Summary
Neutrophils maintain immune homeostasis by engulfing apoptotic cells and debris. We describe the rapid activation of neutrophils after engulfing hemoglobin (Hb)‐activated platelets, which are abundant in the circulation of hemolytic patients. Neutrophils from healthy individuals after engulfing Hb‐activated platelets express elevated CD11b and secrete significant amounts of tumor necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6, myeloperoxidase (MPO) and elastase within 4‐h platelets, but not with free‐Hb only in vitro. These neutrophils exhibit early onset of apoptosis and cell death after engulfing Hb‐activated platelets, but not with free‐Hb only. Further, our data from mice with phenylhydrazine‐induced intravascular hemolysis display a gradual decrease in total neutrophil count, but the number of activated neutrophils and neutrophil–platelet aggregates increases, along with the rise of TNF‐α, IL‐1β, IL‐6 and MPO in circulation. Our data from paroxysmal nocturnal hemoglobinuria (PNH) patients confirmed the observation of decreased total neutrophil counts, but elevated numbers of activated neutrophils, including neutrophil–platelet aggregates, in parallel with elevated expression of TNFA, IL1B and IL6 genes in neutrophils, also increased levels of these cytokines along with MPO in circulation, and this correlated directly with elevated intravascular hemolysis (high free‐Hb in plasma). The patients’ neutrophils displayed significant localization of intracellular Hb and platelets, unlike the counterparts from healthy individuals. Together, therefore, our observations suggest that Hb‐activated platelets, which are abundant in the circulation of patients with hemolytic disorders, including PNH, promotes early onset of neutrophil activation and increases their proinflammatory response and leads to early apoptosis and cell death.
Keywords: hemoglobin, intravascular hemolysis and PNH, neutrophils, platelets
Introduction
Neutrophils constitute 50–70% of our leukocytes and are recruited first at the site of injury for the purpose of immune surveillance to combat infections and clearing apoptotic/dead cells and debris by phagocytosis. Earlier it was believed that neutrophils mediate only cellular immunity and inflammatory response, but recently it has become evident that neutrophils are capable of a vast array of specialized functions, including adaptive immune response 1, 2, 3, 4, 5, 6, 7. Neutrophils have a short circulatory life‐span of approximately 6 h, which demands their daily production in bone marrow. In order to maintain the normal count, our body produces a hundred thousand million neutrophils every day 6, 7, 8. During inflammation, various cytokines and growth factors mediate neutrophil activation and in turn regulate their complex activities, including immune surveillance responses 4, 6, 9, 10.
Neutrophils eliminate pathogens by phagocytosis and/or by forming neutrophil extracellular traps (NETs), which are composed of DNA, histones, enzymes such as myeloperoxidase (MPO) and elastase. Neutrophil migration to the site of injury/infection is the hallmark of inflammatory responses, which are further amplified by the neutrophils themselves through the production of cytokines such as interferon (IFN)‐γ, interleukin (IL)‐1, IL‐8, IL‐17 and tumor necrosis factor (TNF)‐α 4, 5, 11. Studies have shown that the binding of activated platelets to leukocytes, including monocytes and neutrophils, triggers the secretion of various inflammatory cytokines and proteases 2, 3, 6, 10, 12. It has been reported that platelet–neutrophil interactions are mediated by Toll‐like receptor (TLR)‐4 and P‐selectin on platelets and P‐selectin glycoprotein ligand 1 (PSGL‐1) on neutrophils. Activated platelets, which are abundant in the circulation of patients with hemolytic disorders, including paroxysmal nocturnal hemoglobinuria (PNH) and sickle cell disease (SCD), interact with the leukocytes to promote inflammation and vascular occlusion 12, 13, 14, 15. In our recent work, we have described that the binding of extracellular Hb to platelet surface glycoprotein (GP)‐1bα initiated platelet activation promoting prothrombotic complications in patients with PNH and SCD; an estimated 50% of total circulating platelets exist in the activation stages in these patients 16, 17. In another work, we have described that the Hb‐activated platelets are easily targeted by phagocytic immune cells such as monocytes. After engulfing Hb‐activated platelets, monocytes transform into highly proinflammatory subsets. An estimated 40–50% of total monocytes in PNH and 60–70% in SCD patients exist as highly proinflammatory subsets in peripheral circulation 18. Similarly, other studies have described the interaction of activated platelets with neutrophils and their association with the development of thrombotic as well as inflammatory complications in hemolytic patients, including PNH and SCD 15, 18, 19, 20, 21, 22. Although the above studies have described the phenomenon of neutrophil activation under hemolytic disease conditions, our present study clearly explains that the interaction with hyperreactive platelets, which are mainly activated via the binding interaction with free‐Hb (Hb‐activated platelets), closely regulates the function and fate of neutrophils by triggering their proinflammatory responses as well as inducing apoptosis under hemolytic conditions.
