Abstract
Objective
The exact shape of the dose-response relationship between maternal body mass index (BMI) and the risk of congenital heart defects (CHDs) in infants has not been clearly defined yet. This study aims to further clarify the relationship between maternal obesity and the risk of CHDs in infants by an overall and dose-response meta-analysis.
Methods
PubMed, Embase, and Web of Science databases were searched to identify all related studies. The studies were limited to human cohort or case-control studies in English language. Random-effect models and dose-response meta-analysis were used to synthesize the results. Heterogeneity, subgroup analysis, sensitivity analysis, and publication bias were also assessed.
Results
Nineteen studies with 2,416,546 participants were included in our meta-analysis. Compared with the mothers with normal weight, the pooled relative risks (RRs) of infants with CHDs were 1.08 (95% CI=1.03-1.13) in overweight and 1.23 (95% CI=1.17-1.29) in obese mothers. According to the findings from the linear meta-analysis, we observed an increased risk of infants with CHDs (RR=1.07, 95% CI=1.06-1.08) for each 5 kg/m2 increase in maternal BMI. A nonlinear relationship between maternal BMI and risk of infants with CHDs was also found (p=0.012).
Conclusion
The results from our meta-analysis indicate that increased maternal BMI is related to increased risk of CHDs in infants.
1. Introduction
Congenital heart defects (CHDs), which account for nearly one-third of all major congenital anomalies, are the most common birth defects in newborns [1]. As the serious medical problem, CHDs play a very important role in the death of newborns and infants [2, 3]. Epidemiological investigations have documented that the prevalence of CHDs in infants is differentiated in regions with an estimated prevalence of 4 to 10 cases per 1,000 births [4]. It is reported that the number of infants with CHDs worldwide has notably increased with more than one million annually [5]. Identifying modifiable risk factors of infants with CHDs remains important for public health and clinical medicine. The exact etiologies of CHDs are complex, several causes such as genetic factors [6–8], physical and chemical factors [9–12], infection during pregnancy [13, 14], medication during pregnancy [15, 16], and mental health status or diseases during pregnancy [17–20] have been identified. However, there are still some potential risk factors that have not been fully confirmed, such as maternal obesity.
Obesity has become a major public health problem that challenges both developed and developing countries [21–23]. Data from epidemiological research showed that women of childbearing age accounted for a large proportion of obese population [24]. The association between maternal obesity and CHDs in infants has been widely reported, but the results are not consistent. For example, one cohort study by Persson et al. suggested that maternal obesity significantly increased the risk of CHDs in infants, and Brite et al. also confirmed the positive association in their study [25, 26]. However, Rankin et al. and Gharderian et al. demonstrated that there was no significant association between increased maternal BMI and increased CHDs risk in offspring [27, 28]. Therefore, the evidence from these observational studies has been inconsistent.
As the dose-response meta-analysis is a reliable quantitative measure of causality, in our study, we conducted a dose-response meta-analysis on maternal BMI and the risk of CHDs in infants by synthesizing the results of published original studies. Our aim was to clearly delineate the shape of the dose-response relationship between maternal BMI and CHDs in infants and to examine the possibility of the nonlinear relationships.
2. Materials and Methods
2.1. Search Strategy
We systematically searched PubMed, Embase, and Web of Science databases to April 31, 2018, for studies on the relationship between maternal BMI and infants with CHDs. The following search strategy was used: (congenital heart defects OR congenital malformations OR birth defects OR CHD OR CHDs) AND (overweight OR obesity OR body mass index OR BMI). Additional possible relevant publications were identified by reviewing the references lists of retrieved articles and published meta-analysis. The searched studies were strictly limited to human cohort studies or case-control studies in English language.
2.2. Study Selection
Studies satisfying the following criteria were included in our meta-analysis: (1) cohort or case-control study design; (2) having clear BMI categories of prepregnancy or early pregnancy; (3) CHDs or one of the CHD subtypes as outcome; (4) relative risk (RR) or odds ratio (OR) with 95% confidence intervals (CIs) available or having sufficient published data to calculate them. In addition, the study for dose-response analysis had to report the estimates of at least three BMI classifications. The Newcastle-Ottawa Scale in which the star system ranges from 0 to 9 was used to assess the methodological quality of studies, and a study awarded seven or more stars was considered high-quality and was included in the meta-analysis [29, 30]. When multiple studies reported the duplicated data, only the latest one with completed data was included.
2.3. Data Extraction
Data were extracted by 2 independent investigators (X.L. and W.Y.), and any disagreement was resolved through consensus from another author (L.J.). The following variables were collected from each publication: first author's name, publication year, study location, study period, study sample size, number of cases, study design, BMI category and the corresponding risk estimate, confounding factors adjusted in multivariable analysis, and study conclusion. Considering that the rate ratio, risk ratio, and hazard ratio can be used as a valid estimate of the relative risk and the meaning of the odds ratio is similar to the relative risk, then we used the RRs to report the results for convenience. In order to reduce the impact of covariates, the adjusted RRs in multivariate analysis were preferentially extracted.
The average BMI corresponding to each classified RR was calculated by the midpoint of the upper and lower boundary of each category. In the case where the highest category or the lowest category was the open interval, we assumed that they had the same amplitudes as the adjacent category [31]. When a study provided only total number of cases and person-years, the distribution of cases and person-years were estimated through the method described by Aune et al. [32].
2.4. Statistical Analysis
We conducted separate meta-analysis to calculate the pooled RRs and 95% CIs for overweight and obese mothers versus normal-weight mothers. For the category of BMI, we used the classification standard of WHO (underweight, <18.5 kg/m2; normal weight, 18.5-24.9 kg/m2; overweight, 25.0-29.9 kg/m2; obesity, ≥30.0 kg/m2) [33, 34]. The logarithmic transformations for the RRs and the corresponding standard errors extracted from studies were performed to make the variances stabilized and the distributions normalized. A random-effects model was used to combine the estimates [34]. The random-effects model was chosen a priori because it was considered as more conservative than the fixed-effects model, as it accounted for both within- and between-study heterogeneity [35]. The I2 statistic and the Q-test were used to assess the heterogeneity across studies, and I2 values of 0, 25%, 50%, and 75% were considered indicative of no, low, moderate, and high heterogeneity, respectively [36]. Considering that the relationship between maternal obesity and CHDs in infants may be affected by study-specific factors (e.g., study design, study location, study sample size, maternal age, smoking, and education), subgroup analyses were separately conducted based on these possible confounders.
A two-stage random-effect dose-response meta-analysis, which required the variables of cases, person-years, mean level of BMI, and the corresponding RR in each category, was used to depict the trend from the relevant log-RRs estimated across BMI categories, considering the heterogeneity between studies[37]. In the first stage, a generalized least squares regression was used to estimate the restricted cubic spline model with three knots at the 10th, 50th, and 90th percentiles of the distribution, considering the correlation within each set of the published RRs. Then, the estimates value for each study calculated in the previous step was merged to carry out the dose-response relationship between maternal BMI and the risk of infants with CHDs. The null hypothesis that the second spline coefficient is equal to zero was tested to calculate the p value for nonlinearity [38].
