Abstract
Pompe disease is an autosomal recessive disorder caused by a deficiency of acid alpha-glucosidase resulting in intralysosomal glycogen accumulation in multiple tissue types, especially cardiac, skeletal, and smooth muscle. Enzyme replacement therapy (ERT) with alglucosidase alfa has led to improved clinical outcomes and prolonged survival in patients with Pompe disease. While ERT has changed the natural course of Pompe disease, with many long-term survivors, several factors affect the response to ERT. Previous studies in Pompe disease have shown that IgG antibodies to ERT can lead to a decline in muscle strength, pulmonary function, and overall and ventilator-free survival. Additionally, antibody responses to ERT can also cause hypersensitivity reactions. Various strategies to prevent or eliminate the IgG antibody response have been attempted in patients with Pompe disease. A detailed literature search was performed to compile data regarding the consequences of IgG antibodies, clinical approaches to prevent or eliminate IgG antibodies in patients with Pompe disease, and to expand our understanding of new modalities being developed in non-clinical settings. All qualifying articles describing the impact of IgG antibodies on the response to ERT, immunomodulation in patients with Pompe disease, and non-clinical settings identified via a PubMed database search were included in the review. Here, we provide a comprehensive review of combination- and single-agent therapies that have been investigated in the context of immune tolerance induction to ERT in Pompe disease to date. Immunomodulation strategies that successfully induce immune tolerance to ERT have improved overall survival, especially reflected in the decreased number of ventilator-dependent or deceased cross-reactive immunologic material (CRIM)-negative infantile Pompe disease (IPD) patients due to development of IgG antibodies when treated with ERT alone. Immunomodulation in CRIM-positive patients at the time they receive ERT also results in a decrease in the development of IgG antibodies compared to cases treated with ERT alone. Lessons learned from current approaches, alongside results from trials of novel immunomodulation strategies, may provide important insights into the development of next-generation therapies.
Keywords: Pompe disease, alglucosidase alfa, immune tolerance induction, immunomodulation, antidrug antibodies
Introduction
Enzyme replacement therapy (ERT) has transformed the natural history of lysosomal storage disorders (LSDs); yet as with any biopharmaceutical drug, there is a risk of developing of anti-drug antibodies (ADAs) against ERT, which can negatively affect the safety and efficacy of ERT. ADAs have been reported in all LSDs treated with ERT, with the most significant impact appreciated in patients with infantile Pompe disease (IPD). Following the advent of ERT, high and sustained IgG antibodies (HSAT) have been reported in patients with Pompe disease with resultant reduction in treatment efficacy, whereas IgE antibodies are implicated in infusion-associated reactions (IARs) and anaphylaxis.
The severe deleterious effect of HSAT formation warrant effective treatment strategies to avert IgG antibody response in patients with Pompe disease. Mendelsohn and colleagues administered an immunomodulatory regimen consisting of rituximab, methotrexate, and IVIG, in a CRIM-negative IPD patient who developed IgG antibodies (1,600, IgG antibody titer measured by ELISA) to ERT (1). They successfully eliminated these antibodies and demonstrated that immune tolerance to ERT can be achieved in patients with Pompe disease. Following this first success, various clinical approaches have been employed in patients with Pompe disease to prevent and/or eliminate the development of IgG antibodies to ERT. This has been done in patients naïve to ERT (prophylactic approach), as well as in patients who developed antibodies to ERT (therapeutic approach). Prophylactic immunomodulation has included the use of agents such as rituximab, methotrexate, rapamycin, sirolimus, mycophenolate, and intravenous immunoglobulins (IVIGs) in various combinations to target B-cells and T-cells (2-8). This approach has been more successful and safer in comparison to the therapeutic approach, as prophylactic treatment requires a less intensive, shorter duration of immune suppression with an ability to immune tolerize the patients. In therapeutic settings, combination regimens with rituximab, methotrexate, high dose IVIG, plasma exchange, omalizumab, and bortezomib have been administered, yielding varying degrees of success (8-16). In all cases where immune tolerance to ERT has been successfully induced, the mechanism of immune tolerance development has not been established.
Pre-clinical studies have been able to advance our understanding of the immunomodulatory agents currently used in patients with Pompe disease, such as methotrexate and bortezomib. Additionally, current studies have focused on developing novel immunomodulation agents and strategies that aim to induce antigen-specific or antigen targeted tolerance to ERT, rather than employing systemically immunosuppressive agents. More targeted approaches may well improve the efficacy and reduce safety risks associated with agents that are currently applied in the clinical setting. Furthermore, in silico mapping of immunodominant T-cell epitopes and the development of immunological prediction algorithms have advanced our understanding of mechanistic pathways specific to the immune response to ERT in Pompe disease. These tools may facilitate development of more personalized treatments and identify targets for future therapies.