Material and methods
Reagents
Fluorescence labelled monoclonal anti‐human CD66b‐peridinin chlorophyll protein‐cyanin 5.5 (PerCp‐Cy5.5), CD16‐allophycocyanin (APC)‐Cy7, CD41‐fluorescein isothiocyanate (FITC), CD80‐phycoerythrin (PE), CD14‐FITC, CD11c‐V450, human leucocyte antigen D‐related (HLA‐DR)‐PE‐Cy7 and anti‐mouse Ly6C‐V450, Ly6G(GR‐1)‐FITC, CD11b‐PerCp‐Cy5.5, CD45.2‐APC‐Cy7 and CD41‐PE were purchased from BD Biosciences (San Jose, CA, USA), and anti‐human CD11b‐PE from Dako (Agilent Technologies, Glostrup, Denmark). Polyclonal antibody for human neutrophil elastase was purchased from Novous Biologicas LLC (Centennial, CO, USA) and anti‐rabbit Alexa Fluor 594 immunoglobulin (Ig)G and anti‐mouse Alexa Fluor 488 IgG from Life Technologies (Carlsbad, CA, USA). Anti‐human and anti‐mouse cytometric bead array (CBA) kits were bought from BD Biosciences, and anti‐human and anti‐mouse MPO ELISA kits from Biolegend (San Diego, CA, USA) and Thermo Scientific (Waltham, MA, USA), respectively. The adult human erythrocyte purified Hb (HbA) was purchased from Sigma Aldrich (St Louis, MO, USA) with a purity of 98·5%, isolated through Sephadex G‐25 column gel filtration chromatography and CM‐sepharose fast flow column chromatography. Other laboratory reagents were purchased from Sigma Aldrich (St Louis, MO, USA).
Human subjects
Ethics approval was obtained from the Institute Human Ethics Committee of All India Institute of Medical Science, New Delhi and Regional Centre for Biotechnology (RCB), Faridabad for the collection of peripheral blood from PNH patients (approval number RCB/IEC/2017/015). Informed consent was provided according to the recommendations of the declaration of Helsinki. An estimated 5–7 ml of peripheral blood from patients (PNH, n = 15) and healthy control subjects (n = 10) was collected in vacutainers containing anti‐coagulant acid citrate–dextrose (ACD).
Mice with induced hemolysis
Ethics approval was obtained from the Institutional Animal Ethics Committee (IAEC) of RCB (approval number RCB/IAEC/2017/017) and mice experiments were conducted within the guidelines of IAEC. BALB/c mice aged 6–7 weeks of either sex were used for induction of hemolysis. Intravascular hemolysis was induced by intraperitoneal injection with phenylhydrazine (PHZ from Sigma Aldrich) of 1 mg/20 g of body weight concentration, as mentioned 17, 22. The control mice were administered with vehicle.
Experiment 1
After PHZ injection 100 µl blood was collected through retro‐orbital bleed at 24 and 48 h. The counts and activation of neutrophils along with neutrophil–platelet aggregates were measured using flow cytometry.
Experiment 2
Mice were injected with lipopolysaccharide (LPS) (80 µg/20 g body weight) at the peritoneal region of both control and hemolytic mice. Upon LPS injection, inflammation and neutrophil infiltration were assessed using bioluminescence imaging of MPO by injecting luminol (i.p. 20 mg/100 g body weight) (Sigma Aldrich) 30 min prior to imaging using an in‐vivo imaging system (IVIS; Perkin Elmer, Waltham, MA, USA) 4. The neutrophil profiles were measured from the peritoneal lavage of the mice after the treatment, as described.
Neutrophil isolation and culture
Whole blood of healthy donors was processed for Ficoll‐Hypaque (GE Healthcare, Freiburg, Germany) density gradient centrifugation. The lower fraction containing red blood cells (RBCs) and granulocytes was processed further for dextran sedimentation to remove the RBCs. The remaining RBCs were removed using RBC lysis buffer (Sigma Aldrich). The neutrophils were isolated and purity was confirmed by flow cytometry using CD66b and CD16 markers. Neutrophils were cultured under various experimental conditions with free‐Hb, untreated platelets, Hb‐activated platelets or thrombin‐activated platelets for 0, 2, 3, 4 and 6 hr in RPMI‐1640 (Sigma Aldrich) with 10% fetal bovine serum (FBS) and 100 IU/ml penicillin–streptomycin (both from gibco Invitrogen, San Diego, CA, USA) in a cell culture incubator. The unbound platelets were removed after 30 min and the experimental conditions were maintained until the end of the above time‐periods. The neutrophils and culture supernatant were used for flow cytometry or CBA assay. To activate platelets, washed platelets were incubated with 2·5 μM HbA (this corresponds to an in‐vivo concentration in PNH patients with moderate hemolysis 16) for 30 min at 37°C before incubating with neutrophils.
