Abstract
Introduction:
Post-transplant metabolic syndrome (PTMS)—a clustering of hypertension, dyslipidemia, glucose intolerance/diabetes, and obesity—is increasingly recognized as a contributor to long-term morbidity after transplant. We sought to describe pediatric liver transplant center protocols and provider practices in screening for and managing these conditions.
Methods:
Cross-sectional survey of pediatric liver transplant providers from centers that the Studies of Pediatric Liver Transplantation (SPLIT) group. Surveys were completed on-line.
Results:
Of 49 survey respondents from 39 centers, 64% were hepatologists or surgeons, 18% nurses/NPs/PAs, 12% coordinators, 4% other. All providers felt that pediatric liver transplant recipients should be routinely screened for PTMS components. For each condition, at least 70% felt that the liver transplant team should be primarily responsible for routine screening. For each condition, at least 30% of providers reported that their center had no standardized protocol for screening. For diagnostic evaluation and initial management, >60% of providers reported that their center had no standardized protocol for glucose intolerance/diabetes, dyslipidemia, or obesity. Almost 40% had no standardized workup or initial management protocol for hypertension or chronic kidney disease. Of centers that did have screening or workup protocols, most were based on existing center practice, provider consensus, or informal review of published evidence. Screening tools, treatment steps, and thresholds for referral to another specialist varied widely.
Conclusions:
Transplant providers intend to screen for and initiate management of PTMS components in these children, but protocols and practices vary substantially. This highlights opportunities for multi-center collaboration on protocols or interventions to improve screening and management.
Keywords: children, liver transplantation, metabolic syndrome, obesity, hypertension, glucose intolerance, diabetes, dyslipidemia, immunosuppression
INTRODUCTION
To optimize outcomes in pediatric liver transplant recipients, attention to chronic medical conditions that impact long-term morbidity is crucial. Post-transplant metabolic syndrome (PTMS)—a clustering of hypertension, dyslipidemia, glucose intolerance, and increased waist circumference that can occur with or without obesity—is increasingly recognized as a significant contributor to long-term morbidity and mortality after solid-organ transplantation.1,2 In adults after liver transplant, these conditions are associated with long-term cardiovascular morbidity and mortality.3,4
We have recently shown that pediatric liver transplant recipients have a higher risk of pre-hypertension and hypertension, impaired glucose tolerance (pre-diabetes), and low high-density lipoprotein (HDL) than matched peers, even after controlling for obesity and corticosteroid use3. In long-term follow-up of these children, the prevalence of PTMS, indicating 3 or more of the diagnostic features, is estimated to be 14–20%.3–7 These conditions are identifiable in the pre-clinical stage, and early identification with active management may prevent long-term consequences. Recent guidelines from the American Association for the Study of Liver Diseases (AASLD) and the American Society of Transplantation (AST) recommend annual screening for obesity, hypertension, dyslipidemia, and diabetes mellitus with physical exam and fasting blood tests.8 However, implementation of these recommendations has never been investigated. In addition, their adequacy for detecting PTMS and related conditions is not known.
We conducted a cross-sectional survey of pediatric liver transplant providers at Studies of Pediatric Transplantation (SPLIT) centers on their protocols and practices for (1) routine screening for obesity, hypertension, dyslipidemia, and glucose intolerance/diabetes and (2) diagnostic workup and management of these conditions in pediatric liver transplant recipients. We aimed to describe pediatric transplant center practices and to investigate variation across centers.
METHODS
Data for this study were collected in a cross-sectional survey after study approval by the UCSF Committee on Human Research (CHR #18–24303) and by the SPLIT Research Committee. Email addresses for potential participants—medical providers at pediatric liver transplant centers that are members of the SPLIT—were obtained from the SPLIT Data Coordinating Center. Potential participants were e-mailed an introduction to the study and a link to the consent and survey on the Research Electronic Data Capture (REDCap) hosted at https://redcap.ucsf.edu.9 REDCap is a secure, web-based application designed to support data capture for research studies. All survey responses were registered anonymously in the REDCap database. An initial invitation and up to two email reminders were sent to participants who had not yet completed the survey over a two week period in May 2018.
Data analysis was completed using Stata IC14 and Microsoft Excel. Descriptive statistics were primarily used. Differences in protocols by center size were examined using chi-squared testing.