The objectives of this article are to provide a comprehensive review of the deleterious effects of ADA to ERT in the setting of Pompe disease and to describe both the success or failure of various immunomodulation strategies that have been administered to patients to date and novel strategies and mechanistic findings that are under investigation in the non-clinical settings.
Methods
A systemic literature search of the PubMed database was performed using key phrases “Pompe disease immunomodulation”, “Pompe immune tolerance induction”, “Pompe disease immune”, “Pompe disease antibodies”, “Pompe disease immune tolerance”, “Pompe disease tolerance”, and “Pompe disease immune modulation”, and included all the articles published up to March 2019. All publications related to immunomodulation approaches in Pompe disease, in either pre-clinical or clinical settings, were included. Two reviewers determined whether an article met inclusion criteria for the literature review. Articles were excluded from the review if (I) no English translation was available, (II) the article described immune response to ERT, but no immunomodulation was administered, and/or (III) the focus was solely on eliminating IARs through desensitization or alternative dosing regimens, with no other intervention(s) taken. The published articles were stratified based on the following criteria: (I) pre-clinical or clinical setting; (II) timing of immunomodulation initiation—prophylactic or therapeutic setting; and (III) immunomodulation agents administered. Articles that described immunomodulation initiation in multiple settings were included in each of the applicable groups. IgG antibodies were determined by Sanofi Genzyme (Framingham, MA, USA) using enzyme-linked immunosorbent assays and confirmed using radioimmunoprecipitation, as previously described (17) unless specified otherwise.
Results
Following the inclusion/exclusion criteria previously described in the methods section, 61 qualifying articles (out of 85 total results) were identified using the key phrases. Of 61 of these results, 13 articles were excluded due to information overlap. A total of 48 articles published through March 2019 were included in the review. Details regarding the study cohort, IgG antibody titers, B cell recovery, survival, and dosing and administration details of individual immunomodulation regimens are available in Tables 1-3.
Table 1. Overview of safety and efficacy of immunomodulation approaches.
| Publication | Regimen* | Cohort size (No. patients) | CRIM status | Median IgG antibody titers (range) | No. patients with B cell recovery | No. immune tolerant patients | No. patients alive at time of publication | Conclusion and limitations | |
|---|---|---|---|---|---|---|---|---|---|
| Peak IgG antibody titer | IgG antibody titer at study end | ||||||||
| Prophylactic immunomodulation approaches | |||||||||
| Messinger et al. 2012, Banugaria et al. 2013, Kazi et al. 2017 | A | 19 | 19 CN | 200 (0–51,200) | 200 (0–25,600) | 15 | 16 | 14 | • Short 5-week course was able to achieve long-term tolerance in 69% (13/19) of CRIM-negative patients |
| • Additional 16% (3/19) patients were immune tolerized with an additional round of immunomodulation | |||||||||
| Broomfield et al. 2016 | B | 9 | 8 CN; 1 CP | 0 (0–12,800) | 0 | 8 | 8 | 6 | • 88% (7/8) CRIM-negative IPD did not seroconvert and were immune tolerant to ERT |
