Abstract
Background
This pilot study evaluated whether use of evidence-based implementation strategies to integrate care for cannabis and other drug use into primary care (PC) as part of Behavioral Health Integration (BHI) increased diagnosis and treatment of substance use disorders (SUDs).
Methods
Patients who visited the three pilot PC sites were eligible. Implementation strategies included practice coaching, electronic health record decision support, and performance feedback (3/2015–4/2016). BHI introduced annual screening for past-year cannabis and other drug use, a Symptom Checklist for DSM-5 SUDs, and shared decision-making about treatment options. Main analyses tested whether the proportions of PC patients diagnosed with, and treated for, new cannabis or other drug use disorders (CUDs and DUDs, respectively), differed significantly pre- and post-implementation.
Results
Of 39,599 eligible patients, 57% and 59% were screened for cannabis and other drug use, respectively. Among PC patients reporting daily cannabis use (2%) or any drug use (1%), 51% and 37%, respectively, completed an SUD Symptom Checklist. The proportion of PC patients with newly diagnosed CUD increased significantly post-implementation (5 v 17 per 10,000 patients, p<0.0001), but not other DUDs (10 vs 13 per 10,000, p=0.24). The proportion treated for newly diagnosed CUDs did not increase post-implementation (1 vs 1 per 10,000, p=0.80), but did for those treated for newly diagnosed other DUDs (1 vs 3 per 10,000, p=0.038).
Conclusions
A pilot implementation of BHI to increase routine screening and assessment for SUDs was associated with increased new CUD diagnoses and a small increase in treatment of new other DUDs.
Keywords: Primary Care, Screening, Cannabis, Street Drugs, Drug Use Disorders, Quality Improvement
1. Introduction
Cannabis and other drug use is common in the U.S. About 1 in 10 Americans over the age of 12 reports use of cannabis, another drug, or a prescription medication for nonmedical use in the past 30 days, with rates as high as 1 in 4 among young adults ages 18–25 (Ahrnsbrak et al., 2017). Legalization of medical and nonmedical cannabis use may be resulting in increased use (Hasin et al., 2017). Approximately 10–30% of people who use cannabis daily develop cannabis use disorders (CUD) as defined by Diagnostic and Statistical Manual (DSM) (Compton et al., 2009), and cannabis use increases the risk of adverse medical and social outcomes (National Academies of Sciences and Medicine, 2017; Volkow et al., 2014). Other drug use, including misuse of prescription medications (e.g. opioids, benzodiazepines) is less common than cannabis use, but risks of adverse consequences are high (Compton et al., 2007; Goode, 2012; Han et al., 2017; Maynard et al., 2016).
Cannabis and other drug use disorders (CUDs and DUDs, respectively, hereafter), are often not identified by medical providers, and few individuals receive care for these problems (Ahrnsbrak et al., 2017; Becker et al., 2008; Kerridge et al., 2017). Drug use is often hidden due to fear of stigma (Hunt and Derricott, 2001; Room, 2005) and most people who could benefit from care for a DUD do not perceive the need for treatment (Lipari et al., 2016). One approach to increasing diagnosis and access to care for drug use is to integrate screening for and management of DUDs into primary care (PC) settings (Barry et al., 2016; Kerridge et al., 2017).
Experts support the integration of population-based screening for cannabis and other drug use as part of high-quality care in PC settings (Crowley et al., 2015; National Council for Behavioral Health, 2018), because screening identifies drug use and encourages patient/provider dialogue about related health effects. When patients with high-risk cannabis and other drug use are assessed for symptoms of substance use disorders (SUDs), this may enable providers to make CUD and DUD diagnoses and engage patients in discussion about their motivation to change cannabis or other drug use, as well as treatment options, like pharmacotherapy, counseling and specialty addiction treatment (National Council for Behavioral Health, 2018). However, no studies to our knowledge have yet evaluated whether population-based screening and assessment for symptoms of CUD and other DUDs increases rates of diagnosis and treatment.
In 2015, Kaiser Permanente Washington (KPWA), a regional healthcare system, undertook a program called Behavioral Health Integration (BHI). The BHI program was designed by a research/operations partnership and supported partially by a pragmatic trial designed to evaluate the integration of alcohol-related care into PC (Bobb et al., 2017; Glass et al., 2018). KPWA’s BHI program sought to improve clinical care related to depression, alcohol, and drug use, beginning with annual screening of adult patients seen in PC. PC providers requested that screening for cannabis be separated from screening for other drug use given recent legalization of non-medical cannabis use in Washington State (Lapham et al., 2017). Therefore, the BHI program included screening for cannabis and other drug use (including prescription drug misuse) using separate questions, followed by assessment of symptoms of DSM SUDs to support PC providers in diagnosing new CUDs and other DUDs, followed by shared decision-making with their patients about treatment options.
This evaluation compared proportions of PC patients with newly diagnosed CUDs and other DUDs and the proportion treated following a new diagnosis, before and after BHI implementation introduced routine screening and assessment procedures in PC. Further, so that other systems integrating drug screening may benefit from lessons learned during the pilot, we described barriers and facilitators identified in formative evaluation (Stetler et al., 2006) and adaptations made to the implementation strategies. Finally, we described sustainment of cannabis and other drug screening and assessment one year after the pilot ended.