Flow cytometry
Neutrophil and neutrophil–platelet aggregate counts were performed using flow cytometry. The cells were incubated with related antibodies in staining buffer [×1 PBS with 1% bovine serum albumin (BSA) and 0·1% sodium azide] for 30 min. Cells were washed twice with ×1 PBS before flow cytometric analysis. Data were analysed using FlowJo (TreeStar, Ashland, OR, USA).
Polymerase chain reaction (PCR) quantification of cytokines
RNA was isolated from neutrophils from the same pool of patients (n = 8) and healthy individuals (n = 3) using Trizol (Invitrogen, Carlsbad, CA, USA) extraction. cDNA was prepared and the expression of IL1B, TNFA, IL6 and IL10 in neutrophils was analysed using real‐time quantitative PCR (qPCR) using SYBR green (Bio‐Rad, Hercules, CA, USA). Ct value for the expression of these cytokines was normalized with ACTB and relative fold change was calculated with respect to the mean of the healthy controls. The primer sequence for the above‐mentioned cytokines was taken from the OriGene website (Rockville, MD, USA).
CBA for quantifying cytokines
Cytokines such as TNF‐α, IL‐1β, IL‐6, IL‐4 and IL‐10 were measured from culture supernatant of neutrophils from different treatments as well as from plasma of patients and mice using flow cytometry‐based CBA and analyzed by FCAP array software (BD Biosciences, San Jose, CA, USA).
Confocal microscopy
NET formation
Isolated neutrophils from healthy individuals were incubated with various treatments, as mentioned above. The cells were stained for DNA using 5 nM of SYTOX® green (Thermo Fisher Scientific) as well as for elastase using anti‐elastase primary antibody (Enzo Life Sciences, Farmingdale, NY, USA) and Alexa 594‐conjugated anti‐rabbit IgG antibody (Invitrogen, San Diego, CA, USA). Live cell imaging was performed to capture real‐time events during the incubation using a confocal microscope (TCS‐SP5; Leica Microsystems, Wetzlar, Germany).
Intracellular localization of Hb and platelets in neutrophils
To measure intracellular localization of Hb and platelets, the neutrophils (1) treated with Hb‐activated platelets or (2) isolated from PNH patients and healthy subjects were fixed with 4% paraformaldehyde for 20 min at RT and incubated with 0·1% saponin in ×1 PBS with 5% goat serum for 1 h at room temperature (RT) followed by overnight incubation at 4oC with 1 : 1000 dilution of anti‐Hb (Sigma Aldrich) or 1 : 100 CD42b (Abcam, Cambridge, MA, USA) polyclonal antibodies. Cells were washed three times with ×1 PBS and labeled with 10 μg/ml Alexa Fluor 488/594‐conjugated goat anti‐rabbit or anti‐mouse antibody (Invitrogen) for 2 h and washed with ×1 PBS thrice. After nuclear staining by 2 μg/ml 4′,6‐diamidino‐2‐phenylindole (DAPI) (Sigma Aldrich), cells were mounted with anti‐fade ProLong gold mounting reagent (Invitrogen). Imaging was performed using a confocal microscope at ×63.
ELISA for MPO
The MPO was measured from plasma of patients and mice, and also from secretome of treated‐neutrophils using the ELISA kit and analysed according to the manufacturer’s protocol (Biolegend, San Diego, CA, USA).
ELISA for free‐Hb
Sandwich ELISA was used for measuring free‐Hb from plasma of PNH patients and healthy individuals, as mentioned in our previous study 17. Drabkin’s assay was used to measure free‐Hb from plasma of hemolytic as well as healthy mice against mouse Hb standard.
Statistical analysis
Data from at least three experiments are presented as mean ± s.e.m. Statistical analysis was performed using the Mann–Whitney U‐test for patient or mice studies and the paired t‐test for ex‐vivo experiments. Graph Pad Prism version 7.0 software was used for data analysis and P‐values < 0·05 were considered statistically significant.