RESULTS
The survey was completed by 49 providers from 39 pediatric liver transplant centers. Pediatric transplant hepatologists or surgeons accounted for 64% of respondents; 18% were nurses, nurse practitioners, or physician assistants, 12% transplant or research coordinators, and 4% identified as other. Fifty-three percent of respondents had personally worked with pediatric liver transplant recipients for 10 years or longer, 31% for 5–10 years, and 16% for less than 5 years. Ninety percent worked at centers that had been caring for pediatric liver transplant recipients for at least 10 years. Annual center volume of pediatric liver transplants was >20 for 14% of respondents, 11–20 for 39%, 5–10 for 41%, and <5 for 6%.
Responsibility for routine screening
All respondents felt that pediatric liver transplant recipients should be routinely screened for PTMS components (obesity, hypertension, dyslipidemia, glucose intolerance, and diabetes) as well as chronic kidney disease. For each condition, at least 70% of responding providers felt that the liver transplant team should be primarily responsible for this screening. (FIGURE 1) More than one-quarter assigned primary responsibility for obesity screening to the primary care pediatrician.
FIGURE 1:
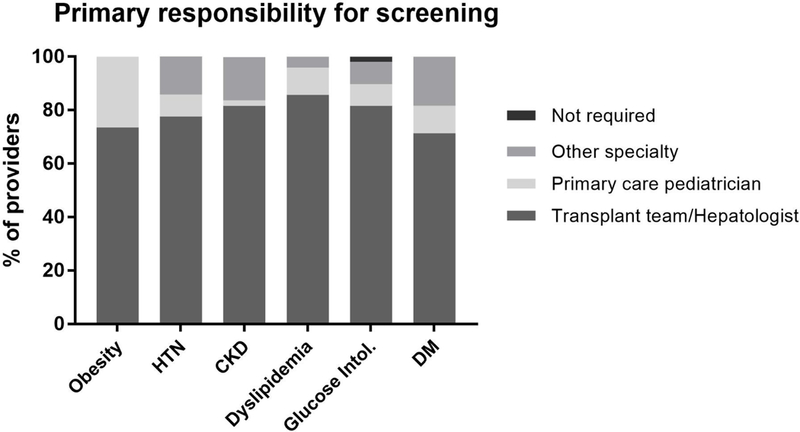
Provider-perceived primary responsibility for screening pediatric liver transplant recipients for components of the metabolic syndrome and chronic kidney disease.
Protocols for routine screening
At least 30% of providers reported that their center had no standardized screening protocol for hypertension, chronic kidney disease, dyslipidemia, or diabetes; 49% had no screening protocol for obesity. Of centers that did have standardized screening protocols, most were based on existing center practice, provider consensus, or informal review of published evidence. A minority were developed through systematic review or other formal process. (FIGURE 2)
FIGURE 2:
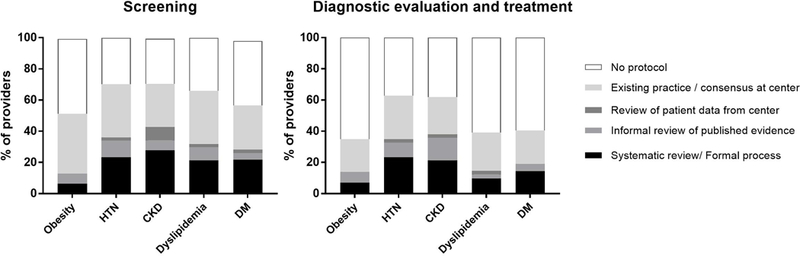
Prevalence of screening practices reported for evaluation and initial treatment of post-transplant metabolic syndrome components in pediatric liver transplant recipients
The percent of providers at centers with screening protocols did not differ significantly by transplant volume (p>0.30 for all 5 conditions). Interestingly, for all conditions low volume centers (<5 transplants per year) and high volume centers (>20 transplants per year) were least likely to have established screening protocols. One example of this trend is that 33% of the low volume centers and 57% of the high volume centers had hypertension screening protocols, compared to 72–79% of medium volume centers (p=0.36).
Among providers that did report having screening protocols at their centers, most providers reported following the protocols “always” or “often,” including 96% for obesity (n=24), 100% for hypertension and chronic kidney disease (n=32), 93% for dyslipidemia (n=31), and 100% for glucose intolerance or diabetes (n=27).
