Abstract
Objective:
The high pill burden of many phosphate binders (PBs) may contribute to increased prevalence of hyperphosphatemia and poor nutritional status observed among patients undergoing maintenance hemodialysis therapy. We examined the real-world effectiveness of sucroferric oxyhydroxide (SO), a PB with low pill burden, in managing serum phosphorus in patients with prevalent hemodialysis over a 1-year period.
Design:
Historical cohort analyses of de-identified electronic medical records.
Subjects:
In-center hemodialysis patients switched from another PB to SO therapy as part of routine care with 12 months of uninterrupted SO prescriptions recorded, and documented serum phosphorus levels were eligible for inclusion. Clinical data were extracted from a pharmacy service, FreseniusRx, database and Fresenius Kidney Care clinical data warehouse.
Main outcome measures:
Comparisons were made between the 91-day period before SO initiation (i.e., baseline) and the 4 consecutive 91-day intervals of SO treatment (Q1-Q4). Clinical measures included achievement of target phosphorus levels (#5.5 mg/dL) and mean number of PB pills/day.
Results:
Among 530 analyzed patients, the proportion achieving target serum phosphorus levels increased by >100% 1 year after switching to SO therapy, that is, from 17.7% at baseline to 24.5%, 30.5%, 36.4%, and 36.0% at Q1 through Q4, respectively (P < .0001 for all). Reductions in serum phosphorus were observed at all follow-up timepoints (P <.0001), irrespective of baseline PB. From a mean baseline PB pill burden of 8.5 pills/day, patients experienced an average 50% pill burden reduction during SO treatment (P <. 0001). Phosphorus-attuned albumin and phosphorus-attuned protein intake (normalized protein catabolic rate) improved significantly after transition to SO (P < .0001). The effectiveness of SO was evident in prespecified subgroups of interest (i.e., black/African-American patients, Hispanic/Latino patients, and women).
Conclusion:
Among patients on hemodialysis, switching to SO resulted in a 2-fold greater likelihood of achieving target phosphorus levels while halving daily PB pill burden. Increases in phosphorus-attuned albumin and protein intake suggest improved nutritional status.
Introduction
HYPERPHOSPHATEMIA IS COMMON among patients with advanced chronic kidney disease and end-stage renal disease (ESRD).1,2 Disrupted phosphorus homeostasis, primarily due to the progressive inability of the kidneys to appropriately excrete phosphorus, leads to accumulation of this mineral.3 High serum phosphorus levels have been associated with increased cardiovascular morbidity and mortality.2,4,5 In a large national study, mortality risk among patients with chronic kidney disease on hemodialysis increased by 6% for each 1 mg/dL increase in serum phosphorus level above the Kidney Disease Outcomes Quality Initiative target of 3.5–5.5 mg/dL.5–7
Oral phosphate binders (PBs), in combination with dialysis and dietary restriction, can be used to lower elevated phosphorus levels. Dietary restriction should aim to limit phosphorous intake without compromising intake of protein and other nutrients.8 Although avoidance of foods containing polyphosphate-based additives (a source of readily absorbable inorganic phosphate) may not pose a risk to patients, avoidance of organic sources of phosphate may impair adequate protein consumption.8,9 Observational studies have suggested that PB treatment is associated with lower mortality when compared with no treatment.10–12 Despite such associations and evidence-based guidelines recommending the lowering of elevated serum phosphorus levels, 35% of US dialysis patients have elevated phosphorus levels (>5.5 mg/dL).7,8,13 Although effective in lowering serum phosphorus concentrations, PBs are traditionally associated with high pill burdens and low regimen adherence.4,14,15 Not surprisingly, reduced adherence to prescribed PB regimens is associated with increased serum phosphorus concentrations.4
Sucroferric oxyhydroxide (SO; Velphoro® [Fresenius Medical Care Renal Therapies Group, Waltham, MA]) is a noncalcium, chewable, iron-based PB indicated for the treatment of hyperphosphatemia in patients on dialysis.14 SO has a high phosphate-binding capacity with minimal iron absorption.2,14,15 Data from multicenter phase II and III clinical studies demonstrated similar efficacy, good tolerability, and lower pill burden of SO when compared to sevelamer carbonate.2,14,16,17 In a 52-week, phase III study comparing SO to sevelamer, equivalent control of serum phosphorus was maintained with a mean (standard deviation [SD]) number of pills per day of 3.3 (1.3) for SO and 8.7 (3.6) for sevelamer.16 As determined by a standardized measure (i.e., PB equivalent dose of 6.0 g/day), 3.75 pills/day of SO are comparable to 10 pills/day of sevelamer carbonate.18
A recent 6-month retrospective analysis of pharmacy data of patients on chronic hemodialysis who were prescribed SO as part of the routine care demonstrated a greater than 50% decrease in PB pill burden and a 95% increase in the proportion of patients with serum phosphorus levels of 5.5 mg/dL or less.19 The objective of the present study was to examine the long-term real-world effectiveness of SO in managing serum phosphorus levels in hemodialysis patients over a 1-year period.
Methods
Study Design
This historical cohort analysis included adult (age ≥ 18 years) in-center hemodialysis patients from Fresenius Kidney Care (FKC) units who switched from another PB to SO therapy as part of routine care between March 2014 and March 2015. All patients were required to have 12 months of uninterrupted SO prescriptions recorded and documented serum phosphorus levels. Retrospective, deidentified electronic medical records were extracted from the FKC clinical data warehouse, and prescription fill information was extracted from the electronic records of FreseniusRx, a renal pharmacy service. SO doses were titrated at the discretion of the treating health-care providers. Treatment periods were divided into consecutive 91-day intervals and were defined as baseline (−Q1; 3 months before SO prescription) and SO follow-up (Q1 through Q4; 12 months of SO prescription). Quarterly comparisons were carried out with the baseline quarter as the reference.
