Abstract
Despite the association between brainstem lesions and coma, a mechanistic understanding of coma pathogenesis and recovery is lacking. We developed a coma model in the rat mimicking human brainstem coma, which allowed multimodal analysis of a brainstem tegmentum lesion’s effects on behavior, cortical electrophysiology, and global brain functional connectivity. After coma induction, we observed a transient period (~1h) of unresponsiveness accompanied by cortical burst-suppression. Comatose rats then gradually regained behavioral responsiveness concurrent with emergence of delta/theta-predominant cortical rhythms in primary somatosensory cortex. During the acute stage of coma recovery (~1 to 8h), longitudinal resting-state functional MRI revealed an increase in functional connectivity between subcortical arousal nuclei in the thalamus, basal forebrain, and basal ganglia and cortical regions implicated in awareness. This rat coma model provides an experimental platform to systematically study network-based mechanisms of coma pathogenesis and recovery, as well as to test targeted therapies aimed at promoting recovery of consciousness after coma.
Keywords: coma, consciousness, brainstem, arousal nuclei, animal model, functional connectivity
Graphical abstract

1. INTRODUCTION
More than one million people worldwide experience a coma every year due to brainstem lesions caused by trauma, stroke, and other severe brain injuries (1, 2). Many die from their injuries or never recover consciousness, remaining in a vegetative state (3). However, recovery of consciousness is possible in comatose patients who retain a sufficient number of subcortical projections to reactivate the cerebral cortex (4–7). For these patients, the cortex remains quiescent until ascending neural activity from subcortical arousal nuclei in the brainstem, thalamus, hypothalamus, or basal forebrain is restored (7–9). Yet, the relative contributions of the brainstem, thalamus, hypothalamus, and basal forebrain to the reemergence of consciousness after brainstem-lesion induced coma are unknown (9). Animal coma models could provide critical experimental platforms to study the neural mechanisms relevant to key brain state dynamic changes, which can help elucidate the neural basis for the reemergence of consciousness.
The neuroanatomic basis of consciousness has historically been studied using a lesional approach in which animal brains are examined under different surgical conditions. Removal of both cerebral hemispheres does not cause coma in rats, cats or dogs (10–13), with animals being able to right themselves (return to the upright position), eat and even groom. These findings demonstrate the challenge of producing an animal coma model, while also highlighting the critical role of subcortical brain lesions for coma induction. Brainstem transection and stimulation studies further demonstrate that, although medullary nuclei modulate vital functions (14), it is the pontomesencephalic tegmentum that is most involved in arousal (15, 16). Transections at this level result in cortical deactivation and the emergence of bursts (15), whereas stimulation of the pontomesencephalic tegmentum results in cortical excitability (16). Numerous animal studies have further verified that brainstem tegmentum lesions or inhibition are associated with altered consciousness (17–24). For example, a rodent model involving chemical lesions of the parabrachial-precoeruleus complex created a coma-like brain state with sub-1 Hz cortical EEG (17). Lesions involving the pontomesencephalic tegmentum have similarly been observed in human patients with coma (25–28).
Despite extensive animal and human evidence for the role of brainstem arousal nuclei in regulation of consciousness, few studies have investigated neurological state changes during coma induction or acute recovery of consciousness following brainstem lesions in patients or in animal models. A transient coma can be caused by administration of neuro-toxins (29–31), anesthetics (18, 32), or cholinergic agonists (33), but the induced coma state is brief (e.g. 5 – 10 min) (30), dose-dependent, and associated with high fatality rates. Hypoxic-ischemic brain injury models in rats (34–41) and dogs (42) can trigger coma, but the global neuronal injury precludes identification of specific brainstem nuclei whose lesioning causes coma and the subcortical circuits that are critical to recovery. Animal models of severe traumatic brain injury (43) provide an opportunity to study coma but involve multifocal lesions and multiple pathophysiological processes (e.g. contusions and axonal shearing). Therefore, new animal models are needed to mimic clinical brainstem injury and to produce a coma of sufficient duration to study mechanisms of recovery.
Here, we describe a diffuse brainstem tegmentum lesion in the rat that reproducibly generates a coma of sufficient duration to enable comprehensive behavioral, electrophysiological, and radiological characterization of the comatose brain. A vasoconstrictor, endothelin-1 (ET-1), was injected into the brainstem to cause ischemic injury to the brainstem tegmentum, resulting in infarction of multiple brainstem arousal nuclei, but preserving diencephalic, basal forebrain and cortical structures, as confirmed by T2-weighted magnetic resonance imaging (MRI) and histopathology. After coma induction, behavioral recovery was tracked quantitatively using a newly developed and validated Tübingen-Boston Rat Coma Scale (RCS). Cortical function and network connectivity were measured using local field potential (LFP) recordings and resting-state functional MRI (rs-fMRI), respectively, for up to 6–8 hours after induction. Using this multimodal approach, we aimed to answer the following questions: What are the initial electrophysiological signs of coma that occur in the cerebral cortex following brainstem injury? How do rats with brainstem coma behave immediately following the injury and during acute recovery? How do the electrophysiological properties of the cerebral cortex evolve in acute brainstem coma? Which subcortical and cortical networks reconnect as rats recover from brainstem coma?
2. METHODS
2. 1. Animal subjects
A total of 76 adult male Sprague Dawley rats (350 ± 50 g) underwent ET-1 injection (ET-1 injection group) into the mid-pons parenchyma (Fig 1C), at approximate coordinates AP −11, ML −0.5, DV −1.3 mm from the ventral surface of the brainstem. The outcomes of these animals are reported in Fig 1J. In summary, 68 rats entered the comatose state, whereas 8 did not, possibly due to technical failure during the injection. Of the 68 rats that entered the comatose state, 32 rats (47.1%) showed behavioral signs of recovery (i.e. the RCS score increased after coma induction) and were thus included in the present study: 6 in the behavior group, 6 in the LFP group, 14 in the rs-fMRI group, and 11 in the anatomic MRI group (5 rats were common to the rs-fMRI and the anatomical MRI groups). Additionally, 3 animals underwent ET-1 injection and concurrent calcium and multi-unit activity (MUA) recording (Ca2+-MUA group) to further characterize the phase of coma induction from a cortical level. Of note, 5 animals belonged simultaneously to both the anatomic and the functional MRI group (9 animals underwent rs-fMRI only, 6 animals underwent anatomic MRI only and 5 animals underwent both rs-fMRI and anatomic MRI). In addition to the animals in the behavior group, behavioral RCS evaluations were performed in the LFP, rs-fMRI, and anatomic MRI groups (albeit at lower temporal resolution and without pupillary light reflex testing in the LFP group), which allowed confirmation of recovery in all animals included in our results.
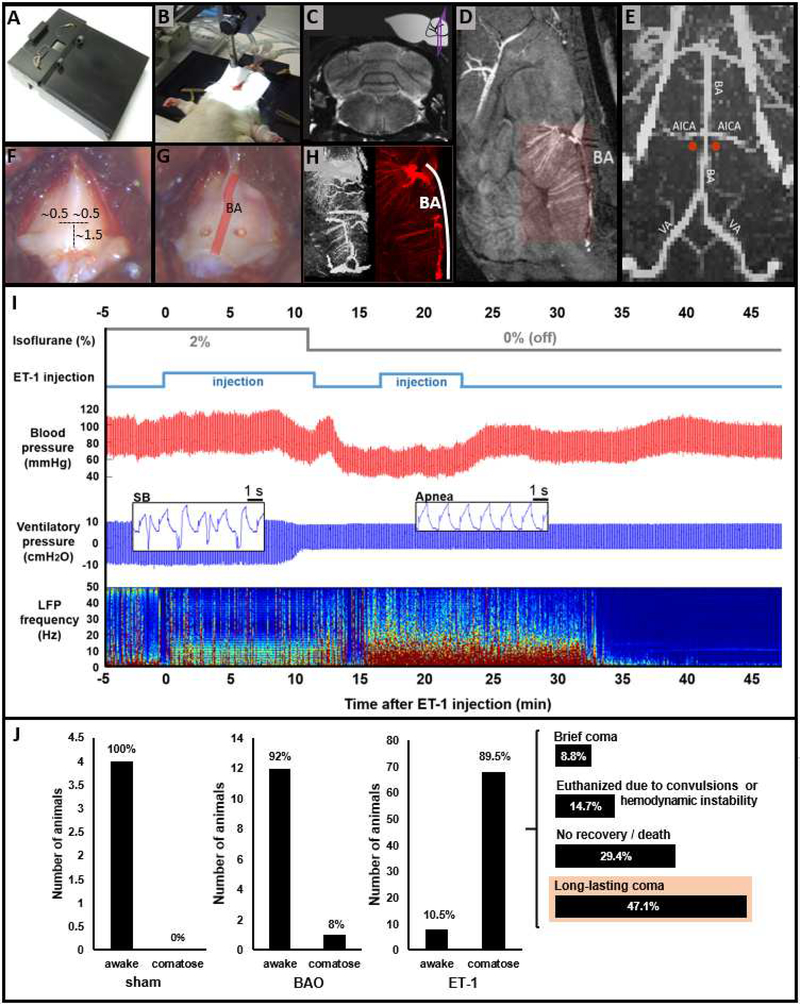
Fig 1. Methodology of coma induction and outcome, compared to two control conditions.
(A) Surgical bed designed for rats with dorsal implants and retractors to expose the basilar part of the occipital bone. (B) Typical setup of an anesthetized, mechanically ventilated rat during injection of endothelin-1 (ET-1) into the brainstem. (C) Approximate point of injection in a T2-weighted MRI image. (D) Sagittal view of an MRA of the rat brain highlighting the BA and penetrating branches. (E) 3D reconstruction of a high resolution MRA of the brainstem, indicating the approximate injection sites in relation to the BA (red dots). (F) Approximate coordinates from the lower edge of the basioccipital (in mm) to drill the craniotomies for ET1 injection. (G) View of the craniotomy sites lateral to the BA, whose inferior-superior course along the midline is shown in schematic form as a red line superimposed on the bone. (H) 3D reconstruction of the MRA of the BA territory observed from different angles. BA: basilar artery; AICA: anterior inferior cerebellar artery; VA: vertebral artery. (I) Physiological monitoring during coma induction in a representative animal. Time 0 represents the beginning of injection of the vasoconstrictor ET-1 into the brainstem parenchyma. Anesthesia, provided with isoflurane, was switched off immediately following detection of apnea and hypotension, which occurred within the first 15 min after ET-1 injection. Apnea was detected based upon a change in shape and amplitude of the ventilatory pressure waveform (spontaneous breathing [SB] inset vs. apnea inset). Cortical activity, as measured by local field potentials (LFP), increased immediately after withdrawal of the anesthetic, then abruptly vanished approximately 35 minutes after ET-1 injection, resulting in an isoelectric line (see Fig 3 for a more detailed description of the electrophysiological data). (J) Behavioral outcome of rats treated with sham surgery (injection of PBS into the brainstem) (‘sham’, N=4), BAO (‘BAO’, N=13), or injection of ET-1 into the brainstem (‘ET-1’, N=76), considered as either awake (fail to induce coma) or comatose. Sham rats showed a normal recovery; rats undergoing BAO awoke with certain abnormalities but not coma in ~92% of the cases; rats treated with ET-1 became comatose in ~90% of the cases (68 out of 76 animals). The long-term behavior of animals treated with ET-1 was variable, with 32 rats (~47% of 68) exhibiting a long-lasting coma with gradual recovery (named just “comatose animals” throughout the manuscript). ‘Brief coma’ defines absence of righting reflex for 1 – 2 hours only, ‘no recovery/death’ defines rats not showing any recovery of the RCS score or dying within a period of 6 hours; ‘long-lasting coma’ defines rats entering the coma state and exhibiting a gradual recovery of neurological function within a period of 6 hours. BAO: basilar artery occlusion.
