Abstract
Semiconducting nanoparticles, more commonly known as quantum dots, possess unique size and shape dependent optoelectronic properties. In recent years, these unique properties have attracted much attention in the biomedical field to enable real-time tissue imaging (bioimaging), diagnostics, single molecule probes, and drug delivery, among many other areas. The optical properties of quantum dots can be tuned by size and composition, and their high brightness, resistance to photobleaching, multiplexing capacity, and high surface-to-volume ratio make them excellent candidates for intracellular tracking, diagnostics, in vivo imaging, and therapeutic delivery. We discuss recent advances and challenges in the molecular design of quantum dots are discussed, along with applications of quantum dots as drug delivery vehicles, theranostic agents, single molecule probes, and real-time in vivo deep tissue imaging agents. We present a detailed discussion of the biodistribution and toxicity of quantum dots, and highlight recent advances to improve long-term stability in biological buffers, increase quantum yield following bioconjugation, and improve clearance from the body. Last, we present an outlook on future challenges and strategies to further advance translation to clinical application.
Keywords: bioimaging, nanotechnology, theranostics, drug delivery, molecular probes
Graphical Abstract
1. Introduction
In the field of biomedical imaging, inorganic particles (e.g., iron oxide, gold, quantum dots) have gained significant interest in the last few decades. Specifically, they are being extensively explored for applications such as DNA-functionalized probes for real-time identification of disease-relevant biomolecules, diagnostic agents for tumor detection through tumor-specific membrane antigens, and molecular probes for simultaneous tracking and monitoring of drug delivery and therapeutic efficacy [1]. This interest is due to the unique optical, electrical, and magnetic properties of inorganic particles, which can be altered through changes in their size, composition, geometry, and structure [2]. Further, inorganic materials offer a relative ease of processing and resistance to degradation under physiological conditions compared to organic materials [2]. To this extent, the tunability and resistance makes inorganic particles a very attractive and enticing modality.
Inorganic particles with nanometer sizes are especially exciting in the biomedical field. Many common proteins and biomolecules exist on a similar size scale, and such small length scales allows the inorganic materials to interact very closely with biological entities on a molecular level. This close interaction can enable a higher detection specificity and sensitivity, as well as the ability for intracellular uptake [3, 4]. In tandem, the nanometer size also increases the particle surface area to volume ratio. The increased ratio, coupled with the wide array of chemistry available, enables easy surface modifications to enhance the biocompatibility, solubility, and reactivity [2, 4, 5].
In bioimaging applications, the nanometer-sized particles also offer a significant advantage towards overcoming the severe toxicity often accompanying inorganic materials. The decreased size can enable increased sensitivity and a lower effective dose, without needing to sacrifice function. Many types of inorganic nanoparticles have been developed in the field, including superparamagnetic nanoparticles, metal-based nanoparticles, and semiconductor nanoparticles. The latter is referred to as quantum nanodots (QDs) in layman terms and is the key focus area of this review.
2. Semiconductor Quantum Dots
The initial discovery of QDs in the early 1980’s has been credited to Brus, Efros, and Ekimov by the Optical Society of America [6–8]. Notably, they display unique size and shape dependent optoelectronic properties that in recent years have found useful application in bioimaging, among many other areas including photodetectors, light-emitting diode (LED) fabrication, solar cell technology or computing. Several types of QDs are currently available commercially, and are listed in Table 1.
Table 1.
Quantum dots available commercially from companies in the U.S.
| Company | Product Line | Composition | Excitation (nm) | Emission (nm) | Solvent | Formulation | Product Notes |
|---|---|---|---|---|---|---|---|
| Cromoz | CDot20 | water | dry powder | conjugated with cancer therapeutic agents | |||
| CDot10 | dry powder | ||||||
| Crystalplex | Trilite ™ | CdSeS | 490-665 | water | solution | composition rather than size dependent; carboxyl, amine, hydroxyl, alkyl functionalized surface | |
| Nanoaxis | AxiCad ™ | CdTe/ZnTe | 500-700 | 530-740 | water | dry powder or solution | conjugated with antibody and peptide |
| NN-Labs, LLC | Line 10 | CdTe | 620 | 640 | organic | solution | photovoltaic application |
| Line 9 | InP/ZnS | 530-650 | nonpolar (chloroform, hexane, toluene) | solution | heavy metal free | ||
| Line 4 | CdSe/ZnS | 520-640 | 530-670 | organic | solution | ||
| Line 3 | CdSe/ZnS | 520-640 | 530-660 | water | solution | ||
| Line 2 | CdSe | 480-640 | 485-670 | organic | solution | ||
| Line 10 | CdS | 360-460 | 365-490 | organic | solution | ||
| NanoTech Ocean | QSP | CdSe/ZnS | 520-630 | nonpolar (chloroform, hexane, toluene) | dry powder | electronic grade | |
| Sigma-Aldrich | Lumidot™ | CdS | 380-480 | toluene | solution | ||
| Lumidot™ | Cd/Se | 480-640 | toluene | solution | |||
| Sun Innovations | PhosphorDots™ | doped Yttrium | 300-980 | 486-650 | aqueous or organic | solution | particularly suited for life sciences; NIR |
2.1. Properties of Quantum Dots
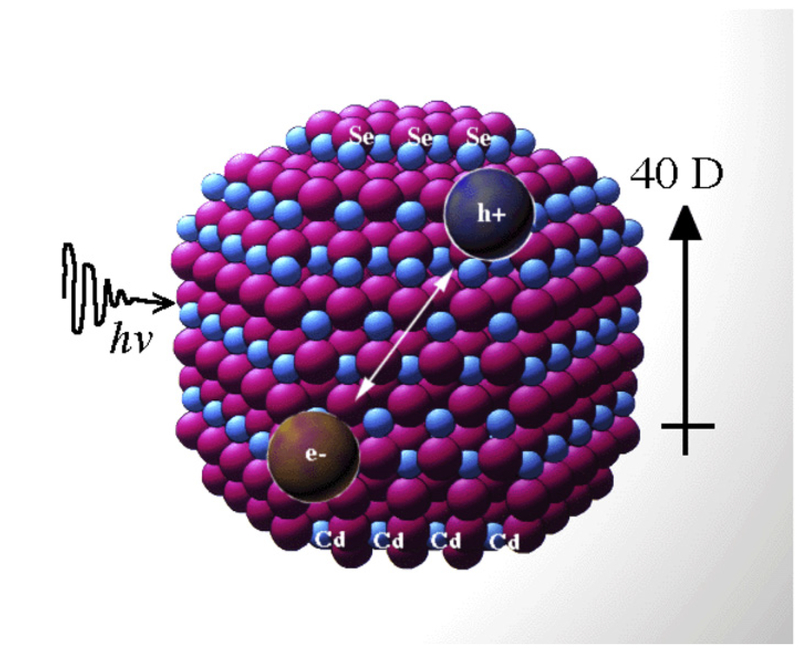
A semiconductor nanocrystal, illustrated in Figure 1, is distinguished by its bandgap energy, which is the energy required to excite an electron from one electronic band into another one at a higher energy level. This excitation scheme ultimately creates an electron-hole pair known as an exciton. Upon relaxation back to its ground state the exciton emits energy in the form of a fluorescent photon [9]. When the semiconducting nanocrystal size becomes similar to that of the bulk Bohr exciton radius, a characteristic length in the range of 2-10 nm, the particle gains unique electrical and optical properties [10].
Figure 1.
Illustrative structure of a CdSe quantum dot. Photon excitation causes electrons to move to a higher energy level, creating an electron-hole pair known as an exciton. If the electron and hole recombine as the electron returns to its ground state, fluorescence is emitted. Adapted from [41] with permission from Elsevier.
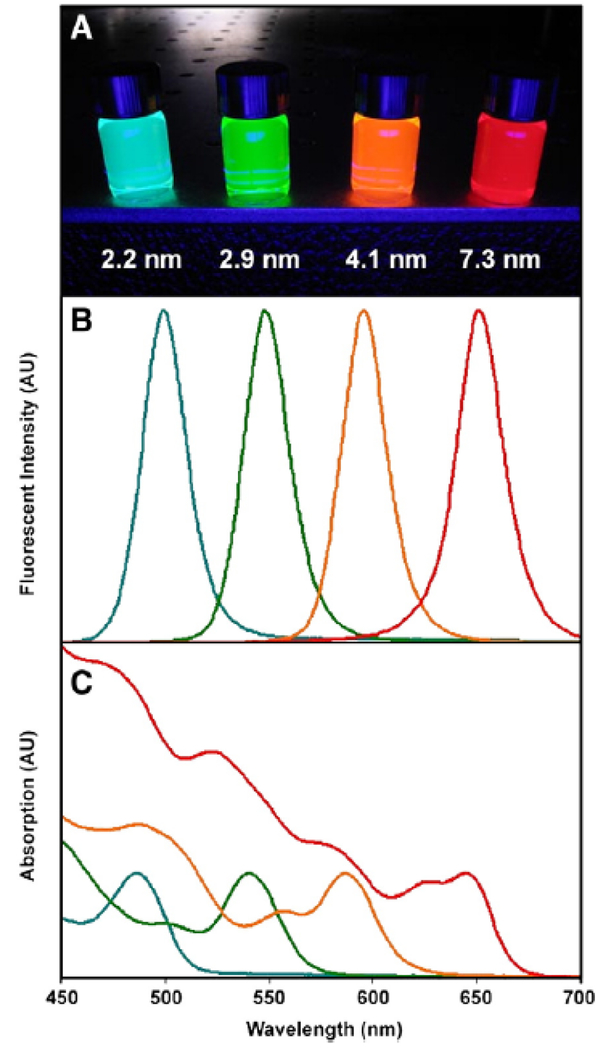
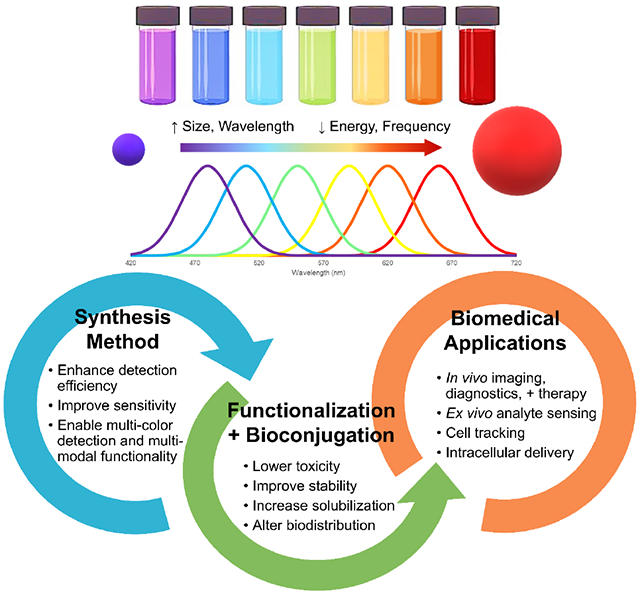
The most well-documented and understood property is the inverse relationship between nanocrystal size and energy band gap; in other words, as the size of the nanocrystal decreases, the energy band gap increases and the corresponding excitation/emission wavelengths decrease. This is known as the quantum size effect [11], and a comparison between excitation and emission profiles of QDs of various sizes [9] is portrayed in Figure 2. In Figure 2A, the fluorescent color emitted by the QDs upon ultraviolet exposure can be tuned simply by varying the size of the particle. In Figures 2B and 2C, respectively, tuning the particle size and distributions of the QDs can enable a wide absorbance range with a highly symmetric and narrow emission spectra.
Figure 2.
Optical properties of CdSe QDs (dispersed in chloroform) can be tuned by the size of the particle, known as the quantum size effect. (A) Fluorescence image of monodisperse QDs after ultraviolet illumination, ranging in size from 2.2 −7.3 nm. (B) Fluorescence spectra of the same four QD samples. Narrow and symmetric emission bands are observed, indicating low variance in particle size. (C) Absorption spectra of the same four QD samples. Broad absorption spectra are observed, enabling a wide range for excitation of the QDs. Adapted from [9] with permission from Elsevier.
2.2. Synthesis Methods
There are two important methodologies behind semiconductor nanoparticle synthesis. This first is a top-down synthesis method, including processing techniques such as molecular beam epitaxy (MBE) and electron beam lithography [10]. The other is achieved by a bottom-up method, where precursor materials self-assemble and react in solution to yield colloidal QDs. As the optical properties relevant to bioimaging are unique to colloidal quantum dots, these top-down synthesis methods will not be discussed in detail here, and we will solely focus on the bottom-up method instead.
A breakthrough in the bottom-up process came in 1993, when Bawendi and coworkers reported a more facile synthetic method yielding nearly monodisperse QDs with a relatively high quantum yield [12]. The organometallic reagents dimethyl cadmium (Me2Cd) and either trioctylphosphine selenide (TOPSe), trioctylphosphine telluride (TOPTe), or bis(trimethylsilyl)selenium [(TMS)2Se] were injected into a solution of coordinating ligand, trioctylphosphine (TOP) or trioctylphosphine oxide (TOPO) heated to ~300°C [12]. The rapid introduction of the cooler organometallic reagents into the reaction vessel induced brief nucleation that ceased as the temperature of the reaction solution dropped abruptly. Nanoparticles were grown by gradual heating and annealing while the coordinating ligand stabilized the crystal solution. Rate of growth and crystal size were dependent upon growth temperature, and once the reaction was quenched by cooling, crystals were separated by size-selective precipitation with 1-butanol and methanol.
This important work by Bawendi et al. resulted in a synthesis method for CdSe QDs with excellent size control and an unprecedented degree of monodispersivity and crystalline order, which enabled researchers to perform detailed investigations of size-dependent optical properties [13]. The method is still largely used today to synthesize QDs, with some changes to the cadmium species and coordinating ligand [14]. However, use of milder reaction conditions to synthesis monodisperse QDs have recently become more common. These methods use lower temperatures (~100°C), green chemicals such as cadmium acetate (Cd(Ac)2), and a thiolated capping agent in aqueous solution to produce CdTe QDs with quantum yields as high as 75% prior to passivation [15].
In these methods, both the size and core composition of the crystal will affect the emission wavelength. For example, CdSe QDs typically emit in the 450-650 nm wavelength range, while CdTe QDs may display an emission wavelength in a slightly higher range (500-750 nm) [9]. Nie and associates have even developed Se:Te alloyed QDs with different morphologies and unique optical properties independent of size by controlling the cadmium feed into the reaction mixture [16]. The ability of CdTe to emit in the near-infrared (NIR) region (~700-1400 nm) is directly applicable to deep tissue imaging, which will be discussed in detail in a later section.
2.3. Passivation
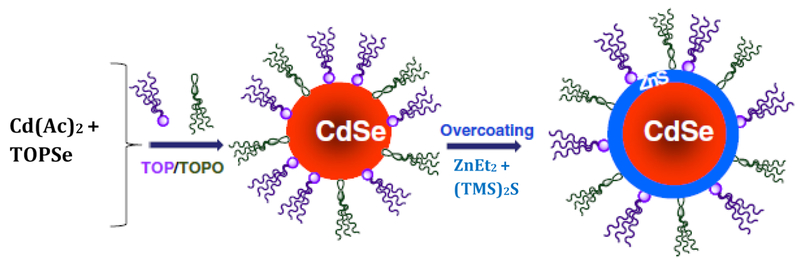
Though there are many advantages attributed to the high surface area to volume ratio of nanoparticles, there is at least one drawback. Coordinating atoms at the surface of the crystal have “dangling” molecular electronic bands that serve to quench the photoluminescence (PL) and decrease the PL quantum yield [9]. Therefore, a passivating layer of a wider bandgap is necessary; this passivated QD is known as a “core-shell’’ structure, shown in Figure 3.
Figure 3.
Schematic depicting the synthesis of a QD and the addition of a passivating layer, resulting in a “core-shell” structure. Typical reagents reported in current literature are shown. Adapted from [10] with permission from Elsevier.
The synthesis developed by Bawendi et al. was at the expense of long fluorescence lifetimes and did not yield a well passivated surface [13]. To this extent, Hines and Guyot-Sionnest developed a key method to cap the core nanocrystal with a higher bandgap inorganic shell to improve the luminescence, stability, and electrical properties more could be observed with an organic capping layer [13]. Inorganic passivating materials are preferred over organic, as they are more robust and amenable to processing conditions. Typical passivating compounds include CdS, CdSe, and ZnS [13, 17]. ZnS is the most common passivating compound, as this coating results in less pronounced red shift than materials of a lower bandgap similar to that of the core material, such as CdS or CdSe [10, 13]. A ZnS shell also prevents oxidation and enhances colloidal stability of the particles [9, 13]. Dabbousi et al. showed that a passivating layer of ZnS can increase the quantum yield to 30-50% from 5-15% for unmodified dots, and that the quantum yield reaches a maximum value with about ~1.3 monolayers of ZnS [17].
The ZnS layer is added to the QD core in a manner similar to the synthesis described earlier, the major difference being the use of diethylzinc (ZnEt2) and bis(trimethylsilyl)sulfide ((TMS)2S) as reagents. The nanoparticles are then purified and precipitated in a size-selective manner with a nonsolvent such as methanol [13, 17]. ZnS shell growth is improved by the addition of a small amount of Cd to promote even coating across the QD surface, achieving particles with a quantum yield of up to 95% [18].
3. Quantum Dots in Biomedical Applications
Historically, QDs have been widely employed in non-biological applications due to their appealing size and composition tunable optical and electrical properties. In the biomedical field, QDs offer many advantages over traditional organic dyes and fluorescent compounds [19–22]. However, there are certain aspects that need to be considered when the materials are intended for contact with biological environments, such as aqueous solubility, stability, and toxicity.