Patient-level demographic characteristics, dialysis vintage, and comorbidity-related characteristics were evaluated at baseline. The mean prescribed number of PB pills was recorded for each patient. Assessed laboratory measures included serum mineral bone disease (MBD) markers (phosphorus, calcium, and intact parathyroid hormone [iPTH]); nutritional and clearance parameters (serum albumin, normalized protein catabolic rate [nPCR], and equilibrated Kt/V); hemoglobin; and iron indices (ferritin and transferrin saturation [TSAT]). Blood samples were drawn as part of routine clinical care using standardized methods at FKC facilities and analyzed at Spectra Laboratories (Rockleigh, NJ). Laboratory tests are measured monthly except for serum ferritin and iPTH, which are assessed quarterly, and hemoglobin, which is measured weekly per standard practice at FKC clinics. Although the schedule varies by clinic, patients at a given site generally have blood samples collected on the same day of each week. Serum calcium was adjusted for serum albumin using an equation validated among patients on hemodialysis.20 Phosphorus-attuned albumin and nPCR values were calculated by dividing serum albumin and nPCR by serum phosphorus levels.21 The use and dose details of intravenous (IV) iron, oral cinacalcet, erythropoiesis-stimulating agent (ESA), and vitamin D were extracted for each quarter. This study was approved by the New England Institutional Review Board (Needham, Massachusetts).
Statistical Analyses
Demographic data are presented as mean (SD) for continuous variables and number of patients (percentage) for categorical variables. Serum phosphorus levels were categorized a priori (≤5.5 mg/dL, 5.6–6.5 mg/dL, 6.6–7.5 mg/dL, 7.6–8.5 mg/dL, and >8.5 mg/dL). Mean baseline and follow-up PB pill burden were calculated for each patient. Monthly assessments are included for descriptive purposes; formal statistical analyses were performed on quarterly data. Monthly clinical data were analyzed using linear mixed effects regression models to account for nonmonotone missing data patterns and to account for nonmonotone missing data patterns and to estimate fixed effects across patients and random effects of individual patients. Laboratory tests repeated within any given month were averaged to overcome short-term measurement variability. Summary statistics were presented as least-squared means and standard errors (SEs), and P values compared estimates across treatment quarters. Cochran’s Q test and McNemar chi-square test were used to compare the proportion of patients who achieved the Kidney Disease Outcomes Quality Initiative–defined serum phosphorus goal (≤5.5 mg/dL).7 Two-tailed P values < .05 were considered to be statistically significant. Prespecified subgroup analyses were conducted for ethnic/racial subgroups of interest (i.e., black/African-American patients and Hispanic/Latino patients), for female patients, for patients achieving serum phosphorus of ≤5.5 mg/dL during at least 2 quarters of SO follow-up, and by baseline PB. All analyses were conducted with SAS (version 9.4; SAS Institute Inc, Cary, NC, USA).
Results
Study Participants
A total of 3,110 patients were switched from another PB to SO therapy during the analysis period. There were 2,580 patients excluded from the current analysis as a result of changing dialysis facilities (n = 201), non-continuous SO prescription days (n = 785), prescription of combination PB therapy with SO (n = 1,024), or switching to a new PB (n = 570). The analytic cohort included 530 adults who received thrice-weekly hemodialysis treatments. Baseline demographics are summarized in Table 1. The cohort included 217 (41.0%) individuals who were identified as black/African American, and 87 (16.4%) who were identified as Hispanic/Latino. During the baseline period, patients were prescribed monotherapy with sevelamer (59.8%), calcium acetate (27.6%), lanthanum carbonate (7.9%), or magnesium carbonate (0.4%) or were switched between these agents over the 3-month period (4.3%).
Table 1.
Patient Characteristics at Baseline
| Demographic Characteristics | Study Cohort (N = 530) |
|---|---|
| Age (years) | 54.5 [17] |
| Dialysis vintage (months) | 45.3 [55.2] |
| Body mass index (kg/m2) | 29.8 [10.7] |
| Female, n (%) | 213 (40.2%) |
| Race, n (%)* | |
| Black/African American | 217 (40.9%) |
| White | 284 (53.6%) |
| Other | 29 (5.5%) |
| Hispanic/Latino, n (%)* | 87 (16.4%) |
| Primary cause of renal failure, n (%) | |
| Diabetes mellitus | 211 (39.8%) |
| Hypertension | 176 (33.2%) |
| Glomerulonephritis | 43 (8.1%) |
| Polycystic kidney | 16 (3%) |
| Other | 66 (12.5%) |
| Unknown | 18 (3.4%) |
| Diabetes mellitus, n (%) | 304 (57.4%) |
| Congestive heart failure, n (%) | 109 (20.6%) |
| Phosphate binder at baseline, n (%) | |
| Sevelamer | 317 (59.8%) |
| Calcium acetate | 146 (27.6%) |
| Lanthanum carbonate | 42 (7.9%) |
| Magnesium carbonate | 2 (0.4%) |
| Other† | 23 (4.3%) |
Values are presented as median [interquartile range], or n (%).
Race/ethnicity was self-reported.
Includes patients who switched between sevelamer, calcium acetate, and/or lanthanum carbonate at baseline.
Serum Phosphorus Levels and Pill Burden in the Overall Cohort
Figure 1 illustrates the monthly distribution of serum phosphorus levels before and after patients were switched to SO. In the month preceding the switch, 18.7% of patients had in-range serum phosphorus concentrations (i.e., ≤5.5 mg/dL). During the SO therapy, as many as 39.8% of patients met this criterion, representing a 113% increase in patients achieving target serum phosphorus goals. The mean (SD) pill burden was 8.7 (4.0) pills/day in the month preceding the switch to SO. Relative to that month, SO therapy was associated with at least a 49% reduction in mean pill burden during each subsequent month. Over the year of SO therapy, the mean (SD) SO pill burden was uptitrated from 4.0 (1.8) at months 1 and 2 to 4.4 (1.9) at month 12.
Figure 1.