Multiple additional groups of rats were studied as controls for comparison with the ET-1 injection group that exhibited the long-lasting coma. A cohort of adult male Sprague Dawly rats (350 ± 50 g) underwent a similar surgical procedure but with injection of PBS instead of ET-1 to serve as a sham group for behavioral purposes (sham group, n=4). A separate group of control animals was anesthetized with 2% isoflurane and scanned for a period of 6 h to serve as a control for the rs-fMRI group (rs-fMRI control group, n=11). A subset of rats in the control rs-fMRI group were also used as controls for the anatomical MRI analysis (anatomic MRI control group, n=9). Another group of rats underwent topical application of ET-1 to the surface of the brainstem without injection into the brainstem parenchyma (topical ET-1 control group, n=4). Finally, a group of rats underwent basilar artery occlusion (BAO) during development of the ET-1 injection model, and these BAO data are reported here as another behavioral control group (BAO control group, n=13). Table S1 provides a description of all groups and the number of animals used in each group.
2. 2. Chemicals
ET-1 (E7764, SIGMA-ALDRICH) was used at a concentration of 400 μM (100μg of ET-1 were diluted in 100μL of PBS). Caution should be considered to store the diluted ET-1 in the −80° freezer (the reported experiments use the ET-1 solution within less than 1 month storage). Also, the delivered ET-1 shows varied potency for vasoconstriction. Depending on the potency of the compound, a total volume ranging from 0.4 to 2 μL was injected into the brainstem through an infusion pump (every time a new batch of ET-1 was diluted, its efficacy was tested in 1 or 2 animals to calibrate the appropriate dosage). ET-1 is an endothelium-derived contracting factor(44, 45) that modifies the vascular tone, hence influencing local blood flow (46). ET-1 has been used to reliably cause stroke (typically cortical) by means of focal cerebral ischemia in multiple animal species (47–49).
2. 3. Animal procedures
All animal procedures were approved by the Animal Protection Committee of Tübingen (Regierungspräsidium Tübingen).
2. 3. 1. Viral injections for GCaMP-mediated calcium imaging studies
4-weeks old animals were anesthetized with 5% isoflurane in chamber. A small amount of an ophthalmic ointment was placed over the eyes to protect from drying. The anesthetized animals were transferred to a stereotaxic frame and kept anesthetized with 1.5–2 % isoflurane. The dorsal skull was exposed. The fissure Bregma was identified and used as a landmark to target the surface of the barrel cortex (AP 2.4, ML 4.9 mm). A ~ 0.8–1 mm diameter hole was drilled on the skull using an electric drill. A micro-injection pump was used to deliver 600 nL of the viral construct AAV5.Syn.GCaMP6f.WPRE.SV40 to the barrel cortex at a depth of 0.8 to 1.2 mm. After the surgery, animals were treated with a painkiller and antibiotic for 3 consecutive days, their weight was monitored and they were allowed to recover for 3–4 weeks, which corresponds to the time needed for the GCaMP to express in the target neuronal population (50, 51). Calcium imaging and coma induction were conducted in the adult animals after this resting period.
2. 3. 2. Animal preparation procedures prior to coma induction
Anesthesia was induced with 5% isoflurane in the adult animal in a chamber. Anesthetized animals were intubated with a 14 Gauge cannula inserted through the oral cavity to access the trachea. Rats were ventilated using an animal ventilator (CW-SAR-830/AP) at 60±1 breaths per minute, with an inspired oxygen concentration of 30%. Anesthesia was maintained with 2 – 2.5 % isoflurane for all surgical procedures until induction of coma. To monitor blood pressure, all animals underwent cannulation of the femoral artery with polyethylene tubing (PE-50, filled with heparin solution to prevent blood coagulation). Ventilatory pressure, end-tidal CO2, arterial blood pressure, heart rate and temperature were monitored continuously throughout the experiments.
2. 3. 3. Procedure for electrode/fiber implantation
For electrophysiology studies, a ~ 1 MΩ impedance and ~100 μm diameter tungsten electrode was implanted into the primary somatosensory cortex (S1 for forepaw or barrel cortex). The LFP recording in the S1 of rats has been routinely practiced with simultaneous fiber optic calcium recording (50, 51). For combined electrophysiology-calcium imaging studies, an optical fiber of ~200 μm diameter was lowered together with a tungsten electrode into the primary somatosensory cortex (barrel cortex). Detailed procedure has been described previously (50, 51). Briefly, the anesthetized rat was positioned in a stereotactic frame, the dorsal skull was exposed and a metal screw was placed on the occipital bone to serve both as reference and ground. The fissure Bregma was identified and used as a landmark to target the surface of the forepaw (AP 0.5, ML 4 mm), or barrel cortex (AP 2.4, ML 4.9 mm). A ~ 1.5 mm diameter burr hole was drilled on the skull and the dura mater was removed. The electrode (or the optical fiber+electrode) was lowered down to 0.9 – 1.2 mm from the brain surface (layer V of S1). An adhesive gel was used to fix the electrode to the skull and the skin was sutured around the implant.
2. 3. 4. Procedure for coma induction
The diffuse brainstem tegmentum coma model relies on vasoconstriction of the arteries irrigating the brainstem by means of injection of ET-1 (Fig S1). Importantly, during development of this ET-1 injection model, we first performed a series of BAO experiments in rats (Supplementary Note 1). In contrast to BAO caused by thrombi in human patients (52), which may result in a comatose state, we found that experimental BAO in rats does not reliably cause a comatose state (Fig 1J and Fig S2). Thus, for our ET-1 injection model, we aimed to target not only the basilar artery (BA), but also its penetrating branches that irrigate the rostral brainstem (see Figs 1D, 1E and H), producing a more diffuse brainstem tegmentum injury.
The procedure to induce coma through ET-1 injection was performed as follows. The anesthetized rat (2–2.5% isoflurane) was positioned in dorsal recumbence in a custom-made rat-surgery bed (Figs 1A and 1B), which allowed surgical procedures to be performed from a ventral approach even in animals with implants on the dorsal skull. Eyes were covered with an eye ointment throughout the experiment to prevent drying (in animals subjected to behavioral tests the eyes were cleaned to test corneal reflex and the ointment was applied again until the next assessment). After shaving the ventral neck, a longitudinal incision was made on the skin, the thyrohyoid muscles were dissected, the trachea was retracted laterally from the midline and the basilar part of the occipital bone (basioccipital) was exposed (Figs 1F and 1G). Two bilateral craniotomies of ~ 0.5 mm were performed on the basilar bone (Fig 1G), at the level of the mid-pons (Fig 1C). The stereotaxic coordinates were estimated based on the landmarks of the basilar bone (Fig 1F) and high resolution MRI confirmed the injection point at the brain parenchyma with approximate coordinates AP −11/11.5, ML ±0.5, DV 9 mm (−1.3 mm from the brain surface), based on the atlas of the rat brain (53). A 33 Gauge needle attached to a microsyringe was lowered with a stereotaxic system between 0.5 and 1.5 mm from the dura mater, and a volume between 0.4 and 2 μL of ET-1 solution was slowly injected into the brainstem (100–200nL/min, using a micro-infusion pump). The needle was left in place for 8 minutes after injection and was carefully removed afterward. A small amount of dental wax was used to cover the craniotomies. The wound was sutured and the analgesic Ketoprofen was administered subcutaneously at 5 mg/kg. The entire procedure for coma induction is shown in Movie S1. The word “post-coma” is used in the text accompanied by the number of minutes or hours to refer to the time elapsing from the moment of coma induction (i.e. withdrawal of anesthesia after observing physiological changes upon injection of ET-1).
2. 4. Behavioral analysis
We developed a coma scale to characterize rat behavior during coma induction and recovery. We aimed for the scale to resemble commonly used human coma scales (i.e. Glasgow Coma Scale (54, 55), the FOUR Score (56), and the Coma Recovery Scale-Revised (57)) while incorporating behavioral observations made by our investigator team in the first subset (20 animals) of rats that underwent coma induction. We name this scale the “Tübingen-Boston Rat Coma Scale” (RCS). The purpose of this scale was to quantitatively discriminate between different neurological states in the rat, from the unconscious unresponsive state to the conscious and responsive state. The following reflexes, physiological responses, and behavioral domains are assessed in the RCS (Table 1): eye blink, motor function (reflexive and purposeful), brainstem reflexes (pupil, corneal and pinna reflex), respiration, righting reflex, auditory response, and whisker movement (detailed discussion in Supplementary Note 2).
Table 1. Tübingen-Boston Rat Coma Scale.
Additional information relevant to the superscripts [1 to 9] can be found in Supplementary Information (Supplementary Note 2).
| TÜBINGEN-BOSTON RAT COMA SCALE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subscales | Minutes Post-Coma | |||||||||
| 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | |
| EYE BLINK SCALE | ||||||||||
| 2 - Blinks spontaneously1 | ||||||||||
| 1 - Blinks upon stimulation2 | ||||||||||
| 0 - No blinking (without directly touching the eye) | ||||||||||
| MOTOR FUNCTION3 | ||||||||||
| 4 - Voluntary walking | ||||||||||
| 3 - Isolated spontaneous movements (e.g. limbs or head) | ||||||||||
| 2 - Withdraws forepaw and/or hindpaw in response to noxious stimulation | ||||||||||
| 1 - Muscle contractions in response to noxious stimulation of the limbs | ||||||||||
| 0 - Absence of motor response to noxious stimulation | ||||||||||
| BRAINSTEM REFLEXES | ||||||||||
| 3 - Pupil4 AND corneal5 AND pinna6 reflexes present | ||||||||||
| 2 - Two of pupil/corneal/pinna reflexes present | ||||||||||
| 1 - Pupil OR corneal OR pinna reflex present | ||||||||||
| 0 - Absence of pupil, corneal and pinna reflexes | ||||||||||
| RESPIRATION | ||||||||||
| 3 - Not intubated, breaths with a regular pattern | ||||||||||
| 2 - Not intubated, breaths with an irregular pattern | ||||||||||
| 1 - Breathes above ventilator rate (initiates spontaneous breaths) | ||||||||||
| 0 - Breathes at ventilator rate or apnea (no spontaneous breaths) | ||||||||||
| RIGHTING REFLEX | ||||||||||
| 2 - Normal righting reflex7 | ||||||||||
| 1 - Partial righting reflex | ||||||||||
| 0 - None | ||||||||||
| AUDITORY RESPONSE | ||||||||||
| 1 - Auditory startle8 | ||||||||||
| 0 - None | ||||||||||
| WHISKER MOVEMENT | ||||||||||
| 1 - Spontaneous whisker movements9 | ||||||||||
| 0 - None | ||||||||||
| TOTAL SCORE | ||||||||||
A detailed characterization of behavioral coma recovery in the rat based on this scale, along with intra- and inter-rater reliability results, is provided in the Supplementary Note 2 and Fig S3. The RCS total score ranges from 0, representing coma, loss of brainstem reflexes, loss of motor function, and apnea, to 16, reflecting a normal conscious rat (i.e. exhibiting all reflexes and voluntary movements observed in healthy animals). The RCS can be completed in 1 minute by a trained investigator (Movie S2). For validation, we tested the RCS in healthy animals anesthetized at different isoflurane levels, the scores of which are reported in Fig 2A and 2D. We used the Wilcoxon-Mann-Whitney test to assess for differences in RCS scores in different experimental groups (Fig 2C). A comprehensive summary of behavioral observations made during development and validation of the RCS is provided in Supplementary Note 2.
Fig 2. Behavioral analysis.
(A) The graph shows the individual (in gray/black) and averaged (in red or blue) RCS scores at different post-surgery times of rats after coma induction (n=6; red color) or after sham surgery (n=4; blue color). Note that all animals were ventilated, so the maximum RCS score possible was 14 (maximum Respiration score was 1). The shadowed areas represent the standard deviation of the data. (B) Averaged category-specific RCS scores before and after coma induction (n=6). Insets show the microscopy-based assessment of pupillary light reflex (a brainstem reflex) at 0.5 and 2 hours after coma induction. (C) RCS scores of rats under 5 different conditions: awake (n=5), anesthesia with 1% isoflurane (1% ISO; n=5), anesthesia with 2.5% isoflurane (2.5% ISO; n=5), 1 hour after coma induction (1h coma; n=6), and 7 hours after coma induction (7h coma, n=6). Error bars represent standard error of the mean. (D) Category-specific RCS score of a representative anesthetized rat under different isoflurane concentrations.