In this work, we discuss several comparative advantages of QDs over conventional organic dyes in biological applications. Further, we discuss current challenges and limitations in the application of QDs, to which we provide possible solutions, advanced techniques, and future perspective in later sections. We concentrate on relatively recent, successful or promising methods. A comparative analysis of quantum dot properties versus conventional organic fluorophores is summarized in Table 2.
Table 2.
Comparative analysis of quantum dot properties versus conventional organic fluorophores in biomedical applications.
| Property | Quantum Dots | Conventional Organic Fluorophores |
|---|---|---|
| Size | Tunable (2-10 nm) | Typically <0.5 nm (protein or small molecule) |
| Absorption Spectra | Broad, discrete bands | Narrow |
| Emission Spectra | Narrow (symmetric, size-tunable) | Broad (asymmetric with red-shift tail) |
| Molar Absorptivity (ε) | Large (typically on the order of 0.5 to 5 × 106 cm−1M−1) | Small (typically on the order of 0.5 to 2.5 × 105 cm−1M−1) |
| Emission Spectra | Narrow (symmetric, size-tunable) | Broad (asymmetric with red-shift tail) |
| Quantum Yield | High (0.1-0.8 in visible range and 0.2-0.7 in NIR) | Low (0.5-1.0 in visible range and 0.05-0.25 in NIR) |
| Fluorescence Excited State Lifetime | Long (10 to 100 nanoseconds with multi-exponential decay) | Short (< 10 nanoseconds with mono-exponential decay) |
| Two-Photon Cross Section | Large (typically on the order of 0.2 to 5 × 10−46 cm4 sec photon−1) | Small (typically on the order of 1 × 10−49 cm4 sec photon−1) |
| Detection Sensitivity | High | Low |
| Resistance to Photo-bleaching | High | Low |
| Aqueous Solubility | Low (need for surface coating or functionalization) | High (control via chemistry) |
| Tissue Penetration Depth | Medium (size dependent) | High |
| Spectral Multiplexing Capability | High | Low (spectral overlap) |
| Quantification Capability | High | Low |
| Toxicity | Potential for accumulation and heavy metal leaching, typically slow metabolism | Chemistry dependent, typically rapid metabolism |
| Key Strengths | Ease of spectra tunability | Typically stable, water-soluble materials |
| Quantum yield (up to 100 times brighter) | Ease of bioconjugation (many available commercially) | |
| High photostability | Deep tissue penetration | |
| Key Challenges | Potential toxicity in vivo | Lack of tunability |
| Poor aqueous solubility and stability | Prone to photo-bleaching | |
| Difficult to alter biodistribution | More difficult to enable in vivo multiplex imaging |
3.1. Comparative Advantages
There are a number of competitive advantages that makes QDs desirable in numerous biomedical applications. In comparison to common organic dyes and luminescent proteins, QDs have broad excitation spectra, narrow emission spectra and large Stokes shifts. QDs also possess superior optical properties including increased intensity (up to nearly 100 times brighter) and significantly improved stability against photo-bleaching [9, 22]. Traditional organic dyes are highly prone to photo-bleaching, and the quantum yield can often be less than 15% in a biological environment [20, 23]. Further, as opposed to most conventional organic label dyes, QDs offer broad optical range of fluorescence, from near ultraviolet to near infrared (NIR). In particular, QDs emitting in the NIR have greatly expanded the potential of fluoresce in biomedicine due to its lower tissue absorption and relatively low autofluorescence [22, 24].
The variety of excitation and emission properties available through the quantum size effect is highly attractive in biomedical applications. For instance, QDs of various sizes can be injected into a patient, excited with a single wavelength, and yield a reproducible and narrow emission with minimal cross-talk between channels and spectral overlap [4, 25]. The reduction in overlap is critical as it enhances detection efficiency, improves sensitivity, and can enable multi-color detection and multi-modal functionality. In contrast, it is challenging to control and modulate the spectral wavelengths of traditional organic dyes as these properties are dependent upon the chemical structure, conformation, and reactivity. They cannot be readily or easily modified to emit different colors, which limits their applicability in multiplex imaging applications [20, 22].
3.2. Current Challenges and Limitations
In spite of these advantages, there still remain several significant limitations in the widespread use of QDs as bioimaging agents. Compared to conventional organic fluorophores, QDs behave as nanocolloids. This complicates their long-term use in biological environments and raises additional safety concerns.[21] Specific challenges include low aqueous solubility, complicated surface chemistry, low biological specificity, poorly controlled biodistribution to target tissues, and the potential for severe long-term toxicity [19, 21, 22].
QDs synthesized using more traditional organic methods typically result in hydrophobic surface properties, leading to a poor aqueous solubility and stability for in vivo applications [26–29]. The surface needs to be modified post-synthesis through encapsulation, ligand exchange, bioconjugation, or other means (detailed discussion in the following section). The type and density of the surface coating strongly affects the achievable fluorescently intensity, target recognition, particle size, solution stability, and biocompatibility [22, 30]. However, many published strategies lack reliable methods to produce the surface coating or neglect to fully characterize the surface modifications.
Additional key limitations include the ability to control the QD biodistribution only to target organs, reduce long-term accumulation in the body, and limit the long-term toxicity [31, 32]. The type of QD chemistry and resulting physicochemical properties will ultimately determine the fate of the nanocolloids in vivo. The particles encounter multiple lines of defense upon administration to the circulatory system and must overcome several significant biological barriers from organ to cellular level (detailed discussion in later section). The absorption, distribution, metabolism, excretion, and toxicity depends on a multitude of properties, and must be studied in careful detail before clinical application [31, 32].
QD composition and properties such as particle size, surface charge, and hydrophilic coating can be rationally designed to slow the body’s natural clearance mechanisms and improve interaction with target cells [21, 22, 27–30, 33]. In particular, surface modification with biological entities targeted to specific receptors (e.g., aptamers, peptides, antibodies, antigens) has shown remarkable success in improving both the aqueous solubility and cell / tissue specificity of imaging and diagnostic modalities. However, despite many published studies, there have been no resulting widespread conclusions to understand all the factors that influence particle biodistribution and target cell interactions [26–30, 33–40].
Ultimately, the field needs more systematic studies to understand the influence QD properties and demonstrate control of biodistribution and long-term safety. Successful strategies to address these limitations will enable the potential of QDs to be fully realized in clinical applications. To the extent, recent progress and potential solutions to major challenges will be discussed in the following sections. We also provide detailed discussion on techniques to improve the long-term stability in biological buffers, increase the quantum yield following bioconjugation, and improve clearance from the body.
4. Surface Modification Strategies to Improve Solubilization, Stability, and Specificity
4.1. Encapsulation
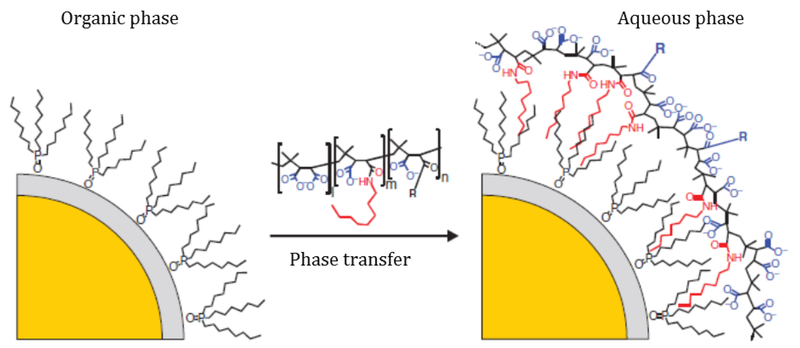
One of the greatest challenges with using QDs in medical and theranostic applications is related to their water-insolubility, as QDs must be water-soluble for proper use in bioimaging applications. In overcoming this limitation, many researchers have focused on encapsulating the QDs in a hydrophilic, soluble material. For instance, QD particles are often encapsulated in polymers or hydrogels. In another strategy, ligand exchange or surface capping can be used to substitute hydrophobic moieties with more water-soluble counterparts. To this extent, Mattoussi et al. have collected a broad list of surface functionalization strategies studied in literature [10]. Ultimately, the choice of coating material and method is primarily driven by the desired application and environmental requirements.
One of the more common general encapsulation methods is the utilization of amphiphilic polymer chains to coat the QD. To achieve the highest quantum yield the original ligands are left intact, and the hydrophobic interactions between the ligands and the hydrophobic regions of the polymer chain result in coating of the QD [41]. The polymer is often crosslinked to create a stable particle with a fully encapsulated core. Quantum yields following solubilization with this method are very high, even nearly identical to the quantum yields prior to encapsulation [42].
Design of the amphiphilic polymer coating has gained much interest, since a facile process that will not alter the colloidal stability of the particles is desirable. Functional groups may even be incorporated into the amphiphilic polymer coating to eliminate a functionalization step and impart the particles with greater utility. An encapsulation protocol employing click chemistry has recently been reported using a functionalized, amphiphilic anhydride-backbone polymer with acetylene groups to coat CdSe QDs (Figure 4). The method is advantageous because it includes common polymeric precursors, simple functionalization of the polymer incorporated into the encapsulation coating, and yields robust, stable, water-soluble particles that require no additional crosslinking or functionalization [43].
Figure 4.
Polymer encapsulation of a CdSe QD. The polymer is a functionalized amphiphilic anhydride, where R represents a functional group (R= -NH2, -OH, etc.). The hydrophobic ligands interact with the hydrophobic regions of the amphiphile, creating a polymer micelle with colloidal stability and solubility in water. Adapted from [43] with permission from Springer Nature.
More recently, multi-dentate polymer coatings have been widely adopted in the field over the last decade to minimize the QD size and increase aqueous stability [44–49]. Multi-dentate polymers are capable of interacting with the QD surface via multiple sites, where the multivalent interactions serve to improve the overall stability of the interface of the QD and ligands [49]. Common example ligands may include bidentate thiol polymers, oligomeric alkyl phosphine polymers, general amine-containing polymers, and thiol-containing polymers [47–54]. These multi-dentate polymer coating strategies typically yield enhanced colloidal stability in solution as well as provide a robust platform for further modification [49]. However, structure analysis, quantification, and control over the spatial distribution of functional groups is often not addressed literature studies [49, 51, 53, 54]. This becomes more important to consider and address with increasing biological complexity of the intended application and with progress towards the clinical application of QDs.
4.2. Ligand Exchange
The other predominant solubilization method is ligand exchange. This technique entails replacement of the organic capping ligand with a bifunctional hydrophilic ligand or polymer that can be anchored to the nanoparticle surface with one end and induce water solubility with the free end. The technique does not greatly increase the hydrodynamic diameter, but it does decrease the PL quantum yield due to direct binding to the QD surface [55]. Examples of hydrophilic ligands include mercaptoundecanoic acid (MUA), dihydrolipoic acid (DHLA), and thiolated agents. Though a simple process, known problems with ligand exchange are the large amounts of ligand required to drive the reaction and the lack of long-term stability of the particles, especially in extreme pH buffers [10].
The ultimate success of this method hinges upon the affinity of the anchoring group to the nanoparticle surface and the degree of colloidal stability promoted by the hydrophilic ligand. It has been shown that hydrophilic ligands incorporating a polyethylene glycol (PEG) chain have increased colloidal stability, high water compatibility, and resistance to immunogenicity and nonspecific interactions. The incorporation of multiple coordinating groups per ligand further enhances colloidal stability over a wide range of pH, from pH 1.1 to pH 13.9, and over various ionic strengths [56].
One modular approach to ligand exchange first uses the unique ability of the amino acid L-histidine to completely exchange the initial TOPO ligand, followed by further ligand exchange with the histidine intermediate in aqueous solution to functionalize the surface [55]. This method resulted in a high degree of phase transfer and a modest preservation of quantum yield, from 53% to 30%, though this is still lower than that of polymer encapsulation techniques. The histidine can be further exchanged with a range of self-assembled monolayers, including DHLA and PEG, in a homogeneous solution [43].
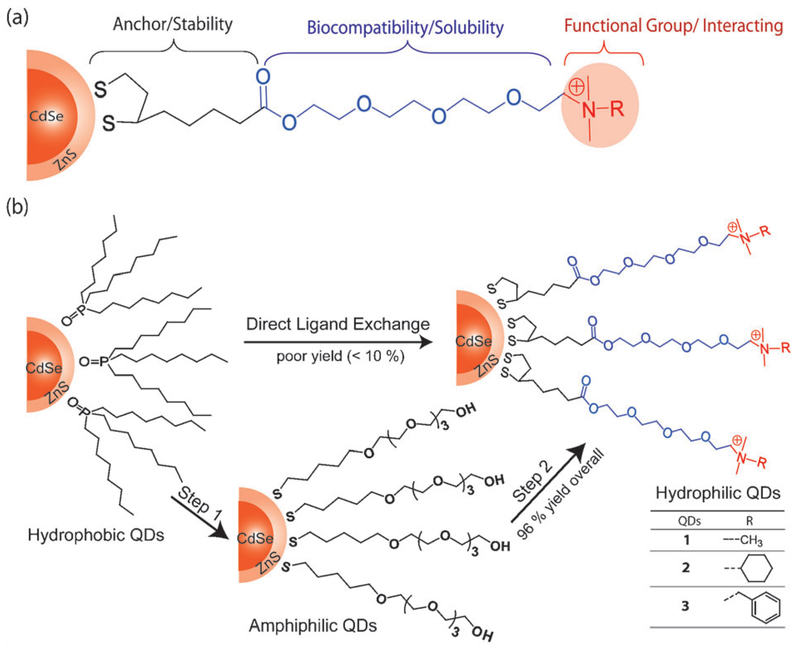
In the specific application of simultaneous imaging and therapeutic delivery, cationic QDs are of interest as they can electrostatically bind with negatively charged proteins and nucleic acids [57]. These particles, shown in Figure 5, resist deprotonation and consequent aggregation at high pH due to quaternary ammonium end groups. Figure 5B shows an overview of the ligand exchange process: hydrophobic QDs are first converted to amphiphilic QDs by exchange with DHLA capped tetra(ethylene glycol) (TEG) chains, then further modified with the quaternary ammonium terminus. The ultimate surface functionality of the particle can be tuned by altering the ammonium head group. The cationic QDs showed high stability under biological conditions, but similar to other ligand exchange methods, preserved only 40-60% of the original quantum yield [57].
Figure 5.
(A) General structure of DHLA-TEG-quaternary ammonium cationic ligand, where R represents a functional group. (B) Two-step ligand exchange scheme. Step 1: Hydrophobic QDs are converted to amphiphilic QDs by exchange with DHLA capped tetra(ethylene glycol) (TEG) chains. Step 2: Addition of the quaternary ammonium terminus, resulting in hydrophilic, cationic QDs. Adapted from [57] with permission from the Royal Society of Chemistry.
4.3. Bioconjugation
Quantum dots were first used as biological labels in 1998, when it was reported that biotin functionalization and protein conjugation of QDs could enable cellular uptake, specific binding, and intracellular imaging [58, 59]. QDs can be conjugated to a number of biomolecules, including proteins, oligonucleotides, antibodies, and small molecule ligands, to direct their in vitro or in vivo pathway to a specific target or to impart therapeutic capability [9, 60].
Conjugation of the QD surface with biomolecules depends heavily upon the ligand chemistries available to provide suitable functional groups for covalent attachment or noncovalent binding [61, 62]. In this respect, conventional organic dyes offer a wider variety of bioconjugation strategies. Controlling the QD surface via passivation and phase transfer of QDs has improved tremendously over the last couple decades. Particularly important are mild processing conditions, long-term stability in a biological buffer, biocompatibility, and maintenance of optimal quantum yield following passivation and solubilization [63–69].
To this extent, conjugation of biomolecules using conventional methods has been fairly successful and various detection and targeting applications have been documented [29, 61–63, 65–72]. However, significant work remains to be done to further improve suitability of QDs to enable more site-specific and a wider variety of bioconjugation strategies [28, 73]. Some additional concerns remaining to be addressed include maintaining high quantum yields after bioconjugation and improved efficiency of bioconjugation, to which research has recently shifted focus [28, 64, 65, 68, 69, 73].