2. Methods
2.1. Setting and BHI Structure
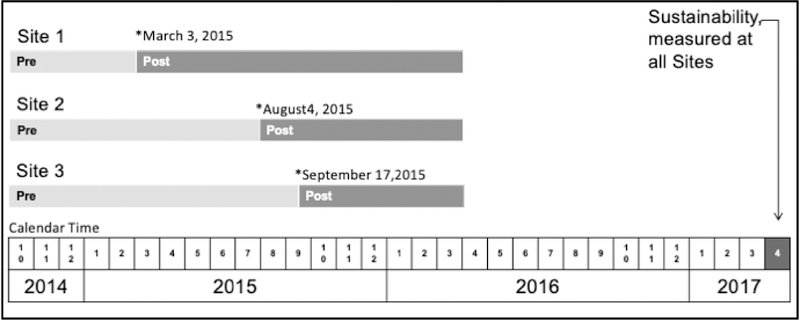
Three large outpatient medical center sites, each with 2–4 separate PC clinics, located in different cities in Western Washington ~30–90 miles apart, were selected by KPWA clinical leaders to pilot BHI based on informal assessment of readiness (e.g. willing/supportive leaders). Sites were initially expected to start implementation at the same time, but due to health system reorganization and staffing transitions, PC site leaders negotiated three separate “BHI launch dates” (Figure 1) when all PC providers in the participating clinics were expected to start screening all their patients. Licensed independent clinical social workers (LICSWs) in the pilot sites, who had previously functioned as medical social workers doing case management, were trained to function as integrated behavioral health clinicians, providing short-term counseling for common behavioral health conditions and linkage to specialty treatment.
Figure 1:
Behavioral Health Integration Quality Improvement Support and Evaluation Timeline
“Pre“ refers to pre-implementation of BHI; “Post” refers to the period after BHI implementation when practice coaches were supporting the clinics. EHR tools and performance monitoring persisted after the Post phase.
2.2. BHI Clinical Care Implemented for Cannabis and Other Drug Use
BHI included routine, annual screening for cannabis and other drug use among all adult PC patients with an in-person visit and assessment for symptoms of SUDs, among patients with high-risk use (defined below). Screening was conducted with a 7-item self-administered paper questionnaire (Supplement S1)*. The cannabis use question read: “How often in the past year have you used marijuana?” (Lapham et al., 2018; Lapham et al., 2017). The drug use question read: “How often in the past year have you used an illegal drug or used a prescription medication for non-medical reasons?” (Smith et al., 2010). For consistency, the questions asked about past-year frequency of use, and response options were “never”, “less than monthly”, “monthly”, “weekly”, and “daily or almost daily”, from the AUDIT-C question #3, which preceded them on the BHI screener (Bradley et al., 2007; Bush et al., 1998). To support clinicians in assessing and diagnosing SUDs, patients who reported cannabis use daily or any other drug use in the past year (hereafter “high-risk use”), were asked by medical assistants (MAs) to complete an “SUD Symptom Checklist”. The SUD Checklist was developed by clinical leaders based on the DSM-5 criteria for SUDs; 2–3, 4–5, and > 6 symptoms are consistent with mild, moderate, and severe CUDs or other DUDs, respectively (Hasin et al., 2013). The SUD Checklist was administered based on different frequencies of cannabis and other drug use due to research suggesting cannabis to be less addictive than other commonly used drugs (opioids, cocaine, amphetamines) (Moss et al., 2012). The clinical performance metric for cannabis and other drug use screening was completion of screening questions on the day of the visit or in the prior year (target: 80% of patients with in-person PC appointments). The clinical performance metric for assessment of SUDs was completion of the SUD Checklist on the day of the visit or in the prior year (target: 80% of patients with high-risk use).
When assessment indicated patients had recurrent symptoms consistent with CUDs and/or DUDs, providers were encouraged to document CUD or DUD diagnoses and offer shared decision-making about treatment options (i.e. pharmacotherapy, counseling, specialty addiction treatment). Providers were also encouraged to connect their newly diagnosed patients to LICSWs trained to provide short-term motivational SUD counselling, shared decision-making, and help patients make connections to pharmacotherapy and treatment programs as desired. When not connected on the day of the visit, LICSWs also outreached to patients with newly diagnosed SUDs from a registry (Glass et al., 2018).
2.3. Implementation Strategies
Three implementation strategies, designed to improve alcohol-related care (Bobb et al., 2017; Glass et al., 2018), were concurrently used to improve care for cannabis and other drug use. These included: 1) front line support of PC teams by practice coaches; 2) electronic health record (EHR) clinical decision support; and 3) performance monitoring and feedback to sites. Practice coaches, trained quality improvement consultants who work with PC teams to improve the quality of care (Baskerville et al., 2012), supported a PC implementation team at each site which included MAs, providers, LICSWs and clinic leaders. Practice coach support began with a 3-day “design event” to plan, pilot and refine workflows that integrated EHR decision support tools, followed bi-weekly to monthly Plan-Do-Check-Adjust (PDCA) quality improvement meetings (Tague, 2005). EHR clinical decision support tools prompted MAs to give the BHI screen to patients due for annual screening (i.e. no past-year screen) and to give SUD Checklists to patients with high-risk use. Performance feedback was provided on rates of screening and SUD Checklist completion monthly, when practice coaches, local implementation teams, and clinical leaders met for PDCA meetings. Both EHR clinical decision support and performance monitoring persisted after the active implementation phase ended.
Additionally, weekly formative evaluation (Stetler et al., 2006) meetings were held during BHI implementation to identify barriers and facilitators and to adapt implementation strategies to overcome barriers and maximize facilitators. Finally, during the one-year period after implementation ended at each site, operational partners provided ongoing monthly performance feedback and offered to have quarterly PDCA quality improvement meetings to review and address quality “gaps” in BHI.