In addition, to study stress levels in the animals, 300 μL of blood was collected via the femoral catheter from 4 comatose animals at 3 hours post-coma and from 4 control animals anesthetized with 1% isoflurane. The blood was centrifuged and plasma was separated and aliquoted in 2 samples (50 μL each) per animal for reproducibility testing purposes. Samples we stored at −80 °C and sent to a steroid analysis laboratory (Steroidlabor im Analysenzentrum, Heidelberg, Germany), where a radioimmunoassay detection method was used to analyze the corticosterone content of the samples.
2. 5. Acquisition and processing of electrophysiological (LFP and MUA) and calcium signals
LFPs were recorded with one of two electrophysiology systems (Biopac-Acknowledge or CED-Spike2), which showed similar results and confirmed the reliability of the recordings. When using Biopac (MP150, EEG100C module), the LFP signals were amplified 5000 times and recorded at 5000 samples per second, while with CED (1401), the LFP signals were pre-amplified 25 times at the headstage followed by a 20 times amplification using an MCP-PLUS Alpha Ohmega amplifier (total amplification = 500), and recorded at 16000 or 25000 samples per second. The GCaMP-dependent fluorescence signal was recorded using CED-Spike2 at 1000 samples per second. LFP signals were originally acquired on the whole spectrum allowed by the given sampling rate and record length (0.0016 to 2500 Hz), but were then filtered between 0.5 Hz and 49 Hz to avoid baseline and electrical noise, respectively. Multi-unit activity (MUA) was acquired through the WaveMark function in Spike2 using a high-pass filter time constant of 3.8 ms and a threshold of 0.1 mV.
An initial electrophysiological record was acquired prior to coma induction to ensure a stable signal. LFP/MUA/calcium recordings were acquired from 3 animals during coma induction (Fig S4) and LFP was obtained from all animals in the electrophysiological study every hour post-coma in runs of 10 minutes. The behavioral state of the animal was assessed every 30 min with the RCS to test associations between behavioral scores and electrophysiological parameters at each post-coma time-point.
The LFP signals were processed using MATLAB (Natick, MA). A power spectrum of the whole LFP recording was computed for frequencies between 0.5 and 49.5 Hz using time-frequency decomposition (timefreq) based on wavelets with 2-second windows (58) (Fig 3A and 3B). The power of the specific electrophysiological bands delta, theta, alpha, beta and gamma was calculated as frequencies between 0.5 to 4, 4 to 7, 7 to 15, 15 to 31, and 31 to 49 Hz, and plotted at different post-coma times (Fig 3D). The raw LFP signal was additionally plotted to detect the electrical waveforms from the cortex (Fig 3C). The correlation between the LFP power and the neurological score was computed using MATLAB (corr2 function) (Fig 3E). The Hilbert transform was applied to specific LFP frequency bands to obtain the phase and the amplitude of the delta, theta and alpha bands. A graph showing the amplitude of theta or alpha at each phase of delta (phase-amplitude coupling) is shown in Fig 3F and Fig 3G. The delta phases at which the amplitude of theta and alpha were largest were used to extract the delta/theta and delta/alpha phase-locked-amplitude coupling at each post-coma time. Finally, the phase-amplitude coupling values extracted from the different comatose animals were averaged at each post-coma time and the standard deviation of the mean was calculated (Fig 3H).
Fig 3. Electrophysiological assessment of comatose rats.
(A) Power spectra of cortical local field potential (LFP) recordings from a healthy anesthetized control rat at different isoflurane (iso) concentrations. (B) Representative LFP power spectra at different times after coma induction (without isoflurane). (C) LFP traces from a representative comatose rat (same as in B) at different times after coma induction. (D) Graph showing the mean LFP power at different frequency bands (delta: 0.5–4 Hz; theta: 4–7 Hz; alpha: 7–15Hz; beta: 15–31 Hz; gamma: 31–49 Hz) during coma recovery (n=6). Error bars represent standard error of the mean. (E) 3-dimensional plot showing the relationship between LFP power, post-coma time and neurological score (different colors represent different animals). (F) Scheme exemplifying the acquisition of phase-amplitude coupling from the LFP data. (G) Phase-amplitude coupling (delta phase / theta amplitude (in blue) and delta phase / alpha amplitude (in green)) at different post-coma times in a representative animal. (H) Group-level phase-coupling analysis along post-coma time. Shadowed areas in H represent standard deviation of the mean.
2. 6. Acquisition of magnetic resonance imaging (MRI) data
All images were acquired with a 14.1 T/26 cm magnet (Magnex, Oxford) interfaced to an Avance III console (Bruker, Ettlingen), and 12 cm diameter gradient capable of providing 100 G/cm with a rise time of 150 μs (Resonance Research). Trans-receiver surface coils with elliptical shape of ~ 2 × 2.7 cm minor and major axis, respectively, were used to acquire rs-fMRI images. Animals studied with MRI underwent an additional preparation step consisting on filling the ear canal with a fluoride paste to reduce the magnetic susceptibility artifacts at the air-tissue interface.
For quantitative analysis and localization of infarcted tissue, anatomical scans of the comatose animals were acquired between 4 and 6 hours after coma induction. A T2-weighted Rapid Acquisition with Relaxation Enhancement (RARE) sequence was used to identify lesions (i.e. cytotoxic edema) in the brain, with the following parameters: effective echo time (TE), 38 ms; repetition time (TR), 2s; matrix size 128×128; in-plane resolution, 200 μm; slice thickness, 1 mm; RARE factor, 8. Typically, 42 times averaged 24 coronal slices were acquired in a scan time of 22 min 24 s.
A gradient-echo Fast Low Angle Shot (FLASH) sequence was used for visualization of the brain vasculature (MR angiography (MRA)) with the following parameters: flip angle, 50°; TE, 1.867 ms; TR, 20 ms.
To study brain connectivity changes during acute recovery from coma, a rs-fMRI scan was performed every ~60 min, starting at hour 1 and up to hour 8 after coma induction. A 3D-EPI sequence was used to map the T2*-weighted MRI signal with the following parameters: TE, 12.5 ms; TR, 1s); matrix size, 48×48×32; resolution, 400×400×600 μm (600 μm slice thickness). Typically, the fMRI signal was recorded for 925 TRs in a scan time of 15 min 25 s. Additionally, an anatomic RARE image (TE, 9 ms; TR, 4000 ms; matrix size 128×128; in-plane resolution, 150 μm; slice thickness, 600 μm; RARE factor, 8) was acquired matching the geometry of the 3D-EPI for registration purposes.
2. 7. Anatomic image processing and analysis
All anatomic images were registered to a template using AFNI software (3dAllineate). MATLAB software was used to quantitatively identify the infarcted regions from the anatomic MRI in the comatose versus anesthetized control rats based on T2 signal intensity. Fourteen regions of interest (ROIs) were defined based on a rat brain atlas (53), including 11 brainstem arousal nuclei (28) (55), 2 additional subcortical nuclei and 1 cortical region. Additionally, an ROI in the visual cortex served as reference or control ROI. Whole brain images (i.e. all slices grouped as a whole) were first normalized using the Matlab function mat2gray, and the average intensity of each ROI was calculated and divided by the intensity of the control ROI, for each individual rat. The final intensity values of each ROI were then averaged among the comatose and control groups to provide a comparative measure of ROI infarction between both conditions, and the standard error was calculated for both groups. Additionally, a percentage map was created by comparing mean MR images of the comatose and control groups. For visualization purposes, a 30% threshold was used on the overlay map (Fig 4A, middle panel), which corresponds, approximately, to two times the standard deviation of the averaged T2-weighted MRI signal over the arousal nuclei in the comatose group (averaged standard deviation over arousal nuclei = 0.16). A 2-sample t-test was computed between the comatose and control animals to assess for significant infarcted areas. Correction for false discovery rate (FDR) included multiple comparisons analysis with randomization and permutation of 10,000 simulations (3dttest++ -Clustsim).
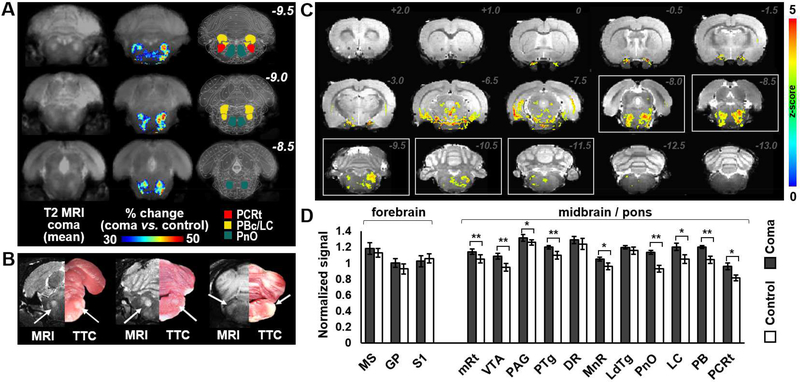
Fig 4. Anatomic correlates of brainstem coma in the rat.
(A) Averaged T2-weighted (T2) MRI of the coronal brainstem images from 11 comatose rats (left column); overlay showing areas with more than 30% T2 signal increase (i.e. infarcted areas, ≥ 2 x standard deviation) in the comatose rats, as compared to 9 healthy anesthetized controls (middle column); and regions of interest overlaid in the rat brain atlas for reference (right column). (B) TTC-stained brain slices of a representative comatose rat compared to its mirrored T2-weighted MRI (right and left panels respectively). Areas of histopathological infarction in the comatose animals correspond to regions of T2 signal increase (arrows). (C) Statistically thresholded overlay (visible voxels present a p-value <0.025, false discovery rate corrected), color-coded based on the Z-score from a 2-sample t-test between the T2-weighted intensity map of the comatose vs. control group. White boxes delineate slices with significant infarcts in arousal nuclei identified in comatose animals. (D) Normalized T2-weighted signal intensity within arousal nuclei of the pons and midbrain, as well as within regions of the forebrain, in comatose rats (n=11) as compared to control rats (n=9). Error bars represent standard error of the mean. Abbreviations: DR: dorsal raphe; GP: globus pallidus; LC: locus coeruleus; LDTg: latero-dorsal tegmental nucleus; MnR: medial raphe; mRt: mesencephalic reticular formation; MS: medial septum; PAG: periaqueductal gray; PBc: parabrachial complex; PCRt: parvicellular reticular nucleus (~ parafacial zone). PnO: pontis oralis (oral part); PTg: pedunculo-pontine tegmental nucleus; S1: primary somatosensory cortex; VTA: ventral tegmental area. **: p-value<0.005, *: p-value<0.01.
2. 8. Histology
To confirm that T2-signal changes on the anatomic MR images represented infarction in the brain, the histopathologic stain TTC (2,3,5-triphenyltetrazolium chloride) was performed in the brains of 4 comatose rats from the anatomical MRI experimental group. Briefly, anesthetized rats were perfused with PBS only and the brain was removed and kept at −80 °C for 10 min, followed by 5 min at −20 °C. The brain was cut in 1 mm slices and exposed to 1% TTC (1g TTC in 100 mL PBS) at 37 °C for ~20 min (until a reddish color was observed in the tissue). Slices were moved to 4% paraformaldehyde solution and a surgical microscope was used for photographing after 5 to 7 days.
2. 9. Functional connectivity image processing and analysis
A combination of AFNI (59) and Lipsia (60) functions was used to process the rs-fMRI datasets. Two types of analysis were performed to identify the nuclei that potentially contributed to recovery from coma: eigenvector centrality mapping (ECM) and traditional region-based correlation analysis.