| Poelman et al. 2017 | C | 3 | 1 CN; 2 CP | 200,000 (6,250–800,000) | N/A | 3 | 1 | 3 | • Prevented antibody formation during B cell suppression |
| • Failed to induce immune tolerance in 66% (2/3) patients | |||||||||
| • Duration and dosage was different from the regimen previously shown to be successful in achieving immune tolerance | |||||||||
| Elder et al. 2013 | D, E, F | 5 | 4 CN; 1 CP | N/A | N/A | 2 | 1 | 4 | • 80% (4/5) patients did not develop antibodies. |
| • Regimen required delay in ERT by at least 3 weeks for initial immunomodulation | |||||||||
| • Regimen required long-term immune suppression (maintenance rituximab) | |||||||||
| Kazi et al. 2018 | G | 14 | 13 CP; 1 CN | 3,200 (50–102,400) | 150 (0–51,200) | Not assessed | 12 | 12 | • Immune tolerance achieved in 86% (12/14) of IPD patients |
| • Long-term follow-up on a larger cohort is needed to assess safety, and to verify whether tolerance to ERT is maintained | |||||||||
| Therapeutic immunomodulation approaches | |||||||||
| Mendelsohn et al. 2009 | H | 1 | 1 CN | 1,600 | 0 | N/A | 1 | 1 | • Achieved immune tolerance |
| • Patient had low antibody titers at time of immune modulation | |||||||||
| Messinger et al. 2012 | I | 2 | 2 CN | 7,200 (1,600–12,800) | 0 | 2 | 2 | 2 | • Successful in inducing immune tolerance in both patients |
| • Required longer duration of immunosuppression | |||||||||
| Markic et al. 2010; Markic et al. 2013 |
J | 1 | 1 CP | 6,400 | 0 | 1 | 1 | 1 | • IgG antibody titers were reduced and IARs were resolved |
| • Resolution of IARs cannot be solely attributed to immunomodulation | |||||||||
| Peak IgG antibody titer | IgG antibody titer at study end | ||||||||
| Rohbarch et al. 2010 | K | 1 | 1 CN | 400 | 400 | N/A | 1 | 1 | • IARs resolved and patient maintained low IgG titers throughout the treatment |
| • It remains unclear how omalizumab affects IgG antibody development, or if the patient would have maintained low titers even without omalizumab | |||||||||
| Deodato et al. 2014 | L | 1 | 1 CN | 25,600 | 100 | 1 | 1 | 1 | • Immune tolerance was achieved |
| • In another reported case plasma exchange was not successful in eliminating high antibodies | |||||||||
| • Efficacy of plasma exchange requires further verification | |||||||||
| Banugaria et al. 2013; Kazi et al. 2016 | M, N, O | 3 | 1 CN; 2 CP | 204,800 (204,800–819,200) | 800 (100–1,600) | 3 | 3 | 3 | • Achieved immune tolerance |
| • Required more than one cycle of immunomodulation to achieve long-term immunomodulation | |||||||||
| Rairikar et al. 2017 | P | 1 | 1 CN | 204,800 | 12,800 | Not assessed | 0 | 1 | • Reduced IgG antibodies but did not induce immune tolerance |
| Owens et al. 2018 | Q, R | 2 | 1 CN; 1 CP | 460,800 (102,400–819,200) | 115,200 (25,600–204,800) | N/A | 0 | 0 | • Did not induce immune tolerance |
| • Could be due to delay in initiation of immunomodulation and/or disease progression | |||||||||
| Poelman et al. 2019 | S | 3 | 2 CN; 1 CP | 156,250 | 31,250 | 3 | 0 | 3 | • Not successful in eliminating IgG antibodies |
| • Combination differed from previously published protocol in terms of dosing, duration and use of rapamycin in place of MTX** | |||||||||