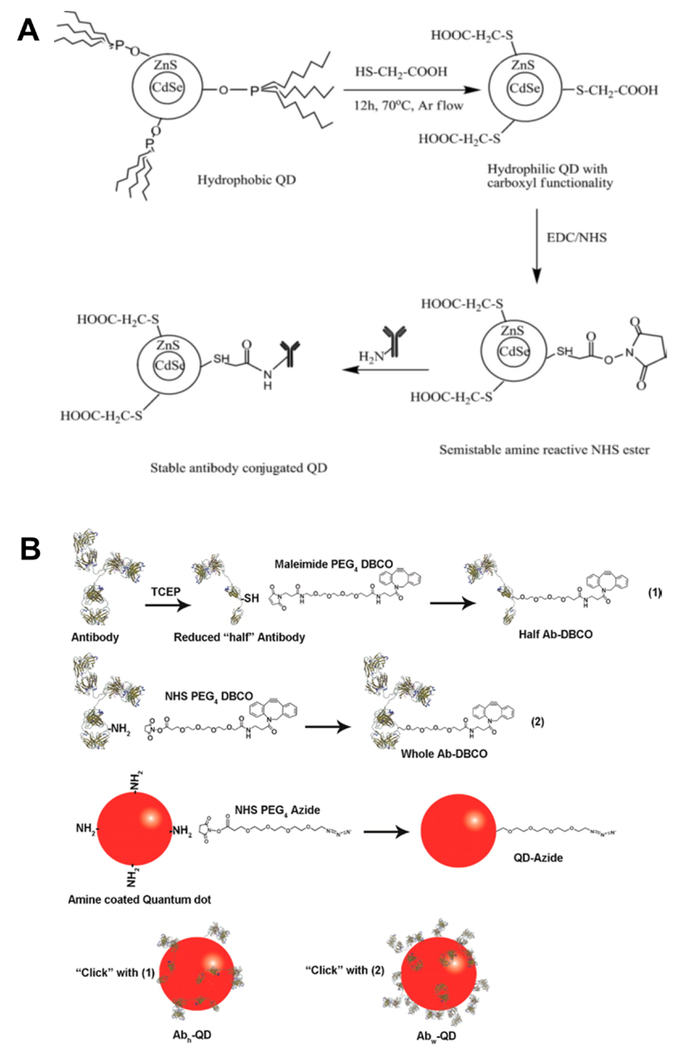
As attachment of biomolecules to QDs relies on available surface ligands, there are limited chemistries commonly used to attach the biomolecule to the QD [61–64, 68, 69]. The first is via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) chemistry, which combines a carboxylic acid-terminated QD to an amine-terminated biomolecule. A second, yet similar method uses Traut’s reagent or N-hydroxysuccinimide (NHS) to fix an amine-terminated QD to amine-terminated biomolecules. Additionally, sulfosuccinimidyl 6-(3′-[2-pyridyldithio]-propionamido)hexanoate has been used to link amine-terminated QDs to thiol-terminated biomolecules.
For example, EDC/NHS chemistry was used to conjugate carboxy-terminated CdSe/ZnS core-shell QDs with an amine-terminated IgG antibody, shown in Figure 6A. A QD to antibody ratio of 50:1 was selected as the optimal formulation based upon fluorescence intensity as well as biological activity, and the size of the particles were in the range of 25-31 nm [74]. The IgG-conjugated QD were then shown to rapidly and specifically detect Salmonella typhi captured on a modified polycarbonate membrane at bacterial concentrations of up to 100 cells/mL. Additionally, the QDs were found to be 100 times more sensitive than a comparable common organic dye labeled with IgG [74].
Figure 6.
Conjugation of the QD surface with biomolecules depends heavily upon the ligand chemistries available to provide suitable functional groups for covalent attachment or noncovalent binding. (A) Illustration of phase transfer followed by EDC/NHS carbodiimide chemistry to conjugate a CdSe/ZnS quantum dot with antibody [74]. (B) Schematic of antibody conjugated QD synthesis using copper free cycloaddition ‘click’ chemistry [221]. Figures adapted from [74] with permission from Elsevier, and [221] with permission from the American Chemical Society.
However, coupling via carbodiimide chemistry is often very inefficient as NHS esters can be readily hydrolyzed in the required conjugation conditions. In an alternative strategy, ligands present during the QD synthesis may be exchanged with biomolecules (e.g., DNA, RNA) to enable electrostatic attachment [61–64, 68, 69]. However, this method may release very toxic or heavy-metal ligands, and must be followed by stringent purification and characterization methods. Most often, this requirement leads to a labor intensive and low yield process. Similarly, ligand exchange with thiol-labeled biomolecules can be used. However, this method is often limited as surface-bound thiols can be readily oxidized or degraded by light. In another common method, the native avidin-biotin interaction can be utilized to attach avidin-functionalized QDs to biotinylated biomolecules [41]. However, this method significantly increases the overall QD size and often limits the applicability to ex vivo studies or imaging of superficial tissues.
As discussed above, these traditional coupling methods have some limitations, including inefficient binding and cross-reactivity [10]. Bioorthogonal reactions and click chemistry (1,3-dipolar cycloaddition between azides and terminal alkynes) are powerful techniques, and advantageous in functionalizing QD with targeted biomolecules as they enable specific reaction sites without undesired side products [75–84]. Many of these methods have been widely adopted and can be found as part of commonly used commercial antibody or protein conjugation kits. Additionally, the stability of terminal alkynes and azides used in click chemistry mean that these methods are compatible with a wide variety of biomolecules (e.g., antibodies, peptides, proteins, enzymes, polysaccharides) and susceptible to hydrolysis or crosslinking as standard coupling reactions often are (e.g., esterification, thiol-maleimide addition) [77, 85]. Importantly, these methods can also be utilized in a high-throughput manner to rapidly produce a library of QDs for subsequent characterization [77].
In click chemistry, reactions can typically occur at room temperature with high yield to form a thermodynamically stable carbon-heteroatom bond [85]. Copper catalyzed azide-alkyne Hüisgen cycloaddition (CuAAC) is commonly used to perform the reaction. However, the successful use of copper in QD functionalization has been not been frequently demonstrated due to the surface trapping of Cu ions and subsequent inhibition of QD photoluminescence, especially in the case of CdSe QDs [85–88].
To address these limitations, methods to enable copper free click chemistry with QDs have progressed rapidly within the last decade [77–79, 89–95]. For example, strain-promoted azide-alkyne cycloaddition (SPAAC) is a very promising and interesting copper free click reaction, with an example reaction scheme shown in Figure 6B. In this reaction, azido-biomolecules can be directly conjugated to QDs surface-functionalized with strained cyclooctyne. Schieber et al. reported the successful use of the copper free cyclooctyne click approach to attach transferrin protein onto the QD surface [78]. The high stability, biocompatibility, and versatility of SPAAC reactions also enables its direct use for dynamic cellular imaging. Using polymeric imidazole ligand-capped QDs prepared through SPAAC reaction, Zhao et al. recently demonstrated the ability to surface label enveloped viruses via the metabolic incorporation of a choline analogue into host cells as a method to facilitate the development of new targeted, therapeutic agents [95].
Additionally, Bawendi et al. developed a tetrazine derivative [3-(4-benzylamino)-1,2,4,5-tetrazine (BAT)] that undergoes click chemistry with strained olefins such as norbornene [96]. To this end, QDs were functionalized with norbornene using polymeric imidazole ligands in a simplistic synthesis. The functionalized QDs were then reacted with BAT-modified biomolecules to conjugate QDs in what has become known as a bio-orthogonal method. After conjugation, the QDs displayed a quantum yield of approximately 60%, maintained approximately the same size as prior to conjugation, and were shown to target endogenously expressed cell receptors [96].
More recently, a novel ligand, 5-norbornene-2-nonanoic acid, has been explored for the flexible functionalization of QDs via click chemistry at nM scale. Interestingly, this new approach avoids the conventional ligand-exchange step by providing a carboxyl at one end of the ligand which binds to the QD surface and providing a norbornene group at the other end of the ligand to enable phase transfer into aqueous solutions and efficient norbornene/tetrazine click reaction [97]. This method is capable of producing water-soluble QDs with a high quantum yield and a small hydrodynamic diameter of around 12 nm at an order of magnitude higher scale than previous methods [97].
QDs can also be derivatized with a modified haloalkane dehalogenase (HaloTag ligand) and subsequently reacted through HaloTag protein mediated site-specific conjugation [80, 98–104]. The haloalkane to HaloTag protein interaction is highly specific and sensitive, with a fast on-rate of ~106 M−1s−1, and can be used to study cellular processes and signaling with minimal toxic effects on regulatory proteins [105]. For example, QDs functionalized with HaloTag ligand conjugates can covalently bind biomolecules tagged with HaloTag protein or to cellular receptors genetically fused to HaloTag protein (i.e. to enable specific labeling of living cells or chemically fixed cells). The Halo-Tag ligand can also react with ligated bromodecanoic acid to form covalent adducts. In another example of dynamic cellular imaging, Liu et al. developed a platform for single-molecule imaging on living cells through QD targeting to cellular proteins using Escherichia coli lipoic acid ligase and HaloTag [106].
5. Approaches to Alter Biodistribution and Toxicity
As discussed in the prior section, substantial progress has been made to improve the surface properties of QDs and improve the solubility and stability in biological environments. However, several challenges still exist in realizing the clinical potential of QDs. Key limitations include the ability to control the QD biodistribution only to target organs, reduce long-term accumulation in the body, and limit the long-term toxicity [22, 28, 30, 32, 73, 107]. Highlighted here is a discussion of potential solutions and recent progress to these ongoing challenges.
5.1. Biodistribution
Investigation of the biodistribution of biological compounds, biomarkers and related systems is of utmost importance in our understanding of the behavior of such systems. For the majority of in vivo applications, QDs are administered directly into the bloodstream via systemic intravenous (i.v.) injection. Since QDs are nanocolloids, their behavior is significantly more complicated than conventional organic dyes in this biological environment. To this extent, the particles encounter multiple lines of defense upon administration to the circulatory system and must overcome several significant biological barriers from organ to cellular level [26–30, 33–40]. These barriers inhibit accumulation and reduce the efficiency of targeted interactions.
First, the bloodstream is comprised of a variety of serum proteins, and those proteins can adsorb to the particle surface through an immune process known as opsonization [36, 38, 40, 107–109]. Consequently, nanocolloids are marked through the formation of a protein corona, and recognized as foreign invading objects by phagocytic cells (predominantly resident macrophages). The particles are then sequestered by the reticuloendothelial system and mononuclear phagocytic system (RES/MPS), and eventually cleared by intracellular lysosome degradation. If not recognized by macrophages, particles can also undergo non-specific uptake by lymphocytes. Additionally, first-pass renal filtering and extraction is another barrier in which foreign particles are quickly removed from circulation. All of these processes combined result in high non-specific and uncontrolled QD accumulation in the liver, spleen, lymph nodes, and kidney [36, 38, 40, 107–109].
QD composition and properties can be rationally designed to slow these clearance mechanisms and increase circulation time in the bloodstream. Gambhir et al. were the first to show the biodistribution of commercially available CdSe QDs in living mice [110]. The results indicated that there was rapid uptake by the liver and spleen, with the most accumulation occurring in the liver on the order of a couple minutes. Once particles entered the RES organs, there was no observation of clearance or metabolism.
Since these studies, there has been significant additional research in controlling and altering the biodistribution of nanocolloids [111–118]. Composition, particle size, particle shape, and surface characteristics are all critical properties that affect the ability to avoid removal by the immune system and extend circulation time in the blood stream [36, 38, 40, 107–109]. In terms of particle size, it has been shown that QDs with sub-10 nm particle sizes are prone to rapid clearance from the body by first-past renal filtration [39, 115, 119, 120]. Additionally, a diameter less than 200 nm is desired to help evade immune recognition and clearance by macrophages [39, 115, 119, 120]. In general, larger particles (in the range of 50–200 nm) have slower removal from the circulation compared with those having smaller size. In terms of particle shape, a rod-like particle shape is advantageous for avoiding protein accumulation, but can lead to a less effective cell internalization compared to spheres [39, 115, 119, 120].
Further, surface charge, hydrophilicity, and material stiffness are very influential for determining the level of protein absorption and play a critical role in determining cell interactions. For example, a net neutral to slightly negative surface charge is desired for circulation as a positive charge enhances uptake by the cells of the phagocytic system and results in a short circulation half-life [39, 115, 119, 120]. In terms of material stiffness, a low elastic modulus is desired to lower the rate of protein accumulation and internalization by macrophages as compared to their stiff counterparts [39, 115, 119, 120].
Coating particles with hydrophilic polymers reduce the clearance rate of particles by the MPS/RES system [39, 121–123]. In particular, polymers can prevent nanoparticles from being recognized as foreign substance, opsonized, and removed from the body. A common strategy is to employ a surface coating of a neutral, soft, hydrophilic polymer, such as polyethylene glycol (PEG).
Depending on the coating density, the PEG can take on a mushroom or extended brush conformation and reduces the interaction with serum proteins [39, 121, 122]. Gambhir et al. found that PEGylation of CdSe QDs did serve to increase particle blood half-life from 20 seconds to 3-4 minutes as well as retard organ uptake to a small extent, from 2 to 6 minutes in the liver. However, it also increased low-level bone uptake. Additionally, all QD formulations tested were still cleared from circulation within 10-20 minutes of injection [110].
The same group expanded their study to investigate the effect of particle size, surface functionalization, and PEGylation on biodistribution of QDs in living mice [124]. Six QD conjugations were studied: two Qdot800 conjugations from Invitrogen (diameter 19-21 nm), one with PEG (2000 MW linear PEG in all instances) and one without; two peptide coated QDs (diameter 11-12 nm), one with PEG and one without; and two indium arsenide (InAs) QDs (diameter 5 nm), one with PEG and one without. Uptake of Qdot800 without PEG by the liver and spleen was rapid, becoming a significant amount in less than 1 minute compared to 6 minutes for the PEGylated Qdot800. Liver activity for both stayed nearly constant for the remainder of the study. Accumulation of peptide coated QDs was slower, taking 19 minutes to reach one standard deviation of the peak liver activity for the PEGylated formulation, and levels in the liver had statistically decreased after 12 hours. The InAs QDs experience rapid accumulation in the liver and spleen, about 2 minutes for both with and without PEG, as well as some activity in the bladder. As with the peptide coated QD, the InAs were cleared from the liver to a significant extent within 6 hours. Different from the other PEGylated QDs, the InAs QDs did not show significant accumulation in the bone. The study concluded that PEGylation and peptide coating both slow RES uptake of QDs, and that the small InAs QDs are able to be renally excreted to some extent [124].
There are additional challenges and biological barriers if the intended QD use is for tumor imaging, diagnostics, or therapy [38, 40, 120, 125]. In this case, a thorough understand and characterization of the tumor microenvironment is important to understand the ability of particles to penetrate and accumulate in the tissue. Abnormal vessels in tumors create a hostile microenvironment: characterized by extreme hypoxia, low pH, and high interstitial fluid pressure. The tumor vessel tortuosity and higher interstitial pressure can lead to an outward convective fluid flow.
Additionally, tumor vasculature grows so rapidly that they develop ~200 nm gaps in the blood vessels. Fortunately, nano-colloid properties can be designed to enable to penetration into the tissue through the gaps and be retained due to poor lymphatic drainage. This passive targeting is commonly referred to as the enhanced permeation and retention (EPR) effect, and is of particular importance in cancer diagnostics and imaging. However, to be able to diffuse through the tumor interstitium and accumulate within organs and tissues, particles should be less than 150 nm in diameter [38, 40, 120].
Ultimately, a key challenge is that many of the strategies used to enhance the biodistribution to target tissues or organs also significantly reduce target cellular interactions and subsequent uptake [36, 108, 113, 120, 126]. For example, a rod-like particle shape is advantageous for avoiding prolonging circulation and passive accumulation in tissue, but it can lead to a significantly lower rate of cellular internalization when compared to spheres or ellipsoids [39, 115, 119, 120]. Additionally, a dense coating of hydrophilic polymer reduces protein accumulation and recognition by the immune system. However, that same coating significantly hinders cell interactions and uptake [39, 121–123]. Surface modification with biological entities targeted to specific receptors (e.g., aptamers, peptides, antibodies, antigens) has shown remarkable success in improving the cell specificity of imaging and diagnostic modalities. Despite many published studies, there have been no resulting widespread conclusions to understand universal factors that influence the biodistribution and target cell interactions [26–30, 33–40].
5.2. Toxicity
The long-term toxicity of QDs is another major concern in biomedical applications, and represents one of the most challenging obstacles to overcome in clinical translation. As discussed above, QD particles have been shown to accumulate and reside in the liver and spleen of living mice [110]. The toxicity stems primarily from four areas: i) heavy metal materials commonly used in the particle core; ii) chemistries with the potential to release toxic components from the surface (i.e. especially for coatings composed of Cd, Se, and Hg); iii) any free radicals or reactive species generated from excitation; and iv) the tissue and nano-colloid interaction in biological environments (e.g., small particle size, positive surface charge, chemical composition) [26–30, 72, 127–132].
Heavy metal toxicity has been well documented in the literature, especially as the metals have shown an extremely long biological half-life (greater than 10 years) and been shown to readily cross the blood-brain barrier [30–32, 107, 133]. Although surface coatings can be used to limit the release of toxic agents from the QDs surface, it has been recently reported that in some occasions the coating itself might be even more toxic [26–29]. The greatest risk of toxic effect is due to the release of Cd2+ ions from the nanoparticles, which can be somewhat controlled by encapsulation of the QDs in a passivating ZnS shell or crosslinked polymer coating [134, 135]. However, adequate long-term in vivo studies have not yet been performed to determine if these measures offer protection against ion release into the body. Consequently, there has been movement toward Cd-free NIR QDs composed of III-V or I-III-VI2 semiconducting metals such as CuInSe [136] and CuInS2 [135].
Pons et al. developed a novel synthesis of CuInS2 using a primary amine precursor [135]. The result was brighter QDs of about 3 nm in diameter with an emission peak around 800 nm and a quantum yield of 10-20%. After addition of a ZnS shell, the diameter increased to about 5.5 nm and quantum yield increased to 30%. After transfer into water by phospholipid micelle encapsulation, the QDs maintained relatively high quantum yields and were stable for multiple weeks. These CuInS2/ZnS QDs, as well as traditional CdTeSe/CdZnS QDs, were then injected subcutaneously into the paws of live mice, and the effect of the QDs on the regional lymph nodes was observed. Both compositions showed accumulation in the lymph nodes, but only the CdTeSe/CdZnS QDs were accompanied by a significant amount of inflammation, which presumably reflects the acute toxicity of the Cd2+ ions [135].