In addition, we conducted sensitivity analysis, in which one study involved in the meta-analysis was eliminated at a time and the rest pooled to evaluate the stability of our results [39]. Evidence of publication bias was appraised through funnel plots and Egger's regression tests [40]. All statistical analyses were performed by Stata 12.0 (Stata Corporation, College Station, TX). A p value less than 0.05 was considered statistically significant, except for the Q-test (p<0.10) because of the low power of the test.
3. Results
3.1. Literature Search and Study Characteristics
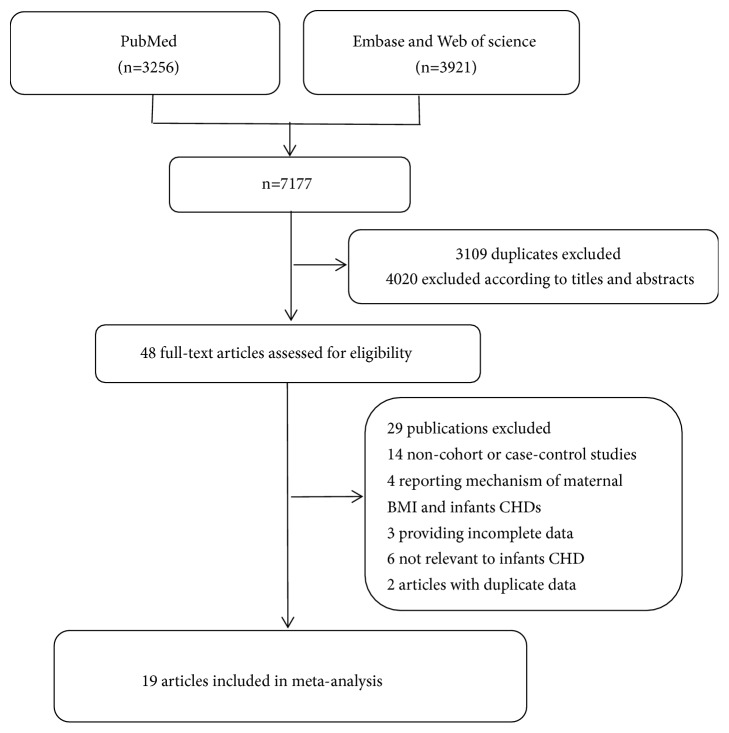
Our meta-analysis included 6 cohort studies [25–27, 41–43] and 13 case-control studies [28, 44–55], which involved 57,172 cases and 2,416,546 participants (Figure 1). Among these studies, 12 were conducted in the North America [26, 28, 41, 43–47, 50, 51, 53, 55], 4 in Europe [25, 27, 42, 54], one in Oceania [48], and 2 in Asia [49, 52]. A total of 10 studies had less than 10,000 participants [28, 44, 48–55] while nine studies had more than 10,000 participants [25–27, 41–43, 45–47]. Eight studies controlled for maternal age [25–27, 46–48, 51, 55] and 7 studies controlled for maternal smoking [25–27, 46, 47, 51, 55]. For the factor of maternal education, it was adjusted in 6 studies [25, 46–48, 51, 55]. Of the included studies, 9 reported that maternal obesity significantly increased the risk of CHDs in infants [25, 26, 42, 44–47, 51, 53], and 10 reported that there was no significant association between increased maternal BMI and increased CHDs risk in offspring [27, 28, 41, 43, 48–50, 52, 54, 55]. The general characteristics of the included studies were shown in Table 1.
Figure 1.
Flowchart of the selection of studies for inclusion in this meta-analysis.
Table 1.
Characteristics of included studies.
| Author (year) | Country | Study period | Study size no | No of cases | Study design | BMI (kg/m2) | RR (95%CI) | Adjustment factors | Study conclusion | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Persson, 2017 | Sweden | 2001-2014 | 1,243,957 | 20,074 | Cohort study | <18.5 18.5-24.9 25.0-29.9 30.0-34.9 35.0-39.9 ≥40.0 |
0.99(0.90-1.09) 1.00 1.05(1.01-1.08) 1.15(1.09-1.20) 1.26(1.16-1.37) 1.44(1.27-1.63) |
Maternal age, height, parity, early pregnancy, smoking status, education level, maternal country of birth, family situation, sex of offspring | Risks of infants CHDs progressively increased with increasing severity of maternal overweight and obesity. | 8 |
|
| ||||||||||
| Warrick, 2015 | The United States | 2005-2011 | 18,226 | 117 | Cohort study | <18.5 18.5-24.9 25.0-29.9 ≥30.0 |
0.61(0.22-1.67) 1.00 0.63(0.39-1.03) 0.90(0.57-1.45) |
NA | No significant differences in maternal obesity between mothers with and without CHDs infants were shown. |
7 |
|
| ||||||||||
| Brite, 2014 | The United States | 2002-2008 | 121,815 | 1,388 | Cohort study | <18.5 18.5-24.9 25.0-29.9 ≥30.0 |
1.08(0.85-1.38) 1.00 1.15(1.01-1.32) 1.26(1.09-1.44) |
Site, maternal age, race, insurance, maternal smoking | Increasing maternal weight class was associated with increased risk for CHDs in infants. | 7 |
|
| ||||||||||
| Rankin, 2010 | England | 2003-2005 | 30,703 | 270 | Cohort study | <18.5 18.5-24.9 25.0-29.9 ≥30.0 |
1.55(0.90-2.66) 1.00 0.75(0.55-1.02) 1.16(0.84-1.59) |
Maternal age, ethnicity, pre-gestational diabetes, cigarette smoking status, index of multiple deprivation. | No significant associations were found between maternal BMI and infants CHDs risk. | 7 |
|
| ||||||||||
| Cedergren, 2006 | Sweden | 1992-2001 | 770,355 | 6,346 | Cohort study | <20.0 20.0-24.9 25.0-29.9 ≥30.0 |
0.97(0.89-1.05) 1.00 1.03(0.97-1.09) 1.18(1.09-1.29) |
NA | Maternal obesity was more common in pregnancies with infants affected by CHDs. | 7 |
|
| ||||||||||
| Moore, 2000 | The United States | 1984-1987 | 22,951 | 60 | Cohort study | <25.0 25.0-27.9 ≥28.0 |
1.00 0.67(0.24-1.86) 0.93(0.37-2.34) |
NA | There was no evidence of an excess risk of CHDs in infants among the obese women. | 7 |
|
| ||||||||||
| Tang, 2015 | The United States | 1997-2008 | 2,147 | 553 | Case-control study | <18.5 18.5-24.9 25.0-29.9 ≥30.0 |
0.64(0.35-1.15) 1.00 1.38(1.09-1.75) 1.56(1.21-2.00) |
NA | The risk of CHDs was closely related to maternal obesity. |
7 |
|
| ||||||||||
| Gharderian, 2013 | The United States | 2011-2012 | 322 | 164 | Case-control study | <18.5 18.5-24.9 25.0-29.9 ≥30.0 |
0.85(0.32-2.27) 1.00 1.28(0.78-2.09) 1.11(0.57-2.16) |
NA | There might not be a relation between maternal BMI and having a child with CHDs. | 7 |
|
| ||||||||||
| Madsen, 2012 | The United States | 1992-2007 | 107,901 | 7,547 | Case-control study | <18.5 18.5-24.9 25.0-29.9 ≥30.0 |