Routine screening methods
Providers were asked about methods used for routine screening of each condition. The following sections summarize responses; providers could choose more than one screening method, so results are reported here based on the most specific/advanced tests utilized.
Obesity
Age and gender-specific percentiles for BMI or weight-for-length were used by 83% of providers to screen for obesity. Only BMI or weight-for-length was used by 13%. Only 2 providers routinely measured waist circumference.
Hypertension
All providers routinely screened for hypertension. Only 13% utilized ambulatory blood pressure monitoring; 20% utilized repeated resting blood pressure or blood pressure percentile for age and gender, and 46% screened using a single blood pressure percentile for age and gender. The remaining 22% of providers reported using only blood pressure, without reference to percentile.
Chronic Kidney Disease
Seventeen percent of providers reported screening for chronic kidney disease with glomerular filtration rate (GFR), and 28% reported using GFR in combination with proteinuria, microalbuminuria and/or cystatin-C. Creatinine plus proteinuria, microalbuminuria and/or cystatin-C was utilized by 33% of providers, and creatinine alone by 20%.
Dyslipidemia
61% of providers routinely used fasting lipid panels to screen for dyslipidemia; 30% relied on random lipid levels, and 7% did not routinely screen.
Glucose Intolerance and/or Diabetes Mellitus
Nine percent reported using oral glucose tolerance testing to screen for glucose intolerance. Most commonly, providers screened using hemoglobin A1c (HbA1c) and fasting glucose (51%); 17% used HbA1c and a random glucose. 11% percent used fasting glucose alone, and 13% relied on a random glucose alone. Only 6% of providers never screened for glucose intolerance or diabetes.
Screening with fasting labs
Fasting labs were ordered for screening annually or at another regular interval by 61% of providers. Twenty percent ordered fasting labs only if the patient was overweight/obese or had another co-morbidity, and 20% never ordered fasting labs for pediatric liver transplant recipients. Of those who did report screening with fasting labs (n=28), 75% had no specific patient age at which they began this screening.
Protocols for evaluation and treatment of suspected disease:
At least 60% of providers reported that their center had no standardized protocol for workup or management of glucose intolerance/diabetes, dyslipidemia, or obesity. (FIGURE 2) Almost 40% had no standardized protocol for workup or management of hypertension or chronic kidney disease. Of those that did have standardized protocols, most were based on existing practice, provider consensus, or informal review of published evidence. There was no association between annual transplant volume and having an evaluation/treatment protocol for any of the conditions (p>0.30 for all 5).
Diagnostic evaluation and treatment prior to referral:
Providers were asked about what steps in diagnostic evaluation and initial treatment of suspected metabolic syndrome components they perform prior to referral to another sub-specialist. Providers both identified which steps they took and ranked the order in which they did those steps. Provider practices are summarized in TABLE 1 and FIGURE 3.
TABLE 1:
Diagnostic evaluation and initial management steps used by pediatric liver transplant providers in workup of children with suspected metabolic syndrome components post-transplant
| % of providers who ever perform* | Rank order in which providers perform this step: Median (IQR) | |
|---|---|---|
| Obesity (n=40†) | ||
| Diet/Exercise counseling | 98% | 1 (1–1) |
| Dietician for diet/exercise counseling | 98% | 2 (1–2) |
| Refer to pediatric weight management clinic | 80% | 3 (3–4) |
| Refer to primary care for evaluation/management | 55% | 3 (3–4) |
| Change immunosuppression | 48% | 4 (2–5) |
| Hypertension (n=39†) | ||
| Refer to nephrologist/hypertension specialist | 95% | 4 (2–6) |
| Add anti-hypertensive medication | 90% | 3 (2–4) |
| Repeat BP at next routine visit | 85% | 1 (1–2) |
| Schedule return visit to re-check BP | 80% | 1 (1–1) |
| Additional kidney function testing | 67% | 3 (2–5) |
| Prescribe ambulatory home BP monitoring | 59% | 2 (2–6) |
| Change immunosuppression | 49% | 5 (4–6) |
| Additional cardiovascular testing | 44% | 4 (3–5) |
| Prescribe low sodium diet | 31% | 3 (3–6) |
| Refer to primary care for evaluation /management | 29% | 5 (4–8) |
| Glucose Intolerance (n=37†) | ||
| Refer to endocrinologist | 95% | 3 (1–5) |
| Fasting glucose | 89% | 1 (1–1) |
| Urinalysis | 73% | 2 (1–3) |
| Other lab testing | 54% | 3 (2–4) |
| Change immunosuppression | 46% | 4 (4–5) |
| Fasting insulin | 43% | 3 (1–3) |
| Prescribe home blood glucose monitoring | 24% | 4 (3–5) |
| Refer to primary care for evaluation /management | 14% | 5 (5–7) |
| Add oral anti-hyperglycemic medication | 11% | 3 (1–8) |
| Start insulin | 11% | 4 (1–8) |
| Dyslipidemia (n=36†) | ||
| Diet/exercise counseling | 89% | 1 (1–2) |
| Repeat lipid panel next visit or blood draw | 81% | 1 (1–2) |
| Refer to lipid specialist | 69% | 3 (2–4) |
| Change immunosuppression | 61% | 3 (3–5) |
| Start an anti-hyperlipidemic medication | 36% | 4 (2–6) |
| Refer to primary care for evaluation /management | 28% | 4 (3–6) |
Percent of providers who perform this treatment options out of total number of providers who selected at least one treatment option for this condition.