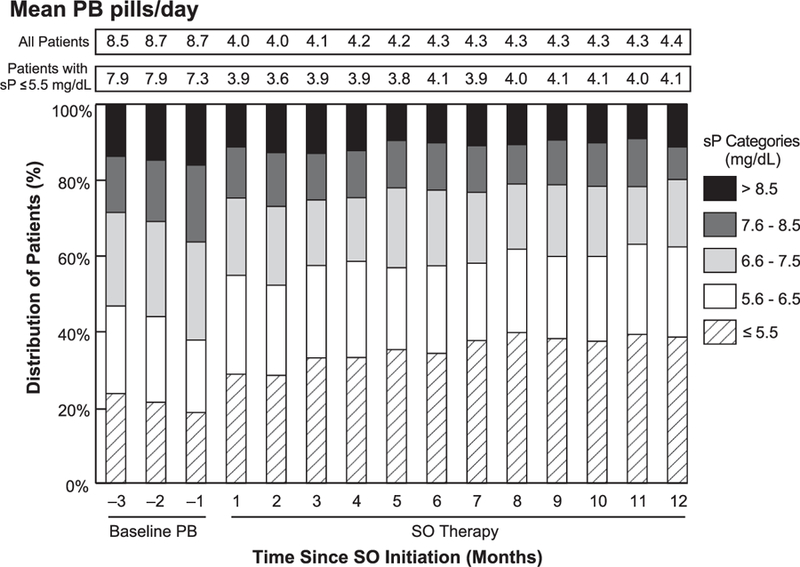
Monthly distribution of patients stratified by serum phosphorus levels. PB, phosphate binder; SO, sucroferric oxyhydroxide; sP, serum phosphorus concentration.
At baseline (−Q1), the mean serum phosphorus concentration was 6.83 mg/dL, and only 17.7% of the cohort had attained a serum phosphorus level of ≤5.5 mg/dL on their PB. After switching to the SO therapy, improvement from baseline in the mean serum phosphorus level was demonstrated at each follow-up quarter (6.54 mg/dL, 6.37 mg/dL, 6.25 mg/L, and 6.19 mg/dL at Q1 through Q4, respectively; P <.0001 vs. baseline). The proportion of patients achieving target serum phosphorus levels (≤5.5 mg/dL) was higher than baseline throughout the SO treatment period: 24.5%, 30.5%, 36.4%, and 36.0% at Q1, Q2, Q3, and Q4, respectively (P < .0001 for each quarter vs. baseline). Additional analyses were conducted to examine the percentage of patients attaining serum phosphorus concentrations ≤4.5 mg/dL. The percentage of patients meeting this cutoff increased from 4.7% at baseline to 6.6%, 11.6%, 12.1%, and 13.7% at SO follow-up Q1, Q2, Q3, and Q4, respectively (P < .0001 for each interval vs. baseline). Overall, PB pill burden was reduced from baseline by approximately 50% for each 91-day interval during the treatment period (Q1 through Q4: 4.0–4.3; P <.0001 for each interval vs. baseline).
Pill Burden by Phosphorus Levels
Table 2 shows the impact of phosphorus levels on PB pill burden. At baseline, patients with increasing severity of hyperphosphatemia were prescribed a higher number of mean daily PB pills, and the attainment of target serum phosphorus goals (≤5.5 mg/dL) was associated with a mean daily pill burden of 7.3 pills. On SO therapy, the pill burden associated with target phosphorus levels was 3.8 to 4.1 pills/day across the treatment period, representing a 44% to 48% reduction. In general, patients with higher on-treatment serum phosphorus concentrations had increased pill burdens. At baseline, patients with the highest serum phosphorus levels (>8.5 mg/dL) were prescribed, on average, 2.5 more pills/day than patients with in-range serum phosphorus levels (≤5.5 mg/dL). In contrast, SO pill number increased by only a mean of 0.4 to 0.8 SO pills/day among patients with the highest serum phosphorus levels (vs. serum phosphorus levels of ≤5.5 mg/dL).
Table 2.
Mean Phosphate Binder Pill Burden Stratified by Serum Phosphorus Categories
| PPD by sP (mg/dL) Categories at Specified Study Quarters |
|||||
|---|---|---|---|---|---|
| Time Period | ≤5.5 | 5.6–6.5 | 6.6–7.5 | 7.6–8.5 | >8.5 |
| Baseline (−Q1) | 7.3 (4.0) | 8.0 (3.9) | 8.8 (4.1) | 9.0 (3.7) | 9.8 (4.4) |
| SO follow-up Q1 | 3.8 (1.7) | 4.0 (1.6) | 4.3 (1.8) | 4.4 (1.7) | 4.2 (1.6) |
| SO follow-up Q2 | 3.9 (1.7) | 3.9 (1.5) | 4.7 (2.1) | 4.4 (2.0) | 4.3 (1.4) |
| SO follow-up Q3 | 4.1 (1.8) | 4.4 (1.9) | 4.4 (1.8) | 4.3 (1.9) | 4.7 (2.2) |
| SO follow-up Q4 | 4.0 (1.8) | 4.5 (1.8) | 4.6 (2.2) | 4.3 (2.0) | 4.8 (2.0) |
PPD, pills per day; SO, sucroferric oxyhydroxide; sP, serum phosphorus.
Summary estimates are presented as mean (standard deviation).
Proportion of Patients Achieving Serum Phosphorus ≤5.5 mg/dL and Pill Burden Stratified by Baseline PB
Among the 317 patients who switched from sevelamer to SO, mean serum phosphorus levels were reduced after SO initiation (6.77 mg/dL at −Q1 to 6.52, 6.31, 6.27, and 6.14 mg/dL during progressive SO-treated quarters, P <.0001). As a result, the percentage of patients achieving serum phosphorus levels of ≤5.5 mg/dL increased significantly during SO follow-up (Figure 2A). This subgroup also demonstrated marked reductions in pill burden from 8.9 sevelamer pills/day at baseline to 4.0, 4.3, 4.4, and 4.4 SO pills/day during quarters 1, 2, 3, and 4, respectively (P <.0001).