1.02(0.91-1.15) 1.00 1.03(0.97-1.10) 1.22(1.15-1.30) |
Gestational diabetes | The significant association between infants CHDs and maternal obesity was confirmed. | 8 |
|
| ||||||||||
| Gilboa, 2010 | The United States | 1998-2003 | 12,113 | 6,440 | Case-control study | <18.5 18.5-24.9 25.0-29.9 30.0-34.9 ≥35.0 |
0.96(0.80-1.16) 1.00 1.16(1.05-1.29) 1.15(1.00-1.32) 1.31(1.11-1.56) |
Maternal age, race-ethnicity, education, hypertension, parity, smoking, folic acid supplement use | Mothers of CHDs infants were more likely than mothers of control infants to be overweight, moderately obese or severely obese. |
7 |
|
| ||||||||||
| Mills, 2010 | The United States | 1993-2003 | 63,696 | 7,392 | Case-control study | <19.0 19.0-24.0 25.0-29.0 ≥30.0 |
1.00(0.91-1.10) 1.00 1.00(0.94-1.06) 1.15(1.07-1.23) |
Maternal age, education, race, smoking, and payment method for health care. | Obese, but not overweight, women are at significantly increased risk of bearing children with CHDs. |
8 |
|
| ||||||||||
| Oddy, 2009 | Australia | 1997-2000 | 529 | 111 | Case-control study | <20.0 20.0-24.9 25.0-29.9 ≥30.0 |
0.74(0.40-1.36) 1.00 0.79(0.45-1.41) 1.34(0.63-2.84) |
Marital status, maternal age, maternal education and periconceptional folic acid supplementation | No significant associations were found between maternal BMI and infants CHDs risk. | 8 |
|
| ||||||||||
| Khalil, 2008 | Saudi Arabia | 1998-2005 | 428 | 214 | Case-control study | 19.0-25.0 30.0-34.9 ≥35.0 |
1.00 0.78(0.51-1.19) 1.57(0.84-2.92) |
NA | No association was found between maternal weight and isolated CHDs in the offspring. | 7 |
|
| ||||||||||
| Shaw, 2008 | The United States | 1999-2004 | 1578 | 278 | Case-control study | <18.5. 20.0-24.9 25.0-29.9 ≥30.0 |
0.84(0.46-1.56) 1.00 1.18(0.87-1.60) 0.75(0.49-1.15) |
NA | The association between maternal BMI and CHDs in infants was not significant. | 7 |
|
| ||||||||||
| Waller, 2007 | The United States | 1997-2002 | 8032 | 4128 | Case-control study | <18.5. 20.0-24.9 25.0-29.9 ≥30.0 |
1.12(0.93-1.36) 1.00 1.13(1.01-1.26) 1.40(1.24-1.59) |
Maternal age, ethnicity, education, parity, smoking in the month prior to conception, and supplemental folic acid intake | Obesity or overweight women had a modest increase in the risk of infants CHDs. | 8 |
|
| ||||||||||
| Martinez, 2005 | Spain | 1976-2001 | 6973 | 813 | Case-control study | ≤20.9 21.0-24.9 25.0-29.9 ≥30.0 |
1.00(0.83-1.20) 1.00 1.17(0.97-1.41) 1.16(0.87-1.56) |
NA | Maternal overweight or obesity did not increase the risk of CHDs in infants. | 7 |
|
| ||||||||||
| Watkins, 2003 | The United States | 1993-1997 | 525 | 195 | Case-control study | <18.5. 20.0-24.9 25.0-29.9 ≥30.0 |
1.70(0.90-3.10) 1.00 2.00(1.20-3.10) 2.00(1.20-3.40) |
NA | The significant association between infants CHDs and maternal obesity was confirmed. | 7 |
|
| ||||||||||
| Cedergren 2002 | Sweden | 1982-1996 | 677 | 231 | Case-control study | <19.8. 19.8-25.9 26.0-28.9 ≥29.0 |
1.46(0.97-2.21) 1.00 1.16(0.64-2.09) 1.68(0.94-3.00) |
NA | The associations between maternal BMI and infants CHDs risk was not confirmed. | 7 |
|
| ||||||||||
| Watkins, 2001 | The United States | 1982-1983 | 3618 | 851 | Case-control study | <16.5. 16.5-19.8 19.9-22.7 22.8-26.0 26.1-29.0 >29.0 |
0.78(0.55-1.11) 0.97(0.81-1.17) 1.00 0.84(0.67-1.06) 1.37(0.92-2.03) 1.24(0.80-1.90) |
Race, birth period, age, education, alcohol use, smoking, chronic illness, and vitamin use | There might not be a relation between maternal BMI and having a child with CHDs. | 8 |
BMI, body mass index; RR, relative risk; CI, confidence interval; NA, not available; NOS, Newcastle-Ottawa Scale.
3.2. Abnormal Maternal BMI and Infants with CHDs
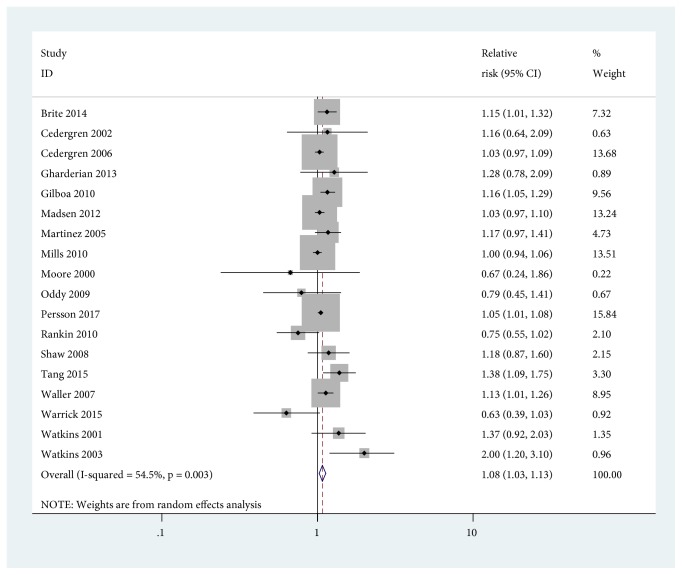
Compared with maternal normal weight, the pooled RR of CHDs in infants was 1.08 (95% CI=1.03-1.13) for maternal overweight and some evidence of heterogeneity across studies was found with I2=54.5% (Figure 2). Subgroup analysis suggested that the pooled association of CHDs in infants among overweight mothers was significantly higher in studies with less than 10,000 participants (RR=1.21, 95% CI=1.10-1.34) than that in studies with more than 10,000 participants (RR=1.04, 95% CI=1.00-1.09). In addition, the corresponding I2 statistics were 16.1% and 54.9%, respectively, which indicated that the heterogeneity was derived from studies with sample sizes more than 10,000. Meanwhile, the pooled RR and the I2 statistic for studies conducted in the United States were 1.12 (95% CI=1.04-1.21) and 62.7%, while the pooled RR and the I2 statistic for studies conducted outside the United States were 1.04 (95% CI=0.99-1.10) and 30.4%, which demonstrated that American studies resulted in the heterogeneity (Table 2).