Results
Rapid activation and elevation of proinflammatory response in neutrophils after engulfing Hb‐activated platelets in vitro
In order to investigate the mechanism of neutrophil activation under a hemolytic environment, freshly isolated neutrophils from peripheral blood of healthy individuals were treated with either free‐Hb, resting platelets or Hb‐activated platelets in vitro. Our data describe the rapid activation as well as the elevated inflammatory response of neutrophils that engulfed Hb‐activated platelets, but not free‐Hb alone. After engulfing the Hb‐activated platelets, neutrophils exhibited maximum CD11b expression between 4 and 6 h (Fig. 1a). These neutrophils displayed rapid elevation in phosphatidylserine (PS) expression and propidium iodide (PI) staining (Fig. 1b), suggesting their early onset of apoptosis and cell death. Our data also showed the rapid activation of neutrophils upon interaction with thrombin‐activated platelets, suggesting clearly the role of activated platelets on neutrophil function independent of any platelet activation stimulus (Fig. 1a–b). In a recent study, we have described that the maximum activation of platelets occurs due to the direct binding interaction of platelet surface GP1bα with extracellular Hb in vitro. An estimated 40–50% of the total circulating platelets existed as Hb‐bound hyperactive states in patients with either PNH or SCD, but not in healthy individuals 16. Further, our microscopy data revealed that the Hb‐activated platelets bound to the neutrophil surface within 15 min of incubation (Fig. 1c) and both Hb and platelets were internalized completely inside neutrophils within 3–4 h (Fig. 1d). After engulfing Hb‐activated platelets, neutrophils displayed elevated secretion of proinflammatory cytokines including IL‐1β, TNF‐α and IL‐6 within 3–4 h compared to the counterparts treated with either free‐Hb only or resting platelets (Fig. 1g). The resting or inactivated platelets did not interact with neutrophils significantly. After engulfing Hb‐activated platelets, neutrophils also showed an elevated secretion of elastase and DNA content (Fig. 1e–f) and MPO (Fig. 1 h) within 3–4 h of incubation compared to the counterparts treated with free‐Hb only.
Figure 1.
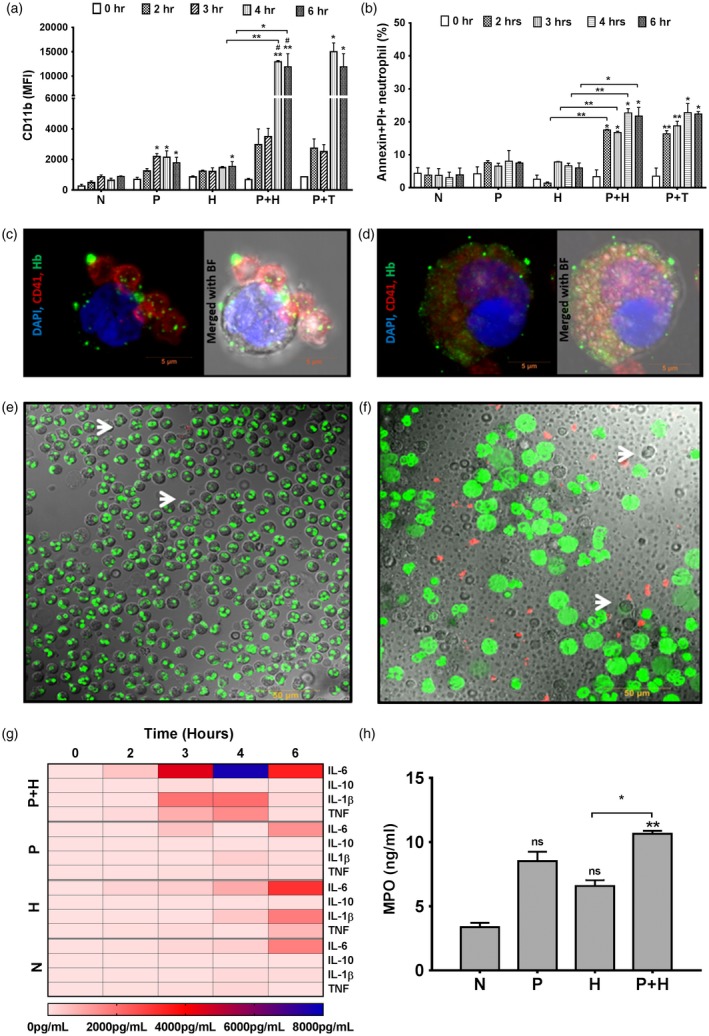
Activation and apoptosis of neutrophils after engulfing hemoglobin (Hb)‐activated platelets in vitro. Freshly isolated neutrophils from whole blood of healthy individuals were cultured with free‐Hb (H), untreated or resting platelets (P), Hb‐activated platelets (P + H), thrombin‐activated platelets (P + T) or without treatment (N) until 6 h. (a) Neutrophil activation (mean fluorescence intensity of CD11b) and (b) apoptosis [% annexin V and propidium iodide (PI)‐positive cells] were analyzed using flow cytometry. Data are the mean ± standard error of the mean (s.e.m.) from three different experiments and P‐values are calculated by paired t‐test, *P < 0·05, **P < 0·01 and non‐significant (n.s.) (without bar) comparision between N versus P/H/P + H/P + T at a particular time‐point; and P‐value with bars represents comparision between groups as indicated. Microscopic image represents (c) binding of Hb‐activated platelet on the neutrophil surface at 15 min and (d) complete internalization of platelets or CD42b (red) and Hb (green) inside neutrophils at 4 h at ×63, image bar ~5 μm. Release of elastase (red, elastase) and DNA content (sytox green) by neutrophils after 3 h of treatment with (e) H or (f) P + H at ×63; arrows indicate the neutrophils; image bar ~50 μm. (g) Secretion of proinflammatory cytokines by neutrophils after encountering Hb‐activated platelets. Secretome was collected from neutrophils from various treatments at 4 h (a) was used for cytokine measurement using the cytometric bead array (CBA) assay kit. The heat‐map shows the higher levels of interleukin (IL)‐6, IL‐1β and tumor necrosis factor (TNF)‐α but not IL‐10 or IL‐4 from P + H treatment. (h) Neutrophils from P + H treatment secreted more myeloperoxidase (MPO) [quantified using enzyme‐linked immunosorbent assay (ELISA)] than the H treatment. Data were calculated and presented in a similar way as mentioned in (a), *P < 0·05, ** P < 0·01 and n.s.