ECM calculates the degree of correlation between each voxel and every other voxel in the network, similar to degree centrality but additionally taking into account the hierarchy of each connection to assign higher values to those voxels that are connected to central hubs within the network (61). From each rs-fMRI scan, ECM generates a map with the relative connectivity values of each voxel within the brain. (61). The ECM analysis was performed on every rs-fMRI scan at each post-coma time (15 min rs-fMRI scans were generated every hour after coma induction), which identified several regions showing an increase in connectivity rank as the function of time (Fig 5). First, all datasets were aligned using AFNI software (3dVolreg, 3dAllineate) and a mask was manually drawn for each slice to cover the midbrain, forebrain and all cortical regions (cerebellum and brainstem excluded). Next, Lipsia software was used to correct and regress motion artifacts (vmovcorrection, vcovariates, vresiduals) within and between runs. A despiking step was also included (vdespike), and time filtering was applied (vpreprocess) to keep frequencies between 0.1 and 0.01 Hz. After pre-processing, ECM was performed (vecm) to measure a relative correlation value of each voxel with the rest of the brain. As scans were acquired progressively after induction of coma, it was possible to calculate the slope of the correlation values acquired at each post-coma time. A linear fitting of the time-dependent correlation values for each voxel was computed in AFNI (3dNLfim). The result was a brain map in which the intensity at each voxel indicates the recovery rate of functional connectivity, which constitutes a novel method to represent connectivity dynamic changes in a single brain map. Only voxels where the error of the fitting was below 5% were included in the future steps of analysis (i.e. averaging or statistical tests). Fig S5 shows a schematic with all the (pre)processing functions used in AFNI and LIPSIA during rs-fMRI analysis. Fig 5A and Fig S5 show the main steps involved in the ECM analysis for acquisition of the slope map.
Fig 5. Analysis of whole brain rs-fMRI in rats recovering from coma, compared to healthy anesthetized control animals.
(A) Experimental design and general principle of the slope map. The text in the middle provides information about the dimensionality of the data and the meaning of the voxel at each step. (B) Averaged slope maps of the comatose rats (top panel) and the control rats (anesthetized with 2% isoflurane, bottom panel). (C) Brain map showing the z-statistic from the two-tailed two-sample t-test between coma and control (anesthetized with 2% isoflurane) slope maps.
For region-based analysis of connectivity at different post-coma times, we analyzed the mean BOLD signal time-courses (pre-processed with motion correction, despiking and 0.1–0.01 Hz time-filtering) of 7 subcortical ROIs that were selected a priori based on the current literature in mammals arousal systems (62): basal forebrain, striatum, globus pallidus, central thalamus, reticular thalamus and posterior lateral hypothalamus. We also performed region-based analysis on association cortical regions that are components of the rat default mode network (Cg and RS) (63). Finally, we used primary somatosensory cortex (S1) to compare the connectivity changes of primary versus higher-order cortices. The corr2 MATLAB function was used to calculate the correlation between seeds for each animal at each time. The average correlation value in rats scanned at specific post-coma times was used to show the time-dependent degree of connectivity between specific ROIs. Fig S6 shows a map with all the regions used during voxel-wise and seed-based rs-fMRI analysis (presented in Fig 6).
Fig 6. Region-specific analysis of longitudinal changes in rs-fMRI connectivity.
(A) Averaged slope (increase/decrease of connectivity during the 6-hour study period) for regions of interest (ROIs) in basal forebrain, basal ganglia, thalamus, hypothalamus and cerebral cortex in rats recovering from coma (dark bars) and in control rats (white bars). (B) Histogram showing the distribution of voxels in the brain of comatose or healthy anesthetized control rats according to their connectivity change (slope value) during the 6-hour study period. (C) Graph summarizing functional connections that increased continuously (solid black lines) and intermittently (dashed grey lines) during the 5-hour period of coma recovery. (D) Seed-based connectivity results from 5 different seeds across 9 brain regions, at different post-coma times. The number at the top of each graph represents the maximum connectivity value observed during the seed-based study. Error bars represent standard error of the mean. Abbreviations: Acb: nucleus accumbens; B: nucleus basalis of Meynert; BF: basal forebrain; C-Pu: caudate-putamen; Cg: cingulate cortex; CThal: central thalamus; DB: diagonal band of Broca; GP: globus pallidus; LH: lateral hypothalamus; MS: medial septum; PO: preoptic nuclei; RS: retrosplenial cortex; RThal: reticular thalamus; SI: substantia innominata; S1: primary somatosensory cortex; VP: ventral pallidum. **: p-value<0.01, *: p-value<0.05
To analyze the motion profiles of the MRI scans, three rotational motion regressors (motion report output from the vmovcorrection function (Lipsia)) were used to acquire a final value representative of the degree of motion (64) detected in each MRI scan. Briefly, the three column vectors (“regressors”) were fitted into the following functionusing MATLAB:
where xi = regressori and d(x) (in MATLAB: diff(x)) returns a new vector of length = length(x)-1 that is the result of subtracting adjacent elements in x.
Control animals anesthetized with 2% isoflurane exhibited variable motion profiles at different scans along 5 hours of scanning. After calculating the motion profile of each scan, the fMRI datasets were re-ordered in an increasing motion profile order, to mimic the motion profile trend of animals evolving from coma, and the slope map was acquired in the same way as for the comatose group (Fig S7).
Finally, in order to test for differences between groups, a two-tailed, two-sample t-test was run on MATLAB (ttest2) for quantification and on AFNI (3dttest++) for visualization (i.e. statistic brain maps). Note that, while false discovery rate was corrected in the anatomical datasets using “–clustsim” (previous section), the functional connectivity slope maps were subjected to a different procedure to ensure reliability of the reported results. As the final measure is not connectivity but increase of connectivity (relative measure from one scan to the next), we masked out all voxels where the linear fitting of the concatenated connectivity values rendered an error above 5%. The final t-test was applied to a set of volumes (slope maps) from which unreliable voxels across different scans had been screened out in a previous step.
3. RESULTS
3. 1. Diffuse injury to the brainstem tegmentum by ET-1 injection produces coma in the rat
Given the high neuroanatomic variability of infarcted areas in brainstem coma patients (25, 52, 65, 66), we injected a vasoconstrictor, ET-1, into the parenchyma of the pontomedullary (PM) junction to alter the vascular network that irrigates the rostral brainstem tegmentum (Figs 1D, 1E and 1H). The diffuse brainstem lesion induced coma in the animals. Figure 1I shows physiological changes in blood pressure, ventilatory pressure and cortical activity observed during coma induction through ET-1 injection. In contrast to other strategies, ET-1-mediated brainstem injury caused coma in ~90% of the animals, with 47% of those exhibiting a long-lasting coma with possibilities of recovery (Figure 1J).
Noteworthy is that all animals subjected to the procedure, independent of the long-term outcome, experienced a transient drop of blood pressure and cessation of spontaneous breathing. The fact that a subgroup of these animals (~10% of a total n=76) woke up within the next 5–15 minutes after withdrawal of isoflurane with focal infarcts in the medulla and caudal pons, but no infarcts in the rostral brainstem tegmentum (Fig. S2C), indicates that coma induction is not caused by ET-1 induced medulla/caudal pons infarcts, or the brief period of hypotension (Fig. 1I). A second control with topical application of ET-1 on the ventral brain surface (i.e., more diffusible to the global cerebrovasculature via the CSF) caused transient vasoconstriction (10–20 min) of the major vessels (e.g., basilar artery (BA) or anterior inferior cerebellar artery (AICA) etc. Fig. S1G), and produced broad infarcts beyond the brainstem regions (Fig. S2D), but did not result in a long-lasting coma, with a much lower incidence of coma onset than the ET-1 injection cases. Also, we observed similar hypotension without causing coma in a subgroup of animals subjected to ET-1 injection that did not result in brainstem infarction (described above and in Supplementary Note 3), in rats undergoing BA occlusion (BAO) and, to an extent, in animals undergoing injection of phosphate buffered saline (PBS) in similar coordinates (Fig S2A-B). While these animals showed comparable hemodynamic changes during surgical manipulation of the brainstem, the behavioral recovery was unambiguously different from that of ET-1 treated rats (Figs S2B and Fig 1J), providing a negative control condition for this model to rule out a generalized hypoperfusion in the brain (similar to global ischemia) as the mechanism of coma. These results demonstrate that the ET-1 injection provides a reliable surgical procedure to induce coma in rats via diffuse injury of the rostral brainstem tegmentum.
3. 2. Comatose animals gradually regain neurological function that is tracked by the Rat Coma Scale
To build upon prior efforts to characterize rat behavior during states of altered consciousness (67, 68), we developed a Rat Coma Scale (RCS) for quantitative assessment of rat behavior (Table 1). The RCS was designed to be similar to the human Glasgow Coma Scale (GCS) (54), Full Outline of Unresponsiveness (FOUR) (56) score, and Coma Recovery Scale-Revised (CRS-R) (57), to optimize the translational relevance of the model (see Methods and Supplementary Material). It is important to acknowledge here that there is no current consensus regarding a definitive, gold-standard test that proves conscious awareness in the rat; hence, we anticipate that the RCS will need to be revised in the future as such evidence becomes available.
Rats in the behavioral study underwent repeated RCS assessments at each time post-coma starting at ~30 min after ET-1 injection and until at least 7 hours post-coma, in intervals of 0.5–1 hours. Prior to injection of ET-1, all rats had a maximal behavioral score on the RCS with clear exploratory whisking behavior. After injection of ET-1, the behavioral changes observed in rats were consistent with the absence of arousal and awareness, with apnea occurring in all rats entering the coma state (artificial ventilation provided continuously). At the time of the first RCS assessment, RCS scores ranged from 0 to 2 (median = 1), with rats showing, at most, motor reflexes (i.e. withdrawal of the limb in response to painful stimulation). The pupillary light reflex was typically absent during the first hour. Between 1 and 2 hours after coma induction, rats recovered spontaneous breathing (but continued to receive mechanical ventilation). At this time, the pupil reflex also returned in most rats. During the following hours (typically at 2 to 3 hours post-coma), the pinna reflex returned, as well as the corneal reflex in at least one eye. At hour 5–6 post-coma, some rats showed spontaneous movement of a limb or lifting up the head, which lasted for 1 or 2 seconds. Although recovery of the righting reflex was not observed in most rats during the first 8 hours, in a few cases it was possible to identify an attempt at keeping an upright posture, which was defined in the RCS as partial righting reflex, characterized by a slow righting movement of part of the body in response to bending the animal around its longitudinal axis. Fig 2A shows RCS scores for comatose rats during the first 7 hours post-coma and for sham animals, which fully recovered within one hour after the sham surgery. Fig 2B shows category-specific RCS scores from 12 comatose rats, and Fig 2D shows the corresponding category-specific RCS scores for a representative anesthetized rat under different anesthesia levels (0 to 4 % isoflurane). At 7 hours, the RCS score of the rats undergoing ET-1 injection was comparable to those of healthy rats anesthetized with 1% isoflurane, which is significantly higher than the RCS score of comatose rats at hour 1 post-coma, indicating a clear sign of recovery (Fig 2C). Two animals were behaviorally assessed at 24 hours, showing partial or complete righting reflex and weak attempts to initiate walking, suggesting ongoing recovery in neurological function. A video containing all reflexes tested in the RCS is provided as Movie S2. Movie S3 shows an example of a comatose rat recovering during the first 4 hours.
Double-blinded analysis was performed to assess the RCS scores from multiple examiners to test the reliability of behavioral assessment (Fig S3, details in methods section).
To assess the level of stress in the animals, the blood corticosterone content was measured at 3 hours post-coma (RCS = 5–6), with levels comparable to those of the corticosterone measured in rats anesthetized with 1 % isoflurane (125.4 ± 13.5 and 130.8 ± 9.7 ng/mL, respectively).