2.4. Research Design, Sample and Data Collection
This evaluation used an observational pre-post design, evaluating rates of cannabis and drug use screening, completion of SUD Checklists, and new diagnosis and treatment of CUDs and other DUDs. The evaluation (October 3, 2014 to April 1, 2016) lasted from six months prior to the BHI launch date at site 1, to six months after the BHI launch date at site 3 (Figure 1). The three PC sites elected to implement BHI only in some of their PC clinics due to LICSW staffing (Bobb et al., 2017). This evaluation focused on care in participating clinics. The patient sample for this pilot study included adult patients (≥ 18 years) with an in-person PC visit during the study periods at a participating clinic at one of the three sites, before or after the agreed launch date—classified as “pre” or “post”, respectively. Data for the eligible sample were obtained from the EHR and insurance claims, which included demographic information, diagnoses, dispensed medications, and results of screening and SUD Symptom Checklists. Data are presented for each patient’s first PC visit in the pre- and post-implementation periods, unless otherwise specified. The KPWA Institutional Review Board approved this study, including waivers of consent and HIPAA authorization.
2.5. Measures
2.5.1. Screening and Assessment
Consistent with performance metrics above, patients were considered “screened” for cannabis or other drug use, if they had screening documented in the EHR on the day of the first PC visit in each period or in the prior year. Similarly, patients with high-risk cannabis or other drug use were considered assessed for SUD symptoms if they had a SUD Checklist documented in the EHR on the day of the visit or in the prior year.
2.5.2. New CUD and DUD Diagnoses and Treatment
We identified whether the patient had a new CUD or DUD diagnosis on the date of the first PC visit in each period. A new CUD or DUD diagnosis was defined as a diagnosis without any prior CUD or DUD diagnosis, respectively, within the past year using International Classification of Disease (ICD) codes from the U.S. National Committee for Quality Assurance’s (NCQA’s) Healthcare Effectiveness Data and Information Set (HEDIS) measure for the Initiation and Engagement (IET) of Alcohol and Other Drug Dependence Treatment. If the patient had a new CUD or DUD diagnosis on that visit, we identified whether the patient was treated for CUD or DUD within the following 14 days based on data from the EHR or claims for outside care. Medication fills for buprenorphine were also used to define DUD treatment. Our primary time window of interest was 14 days consistent with the NCQA HEDIS IET measure, but we also considered a 30-day and 90-day window to assess whether BHI implementation was associated with increased treatment of new CUDs or DUDs over a longer timeframe.
2.6. Statistical Analyses
We described demographic and clinical characteristics of patients separately within the pre- and post-implementation periods. Descriptive results of cannabis and other drug screening and assessment of SUD symptoms were presented for the post-implementation period only because these measures were introduced as part of BHI. We described the proportion of patients completing each measure, the proportion reporting each frequency of past-year cannabis and other drug use, and the proportion with, mild, moderate, and severe SUD symptoms. For descriptive purposes, we also plotted the proportion of visits in which patients due for screening were screened over weekly time intervals during the study period using visit-based binary indicators, separately for each clinic.
Generalized estimating equations (GEE) were used to test for a significant difference—comparing pre-implementation to post-implementation—in the proportions of PC patients with newly diagnosed CUDs or DUDs, or with treatment for newly diagnosed CUDs or DUDs, accounting for correlation of repeated visits if the same patient had a visit in both periods (Zeger et al., 1988). Specifically, a separate logistic GEE model was fit for each of the binary outcomes regressed on an indicator for whether the visit occurred in the pre- versus post-implementation period. We used an independent working covariance structure and the robust sandwich variance estimator; p values were calculated using the Wald test (two tailed α = 0.05). The sample for these analyses was all patients who visited participating PC clinics (rather than subsets who were screened, screened positive, or had a relevant diagnosis), to avoid potential identification bias that can result when the denominator may be affected by the intervention (Eldridge et al., 2009; Hernan et al., 2004). We also stratified analyses to examine site-specific outcomes. Finally, sensitivity analysis, using the last patient visit during the pre- and post-implementation periods, assessed whether allowing more time for BHI processes to go into effect at each site potentially changed our results.
2.7. Formative Evaluation Data Collection and Qualitative Analysis
Detailed notes were taken electronically by research staff at all weekly formative evaluation meetings and at the weekly to monthly quality improvement meetings with clinics. Preliminary template analyses (Hsieh and Shannon, 2005) were conducted using a rapid process (Beebe, 2001) in which a researcher trained in qualitative methods summarized qualitative data related to barriers and facilitators related to integration of care for cannabis and other drug use in PC using domains from Greenhalgh’s conceptual framework for dissemination of innovations (Greenhalgh et al., 2004). Members of the larger research team met twice to review the summary in comparison with more detailed notes and check conclusions against the experience of practice coaches.
2.8. Sustainment
Post hoc analyses of the sustainment of screening and assessment evaluated performance monitoring data from the month of April 2017, which was 18, 14, and 13 months after practice coaching ended at sites 1, 2, and 3, respectively. We report the proportions of PC patients who had visits to each site in April 2017 who completed screening and the proportion of patients with high-risk use who completed SUD Symptom Checklists. For the analysis of sustainment, all PC clinics at each site were included in performance monitoring data (i.e. not limited to the PC clinics that implemented BHI during the pilot).
3. Results
3.1. Study Sample
Overall 53,133 patients were eligible for this pilot study: 32,295 had visits pre-implementation and 39,599 had visits post-implementation (18,761 had visits both periods). Patients had a mean of 2.6 visits in both the pre- and post-implementation periods. Patients with a visit in the pre-implementation period were predominantly female (62%), non-Hispanic (92%), and white (82%), with mean age 55 years (Table 1). The prevalence of past-year mental health diagnoses documented in the EHR or claims included 2% with an alcohol use disorder, 18% with depression, and 15% with anxiety. Demographic and mental health-related characteristics were similar among patients with a visit in post-implementation.
Table 1.