Figure 2.
Forest plot of RRs of maternal overweight versus maternal normal weight for BMI with CHDs risk in infants. RR, relative risk; CI, confidence interval; BMI, body mass index.
Table 2.
Subgroup analysis of maternal BMI and CHDs risk in infants.
| Study | Overweight | Obesity | ||||
|---|---|---|---|---|---|---|
| No.of studies | RR (95%CI) | I 2(%) | No.of studies | RR (95%CI) | I 2(%) | |
| All studies | 18 | 1.08(1.03-1.13) | 54.5 | 19 | 1.23(1.17-1.29) | 48.3 |
| Study design | ||||||
| Cohort | 6 | 1.03(0.96-1.11) | 56.9 | 6 | 1.22(1.15-1.31) | 53.2 |
| Case-control | 12 | 1.13(1.05-1.21) | 56.1 | 13 | 1.24(1.15-1.33) | 48.7 |
| Study location | ||||||
| The United States | 12 | 1.12(1.04-1.21) | 62.7 | 12 | 1.24(1.15-1.32) | 48.3 |
| Not the United States | 6 | 1.04(0.99-1.10) | 30.4 | 7 | 1.22(1.14-1.32) | 52.1 |
| Sample sizes | ||||||
| Less than 10000 | 9 | 1.21(1.10-1.34) | 16.1 | 10 | 1.27(1.08-1.49) | 49.3 |
| More than 10000 | 9 | 1.04(1.00-1.09) | 54.9 | 9 | 1.21(1.16-1.26) | 38.8 |
| Adjustment factors | ||||||
| Maternal age | ||||||
| Yes | 8 | 1.07(1.01-1.14) | 58.3 | 8 | 1.24(1.17-1.31) | 54.1 |
| No | 10 | 1.11(1.01-1.22) | 56.2 | 11 | 1.20(1.08-1.33) | 47.0 |
| Maternal smoking | ||||||
| Yes | 7 | 1.07(1.01-1.14) | 62.1 | 7 | 1.24(1.17-1.31) | 58.5 |
| No | 11 | 1.10(1.00-1.21) | 53.5 | 12 | 1.20(1.09-1.33) | 42.4 |
| Maternal education | ||||||
| Yes | 6 | 1.07(1.01-1.13) | 52.4 | 6 | 1.24(1.17-1.33) | 62.5 |
| No | 12 | 1.09(1.00-1.19) | 59.1 | 13 | 1.21(1.11-1.31) | 38.6 |
BMI, body mass index; CHDs, congenital heart defects; RR, relative risk; CI, confidence interval.
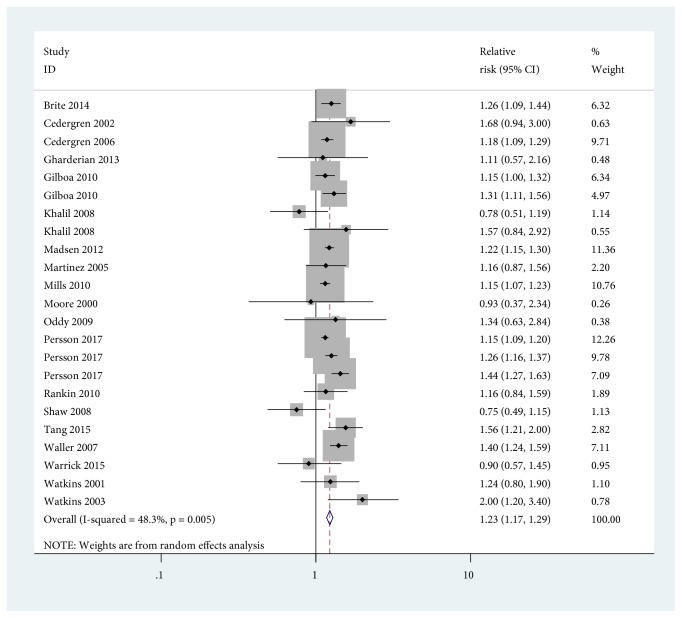
Using mothers with normal BMI as the reference category, we found that maternal obesity increased the risk of CHDs in infants (RR=1.23, 95% CI=1.17-1.29). No evidence of high heterogeneity was found for the category of obesity (I2 = 48.3%) (Figure 3). When stratified by study design, the pooled RR of infants with CHDs among obese mothers was 1.22 (95% CI=1.15-1.31) compared with mothers with normal weight in cohort studies, and the pooled RR among obese mothers was 1.24 (95% CI=1.15-1.33) compared with mothers with normal weight in case-control studies. It was noted that the effect differences were not observed for study design, study location, study sample sizes, and other adjustment factors (e.g., maternal age, maternal smoking, and maternal education) (Table 2).
Figure 3.
Forest plot of RRs of maternal obesity versus maternal normal weight for BMI with CHDs risk in infants. RR, relative risk; CI, confidence interval; BMI, body mass index.
3.3. Dose-Response Meta-Analysis
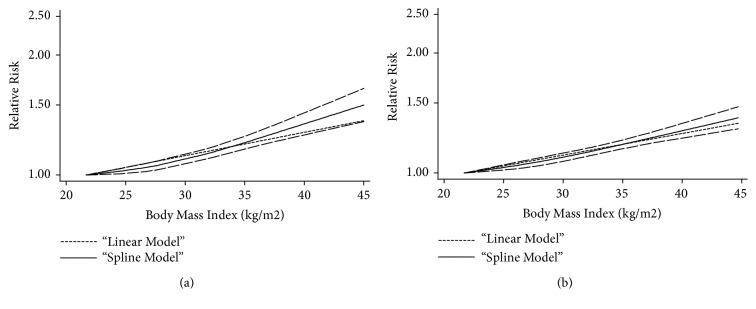
All of the above 19 studies were included in the dose-response meta-analysis of maternal BMI and risk of infants with CHDs. As shown in Figure 4, an increased risk of CHDs in infants (RR=1.07, 95% CI=1.06-1.08) for each 5 kg/m2 increase in maternal BMI was shown in this meta-analysis. When stratified by study design, it was found that the risk of infants with CHDs increased by 7% for every 5 kg/m2 increase of maternal BMI, in both cohort studies (RR=1.07, 95% CI=1.06-1.08) and case-control studies (RR=1.07, 95% CI=1.05-1.09) (Figure 5).