n = number of providers who selected at least one treatment option for that condition.
FIGURE 3:
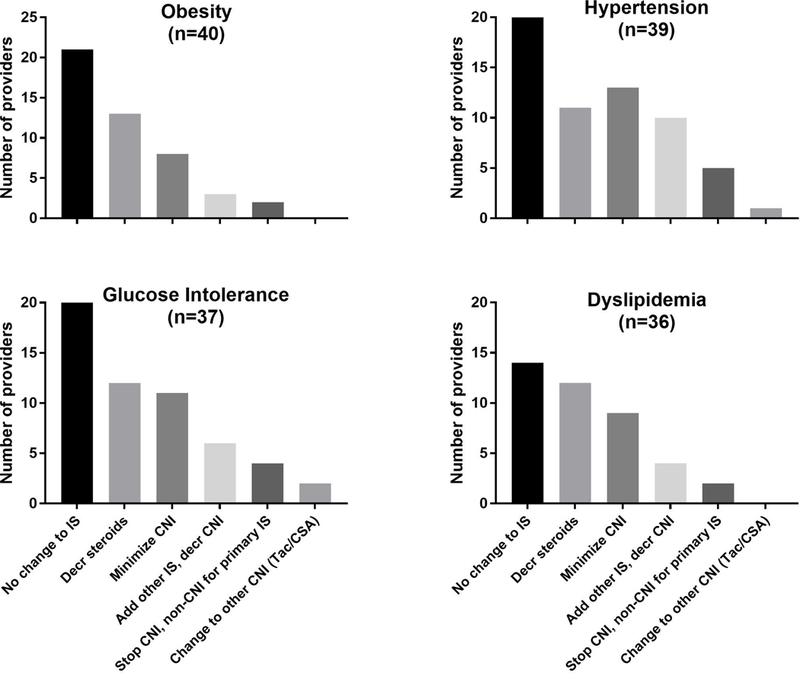
Immunosuppression changes to address components of the metabolic syndrome. Providers selected changes to immunosuppression by condition. Total n for each condition indicates number of providers who indicated at least one choice for workup of that condition.
Obesity
Nearly all providers gave diet and exercise counseling themselves or via a dietitian on the transplant team. Referral for treatment was most commonly to weight management clinics (80% of providers) or to the patient’s primary care provider (55%). Almost half reported that obesity could prompt changes in immunosuppression, most commonly by weaning or stopping corticosteroids. Several noted calcineurin inhibitor minimization in obese patients. (FIGURE 3)
Hypertension
In children with suspected hypertension, providers most commonly measured repeated blood pressures as an initial step (TABLE 1). 53% ordered additional kidney function testing; 89% of these providers did so prior to or in conjunction with nephrology/hypertension specialist referral. Ambulatory blood pressure monitoring was prescribed by 37% of providers; again 89% prior to or in conjunction with specialist referral. Addition of an anti-hypertensive medication before or with referral to a specialist was reported by 55%. Seven providers (14%) ordered echocardiograms for hypertensive children, most commonly after referral to another sub-specialist. Almost all providers referred to a nephrologist at some point (TABLE 1); only 2 providers reported referring to the primary care pediatrician for hypertension management or treatment.