Figure 2.
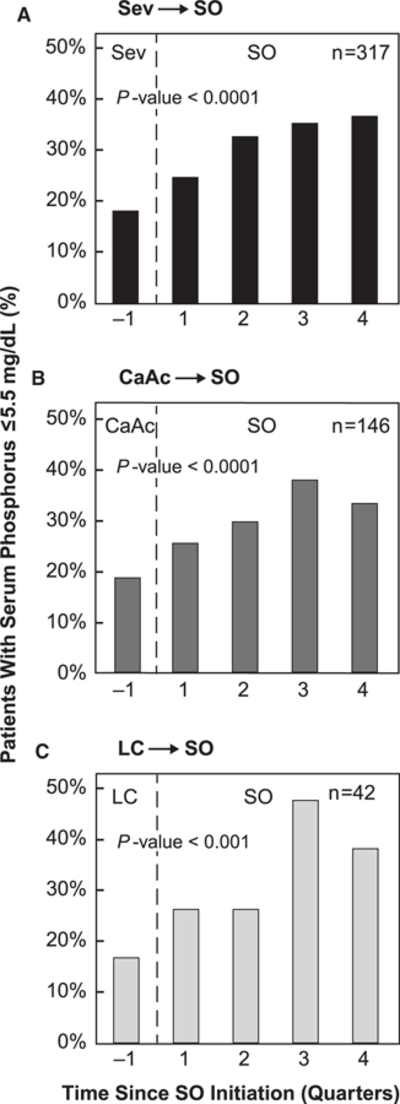
Percentage of patients achieving serum phosphorus ≤5.5 mg/dL stratified by baseline phosphate binder type. Figure does not include 25 patients who switched between sevelamer, calcium acetate, and/or lanthanum carbonate at baseline and those on magnesium carbonate. CaAc, calcium acetate; LC, lanthanum carbonate; Sev, sevelamer; SO, sucroferric oxyhydroxide.
Similarly, among former calcium acetate patients prescribed with calcium acetate (n = 146), a notable reduction in serum phosphorus (6.90 mg/dL at baseline to 6.55, 6.49, 6.28, and 6.31 mg/dL during SO-treated quarters, P< .0001) and a significant increase in the percentage of patients achieving in-range serum phosphorus were observed (Figure 2B). Mean daily pill burden among this subgroup was reduced by more than 50% (8.9 calcium acetate pills/day to 3.9–4.0 SO pills/day; P <.0001).
Patients who were initially prescribed with lanthanum carbonate (n = 42) demonstrated a marked improvement in serum phosphorus after switching to SO (6.71 mg/dL at baseline; 6.38, 6.28, 5.79, and 5.93 mg/dL at SO Q1 through Q4, respectively; P < .0001). The percentage of patients achieving target serum phosphorus levels significantly increased from 16.7% at baseline to as high as 47.6% at Q3 (Figure 2C). In contrast to other PB, patients receiving lanthanum carbonate at baseline did not exhibit a decrease in pill burden after switching to SO (4.4 lanthanum pills/day at baseline to 4.5–4.7 SO pills/day; P =.56).
Among patients who were on magnesium carbonate (n = 2) and those who switched between PBs at baseline (n = 23), reductions in pill burden (8.5 pills/day [−Q1] to 4.3 pills/day [Q4]) and serum phosphorus (6.8 mg/dL [−Q1] to 6.2 mg/dL [Q4]) were observed during SO treatment (P < .0001 for all comparisons). This subgroup also demonstrated marked increases in the proportion of patients achieving in-range serum phosphorus levels after the switch to SO (12.0% [−Q1] to 41.7% [Q4]; P = .01).
Proportion of Patients Achieving Serum Phosphorus ≤5.5 mg/dL and Pill Burden in Predefined Subgroups
Given the increased burden of ESRD among the black/African-American population,22 we examined the changes in serum phosphorus and PB pill burden in this subgroup (n = 217). Before initiating the SO therapy, this subgroup was prescribed a mean 8.9 PB pills/day, with 14.3% achieving serum phosphorus target goals (≤5.5 mg/dL). After switching to SO, there was a decrease in PB pill number (4.1 [Q1], 4.3 [Q2], 4.4 [Q3], and 4.5 [Q4] SO pills/day, P < .0001) and a notable increase in the proportion of patients achieving in-range serum phosphorus levels (23.0%, 30.4%, 34.1%, and 32.7% in Q1 through Q4, respectively; P ≤.004).
Among Hispanic/Latino patients (n = 87), 18.4% attained target phosphorus concentrations on baseline therapy. After a switch to SO treatment, there was a greater than 55% increase in the proportion of Hispanic/Latino patients achieving target serum phosphorus levels (32.2% [Q1], 28.7% [Q2], 39.1% [Q3], and 35.3% [Q4]; P < .02 for Q1, Q3, and Q4). This subgroup also demonstrated a greater than 50% reduction in their mean daily PB pill burden (8.9 pills/day [−Q1] to 4.1–4.4 SO pills/day; P <.0001).
The effectiveness of SO in lowering serum phosphorus was also observed among the 213 women in the cohort. Serum phosphorus levels were reduced from 6.77 mg/dL (−Q1) to 6.48 mg/dL, 6.25 mg/dL, 6.24 mg/dL, and 6.10 mg/dL at SO follow-up (Q1 through Q4, respectively, P < .0001). Progressive increases were seen in the proportion of women who achieved target phosphorus levels (P =.03 [Q1] and P <.0001 [Q2–Q4]). At baseline, 17.4% of women had in-range serum phosphorus concentrations; by Q4, this increased by 119% to 38.0%. Women also experienced marked reductions in mean PB pill burden from 8.4 pills/day (–Q1) to 4.0 to 4.3 pills/day during SO follow-up (P < .0001).