Elevated neutrophil–platelet aggregates and MPO in circulation as well as locally at the site of LPS injection in the peritoneum of mice with intravascular hemolysis
To investigate the in‐vivo phenotype and function of neutrophils in the hemolytic environment, we used the PHZ‐induced hemolytic mouse model (Fig. 2a). The injection of PHZ (1 mg/20 g body weight) induced moderate intravascular hemolysis (an estimated 3 μM free‐Hb in plasma; Supporting information, Fig. S1a) along with thrombocytopenia (Supporting information, Fig. S1b) and neutropenia (Fig. 2b) without affecting the normal activity of these mice. An elevated count of activated neutrophils (CD11b+, Fig. 2d), including neutrophil–platelet aggregates (Fig. 2c), existed in the peripheral blood of hemolytic mice, greater at 48 than 24 h after hemolysis. The hemolytic mice showed a gradual increase in proinflammatory cytokines such as TNF‐α, IL‐1β and IL‐6 (Fig. 2e) and MPO (Fig. 2f) in plasma during 48 rather than 24 h. Further, to investigate the neutrophil migration to the site of LPS injection, the mice were injected with mild LPS at peritoneum. Our data showed the significant accumulation of activated neutrophils (Fig. 2i–j), including neutrophil–platelet aggregates (Fig. 2k), at peritoneum within 3 h of LPS treatment. Neutrophils released elevated levels of MPO at the LPS injection site measured by in‐vivo imaging (Fig. 2g–h). Therefore, the above data together suggest a direct correlation between neutrophil–platelet interactions and elevated accumulation of proinflammatory cytokines/enzymes in circulation, as well as at the local LPS injection sites in mice with intravascular hemolysis compared to counterparts without hemolysis.
Figure 2.
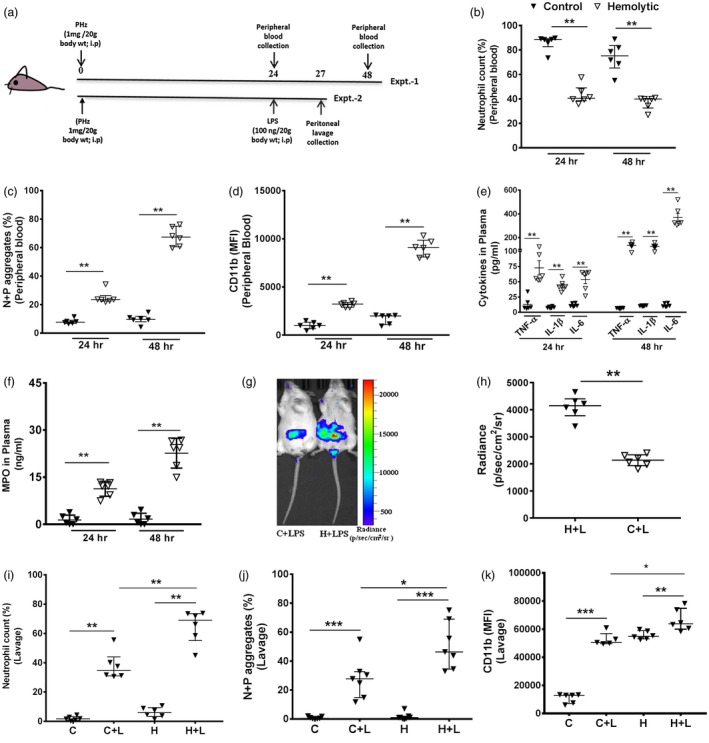
Neutrophil phenotype and function from mice with induced hemolysis. (a) Schematic representation of experiments 1 and 2; 6–7‐week‐old BALB/c mice were injected with phenylhydrazine (PHZ) to induce intravascular hemolysis. Experiment 1: blood collected at 24 and 48 h after PHZ treatment. (b) Neutrophil count, (c) neutrophil‐platelet aggregates (N + P, CD41+Ly6G+) and (d) neutrophil activation (CD11b mean fluorescence intensity) were analyzed using flow cytometry. The detail gating strategy is mentioned in Supporting information, Fig. S2a–d. (e) Cytokines such as tumor necrosis factor (TNF)‐α, interleukin (IL)‐1β and IL‐6 and (f) myeloperoxidase (MPO) were quantified from mice plasma from experiment 1. Data are the median with interquartile range (IQR) from six mice in each group from two different experiments and P‐values are calculated by Mann–Whitney U‐test; * P < 0·05, ** P < 0·01. (b–f) Black triangles represent control and open triangles represent hemolytic mice. Experiment 2: after 24 h of PHZ treatment, mice were further injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) locally at peritoneal cavity. At 3 h after LPS treatment (g) neutrophil activation was measured by assessing luminescence intensity from reaction of MPO with luminol using in‐vivo imaging. The inflamed area was quantified as (h) mean radiance, C + L~control mice with LPS, H + L~hemolytic mice with LPS. (i) The neutrophil count (j) neutrophil–platelet aggregates (N + P, CD41+Ly6G+) and (k) neutrophil activation (CD11b) were analyzed from peritoneal lavage using flow cytometry. Data are the median with IQR from six mice in each group from to different experiments and P‐value are calculated by Mann–Whitney U‐test; *P < 0·05, **P < 0·01, ***P < 0·001.