Immunosuppression changes were utilized by 40% of providers for hypertensive children. (FIGURE 3) Two-thirds of providers reported adding an anti-hypertensive prior to changing immunosuppression. Conversely, 35% of providers report adding an anti-hypertensive but not changing immunosuppression for hypertension.
Glucose intolerance/diabetes mellitus
For children with suspected glucose intolerance or diabetes, providers reported checking a fasting glucose as their most common initial step, followed by urinalysis, HbA1c, and fasting insulin. (TABLE 1) Only one provider reported ordering oral glucose tolerance testing for workup of suspected diabetes. All providers answering this question reported referring to an endocrinologist, but the timing and trigger for the referral varied. Only 35% of providers reported changing immunosuppression in children with suspected glucose intolerance or diabetes; again most common were steroid changes and calcineurin inhibitor minimization. (FIGURE 3)
Dyslipidemia
Diet and exercise counseling was providers’ most common initial step, followed by repeating the lipid panel. Lipid specialist referrals were reported by 51% of all providers, and primary care referrals for lipid management by 20%. (TABLE 1) Among providers that reported changing immunosuppression for dyslipidemia, changes to corticosteroids and calcineurin inhibitor minimization were most commonly reported.
Of all changes to immunosuppression reported by providers for these four conditions, calcineurin inhibitor minimization or calcineurin inhibitor-sparing changes were reported by 41% of providers. No providers reported withdrawing all immunosuppression. Stopping calcineurin inhibitors was rarely reported—by 5 providers for hypertension and 4 for glucose intolerance/diabetes.
Thresholds for referral to other sub-specialties:
Thresholds for referral were described by 53–59% of all providers that answered the survey, as detailed below. Descriptions below represent a summary of free text comments, so not all percentages sum to 100%.
Obesity
Of 28 respondents who described specific thresholds for referral, 43% referred for lack of response to counseling or other attempted diet and lifestyle changes, and 29% for elevated BMI or BMI percentile (ranging from BMI 85th to 99th percentile). Only 11% never referred to a sub-specialist for obesity management, and the remainder referred for obesity plus co-morbidities.
Hypertension (n=27)
Elevated blood pressure prompted referral for 41%. Another 44% referred for persistent blood pressure elevation despite one anti-hypertensive medication. One provider reported referring when more than 2 anti-hypertensives had already been added. Two (7.5%) referred for hypertension with evidence of chronic kidney disease or renal insufficiency, and one has a nephrologist embedded in the liver transplant program.
Chronic kidney disease (n=28)
Providers most commonly referenced GFR (36%) or chronic kidney disease stage (as defined by GFR and proteinuria, 11%) thresholds for referral, with GFR threshold ranging from 50–90 mL/min/1.73m2. Twenty-one percent used creatinine elevation and/or proteinuria. Cystatin C was also used to prompt referral in 11%. The remaining providers used “abnormal” values but did not specify which test or quantitative thresholds.
Glucose intolerance/diabetes mellitus (n=29)
HbA1c alone was reported as a referral trigger for 24% of providers. An additional 24% relied on HbA1c in conjunction with glucose and/or insulin levels. Ten percent relied on fasting or random glucose levels alone. Fasting glucose levels that triggered referral ranged from 100–250 mg/dL. Referral for diabetes or pre-diabetes was noted by 17% of providers with lab thresholds specified. Other thresholds mentioned by single providers included: insulin requirement, steroid-induced diabetes, and lack of response to decrease in tacrolimus.
Dyslipidemia (n=26)
Lipid levels triggered referral for 58% of answering providers. Most described their threshold as “abnormal,” “elevated,” or “above upper limit of normal.” Fasting lipids were specified by 15%, and repeated evaluations prior to referral by 15%. Patients were referred for lack of treatment response by 19% of providers. Treatments noted included dietary counseling, fish oil, changing immunosuppression, and “medication” not otherwise specified. An additional 19% of answering providers never referred patients with dyslipidemia to another sub-specialty for treatment.