Additional Clinical Parameters
Multiple laboratory parameters were extracted from the electronic records of the analyzed cohort. In addition to the significant reduction in the serum phosphorus level previously described, reductions in corrected serum calcium and increases in iPTH were observed over the 1-year follow-up period (Table 3). Significant reductions in corrected calcium concentrations were also observed across the racial/ethnic/sex subgroups described previously. Changes in serum iPTH were not associated with SO initiation among black/African-American patients or women but increased significantly among Hispanic patients. Although small decreases in albumin and nPCR were observed for the overall cohort, once these variables were adjusted for serum phosphorus (i.e., phosphorus-attuned albumin and phosphorus-attuned nPCR), improvements (i.e., increases) in both parameters were observed (P < .0001). As detailed in Table 3, minimal changes in other nutritional and clearance parameters were observed in the overall cohort.
Table 3.
Comparison of Changes in Clinical Parameters and CKD-MBD Medication Use
| Parameter | Baseline (−Q1; ref) | SO Therapy |
||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P Value | ||
| CKD-MBD biochemical markers | ||||||
| Serum phosphorus (mg/dL) | 6.82 (0.05) | 6.54 (0.05)*** | 6.37 (0.05)*** | 6.25 (0.05)*** | 6.19 (0.05)*** | <.0001 |
| Serum phosphorus ≤5.5 mg/dL (%) | 17.7 | 24.5*** | 30.5*** | 36.4*** | 36.0*** | <.0001 |
| Corrected calcium (mg/dL)† | 9.25 (0.03) | 9.21 (0.03)* | 9.16 (0.03)*** | 9.16 (0.03)*** | 9.1 (0.03)*** | <.0001 |
| iPTH (pg/mL) | 611 (23) | 627 (23) | 622 (23) | 636 (23) | 643 (23)* | .16 |
| CKD-MBD medications | ||||||
| Phosphate binder pills/day | 8.5 (0.08) | 4.0 (0.07)*** | 4.1 (0.07)*** | 4.2 (0.07)*** | 4.3 (0.07)*** | <.0001 |
| Cinacalcet use (%) | 38.5 | 40.8* | 44.2*** | 45.7*** | 46.0*** | <.0001 |
| Cinacalcet dose (mg/day) | 60.1 (4.1) | 63.4 (4.1)** | 63.9 (4.1)*** | 63.0 (4.1)* | 62.0 (4.1) | .0002 |
| IV active vitamin D‡ use (%) | 74.2 | 69.6* | 62.5*** | 53.4*** | 43.2*** | <.0001 |
| IV doxercalciferol dose (mcg/week) | 3.7 (0.1) | 3.9 (0.1)* | 4.0 (0.1)** | 4.2 (0.1)*** | 4.3 (0.1)*** | <.0001 |
| Oral active vitamin D§ use (%) | 15.7 | 24.2*** | 34.5*** | 42.6*** | 47.2*** | <.0001 |
| Oral calcitriol dose (mcg/week) | 0.62 (0.03) | 0.65 (0.02)* | 0.70 (0.02)*** | 0.75 (0.02)*** | 0.84 (0.02)*** | <.0001 |
| Nutritional and clearance parameters | ||||||
| Serum albumin (g/dL) | 3.96 (0.01) | 3.97 (0.01) | 3.97 (0.01) | 3.95 (0.01) | 3.92 (0.01)*** | <.0001 |
| Phosphorus-attuned albumin, ×103 | 0.62 (0.01) | 0.65 (0.01)*** | 0.68 (0.01)*** | 0.69 (0.01)*** | 0.69 (0.01)*** | <.0001 |
| Predialysis weight (kg) | 90.3 (1.0) | 90.8 (1.0)*** | 90.9 (1.0)*** | 90.8 (1.0)*** | 90.7 (1.0)*** | <.0001 |
| nPCR (g/kg/day) | 0.96 (0.01) | 0.96 (0.01) | 0.95 (0.01) | 0.94 (0.01)* | 0.94 (0.01)** | .002 |
| Phosphorus-attuned nPCR, ×103 dL/kg/day | 0.15 (0.002) | 0.16 (0.002)*** | 0.16 (0.002)*** | 0.16 (0.002)*** | 0.16 (0.002)*** | <.0001 |
| Equilibrated Kt/V | 1.46 (0.01) | 1.47 (0.01) | 1.46 (0.01) | 1.47 (0.01) | 1.46 (0.01) | .23 |
CKD-MBD, chronic kidney disease–related mineral and bone disorders; iPTH, intact parathyroid hormone; IV, intravenous; nPCR, normalized protein catabolic rate; ref, referent; SO, sucroferric oxyhydroxide.
Values are presented as least-squared mean (standard error). P values compare summary estimates across time with −Q1 as the reference. Overall P values were calculated using linear mixed effects regression (continuous variables) or Cochran’s Q test (categorical variables).
P < .05
P < .001
P < .0001 (vs. baseline).
Corrected calcium = serum calcium + [0.0176 × (34 − serum albumin)].
IV vitamin D use includes doxercalciferol, calcitriol, and paricalcitol.
Oral vitamin D use includes calcitriol and doxercalciferol.
Consistent with current recommendations to prevent the progression of secondary hyperparathyroidism, some patients were prescribed calcimimetics and/or active vitamin D analogs as part of routine clinical care during the study period. The temporal changes in these utilization parameters are detailed in Table 3.
At baseline, 78.9% of patients received IV iron (mean [SE] dose: 75.4 [1.1] mg/month) and 87.4% received IV ESA (mean [SE] epoetin alfa dose: 5085 [192] units/week). During SO-treated follow-up, there was a small but statistically significant decrease in prescription of anemia therapy medications and a minimal reduction in mean IV iron and ESA doses (Table 4). Analyzing anemia and iron indices, we observed an increase in the mean ferritin level from 987 ng/mL (baseline) to 1096 ng/mL (Q4; P < .0001). Small increases in TSAT (P = .0001) and hemoglobin (P < .0001) were observed during the SO follow-up period.
Table 4.