Elevated activation and expression of proinflammatory cytokines in neutrophils correlated directly with intracellular staining of both platelets and Hb in these cells and also with intravascular hemolysis in patients
We measured the elevated percentage of activated neutrophils, including neutrophil–platelet aggregates, but a reduction in the total number of both cell types (Fig. 3a–c) from the peripheral blood of PNH patients with high intravascular hemolysis (represented by elevated plasma free‐Hb, Fig. 3d). Our data also showed intracellular localization of both platelets and Hb in patients’ neutrophils, but not in healthy individuals (Fig. 3e). The patients’ neutrophils exhibited elevated gene expression of proinflammatory cytokines such as TNFA, IL1B and IL6 (Fig. 3f) in parallel with increased levels of these cytokines in plasma (Fig. 3g). An elevated level of plasma MPO correlated directly with neutrophil activation in these patients (Fig. 3h). Also, patients’ neutrophils displayed significant expression of apoptotic markers such as phosphatidylserine and increased uptake of PI, indicating premature cell death (Fig. 3i). Although we could not directly measure the platelet activation in these patients, our recent study described the presence of activated platelets (activation percentage ranging between 40 and 50%) in PNH patients 17. Taken together, therefore, our data from PNH patients suggest direct correlations among (1) intravascular hemolysis, (2) Hb‐mediated platelet activation, (3) platelet‐mediated neutrophil stimulation, (4) proinflammatory response by neutrophils and (5) premature neutrophil death or neutropenia.
Figure 3.
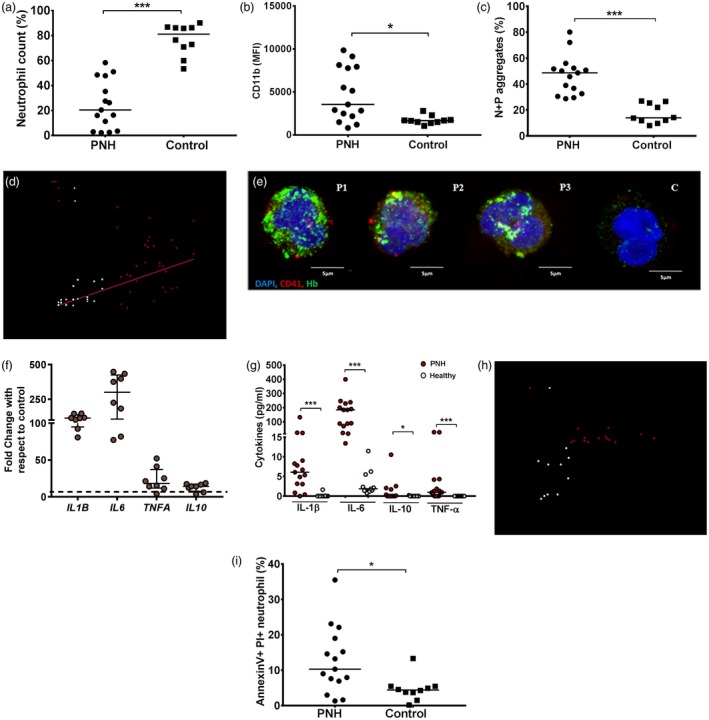
Neutrophil phenotype and function analysis in paroxysmal nocturnal hemoglobinuria (PNH) patients. Neutrophils were isolated from whole blood of PNH patients (n = 15) or healthy individuals (n = 10) and analyzed for (a) neutrophil count, (b) neutrophil activation (CD11b) and (c) neutrophil–platelet (N + P) aggregates (CD41+CD11b+) using flow cytometry. The detail gating strategy is mentioned in Supporting information, Fig. S3a–b. (d) Correlation plots show direct association between activated–neutrophil count (Fig. 3b)/N + P aggregates (c) and plasma free‐Hb concentration, r~ correlation coefficient. (e) The intracellular localization of platelets (red, CD42b), Hb (green, Hb) and nucleus [blue, 4′,6‐diamidino‐2‐phenylindole (DAPI)] was analyzed in neutrophils collected from peripheral blood of three PNH patients (P1–P3) and one healthy individual (c), at ×63, image bar ~5 μm. (f) TNFA, IL1B, IL6 and IL10 genes were quantified in neutrophil using quantitative polymerase chain reaction (qPCR). Data represent the fold‐change compared to mean of respective parameters from control individuals (n = 3). (g) Cytokines in plasma were quantified using the cytometric bead array (CBA) assay. (h) Correlation plots show direct association between N + P aggregates (Fig. 3c) and plasma MPO levels in peripheral blood of PNH patients and healthy individuals. (i) Neutrophil apoptosis was measured using annexin V and propidium iodide (PI) bindings using flow cytometry. Each dot represents each individual value. Data are the median with IQR; and P‐values are calculated by Mann–Whitney U‐test; *P < 0·05, ***P < 0·001.
Discussion