Another report described CuInxSey/ZnS QDs with a much higher quantum yield of 40-60% following passivation [136]. However, the stability of these QDs was limited to 1-2 days, mainly due to aggregation. Modification of the coordinating ligand improved the stability to 10-14 days, but this is still not optimal for commercialization. These QDs were coated in a lipid layer and injected into mice; after 90 minutes, fluorescence intensity started to decay. At 48 hours, the fluorescence level had returned to the baseline, indicating destabilization of the particles or clearance from the body. There were no observed bright spots, suggesting that no aggregation or accumulation in organs occurred, but this needs to be confirmed by further in vivo studies [136]. Though there is need for improvement in the synthesis, quantum yields, and stability of these cadmium-free QDs, preliminary studies indicate favorable toxicity over traditional cadmium QDs and further investigation is worth pursuit.
To further advance in reducing toxicity of QDs, it is recommended to fabricate the semiconductor nanoparticles with optimized colloidal stability under physiological conditions, to control QD size and to improve surface properties for better cellular uptake. Moreover, QDs can be passivated or bioconjugated with antibodies, polymers such as polyethylene glycol (PEG) or nanoparticles like micelles. The latter reduce QDs toxicity without altering their surface and optoelectronic properties. Last but not least, it should be reminded that toxicity of QDs could also be helpful for cancer treatment. For instance, photo-induced toxicity of QDs coupled to molecular photosensitizers has been explored for causing apoptosis of cancer cells [137].
6. Recent Progress and Trends in Biomedical Applications
From the time quantum dots were first conjugated to a biological molecule in 1998, they have evolved into a potent tool for fluorescent bioimaging, including intracellular and single molecule imaging. Highlighted here is a selection of recent and progressive reports of QD development and application to biological imaging, diagnostic tools, and therapeutic application.
6.1. Ex vivo Analyte Detection and Molecular Diagnostics
Recent studies in literature have also focused on the development of functionalized QDs to create diagnostic tools to enable more sensitive ex vivo identification of disease-relevant biomolecules [138–150].
In a recent study, Zhang et al. reported the development of dopamine-functionalized QDs for use in a redox-mediated immunoassay in order to detect the cancer biomarker alpha-fetoprotein (AFP) in human serum samples [70]. The authors developed ~12 nm CdSe/ZnS QDs functionalized with dopamine through self-assembly of surface-capped ligand dopamine onto the QD surface through the sulfhydryl group. In the sandwich redox-mediated indirect fluorescence immunoassay (RMFIA), the authors used tyrosinase in order to catalyze enzymatic oxidation of dopamine on the surface of the functionalized QDs, leading to fluorescence quenching in the presence of the analyte AFP. The authors successfully demonstrated the ability to detect AFP concentrations as low as 10 pM, concluding that this assay has great potential as a rapid, sensitive detection method [70]. Further, the authors subsequently demonstrated clinical application through the detection with serum samples collected from hepatocellular carcinoma patients versus normal subjects, confirming the method applicability in clinical ex vivo biological testing.
Avidin-biotin binding has also been used in a similar application of QDs in competitive fluoroimmunoassay development for the detection of human serum albumin (HSA) in human urine [151]. Biotinylated QDs were prepared via the bioconjugation of CdTe QDs with denatured bovine serum albumin (dBSA) at the QD surface. The conjugation strategy employed also allowed for flexibility in further surface modification, as the albumin can be used as a bridge for additional ligand functionalization [151]. It was observed that for this application, an excess of 6.7 dBSA to QD was required for complete derivatization of QDs, and the highest quantum yield of 15% was achieved by the resulting 400 nm diameter bioconjugated QDs. These were much larger particles with a lower quantum yield compared to unconjugated QD, but they were shown to be stable for up to two months and able to detect increased levels of HAS in urine using a fluorescence assay [151]. The authors successfully demonstrated the ability to detect HSA concentrations in urine as low as 1.0 μg/mL, with a relatively broad working range from 1.7 to 10 μg/mL.
Qiu et al. developed a bioresponsive QD-paper-based analytical device as a high-performance molecular diagnostic method to monitor disease-relevant levels of carcinoembryonic antigen (CEA) analyte in human serum samples [71]. In this research, the authors used CdTe/CdSe QDs and glucose oxidase immobilized on paper to develop a visual fluorescence assay based on CEA aptamer/complementary DNA (cDNA)-gated release of glucose from mesoporous silica nanocontainers. The glucose is released from the nanocontainers, which are oxidized by the glucose oxidase and produce hydrogen peroxide to quench the fluorescence of the CdTe/CdSe QDs. The authors demonstrated that the fluorescence detection could be visualized by the naked eye and on a commercial spectrometer. Sensitive and accurate discrimination of CEA was reported with a linear range of 0.05-20 ng mL−1 and high reproducibility [71].
To enable a more rapid and accurate approach for colorectal cancer diagnosis, Park et al. developed a multiplexed detection approach using the combination of an enzyme-sensitive fluorescence dye and antibody-QD conjugates [152]. In this study, a cresyl violet-glutamic acid (CV–Glu) derivative was used as the fluorescent molecular probe, as it is capable of rapidly switching between two fluorescent colors in response to enzymatic activity of λ-glutamyltranspeptidase (GGT). A complementary QD probe was developed through bioconjugation of matrix metalloproteinase-14 antibody (MMP14) to biocompatible, 690 nm emitting AgInS2/ZnS (core/shell) QD. The probes were tested in both in vitro and ex vivo applications, using human colorectal cancer cell lines, ex vivo murine model tissues, and ex vivo patient tumor colon specimens. The authors used two-photon microscopy to demonstrate that co-application of both probes were able to rapidly permeate from the tissue surface at 10-20 μm depths. Ultimately, the study showed that co-application of both probes enabled rapid (within 5 minutes) and accurate visualization of tumor lesions difficult to detect via conventional colonoscopy techniques [152]. Additionally, the dual-probe technique was able to identify regions with hyperplastic polyps and adenomas possibly in the early stages of colorectal cancer [152].
Molecular changes in tissue are extremely complex and difficult to study the progression of disease. This represents a significant and major challenge still remaining in the biomedical field, particularly in cancer [153]. Additionally, heterogeneity in tumors leads to significant challenges in the development of targeted therapeutics. To this extent, the use of QDs has shown great promise to enable noninvasive ex vivo cell labeling and diagnostic imaging in tumor specimens, and ex vivo investigation of the molecular markers and major dynamic processes of cancer – migration, invasion, and metastasis [19, 142, 154–160].
In particular, one study explored the effect of breast cancer invasion on the extracellular matrix (ECM) using QD-based multicolor imaging of human epidermal growth factor (HER2) and type IV collagen [161]. It is difficult to achieve multicolor imaging with traditional stains and organic molecules due to unreliable specificity and photo-bleaching caused by excitation over many different wavelengths, but different color QDs can be excited by a single wavelength and have more consistent biomarker quantification relationships, alleviating these issues to some extent [161]. Clinical human HER2 positive breast cancer tissue was incubated with HER2 and type IV collagen primary antibodies, followed by incubation with QDs conjugated with the secondary antibody, and imaged using a fluorescent microscope. The researchers were able to visually show that visually that high levels of HER2 expression is in fact correlated with increased ECM destruction, breast cancer cell invasion, and possibly even angiogenesis. The researchers concluded that this imaging modality could provide a beneficial method of diagnosis and aid in determination of treatment strategy [161].
In another recent study, multiplexed QDs-antibody conjugates and wavelength-resolved spectral imaging were used to enable high-throughput molecular mapping of tumor heterogeneity using clinical prostate cancer specimens [153]. Here, a sequential staining method of primary antibodies with QD-secondary antibody conjugates was used to build the degrees of multiplexing. The study evaluated four protein biomarkers (E-cadherin, high-molecular-weight cytokeratin, p63, α-methylacyl CoA racemase), and demonstrated accurate detection and identification of structurally distinct prostate glands and single malignant tumor cells from human specimens [153]. Further, the authors used high-throughput molecular mapping to create profiles of the tumor tissue, revealing the extensive heterogeneity at both the molecular, cellular, and architectural levels. Fundamentally, the study demonstrated the ability to visualize gland morphological and structural transitions during prostate cancer progression, from a double layer of basal and luminal cells to a single layer of malignant cells [153].
6.2. Real-Time in vivo Imaging
In order to be more applicable in real-time biomedical imaging, diagnosis, and image-guided surgical applications, imaging probes need to be capable of deep tissue imaging [20, 22, 26–28, 30]. Light in the visible range (below 700 nm) is not capable of penetrating into deep tissue due to high levels of scattering from natural absorbers (e.g., lipids, deoxyhemoglobin, water) [23, 26]. QDs have a large two-photon cross section, meaning it is possible to use near-infrared (NIR) irradiation with nanoparticles that emit around 650-900 nm wavelengths [135]. This is known as the tissue transparency window, as absorption by water and tissue is very weak in this range, making deep tissue imaging possible. Further, QDs make excellent probes for real-time in vivo imaging as they are 100 to 1,000 times more intense than conventional organic fluorophores and are capable of multiphoton imaging with excitation by a single wavelength [22, 135].
Recently, there has been significant success reported using QDs for real-time imaging application and image-guided surgery [155–160, 162–165]. In particular, near-infrared (NIR) QDs have emerged as a remarkable in vivo imaging modality [157, 166–180]. Compared to the visible spectral window commonly employed for conventional fluorescence imaging, the ideal spectrum to enable deep in vivo imaging needs to have reduced scattering, negligible tissue or biological autofluorescence, minimal absorption, and yield a higher signal-to-noise ratio [166, 172, 176, 179]. To this extent, both the first NIR (NIR-I, 700 to 900 nm) and the second NIR (NIR-II, 1000 to 1700 nm) spectral windows offer significant advantages to enable ultrahigh spatial resolution with increased penetration depths [166, 176, 177].
Comparably, the NIR-II window is more advantageous than NIR-I as it can yield higher imaging clarity than achievable in NIR-I, owed in part to the dramatically reduced photon scattering, absorbance, and tissue autofluorescence at the higher wavelengths [166, 176, 177]. Though the NIR-II range is defined broadly, imaging in the NIR-IIa (1300 to 1400 nm) and NIR-IIb (1500 to 1700 nm) windows can result in additional improvements [166, 181, 182]. However, imaging in the NIR-II window is a relatively newer research area as it has only been readily achievable within the last decade [166, 168, 172, 176, 177, 179, 180]. Recent advances in both chemistry and optical imaging capabilities have enabled the development of NIR fluorophores and QDs with longer wavelengths as well as the development of more advanced photodetectors with sufficient sensitivity and efficiency for NIR-II imaging [157, 166–171, 173–175, 178, 183–185].
Recently, Bruns et al. have reported the development of next-generation NIR-II QDs capable of deep tissue penetration, high spatial imaging resolution, multicolor imaging, and rapid acquisition for a variety of in vivo imaging applications [185]. In this work, a class of heavy metal-free indium/arsenide (core/shell) QDs was developed and demonstrated to exhibit a narrow, size-tunable emission and significantly higher quantum yield (up to 30%) than other literature reports. Further, the authors demonstrated composition-tunability of the QDs and showed that the surface is easily modified with a variety of functional ligands for broad use in applications such as bioimaging, molecular tracking, and structural mapping. Applicability of the QDs was demonstrated using an in vivo murine model to enable the real-time imaging and quantification of lipoprotein metabolic turnover rates, heartbeat and breathing rates, and three-dimensional quantitative flow maps of the mouse brain vasculature [185].
In a similar application, Li et al. reported the ability to visualize tissue blood flow and angiogenesis real-time in vivo through the development of NIR-II QDs [186]. Hydrophilic, PEGylated silver sulfide (Ag2S) QDs were synthesized through ligand exchange and carbodiimide coupling, resulting in peak emission at 1200 nm and a quantum yield of up to 16%. Mouse studies confirmed the high stability and long circulation time of the QDs in vivo, and the QDs were capable of deep tissue penetration with ultrahigh spatial and temporal resolution (~40 μm) [186]. Ultimately, the study demonstrated the ability to perform real-time, noninvasive and dynamic visualization of lymphatic drainage and vascular networks, which may aid in image-guided surgery as well in assessment of blood supply in tissues and organs and the screening of anti-angiogenic therapeutic treatment efficiency [186].
Another of the remaining challenges in the in vivo application of QDs has been in the successful engineering of water-soluble, biocompatible QDs that emit in the 650-900 nm range [20, 23, 30]. In one approach, CdTe/CdSe QDs were loaded into poly(lactic-co-glycolic acid) (PLGA) nanospheres for the ultimate purpose of tumor imaging [134]. Unmodified QDs were encapsulated so that the highest possible quantum efficiency could be preserved, while the PLGA coating created a stable, highly water-soluble shell. PLGA also has a high degree of biocompatibility and can biodegrade. The particles had an emission peak of 760 nm, and exhibited a quantum yield of approximately 50% [134]. These are ideal properties for NIR bioimaging. It was also shown that pH range had minimal effect on the stability PLGA encapsulated QDs, and that exposure to proteins in biological serum did not greatly change the optical properties of the QDs. The results indicate that there is very little escape of QDs from the polymer nanosphere, causing insignificant cytotoxicity on the order of two days, but further studies are needed to assess the effect of PLGA biodegradation over an extended period of time [134]. Confocal laser scanning microscopy revealed high cellular uptake of the particles by two different cell lines [134]. These are promising results for transforming QDs into a real-time in vivo tissue imaging tool, with the acknowledgement that a deeper investigation into long-term, repeated toxicity and biodistribution still remains.
Another major application of quantum dots is targeted tumor imaging, as the small size of QDs facilitates tumor accumulation by leaky vasculature around tumor sites due to the enhanced permeability and retention (EPR) effect [2]. With only passive accumulation, applications are limited by poor tumor specificity. To this extent, the use of the previously discussed bioconjugation strategies with cancer-specific targeting moieties has significantly enhanced the targeting efficiency.
For example, Liu et al. recently reported the development of biocompatible NIR QDs capable of in vivo cation exchange to enable tumor-specific imaging [187]. The system was designed to achieve tumor-specific imaging first through passive accumulation of the QDs in tumor tissue, followed by induction of cation exchange in excess QDs remaining in the circulation to quench any remaining background signals [187]. The QD core was comprised from Zn2+, Hg2+, Se2−, and S2−, and PEGylated to reduce rapid clearance by the MPS system. A membrane-impermeable etchant was developed from Ag+ ions stabilized with the metal chelator thiosulfate (TS) to serve as a cation donor to the QDs, where the etchant can provide silver ions to rapidly quench the QD photoluminescence when exchanged with the QD cations (Zn2+, Hg2+) [187]. Using orthotopic breast and pancreatic tumors in mice, the study demonstrated a highly tumor-specific signal with minimal toxicity, demonstrating in vivo cation exchange as a promising strategy to enhance the specificity of QDs in tumor imaging [187].
6.3. Intracellular Imaging, Delivery, and Therapeutic Modalities
In the therapeutic application of QDs, photodynamic therapy (PDT) has been extensively studied [188–208]. PDT utilizes the application of light to activate a photosensitizing agent, which in turn transfers its triplet state energy to form reactive singlet oxygen species (ROS) [189]. These reactive species can then initiate apoptosis of local cancer cells. PDT has been widely studied due to its potential for more selective and specific treatment than conventional chemotherapy [189]. However, application of conventional PDT agents is often limited by low singlet oxygen quantum yields, photo-bleaching, and in vivo toxicity [209].
The use of QDs as photosensitizing agents has demonstrated the potential to overcome some of these limitations. QDs comprised from CdSe, CdTe, carbon-based materials, and others have been studied in the literature for PDT applications as either QDs alone, QDs in the presence of other photosensitizing agents (e.g., phthalocyanine, phthalocyanine derivatives, porphyrin, chlorine e6), or QDs conjugated to another photosensitizing agent [189, 194, 196, 198, 200, 203–208]. QDs have been shown to display superior photostability and aqueous solubility, but the clinical translation has been hindered by significant toxicity to health tissue and low ROS-generation efficiency [196, 198, 200, 204, 209].
To this extent, Ge et al. have reported the development of a novel PDT agent with high singlet oxygen generation based on highly water-dispersible graphene QDs [209]. The graphene QDs were synthesized through a hydrothermal method using polythiophene derivatives as a carbon source [209]. The QDs were able to produce ROS through a multi-state sensitization process, exhibited a broad absorption band with a strong emission peaking at 680 nm, displayed superior photostability to the more conventional CdTe QDs, and resulted in a ROS quantum yield (~1.3) significantly higher than similar studies reported in literature. An in vivo murine xenograft model was used to evaluate the graphene QDs for dual PDT and bioimaging in breast cancer. In terms of real-time tumor imaging, the study demonstrated a high signal-to-noise ratio of 229.5, and no apparent fluorescence intensity decay or diffusion observed at the injection sites, even 1 week after injection [209]. In terms of efficacy, tumors began to decompose 9 days after light exposure and QD activation, and were fully destroyed after 17 days with no tumor regrowth observed for up to 50 days [209]. In a second study, the authors further demonstrated the applicability of the graphene QDs as multifunctional fluorescent, photoacoustic, and thermal theranostics for simultaneous diagnosis and therapy of cancer [210].
Using nitrogen-doped graphene-based QDs, Kuo et al. reported dual photodynamic antimicrobial therapy and bioimaging [211]. The developed nitrogen-doped QDs were capable of generating a higher amount of reactive oxygen species than a nitrogen-free QDs after only 3 minutes of photoexcitation (670 nm laser, 0.1 W cm−2), and demonstrated an increase in PDT antimicrobial efficiency with higher nitrogen-binding compositions [211]. Additionally, the study demonstrated that the NIR and high photostability properties of the graphene QDs enabled better contrast and imaging of the bacteria.
