical assistance; Dr. Kimberly Signs of the MDHHS, Dr. James Kazmierczak of the Wisconsin Division of Public Health, Dr. Howard Pue of the Missouri Department of Health and Senior Services, and the Surveillance & Statistics Section of the Infectious Diseases Branch of the California Department of Public Health for information on cases of human infection with B canis; and Jessica Gevers for graphic design.
ABBREVIATIONS
- 2ME-RSAT
Rapid slide agglutination test with 2-mercaptoethanol
- AGIDcpa
Agar gel immunodiffusion with Brucella-specific cytoplasmic antigen
- IFA
Indirect fluorescent antibody assay
- MDARD
Michigan Department of Agriculture and Rural Development
- MDHHS
Michigan Department of Health and Human Services
- MSU
Michigan State University
- MSUVDL
Michigan State University Veterinary Diagnostic Laboratory
- RSAT
Rapid slide agglutination test
Footnotes
MSUVDL, formerly MSU Diagnostic Center for Public and Animal Health (until June 2017).
Missouri Animal Health Laboratory, Jefferson City, Mo.
Animal Health Diagnostic Center, Cornell University, Ithaca, NY.
D-TEC CB, Synbiotics Corp, Kansas City, Mo.
Brucellosis canis IFA slides, conjugate and control, Veterinary Medical Research & Development, Pullman, Wash.
Idexx Laboratories Inc, Westbrook, Me.
Antech Diagnostics, Fountain Valley, Calif.
Marshfield Labs, Marshfield, Wis.
Kharod GA, CDC Bacterial Special Pathogens Branch: Personal communication, 2017.
Murphy J, National Association of State Public Health Veterinarians. Brucella canis State Regulatory and Response Practices Survey, 2016: Unpublished data distributed to the National Assembly of State Animal Health Officials, February 2017.
Missouri Department of Agriculture, Division of Animal Health. Canine Brucellosis-free certification. Available at: agriculture.mo.gov/animals/pdf/CanineBrucellosisCertification.pdf. Accessed Apr 11, 2018.
Contributor Information
Cheri A. Johnson, Department of Small Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48 8 24.
Todd D. Carter, Department of Small Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48 8 24.
John R. Dunn, Michigan Department of Agriculture and Rural Development, 525 W Allegan St, Lansing, MI 48933.
Susan R. Baer, Michigan Department of Agriculture and Rural Development, 525 W Allegan St, Lansing, MI 48933.
Michele M. Schalow, Michigan Department of Agriculture and Rural Development, 525 W Allegan St, Lansing, MI 48933.
Yvonne M. Bellay, Wisconsin Department of Agriculture, Trade and Consumer Protection, 2811 Agriculture Dr, Madison, WI.
Marta A. Guerra, Wisconsin Department of Agriculture, Trade and Consumer Protection, 2811 Agriculture Dr, Madison, WI CDC, 1600 Clifton Rd, Atlanta, GA 30333.
Nancy A. Frank, Michigan Department of Agriculture and Rural Development, 525 W Allegan St, Lansing, MI 48933.