3. 3. Cortical activity evolves from isoelectricity to slow wave-predominant rhythms during the first 4 hours after coma induction
Cortical electrophysiological assessment was performed to confirm the comatose state and rule out the possibility of a locked-in state caused by damage to the descending motor pathways. Like the comatose state, a locked-in state may be characterized by an absence of behavioral responses (69). However, individuals who are locked-in retain arousal and awareness, and hence show normal or near-normal cortical electrophysiological features (70, 71). In the rats studied here, electrophysiological data recorded from primary somatosensory cortex (S1) demonstrated profound cortical suppression, which rules out the possibility of a locked-in state. During surgery, prior to ET-1 injection, rats showed burst suppression events (Fig. 1I, lower trace), typical of deep anesthesia. Approximately 10 to 15 minutes after ET-1 injection, the isoflurane was withdrawn (after blood pressure drop and cease of spontaneous breath), and cortical activity increased (Fig 1I, lower trace), with a spectrum that resembled EEG patterns typical from awake or lightly anesthetized animals, although rats were completely unresponsive at this stage and had no spontaneous breathing with short-term hypotension (~15min, under ventilation and with sufficient O2 supplies). The paradoxical increase in cortical activity remained until approximately 20–35 minutes after ET-1 injection. At this moment, the cortex became isoelectric or transited to a burst-suppression pattern (Fig. 1I, lower trace and Fig 3B and 3C). Cortical silence/inactivation was preceded by spreading depression/depolarization in 3 comatose animals that were subjected to recording of intracellular calcium and multi-unit activity during the induction of coma (Fig S4). Interestingly, iso-electrical lines were observed after cessation of the ET-1 vasoconstriction effect (Fig S1G) and the return to baseline blood pressure (Fig. 1I), indicating a delayed coma onset following the immediate physiological state changes. The timing of cortical deactivation after injury to the tegmentum in this rat coma model is different from the fast onset of isoelectricity (in 2–3 min following insults) observed after global ischemia (i.e. occlusion of common carotid arteries (72, 73) or cardiac arrest (37, 39, 74)). The initial period of isoelectricity (20–35 minutes after ET-1 injection) (75, 76) was observed in 83% of the rats. In the other 17%, sparse bursting spikes (77) were observed in the initial recordings, although a short period of isoelectricity might have occurred before starting the acquisition. The frequency of the bursting spikes increased as a function of post-coma time, similar to the burst suppression pattern observed in rats anesthetized with 2 – 2.5% isoflurane (Fig 3A), although the comatose burst spikes were smaller in amplitude. Within 2 to 4 hours, this pattern evolved in most rats to a background of mainly delta and theta activity (Fig 3D). Fig 3C shows the LFP recordings of a representative rat (same animal as in Fig 3B). Increased RCS scores correlated with LFP power during coma recovery, with a correlation coefficient of 0.73 ± 0.06 (Fig 3E). Phase-coupling analysis (Fig 3F) demonstrated an increase in the synchronization of the theta and alpha bands with delta phase, as animals recovered behavioral responses during the first 6 hours post-coma (Fig 3G-H). These results demonstrate that cortical activity is regained in a gradual manner after brainstem coma, with the most pronounced changes occurring within the first 3–4 hours.
3. 4. Coma is associated with a diffuse brainstem tegmentum injury
To localize the brainstem lesions that caused coma, anatomical MRI was performed in comatose rats 4 to 6 hours after the surgery (ET-1 long-lasting coma) and in healthy anesthetized control animals. Focal T2-hyperintense lesions were detected in the comatose animals in several pontine and midbrain arousal nuclei, including the mesencephalic reticular formation, pedunculopontine tegmental nucleus, locus coeruleus, parabrachial nuclear complex, parvicellular reticular nucleus (parafacial zone), ventral tegmental area and the pontis oralis (pontine reticular formation), among others (Fig 4A and 4C). Fig 4D shows the differences in T2-weighted signal in specific regions of interest (ROIs) in the comatose rats as compared to healthy anesthetized animals. Histopathological stains of brainstem tissue with triphenyl tetrazolium chloride (TTC) in rats that had been scanned with MRI showed lesion patterns consistent with the MRI findings (Fig 4B), confirming the validity of the T2-weighted MR images for detection of infarcted brain tissue. In contrast to the brainstem injury, the basal forebrain, basal ganglia, thalamus, hypothalamus and cerebral cortex remained highly preserved (Figs 4C and 4D), allowing these structures to be functionally studied during coma recovery. These results indicate that lesions involving arousal nuclei at the rostral brainstem tegmentum are crucial for the coma induction observed in the rat coma model.
3. 5. Subcortical nuclei reconnect during the acute phase of coma recovery
Rs-fMRI was performed in the comatose animals at several post-coma times and in a healthy anesthetized control group at similar time intervals to track potential changes in brain connectivity during early recovery from coma. Longitudinal voxel-wise (ECM-based) and ROI-based connectivity analysis was performed on the acquired functional scans (see Methods).
Animals anesthetized with constant 2% isoflurane showed low global connectivity fluctuation over time. In contrast, rats recovering from coma showed several brain areas with a significant longitudinal increase in signal temporal correlation (i.e. connectivity) with the rest of the brain (Fig 5C and Figs 6A and 6B). This increase in connectivity was pronounced in the thalamus, the basal forebrain and the basal ganglia, with significant differences observed in the reticular thalamus, nucleus accumbens, nucleus basalis of Meynert and striatum (i.e. caudate-putamen) (Fig 5C and Fig 6A).
Similarly, in an ROI-specific seed-based analysis, a continuous increase of correlation was identified between the paired basal ganglia to central thalamus, or basal ganglia to reticular thalamic nuclei during the acute phase of coma recovery, suggesting a crucial involvement of the striato-pallidal-thalamic network (Figs 6C and 6D). In addition, the central thalamus gradually connected with the basal forebrain and with the primary somatosensory cortex (S1) (Figs 6C and 6D). The S1 and cingulate cortex (Cg) experienced their most significant increase of correlation at 4–5 hours post-coma with the thalamic nuclei and the globus pallidus. An increase in the correlation with the central thalamus at this time was also observed from the retrosplenial cortex, although the latter did not show net increases of connectivity with other regions. Notably, the posterior aspect of the lateral hypothalamus did not show an increase of connectivity with the other studied ROIs. All ROI-specific connectivity changes during acute coma recovery are summarized in Fig 6C.
4. DISCUSSION
We report a brainstem tegmentum lesion model in the rat that creates a comatose state of sufficient duration to enable longitudinal analysis of animal behavior, cortical electrophysiology, and brain network functional connectivity during coma recovery. (A cost-benefit assessment of the model is provided as Supplementary Note 4.) We developed and validated a behavioral scale, the Tübingen-Boston Rat Coma Scale, to quantitatively track coma recovery in the rat, analogous to coma scales that are commonly used in clinical practice to track recovery in humans. We found that increasing RCS scores were associated with increasing electrophysiological activity in the cerebral cortex, which evolved from transient isoelectricity to bursting spikes during the first hour post-coma, and to continuous slow wave (i.e. delta-theta) activity within the first 3 hours of recording. Concurrently, we observed an increase in global functional connectivity in the thalamus, basal forebrain, and basal ganglia during coma recovery. Seed-based correlation analysis revealed a reemergence of connectivity between the central thalamus and the striato-pallidal complex, as well as between the thalamus, basal forebrain, cingulate cortex and retrosplenial cortex. These findings suggest an essential role of the thalamus, basal forebrain, and basal ganglia in reactivating the cerebral cortex and restoring behavioral responsiveness after ascending brainstem inputs are disrupted.
Relevance of a hyperacute model of coma and early electrophysiological changes
The brainstem tegmentum lesion model of coma described here provides a platform to elucidate mechanisms of coma pathogenesis and recovery within a hyperacute 6–8 hour time window that has particular clinical-translational relevance. This is a time window during which therapeutic interventions aimed at mitigating neural network disruption and restoring consciousness are actively being investigated in patients with brainstem injuries (78). Yet little is known about acute changes in brain electrophysiology and functional connectivity in the immediate aftermath of brainstem coma, because electrophysiological and fMRI brain mapping studies are typically not feasible or safe to perform during the initial resuscitation and stabilization of a critically ill comatose patient (79). This hyperacute time window (i.e. the first two hours) is particularly relevant to the interpretation of our electrophysiological recordings in the rat cortex, which revealed isoelectricity followed by burst spikes. These findings are uncommon in human comatose patients, except those whose coma is caused by global hypoxic-ischemic injury (80–82). However, a few exceptional case reports support the hypothesis that a burst-suppression pattern may transiently occur after brainstem injury. In one case, a patient experienced recurrent coma that manifested electrophysiologically with a burst-suppression pattern and frontal intermittent rhythmic delta activity upon BA spasm and rostral brainstem ischemia (83). It is also notable that a burst suppression pattern can be observed when the cerebral cortex is intact but isolated from posterior structures in animals (84) and human subjects (85, 86). It is possible that isoelectricity and bursting spikes have not been observed in human patients with brainstem coma because EEG data are not typically acquired during the hyperacute stage of injury. Alternatively, it is possible that our observations reflect species-specific electrophysiological dynamics within the rat brain in the setting of a brainstem lesion. EEG studies of human patients in the hyperacute stage of brainstem coma are needed to clarify whether the human cortex undergoes a similar progression from isoelectricity to burst suppression before the emergence of a continuous delta-theta rhythm. The immediate transient period of cortical excitability following withdrawal of anesthesia after ET-1 injection (Fig 1I) possibly reflects a delay in cortical deactivation upon a potential ischemic insult. Calcium imaging and multi-unit recording in three animals during coma induction showed increased MUA firing rate followed by spreading depression, and then full cortical silence (Fig S4). The nature of this delayed cortical deactivation with respect to the physiological changes that mark the onset of coma in our model will be further investigated.
Diffuse brainstem damage as the underlying cause of coma
The rat brainstem coma model is based upon injection of the vasoconstrictor ET-1 into the dorsomedial brainstem tegmentum, which leads to reduction in the diameter of pontine penetrator vessels branching from the BA (87). The vasoconstriction effect of ET-1 is mediated by ET-1 receptors in the smooth muscle and endothelial cells of arteries (88, 89). The resulting ischemic insult is targeted to monoaminergic, cholinergic, and glutamatergic nuclei within the brainstem tegmentum that are known to mediate arousal, and hence consciousness, in animals and humans (90). Importantly, neurons and astrocytes both express ET-1 receptors (91, 92), and therefore ET-1 interaction with the local neuro-glial network may contribute to neuronal dysfunction. Nevertheless, ischemic infarction of the brainstem tegmentum, as verified by radiological and histopathological analyses in this study, is believed to be the primary mechanism of coma induction. A transient period of global brain hypoperfusion cannot be ruled out in the present study, but, if present, it is not the source of coma, as a group of animals subjected to the same procedure and exhibiting the same hypotension but without dorsal/rostral brainstem infarction did not enter the comatose state. Further supporting the brainstem-specific cause of coma is the fact that animals in which the ET-1 diffused from the brainstem to broader regions, causing massive injuries in the forebrain (Fig. S2D), had a lower incidence of coma or experienced a much faster recovery.