DISCUSSION:
This is the first systematic survey of how providers screen for, evaluate, and manage components of post-transplant metabolic syndrome in pediatric liver transplant recipients. All providers who answered this survey felt that these children should be screened for metabolic syndrome components. Most felt that the pediatric transplant team should provide this screening. However, more than 30% of providers reported that their center does not have standardized screening protocols for these conditions. An even higher percentage lack protocols for workup and management in children suspected to have these conditions. Of centers that do have protocols, most are based on existing local practice, provider consensus, or informal literature review.
We also asked providers about their actual practices for screening, evaluation, and treatment of these conditions. Although initial screening steps were relatively consistent across providers, we found significant variability in subsequent steps, treatments started in the liver transplant clinic, and thresholds for referral to other specialists. Some of this variability likely reflects variation in individual experience and resource/subspecialty availability at different centers.
But it also highlights opportunities for developing and testing guidelines for more standardized approaches. Current AASLD/AST recommendations8 for long-term management of pediatric liver transplant recipients mention “regularly” screening GFR. A significant minority of our providers reported using GFR, as well as protein/creatinine ratios and Cystatin C, to evaluate for chronic kidney disease and hypertension sequelae. But the majority did not. Updated prospective research on the predictive utility of these markers across centers, or extrapolation from populations with similar risk factors like renal transplant recipients or obese children, could help with development or testing of more evidence-driven guidelines.
Additional steps taken by some providers—but again not a majority—included ambulatory blood pressure monitoring and prescribing an initial anti-hypertensive. American Academy of Pediatrics guidelines on hypertension detection and treatment recommend considering ambulatory blood pressure monitoring for children after solid-organ transplant, given their risk factors and previously reported high prevalence of masked and nocturnal hypertension.10 Shared or multicenter consensus protocols, or additional provider education, might increase adoption of these practices. This could help accelerate the diagnosis and treatment of hypertension.
The AASLD/AST guidelines also recommend annual screening with fasting glucose in children 5 years and older4. Half of providers surveyed relied on fasting glucose and HbA1c to screen for pre-diabetes. One-quarter relied on HbA1c to trigger referral to endocrinology for additional workup. However, our previous research demonstrated that these tests are insufficiently sensitive in pediatric liver transplant recipients. In our cohort, of the 27% with glucose intolerance by oral glucose tolerance testing, only 2 had abnormal fasting glucose and all had normal hemoglobin A1c (<5.7%).7,11. Oral glucose tolerance testing may be worth exploring as a screening test for pre-diabetes in these children; it is widely available but not commonly used in current practice.
Another area of practice variation—and potential for innovation—is immunosuppression adjustment as a tool to prevent or treat PTMS components in children. Calcineurin-inhibitors and corticosteroids can both contribute to hypertension, chronic kidney disease and glucose intolerance, albeit by different mechanisms. Corticosteroids can also directly lead to central obesity and insulin resistance, with PTMS components developing as a downstream effect. Over the last several years, evidence is building about the feasibility of calcineurin inhibitor minimization,12 calcineurin inhibitor-sparing regimens,13 and even calcineurin inhibitor withdrawal14 in pediatric liver transplant recipients. Safe and effective use of these options requires additional research and careful prospective follow-up. This work would serve as a foundation for clinical guidelines optimizing immunosuppression—to protect both the liver and the long-term health of other organs.
Although we have summarized protocols and practices across a large number of pediatric transplant centers, this report is not intended as a recommendation about how these conditions should be handled. Developing such recommendations would require additional collaboration between centers, with work towards some consensus on what the goals of screening and management of these conditions should be and how to balance protection of the allograft with attention to the patient’s overall health. It will also require analysis of existing data, and acknowledgement of gaps in that evidence base, to establish what recommendations can be made which confidence and which cannot.
We relied on provider self-report of their center protocols and practices; we did not collect printed protocols or study ordering practices to confirm these data. We did have some centers with more than one answering provider, and this was not a comprehensive survey of all centers that perform pediatric liver transplantation in North America. The majority of answering providers were physicians, so we could not consider variation in practice by provider type. We asked only about screening and management protocols for PTMS components, so we do not know if pediatric liver transplant centers more generally have a low prevalence of protocol-driven long-term surveillance and management strategies, or if this represents a specific gap in PTMS. We have not reported on prevalence of these PTMS components by center, or changes in their prevalence with time from transplant, to evaluate whether currently used protocols and practices are differentially effective. In addition, we may not have fully captured other metabolic or nutritional markers that providers routinely send, for example uric acid, Vitamin D or measures of insulin resistance. These would all be helpful inquiries for future research.