Figure 4.
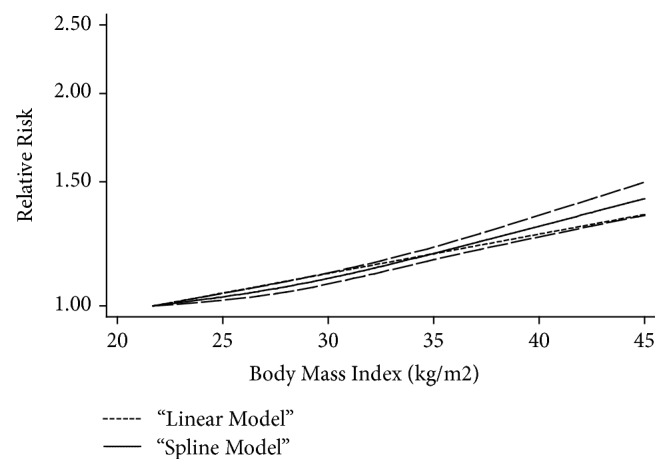
The dose-response analysis between maternal BMI and CHDs risk in infants with restricted cubic splines in a multivariate random-effects dose-response model. The solid line and the long dash line represent the estimated RR and its 95% CI. Short dash line represents the linear relationship (per 5 kg/m2 increment). RR, relative risk; CI, confidence interval; BMI, body mass index.
Figure 5.
The dose-response analysis between maternal BMI and CHDs risk in infants by adjustment of study design. (a) Cohort studies; (b) case-control studies. The solid line and the long dash represented RR and its 95% CI. Short dash line represents the linear relationship (per 5 kg/m2 increment). RR, relative risk, CI, confidence interval; BMI, body mass index.
As shown in Figure 4, it was found that there was a nonlinear relationship between maternal BMI and risk of CHDs in infants (p=0.012). Compared with BMI=22.05 kg/m2, the pooled RRs (95% CIs) of infants with CHDs were 1.03 (95% CI=1.02-1.04), 1.08 (95% CI=1.06-1.10), 1.18 (95% CI=1.16-1.21), 1.36 (95% CI=1.30-1.42), and 1.42 (95% CI=1.34-1.50) for BMI=25, 30, 35, 40, and 45 kg/m2, respectively. The evidence of significant nonlinear relationship was also observed in cohort studies (p=0.015) when adjusting the factor of study design. At the points of BMI=25, 30, 35, 40, and 45 kg/m2, the corresponding RRs (95% CIs) for cohort studies were 1.02 (95% CI=1.01-1.04), 1.13 (95% CI=1.10-1.16), 1.21 (95% CI=1.16-1.25), 1.42 (95% CI=1.31-1.54), and 1.50 (95% CI=1.36-1.65), respectively (Figure 5).
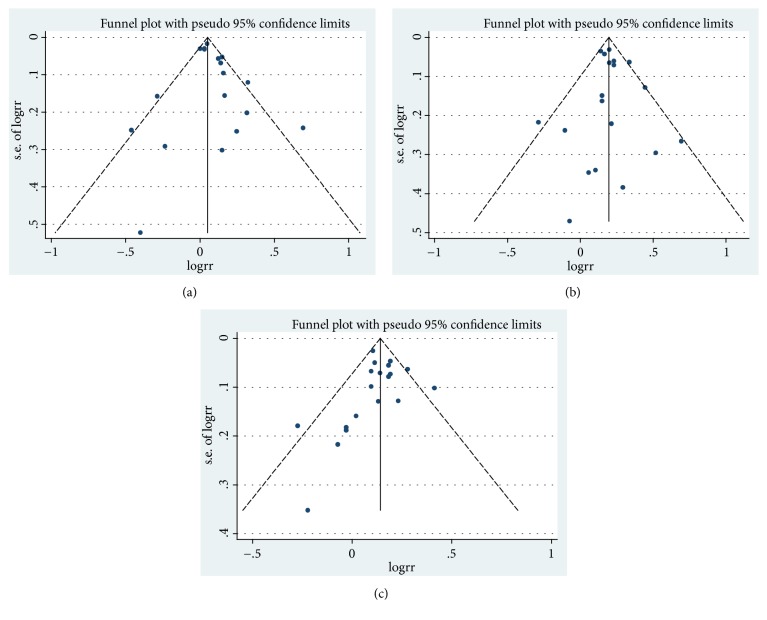
3.4. Publication Bias
Egger's regression tests showed no evidence of publication bias in the literature about maternal BMI and risk of infants with CHDs in maternal overweight group (p=0.346), maternal obesity group (p=0.744), and dose-response group (p=0.605) (Figure 6).
Figure 6.
Funnel plot corresponding to the random-effects meta-analysis of the relationship between (a) maternal overweight and infants CHDs risk (p=0.346, by Egger's test); (b) maternal obesity and infants CHDs risk (p=0.744, by Egger's test); (c) funnel plot corresponding to the dose-response meta-analysis of the relationship between maternal BMI and infants CHDs risk (p=0.605, by Egger's test). BMI, body mass index; CHD, congenital heart defects.
3.5. Sensitivity Analysis
In a sensitivity analysis in which one study at a time was eliminated and the remaining analyzed, the pooled RRs of infants with CHDs ranged from 1.07 to 1.09 for maternal overweight group, from 1.21 to 1.24 for maternal obesity group, and from 1.15 to 1.17 for dose-response analysis group separately, which demonstrated that the pooled estimates were steady and not affected by a single study.
4. Discussion
In the present meta-analysis, we discovered an increase of 8% risk of infants with CHDs in maternal overweight group and an increase of 23% risk in maternal obesity group compared with the mothers with normal weight. Subgroup analysis by study design showed that the significant association between maternal overweight and increased risk of infants with CHDs existed only in case-control studies, while the significant association between maternal obese and increased risk of infants with CHDs existed in both cohort studies and case-control studies. Dose-response meta-analysis showed that each 5 kg/m2 increase of maternal BMI is accompanied by a 7% increment of risk of infants with CHDs, and a significantly nonlinear relationship between maternal BMI and infants with CHDs risk was observed (p=0.012). When stratified by study design, the pooled RR of infants with CHDs increased by 7% per 5 kg/m2 increase of maternal BMI, for both cohort and case-control studies. The evidence of significant nonlinear relationship between maternal BMI and risk of infants with CHDs was also found in cohort studies (p=0.015).