Using the ET-1 approach to disrupt the brainstem vasculature, we found that the neuroanatomic localization of the coma-causing lesion in our rat model was consistent with that of coma-causing brainstem lesions in prior animal and human studies, which have consistently highlighted the critical role of the brainstem tegmentum in consciousness (17, 25, 28). Specifically, we found radiological and histopathological evidence of infarction within the mesencephalic reticular formation, ventral tegmental area, pedunculotegmental nucleus, median raphe, locus coeruleus, parabrachial nucleus, pontis oralis and parvocellular reticular nucleus (i.e. the parafacial zone (93, 94)) (Supplementary Note 5). In contrast to the pathophysiological contribution of this diffuse brainstem tegmentum lesion to coma induction, the infarction observed ventrally near the point of ET-1 injection is not believed to contribute to coma induction, as animals subjected to ET-1 treatment but not entering the coma state (ET-1 control cases, Fig 1J “awake”) showed local infarction at the level where the vasoconstrictor was injected (i.e., the caudal pons, Fig S2C and Supplementary Note 3). The specific contribution of each lesioned arousal nucleus to coma induction remains to be further investigated, which is why we refer to the model as a “diffuse brainstem tegmentum lesion” model of coma. In contrast to prior studies targeting individual arousal nuclei within the brainstem tegmentum, our coma model involves a large “diffuse” lesion that encompasses multiple arousal nuclei, which induced a robust comatose state verified by behavioral and physiological tests. The fact that lesions involving individual arousal nuclei did not reliably cause coma in previous works (e.g. lesions of the ventral tegmental area, locus coeruleus, tuberomammillary nucleus, basal forebrain or suprachiasmatic nucleus caused alteration of the sleep-wake cycle but not coma (95–97)) further suggests that a diffuse lesion involving multiple brainstem arousal nuclei may be key to the pathogenesis of a long-lasting comatose state. The similar anatomic boundaries of the infarcted brainstem region in this rat coma model to that of coma-causing brainstem lesions in humans (25–28) suggests that this animal model is well suited for use in future translational studies that will aim to identify the subcortical circuits that facilitate recovery of consciousness in rats and humans with coma-causing brainstem tegmentum lesions. Whether the diffuse topography of brainstem tegmentum lesions is necessary for the development of long-lasting coma will be investigated in future animal studies in which focal lesions to different combinations of individual arousal nuclei will be tested for their behavioral outcomes.
Connectivity changes during early recovery post-coma
Our rs-fMRI functional connectivity results provide compelling evidence of the neuroscientific and translational opportunities that are created by the new coma model. Specifically, our findings add to a growing body of evidence about the critical role of a striato-pallidal-thalamic-basal forebrain network in promoting recovery from coma. Consistent with the mesocircuit hypothesis (9), rats recovering from coma experienced a continuous increase in connectivity between the striatum, globus pallidus and thalamus, accompanied by an increase in whole brain connectivity of these and basal forebrain nuclei. The contribution of the basal ganglia to recovery of consciousness has also been observed in humans emerging from anesthesia-induced coma (98, 99). In this work, the additional reconnection of the basal forebrain with the central thalamus suggests a potential role of basal forebrain arousal nuclei in coma recovery, particularly from the nucleus accumbens and nucleus basalis of Meynert (although probably to a lesser extent than the thalamic-basal ganglia interactions, as reported by the ROI-based analysis, Fig. 6D: central thalamus graph). This observation is consistent with animal studies showing that the basal forebrain plays a crucial role in emergence from anesthesia (100), in modulating sleep-wake circadian rhythms (101) and in the overall arousal level of the brain (17, 102).
In addition to reintegration of a striato-pallidal-thalamic-basal forebrain network, we also observed reintegration of a thalamocortical network (103) during coma recovery in the rat. This finding builds upon prior studies showing that thalamocortical networks contribute to recovery of consciousness after severe brain injury and anesthesia (8, 104, 105). However, while thalamocortical networks are widely considered to be a substrate of consciousness (8, 9, 104, 106), there is ongoing debate as to whether these networks are crucial for arousal or whether they primarily mediate higher-order cognition (17, 98). Interestingly, we observed a longitudinal increase in cortical connectivity not only in the central thalamic nuclei, which are a key node of thalamocortical networks and whose electrical stimulation promotes cognitive enhancement in animals (107) and humans (7, 108), but also in the reticular thalamic nucleus, which encapsulates the thalamus and gates thalamocortical signaling (109). Indeed, the reticular thalamic nucleus exhibited one of the most significant increases in connectivity during coma recovery of all the subcortical nodes that were studied (Fig 6). Given that the reticular thalamic nucleus contains GABAergic inhibitory neurons whose optogenetic stimulation during the awake state results in a slow-wave EEG (110), thalamic bursts and cortical spindles (111), our observed correlation between reticular thalamic nucleus connectivity and coma recovery may seem counterintuitive. However, the reticular nucleus of the thalamus not only inhibits excitatory thalamocortical neurons during sleep, but also inhibits local inhibitory thalamic neurons during wakefulness, generating a net increase in cortical activity (109). A circuit-based electrophysiological interpretation of our results is beyond the scope of the present study, but our connectivity results suggest that during cortical reactivation and coma recovery, inhibition of local inhibitory thalamic neurons by the thalamic reticular nucleus predominates over its inhibition of excitatory thalamocortical neurons. Also noteworthy is that the recovery of arousal during the acute post-coma stage may depend, to an extent, on thalamocortical connectivity changes. Multi-electrode recordings will be needed to further clarify the cortical neuronal synchronization and desynchronization patterns during post-coma recovery.
Unexpectedly, we did not detect an increase in connectivity between the hypothalamus and cerebral cortex during coma recovery. The hypothalamus is a key hub in the arousal network (16, 112–114), a homeostatic control center (115), and has been shown to connect extensively with the cerebral cortex (116). One potential explanation for this reduced participation of the hypothalamus in recovery from brainstem tegmentum coma is the inhibitory inputs it receives from the reticular thalamus (110) and the basal forebrain (111). Alternatively, the hypothalamus is a large and heterogeneous region that contains diverse neuronal populations with broadly different homeostatic functions (115, 117, 118). The challenges of imaging the hypothalamus in small animals have been previously reported (119). The lack of spatial specificity in rs-fMRI global connectivity mapping may not detect small clusters of hypothalamic neurons that contribute to coma recovery. It is also notable that the ventral location of the hypothalamus within the diencephalon is especially vulnerable to potential magnetic field inhomogeneities derived from the active breathing-induced resonance frequency offset (120, 121). Finally, it is possible that the brainstem tegmentum lesion extended into the posterior aspect of the hypothalamus without being detected by the T2-weighted MRI scan. The use of more specific techniques, such as calcium imaging, that target specific neuronal populations (e.g. GABAergic or orexinergic neurons) and that are free of potential magnetic field artifacts may clarify the role of hypothalamic networks in rats recovering from brainstem coma.
An additional insight regarding brainstem coma recovery in the rat was generated by comparing the dynamic functional connectivity changes across the evaluation periods in subcortical arousal nuclei and different regions of the cerebral cortex. Whereas thalamic, basal forebrain, and striato-pallidal nuclei showed a continuous increase in connectivity during recovery, a consistent increase in connectivity between subcortical arousal nuclei with association cortices – particularly Cg - was only observed after hour 4 post-coma, which corresponds to an epoch within the recovery period when more complex behavioral responses may become noticeable (e.g. righting reflex) and when background activity reappears in the electrophysiological recordings. Interestingly, a recent study reported a critical role of the prefrontal cortex (specifically the prelimbic cortex, an area immediately ventral to the cingulate cortex in the rat) on the transition to wakefulness from general anesthesia (122). Given that the Cg is an integrative hub for cognition (123, 124) and a key node of the rat default mode network (DMN) (63), our results suggest a link in the rat brain between reemergence of behavioral responses in the later phase of coma recovery and reintegration of subcortical and cortical networks that support arousal and awareness. Though the connectivity properties of the rat DMN are only beginning to be understood, there is compelling evidence in humans that reemergence of DMN connectivity is essential for recovery of consciousness after severe brain injury (125, 126). It is also notable that the region of association cortex that is believed to represent the hub node of the DMN differs in animals and humans. Whereas human rs-fMRI and diffusion MRI studies indicate that the posterior Cg and precuneus (i.e. posteromedial complex) comprise the hub node of the DMN, it is the retrosplenial cortex in rats that is believed to play this role. The Cg has been identified as a region of association cortex that modulates cognition in rats (127–131), but evolutionary differences in Cg anatomy (e.g. rats do not possess Brodmann areas 23 and 31), and phenomenological differences between rodent and human consciousness (132) indicate that there are likely fundamental differences between the rat DMN and human DMN. Inferences pertaining to the translational human relevance of connectivity findings in the rat DMN must therefore be interpreted with caution. Nevertheless, our multimodal observations of cortical reactivation after brainstem coma provide evidence for a reintegration of subcortical arousal networks and the DMN in the rat brain. This cortical reactivation, and its accompanying behavioral improvement, appears to rely upon a constellation of subcortical arousal nodes within the thalamus, basal forebrain, and basal ganglia, along with cortical nodes within association regions of cerebral cortex.
5. LIMITATIONS AND FUTURE DIRECTIONS
Several limitations pertaining to the multimodal analysis of brain function should be considered when interpreting the results of this study. Although the use of rs-fMRI to study brain function has been applied to rodents (133, 134), it remains challenging to account for potential artifacts associated with spontaneous breathing in non-anesthetized rodents. This spontaneous breathing could lead to B0 field inhomogeneity, such as the voluntary respiration-induced resonance offset, and special care is needed to dampen these motion artifacts (120, 135). Although several regression steps were used to acquire a reliable analysis, motion artifact remains an issue in the acquisition of rs-fMRI once the animal recovers spontaneous breathing. To investigate the degree of interference between motion and the observed results, we calculated the motion profiles of the fMRI scans acquired from the comatose rats and from control rats anesthetized with 2% isoflurane. The varying motion profile observed in the control rats was in a range similar to that observed in the comatose rats, which allowed us to re-order the scans in a way that the motion content at each time matched that observed during coma recovery. The slope map for the re-ordered control scans was then re-calculated, which provided a “motion reference” map (Fig S7). This motion reference map was substantially different from the coma recovery slope map, diminishing concern about motion interference in our data.
It is also important to emphasize that the network-based connectivity results reported here are based upon macroscale measurements of large groups of neurons. Circuit-based insights into the connectivity and functional dynamics of individual subcortical neurons within the arousal network will require future studies with neuron-specific techniques. This limitation is particularly relevant to the interpretation of our hypothalamic connectivity results, which unexpectedly did not show an increase in global connectivity during coma recovery, as discussed above. With regard to the rs-fMRI analysis of brain network function, it should also be noted that the whole-brain ECM-based connectivity was a relative measure among brain voxels. The highlighted voxels in the slope connectivity map are the ones in which the dynamic connectivity degree increased the most in comparison with others (the ECM analysis provides a rank, not an absolute value). Thus, functional nuclei that contribute to coma recovery might not have been readily identified here if they were overshadowed by the more significant connectivity changes observed in the thalamus, basal forebrain, and basal ganglia (see Supplementary Note 6 for a more detailed discussion about the interpretation of the ECM analysis).
Although converging evidence from our control studies suggests that coma induction is attributable to the diffuse brainstem tegmentum lesion, we acknowledge that a pathophysiologic contribution to coma induction from global cerebral hypoperfusion cannot be definitively ruled out. Future studies should clarify the potential existence and extent of such injury in the present model. Nevertheless, the observation that 10% of the ET-1 treated cases (i.e. waking animals) experienced similar systemic hypotension and even, in some cases, diffuse ET-1-induced infarcts through the brain, demonstrates that an episode of global hypoperfusion, if present, is not the main cause of coma in this rat coma model.
6. CONCLUSION
We developed a method to induce coma via a diffuse brainstem tegmentum lesion in adult rats and provided verification of brainstem lesion anatomy, behavioral assessment and examination of brain electrophysiology and network connectivity. Unlike other approaches, this animal model makes it possible to perform multimodal analysis longitudinally during the hyperacute phase (first 6–8 hours) of natural coma recovery in the rat, which is currently not feasible in human patients in the clinical environment. The rs-fMRI connectivity analysis identified a crucial interaction between the thalamus, basal forebrain, and basal ganglia in reactivating the cerebral cortex during coma recovery. Future lines of study dedicated to stimulating specific thalamic, basal forebrain, and basal ganglionic subcortical arousal nuclei will aim to clarify their relative contributions to coma recovery. Establishing a reliable coma model in the rat may ultimately facilitate the development of targeted therapies aimed at promoting recovery of consciousness in humans with brainstem injuries.
Supplementary Material
Legend of the RCS:
The observation time for assessment of spontaneous blink should be of around 3 minutes, in the absence of stimulation (in healthy rats it ranges from several seconds to 2–3 minutes).
Stimulation to assess evoked blink consists of gently stimulating any part of the animal (without touching the eye –corneal reflex is assessed separately). Some rats blink easily in response to stimulation of the pinna (with or without parallel shaking of the head), therefore attention must be put on the eye response when testing the pinna reflex in order to score a positive evoked blink.