Our study provides new insight into the phenomenon of neutrophil activation in the hemolytic environment. The data show significant elevation in the proinflammatory response of neutrophils after engulfment of Hb‐activated platelets, but not free‐Hb alone. In a recent work, we have described that the high level of extracellular Hb (2–7 μM) co‐exists with an increased percentage of activated platelets (an estimated 40–50% of total platelets and are mainly Hb‐bound) in peripheral blood of PNH patients 17. We have previously described that the binding interaction of extracellular Hb with platelet surface GP1bα is the major adopter for platelet activation in the hemolytic environment 12. As a result, the Hb‐activated platelets are easily targeted and encountered by the phagocytic immune cells, including monocytes and neutrophils. Our in‐vitro data show that freshly isolated neutrophils from healthy individuals expressing elevated CD11b secrete significant amounts of proinflammatory cytokines such as IL‐1β, TNF‐α and IL‐6 and enzymes such as elastase and MPO within 3 h after engulfing Hb‐activated platelets in comparison to their counterparts treated with free‐Hb only. Several other studies have also described the phenomenon of neutrophil activation upon interaction with platelets under hemolytic diseases, including PNH 12, 13. However, our study indicates a clear mechanism for the rapid activation of neutrophils, which is mediated mainly by the Hb‐activated platelets but not free‐Hb only under the hemolytic environment. Our in‐vitro data also demonstrate the phenomenon of early‐onset apoptosis and cell death in neutrophils after engulfing Hb‐activated platelets versus their counterparts treated with free‐Hb only.
Further, our data from the mice experiments support our above observations describing the gradual elevation in the count of activated neutrophils including neutrophil–platelet aggregates in the peripheral blood after 48 h compared to 24 h after the induction of intravascular hemolysis. The MPO, which is secreted abundantly from activated neutrophils, shows gradual elevation in the circulation after the induction of intravascular hemolysis. Furthermore, our data describe that upon interaction with platelets, neutrophils (as platelet–neutrophil aggregates) displayed significant transmigration to the site of LPS injection in the peritoneum of hemolytic mice compared to untreated counterparts. The accumulated neutrophils secreted a significant amount of MPO in the peritoneum of hemolytic mice compared to the similar event in normal counterparts without hemolysis. The total neutrophil count decreased significantly, further indicating the phenomenon of neutropenia in these mice with intravascular hemolysis. Furthermore, our data from PNH patients confirm the above observations showing a direct correlation between elevated intravascular hemolysis with increased counts of activated neutrophils along with neutrophil–platelet aggregates in the peripheral blood. The patients’ neutrophils display significant intracellular staining for both platelet and Hb, highlighting once again the role of the Hb–platelet axis in neutrophil activation. These neutrophils exhibit an elevated expression of TNFA, IL1B and IL6 genes in parallel with the increase in levels of these proinflammatory cytokines in patients’ plasma. The elevated MPO in plasma correlated directly with neutrophil activation in these patients, therefore suggesting a unique in‐vivo correlation between platelet–neutrophil interactions and activation as well as a proinflammatory response of neutrophils in hemolytic environments in PNH. The patients’ neutrophils display significant apoptosis and cell death, further suggesting the possible cause of neutropenia, a clinical condition commonly observed in hemolytic disorders including PNH.