Our findings are similar to meta-analysis by Cai et al., who examined the association between maternal BMI and CHDs in offspring and reported a similar summary for overweight and obese individuals [56]. However, that meta-analysis only included 14 studies and the possibility of nonlinear association between maternal BMI and infants with CHDs was not reported. In another meta-analysis, a slightly lower significant association between maternal overweight and increased CHDs risk in infants and a significant association between maternal obesity and CHDs in their offspring were observed [57]. Nevertheless, the dose-response relationship was also not examined in their meta-analysis. Our results, based on 20 studies, were generally in line with the results of previous meta-analysis [56, 57]. Moreover, the statistically nonlinear dose-response relationship between maternal BMI increase and risk of infants with CHDs was also found in our study. In addition, we conducted subgroup analysis through possible confounding factors such as study design, study sample sizes and adjustment factors, which made our result more abundant.
Maternal obesity might be associated with increased risk of infants with CHDs through several mechanisms. Data from epidemiology research suggest that folate, glutathione, and homocysteine metabolism related genetic variants in maternal and fetal may have great impact on the heart development [57]. It had been reported that obesity mothers who carried mutant genotype AC for glutamate-cysteine ligase, catalytic subunit (GCLC) gene (rs6458939) significantly increased the risk of conotruncal defects (CTDs) in infants, compared with those obesity mothers who carried the CC genotype [58]. Another possible mechanism is that maternal metabolic environment plays an important role in fetal developments [59]. Decreased intake of folate and glutathione and increased intake of homocysteine caused by maternal obesity may lead to abnormal in utero environment, which contribute to the onset and development of impaired fetal developments [60–64]. Additionally, some animal studies have reported on possible ways of maternal obesity-mediated offspring CHDs. Firstly, through changing the signal path, Wang et al. reported that diabetes-induced heart defects may be affected by apoptosis signal-regulating kinase 1 (ASK1), which can be attributed to the activation of ASK1 on c-Jun NH2-terminal kinase 1/2 (JNK 1/2)-endoplasmic reticulum (ER) stress pathway, inhibition of ASK1 on cell cycle progression, and mediation of ASK1 on teratogenicity of diabetes [65]. Another study demonstrated that maternal obesity in sheep pregnancy can alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart [66]. Huang et al. indicated that maternal obesity results in greater fetal heart connective tissue accumulation associated with an upregulated TGF-β/p38 signaling pathway at late gestation, and such changes may negatively impact offspring heart function [67]. Secondly, it was reported that maternal obesity may impair fetal cardiomyocyte contractility and affect cardiac development by altering intracellular Ca2+ treatment, overloading fetal Ca2+, and abnormal myofibrillar proteins [68]. Thirdly, maternal obesity significantly enhances TLR4, IL-1a, IL-1b, and IL-6 expression, promotes phosphorylation of I-κB, decreases cytoplasmic NF-κB levels, and increases neutrophil and monocyte infiltration, eventually leading to inflammation in the fetal heart and altering fetal cardiac morphometry [69]. Furthermore, a mini-review by Dong et al. reported that lipotoxicity resulting from maternal obesity is capable of activating a number of stress signaling cascades including proinflammatory cytokines and oxidative stress to exacerbate cardiovascular complications [70].
The present meta-analysis had some advantages. Firstly, more relevant original studies and a large number of participants and cases were included, which significantly improved the statistical power of the analysis. Meanwhile, we conducted a quality assessment of eligible studies using the Newcastle-Ottawa Scale, and the included studies can be considered as high-quality because they all awarded seven or more stars. Secondly, the dose-response meta-analysis was performed, and the possibility of nonlinear relationship was evaluated in our study, which made the association between maternal BMI and the risk of CHDs in infants better described. In spite of these strengths, the interpretation of the results in our meta-analysis may be affected by several potential limitations. First, most studies included in our meta-analysis were case-control studies and it is reported that the information bias might be more prone to occur in case-control studies than cohort studies. Then, some confounding factors (e.g., maternal age, maternal smoking, and maternal education) only were adjusted in very few included studies, which may lead to an overestimation of the true association between maternal obesity and risk of CHDs in offspring. Finally, it is impossible to completely exclude the potential publication bias because some studies with invalid results tend not to be published.
5. Conclusion
In conclusion, our overall and dose-response meta-analysis indicate that increased maternal BMI is related to increased risk of CHDs in infants. The measures of maternal weight control before they plan to conceive are necessary to decrease the risk of CHDs in infants. The findings from our meta-analysis need to be confirmed in well-designed intervention studies in the future.
Acknowledgments
We thank all the investigators for their contributions to this study. This study was supported by the National Natural Science Foundation of China (No. 81773527), Shandong Natural Science Foundation of China (No. ZR2017MH007), and the Project of Shandong Province Higher Educational Science and Technology Program (No. J16LL09).
Contributor Information
Long Ji, Email: lji@tsmc.edu.cn.
Dong Li, Email: tsmcdongli@163.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Xuezhen Liu, Guoyong Ding, and Weili Yang contributed equally to this work.
Supplementary Materials
Scheme: the scheme of the meta-analysis. Supplementary Figure: figures of sensitivity analysis. Supplementary Table: the checklist of PRISMA Statement.