QDs can also be utilized to deliver and monitor the efficacy of a therapeutic payload, as attached cargo quenches the fluorescence of the particles by fluorescence resonance energy transfer (FRET) [60]. These platforms hold great promise to enable the study of the pharmacokinetic and pharmacodynamic behavior of chemotherapeutic agents, and monitoring of disease progression and the therapeutic effect.
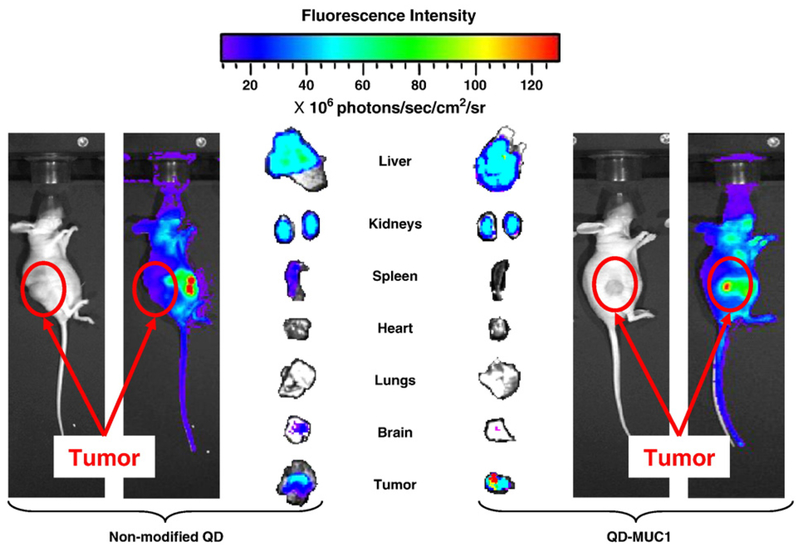
To this extent, Minko and colleagues recently reported a QD carrier with doxorubicin (DOX) conjugated via a pH sensitive hydrazone bond, coupled with a DNA aptamer targeted to MUC1 [60]. This system was engineered to advantageously increase delivery into ovarian cancer cells, avoid systemic effects of chemotherapy by employing targeted delivery, and provide an effective imaging modality to monitor drug release. Increased uptake of the QD-MUC1-DOX particles over unmodified QD by DOX-resistant A2780-AD human ovarian cancer cells was confirmed, as was release of the anti-cancer agent DOX within the cells. In vivo distribution of the targeted and non-targeted QD, evaluated in a nude mouse model, indicated that the targeted QD conjugate showed higher accumulation in the tumor and less accumulation in other organs, compared to the non-targeted QD, shown in Figure 7 [60].
Figure 7.
Real-time in vivo imaging of non-targeted versus targeted QD biodistribution in a nude mouse model 24 hours after intravenous injection of QDs. Fluorescence intensity reflects accumulation of QDs; blue represents lowest intensity and red represents highest. Targeted QDs showed greater accumulation at the tumor site than non-targeted QDs. Adapted from [60] with permission from Elsevier.
QDs represent an exciting candidate to enable tumor theranostic platforms, and developing nanocarriers co-loaded with chemotherapeutic agents and QDs has been a prominent goal in recent literature. For example, paclitaxel along with CdTe/CdS/ZnS QDs loaded in nanostructured lipid carriers showed an encapsulation efficiency of around 80%, drug loading of 4.7% and a tumor growth inhibition rate of more than 77% [154]. Other additional recent approaches include quercetin loaded CdSe/ZnS QDs as efficient antibacterial and anticancer nanoplatforms [212] or 5-Fluorouracil loaded onto Mn-ZnS QDs encapsulated with chitosan biopolymer and conjugated with folic acid to enhance targeting efficacy towards folate receptors expressing malignant tissues [213].
QDs have also been utilized to facilitate gene therapy through intracellular delivery and imaging of treatment with small interfering RNA (siRNA). siRNA are short chain s of double-stranded RNA that have the ability to specifically silence genes via a endogenous pathway known as RNA interference (RNAi) [214]. RNAi is defined as the process of messenger RNA cleavage by sequence-specific complementary siRNA, and may be applied as a therapeutic for diseases that result from aberrant gene expression. For RNA interference to occur, siRNA must be delivered into the cell cytoplasm.
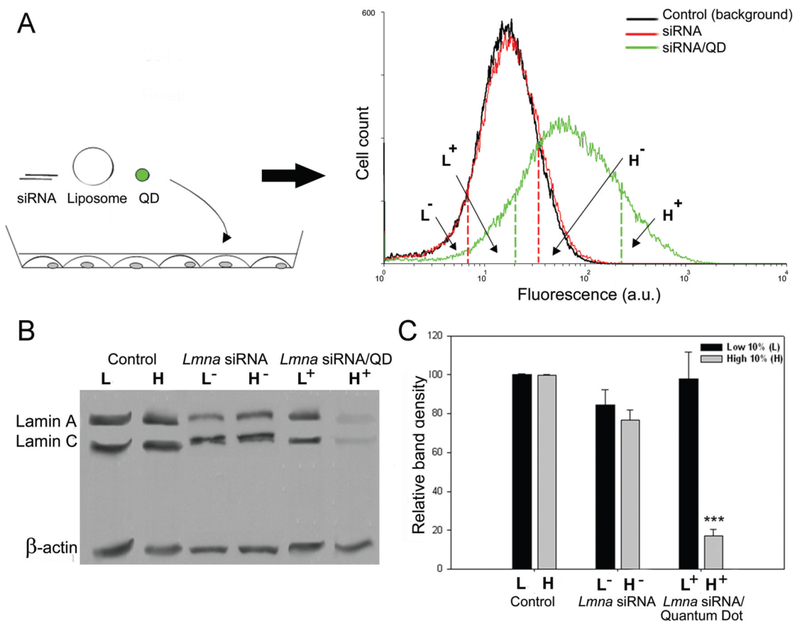
Typical intracellular vehicles for siRNA include cationic polymer nanoparticles and micelles, as well as liposomes. One of the earliest reports appeared in 2005, in which QDs were used to observe siRNA transfection in vitro [215]. In this study, CdSe-core QDs, ZnS-shell QDs, and/or siRNA (Lamin A/C gene target) were encapsulated in the commercially-available cationic-lipid reagent, Lipofectamine (Invitrogen). Subsequently, fibroblasts were transfected, and siRNA uptake and silencing were analyzed via flow cytometry, western blotting, and immunofluorescence staining. As shown in Figure 8, the study showed a strong correlation between gene knockdown and fluorescent intensity in cells transfected with both the QDs and siRNA, demonstrating that QDs are a suitable probe to track siRNA delivery. Further, the study also demonstrated that the QD-mediated siRNA delivery was able to increase the in vitro gene silencing efficiency [215]. The formulation delivering both the QDs and siRNA together resulted in a 90% silencing efficiency. This was significantly higher than the control formulation delivering only siRNA, which resulted in 20-30% silencing efficiency.
Figure 8.
Cd/Se core-Zn/S shell QD/siRNA complexes enable sorting of transfected cell populations. (A) A schematic representing the transfection of fibroblast cells with liposome/siRNA/QD complexes. Fluorescence was measured using flow cytometry, and the histograms show control cells, siRNA-treated cells, and QD/siRNA-treated cells. (B) Western blot of Lamin A/C expression after transfection (β-actin as loading control). Cells with a high level siRNA/QD fluorescence show knockdown of the Lamin genes. (C) Band densitometry analysis of the western blots. Adapted from [215] with permission from Oxford University Press.
QDs with a positive surface charge have also been used as carriers of siRNA themselves, eliminating the need for an additional transfection agent such as Lipofectamine. In one reported study, L-arginine (L-arg)-modified CdSe/ZnSe QDs, both with and without β-cyclodextrin (β-CD), were used as siRNA delivery vehicles to target gene silencing of HPV18 E6 [216]. The purpose of modifying the QD surface with L-arg was to provide a large, positively charged surface area onto which negatively charged siRNA was complexed via electrostatic attraction. Subsequently, the QD and siRNA complexes were transfected into HeLa cells. Cell uptake and trafficking was monitored via flow cytometry and confocal microcopy, respectively, and efficacy was analyzed via RT-PCR and western blotting. The study demonstrated that the complexes were cytocompatible (defined in the study as greater than 70% viability) at QD concentrations less than 70 μg/mL after 24 hours of exposure. Further, the QD and siRNA complexes were able to achieve nearly 80% gene knockdown and approximately 80% target protein suppression [216].
More recently, cadmium sulphoselenide/zinc sulfide quantum dots (CdSSe/ZnS QDs)-based nanocarriers loaded with siRNA have been evaluated for targeting human telomerase reverse transcriptase (TERT) of central nervous system tumor cells [127]. Results showed high gene transfection efficiency (>80%) on two glioblastoma cell lines. Furthermore, silencing of TERT gene expression significantly inhibited the proliferation of glioblastoma cells.
Another intriguing approach involves using QDs and siRNA for exploring the role of genes such as SOX9 in the chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Authors fabricated functional quantum dot (QD) nanoplexes by sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC) activation of PEG-coated CdSe/ZnS QDs as the gene carrier of siRNA to explore the effect of SOX9 RNA interference on MSCs [217]. Interestingly, SOX9 knockdown inhibited the expression of cartilage-specific markers in MSCs, delaying cartilage repair.
7. Conclusions and Future Outlook
Quantum dots (QDs) present a versatile tool to enable more accurate diagnostic tools and fluoroimmunoassays, multiplexed imaging, dual imaging and therapeutic platforms, real-time in vivo and cellular process imaging, and tracking of single cells and biological molecules. With the widely increased interest in inorganic particles over the last decade, the design and development of QDs has advanced significantly.
Recent advances in synthesis and bioconjugation strategies have rapidly progressed the ability to ensure accurate control over QD size, polydispersity, quantum yield, and surface properties. The developed strategies are versatile and flexible, enabling QDs to also be used in more complex hybrid nanomaterials. Additionally, these strategies can yield enhanced colloidal stability as well as provide a robust platform for further bioconjugation. However, investigation, quantification, and control over the spatial distribution of functional groups is often not addressed literature reports. This will become more important to consider and address with increasing biological complexity of the intended application.
While QDs as biological probes have demonstrated their potential across a variety of fields, the most promising reports to date are limited to in vitro and ex vivo applications. In these applications, QDs can be readily substituted for and are significantly more advantageous than traditional fluorophores, outperforming in nearly every aspect. For example, the development of novel and advanced QD conjugation strategies with a wide variety of biologically-active molecules has opened up several avenues towards creating advanced bioimaging techniques. As discussed previously, conjugation with molecules such as proteins, oligonucleotides, and cell-targeted ligands can enable profound discoveries in the study of cellular mechanisms and molecular structures. Additionally, QD applications with ex vivo patient tissues and fluids have the potential to significantly improve our understanding of disease progression, enable more rapid and accurate diagnosis, and create improved tools to aid in the identification and design of new drugs for a variety of diseases.
As acute and long term toxicity are not major concerns, in vitro and ex vivo applications of QD-based approaches are likely to continue progressing rapidly in the next decade. With further validation of the technologies, these QD-based approaches could also make significant strides towards enabling the pre-treatment analysis and prediction of therapeutic efficacy in specific patients – a significant goal towards the realization of personalized medicine.
In spite of these advances, there still remain several significant limitations and regulatory issues that need to be addressed before the application of QDs in medicine and in vivo imaging in human subjects [218]. A key challenge remaining is the inefficient delivery from low biological specificity and poorly controlled biodistribution to target tissues. The biodistribution of current QD compositions show inadequate clearance from real-time in vivo studies, and the long-term accumulation of QDs is still largely unknown.
However, the greatest challenge to the clinical translation of QDs is the risk of severe acute and long-term toxicity. More specifically, there is limited understanding of the biological interaction of QDs reported in literature, and there is a critical need to develop a detailed understanding of the relationship between then QD physicochemical properties and their pharmacokinetic and pharmacodynamic behavior. In an effort to reduce safety concerns, research should also focus towards a better replacement of the heavy metal-based QDs, as these materials have dominated current bioimaging studies especially with cadmium being used a prominent core material [219, 220].
The ultimate clinical success of QDs will depend on the ability of future research overcome these limitations. If research does not continue to work towards addressing these limitations, QDs will remain only for use in animal and laboratory research. The advent of advanced synthesis routes, improved heavy-metal-free QDs, and a deeper understanding of the structure-function relationship of QDs at biological interfaces are critical. Ultimately, we believe that researchers will be able to address these challenges and continue to push QDs forward towards their clinical potential.
Acknowledgements
A.M.W. and J.M.K. contributed equally to this work. The work was supported in part by a grant from the National Institutes of Health (EB000246, EB012726, EB022025, GM56321) and the Cockrell Family Regents Chair in Engineering (UT Austin). A.M.W. and J.M.K. were supported by the National Science Foundation Graduate Research Fellowship (DGE-1610403) and the Philanthropic Educational Organization Scholar Award. A.M.W. was also supported by the S.E.S.H.A. Endowed Graduate Fellowship in Engineering (UT Austin) and the Marion Johnson South Texas Section Society of Plastics Engineers Endowed Presidential Scholarship in Chemical Engineering.
Abbreviations
- QD
quantum dot
- MBE
molecular beam epitaxy
- Me2Cd
dimethyl cadmium
- TOPSe
trioctylphosphine selenide
- TOPTe
trioctylphosphine telluride
- (TMS)2Se
bis(trimethylsilyl)selenium
- TOP
trioctylphosphine
- TOPO
trioctylphosphine oxide
- CdS
cadmium sulfide
- Cd(Ac)2
cadmium acetate
- CdSe
cadmium selenide
- CdTe
cadmium telluride
- NIR
near-infrared
- PL
photoluminescence
- ZnS
zinc sulfide
- ZnEt2
diethylzinc
- (TMS)2S
bis(trimethylsilyl)sulfide
- MUA
mercaptoundecanoic acid
- DHLA
dihydrolipoic acid
- PEG
polyethylene glycol
- TEG
tetra(ethylene) glycol
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide)
- NHS
N-hydroxysuccinimide
- dBSA
denatured bovine serum albumin
- BAT
3-(4-benzylamino)-1,2,4,5-tetrazine
- siRNA
small interfering RNA
- RNAi
RNA interference
- L-Arg
L-arginine
- β-CD
β-cyclodextrin
- EPR
enhanced permeability and retention
- FRET
fluorescence resonance energy transfer
- DOX
doxorubicin
- ECM
extracellular matrix
- HER2
human epidermal growth factor
- PLGA
poly(lactic-co-glycolic acid)
- RES
reticuloendothelial system
- InAs
indium arsenide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cormode DP, Skajaa T, Fayad ZA, Mulder WJM, Nanotechnology in Medical Imaging: Probe Design and Applications, Atertio. Thromb. Vasc. Biol 29(7) (2008) 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cho EC, Glaus C, Chen J, Welch MJ, Xia Y, Inorganic nanoparticle-based contrast agents for molecular imaging, Trends Mol. Med 16(12) (2010) 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lim CT, Han J, Guck J, Espinosa H, Micro and nanotechnology for biological and biomedical applications, Med Biol Eng Comput 48(10) (2010) 941–943. [DOI] [PubMed] [Google Scholar]
- [4].West JL, Halas NJ, ENGINEEREDNANOMATERIALS FORBIOPHOTONICSAPPLICATIONS: Improving Sensing, Imaging, and Therapeutics, Annu. Rev. Biomed. Eng 5(1) (2003) 285–292. [DOI] [PubMed] [Google Scholar]
- [5].Yezhelyev MV, Qi L, O’Regan RM, Nie S, Gao X, Proton-sponge coated quantum dots for siRNA delivery, Journal of American Chemical Society 130(9006-9012) (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].TWENTY ATTAIN 2006 TOP HONORS FROM THE OPTICAL SOCIETY OF AMERICA, 2006. http://www.osa.org/about_osa/newsroom/news_releases/releases/09.2006/awards.aspx (Accessed November 30, 2011.