As pediatric liver transplant recipients expand in number and age into adulthood, we expect that screening for and care of PTMS components will remain an important part of post-transplant care. This survey demonstrates transplant provider interest in screening for and initiating management of these conditions, but also significant variation in their approaches. It also highlights opportunities for multi-center collaboration on identifying and testing interventions to improve screening and management—with an ultimate goal of improving long-term outcomes for all of these children.
ACKNOWLEDGEMENTS:
Thank you to the Studies of Pediatric Liver Transplantation (SPLIT) Consortium members who completed the provider survey and to EMMES and Jeff Mitchell, who assisted with dissemination of the survey. This work is supported in part by NIH-NIDDK K23 DK0990253-A101 (ERP), the American Gastroenterological Association Emmet B. Keeffe Career Development Award in Liver Disease (ERP), UCSF Liver Center Pilot Funding (ERP, P30 DK026743), UCSF and by the NIH-National Center for Advancing Translational Sciences (UCSF-CTSI Grant UL1 TR000004). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the NIH, AGA, or other agencies.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- AST
American Society of Transplantation
- BMI
Body mass index
- GFR
Glomerular filtration rate (GFR)
- HbA1c
Hemoglobin A1c
- HDL
High-density lipoprotein
- PTMS
Post-transplant metabolic syndrome
- SPLIT
Studies of Pediatric Liver Transplantation
REFERENCES:
- 1.Thoefner LB, Rostved AA, Pommergaard H, Rasmussen A. Risk factors for metabolic syndrome after liver transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando) 2018;32(1):69–77. [DOI] [PubMed] [Google Scholar]
- 2.Prasad GVR, Huang M, Silver SA, et al. Metabolic syndrome definitions and components in predicting major adverse cardiovascular events after kidney transplantation. Transpl Int 2015;28(1):79–88. [DOI] [PubMed] [Google Scholar]
- 3.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl 2011;17(1):15–22. [DOI] [PubMed] [Google Scholar]
- 4.Laryea M, Watt KD, Molinari M, et al. Metabolic Syndrome in Liver Transplant Recipients: Prevalence and Association With Major Vascular Events. Liver Transpl 2007. [DOI] [PubMed]
- 5.Baskar S, George PL, Eghtesad B, et al. Cardiovascular risk factors and cardiac disorders in long-term survivors of pediatric liver transplantation. Pediatr Transplant 2015;19(1):48–55. [DOI] [PubMed] [Google Scholar]
- 6.Dagher M, Ng VL, Carpenter A, et al. Overweight, central obesity, and cardiometabolic risk factors in pediatric liver transplantation. Pediatr Transplant 2015;19(2):175–181. [DOI] [PubMed] [Google Scholar]
- 7.Perito ER, Lustig RH, Rosenthal P. Metabolic Syndrome Components After Pediatric Liver Transplantation: Prevalence and the Impact of Obesity and Immunosuppression. Am J Transplant 2016;16(6):1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly DA, Bucuvalas JC, Alonso EM, et al. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013;19(8):798–825. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JT, Kaelber DC, Baker-Smith CM et al. Subcommittee on screening and management of high blood pressure in children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017;140(6):e20173035. [DOI] [PubMed] [Google Scholar]
- 11.Perito ER, Lustig RH, Rosenthal P. Prediabetes in Pediatric Recipients of Liver Transplant: Mechanism and Risk Factors. J Pediatr 2017;182:223–231.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin HC, Melin-Aldana H, Mohammad S, Ekong UD, Alonso EM. Extended follow-up of pediatric liver transplantation patients receiving once daily calcineurin inhibitor. Pediatr Transplant 2015;19(7):709–715. [DOI] [PubMed] [Google Scholar]
- 13.Ganschow R, Ericzon B-G, Dhawan A, et al. Everolimus and reduced calcineurin inhibitor therapy in pediatric liver transplant recipients: Results from a multicenter, prospective study. Pediatr Transplant 2017;21(7):e13024. [DOI] [PubMed] [Google Scholar]
- 14.Feng S, Ekong UD, Lobritto SJ, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA 2012;307(3):283–293. [DOI] [PubMed] [Google Scholar]