References
- 1.Dentinger CM, Jacob K, Lee LV, et al. Human Brucella canis infection and subsequent laboratory exposures associated with a puppy, New York City, 2012. Zoonoses Public Health; 2015;62:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene CE, Carmichael LE. Canine brucellosis In: Green CE, ed. Infectious diseases of the dog and cat. 4th ed. St Louis: Elsevier, 2012:398–411. [Google Scholar]
- 3.Keid LB, Chiebao DP, Batinga MC, et al. Brucella canis infection in dogs from commercial breeding kennels in Brazil. Transbound Emerg Dis 2017;64:691–697. [DOI] [PubMed] [Google Scholar]
- 4.Reynes E, Lopez G, Ayala SM, et al. Monitoring infected dogs after a canine brucellosis outbreak. Comp Immunol Microbiol Infect Dis 2012;35:533–537. [DOI] [PubMed] [Google Scholar]
- 5.Burkert B, Kerwin SC, Hosgood GL, et al. Signalment and clinical features of diskospondylitis in dogs: 513 cases (1980–2001). J Am Vet Med Assoc 2005;227:268–275. [DOI] [PubMed] [Google Scholar]
- 6.Kerwin SC, Lewis DD, Hribernik TN, et al. Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980–1991). J Am Vet Med Assoc 1992;201:1253–1257. [PubMed] [Google Scholar]
- 7.Hollett RB. Canine brucellosis: outbreaks and compliance. Theriogenology 2006;66:575–587. [DOI] [PubMed] [Google Scholar]
- 8.Brower A, Okwumabua O, Massengill C, et al. Investigation of the spread of Brucella canis via the US interstate dog trade. Int J Infect Dis 2007;11:454–458. [DOI] [PubMed] [Google Scholar]
- 9.Jones RL. Canine brucellosis in a commercial breeding kennel. J Am Vet Med Assoc 1984;184:834–835. [PubMed] [Google Scholar]
- 10.Glynn MK, Lynn TV. Zoonosis update: brucellosis. J Am Vet Med Assoc 2008;233:900–908. [DOI] [PubMed] [Google Scholar]
- 11.Olsen SC, Palmer MV Advancement of knowledge of Brucella over the past 50 years. Vet Pathol 2014;5:1076–1089. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael LE, Kenney RM. Canine abortion caused by Brucella canis. J Am Vet Med Assoc 1968;152:605–616. [PubMed] [Google Scholar]
- 13.Carmichael LE. Canine brucellosis: An annotated review with selected cautionary comments. Theriogenology 1976;6:105–116. [Google Scholar]
- 14.Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med 2005;352:2325–2336. [DOI] [PubMed] [Google Scholar]
- 15.Carmichael LE, Joubert JC. Transmission of Brucella canis by contact exposure. Cornell Vet 1988;78:63–73. [PubMed] [Google Scholar]
- 16.Johnson CA, Walker RD. Clinical signs and diagnosis of Brucella canis infection. Compend Contin Educ Pract Vet 1992;14:763–772. [Google Scholar]
- 17.Yang Y, Wang Y, Poulsen E, et al. Genotyping Brucella canis isolates using highly discriminatory multilocus variable-number tandem-repeat analysis (MLVA) assay. Sci Rep 2017;7:1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson AP, Sykes JE. Canine brucellosis In: Sykes JE, ed. Canine and feline infectious diseases. St Louis: Elsevier, 2012:512–519. [Google Scholar]
- 19.Lucero NE, Corazza R, Almuzara MN, et al. Human Brucella canis outbreak linked to infection in dogs. Epidemiol Infect 2010;138:280–285. [DOI] [PubMed] [Google Scholar]
- 20.Krueger WS, Lucero NE, Bower A, et al. Evidence for un-apparent Brucella canis infections among adults with occupational exposure to dogs. Zoonoses Public Health 2014;61:509–518. [DOI] [PubMed] [Google Scholar]
- 21.Pappas G, Panagopoulou P, Christou L, et al. Brucella as a biological weapon. Cell Mol Life Sci 2006;23:2229–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzetti S, Carranza C, Roncallo M, et al. Recent trends in human Brucella canis infection. Comp Immunol Microbiol Infect Dis 2013;36:55–61. [DOI] [PubMed] [Google Scholar]
- 23.Polt SS, Dismukes WE, Flint A, et al. Human brucellosis caused by Brucella canis. Ann Intern Med 1982;97:717–719. [DOI] [PubMed] [Google Scholar]
- 24.Swenson RM, Carmichael LE, Cundy KR. Human infection with Brucella canis. Ann Intern Med 1972;76:435–438. [DOI] [PubMed] [Google Scholar]
- 25.Munford RS, Weaver RE, Patton C, et al. Human disease caused by Brucella canis: a clinical and epidemiological study of two cases. J Am Med Assoc 1975;231:1267–1269. [PubMed] [Google Scholar]
- 26.Blankenship RM, Sanford JP. Brucella canis: a cause of undulant fever. Am J Med 1975;59:424–426. [DOI] [PubMed] [Google Scholar]