Demographic and Clinical Characteristics of Adult Primary Care Patients Pre- & Post-Implementation of Behavioral Health Integration
| Measure | Pre-implementation N=32,295 | Post-implementation N=39,599 | p-value | ||
|---|---|---|---|---|---|
| Age, mean (SD)*** | 54.5 | (18.4) | 55.2 | (17.9) | < 0.0001 |
| Male, n (%)* | 12,282 | (38.0) | 15,875 | (40.1) | < 0.0001 |
| Race, n (%)** | < 0.0001 | ||||
| White | 26,504 | (82.1) | 32,912 | (83.1) | |
| Black | 772 | (2.4) | 898 | (2.3) | |
| Asian | 1,899 | (5.9) | 2,021 | (5.1) | |
| Other/Multiracial | 2,231 | (6.9) | 2,608 | (6.6) | |
| Unknown | 889 | (2.8) | 1,160 | (2.9) | |
| Hispanic, n (%)* | 0.022 | ||||
| No | 29,779 | (92.2) | 36,486 | (92.1) | |
| Yes | 1,639 | (5.1) | 1,928 | (4.9) | |
| Unknown | 877 | (2.7) | 1,185 | (3.0) | |
| Diagnoses in prior year, n (%)*† | |||||
| Alcohol use disorder | 578 | (1.8) | 699 | (1.8) | 0.014 |
| Major Depression | 5,878 | (18.2) | 6,778 | (17.1) | < 0.0001 |
| Anxiety disorders‡ | 4,855 | (15.0) | 5,684 | (14.4) | < 0.0001 |
| Other mental health diagnosis | 2,956 | (9.2) | 3,680 | (9.3) | 0.002 |
P value obtained from Fisher’s exact test (*), analysis of variance (**) or Wilcoxon rank sum test (***). Patients with visits in both the pre- & post-implementation periods were excluded from these statistical tests due to these tests’ assumption of independence.
Assessed in the year prior to initial visit to pilot clinic, does not include “in remission” codes
Post traumatic stress disorder; panic disorder, generalized anxiety disorder, obsessive compulsive disorder, other anxiety
3.2. Cannabis and Other Drug Use Screening
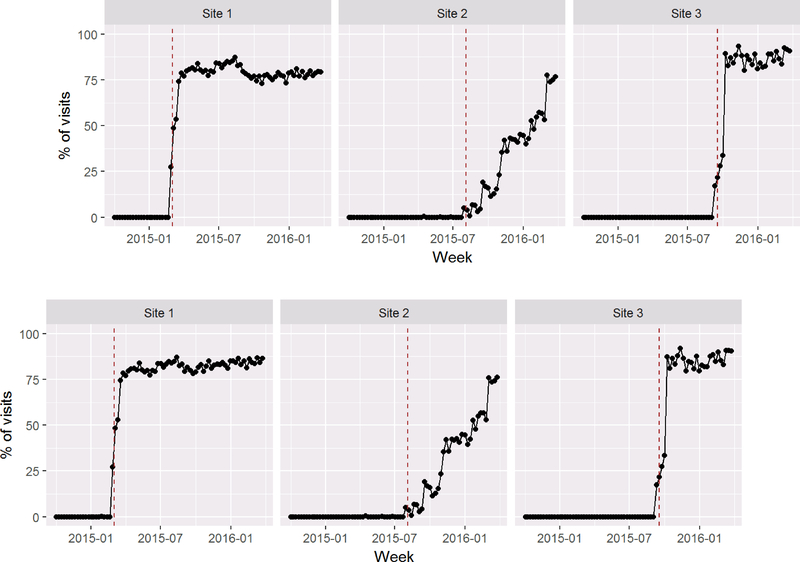
Approximately 57% and 59% of PC patients were screened for cannabis and other drug use, respectively, at their first visit after BHI launch (Table 2) reflecting the fact that screening increased quickly to near target following implementation at sites 1 and 3, but much more slowly at site 2 which chose to implement BHI gradually starting with one provider and adding more providers over time (Figure 2A and 2B). Across all patients in the three sites, 2% reported daily cannabis use, and 1% any other drug use (3% and 2% of those screened, respectively).
Table 2:
Cannabis and Other Drug Use Screening, SUD Symptom Checklists, Diagnosis & Treatment Pre- & Post-Behavioral Health Integration Implementation (using first visit in each period)
| Measure | Pre N=32,295 | Post N=39,599 | p-value† | ||
|---|---|---|---|---|---|
| *Screened for Cannabis Use, n (%) | 22457 | (56.71) | * | ||
| Report daily use‡ | 723 | (1.83) | * | ||
| *SUD Symptom Checklist, n (%) | 366 | (0.92) | * | ||
| **New CUD diagnosis, n (%) | 17 | (0.05) | 68 | (0.17) | <0.0001 |
| **New CUD treatment, n (%) | |||||
| Within 14 days | 3 | (0.01) | 3 | (0.01) | 0.80 |
| Within 30 days | 4 | (0.01) | 6 | (0.02) | 0.75 |
| Within 90 days | 6 | (0.02) | 12 | (0.03) | 0.33 |
| *Screened for Other Drug Use, n (%) | 23185 | (58.55) | * | ||
| Report some/any use‡ | 364 | (0.92) | * | ||
| *SUD Symptom Checklist, n (%) | 136 | (0.34) | * | ||
| **New DUD diagnosis, n (%) | 32 | (0.10) | 51 | (0.13) | 0.24 |
| **New DUD treatment, n (%) | |||||
| Within 14 days | 2 | (0.01) | 12 | (0.03) | 0.038 |
| Within 30 days | 5 | (0.02) | 15 | (0.04) | 0.083 |
| Within 90 days | 8 | (0.02) | 22 | (0.06) | 0.050 |
P values were calculated using Wald test from fitting Generalized Estimating Equations (GEE) clustered on the patient and using robust variance estimator
Screening & assessments were introduced as part of the pilot implementation.