Stimulation consists of pinching forepaw/hindpaw, applying a force of ~3 N for less than 0.5 s. Positive reactions may occur as muscle contractions or withdrawal of the limb.
Pupil contraction is observed with optical microscope. The eye is subjected to different light intensities from darkness (eye is covered) to full brightness (increasing the intensity of the microscope light source). Pupil reflex is considered present if the eye exhibits a decrement in pupil diameter upon light intensification.
The tip of a cotton swab is approached to the corneal epithelium, medial and lateral canthus of the eye. The reflex is considered present if a blink follows stimulation.
A thin (~ 1 mm diameter) semi-flexible rod with blunt tip (to prevent damage) is used to lightly touch or tickle the pinna of the animal. The reflex is positive if the animal flicks ears or shakes head after stimulation.
The test consists of bending the body of the animal to a side (taking it out of its normal upright position). Righting reflex is present if the animal is able to reorient itself (integrating movements of several muscles in head, trunk and limbs). Partial righting reflex is considered when only the upper or the lower body rotates to recover the upright position, in contrast to the synchronized whole-body repositioning observed in a normal righting reflex.
Stimulation is provided with hand clapping over the head of the animal. Startle reflex is considered present if the animal shows any reaction to the sound, e.g. body trembles.
Natural whisker movement defined as a relatively high amplitude movement of all the whiskers in one pad.
Acknowledgements:
This research was supported by internal funding from Max Planck Society for X.Y., the United States National Institute of Neurological Disorders and Stroke (K23NS094538) and the James S. McDonnell Foundation for B.L.E., and the graduate training center of neuroscience in Tuebingen for P.P. We thank Dr. N. Avdievitch and Ms. H. Schulz for technical support, Drs. E. Weiler, Y. Tang and Ms. S. Fischer for animal protocol and maintenance support, and Dr. G. Lohmann and the AFNI team for the software support.
Footnotes
Additional notes: Reflexes that can be tested on both sides (e.g. those concerning eyes, ears or paws) are considered present if one of the two (left or right) counterparts exhibits the positive response. If one reflex is tested several times, the best score counts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. [DOI] [PubMed] [Google Scholar]
- 2.Edlow JA, Rabinstein A, Traub SJ, Wijdicks EF. Diagnosis of reversible causes of coma. Lancet. 2014;384(9959):2064–76. [DOI] [PubMed] [Google Scholar]
- 3.Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys Med Rehabil. 2018. [DOI] [PubMed] [Google Scholar]
- 4.Thengone DJ, Voss HU, Fridman EA, Schiff ND. Local changes in network structure contribute to late communication recovery after severe brain injury. Sci Transl Med. 2016;8(368):368re5. [DOI] [PubMed] [Google Scholar]
- 5.Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog Brain Res. 2009;177:73–88. [DOI] [PubMed] [Google Scholar]
- 6.Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10(2):99–114. [DOI] [PubMed] [Google Scholar]
- 7.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448(7153):600–3. [DOI] [PubMed] [Google Scholar]
- 8.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355(9217):1790–1. [DOI] [PubMed] [Google Scholar]
- 9.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bignall KE, Schramm L. Behavior of chronically decerebrated kittens. Exp Neurol. 1974;42(3):519–31. [DOI] [PubMed] [Google Scholar]
- 11.Bell DJ, Horne EA, Magee HE. The decerebrate rat. J Physiol. 1933;78(2):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods JW. Behavior of Chronic Decerebrate Rats. J Neurophysiol. 1964;27:635–44. [DOI] [PubMed] [Google Scholar]
- 13.Tonkovic-Capin M, Krolo M, Stuth EA, Hopp FA, Zuperku EJ. Improved method of canine decerebration. J Appl Physiol (1985). 1998;85(2):747–50. [DOI] [PubMed] [Google Scholar]
- 14.Gromysz H, Karczewski WA. The effects of brainstem transection on respiratory activity in the rabbit. Acta Neurobiol Exp (Wars). 1981;41(2):225–35. [PubMed] [Google Scholar]
- 15.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr Clin Neurophysiol. 1949;1(4):475–86. [PubMed] [Google Scholar]
- 16.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–73. [PubMed] [Google Scholar]
- 17.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(5):933–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abulafia R, Zalkind V, Devor M. Cerebral activity during the anesthesia-like state induced by mesopontine microinjection of pentobarbital. J Neurosci. 2009;29(21):7053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsley DB, Schreiner LH, Knowles WB, Magoun HW. Behavioral and EEG changes following chronic brain stem lesions in the cat. Electroencephalogr Clin Neurophysiol. 1950;2(4):483–98. [DOI] [PubMed] [Google Scholar]
- 20.Hayes RL, Pechura CM, Katayama Y, Povlishock JT, Giebel ML, Becker DP. Activation of pontine cholinergic sites implicated in unconsciousness following cerebral concussion in the cat. Science. 1984;223(4633):301–3. [DOI] [PubMed] [Google Scholar]
- 21.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458(2):285–302. [DOI] [PubMed] [Google Scholar]
- 22.Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124(3):473–96. [DOI] [PubMed] [Google Scholar]
- 23.Hobson JA. The Effects of Chronic Brain-Stem Lesions on Cortical and Muscular Activity during Sleep and Waking in the Cat. Electroencephalogr Clin Neurophysiol. 1965;19:41–62. [DOI] [PubMed] [Google Scholar]
- 24.Yang SR, Hu ZZ, Luo YJ, Zhao YN, Sun HX, Yin D, et al. The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol. 2018;16(4):e2002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126(Pt 7):1524–36. [DOI] [PubMed] [Google Scholar]
- 26.Rosenblum WI. Immediate, irreversible, posttraumatic coma: a review indicating that bilateral brainstem injury rather than widespread hemispheric damage is essential for its production. J Neuropathol Exp Neurol. 2015;74(3):198–202. [DOI] [PubMed] [Google Scholar]
- 27.Edlow BL, Haynes RL, Takahashi E, Klein JP, Cummings P, Benner T, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72(6):505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87(23):2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonobe T, Chenuel B, Cooper TK, Haouzi P. Immediate and Long-Term Outcome of Acute H2S Intoxication Induced Coma in Unanesthetized Rats: Effects of Methylene Blue. PLoS One. 2015;10(6):e0131340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clin Toxicol (Phila). 2015;53(6):525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamaoki S, Suzuki H, Okada M, Fukui N, Isobe M, Saito T. Development of an experimental rat model of hyperammonemic encephalopathy and evaluation of the effects of rifaximin. Eur J Pharmacol. 2016;779:168–76. [DOI] [PubMed] [Google Scholar]
- 32.Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94(1):101–12. [DOI] [PubMed] [Google Scholar]
- 33.Katayama Y, Tsubokawa T, Abekura M, Hayes RL, Becker DP. Coma induced by cholinergic activation of a restricted region in the pontine reticular formation--a model of reversible forms of coma. Neurol Med Chir (Tokyo). 1986;26(1):1–10. [DOI] [PubMed] [Google Scholar]
- 34.Shoykhet M, Simons DJ, Alexander H, Hosler C, Kochanek PM, Clark RS. Thalamocortical dysfunction and thalamic injury after asphyxial cardiac arrest in developing rats. J Neurosci. 2012;32(14):4972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manole MD, Kochanek PM, Bayir H, Alexander H, Dezfulian C, Fink EL, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr Res. 2014;75(2):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15(6):1032–9. [DOI] [PubMed] [Google Scholar]
- 37.Jia X, Koenig MA, Shin HC, Zhen G, Yamashita S, Thakor NV, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111(1):166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai K, Nitecka L, Ruetzler CA, Nagashima G, Joo F, Mies G, et al. Global cerebral ischemia associated with cardiac arrest in the rat: I. Dynamics of early neuronal changes. J Cereb Blood Flow Metab. 1992;12(2):238–49. [DOI] [PubMed] [Google Scholar]
- 39.Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord. 2000;15 Suppl 1:14–21. [DOI] [PubMed] [Google Scholar]
- 40.Muthuswamy J, Kimura T, Ding MC, Geocadin R, Hanley DF, Thakor NV. Vulnerability of the thalamic somatosensory pathway after prolonged global hypoxic-ischemic injury. Neuroscience. 2002;115(3):917–29. [DOI] [PubMed] [Google Scholar]
- 41.Liachenko S, Tang P, Hamilton RL, Xu Y. A reproducible model of circulatory arrest and remote resuscitation in rats for NMR investigation. Stroke. 1998;29(6):1229–38; [DOI] [PubMed] [Google Scholar]
- 42.Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10(1):57–70. [DOI] [PubMed] [Google Scholar]
- 43.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. [DOI] [PubMed] [Google Scholar]
- 45.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6(4):S188–91. [DOI] [PubMed] [Google Scholar]
- 46.Kramer BK, Ackermann M, Kohler SM, Riegger GA. Role of endothelin in hypertension. Clin Investig. 1994;72(2):88–93. [DOI] [PubMed] [Google Scholar]
- 47.Ansari S, Azari H, Caldwell KJ, Regenhardt RW, Hedna VS, Waters MF, et al. Endothelin-1 induced middle cerebral artery occlusion model for ischemic stroke with laser Doppler flowmetry guidance in rat. J Vis Exp. 2013(72). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virley D, Hadingham SJ, Roberts JC, Farnfield B, Elliott H, Whelan G, et al. A new primate model of focal stroke: endothelin-1-induced middle cerebral artery occlusion and reperfusion in the common marmoset. J Cereb Blood Flow Metab. 2004;24(1):24–41. [DOI] [PubMed] [Google Scholar]
- 49.Roome RB, Bartlett RF, Jeffers M, Xiong J, Corbett D, Vanderluit JL. A reproducible Endothelin-1 model of forelimb motor cortex stroke in the mouse. J Neurosci Methods. 2014;233:34–44. [DOI] [PubMed] [Google Scholar]
- 50.He Y, Wang M, Chen X, Pohmann R, Polimeni JR, Scheffler K, et al. Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI Spatiotemporal Dynamics and Their Correlation with Neuronal Intracellular Calcium Signals. Neuron. 2018;97(4):925–39 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M, He Y, Sejnowski TJ, Yu X. Brain-state dependent astrocytic Ca(2+) signals are coupled to both positive and negative BOLD-fMRI signals. Proc Natl Acad Sci U S A. 2018;115(7):E1647–E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Campe G, Regli F, Bogousslavsky J. Heralding manifestations of basilar artery occlusion with lethal or severe stroke. J Neurol Neurosurg Psychiatry. 2003;74(12):1621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam; Boston;: Academic Press/Elsevier; 2007. [Google Scholar]
- 54.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4. [DOI] [PubMed] [Google Scholar]
- 55.Okamura K Glasgow Coma Scale flow chart: a beginner’s guide. Br J Nurs. 2014;23(20):1068–73. [DOI] [PubMed] [Google Scholar]
- 56.Wijdicks EF, Rabinstein AA, Bamlet WR, Mandrekar JN. FOUR score and Glasgow Coma Scale in predicting outcome of comatose patients: a pooled analysis. Neurology. 2011;77(1):84–5. [DOI] [PubMed] [Google Scholar]
- 57.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–9. [DOI] [PubMed] [Google Scholar]
- 58.van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. J Neurosci Methods. 2007;162(1–2):49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 60.Lohmann G, Muller K, Bosch V, Mentzel H, Hessler S, Chen L, et al. LIPSIA--a new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph. 2001;25(6):449–57. [DOI] [PubMed] [Google Scholar]