Comparison of Changes in Anemia and Iron Indices and Anemia Therapies
| SO Therapy |
||||||
|---|---|---|---|---|---|---|
| Parameter | Baseline (−Q1; ref) | Q1 | Q2 | Q3 | Q4 | P Value |
| Anemia and iron indices | ||||||
| Ferritin (ng/mL) | 988 (22) | 1056 (22)** | 1075 (21)*** | 1089 (22)*** | 1096 (21)*** | <.0001 |
| Transferrin saturation (%) | 34.4 (0.5) | 35.7 (0.5)* | 35.9 (0.5)** | 36.3 (0.5)*** | 35.8 (0.5)* | .0001 |
| Hemoglobin (g/dL) | 10.9 (0.05) | 10.9 (0.05) | 10.9 (0.05)*** | 10.9 (0.05) | 10.9 (0.05) | <.0001 |
| Anti-anemia therapy | ||||||
| IV iron sucrose use (%) | 78.9 | 77.7 | 75.7 | 69.6** | 72.1* | .0002 |
| IV iron sucrose dose (mg/month) | 75.4 (1.1) | 73.5 (1.1) | 72.7 (1.1)* | 71.0 (1.1)*** | 73.0 (1.1)* | .001 |
| IV ESA use† (%) | 87.4 | 85.1 | 84.5* | 84.3* | 83.2* | .03 |
| IV epoetin alfa dose (IU/week) | 5085 (192) | 4675 (193)** | 4790 (195)* | 4830 (202)* | 5012 (215) | .003 |
ESA, erythropoietin-stimulating agents; IV, intravenous; ref, referent; SO, sucroferric oxyhydroxide.
Values are presented as least-squared mean (standard error) or n (%). P values compare summary estimates across time with −Q1 as the reference. Overall P values were calculated using linear mixed effects regression (continuous variables) or Cochran’s Q test (categorical variables).
P < .05
P < .001
P < .0001 (vs. baseline).
IV ESA use includes epoetin alfa, epoetin beta and methoxy polyethylene glycol, and darbepoetin alfa.
Patients With In-Range Serum Phosphorus for ≥50% of SO Treatment Period
A prespecified analysis of patients (n = 197) who achieved serum phosphorus ≤5.5 mg/dL during at least half of the SO treatment period (i.e., ≥2 quarters) was performed to evaluate the effects of achieving target phosphorus goals on other MBD markers and PB pill burden. Within this subgroup, PB pill burden decreased from a baseline mean of 7.8 pills/day to a mean of 3.8, 3.9, 4.0, and 4.0 pills/day during Q1 through Q4 of SO follow-up, respectively (P <.0001). A reduction in mean serum phosphorus concentrations from baseline (5.9 mg/dL) was observed (5.4, 5.1, 5.0, and 4.9 mg/dL for SO follow-up from Q1 through Q4, respectively; P <.0001 for each). Treatment with SO was also associated with small reductions in mean corrected calcium levels at later follow-up timepoints (9.35 mg/dL [2Q1] to 9.16 mg/dL [Q4]; P <.0001). Finally, at Q4, small increases in mean iPTH were observed in this subgroup (baseline: 442 pg/mL; Q4: 477 pg/mL; P <.05).
Nearly two-third of patients (127/197) who achieved in-range serum phosphorus levels during the SO follow-up period for 2 or more quarters had a baseline serum phosphorus level > 5.5 mg/dL. Among this subgroup, we observed improvement in mean serum phosphorus (6.47 mg/dL at baseline to 5.56, 5.18, 5.07, and 5.08 mg/dL at SO follow-up Q1 through Q4, respectively; P < .0001) and substantial reductions in PB pill burden from baseline (8.0 pills/day) to SO follow-up (4.0–4.2 SO pills/day; P < .0001). In this subgroup, the significant improvements in phosphorus-attuned albumin and nPCR were more than 2.5-fold greater than those observed in the overall cohort at each quarter (vs. baseline). During SO treatment, reductions in mean corrected calcium levels were recorded (9.34 mg/dL [−Q1] to 9.16 mg/dL [Q4]; P < .0001), but no consistent change in iPTH was observed in this subgroup.
Discussion
In this present retrospective study, 1 year of SO treatment effectively reduced the number of mean prescribed PB pills by approximately 50% (8.5 pills/day to 4.0–4.3 pills/day) among patients on hemodialysis for whom the clinical decision was made to switch baseline PBs to SO. In addition, when analyzed by the 91-day treatment period, 1 year of SO therapy was associated with a 2-fold increase in the proportion of patients achieving target serum phosphorus goals of ≤5.5 mg/dL (17.7% to 36.0%). Because pill burden is associated with adherence, the observed reductions in serum phosphorus associated with the real-world use of SO may, in part, result from increased adherence to a therapy with a lower pill burden. Such a mechanism is supported by findings from a prior randomized clinical trial in which SO was noninferior to sevelamer.23 The results of the present analyses are consistent with a previous study, where an improvement in pill burden and serum phosphorus was observed in a hemodialysis population over 6 months of SO treatment.19 Also, reductions in serum phosphorus levels observed with SO in the present study were similar to those documented in prospective clinical trials of SO in patients on dialysis.2,16,17,23
In the setting of inadequate renal excretion of phosphorus, restriction of dietary phosphorus is often difficult for patients to implement and may be inadequate to control serum phosphorus levels. In clinical practice, modification, including up-titration, of PB is often carried out when patients present with uncontrolled serum phosphorus levels. Baseline data from this cohort suggest considerable variations in the number of prescribed PB pills/day. Patients with the highest serum phosphorus levels were prescribed an average of 2.5 more pills/day than those with target serum phosphorus concentrations. In contrast, SO doses exhibited less variation across serum phosphorus categories. It is unclear why higher doses of SO were not more commonly prescribed to patients not achieving target phosphorus levels on starting doses of the medication. In a controlled trial, dose-dependent improvements in serum phosphorus levels were observed with SO doses of up to 3,000 mg (6 tablets) per day,17 suggesting that increased control could have been achieved with higher doses of SO.