Defined as warranting further assessment
New CUD & DUD diagnosis and CUD & DUD treatment are defined in text
Figure 2:
Screening for Cannabis (A) and Other Drug Use (B) Following Behavioral Health Integration Implementation at Three Primary Care Sites
Rates of screening over time, calculated as the proportion of all PC visits in each week in which the patients due for annual screening were screened until the end of active implementation. The dotted vertical lines show the “launch date” of each clinic.
3.3. Completion of SUD Symptom Checklists
Among 723 patients reporting daily cannabis use, about half (51%) completed SUD Checklists. Among those, 69% reported 0–1 of the 11 DSM-5 symptoms on the SUD Checklist (Table 3), and 14% reported 4 or more. Among 364 patients reporting any other drug use, about one-third (37%) completed the SUD Checklist. Among those, 62% reported 0–1 symptoms and 30% reported 4 or more.
Table 3.
Cannabis and Other Drug Use Screening & SUD Symptom Checklist Results Post-BHI Implementation
| Cannabis Use Screening Results, n (%) | N=22457 | ||
|---|---|---|---|
| Never | 18,898 | (84.2) | |
| Less than monthly | 1,759 | (7.8) | |
| Monthly | 550 | (2.4) | |
| Weekly | 527 | (2.3) | |
| Daily or Almost Daily | 723 | (3.2) | |
| SUD Symptom Checklist among those reporting daily cannabis use, n (%) | N=366 | ||
| 0–1 symptoms | 254 | (69.4) | |
| 2–3 symptoms | 61 | (16.7) | |
| 4–5 symptoms | 26 | (7.1) | |
| 6+ symptoms | 25 | (6.8) | |
| Other Drug Use Screening Results, n (%) | N=23185 | ||
| Never | 22,821 | (98.4) | |
| Less than monthly | 267 | (1.2) | |
| Monthly | 33 | (0.1) | |
| Weekly | 22 | (0.1) | |
| Daily or Almost Daily | 42 | (0.2) | |
| SUD Symptom Checklists among those reporting any drug use, n (%) | N=136 | ||
| 0–1 symptoms | 84 | (61.8) | |
| 2–3 symptoms | 11 | (8.1) | |
| 4–5 symptoms | 11 | (8.1) | |
| 6+ symptoms | 30 | (22.1) |
3.4. New CUD and DUD Diagnosis and Treatment
The proportion of PC patients newly diagnosed with CUD increased from pre- to post-implementation (5 vs 17 per 10,000 patients, p<0.0001), but there was no significant difference in CUD treatment within 14, 30 or 90 days of new diagnoses (Table 2). There was no significant difference in the proportion of patients newly diagnosed with other DUDs pre-implementation compared to pre-implementation (10 vs 13 per 10,000 patients; p 0.24). However, a greater proportion of patients newly diagnosed received DUD treatment within 14 days of a new DUD diagnosis post-implementation (1 vs 3 patients per 10,000 patients; p 0.038), as well as within 90 days of a new DUD diagnosis (2 vs 6 patients per 10,000, p=0.05) (Table 2). Site specific results were similar for new CUD and DUD diagnoses, but due to small sample sizes we were unable to assess statistical significance of new CUD and DUD treatment (Supplement S2)*. Sensitivity analyses, using the last visit completed by each patient indicated the increase in the prevalence of newly diagnosed CUD persisted, but there was no increase in treatment of DUDs (Supplement S3)*.
3.5. Barriers and Facilitators of Implementation
The formative evaluation identified key barriers and facilitators, and related adaptations to BHI strategies that focused on implementation of care for cannabis and other drugs (Table 4). In summary, provider knowledge gaps and stigma related to cannabis and other drug use were key barriers, while the high prevalence of cannabis use and stories of patients who appreciated the care they received were key facilitators. PC providers had little training or knowledge about cannabis use risks and benefits. At their request, a handout on cannabis use and health was developed (Supplement S4)†, and a cannabis-specific training was offered to providers at site 3. Stigma was also an important barrier—MAs and PC providers were fearful of making patients upset or uncomfortable, and MAs worried that asking patients to complete the SUD Symptom Checklist they might make them feel like they were “in trouble.” As a result, scripting was developed for MAs to facilitate introduction of symptom assessment. Alternatively, a key facilitator was the relatively high prevalence of cannabis use identified during screening; as PC providers saw how common cannabis use was among their patients, they placed more importance on offering screening and symptom assessment. Finally, when PC teams had regularly scheduled PDCA meetings, they shared local stories with one another and practice coaches about patients who had expressed appreciation for help addressing cannabis and other drug use. For example, one provider reported being able to engage a young woman in care for an anxiety disorder following discussions about her cannabis use, which reinforced the value of screening. Another provider reported helping a patient with complications of diabetes cut down on cocaine use.
Table 4.