*Immunomodulation administration details: A. RTX (375 mg/m2) ×4 weekly doses initiated at ERT week 0, MTX (0.4 mg/kg) ×3 doses every other week (9–17 doses total) initiated at week 0, IVIG (0.5g/kg) initiated at week 0; B. RTX 1-4 doses, MTX 10–18 doses; C. RTX (375 mg/m2) immediately followed by MTX (1 mg/kg) ×4 weekly doses each, both drugs initiated at week 0, IVIG (400 mg/kg); D. Induction RTX (750 mg/m2) ×2 doses 10–14 days apart initiated at week 0, daily sirolimus (0.6–1 mg/m2/day) initiated AFTER induction RTX, monthly IVIG (500–1,000 mg/kg), maintenance RTX (375 mg/m2) every 12 weeks; E. Induction RTX (375 mg/m2) ×3 weekly doses initiated at week 0, daily sirolimus (0.6–1 mg/m2/day) initiated AFTER induction RTX , monthly IVIG (500–1,000 mg/kg), maintenance RTX (375 mg/m2) every 12 weeks; F. Induction RTX (750 mg/m2) ×2 doses 10–14 days apart initiated at week 0, daily sirolimus (0.6–1 mg/m2/day) initiated AFTER induction RTX, monthly IVIG (500–1,000 mg/kg), maintenance RTX (375 mg/m2) every 12 weeks; G. MTX (0.4 mg/kg) for 3 cycles (3 doses/cycle) initiated with first 3 ERT infusions; H. Induction RTX (375 mg/m2) ×4 weekly doses, followed by maintenance RTX every 4–12 weeks, MTX (0.5 mg/kg) weekly initiated at week 7, IVIG (500 mg/kg) every 4 weeks; I. Induction RTX (375 mg/m2) ×4 weekly doses, followed by maintenance RTX every 4 weeks, MTX (0.5 mg/kg) weekly given enterally, IVIG (0.5 g/kg) every 4 weeks; J. Induction RTX (375 mg/m2) ×4 weekly doses, followed by maintenance RTX (4 doses, every 5–12 weeks), MTX (0.5 mg/kg) weekly initiated at week 5, IVIG (500 mg/kg) every 4 weeks; K. Omalizumab initiated at 4 months on ERT and continued for >1 year; L. Plasma exchange ×3 sessions 2 months after ERT discontinued, followed by RTX (375 mg/m2) ×1 dose, then IVIG (dose unknown) ×4 doses; M. Bortezomib (1.3 mg/m2) for 3 cycles (4 doses/cycle), RTX (375 mg/m2) ×4 weekly doses initiated after second cycle of BTZ, followed by monthly maintenance RTX and biweekly MTX (15 mg/m2). Monthly IVIG (400–500 mg/kg); N. Bortezomib (1.3 mg/m2) for 3 cycles (4 doses/cycle), RTX (375 mg/m2) ×4 weekly doses initiated after first cycle of BTZ, followed by monthly maintenance RTX and biweekly MTX (15 mg/m2). Monthly IVIG (400–500 mg/kg); O. Bortezomib (1.3 mg/m2) for 6 cycles (4 doses/cycle), monthly maintenance RTX (375 mg/m2) thereafter with the exception of RTX ×4 weekly doses following first and third cycles of BTZ, biweekly MTX (15 mg/m2) initiated after first cycle BTZ. Monthly IVIG (400–500 mg/kg); P. Weekly IVIG (1g/kg) for 20 weeks; Q. RTX ×4 weekly doses, followed by weekly MTX and monthly IVIG, then BTZ ×4 weekly doses; R. Weekly MTX and monthly IVIG, followed by RTX and BTZ ×4 weekly doses each; S. RTX (375 mg/m2) ×3 weekly doses, BTZ (1.3 mg/m2) twice a week ×6 doses, monthly IVIG (first dose 1.0 g/kg, subsequent doses 0.5 g/kg), all initiated simultaneously. Rapamycin (10–20 kg, 1.0–1.5 mg/day; 20–30 kg, 1.5–2.0 mg/day); double dose on first day of rapamycin. CRIM, cross-reactive immunologic material; CP, CRIM-positive; CN, CRIM-negative; MTX, methotrexate; RTX, rituximab; BTZ, bortezomib; IVIG, intravenous immunoglobulin; IPD, infantile Pompe disease; ERT, enzyme replacement therapy; IgG, immunoglobulin G; N/A, not available; IARs, infusion associated reactions.
Table 2. Overview of prophylactic immunomodulation approaches.
| Publication | Patient code | Complementary DNA change | CRIM status | Alive at end of study? | IgG antibody titers (time on ERT) | B cell recovery | ||
|---|---|---|---|---|---|---|---|---|
| GAA, Variant 1 | GAA, Variant 2 | Peak IgG antibody titer | IgG antibody titer at study end | |||||