Taken together, the above observations suggest that the Hb‐activated platelets, which are abundant in the circulation of hemolytic patients, closely regulate the function and fate of neutrophils by triggering their activation as well as proinflammatory functions in correlation with the severity of intravascular hemolysis. These phagocytic cells also lose survivability upon frequent encounter with Hb‐activated platelets in the hemolytic environment, predisposing towards clinical conditions such as neutropenia in patients with hemolytic disorders, including PNH.
Author contributions
A. B., G. K. A. and P. G. designed the experiments. A. B. performed the flow cytometry assay. T. S. recruited patients, and collected and analyzed the clinical data. S. S. processed the clinical samples and performed assays. N. S. and G. K. A. performed microscopy. A. B., G. K. A., N. S. and S. G. performed mice experiments and analyzed the data. A. B., G. K. A. and P. G. analyzed all data and prepared the manuscript. P. G. was responsible for oversight of the project and preparation of the final manuscript.
Disclosures
The authors declare no commercial or financial conflicts of interest.
Supporting information
Fig. S1 . Assessment of cell‐free Hb and platelet counts in peripheral blood of control and hemolytic mice.(a) Plasma Hb level was measured from plasma of hemolytic (h) and control (c) mice using drabkin's assay against mouse Hb standard. (b) Platelet count in peripheral blood was measured using flow cytometry. Data are the mean ± SEM and p value is calculated by un‐paired t‐test. ***P < 0·001.
Fig. S2 . Gating strategy to analyze neutrophil and neutrophil‐platelet (N+P) aggregates in peripheral blood and peritoneal lavage of control and hemolytic mice using flow cytometry.
Fig. S3 . Gating strategy to analyse neutrophil and neutrophil‐platelet (N+P) aggregates in PNH patients (a) and healthy individuals (b) using flow cytometry.
Acknowledgements
This study was supported financially by grants BT/PR8591 and BT/PR22985 from the Department of Biotechnology (DBT), Government of India (GoI) and from the Regional Centre for Biotechnology grants‐in‐aid from the DBT, GoI to P. G.
References
- 1. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 2. Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med 2011; 17:1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 2017; 17:248–61. [DOI] [PubMed] [Google Scholar]
- 4. Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol 2015; 98:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol 2011; 32:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol 2010; 31:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol 2014; 9:181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newburger PE. Disorders of neutrophil number and function. Hematology Am Soc Hematol Educ Program 2006; 104–10. [DOI] [PubMed] [Google Scholar]
- 9. Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010; 33:657–70. [DOI] [PubMed] [Google Scholar]
- 10. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012; 30:459–89. [DOI] [PubMed] [Google Scholar]
- 11. Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 2013; 210:1283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost 2014; 12:1764–75. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Kim K, Barazia A, Tseng A, Cho J. Platelet–neutrophil interactions under thromboinflammatory conditions. Cell Mol Life Sci 2015; 72:2627–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol 2012; 590:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polanowska‐Grabowska R, Wallace K, Field JJ et al P‐selectin‐mediated platelet–neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol 2010; 30:2392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Annarapu GK, Singhal R, Gupta A et al HbS binding to GP1balpha activates platelets in sickle cell disease. PLOS ONE 2016; 11:e0167899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singhal R, Annarapu GK, Pandey A et al Hemoglobin interaction with GP1balpha induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis. Haematologica 2015; 100:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lum AF, Wun T, Staunton D, Simon SI. Inflammatory potential of neutrophils detected in sickle cell disease. Am J Hematol 2004; 76:126–33. [DOI] [PubMed] [Google Scholar]
- 19. Manwani D, Frenette PS. Vaso‐occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 2013; 122:3892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood 2016; 127:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jimenez MA, Kato GJ, Neutrophil‐Platelet SP. Aggregation enables vaso‐occlusion in sickle cell disease. Blood 2016; 128:1295. [Google Scholar]
- 22. Bennewitz MF, Jimenez MA, Vats R, et al Lung vaso‐occlusion in sickle cell disease mediated by arteriolar neutrophil‐platelet microemboli. JCI Insight 2017; 2:e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 . Assessment of cell‐free Hb and platelet counts in peripheral blood of control and hemolytic mice.(a) Plasma Hb level was measured from plasma of hemolytic (h) and control (c) mice using drabkin's assay against mouse Hb standard. (b) Platelet count in peripheral blood was measured using flow cytometry. Data are the mean ± SEM and p value is calculated by un‐paired t‐test. ***P < 0·001.
Fig. S2 . Gating strategy to analyze neutrophil and neutrophil‐platelet (N+P) aggregates in peripheral blood and peritoneal lavage of control and hemolytic mice using flow cytometry.
Fig. S3 . Gating strategy to analyse neutrophil and neutrophil‐platelet (N+P) aggregates in PNH patients (a) and healthy individuals (b) using flow cytometry.