In this retrospective analysis of patients who had their PB switched to SO, we observed that SO therapy was associated with a mean pill burden similar to that of lanthanum carbonate but significantly less than that of sevelamer and calcium acetate. Patients on sevelamer or calcium acetate at baseline experienced a greater than 50% reduction in prescribed PB pill burden 1 year after switching to SO. When patients were switched from sevelamer or lanthanum carbonate to SO, the proportion of patients achieving in-range serum phosphorus increased by more than 100% from baseline to Q4; an increase of nearly 80% was observed for patients switching from calcium acetate to SO. These findings are consistent with the results from previous studies.2,16,23 Irrespective of the baseline PB, SO was effective in lowering serum phosphorus.
In subgroup analyses, we found that SO improved serum phosphorus levels in black/African-American and Hispanic/Latino patients on hemodialysis while also providing a reduced pill burden. The United States Renal Data System indicates that in 2015, blacks were 3 times more likely to develop ESRD than whites, whereas Hispanics were 1.3 times more likely to develop ESRD than non-Hispanics.22 Moreover, these subgroups may exhibit differences in phosphorus levels and/or other markers of mineral metabolism compared with reference groups.24,25 One study found that the impact of elevated levels of serum phosphorus on survival appears to be greater for African Americans than for reference groups.24 This finding, however, has not been replicated.26
Although the incidence of ESRD is considerably lower among women than men,22 sex-related differences in phosphorus homeostasis exist,26 theoretically impacting the treatment needs of this subgroup. Data from the Dialysis Outcomes and Practice Patterns Study have demonstrated that PB use was an independent predictor of improved survival among women.27 The effect of PB use on mortality was greater than that observed in men. In the present analysis, among women, the effects of switching to SO were consistent with those observed for the overall cohort (i.e., a significant decrease in serum phosphorus and PB pill burden). The effectiveness of SO in lowering the serum phosphorus level across subgroups defined by race, ethnicity, and sex is consistent with the findings of controlled clinical trials.28
Given that SO is an iron-based PB, we examined temporal changes in anemia and iron indices. Ferritin concentrations and TSAT were higher during SO follow-up than at baseline. Such increases were observed in the setting of reduced IV iron and ESA use. Similar results were reported in a phase III study in which changes were attributed to concomitant IV iron use, suggesting minimal iron absorption from SO.29
In this real-world evaluation of patients who switched to SO, changes were also observed in several MBD markers and medications over time. There was an observed increase in oral calcitriol use and concurrent decrease in IV doxercalciferol use. In the year after initiation of the SO therapy, patients also demonstrated decreases in calcium and increases in iPTH. The magnitude of these changes was similar to that observed in the SO and active control (sevelamer) arms of a 1-year clinical trial16 and may reflect progression of hyperparathyroidism. Such changes may reflect alterations in health status rather than direct therapy effects. It is worth noting that the present analyses were not adjusted for changes in therapy (other than the switch to SO), comorbid illness, or other laboratory values. Similarly, patients were not excluded from the study based on any baseline laboratory parameters, medications, or health status.
Among patients undergoing hemodialysis, the restriction of dietary protein intake for the purposes of preventing hyperphosphatemia should be balanced against the risk of malnutrition.30 Several analyses have failed to demonstrate a survival benefit with dietary restriction of phosphorus, and in some subgroups, stringent dietary restriction of phosphorus and resultant decreases in protein intake (as assessed by nPCR) were actually associated with increased mortality.30,31 Reduced protein intake is also associated with reductions in albumin concentrations. In a large cohort of patients on maintenance hemodialysis, higher nPCR and temporal increases in albumin was independently associated with greater survival.32 In the present study, there were small, but significant, reductions in nPCR and serum albumin levels observed after the switch to SO. To assess the impact of lowering serum phosphorus without further restricting dietary protein, serum albumin and nPCR were each divided by serum phosphorus. Both phosphorus-attuned variables demonstrated significant increases after the switch to SO, suggesting an overall improvement in the nutritional status. These findings further suggest that observed reductions in phosphorus were the result of SO, not decreases in protein intake or substantial changes in dietary habits. Hemodialysis adequacy also remained unchanged during the analysis period. It is worth noting that the initiation of SO therapy for the management of hyperphosphatemia does not obviate the need for continued dietary phosphorus management. A high dietary phosphorus burden, particularly one containing excess consumption of absorbable inorganic phosphorus, can undermine the effects of therapy with any PB.9
The strengths of the present study include its broad patient population, making the results generalizable to the ‘‘real-world’’ hemodialysis patient population encountered in clinical practice. In addition, the year-long treatment period extends the findings of a prior 6-month analysis19 and supports the long-term effectiveness of SO in managing serum phosphorus. The results of the present analysis should, however, be viewed in the context of several limitations. It is retrospective in nature and lacks a comparator group followed up over the same study period. Patient-level data were extracted from electronic medical records and were not collected for research purposes; there may be incomplete or missing variables of interest (e.g., tolerability, reasons for medication changes). The absence of side effect data prevents one from drawing conclusions regarding the safety and tolerability of SO from this retrospective analysis, but the safety profile of SO has been previously characterized in controlled trials.2,23,28 The decision to discontinue baseline PBs and switch to SO was made on a clinical basis, and the reasons underlying this change were not available. Possible reasons for discontinuation of baseline PBs include lack of effectiveness, nonadherence, nontolerability, insurance coverage, and out-of-pocket costs. Those patients who did not continue PB monotherapy with SO for 12 months were excluded from the present analysis. In addition, although markers of nutritional status were analyzed, we do not account for specific nutritional education/advice provided to patients by dietitians, including those provided alongside the PB switch to SO, nor do we have data on the use of protein supplements. Finally, prescription data provide accurate insight into prescribed pill burden but cannot be used as a surrogate for patient adherence with prescribed regimens.