References
- 1.Van der Linde D., Konings E. E. M., Slager M. A., et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Fahed A. C., Gelb B. D., Seidman J. G., Seidman C. E. Genetics of congenital heart disease: the glass half empty. Circulation Research. 2013;112(4):707–720. doi: 10.1161/circresaha.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meberg A., Otterstad J., Frøland G., Lindberg H., Sørland S. Outcome of congenital heart defects--a population-based study. Acta Paediatrica. 2000;89(11):1344–1351. doi: 10.1111/j.1651-2227.2000.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 4.Oyarzun I., Claveria C., Larios G., Le Roy C. Nutritional recovery after cardiac surgery in children with congenital heart disease. Revista Chilena de Pediatria. 2018;89(1):24–31. doi: 10.4067/S0370-41062018000100024. [DOI] [PubMed] [Google Scholar]
- 5.Bernier P., Stefanescu A., Samoukovic G., Tchervenkov C. I. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 2010;13(1):26–34. doi: 10.1053/j.pcsu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Martínez Olorón P., Romero Ibarra C., Alzina de Aguilar V. Incidence of congenital heart disease in navarra (1989–1998) Revista Española de Cardiología. 2005;58(12):1428–1434. doi: 10.1016/S1885-5857(06)60750-0. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman J. I. E., Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39(12):1890–1900. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 8.Varadkar P., Kraman M., Despres D., Ma G., Lozier J., McCright B. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2008;237(4):1144–1152. doi: 10.1002/dvdy.21502. [DOI] [PubMed] [Google Scholar]
- 9.Stoupel E., Birk E., Kogan A., et al. Congenital heart disease: correlation with fluctuations in cosmophysical activity, 1995-2005. International Journal of Cardiology. 2009;135(2):207–210. doi: 10.1016/j.ijcard.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Kučienė R., Dulskienė V. Selected environmental risk factors and congenital heart defects. Medicina (Kaunas, Lithuania) 2008;44(11):827–832. doi: 10.3390/medicina44110104. [DOI] [PubMed] [Google Scholar]
- 11.Rogers J. M. Tobacco and pregnancy. Reproductive Toxicology. 2009;28(2):152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Gianicolo E. A., Cresci M., Ait-Ali L., Foffa I., Andreassi M. G. Smoking and congenital heart disease: the epidemiological and biological link. Current Pharmaceutical Design. 2010;16(23):2572–2577. doi: 10.2174/138161210792062849. [DOI] [PubMed] [Google Scholar]
- 13.Patel S. S., Burns T. L. Nongenetic risk factors and congenital heart defects. Pediatric Cardiology. 2013;34(7):1535–1555. doi: 10.1007/s00246-013-0775-4. [DOI] [PubMed] [Google Scholar]
- 14.Malinowska-Polubiec A., Czajkowski K., Sotowska A., Sieinko J. Course of pregnancy and delivery in patients with pregestational diabetes mellitus. Ginekologia polska. 2005;76(4):264–269. [PubMed] [Google Scholar]
- 15.Hernández-Díaz S., Werler M. M., Walker A. M., Mitchell A. A. Folic acid antagonists during pregnancy and the risk of birth defects. The New England Journal of Medicine. 2000;343(22):1608–1641. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 16.Czeizel A. E., Rockenbauer M., Sørensen H. T., Olsen J. The teratogenic risk of trimethoprim-sulfonamides: a population based case-control study. Reproductive Toxicology. 2001;15(6):637–646. doi: 10.1016/S0890-6238(01)00178-2. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Sulaiman R. M., Subaih B. Congenital heart disease in infants of diabetic mothers: echocardiographic study. Pediatric Cardiology. 2004;25(2):137–140. doi: 10.1007/s00246-003-0538-8. [DOI] [PubMed] [Google Scholar]
- 18.Petry P., Polli J. B., Mattos V. F., et al. Clinical features and prognosis of a sample of patients with trisomy 13 (Patau syndrome) from Brazil. American Journal of Medical Genetics Part A. 2013;161(6):1278–1283. doi: 10.1002/ajmg.a.35863. [DOI] [PubMed] [Google Scholar]
- 19.Rosa R. F., Rosa R. C., Zen P. R., Graziadio C., Paskulin G. A. Trisomy 18: review of the clinical, etiologic, prognostic, and ethical aspects. Revista Paulista de Pediatria : Orgao Oficial Da Sociedade De Pediatria De Sao Paulo. 2013;31(1):111–120. doi: 10.1590/s0103-05822013000100018. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho A. B., Guerra Júnior G., Baptista M. T., Faria A. P., Marini S. H., Guerra A. T. Cardiovascular and renal anomalies in Turner syndrome. Revista da Associação Médica Brasileira. 2010;56(6):655–659. doi: 10.1590/S0104-42302010000600012. [DOI] [PubMed] [Google Scholar]
- 21.Gundogan K., Bayram F., Gedik V., et al. Metabolic syndrome prevalence according to ATP III and IDF criteria and related factors in Turkish adults. Archives of Medical Science: AMS. 2013;9(2):243–253. doi: 10.5114/aoms.2013.34560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Januszek-Trzciąkowska A., Małecka-Tendera E., Klimek K., Matusik P. Obesity risk factors in a representative group of Polish prepubertal children. Archives of Medical Science: AMS. 2014;10(5):880–885. doi: 10.5114/aoms.2013.33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyriazis I., Rekleiti M., Saridi M., et al. Prevalence of obesity in children aged 6-12 years in Greece: nutritional behaviour and physical activity. Archives of Medical Science: AMS. 2012;8(5):859–864. doi: 10.5114/aoms.2012.31296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. Prevalence and trends in obesity among US adults, 1999-2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 25.Persson M., Cnattingius S., Villamor E., et al. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. British Medical Journal. 2017;357, article j2563 doi: 10.1136/bmj.j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brite J., Laughon S. K., Troendle J., Mills J. Maternal overweight and obesity and risk of congenital heart defects in offspring. International Journal of Obesity. 2014;38(6):878–882. doi: 10.1038/ijo.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin J., Tennant P. W., Stothard K. J., Bythell M., Summerbell C. D., Bell R. Maternal body mass index and congenital anomaly risk: a cohort study. International Journal of Obesity. 2010;34(9):1371–1380. doi: 10.1038/ijo.2010.66. [DOI] [PubMed] [Google Scholar]
- 28.Ghaderian M., Emami-Moghadam A. R., Khalilian M. R., Riahi K., Ghaedi F. Prepregnancy maternal weight and body mass index of children with and without congenital heart disease. Iranian Journal of Pediatrics. 2014;24(3):313–318. [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q. J., Yang Y., Vogtmann E., et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2013;24(4):1079–1087. doi: 10.1093/annonc/mds601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., Wu Z., Han B., et al. The association between BMI and gallbladder cancer risk: a meta-analysis. Oncotarget. 2016;7(28):43669–43679. doi: 10.18632/oncotarget.9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F., Xu Y. Body mass index and risk of renal cell cancer: A dose-response meta-analysis of published cohort studies. International Journal of Cancer. 2014;135(7):1673–1686. doi: 10.1002/ijc.28813. [DOI] [PubMed] [Google Scholar]
- 32.Aune D., Greenwood D. C., Chan D. S. M., et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Annals of Oncology. 2012;23(4):843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 33.Obesity: preventing and managing the global epidemic. report of a WHO consultation. World Health Organization Technical Report Series. 2000;894:1–253. [PubMed] [Google Scholar]