- [7].Efros AL, Efros AL, Interband absorption of light in a semiconductor sphere, Soviet Physics Semiconductors Ussr 16 (1982) 772–775. [Google Scholar]
- [8].Ekimov AI, Onushchenko AA, Quantum size effect in three-dimensional microscopic semiconductor crystals, Journal of Experimental and Theoretical Physics 34 (1981) 363–366. [Google Scholar]
- [9].Smith A, Duan H, Mohs A, Nie S, Bioconjugated quantum dots for in vivo molecular and cellular imaging, Adv. Drug Del. Rev 60(11) (2008) 1226–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mattoussi H, Palui G, Na HB, Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes, Adv. Drug Del. Rev (2011). [DOI] [PubMed] [Google Scholar]
- [11].Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T, Quantum dots versus organic dyes as fluorescent labels, Nature Methods 5(9) (2008) 763–775. [DOI] [PubMed] [Google Scholar]
- [12].Murray CB, Norris DJ, Bawendi MG, Synthesis and characterization of nearly monodisperse CdE (E=S, Se, Te) semiconductor nanocrystallites, Journal of American Chemical Society 115 (1993) 8706–8715. [Google Scholar]
- [13].Hines MA, Guyot-Sionnest P, Synthesis and Characterization of Strongly Luminescing ZnS-Capped CdSe Nanocrystals, The Journal of Physical Chemistry 100(2) (1996) 468–471. [Google Scholar]
- [14].Qu L, Peng ZA, Peng X, Alternative routes toward high quality CdSe nanocrystals, Nano Lett 1(333-337) (2001). [Google Scholar]
- [15].Yong K-T, Law W-C, Roy I, Jing Z, Huang H, Swihart MT, Prasad PN, Aqueous phase synthesis of CdTe quantum dots for biophotonics, Journal of Biophotonics 4(1-2) (2011) 9–20. [DOI] [PubMed] [Google Scholar]
- [16].Bailey RE, Nie S, Alloyed Semiconductor Quantum Dots: Tuning the Optical Properties without Changing the Particle Size, Journal of American Chemical Society 125 (2003) 7100–7106. [DOI] [PubMed] [Google Scholar]
- [17].Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG, (CdSe)ZnS Core-shell Quantum Dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites, J. Phys. Chem. B 101 (1997) 9463–9475. [Google Scholar]
- [18].Mcbride J, Treadway J, Feldman LC, Pennycook SJ, Rosenthal SJ, Structural basis for nearly unity quantum yield core/shell nanostructures, Nano Lett 6 (2006) 1496–1501. [DOI] [PubMed] [Google Scholar]
- [19].M.K. J, Lihong J, B.A. M, Surangi J, Wen T, Mingyuan G, Robert L, Ana J, Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents, Adv. Mater 30(18) (2018) 1706356. [DOI] [PubMed] [Google Scholar]
- [20].Aswathy RG, Yoshida Y, Maekawa T, Kumar DS, Near-infrared quantum dots for deep tissue imaging, Anal. Bioanal. Chem 397(4) (2010) 1417–1435. [DOI] [PubMed] [Google Scholar]
- [21].Kumar S, Nanomaterials for Tumor Targeting Theranostics. A Proactive Clinical Perspective. Edited by Mingqian Tan and Aiguo Wu. World Scientific, 2016. Pp. 432. Price GBP 120.00 (Hardcover). ISBN 978-981-4635-41-7, Acta Crystallographica Section D 73(7) (2017) 626–627. [Google Scholar]
- [22].Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T, Quantum dots versus organic dyes as fluorescent labels, Nat. Methods 5 (2008) 763. [DOI] [PubMed] [Google Scholar]
- [23].Frangioni JV, In vivo near-infrared fluorescence imaging, Curr. Opin. Chem. Biol 7(5) (2003) 626–634. [DOI] [PubMed] [Google Scholar]
- [24].Li C, Zhang Y, Wang M, Zhang Y, Chen G, Li L, Wu D, Wang Q, In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window, Biomaterials 35(1) (2014) 393–400. [DOI] [PubMed] [Google Scholar]
- [25].Wang D, Rogach AL, Caruso F, Semiconductor quantum dot-labeled microsphere bioconjugates prepared by stepwise self-assembly, Nano Lett 2 (2002) 857–861. [Google Scholar]
- [26].Mukherjee A, Shim Y, Myong Song J, Quantum dot as probe for disease diagnosis and monitoring, Biotechnology journal 11(1) (2016) 31–42. [DOI] [PubMed] [Google Scholar]
- [27].Bilan R, Nabiev I, Sukhanova A, Quantum Dot-Based Nanotools for Bioimaging, Diagnostics, and Drug Delivery, ChemBioChem 17(22) (2016) 2103–2114. [DOI] [PubMed] [Google Scholar]
- [28].Pohanka M, Quantum Dots in the Therapy: Current Trends and Perspectives, Mini reviews in medicinal chemistry 17(8) (2017) 650–656. [DOI] [PubMed] [Google Scholar]
- [29].Manshian BB, Jimenez J, Himmelreich U, Soenen SJ, Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity, Biomaterials 127 (2017) 1–12. [DOI] [PubMed] [Google Scholar]
- [30].Medintz IL, Mattoussi H, Clapp AR, Potential clinical applications of quantum dots, Int. J. Nanomed 3(2) (2008) 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hardman R, A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors, Environ. Health Perspect 114(2) (2006) 165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nel A, Xia T, Madler L, Li N, Toxic potential of materials at the nanolevel, Science 311(5761) (2006) 622–7. [DOI] [PubMed] [Google Scholar]
- [33].Derfus AM, Chan WCW, Bhatia SN, Probing the Cytotoxicity Of Semiconductor Quantum Dots, Nano Lett 4(1) (2004) 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Owens DE, Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, International Journal Of Pharmaceutics 307(1) (2006) 93–102. [DOI] [PubMed] [Google Scholar]
- [35].Brigger I, Dubemet C, Couvreur P, Nanoparticles in cancer therapy and diagnosis, Adv. Drug Del. Rev 54(5) (2002) 631–651. [DOI] [PubMed] [Google Scholar]
- [36].Anselmo AC, Mitragotri S, Nanoparticles in the clinic, Bioengineering & Translational Medicine 1(1) (2016) 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S, Size-Dependent Endocytosis of Nanoparticles, Advanced materials (Deerfield Beach, Fla.) 21 (2009) 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brigger I, Dubemet C, Couvreur P, Nanoparticles in cancer therapy and diagnosis, Advanced drug delivery reviews 54(5) (2002) 631–51. [DOI] [PubMed] [Google Scholar]
- [39].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nature biotechnology 33(9) (2015) 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang AZ, Langer R, Farokhzad OC, Nanoparticle delivery of cancer drugs, Annual review of medicine 63 (2012) 185–98. [DOI] [PubMed] [Google Scholar]
- [41].Rosenthal SJ, Chang JC, Kovtun O, McBride JR, Tomlinson ID, Biocompatible Quantum Dots for Biological Applications, Chem. Biol 18(1) (2011) 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu WW, Chang E, Falkner JC, Zhang J, Al-Solmali AM, Sayes CM, Johns J, Drezek R, Colvin VL, Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers, Journal of American Chemical Society 129 (2007) 2871–2879. [DOI] [PubMed] [Google Scholar]
- [43].Jańczewski D, Tomczak N, Han M-Y, Vancso GJ, Synthesis of functionalized amphiphilic polymers for coating quantum dots, Nature protocols 6(10) (2011) 1546–1553. [DOI] [PubMed] [Google Scholar]
- [44].Smith AM, Nie S, Minimizing the Hydrodynamic Size of Quantum Dots with Multifunctional Multidentate Polymer Ligands, J. Am. Chem. Soc 130(34) (2008) 11278–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu W, Greytak AB, Lee J, Wong CR, Park J, Marshall LF, Jiang W, Curtin PN, Ting AY, Nocera DG, Fukumura D, Jain RK, Bawendi MG, Compact Biocompatible Quantum Dots via RAFT-Mediated Synthesis of Imidazole-Based Random Copolymer Ligand, J. Am. Chem. Soc 132(2) (2010) 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhan N, Palui G, Safi M, Ji X, Mattoussi H, Multidentate Zwitterionic Ligands Provide Compact and Highly Biocompatible Quantum Dots, J. Am. Chem. Soc 135(37) (2013) 13786–13795. [DOI] [PubMed] [Google Scholar]
- [47].Ma L, Tu C, Le P, Chitoor S, Lim SJ, Zahid MU, Teng KW, Ge P, Selvin PR, Smith AM, Multidentate Polymer Coatings for Compact and Homogeneous Quantum Dots with Efficient Bioconjugation, J. Am. Chem. Soc 138(10) (2016) 3382–3394. [DOI] [PubMed] [Google Scholar]
- [48].Gui R, Wan A, Liu X, Yuan W, Jin H, Water-soluble multidentate polymers compactly coating Ag2S quantum dots with minimized hydrodynamic size and bright emission tunable from red to second near-infrared region, Nanoscale 6(10) (2014) 5467–5473. [DOI] [PubMed] [Google Scholar]
- [49].Tomczak N, Liu R, Vancso JG, Polymer-coated quantum dots, Nanoscale 5(24) (2013) 12018–12032. [DOI] [PubMed] [Google Scholar]
- [50].Mei BC, Susumu K, Medintz IL, Mattoussi H, Polyethylene glycol-based bidentate ligands to enhance quantum dot and gold nanoparticle stability in biological media, Nature protocols 4(3) (2009) 412–23. [DOI] [PubMed] [Google Scholar]
- [51].Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H, Synthesis of Compact Multidentate Ligands to Prepare Stable Hydrophilic Quantum Dot Fluorophores, J. Am. Chem. Soc 127(11) (2005) 3870–3878. [DOI] [PubMed] [Google Scholar]
- [52].Susumu K, Mei BC, Mattoussi H, Multifunctional ligands based on dihydrolipoic acid and polyethylene glycol to promote biocompatibility of quantum dots, Nature protocols 4(3) (2009) 424–36. [DOI] [PubMed] [Google Scholar]
- [53].Kim S, Bawendi MG, Oligomeric ligands for luminescent and stable nanocrystal quantum dots, J. Am. Chem. Soc 125(48) (2003) 14652–3. [DOI] [PubMed] [Google Scholar]
- [54].Aldana J, Wang YA, Peng X, Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols, J. Am. Chem. Soc 123(36) (2001) 8844–50. [DOI] [PubMed] [Google Scholar]
- [55].Zylstra J, Amey J, Miska NJ, Pang L, Hine CR, Langer J, Doyle RP, Maye MM, A Modular Phase Transfer and Ligand Exchange Protocol for Quantum Dots, Langmuir : the ACS journal of surfaces and colloids 27(8) (2011) 4371–4379. [DOI] [PubMed] [Google Scholar]
- [56].Stewart MH, Susumu K, Mei BC, Medintz IL, Delehanty JB, Blanco-Canosa JB, Dawson PE, Mattoussi H, Multidentate Poly(ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions, Journal of American Chemical Society 132 (2010) 98004–9813. [DOI] [PubMed] [Google Scholar]
- [57].Yeh Y-C, Patra D, Yan B, Saha K, Miranda OR, Kim CK, Rotello VM, Synthesis of cationic quantum dots via a two-step ligand exchange process, Chem. Commun 47(11) (2011) 3069. [DOI] [PubMed] [Google Scholar]
- [58].Bruchez M Jr, Semiconductor Nanocrystals as Fluorescent Biological Labels, Science 281(5385)(1998)2013–2016. [DOI] [PubMed] [Google Scholar]
- [59].Chan WCW, Nie S, Quantum dot bioconjugates for ultrasnesitive nonisotopic detection, Science 281 (1998) 2016–2018. [DOI] [PubMed] [Google Scholar]
- [60].Savla R, Taratula O, Garbuzenko O, Minko T, Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer, Journal of Controlled Release 153(1)(2011)16–22. [DOI] [PubMed] [Google Scholar]
- [61].Wang Q, Liu Y, Ke Y, Yan H, Quantum dot bioconjugation during core-shell synthesis, Angew. Chem. Int. Ed. Engl 47(2) (2008) 316–9. [DOI] [PubMed] [Google Scholar]
- [62].Mansur HS, Gonzalez JC, Mansur AA, Biomolecule-quantum dot systems for bioconjugation applications, Colloids Surf. B. Biointerfaces 84(2) (2011) 360–8. [DOI] [PubMed] [Google Scholar]
- [63].Michalet X, Pinaud F, Lacoste TD, Dahan M, Bruchez MP, Alivisatos AP, Weiss S, Properties of fluorescent semiconductor nanocrystals and their application to biological labeling, Single Mol 2(4) (2001) 261–276. [Google Scholar]
- [64].Erathodiyil N, Ying JY, Functionalization of Inorganic Nanoparticles for Bioimaging Applications, Acc. Chem. Res 44(10) (2011) 925–935. [DOI] [PubMed] [Google Scholar]
- [65].Gao XH, Chan WCW, Nie SM, Quantum-dot nanocrystals for ultrasensitive biological labeling and multicolor optical encoding, J. Biomed. Opt 7(4) (2002) 532–537. [DOI] [PubMed] [Google Scholar]
- [66].Selvan ST, Patra PK, Ang CY, Ying JY, Synthesis of silica-coated semiconductor and magnetic quantum dots and their use in the imaging of live cells, Angew. Chem.-Int. Edit 46(14) (2007) 2448–2452. [DOI] [PubMed] [Google Scholar]
- [67].Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak WJ, Chang WH, Synthesis, Characterization, and Bioconjugation of Fluorescent Gold Nanoclusters toward Biological Labeling Applications, ACS nano 3(2) (2009) 395–401. [DOI] [PubMed] [Google Scholar]
- [68].Sperling RA, Parak WJ, Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles, Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci 368(1915) (2010)1333–1383. [DOI] [PubMed] [Google Scholar]
- [69].Medintz IL, Uyeda HT, Goldman ER, Mattoussi H, Quantum dot bioconjugates for imaging, labelling and sensing, Nature materials 4(6) (2005) 435–446. [DOI] [PubMed] [Google Scholar]
- [70].Zhang WH, Ma W, Long YT, Redox-Mediated Indirect Fluorescence Immunoassay for the Detection of Disease Biomarkers Using Dopamine-Functionalized Quantum Dots, Anal. Chem 88(10) (2016) 5131–6. [DOI] [PubMed] [Google Scholar]
- [71].Qiu Z, Shu J, Tang D, Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper, Anal. Chem 89(9) (2017) 5152–5160. [DOI] [PubMed] [Google Scholar]
- [72].Qu W, Zuo W, Li N, Hou Y, Song Z, Gou G, Yang J, Design of multifunctional liposome-quantum dot hybrid nanocarriers and their biomedical application, J Drug Target 25(8) (2017) 661–672. [DOI] [PubMed] [Google Scholar]
- [73].Pisanic TR 2nd, Zhang Y, Wang TH, Quantum dots in diagnostics and detection: principles and paradigms, The Analyst 139(12) (2014) 2968–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jackeray R, Zainul Abid CKV, Singh G, Jain S, Chattopadhyaya S, Sapra S, Shrivastav TG, Singh H, Selective capturing and detection of Salmonella typhi on polycarbonate membrane using bioconjugated quantum dots, Talanta 84(3) (2011) 952–962. [DOI] [PubMed] [Google Scholar]
- [75].Hawker CJ, Wooley KL, The convergence of synthetic organic and polymer chemistries, Science 309(5738) (2005) 1200–1205. [DOI] [PubMed] [Google Scholar]
- [76].Han HS, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG, Development of a Bioorthogonal and Highly Efficient Conjugation Method for Quantum Dots Using Tetrazine-Norbornene Cycloaddition, J. Am. Chem. Soc 132(23) (2010) 7838-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bernardin A, Cazet A, Guyon L, Delannoy P, Vinet F, Bonnaffe D, Texier I, Copper-Free Click Chemistry for Highly Luminescent Quantum Dot Conjugates: Application to in Vivo Metabolic Imaging, Bioconjugate Chem 21(4) (2010) 583–588. [DOI] [PubMed] [Google Scholar]
- [78].Schieber C, Bestetti A, Lim JP, Ryan AD, Nguyen TL, Eldridge R, White AR, Gleeson PA, Donnelly PS, Williams SJ, Mulvaney P, Conjugation of Transferrin to Azide-Modified CdSe/ZnS Core-Shell Quantum Dots using Cyclooctyne Click Chemistry, Angew. Chem.-Int. Edit 51(42) (2012) 10523–10527. [DOI] [PubMed] [Google Scholar]
- [79].Mattoussi H, Palui G, Na HB, Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes, Adv. Drug Del. Rev 64(2) (2012) 138–166. [DOI] [PubMed] [Google Scholar]
- [80].Algar WR, Prasuhn DE, Stewart MH, Jennings TL, Blanco-Canosa JB, Dawson PE, Medintz IL, The Controlled Display of Biomolecules on Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry, Bioconjugate Chem 22(5) (2011) 825–858. [DOI] [PubMed] [Google Scholar]
- [81].Zhang PF, Liu SH, Gao DY, Hu DH, Gong P, Sheng ZH, Deng JH, Ma YE, Cai LT, Click-Functionalized Compact Quantum Dots Protected by Multi dentate-imidazole Ligands: Conjugation-Ready Nanotags for Living-Virus Labeling and Imaging, J. Am. Chem. Soc 134(20) (2012) 8388–8391. [DOI] [PubMed] [Google Scholar]
- [82].Karakoti AS, Shukla R, Shanker R, Singh S, Surface functionalization of quantum dots for biological applications, Adv. Colloid Interface Sci 215 (2015) 28–45. [DOI] [PubMed] [Google Scholar]
- [83].Cheng XY, Gondosiswanto R, Ciampi S, Reece PJ, Gooding JJ, One-pot synthesis of colloidal silicon quantum dots and surface functionalization via thiol-ene click chemistry, Chem. Commun 48(97) (2012) 11874–11876. [DOI] [PubMed] [Google Scholar]
- [84].Blanco-Canosa JB, Wu M, Susumu K, Petryayeva E, Jennings TL, Dawson PE, Algar WR, Medintz IL, Recent progress in the bioconjugation of quantum dots, Coord. Chem. Rev 263 (2014) 101–137. [Google Scholar]