Summary of Qualitative Barriers and Facilitators to Integration of Care for Cannabis and Other Drug Use in PC Identified During Formative Evaluation and Resulting Adaptions to Implementation Organized by Greenhalgh (Greenhalgh et al., 2004) Domain
| Innovation Characteristics | ||
| Barrier | PC staff who roomed patients (usually Medical Assistants) felt like they were making patients uncomfortable (i.e. “in trouble”) when they gave them the SUD Symptom Checklist following report of daily cannabis use or other drug use. | Adaptation: Scripting developed to help staff who roomed patients hand the patient the Substance Use Symptom Checklist |
| Barrier | Screening & assessment tools for cannabis and drug use were new to PC staff (unlike some depression and alcohol use tools). | Adaptation: Guide developed to help staff use these tools (i.e. BHI Getting Started Guide) |
| Barrier | When patients reported using multiple drugs clinicians had difficulty assessing role of specific drugs on symptoms from DSM-5 Checklist. | Adaptation: Clinicians encouraged not to try to link specific drugs to specific symptoms |
| Facilitator and Barrier | Prompts built in the EHR for annual screening helped MAs conduct screening, but follow-up assessments were often missed. | Adaptation: EHR clinical decision support developed to prompt MAs to give patients the SUD Symptom Checklist |
| Facilitator | Providers valued the Symptom Checklist for SUDs for their diagnostic utility. | Adaptation: Encouraged MAs to enter SUD Symptom Checklist results in EHR during rooming to support providers during the visit |
| Facilitator | Another quality improvement project, which trained social workers to outreach to patients with new substance use disorders (supported by an EHR registry) developed social workers’ skills to support PC providers and their patients with CUD or DUDs. | Adaptation: LICSWs encouraged warm hand-offs and modeled patient-centered, non-judgmental conversations with patients about CUDs and other DUDs. |
| Implementation Process | ||
| Facilitator | Routine sharing of positive stories during local implementation team meetings with practice coaches helped front-line PC teams value the work | Adaptation: Positive stories were used to spread knowledge of benefit in clinic huddles and other meetings with other PC staff |
| Facilitator and Barrier | Providers used training opportunities to ask questions about cannabis use, including how to diagnose CUD, and indicated it was a problem that they had no patient handout. | Adaptation: Handout on cannabis use and health was developed (Supplement S4). |
| Facilitator | Performance reports on rates of screening & use of SUD Symptom Checklist helped clinics identify “care gaps”. | Adaptation: Practice coaches provided teams with more frequent, regular performance reports |
| Adopters | ||
| Barrier | Asking patients about cannabis and other drug use was sometimes inconsistent with the values of front-line PC personnel to protect patient autonomy and privacy. For example, one provider complained asking patients about drug use felt too “big brother.” | Adaptation: Practice coaches stressed patient-centered approaches to shared decision-making and the relationship between health and cannabis and/or other drug use. |
| Facilitator | Pilot goal of improving access to care related to cannabis and other drug use (i.e. “no wrong door”) was consistent with the goal of front-line PC personnel to provide patient-centered care to their patients. | Adaptation: Cannabis and drug screening questions were added to patient questionnaires for use in behavioral health specialty and urgent care departments |
| Outer Context | ||
| Barrier | Patient concerns that because cannabis and other drug use remain illegal at federal level it may increase their risk to record this information in EHRs. | Adaptation: Staff were encouraged to validate patient privacy concerns and indicate that completing screening questions was not required |
| Facilitator | New trainings & educational tools developed by Washington State organizations about marijuana use (University of Washington Alcohol & Drug Abuse Institute, 2015). | Adaptation: This information was used in developing our handout. |
| Facilitator | Growing socio-political support for integration of care for addressing drug use in PC settings, due in part to high visibility research on increasing mortality associated with substance use (Crowley et al., 2015; Kolata, 2015). | Adaptation: Increased visibility of efforts to address cannabis and other drug use through behavioral health integration in PC. |
3.6. Sustainment
In April 2017, a year or more after practice coaching ended at all three sites, ongoing performance monitoring revealed that 81% of all PC patients completed annual cannabis and other drug screening. Site specific screening proportions at sites 1, 2 and 3 were 76%, 86% and 86% for cannabis use and 76%, 85% and 86% for other drug use, respectively. Symptom Checklists for SUDs were completed by 46% of patients who reported daily cannabis use (16%, 72% and 71% for sites 1–3 respectively) and 53% of patients who reported any other drug use (19%, 77% and 67%, for sites 1–3, respectively).
4. Discussion
This is the first study, to our knowledge, to describe implementation of cannabis and other drug screening followed by routine assessment for DSM-5 symptoms of SUDs as part of general BHI. The central objective was to determine whether BHI implementation was associated with increases in newly diagnosed CUDs or DUDs, and treatment of patients newly diagnosed. Compared to the pre-implementation period, the prevalence of newly diagnosed CUDs increased post-implementation, but not for other newly diagnosed DUDs. In contrast, treatment of patients with newly recognized CUDs did not increase post-implementation, but there was a small increase in treatment of patients with new other DUDs. This may reflect the finding that the majority (86%) of patients assessed following report of daily cannabis use endorsed 3 symptoms or less, while 1 in 5 (22%) patients assessed following report of other drug use reported 6+ symptoms. This evaluation did not assess patients’ treatment desires or needs, but these results suggest patients using drugs other than cannabis experienced more symptoms, which perhaps resulted in greater perceived need for treatment. The finding of increased treatment for newly diagnosed DUDs did not persist in sensitivity analyses using the last (versus the first) patient visit in the post-implementation period, which we suspect was a result of the shorter observation period (i.e. using the first visit allowed more time to observe treatment following screening). Nevertheless, a rigorous, randomized trial of the effectiveness of this approach to implementing cannabis- and drug-related care is needed to determine whether it increases new diagnoses and treatment.
These evaluation results also suggest BHI implementation resulted in 57 to 59% of PC patients being screened for cannabis and other drug use, respectively, and 37 to 51% of those were assessed for DSM-5 symptoms of SUDs during active implementation. Thus, a significant proportion of patients who may have benefitted from symptom assessment were missed post-implementation. Formative evaluation suggested fear of upsetting patients was a major barrier to administration of the SUD Symptom Checklist. Rates of screening and assessment also varied over time by site. For example, while staff at site 2 were slower to begin implementing BHI they ultimately achieved high rates of screening (>80%) and assessment (>70%) during the sustainment period. Formative evaluation suggested sites 2 and 3 benefited from adaptations made during implementation at site 1. One important adaptation (Bobb et al., 2017), was that unlike site 1, PDCA meeting time with practice coaches was proactively scheduled with implementation teams in sites 2 and 3. These teams appeared to benefit from the additional time to share stories and reflect on the value of integrating care for cannabis and other drug use into routine PC workflow (Barry, 2017; Crabtree et al., 2011), which may have facilitated continued quality improvement after the active implementation phase. A year after the pilot ended, rates of screening and assessment had improved overall, suggesting that implementation of BHI takes time, and may improve over time with ongoing support.