- 34.DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Riley R. D., Higgins J. P., Deeks J. J. Interpretation of random effects meta-analyses. British Medical Journal. 2011;342, article d549 doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J. P., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American Journal of Epidemiology. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desquilbet L., Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in Medicine. 2010;29(9):1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 39.Patsopoulos N. A., Evangelou E., Ioannidis J. P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International Journal of Epidemiology. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warrick C. M., Hart J. E., Lynch A. M., Hawkins J. A., Bucklin B. A. Prevalence and descriptive analysis of congenital heart disease in parturients: obstetric, neonatal, and anesthetic outcomes. Journal of Clinical Anesthesia. 2015;27(6):492–498. doi: 10.1016/j.jclinane.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Cedergren M. I., Källén B. A. Obstetric outcome of 6346 pregnancies with infants affected by congenital heart defects. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2006;125(2):211–216. doi: 10.1016/j.ejogrb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Moore L. L., Singer M. R., Bradlee M. L., Rothman K. J., Milunsky A. A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology. 2000;11(6):689–694. doi: 10.1097/00001648-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Tang X., Cleves M. A., Nick T. G., et al. Obstructive heart defects associated with candidate genes, maternal obesity, and folic acid supplementation. American Journal of Medical Genetics Part A. 2015;167(6):1231–1242. doi: 10.1002/ajmg.a.36867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madsen N. L., Schwartz S. M., Lewin M. B., Mueller B. A. Prepregnancy body mass index and congenital heart defects among offspring: a population-based study. Congenital Heart Disease. 2013;8(2):131–141. doi: 10.1111/j.1747-0803.2012.00714.x. [DOI] [PubMed] [Google Scholar]
- 46.Gilboa S. M., Correa A., Botto L. D., et al. Association between prepregnancy body mass index and congenital heart defects. American Journal of Obstetrics & Gynecology. 2010;202(1):51.e1–51.e10. doi: 10.1016/j.ajog.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Mills J. L., Troendle J., Conley M. R., Carter T., Druschel C. M. Maternal obesity and congenital heart defects: a population-based study. American Journal of Clinical Nutrition. 2010;91(6):1543–1549. doi: 10.3945/ajcn.2009.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oddy W. H., De Klerk N. H., Miller M., Payne J., Bower C. Association of maternal pre-pregnancy weight with birth defects: evidence from a case-control study in Western Australia. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 2009;49(1):11–15. doi: 10.1111/j.1479-828X.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- 49.Khalil H. S., Saleh A. M., Subhani S. N. Maternal obesity and neonatal congenital cardiovascular defects. International Journal of Gynecology & Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics. 2008;102(3):232–236. doi: 10.1016/j.ijgo.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Shaw G. M., Carmichael S. L. Prepregnant obesity and risks of selected birth defects in offspring. Epidemiology. 2008;19(4):616–620. doi: 10.1097/EDE.0b013e3181761fa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waller D. K., Shaw G. M., Rasmussen S. A., Hobbs C. A., Canfield M. A., Siega-Riz A. M. Prepregnancy obesity as a risk factor for structural birth defects. Archives of Pediatrics & Adolescent Medicine. 2007;161(8):745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Frias M. L., Frias J. P., Bermejo E., Rodriguez-Pinilla E., Prieto L., Frias J. L. Pre-gestational maternal body mass index predicts an increased risk of congenital malformations in infants of mothers with gestational diabetes. Diabetic Medicine: A Journal of The British Diabetic Association. 2005;22(6):775–781. doi: 10.1111/j.1464-5491.2005.01492.x. [DOI] [PubMed] [Google Scholar]
- 53.Watkins M. L., Rasmussen S. A., Honein M. A., Botto L. D., Moore C. A. Maternal obesity and risk for birth defects. Pediatrics. 2003;111 (5 Pt 2):1152–1158. [PubMed] [Google Scholar]
- 54.Cedergren M. I., Selbing A. J., Kallen B. A. Risk factors for cardiovascular malformation--a study based on prospectively collected data. Scandinavian Journal of Work, Environment & Health. 2002;28(1):12–17. doi: 10.5271/sjweh.641. [DOI] [PubMed] [Google Scholar]
- 55.Watkins M. L., Botto L. D. Maternal prepregnancy weight and congenital heart defects in offspring. Epidemiology. 2001;12(4):439–446. doi: 10.1097/00001648-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Cai G., Sun X., Zhang L., Hong Q. Association between maternal body mass index and congenital heart defects in offspring: a systematic review. American Journal of Obstetrics & Gynecology. 2014;211(2):91–117. doi: 10.1016/j.ajog.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y., Chen Y., Feng Y., Yu D., Mo X. Association between maternal body mass index and congenital heart defects in infants: a meta-analysis. Congenital Heart Disease. 2018;13(2):271–281. doi: 10.1111/chd.12567. [DOI] [PubMed] [Google Scholar]
- 58.Tang X., Nick T. G., Cleves M. A., et al. Maternal obesity and tobacco use modify the impact of genetic variants on the occurrence of conotruncal heart defects. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0108903.e108903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catalano P. M. The impact of gestational diabetes and maternal obesity on the mother and her offspring. Journal of Developmental Origins of Health and Disease. 2010;1(4):208–215. doi: 10.1017/S2040174410000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen S., Iyer C., Meydani S. N. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. Journal of Perinatology: Official Journal of the California Perinatal Association. 2014;34(2):105–111. doi: 10.1038/jp.2013.153. [DOI] [PubMed] [Google Scholar]
- 61.Amirkhizi F., Siassi F., Djalali M., Shahraki S. H. Impaired enzymatic antioxidant defense in erythrocytes of women with general and abdominal obesity. Obesity Research & Clinical Practice. 2014;8(1):e26–e34. doi: 10.1016/j.orcp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Igosheva N., Abramov A. Y., Poston L., et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010074.e10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vayá A., Rivera L., Hernández-Mijares A., et al. Homocysteine levels in morbidly obese patients: its association with waist circumference and insulin resistance. Clinical Hemorheology and Microcirculation. 2012;52(1):49–56. doi: 10.3233/CH-2012-1544. [DOI] [PubMed] [Google Scholar]
- 64.Sánchez-Margalet V., Valle M., Ruz F. J., Gascón F., Mateo J., Goberna R. Elevated plasma total homocysteine levels in hyperinsulinemic obese subjects. The Journal of Nutritional Biochemistry. 2002;13(2):75–79. doi: 10.1016/S0955-2863(01)00197-8. [DOI] [PubMed] [Google Scholar]
- 65.Wang F., Wu Y., Quon M. J., Li X., Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. American Journal of Physiology-Endocrinology and Metabolism. 2015;309(5):E487–E499. doi: 10.1152/ajpendo.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J., Ma H., Tong C., et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2010;24(6):2066–2076. doi: 10.1096/fj.09-142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y., Yan X., Zhao J. X., et al. Maternal obesity induces fibrosis in fetal myocardium of sheep. American Journal of Physiology-Endocrinology and Metabolism. 2010;299(6):E968–E975. doi: 10.1152/ajpendo.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Q., Zhu C., Sun M., et al. Maternal obesity impairs fetal cardiomyocyte contractile function in sheep. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2019;33(2):2587–2598. doi: 10.1096/fj.201800988R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandadi M. R., Hua Y., Zhu M., et al. Influence of gestational overfeeding on myocardial proinflammatory mediators in fetal sheep heart. The Journal of Nutritional Biochemistry. 2013;24(11):1982–1990. doi: 10.1016/j.jnutbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Dong M., Zheng Q., Ford S. P., Nathanielsz P. W., Ren J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. Journal of Molecular and Cellular Cardiology. 2013;55(1):111–116. doi: 10.1016/j.yjmcc.2012.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scheme: the scheme of the meta-analysis. Supplementary Figure: figures of sensitivity analysis. Supplementary Table: the checklist of PRISMA Statement.