In conclusion, results from this study demonstrate that completion of 1 year of treatment with SO was effective in controlling hyperphosphatemia in patients on hemodialysis with fewer number of prescribed pills per day than other PBs.
Practical Application
Clinicians have an increasing armamentarium of pharmacologic options for lowering serum phosphorus levels among patients with ESRD undergoing maintenance hemodialysis. This analysis suggests that switching patients with hyperphosphatemia to SO is an effective means of achieving further phosphorus control while simultaneously reducing the pill burden experienced by patients and improving the nutritional status.
Acknowledgments
Medical writing and editing support was provided by Adam Perahia, MD, of NorthStar Strategic Consulting, LLC, via funding by Fresenius Medical Care Renal Therapies Group.
Footnotes
Support and financial disclosure declaration: This retrospective database analysis was funded by Fresenius Medical Care Renal Therapies Group. J.K. has participated in advisory boards for Fresenius Medical Care Renal Therapies Group. L.H.F., V.P., C.M., and R.J.K. are employees of Fresenius Medical Care Renal Therapies Group. N.J.O. and S.D. are employees of Fresenius Medical Care North America. S.D. is a speaker and advisor to Fresenius Medical Care Renal Therapies Group. C.M. and R.J.K. own stock in the company. R.J.K. is on the Board of Directors of Advanced Renal Technologies. K.K.Z. has received honoraria and/or support from Abbott, AbbVie, Alexion, Amgen, ASN (American Society of Nephrology), Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, IFKF (International Federation of Kidney Foundations), ISH (International Society of Hemodialysis), International Society of Renal Nutrition & Metabolism, (ISRNM), JSDT (Japanese Society of Dialysis Therapy), Hospira, Kabi, Keryx, Novartis, OPKO, NIH (National Institutes of Health), NKF (National Kidney Foundations), Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZS-Pharma.
References
- 1.Joson CG, Henry SL, Kim S, et al. Patient-reported factors associated with poor phosphorus control in a maintenance hemodialysis population. J Ren Nutr 2016;26:141–148. [DOI] [PubMed] [Google Scholar]
- 2.Koiwa F, Yokoyama K, Fukagawa M, Terao A, Akizawa T. Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphatemia: a randomized, open-label, multicentre, 12-week phase III study. Nephrology (Carlton) 2017;22:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaman AM, Kowalski SR. Hyperphosphatemia management in pa- tients with chronic kidney disease. Saudi Pharm J 2016;24:494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016;20:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locatelli F, Del Vecchio L, Violo L, Pontoriero G. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profiles. Expert Opin Drug Saf 2014;13:551–561. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998;31:607–617. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease– mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupisti A, Kalantar-Zadeh K. Management of natural and added dietary phosphorus burden in kidney disease. Semin Nephrol 2013;33:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208–2218. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictabil- ity of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006;70:771–780. [DOI] [PubMed] [Google Scholar]
- 12.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 2005;16:1788–1793. [DOI] [PubMed] [Google Scholar]
- 13.US-DOPPS (Dialysis and Advanced CKD Outcomes and Practice Patterns Study Program) Practice Monitor. Serum phosphorous (most recent) Available at: http://www.dopps.org/dpm/. Accessed December 20, 2017.
- 14.Floege J, Covic AC, Ketteler M, et al. One-year efficacy and safety of the iron-based phosphate binder sucroferric oxyhydroxide in patients on peritoneal dialysis. Nephrol Dial Transpl 2017;32:1918–1926. [DOI] [PubMed] [Google Scholar]
- 15.Kendrick J, Chonchol M. Novel therapeutic options for the treatment of mineral metabolism abnormalities in end stage renal disease. Semin Dial 2015;28:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floege J, Covic AC, Ketteler M, et al. Long-term effects of the iron- based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transpl 2015;30:1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koiwa F, Terao A. Dose-response efficacy and safety of PA21 in Japanese hemodialysis patients with hyperphosphatemia: a randomized, placebo-controlled, double-blind, Phase II study. Clin Exp Nephrol 2017;21:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutekunst L An update on phosphate binders: a dietitian’s perspective. J Ren Nutr 2016;26:209–218. [DOI] [PubMed] [Google Scholar]
- 19.Coyne DW, Ficociello LH, Parameswaran V, et al. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: a retrospective analysis of pharmacy data. Clin Nephrol 2017;88:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clase CM, Norman GL, Beecroft ML, Churchill DN. Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transpl 2000;15:1841–1846. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Parameswaran V, Ficociello LH, et al. Real-world scenario improvements in serum phosphorus levels and pill burden in peritoneal dialysis patients treated with sucroferric oxyhydroxide. Am J Nephrol 2018;47:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Renal Data System. 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017. [Google Scholar]
- 23.Floege J, Covic AC, Ketteler M, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014;86:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scialla JJ, Parekh RS, Eustace JA, et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol 2015;42:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar VA, Tilluckdharry N, Xue H, Sidell MA. Serum phosphorus levels, race, and socioeconomic status in incident hemodialysis patients. J Ren Nutr 2016;26:10–17. [DOI] [PubMed] [Google Scholar]
- 26.Ho LT, Sprague SM. Women and CKD-mineral and bone disorder. Adv Chronic Kidney Dis 2013;20:423–426. [DOI] [PubMed] [Google Scholar]
- 27.Hecking M, Bieber BA, Ethier J, et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 2014;11:e1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velphoro (Sucroferric Oxyhydroxide) [package insert] Waltham, MA: Fresenius Medical Care North America; 2017. [Google Scholar]
- 29.Covic AC, Floege J, Ketteler M, et al. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transpl 2017;32:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinaberger CS, Greenland S, Kopple JD, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 2008;88:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch KE, Lynch R, Curhan GC, Brunelli SM. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol 2011;6:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriguchi R, Obi Y, Streja E, et al. Longitudinal associations among renal urea clearance-corrected normalized protein catabolic rate, serum albumin, and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2017;12:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]