While some health systems have implemented population-based alcohol screening (Bradley et al., 2006), few assess patients for SUD symptoms (Oslin et al., 2006), and most health systems do not routinely screen for cannabis and other drug use or assess high-risk patients for SUD symptoms. Further, most prior drug screening implementation efforts have focused on screening, brief intervention and referral to treatment (SBIRT) (Aldridge et al., 2017; National Council for Behavioral Health, 2018), despite rigorous trials demonstrating the lack of efficacy of preventive brief interventions (Roy-Byrne et al., 2014; Saitz et al., 2014) and the fact that most patients offered referral to specialty drug treatment programs do not accept referral (Lipari et al., 2016).
There are important limitations of this observational pre-post pilot study. First, the evaluation of sustainment was not part of the pilot when it was initially planned, and future evaluations are needed to confirm results. Second, while the screening questions used have face validity and the drug screen was based on a validated single-item screen (Smith et al., 2010), the exact questions have not been validated. Similarly, though the SUD Symptom Checklist mirrors the 11 symptoms of DSM-5 substance use disorders, it has not been validated and did not assess recurrence of symptoms. Moreover, drug-specific data was not available due to patients’ difficulty attributing symptoms to a single substance. Third, limiting use of the SUD Checklist to patients reporting daily cannabis use may have missed patients with CUD. Relatedly, patients may have under-reported substance use and symptoms due to fear of legal consequences or social desirability bias. However, there was ample report of cannabis and drug use, suggesting that patients will answer these questions at a PC visit. Future qualitative research should explore PC patients’ perceptions of these questions, including their perceived needs for SUD-related care. Finally, this study was conducted in one integrated health system; results may not generalize to other health systems that lack resources for implementation and/or support by clinical leadership.
Conclusion
This pilot study in 3 PC sites found that implementation of screening and assessment for cannabis and other drug use as part of BHI was associated with increased diagnoses of CUD and a small increase in treatment for other new DUDs. While experts have recommended integration of improved care for cannabis and other drug use as part of BHI (National Council for Behavioral Health, 2018), these findings need to be replicated in larger studies and other health care systems, in more rigorous trials.
Supplementary Material
Highlights.
Study evaluated implementation of care for cannabis and drug use in primary care
Screening for cannabis and drug use increased after implementation
Diagnoses of new cannabis use disorders increased after implementation
Treatment of newly diagnosed drug use disorders increased after implementation
Evaluation of barriers, facilitators and adaptations may help other organizations
Acknowledgements
The authors would like to thank the leaders and clinicians of KP Washington who supported or participated in this pilot, in particular KPWA Medical Director of Behavioral Health Services Larry Marx, and Tory Gildred. And finally, we would like to thank the KP Washington primary care providers, staff and patients that made this work possible.
Author Disclosures
Role of Funding Sources
This research was supported by National Institute on Drug Abuse of the National Institutes of Health under Award Numbers UG1 DA01581, UG1DA040314 (CTN-0065Ot) and the Agency for Healthcare Research and Quality Award Number R18 HS023173. Dr. Bradley is supported by a mid-career mentorship award from National Institute for Alcohol Abuse and Alcoholism (NIAAA; K24-AA022128) and Dr. Williams is supported by a Career Development Award from VA Health Services Research & Development (CDA 12-276), and Dr. Glass is supported by a Career Development award from NIAAA (K01AA023859). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
No conflict declared.
Supplementary material can be found by accessing the online version of this paper.
Supplementary material can be found by accessing the online version of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrnsbrak R, Bose J, Hedden S, Lipari R, Park-Lee E, 2017. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration (SAMHSA) https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm [Google Scholar]
- Aldridge A, Linford R, Bray J, 2017. Substance use outcomes of patients served by a large US implementation of Screening, Brief Intervention and Referral to Treatment (SBIRT). Addiction 112, S43–S53. [DOI] [PubMed] [Google Scholar]
- Barry AR, 2017. Patient-Centred Care through Storytelling. Can. J. Hosp. Pharm 70, 322–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CL, Epstein AJ, Fiellin DA, Fraenkel L, Busch SH, 2016. Estimating demand for primary care-based treatment for substance and alcohol use disorders. Addiction 111, 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville NB, Liddy C, Hogg W, 2012. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann. Fam. Med 10, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA, Merrill JO, Schulman B, Finkelstein R, Olsen Y, Busch SH, 2008. Opioid use disorder in the United States: insurance status and treatment access. Drug Alcohol Depend. 94, 207–213. [DOI] [PubMed] [Google Scholar]
- Beebe J, 2001. Rapid assessment process: An introduction. AltaMira Press. [Google Scholar]
- Bobb JF, Lee AK, Lapham GT, Oliver M, Ludman E, Achtmeyer C, Parrish R, Caldeiro RM, Lozano P, Richards JE, Bradley KA, 2017. Evaluation of a Pilot Implementation to Integrate Alcohol-Related Care within Primary Care. Int. J. Environ. Res. Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR, 2007. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin. Exp. Res 31, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR, 2006. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am. J Manag. Care 12, 597–606. [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, 1998. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med 158, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Compton WM, Saha TD, Conway KP, Grant BF, 2009. The role of cannabis use within a dimensional approach to cannabis use disorders. Drug Alcohol Depend. 100, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF, 2007. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry 64, 566–576. [DOI] [PubMed] [Google Scholar]