- [85].Lallana E, Femandez-Megia E, Riguera R, Surpassing the use of copper in the click functionalization of polymeric nanostructures: a strain-promoted approach, J. Am. Chem. Soc 131(16) (2009) 5748–5750. [DOI] [PubMed] [Google Scholar]
- [86].Binder WH, Sachsenhofer R, Straif CJ, Zirbs R, Surface-modified nanoparticles via thermal and Cu (I)-mediated “click” chemistry: generation of luminescent CdSe nanoparticles with polar ligands guiding supramolecular recognition, J. Mater. Chem 17(20) (2007) 2125–2132. [Google Scholar]
- [87].Bernardin A, Cazet A, Guyon L, Delannoy P, Vinet F, Bonnaffé D, Texier I, Copper-free click chemistry for highly luminescent quantum dot conjugates: application to in vivo metabolic imaging, Bioconjugate Chem 21(4) (2010) 583–588. [DOI] [PubMed] [Google Scholar]
- [88].Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG, Development of a bioorthogonal and highly efficient conjugation method for quantum dots using tetrazine-norbomene cycloaddition, J. Am. Chem. Soc 132(23) (2010) 7838–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hao J, Huang LL, Zhang R, Wang HZ, Xie HY, A Mild and Reliable Method to Label Enveloped Virus with Quantum Dots by Copper-Free Click Chemistry, Anal. Chem 84(19) (2012) 8364–8370. [DOI] [PubMed] [Google Scholar]
- [90].Lee DE, Na JH, Lee S, Kang CM, Kim HN, Han SJ, Kim H, Choe YS, Jung KH, Lee KC, Choi K, Kwon IC, Jeong SY, Lee KH, Kim K, Facile Method To Radiolabel Glycol Chitosan Nanoparticles with Cu-64 via Copper-Free Click Chemistry for MicroPET Imaging, Molecular Pharmaceutics 10(6) (2013) 2190–2198. [DOI] [PubMed] [Google Scholar]
- [91].Zhang HY, Feng GQ, Guo Y, Zhou DJ, Robust and specific ratiometric biosensing using a copper-free clicked quantum dot-DNA aptamer sensor, Nanoscale 5(21) (2013) 10307–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kotagiri N, Li ZY, Xu XX, Mondal S, Nehorai A, Achilefu S, Antibody Quantum Dot Conjugates Developed via Copper-Free Click Chemistry for Rapid Analysis of Biological Samples Using a Microfluidic Microsphere Array System, Bioconjugate Chem 25(7) (2014) 1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang WT, Kapur A, Ji X, Zeng BR, Mishra D, Mattoussi H, Multifunctional and High Affinity Polymer Ligand that Provides Bio-Orthogonal Coating of Quantum Dots, Bioconjugate Chem 27(9) (2016) 2024–2036. [DOI] [PubMed] [Google Scholar]
- [94].Lee-Montiel FT, Li P, Imoukhuede PI, Quantum dot multiplexing for the profiling of cellular receptors, Nanoscale 7(44) (2015) 18504–18514. [DOI] [PubMed] [Google Scholar]
- [95].Zhao X, Shen Y, Adogla EA, Viswanath A, Tan R, Benicewicz BC, Greytak AB, Lin Y, Wang Q, Surface labeling of enveloped virus with polymeric imidazole ligand-capped quantum dots via the metabolic incorporation of phospholipids into host cells, Journal of Materials Chemistry B 4(14) (2016) 2421–2427. [DOI] [PubMed] [Google Scholar]
- [96].Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG, Development of a bioorthogonal and highly efficient conjugation method for quantum dots using tetrazine-norbornene cycloaddition, Journal of American Chemical Society 132 (2010) 7838–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chen Y, Cordero JM, Wang H, Franke D, Achorn OB, Freyria FS, Coropceanu I, Wei H, Chen O, Mooney DJ, Bawendi MG, A Ligand System for the Flexible Functionalization of Quantum Dots via Click Chemistry, Angew. Chem. Int. Ed. Engl 57(17) (2018) 4652–4656. [DOI] [PubMed] [Google Scholar]
- [98].Zhang Y, So MK, Loening AM, Yao HQ, Gambhir SS, Rao JH, HaloTag protein-mediated site-specific conjugation of bioluminescent proteins to quantum dots, Angew. Chem.-Int. Edit 45(30) (2006) 4936–4940. [DOI] [PubMed] [Google Scholar]
- [99].So MK, Yao HQ, Rao JH, HaloTag protein-mediated specific labeling of living cells with quantum dots, Biochem. Biophys. Res. Commun 374(3) (2008) 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Popa I, Berkovich R, Alegre-Cebollada J, Badilla CL, Rivas-Pardo JA, Taniguchi Y, Kawakami M, Fernandez JM, Nanomechanics of HaloTag Tethers, J. Am. Chem. Soc 135(34) (2013) 12762–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu DS, Phipps WS, Loh KH, Howarth M, Ting AY, Quantum Dot Targeting with Lipoic Acid Ligase and Halo Tag for Single-Molecule Imaging on Living Cells, ACS nano 6(12) (2012) 11080–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hong H, Benink HA, Zhang Y, Yang YN, Uyeda HT, Engle JW, Severin GW, McDougall MG, Barnhart TE, Klaubert DH, Nickles RJ, Fan F, Cai WB, HaloTag: a novel reporter gene for positron emission tomography, American Journal of Translational Research 3(4) (2011) 392–403. [PMC free article] [PubMed] [Google Scholar]
- [103].Liu AA, Zhang ZF, Sun EZ, Zheng ZH, Zhang ZL, Hu QX, Wang HZ, Pang DW, Simultaneous Visualization of Parental and Progeny Viruses by a Capsid-Specific HaloTag Labeling Strategy, ACS nano 10(1) (2016) 1147–1155. [DOI] [PubMed] [Google Scholar]
- [104].Hatakeyama H, Nakahata Y, Yarimizu H, Kanzaki M, Live-cell single-molecule labeling and analysis of myosin motors with quantum dots, Molecular Biology of the Cell 28(1) (2017) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV, HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis, ACS Chemical Biology 3(6) (2008) 373–382. [DOI] [PubMed] [Google Scholar]
- [106].Liu DS, Phipps WS, Loh KH, Howarth M, Ting AY, Quantum Dot Targeting with Lipoic Acid Ligase and HaloTag for Single-Molecule Imaging on Living Cells, ACS nano 6(12) (2012) 11080–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hoet PH, Bruske-Hohlfeld I, Salata OV, Nanoparticles - known and unknown health risks, Journal of nanobiotechnology 2(1) (2004) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nature Reviews Materials 1 (2016) 16014. [Google Scholar]
- [109].Byrne JD, Betancourt T, Brannon-Peppas L, Active targeting schemes for nanoparticle systems in cancer therapeutics, Advanced drug delivery reviews 60(15) (2008) 1615–26. [DOI] [PubMed] [Google Scholar]
- [110].Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J, Chen X, Wu AM, Weiss S, Gambhir SS, microPET-Based Biodistribution of Quantum Dots in Living Mice, Journal of Nuclear Medicine 48(9) (2007) 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Park K, The drug delivery field at the inflection point: Time to fight its way out of the egg, Journal of Controlled Release 267 (2017) 2–14. [DOI] [PubMed] [Google Scholar]
- [112].Park K, Facing the Truth about Nanotechnology in Drug Delivery, ACS nano 7(9) (2013) 7442–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat. Biotechnol 33(9) (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mora-Huertas CE, Fessi H, Elaissari A, Polymer-based nanocapsules for drug delivery, Int. J. Pharm 385(1-2) (2010) 113–42. [DOI] [PubMed] [Google Scholar]
- [115].Fisher OZ, Khademhosseini A, Peppas NA, Drug Delivery: Nanoscale Devices, Encyclopedia of Materials: Science and Technology, Elsevier, 2010, pp. 1–9. [Google Scholar]
- [116].Mills JK, Needham D, Targeted drug delivery, Expert Opinion on Therapeutic Patents 9(11) (1999)1499–1513. [Google Scholar]
- [117].Bae YH, Park K, Targeted drug delivery to tumors: Myths, reality and possibility, Journal of Controlled Release 153(3) (2011) 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Park K, Facing the truth about nanotechnology in drug delivery, ACS nano 7(9) (2013) 7442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Haley B, Frenkel E, Nanoparticles for drug delivery in cancer treatment, Urologic Oncology: Seminars and Original Investigations 26(1) (2008) 57–64. [DOI] [PubMed] [Google Scholar]
- [120].M WA, S SD, A PN, Advanced architectures in the design of responsive polymers for cancer nanomedicine, Journal of Applied Polymer Science 135(24) (2018) 46154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Liechty WB, Kryscio DR, Slaughter BV, Peppas NA, Polymers for Drug Delivery Systems, Annu Rev Chem Biomol Eng 1 (2010) 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Perez RA, Kim H-W, Core–shell designed scaffolds for drug delivery and tissue engineering, Acta Biomater 21 (2015) 2–19. [DOI] [PubMed] [Google Scholar]
- [123].Wagner AM, Gran MP, Peppas NA, Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery, Acta Pharmaceutica Sinica B 8(2) (2018) 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, Chen X, Banin U, Wu AM, Sinclair R, Weiss S, Gambhir SS, Particle Size, Surface Coating, and PEGylation Influence the Biodistribution of Quantum Dots in Living Mice, Small (Weinheim an der Bergstrasse, Germany) 5(1) (2009) 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC, Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology, Advanced drug delivery reviews 66 (2014) 2–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Petros RA, DeSimone JM, Strategies in the design of nanoparticles for therapeutic applications, Nat. Rev. Drug Discov 9(8) (2010) 615–27. [DOI] [PubMed] [Google Scholar]
- [127].Lin G, Chen T, Zou J, Wang Y, Wang X, Li J, Huang Q, Fu Z, Zhao Y, Lin MC, Xu G, Yong KT, Quantum Dots-siRNA Nanoplexes for Gene Silencing in Central Nervous System Tumor Cells, Frontiers in pharmacology 8 (2017) 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y, Quantum dots as photosensitizers?, Nat. Biotechnol 22 (2004) 1360. [DOI] [PubMed] [Google Scholar]
- [129].Bakalova R, Zhelev Z, Ohba H, Baba Y, Quantum Dot-Conjugated Hybridization Probes for Preliminary Screening of siRNA Sequences, J. Am. Chem. Soc 127(32) (2005) 113280–11335. [DOI] [PubMed] [Google Scholar]
- [130].Ye L, Hu R, Liu L, Liu J, Liu J, Chen H, Hu Y, Liu Y, Liu X, Liu C, Tng DJH, Meng Y, Qu J, Swihart MT, Yong KT, Comparing Semiconductor Nanocrystal Toxicity in Pregnant Mice and Non-Human Primates, Nanotheranostics 3(1) (2019) 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mo D, Hu L, Zeng G, Chen G, Wan J, Yu Z, Huang Z, He K, Zhang C, Cheng M, Cadmium-containing quantum dots: properties, applications, and toxicity, Appl. Microbiol. Biotechnol 101(7) (2017) 2713–2733. [DOI] [PubMed] [Google Scholar]
- [132].Sharma VK, McDonald TJ, Sohn M, Anquandah GAK, Pettine M, Zboril R, Assessment of toxicity of selenium and cadmium selenium quantum dots: A review, Chemosphere 188 (2017) 403–413. [DOI] [PubMed] [Google Scholar]
- [133].Colvin VL, The potential environmental impact of engineered nanomaterials, Nature biotechnology 21(10) (2003) 1166–70. [DOI] [PubMed] [Google Scholar]
- [134].Kim JS, Cho KJ, Tran TH, Nurunnabi M, Moon TH, Hong SM, Lee Y.-k., In vivo NIR imaging with CdTe/CdSe quantum dots entrapped in PLGA nanospheres, J. Colloid Interface Sci 353(2) (2011) 363–371. [DOI] [PubMed] [Google Scholar]
- [135].Pons T, Pic E, Lequeux N, Cassette E, Bezdetnaya L, Guillemin F, Marchal F, Dubertret B, Cadmium-free CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity, American Chemical Society Nano 4 (2010) 2531–2538. [DOI] [PubMed] [Google Scholar]
- [136].Park J, Dvoracek C, Lee KH, Galloway JF, Bhang H.-e.C., Pomper MG, Searson PC, CuInSe/ZnS Core/Shell NIR Quantum Dots for Biomedical Imaging, Small (Weinheim an der Bergstrasse, Germany) 7(22) (2011) 3148–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Charron G, Stuchinskaya T, Edwards DR, Russell DA, Nann T, Insights into the Mechanism of Quantum Dot-Sensitized Singlet Oxygen Production for Photodynamic Therapy, The Journal of Physical Chemistry C 116(16) (2012) 9334–9342. [Google Scholar]
- [138].Amelia M, Lavie-Cambot A, McClenaghan ND, Credi A, A ratiometric luminescent oxygen sensor based on a chemically functionalized quantum dot, Chem. Commun 47(1) (2011) 325–327. [DOI] [PubMed] [Google Scholar]
- [139].Roberti MJ, Morgan M, Menendez G, Pietrasanta LI, Jovin TM, Jares-Erijman EA, Quantum Dots As Ultrasensitive Nanoactuators and Sensors of Amyloid Aggregation in Live Cells, J. Am. Chem. Soc 131(23) (2009) 8102–8107. [DOI] [PubMed] [Google Scholar]
- [140].Sapsford KE, Farrell D, Sun S, Rasooly A, Mattoussi H, Medintz IL, Monitoring of enzymatic proteolysis on a electroluminescent-CCD microchip platform using quantum dot-peptide substrates, Sens. Actuator B-Chem 139(1) (2009) 13–21. [Google Scholar]
- [141].Sapsford KE, Granek J, Deschamps JR, Boeneman K, Blanco-Canosa JB, Dawson PE, Susumu K, Stewart MH, Medintz IL, Monitoring Botulinum Neurotoxin A Activity with Peptide-Functionalized Quantum Dot Resonance Energy Transfer Sensors, ACS nano 5(4) (2011) 2687–2699. [DOI] [PubMed] [Google Scholar]
- [142].Lee H, Lee K, Kim IK, Park TG, Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes, Biomaterials 29(35) (2008) 4709–4718. [DOI] [PubMed] [Google Scholar]
- [143].Zhang CY, Hu J, Single Quantum Dot-Based Nanosensor for Multiple DNA Detection, Anal. Chem 82(5) (2010) 1921–1927. [DOI] [PubMed] [Google Scholar]
- [144].Freeman R, Liu XQ, Willner I, Amplified Multiplexed Analysis of DNA by the Exonuclease III-Catalyzed Regeneration of the Target DNA in the Presence of Functionalized Semiconductor Quantum Dots, Nano Lett 11(10) (2011) 4456–4461. [DOI] [PubMed] [Google Scholar]
- [145].Xia YS, Ye JJ, Tan KH, Wang JJ, Yang G, Colorimetric Visualization of Glucose at the Submicromole Level in Serum by a Homogenous Silver Nanoprism-Glucose Oxidase System, Anal. Chem 85(13) (2013) 6241–6247. [DOI] [PubMed] [Google Scholar]
- [146].Ghanbari H, Cousins BG, Seifalian AM, A Nanocage for Nanomedicine: Polyhedral Oligomeric Silsesquioxane (POSS), Macromol. Rapid Commun 32(14) (2011) 1032–1046. [DOI] [PubMed] [Google Scholar]
- [147].Jokerst JV, Raamanathan A, Christodoulides N, Floriano PN, Pollard AA, Simmons GW, Wong J, Gage C, Furmaga WB, Redding SW, McDevitt JT, Nano-bio-chips for high performance multiplexed protein detection: Determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels, Biosensors & Bioelectronics 24(12) (2009) 3622–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhou J, Yang Y, Zhang C.-y., Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application, Chem. Rev 115(21) (2015) 11669–11717. [DOI] [PubMed] [Google Scholar]
- [149].Jing L, Kershaw SV, Li Y, Huang X, Li Y, Rogach AL, Gao M, Aqueous Based Semiconductor Nanocrystals, Chem. Rev 116(18) (2016) 10623–10730. [DOI] [PubMed] [Google Scholar]
- [150].Hildebrandt N, Spillmann CM, Algar WR, Pons T, Stewart MH, Oh E, Susumu K, Diaz SA, Delehanty JB, Medintz IL, Energy Transfer with Semiconductor Quantum Dot Bioconjugates: A Versatile Platform for Biosensing, Energy Harvesting, and Other Developing Applications, Chem. Rev 117(2) (2017) 536–711. [DOI] [PubMed] [Google Scholar]
- [151].Hlavacek A, Bouchal P, Skládal P, Biotinylation of quantum dots for application in fluoroimmunoassays with biotin-avidin amplification, Microchimica Acta (2011). [Google Scholar]
- [152].Park Y, Ryu Y-M, Wang T, Jung Y, Kim S, Hwang S, Park J, Bae D-J, Kim J, Moon H, Lim H-S, Kim S-Y, Chung E, Kim KH, Kim S, Myung S-J, Colorectal Cancer Diagnosis Using Enzyme-Sensitive Ratiometric Fluorescence Dye and Antibody-Quantum Dot Conjugates for Multiplexed Detection, Adv. Funct. Mater 28(4) (2018) 1703450. [Google Scholar]
- [153].Liu J, Lau SK, Varma VA, Moffitt RA, Caldwell M, Liu T, Young AN, Petros JA, Osunkoya AO, Krogstad T, Leyland-Jones B, Wang MD, Nie S, Molecular mapping of tumor heterogeneity on clinical tissue specimens with multiplexed quantum dots, ACS nano 4(5) (2010) 2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Olerile LD, Liu Y, Zhang B, Wang T, Mu S, Zhang J, Selotlegeng L, Zhang N, Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic, Colloids Surf. B. Biointerfaces 150 (2017) 121–130. [DOI] [PubMed] [Google Scholar]