- 27.Ying W, Nguyen MQ, Jahre JA. Brucella canis endocarditis: case report. Clin Infect Dis 1999;29:1593–1594. [DOI] [PubMed] [Google Scholar]
- 28.McKee MA, Ballard JL. Mycotic aneurysms of the tibioperoneal arteries. Ann Vasc Surg 1999;13:188–190. [DOI] [PubMed] [Google Scholar]
- 29.Piampiano P, McLeary M, Young LW, et al. Brucellosis: unusual presentations in two adolescent boys. Pediatr Radiol 2000;30:355–357. [DOI] [PubMed] [Google Scholar]
- 30.Wallach JC, Giambartolomai GH, Baldi PC, et al. Human infection with M-strain of Brucella canis. Emerg Infect Dis 2004;10:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucero NE, Jacob NO, Ayala SM, et al. Unusual clinical presentation of brucellosis caused by Brucella canis. J Med Microbiol 2005;54:505–508. [DOI] [PubMed] [Google Scholar]
- 32.Lucero NE, Maldonado PI, Kaufman S, et al. Brucella canis causing infection in an HIV-infected patient. Vector Borne Zoonotic Dis 2010;10:527–529. [DOI] [PubMed] [Google Scholar]
- 33.Nomura A, Imaoka K, Imanishi A, et al. Human Brucella canis infections diagnosed by blood culture. Emerg Infect Dis 2010;16:1183–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawaczek E, Toporek J, Cwikla J, et al. Brucella canis in a HIV-infected patient. Zoonoses Public Health 2011;58:150–152. [DOI] [PubMed] [Google Scholar]
- 35.Javeri H, Jamieson S, Sehgal R, et al. Brucella canis peritonitis. Infection 2014;42:195–197. [DOI] [PubMed] [Google Scholar]
- 36.National Association of State Public Health Veterinarians. Veterinary Infection Control Committee 2015. Compendium of veterinary standard precautions for zoonotic disease prevention in veterinary personnel. J Am Vet Med Assoc 44. 2015;247:1252–1277. [DOI] [PubMed] [Google Scholar]
- 37.Kauffman LK, Bjork JK, Gallup JM. Early detection of Brucella canis via quantitative polymerase chain reaction analysis. Zoonoses Public Health 2014;61:48–54. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Ke Y, Zhen Q, et al. Complete genome sequence of Brucella canis BCB018, a strain isolated from a human patient. J Bacteriol 2012;194:6697–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmichael LE, Shin SJ. Canine brucellosis: a diagnostician’s 46. dilemma. Semin Vet Med Surg (Small Anim) 1996;11:161–165. [DOI] [PubMed] [Google Scholar]
- 40.Batinga MCA, dos Santos JC, Lima JTR, et al. Comparison of three methods for recovery of Brucella canis DNA from canine blood samples. J Microbiol Methods 2017;143:26–31. [DOI] [PubMed] [Google Scholar]
- 41.Kaden R, Ågren J, Båverud V, et al. Brucellosis outbreak in a Swedish kennel 2013: determination of genetic markers for source tracing. Vet Microbiol 2014;174:523–530. [DOI] [PubMed] [Google Scholar]
- 42.Moore JA, Gupta BN. Epizootiology, diagnosis, and control of Brucella canis. J Am Vet Med Assoc 1970;156:1737–1740. [PubMed] [Google Scholar]
- 43.Johnson CA, Bennett M, Jensen RK, et al. Effect of combined antibiotic therapy on fertility in brood bitches infected with Brucella canis. J Am Vet Med Assoc 1982;180:1330–1333. [PubMed] [Google Scholar]
- 44.Wanke MM, Delpino MV, Baldi PC. Use of enrofloxacin in the treatment of canine brucellosis in a dog kennel (clinical trial). Theriogenology 2006;66:1573–1578. [DOI] [PubMed] [Google Scholar]
- 45.Serikawa T, Iwaki S, Mori M, et al. Purification of a Brucella canis cell wall antigen by using immunosorbent columns and use of antigen in enzyme-linked immunosorbent assay for specific diagnosis of canine brucellosis. J Clin Microbiol 1989;27:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickerill PA, Carmichael LE. Canine brucellosis: control programs in commercial kennels and effect on reproduction. J Am Vet Med Assoc 1972;160:1607–1615. [PubMed] [Google Scholar]
- 47.Council of State and Territorial Epidemiologists. Enhanced laboratory capability and public health reporting for Brucella canis infections. CSTE position statement 12-ID-03. Available at: www.cste.org/resource/resmgr/PS/12-ID-03FINAL.pdf. Accessed Jul 10, 2015. [Google Scholar]
- 48.AVMA. Canine brucellosis. Available at: www.avma.org/KB/Policies/Pages/Canine-Brucellosis.aspx. Accessed Oct 7, 2015. [Google Scholar]