- Crabtree BF, Nutting PA, Miller WL, McDaniel RR, Stange KC, Jaen CR, Stewart E, 2011. Primary care practice transformation is hard work: insights from a 15-year developmental program of research. Med. Care 49, S28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley RA, Kirschner N, Health, Public Policy Committee of the American College of, P., 2015. The integration of care for mental health, substance abuse, and other behavioral health conditions into primary care: executive summary of an American College of Physicians position paper. Ann. Intern. Med 163, 298–299. [DOI] [PubMed] [Google Scholar]
- Eldridge S, Kerry S, Torgerson DJ, 2009. Bias in identifying and recruiting participants in cluster randomised trials: what can be done? BMJ 339, b4006. [DOI] [PubMed] [Google Scholar]
- Glass JE, Bobb JF, Lee AK, Richards JE, Lapham GT, Ludman E, Achtmeyer C, Caldeiro RM, Parrish R, Williams EC, Lozano P, Bradley KA, 2018. Study protocol: a cluster-randomized trial implementing Sustained Patient-centered Alcohol-related Care (SPARC trial). Implement. Sci. 13, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode E, 2012. DRUGS in American Society, 8th ed. McGraw-Hill, New York, NY. [Google Scholar]
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, 2004. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 82, 581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med 167, 293–301. [DOI] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF, 2013. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry 170, 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Cerda M, Keyes KM, Stohl M, Galea S, Wall MM, 2017. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry 74, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM, 2004. A structural approach to selection bias. Epidemiology 15, 615–625. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE, 2005. Three approaches to qualitative content analysis. Qual. Health Res. 15, 1277–1288. [DOI] [PubMed] [Google Scholar]
- Hunt N, Derricott J, 2001. Smackheads, crackheads and other junkies: dimensions of the stigma of drug use, in: Carlisle C, Mason T, Watkins C, Whitehead (Eds.), Stigma and Social Exclusion in Healthcare. Routledge, London, p. 190. [Google Scholar]
- Kerridge BT, Mauro PM, Chou SP, Saha TD, Pickering RP, Fan AZ, Grant BF, Hasin DS, 2017. Predictors of treatment utilization and barriers to treatment utilization among individuals with lifetime cannabis use disorder in the United States. Drug Alcohol Depend. 181, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata G, 2015. Death rates rising for middle-aged white Americans, study finds, The New York Times; New York: https://www.nytimes.com/2015/11/03/health/death-rates-rising-for-middle-aged-white-americans-study-finds.html [Google Scholar]
- Lapham GT, Lee AK, Caldeiro RM, Glass JE, Carrell DS, Richards JE, Bradley KA, 2018. Prevalence of behavioral health conditions across frequency of cannabis use among adult primary care patients in Washington State. J. Gen. Intern. Med 33, 1833–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapham GT, Lee AK, Caldeiro RM, McCarty D, Browne KC, Walker DD, Kivlahan DR, Bradley KA, 2017. Frequency of Cannabis Use Among Primary Care Patients in Washington State. J. Am. Board Fam. Med 30, 795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari RN, Park-Lee E, Van Horn S, 2016. America’s Need for and Receipt of Substance Use Treatment in 2015. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Maynard C, Graves MC, West II, Bumgardner K, Krupski A, Roy-Byrne P, 2016. Drug use severity, mortality, and cause of death in primary care patients with substance use disorders. SAGE; Open 6, 10.1177/2158244015626225 [DOI] [Google Scholar]
- Moss HB, Chen CM, Yi HY, 2012. Measures of substance consumption among substance users, DSM-IV abusers, and those with DSM-IV dependence disorders in a nationally representative sample. J. Stud. Alcohol Drugs 73, 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., Medicine, 2017. The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. National Academies Press; 10.17226/24625. [DOI] [PubMed] [Google Scholar]
- National Council for Behavioral Health, 2018. Implementing care for alcohol and other drug use in medical settings: an extension of SBIRT. Available at https://www.thenationalcouncil.org/wp-content/uploads/2018/03/021518_NCBH_ASPTReport-FINAL.pdf
- Oslin DW, Ross J, Sayers S, Murphy J, Kane V, Katz IR, 2006. Screening, assessment, and management of depression in VA primary care clinics. The Behavioral Health Laboratory. J. Gen. Intern. Med 21, 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room R, 2005. Stigma, social inequality and alcohol and drug use. Drug Alcohol Rev. 24, 143–155. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, West II, Maynard C, Atkins DC, Graves MC, Joesch JM, Zarkin GA, 2014. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA 312, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Chaisson CE, Samet JH, 2014. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA 312, 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R, 2010. A single-question screening test for drug use in primary care. Arch. Intern. Med 170, 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler CB, Legro MW, Wallace CM, Bowman C, Guihan M, Hagedorn H, Kimmel B, Sharp ND, Smith JL, 2006. The role of formative evaluation in implementation research and the QUERI experience. J. Gen. Intern. Med 21, S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tague NR, 2005. Plan-Do-Check-Act (PDCA) Cycle, The quality toolbox. ASQ Quality Press; Milwaukee, pp. 390–392. [Google Scholar]
- University of Washington Alcohol and Drug Abuse Institute, 2015. Medicinal cannabis and chronic pain: science-based education in times of legalization. http://adai.uw.edu/mcacp/. Accessed October 2, 2017.
- Volkow ND, Baler RD, Compton WM, Weiss SR, 2014. Adverse health effects of marijuana use. N. Engl. J. Med 370, 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS, 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44, 1049–1060. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.