| Messinger et al. 2012, Banugaria et al. 2013, Kazi et al. 2017 | 1* | c.341insT | c.341insT | Negative | Yes | 1,600 (38 weeks) | 200 (103 weeks) | Yes |
| 2* | c.1548G>A | c.525delT | Negative | No | 0 (68 weeks) | 0 (68 weeks) | Yes | |
| 3** | c.2608C>T | c.2608C>T | Negative | Yes | 0 | 0 (281 weeks) | Yes | |
| 4** | c.546+2T>C | c.546+2T>C | Negative | Yes | 0 | 0 (285 weeks) | Yes | |
| 5** | c.236_246del | c.236_246del | Negative | Yes | 0 | 0 (89 weeks) | Yes | |
| 6** | c.525delT | c.2560C>T | Negative | Yes | 0 | 0 (70 weeks) | Not done | |
| 7** | c.2560C>T | c.2560C>T | Negative | No | 6,400 (31 weeks) | 6,400 (59 weeks) | Yes | |
| 8** | c.525_526delTG | c.525_526delTG | Negative | No | 6,400 (20 weeks) | 6,400 (46 weeks) | Yes | |
| 9** | c.2560C>T | c.2560C>T | Negative | No | 1,600 (39 weeks) | 800 (46 weeks) | Yes | |
| 10 | c.2560C>T | c.1292_1295dupTGCA | Negative | Yes | 25,600 (199 weeks) | 25,600 (199 weeks) | Yes | |
| 11 | c.2560C>T | c.2560C>T | Negative | Yes | 0 | 0 (214 weeks) | Yes | |
| 12 | c.2560C>T | c.2560C>T | Negative | No | 25,600 (39 weeks) | 6,400 (57 weeks) | Not done | |
| 13 | c.2560C>T | c.2560C>T | Negative | Yes | 0 | 0 (11 weeks) | NA | |
| 14 | c.258dupC | c.2227C>T | Negative | Yes | 25,600 (96 weeks) | 800 (152 weeks) | Yes | |
| 15 | c.1754+2T>A | c.1822C>T | Negative | Yes | 51,200 (71 weeks) | 6,400 (184 weeks) | Continued ITI | |
| 16 | c.2237G>A | c.437delT | Negative | Yes | 200 (81 weeks) | 200 (81 weeks) | Yes | |
| 17 | c.2560C>T | c.2560C>T | Negative | Yes | 200 (3 weeks) | 200 (32 weeks) | Yes | |
| 18 | c.2560C>T | c.2560C>T | Negative | Yes | 0 | 0 (58 weeks) | Yes | |
| 19 | c.2560C>T | c.525delT | Negative | Yes | 3,200 (20 weeks) | 3,200 (24 weeks) | Yes | |
| Broomfield et al. 2016 | 6 | c.525del | c.2608C>T | Negative | No | 0 | 0 | Yes |
| 7 | c.2608C>T | c.2608C>T | Negative | Yes | 0 (0.6 years) | 0 | Not followed beyond 6 months | |
| 8 | c.2237G>A | c.2237G>A | Negative | No | 0 | 0 | Yes | |
| 12 | c.2078dup | c.2078dup | Negative | Yes | 0 (0.9 years) | 0 | Yes | |
| 14 | c.2560C>T | c.2560C>T | Negative | No | 0 | 0 | Yes | |
| 16 | c.2237G>A | c.2237G>A | Negative | Yes | 0 | 0 | Yes | |
| 17 | c.2560C>T | c.2560C>T | Negative | Yes | 0 (1.8 years) | 0 | Yes | |
| 19 | c.525del | c.2481+102_2646+31del535 | Negative | Yes | 12,800 (1.3 years) | Unknown | Yes | |
| 22 | c.877G>A | c.877G>A | Positive | Yes | 0 (2.5 years) | 0 | Yes | |
| Poelman et al. 2017 | 1 | c.525delT | c.525delT | Negative | Yes | 800,000 (13 months) | Remained high | Yes (6.7 mo on ERT) |
| 2 | c.1551+1G>A | c.1551+1G>A | Positive | Yes | 6,250 (14 months) | Intermediate levels | Yes (6.7 mo on ERT) | |
| 3 | c.525delT | c.2481+102_2646+31del | Positive | Yes | 200,000 (14 months) | Remained high | Yes (7.7 mo on ERT) | |
| Elder et al. 2013 | A | c.2560C>T | c.2560C>T | Negative | No | 500,000 (5 weeks) | Very high | Yes |
| B | c.1396delG | c.1705dupT | Negative | Yes | 0 | 0 | No, continued RTX | |
| C | c.925G>A | c.925G>A | Negative | Yes | 0 | 0 | No, continued RTX | |
| D | c.1548G>A | not found | Negative | Yes | 0 | 0 | No, continued RTX | |
| E | c.1933G>A | c.2501_2502del | Positive | Yes | 0 | 0 | No, continued RTX | |
| Kazi et al. 2018 | IOPD1 | c.953T>C | c.1292_1295dupTGCA | Positive | Yes | 3,200 (4 weeks) | 0 (89 weeks) | N/A |
| IOPD2 | c.1004G>A | c.1841C>A | Positive | Yes | <100 (31 weeks) | 0 (122 weeks) | N/A | |