- [155].Bhang SH, Won N, Lee TJ, Jin H, Nam J, Park J, Chung H, Park HS, Sung YE, Hahn SK, Kim BS, Kim S, Hyaluronic Acid-Quantum Dot Conjugates for In Vivo Lymphatic Vessel Imaging, ACS nano 3(6) (2009) 1389–1398. [DOI] [PubMed] [Google Scholar]
- [156].Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS, Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature, Nano Lett 8(9) (2008) 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Li CY, Zhang YJ, Wang M, Zhang Y, Chen GC, Li L, Wu DM, Wang QB, In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window, Biomaterials 35(1) (2014) 393–400. [DOI] [PubMed] [Google Scholar]
- [158].Soltesz EG, Kim S, Laurence RG, DeGrand AM, Parungo CP, Dor DM, Cohn LH, Bawendi MG, Frangioni JV, Mihaljevic T, Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots, Ann. Thorac. Surg 79(1) (2005) 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tada H, Higuchi H, Wanatabe TM, Ohuchi N, In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice, Cancer Res 67(3) (2007)1138–1144. [DOI] [PubMed] [Google Scholar]
- [160].Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV, Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping, Nat. Biotechnol 22(1) (2004) 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Liu X-L, Peng C-W, Chen C, Yang X-Q, Hu M-B, Xia H-S, Liu S-P, Pang D-W, Li Y, Quantum dots-based double-color imaging of HER2 positive breast cancer invasion, Biochem. Biophys. Res. Commun 409(3) (2011) 577–582. [DOI] [PubMed] [Google Scholar]
- [162].Morgan NY, English S, Chen W, Chernomordik V, Russo A, Smith PD, Gandjbakhche A, Real time in vivo non-invasive optical imaging using near-infrared fluorescent quantum dots, Acad. Radiol 12(3) (2005) 313–323. [DOI] [PubMed] [Google Scholar]
- [163].Tang J, Kong B, Wu H, Xu M, Wang YC, Wang YL, Zhao DY, Zheng GF, Carbon Nanodots Featuring Efficient FRET for Real-Time Monitoring of Drug Delivery and Two-Photon Imaging, Adv. Mater 25(45) (2013) 6569–6574. [DOI] [PubMed] [Google Scholar]
- [164].de Chermont QL, Chaneac C, Seguin J, Pelle F, Maitrejean S, Jolivet JP, Gourier D, Bessodes M, Scherman D, Nanoprobes with near-infrared persistent luminescence for in vivo imaging, Proc. Natl. Acad. Sci. U.S.A 104(22) (2007) 9266–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S, Quantum dots for live cells, in vivo imaging, and diagnostics, Science 307(5709) (2005) 538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Hong G, Antaris AL, Dai H, Near-infrared fluorophores for biomedical imaging, Nature Biomedical Engineering 1 (2017) 0010. [Google Scholar]
- [167].Zhang Y, Hong GS, Zhang YJ, Chen GC, Li F, Dai HJ, Wang QB, Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second Near-Infrared Window, ACS nano 6(5) (2012) 3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zhu CN, Jiang P, Zhang ZL, Zhu DL, Tian ZQ, Pang DW, Ag2Se Quantum Dots with Tunable Emission in the Second Near-Infrared Window, Acs Applied Materials & Interfaces 5(4) (2013) 1186–1189. [DOI] [PubMed] [Google Scholar]
- [169].Zhang Y, Zhang YJ, Hong GS, He W, Zhou K, Yang K, Li F, Chen GC, Liu Z, Dai HJ, Wang QB, Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice, Biomaterials 34(14) (2013) 3639–3646. [DOI] [PubMed] [Google Scholar]
- [170].Dong BH, Li CY, Chen GC, Zhang YJ, Zhang Y, Deng MJ, Wang QB, Facile Synthesis of Highly Photoluminescent Ag2Se Quantum Dots as a New Fluorescent Probe in the Second Near-Infrared Window for in Vivo Imaging, Chem. Mater 25(12) (2013) 2503–2509. [Google Scholar]
- [171].Chen GC, Tian F, Zhang Y, Zhang YJ, Li CY, Wang QB, Tracking of Transplanted Human Mesenchymal Stem Cells in Living Mice using Near-Infrared Ag-2 S Quantum Dots, Adv. Funct. Mater 24(17) (2014) 2481–2488. [Google Scholar]
- [172].Hong GS, Antaris AL, Dai HJ, Near-infrared fluorophores for biomedical imaging, Nature Biomedical Engineering 1(1) (2017). [Google Scholar]
- [173].Yang QL, Ma ZR, Wang HS, Zhou B, Zhu SJ, Zhong YT, Wang JY, Wan H, Antaris A, Ma R, Zhang X, Yang JY, Zhang XD, Sun HT, Liu WQ, Liang YY, Dai HJ, Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window, Adv. Mater 29(12) (2017). [DOI] [PubMed] [Google Scholar]
- [174].Li CY, Li F, Zhang YJ, Zhang WJ, Zhang XE, Wang QB, Real-Time Monitoring Surface Chemistry-Dependent In Vivo Behaviors of Protein Nanocages via Encapsulating an NIR-II Ag2S Quantum Dot, ACS nano 9(12) (2015) 12255–12263. [DOI] [PubMed] [Google Scholar]
- [175].Yang HY, Zhao YW, Zhang ZY, Xiong HM, Yu SN, One-pot synthesis of water-dispersible Ag2S quantum dots with bright fluorescent emission in the second near-infrared window, Nanotechnology 24(5) (2013). [DOI] [PubMed] [Google Scholar]
- [176].Wang R, Zhang F, NIR luminescent nanomaterials for biomedical imaging, Journal of Materials Chemistry B 2(17) (2014) 2422–2443. [DOI] [PubMed] [Google Scholar]
- [177].Sun Y, Ding MM, Zeng XD, Xiao YL, Wu HP, Zhou H, Ding BB, Qu CR, Hou W, Er-bu AGA, Zhang YJ, Cheng Z, Hong XC, Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery, Chemical Science 8(5) (2017)3489–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kong YF, Chen J, Fang HW, Heath G, Wo Y, Wang WL, Li YX, Guo Y, Evans SD, Chen SY, Zhou DJ, Highly Fluorescent Ribonuclease-A-Encapsulated Lead Sulfide Quantum Dots for Ultrasensitive Fluorescence in Vivo Imaging in the Second Near-Infrared Window, Chem. Mater 28(9) (2016) 3041–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Xu SY, Cui JB, Wang LY, Recent developments of low-toxicity NIR II quantum dots for sensing and bioimaging, Trac-Trends in Analytical Chemistry 80 (2016) 149–155. [Google Scholar]
- [180].Li BH, Lu LF, Zhao MY, Lei ZH, Zhang F, An Efficient 1064 nm NIR-II Excitation Fluorescent Molecular Dye for Deep-Tissue High-Resolution Dynamic Bioimaging, Angew. Chem.-Int. Edit 57(25) (2018) 7483–7487. [DOI] [PubMed] [Google Scholar]
- [181].Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI, Through-skull fluorescence imaging of the brain in a new near-infrared window, Nature Photonics 8(9) (2014) 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Diao S, Blackburn JL, Hong G, Antaris AL, Chang J, Wu JZ, Zhang B, Cheng K, Kuo CJ, Dai H, Fluorescence imaging in vivo at wavelengths beyond 1500 nm, Angew. Chem. Int. Ed 54(49) (2015) 14758–14762. [DOI] [PubMed] [Google Scholar]
- [183].Franke D, Harris DK, Chen O, Bruns OT, Carr JA, Wilson MWB, Bawendi MG, Continuous injection synthesis of indium arsenide quantum dots emissive in the short-wavelength infrared, Nature communications 7 (2016) 12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Zamberlan F, Turyanska L, Patanè A, Liu Z, Williams HEL, Fay MW, Clarke PA, Imamura Y, Jin T, Bradshaw TD, Thomas NR, Grabowska AM, Stable DHLA–PEG capped PbS quantum dots: from synthesis to near-infrared biomedical imaging, Journal of Materials Chemistry B 6(4) (2018) 550–555. [DOI] [PubMed] [Google Scholar]
- [185].Bruns OT, Bischof TS, Harris DK, Franke D, Shi Y, Riedemann L, Bartelt A, Jaworski FB, Carr JA, Rowlands CJ, Wilson MWB, Chen O, Wei H, Hwang GW, Montana DM, Coropceanu I, Achorn OB, Kloepper J, Heeren J, So PTC, Fukumura D, Jensen KF, Jain RK, Bawendi MG, Next-generation in vivo optical imaging with short-wave infrared quantum dots, Nature Biomedical Engineering 1 (2017) 0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Li C, Zhang Y, Wang M, Zhang Y, Chen G, Li L, Wu D, Wang Q, In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window, Biomaterials 35(1) (2014) 393–400. [DOI] [PubMed] [Google Scholar]
- [187].Liu X, Braun GB, Qin M, Ruoslahti E, Sugahara KN, In vivo cation exchange in quantum dots for tumor-specific imaging, Nature communications 8(1) (2017) 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sarnia ACS, Chen X, Burda C, Semiconductor Quantum Dots for Photodynamic Therapy, J. Am. Chem. Soc 125(51) (2003) 15736–15737. [DOI] [PubMed] [Google Scholar]
- [189].Viana OS, Ribeiro MS, Fontes A, Santos BS, Quantum Dots in Photodynamic Therapy, in: Batinić-Haberle I, Rebouças JS, Spasojević I (Eds.), Redox-Active Therapeutics, Springer International Publishing, Cham, 2016, pp. 525–539. [Google Scholar]
- [190].Kim J, Piao Y, Hyeon T, Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy, Chem. Soc. Rev 38(2) (2009) 372–390. [DOI] [PubMed] [Google Scholar]
- [191].Chatterjee DK, Fong LS, Zhang Y, Nanoparticles in photodynamic therapy: An emerging paradigm, Adv. Drug Del. Rev 60(15) (2008) 1627–1637. [DOI] [PubMed] [Google Scholar]
- [192].Kelkar SS, Reineke TM, Theranostics: Combining Imaging and Therapy, Bioconjugate Chem 22(10) (2011) 1879–1903. [DOI] [PubMed] [Google Scholar]
- [193].Samia ACS, Chen XB, Burda C, Semiconductor quantum dots for photodynamic therapy, J. Am. Chem. Soc 125(51) (2003) 15736–15737. [DOI] [PubMed] [Google Scholar]
- [194].Park Y, Kim HM, Kim JH, Moon KC, Yoo B, Lee KT, Lee N, Choi Y, Park W, Ling D, Na K, Moon WK, Choi SH, Park HS, Yoon SY, Suh YD, Lee SH, Hyeon T, Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous In Vivo Dual-Modal Imaging and Photodynamic Therapy, Adv. Mater 24(42) (2012) 5755–5761. [DOI] [PubMed] [Google Scholar]
- [195].Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei BW, Burda C, Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer, J. Am. Chem. Soc 130(32) (2008) 10643–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ge JC, Lan MH, Zhou BJ, Liu WM, Guo L, Wang H, Jia QY, Niu GL, Huang X, Zhou HY, Meng XM, Wang PF, Lee CS, Zhang WJ, Han XD, A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation, Nature communications 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Chen WS, Ouyang J, Liu H, Chen M, Zeng K, Sheng JP, Liu ZJ, Han YJ, Wang LQ, Li J, Deng L, Liu YN, Guo SJ, Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer, Adv. Mater 29(5) (2017). [DOI] [PubMed] [Google Scholar]
- [198].Juzenas P, Chen W, Sun YP, Coelho MAN, Generalov R, Generalova N, Christensen IL, Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer, Adv. Drug Del. Rev 60(15) (2008) 1600–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Fan WP, Bu WB, Shen B, He QJ, Cui ZW, Liu YY, Zheng XP, Zhao KL, Shi JL, Intelligent MnO2 Nanosheets Anchored with Upconversion Nanoprobes for Concurrent pH-/H2O2-Responsive UCL Imaging and Oxygen-Elevated Synergetic Therapy, Adv. Mater 27(28) (2015) 4155–4161. [DOI] [PubMed] [Google Scholar]
- [200].Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y, Quantum dots as photosensitizers?, Nat. Biotechnol 22(11) (2004) 1360–1361. [DOI] [PubMed] [Google Scholar]
- [201].Zheng M, Liu S, Li J, Qu D, Zhao HF, Guan XG, Hu XL, Xie ZG, Jing XB, Sun ZC, Integrating Oxaliplatin with Highly Luminescent Carbon Dots: An Unprecedented Theranostic Agent for Personalized Medicine, Adv. Mater 26(21) (2014) 3554–3560. [DOI] [PubMed] [Google Scholar]
- [202].Lai CW, Wang YH, Lai CH, Yang MJ, Chen CY, Chou PT, Chan CS, Chi Y, Chen YC, Hsiao JK, Iridium-complex-functionalized Fe3O4/SiO2 core/shell nanoparticles: A facile three-in-one system in magnetic resonance imaging, luminescence imaging, and photodynamic therapy, Small (Weinheim an der Bergstrasse, Germany) 4(2) (2008) 218–224. [DOI] [PubMed] [Google Scholar]
- [203].Bakalova R, Ohba H, Zhelev Z, Nagase T, Jose R, Ishikawa M, Baba Y, Quantum dot anti-CD conjugates: Are they potential photosensitizers or potentiators of classical photosensitizing agents in photodynamic therapy of cancer?, Nano Lett 4(9) (2004) 1567–1573. [Google Scholar]
- [204].Markovic ZM, Ristic BZ, Arsikin KM, Klisic DG, Harhaji-Trajkovic LM, Todorovic-Markovic BM, Kepic DP, Kravic-Stevovic TK, Jovanovic SP, Milenkovic MM, Milivojevic DD, Bumbasirevic VZ, Dramicanin MD, Trajkovic VS, Graphene quantum dots as autophagy-inducing photodynamic agents, Biomaterials 33(29) (2012) 7084–7092. [DOI] [PubMed] [Google Scholar]
- [205].Tsay JM, Trzoss M, Shi LX, Kong XX, Selke M, Jung ME, Weiss S, Singlet oxygen production by peptide-coated quantum dot-photosensitizer conjugates, J. Am. Chem. Soc 129(21) (2007) 6865–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Shi LX, Hernandez B, Selke M, Singlet oxygen generation from water-soluble quantum dot-organic dye nanocomposites, J. Am. Chem. Soc 128(19) (2006) 6278–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Yong Y, Cheng XJ, Bao T, Zu M, Yan L, Yin WY, Ge CC, Wang DL, Gu ZJ, Zhao YL, Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy, ACS nano 9(12) (2015) 12451–12463. [DOI] [PubMed] [Google Scholar]
- [208].Choi Y, Kim S, Choi MH, Ryoo SR, Park J, Min DH, Kim BS, Highly Biocompatible Carbon Nanodots for Simultaneous Bioimaging and Targeted Photodynamic Therapy In Vitro and In Vivo, Adv. Funct. Mater 24(37) (2014) 5781–5789. [Google Scholar]
- [209].Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, Jia Q, Niu G, Huang X, Zhou H, Meng X, Wang P, Lee C-S, Zhang W, Han X, A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation, Nature communications 5 (2014) 4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Ge J, Jia Q, Liu W, Guo L, Liu Q, Lan M, Zhang H, Meng X, Wang P, Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice, Adv. Mater 27(28) (2015) 4169–4177. [DOI] [PubMed] [Google Scholar]
- [211].Kuo W-S, Chen H-H, Chen S-Y, Chang C-Y, Chen P-C, Hou Y-I, Shao Y-T, Kao H-F, Lilian Hsu C-L, Chen Y-C, Chen S-J, Wu S-R, Wang J-Y, Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging, Biomaterials 120 (2017) 185–194. [DOI] [PubMed] [Google Scholar]
- [212].Yang X, Zhang W, Zhao Z, Li N, Mou Z, Sun D, Cai Y, Wang W, Lin Y, Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials, J. Inorg. Biochem 167 (2017) 36–48. [DOI] [PubMed] [Google Scholar]
- [213].Bwatanglang IB, Mohammad F, Yusof NA, Abdullah J, Alitheen NB, Hussein MZ, Abu N, Mohammed NE, Nordin N, Zamberi NR, Yeap SK, In vivo tumor targeting and antitumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system, J. Colloid Interface Sci 480 (2016) 146–158. [DOI] [PubMed] [Google Scholar]
- [214].Whitehead KA, Langer R, Anderson DG, Knocking down barriers: advances in siRNA delivery, Nature Reviews Drug Discovery 8(2) (2009) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Chen AA, Quantum dots to monitor RNAi delivery and improve gene silencing, Nucleic Acids Res 33(22) (2005) e190–e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Li J-M, Zhao M-X, Su H, Wang Y-Y, Tan C-P, Ji L-N, Mao Z-W, Multifunctional quantum-dot-based siRNA delivery for HPV18 E6 gene silence and intracellular imaging, Biomaterials 32(31) (2011) 7978–7987. [DOI] [PubMed] [Google Scholar]
- [217].Wu Y, Zhou B, Xu F, Wang X, Liu G, Zheng L, Zhao J, Zhang X, Functional quantum dot-siRNA nanoplexes to regulate chondrogenic differentiation of mesenchymal stem cells, Acta Biomater 46 (2016) 165–176. [DOI] [PubMed] [Google Scholar]
- [218].Ornes S, Core Concept: Quantum dots, Proceedings of the National Academy of Sciences 113(11) (2016) 2796–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Zhang B, Wang Y, Hu R, Roy I, Yong K-T, Cadmium-Free Quantum Dots for Biophotonic Imaging and Sensing, in: Ho AH-P, Kim D, Somekh MG (Eds.), Handbook of Photonics for Biomedical Engineering, Springer Netherlands, Dordrecht, 2017, pp. 841–870. [Google Scholar]
- [220].Xu G, Zeng S, Zhang B, Swihart MT, Yong K-T, Prasad PN, New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine, Chem. Rev 116(19) (2016) 12234–12327. [DOI] [PubMed] [Google Scholar]
- [221].Kotagiri N, Li Z, Xu X, Mondal S, Nehorai A, Achilefu S, Antibody Quantum Dot Conjugates Developed via Copper-Free Click Chemistry for Rapid Analysis of Biological Samples Using a Microfluidic Microsphere Array System, Bioconjugate Chem 25(7) (2014) 1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]