- 61.Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One. 2010;5(4):e10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scammell TE, Arrigoni E, Lipton JO. Neural Circuitry of Wakefulness and Sleep. Neuron. 2017;93(4):747–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012;109(10):3979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spisak T, Jakab A, Kis SA, Opposits G, Aranyi C, Berenyi E, et al. Voxel-wise motion artifacts in population-level whole-brain connectivity analysis of resting-state FMRI. PLoS One. 2014;9(9):e104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demel SL, Broderick JP. Basilar Occlusion Syndromes: An Update. Neurohospitalist. 2015;5(3):142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolukisa M, Ozdemir Gultekin T, Eryigit Baran G, Aralasmak A, Kocaman G, Gursoy AE, et al. One-year follow-up in patients with brainstem infarction due to large-artery atherothrombosis. Neuropsychiatr Dis Treat. 2015;11:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reed SJ, Plourde G, Tobin S, Chapman CA. Partial antagonism of propofol anaesthesia by physostigmine in rats is associated with potentiation of fast (80–200 Hz) oscillations in the thalamus. Br J Anaesth. 2013;110(4):646–53. [DOI] [PubMed] [Google Scholar]
- 68.Taylor NE, Van Dort CJ, Kenny JD, Pei J, Guidera JA, Vlasov KY, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsao JW, Hemphill JC s, Johnston SC, Smith WS, Bonovich DC. Initial Glasgow Coma Scale score predicts outcome following thrombolysis for posterior circulation stroke. Arch Neurol. 2005;62(7):1126–9. [DOI] [PubMed] [Google Scholar]
- 70.Hawkes CH, Bryan-Smyth L. The electroencephalogram in the “locked-in” syndrome. Neurology. 1974;24(11):1015–8. [DOI] [PubMed] [Google Scholar]
- 71.Zeman A What is consciousness and what does it mean for the persistent vegetative state? Adv Clin Neurosci Rehabil 2003;3:12–4. [Google Scholar]
- 72.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10(3):267–72. [DOI] [PubMed] [Google Scholar]
- 73.Fortuna S, Pestalozza S, Lorenzini P, Bisso GM, Morelli L, Michalek H. Transient global brain hypoxia-ischemia in adult rats: neuronal damage, glial proliferation, and alterations in inositol phospholipid hydrolysis. Neurochem Int. 1997;31(4):563–9. [DOI] [PubMed] [Google Scholar]
- 74.Bauer G, Trinka E, Kaplan PW. EEG patterns in hypoxic encephalopathies (post-cardiac arrest syndrome): fluctuations, transitions, and reactions. J Clin Neurophysiol. 2013;30(5):477–89. [DOI] [PubMed] [Google Scholar]
- 75.Robert F, Mumenthaler M. [Criteria of brain death. Spinal reflexes in 45 personal studies]. Schweiz Med Wochenschr. 1977;107(10):335–41. [PubMed] [Google Scholar]
- 76.Kroeger D, Florea B, Amzica F. Human brain activity patterns beyond the isoelectric line of extreme deep coma. PLoS One. 2013;8(9):e75257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis LD, Ching S, Weiner VS, Peterfreund RA, Eskandar EN, Cash SS, et al. Local cortical dynamics of burst suppression in the anaesthetized brain. Brain. 2013;136(Pt 9):2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith DH, Hicks R, Povlishock JT. Therapy development for diffuse axonal injury. J Neurotrauma. 2013;30(5):307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edlow BL, Chatelle C, Spencer CA, Chu CJ, Bodien YG, O’Connor KL, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Synek VM. Prognostically Important Eeg Coma Patterns in Diffuse Anoxic and Traumatic Encephalopathies in Adults. Journal of Clinical Neurophysiology. 1988;5(2):161–74. [DOI] [PubMed] [Google Scholar]
- 81.Bagnato S, Boccagni C, Prestandrea C, Sant’Angelo A, Castiglione A, Galardi G. Prognostic value of standard EEG in traumatic and non-traumatic disorders of consciousness following coma. Clin Neurophysiol. 2010;121(3):274–80. [DOI] [PubMed] [Google Scholar]
- 82.Brenner RP. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11(5):271–84. [DOI] [PubMed] [Google Scholar]
- 83.Frequin ST, Linssen WH, Pasman JW, Hommes OR, Merx HL. Recurrent prolonged coma due to basilar artery migraine. A case report. Headache. 1991;31(2):75–81. [DOI] [PubMed] [Google Scholar]
- 84.Ingvar DH. Electrical activity of isolated cortex in the unanesthetized cat with intact brain stem. Acta Physiol Scand. 1955;33(2–3):151–68. [DOI] [PubMed] [Google Scholar]
- 85.Henry CE, Scoville WB. Suppression-burst activity from isolated cerebral cortex in man. Electroencephalogr Clin Neurophysiol. 1952;4(1):1–22. [DOI] [PubMed] [Google Scholar]
- 86.Kharoshankaya L, Filan PM, Bogue CO, Murray DM, Boylan GB. Global suppression of electrocortical activity in unilateral perinatal thalamic stroke. Dev Med Child Neurol. 2014;56(7):695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujii K, Heistad DD, Faraci FM. Role of the basilar artery in regulation of blood flow to the brain stem in rats. Stroke. 1991;22(6):763–7. [DOI] [PubMed] [Google Scholar]
- 88.Dashwood M, Turner M, Jacobs M. Endothelin-1: contractile responses and autoradiographic localization of receptors in rabbit blood vessels. J Cardiovasc Pharmacol. 1989;13 Suppl 5:S183–5. [PubMed] [Google Scholar]
- 89.Loesch A Localisation of endothelin-1 and its receptors in vascular tissue as seen at the electron microscopic level. Curr Vasc Pharmacol. 2005;3(4):381–92. [DOI] [PubMed] [Google Scholar]
- 90.Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79(1–2):135–60. [DOI] [PubMed] [Google Scholar]
- 91.Schinelli S Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006;13(6):627–38. [DOI] [PubMed] [Google Scholar]
- 92.Dashwood MR, Loesch A. Endothelin-1 as a neuropeptide: neurotransmitter or neurovascular effects? J Cell Commun Signal. 2010;4(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anaclet C, Griffith K, Fuller PM. Activation of the GABAergic Parafacial Zone Maintains Sleep and Counteracts the Wake-Promoting Action of the Psychostimulants Armodafinil and Caffeine. Neuropsychopharmacology. 2018;43(2):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anaclet C, Ferrari L, Arrigoni E, Bass CE, Saper CB, Lu J, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17(9):1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27(51):14041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerashchenko D, Blanco-Centurion CA, Miller JD, Shiromani PJ. Insomnia following hypocretin2-saporin lesions of the substantia nigra. Neuroscience. 2006;137(1):29–36. [DOI] [PubMed] [Google Scholar]
- 97.Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci Lett. 1983;42(1):49–54. [DOI] [PubMed] [Google Scholar]
- 98.Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30(27):9095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crone JS, Lutkenhoff ES, Bio BJ, Laureys S, Monti MM. Testing Proposed Neuronal Models of Effective Connectivity Within the Cortico-basal Ganglia-thalamo-cortical Loop During Loss of Consciousness. Cereb Cortex. 2017;27(4):2727–38. [DOI] [PubMed] [Google Scholar]
- 100.Zhang LN, Yang C, Ouyang PR, Zhang ZC, Ran MZ, Tong L, et al. Orexin-A facilitates emergence of the rat from isoflurane anesthesia via mediation of the basal forebrain. Neuropeptides. 2016;58:7–14. [DOI] [PubMed] [Google Scholar]
- 101.Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18(11):1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steriade M Brainstem activation of thalamocortical systems. Brain Res Bull. 1999;50(5–6):391–2. [DOI] [PubMed] [Google Scholar]
- 104.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19(2 Pt 1):402–11. [DOI] [PubMed] [Google Scholar]
- 105.Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107(52):22665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akeju O, Loggia ML, Catana C, Pavone KJ, Vazquez R, Rhee J, et al. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife. 2014;3:e04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103(45):17007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM. Non-Invasive Ultrasonic Thalamic Stimulation in Disorders of Consciousness after Severe Brain Injury: A First-in-Man Report. Brain Stimul. 2016;9(6):940–1. [DOI] [PubMed] [Google Scholar]
- 109.Steriade M, Domich L, Oakson G. Reticularis Thalami Neurons Revisited - Activity Changes during Shifts in States of Vigilance. Journal of Neuroscience. 1986;6(1):68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis LD, Voigts J, Flores FJ, Schmitt LI, Wilson MA, Halassa MM, et al. Thalamic reticular nucleus induces fast and local modulation of arousal state. Elife. 2015;4:e08760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14(9):1118–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Economo Cv. Sleep as a problem of localization. The Journal of Nervous and Mental Disease. 1930;71(3). [Google Scholar]
- 113.Nauta WJ. Hypothalamic regulation of sleep in rats; an experimental study. J Neurophysiol. 1946;9:285–316. [DOI] [PubMed] [Google Scholar]
- 114.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–31. [DOI] [PubMed] [Google Scholar]
- 115.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–63. [DOI] [PubMed] [Google Scholar]
- 116.Saper CB. Organization of Cerebral Cortical Afferent Systems in the Rat .2. Hypothalamocortical Projections. Journal of Comparative Neurology. 1985;237(1):21–46. [DOI] [PubMed] [Google Scholar]
- 117.Luthi A Sleep: Switching Off the Off-Switch. Curr Biol. 2016;26(16):R765–7. [DOI] [PubMed] [Google Scholar]
- 118.Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25(3):473–504. [DOI] [PubMed] [Google Scholar]
- 119.Van der Linden A, Van Camp N, Ramos-Cabrer P, Hoehn M. Current status of functional MRI on small animals: application to physiology, pathophysiology, and cognition. NMR Biomed. 2007;20(5):522–45. [DOI] [PubMed] [Google Scholar]
- 120.Van de Moortele PF, Pfeuffer J, Glover GH, Ugurbil K, Hu X. Respiration-induced B0 fluctuations and their spatial distribution in the human brain at 7 Tesla. Magn Reson Med. 2002;47(5):888–95. [DOI] [PubMed] [Google Scholar]
- 121.Pais-Roldán PBB, Scheffler K, Yu X. Identifying respiration-related aliasing artifacts in the rodent resting-state fMRI. Frontiers in Neuroscience. 2018;(under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pal D, Dean JG, Liu T, Li D, Watson CJ, Hudetz AG, et al. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol. 2018;28(13):2145–52 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newman LA, Creer DJ, McGaughy JA. Cognitive control and the anterior cingulate cortex: how conflicting stimuli affect attentional control in the rat. J Physiol Paris. 2015;109(1–3):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lavin C, Melis C, Mikulan E, Gelormini C, Huepe D, Ibanez A. The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Front Neurosci. 2013;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Threlkeld ZD, Bodien YG, Rosenthal ES, Giacino JT, Nieto-Castanon A, Wu O, et al. Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78(3):175–81. [DOI] [PubMed] [Google Scholar]
- 127.Ebitz RB, Platt ML. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 2015;85(3):628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Joshi S, Li Y, Kalwani RM, Gold JI. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron. 2016;89(1):221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cao B, Wang J, Shahed M, Jelfs B, Chan RH, Li Y. Vagus Nerve Stimulation Alters Phase Synchrony of the Anterior Cingulate Cortex and Facilitates Decision Making in Rats. Sci Rep. 2016;6:35135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30(43):14543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu D, Deng H, Xiao X, Zuo Y, Sun J, Wang Z. Persistent Neuronal Activity in Anterior Cingulate Cortex Correlates with Sustained Attention in Rats Regardless of Sensory Modality. Sci Rep. 2017;7:43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boly M, Seth AK, Wilke M, Ingmundson P, Baars B, Laureys S, et al. Consciousness in humans and non-human animals: recent advances and future directions. Front Psychol. 2013;4:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, et al. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59(5):1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shim WH, Baek K, Kim JK, Chae Y, Suh JY, Rosen BR, et al. Frequency distribution of causal connectivity in rat sensorimotor network: resting-state fMRI analyses. J Neurophysiol. 2013;109(1):238–48. [DOI] [PubMed] [Google Scholar]
- 135.Pais-Roldan P, Biswal B, Scheffler K, Yu X. Identifying Respiration-Related Aliasing Artifacts in the Rodent Resting-State fMRI. Front Neurosci. 2018;12:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.