| IOPD3 | c.1118T>G | c.1118T>G | Positive | No | 12,800 (5 weeks) | 400 (49 weeks) | N/A | |
| IOPD4 | c.2560C>T | c.1466A>G | Positive | Yes | 3,200 (11 weeks) | 400 (81 weeks) | N/A | |
| IOPD5 | c.665T>G | c.1437+2T>C | Positive | Yes | 200 (19 weeks) | 0 (60 weeks) | N/A | |
| IOPD6 | c.1114C>T | c.1979G>A | Positive | Yes | 800 (22 weeks) | 0 (61 weeks) | N/A | |
| IOPD7 | c.2456G>C | c.2456G>C | Positive | Yes | 800 (60 weeks) | <100 (90 weeks) | N/A | |
| IOPD8 | c.525delT | c.2297A>C | Positive | Yes | 3,200 (7 weeks) | 100 (39 weeks) | N/A | |
| IOPD9 | c.1A>G | c.1A>G | Negative | Yes | 6,400 (91 weeks) | 6,400 (91 weeks) | N/A | |
| IOPD10 | c.1942G>A | c.1942G>A | Positive | Yes | 800 (7 weeks) | 100 (42 weeks) | N/A | |
| IOPD11 | c.1447G>A | c.2560C>T | Positive | Yes | 3,200 (28 weeks) | 400 (118 weeks) | N/A | |
| IOPD12 | c.1A>G | c.2234T>C | Positive | Yes | 200 (93 weeks) | 200 (93 weeks) | N/A | |
| IOPD13 | c.1979G>A | c.2560C>T | Positive | Yes | 102,400 (76 weeks) | 51,200 (85 weeks) | N/A | |
| IOPD14 | c.525delT | c.1979G>A | Positive | Yes | 25,600 (28 weeks) | 6,400 (36 weeks) | N/A | |
*, patients 3 and 4 in Messinger et al. [2012], patients 1 and 2 in Kazi et al. [2017]; **, patients 1–7 in Banugaria et al. [2013], patients 3–9 in Kazi et al. [2017]. CRIM, cross-reactive immunologic material; IgG, immunoglobulin G; N/A, not available, ERT, enzyme replacement therapy; ITI, immune tolerance induction; GAA, gene encoding acid alpha-glucosidase; RTX, rituximab.
Table 3. Overview of therapeutic immunomodulation approaches.
| Publication | Patient Code | Complementary DNA change | CRIM status | Alive at end of study? | IgG antibody titers (time on ERT) | B cell recovery | ||
|---|---|---|---|---|---|---|---|---|
| GAA, Variant 1 | GAA, Variant 2 | Peak IgG antibody titer before ITI | IgG antibody titer at study end | |||||
| Mendelsohn et al. 2009 | N/A | c.2560C>T | c.2560C>T | Negative | Yes | 1,600 (16 weeks) | 0 | Unknown |
| Messinger et al. 2012 | 1 | c.2560C>T | c.2560C>T | Negative | Yes | 1,600 (4 months) | 0 | Yes |
| 2 | c.1128_1129delinsC | c.2237G>A | Negative | Yes | 12,800 (1 month) | 0 | Yes | |
| Markic et al. 2010 & Markic et al. 2013 | N/A | c.925G>A | c.2269C>T | Positive | Yes | 6,400 (6 weeks) | 0 (3 years) | Yes |
| Rohbarch et al. 2010 | N/A | c.1157insA | c.1157insA | Negative | Yes | 0 (6 months) | 400 (44 months) | N/a |
| Deodato et al. 2014 | N/A | c.1833_1839del; c.1846G>T; c.1847_1848insT | c.1833_1839del; c.1846G>T; c.1847_1848insT | Negative | Yes | 25,600 (16 weeks) | 100 (16 months) | Yes |
| Banugaria et al. & Kazi et al. 2016 | 1 | c.307T>G | c.2481+102_2646+31del | Positive | Yes | 204,800 (64 weeks) | 100 (383 weeks) | Yes |
| 2 | c.2560C>T | c.1654delC | Negative | Yes | 819,200 (87 weeks) | 1,600 (423 weeks) | Yes | |
| 3 | c.1655T>C | c.1655T>C | Positive | Yes | 204,800 (64 weeks) | 800 (354 weeks) | Yes | |
| Rairikar et al. 2017 | N/A | c.1195-18_2190-20del | c.1195-18_2190-20del | Negative | Yes | 204,800 (265 weeks) | 12,800 (374 weeks) | Unknown |
| Owens et al. 2018 | 2 | Novel homozygous deletion spanning exons 19-20 | Negative | No | 819,000 (9 months) | 204,800 (14 months) | Unknown | |
| 4 | c.1942G>A | c.1942G>A | Positive | No | 102,400 (9 months) | 25,600 (12 months) | Unknown | |
| Poelman et al. 2019 | 1 | c.2481 + 102_2646 + 31del538 | c.2481 + 102_2646 + 31del538 | Positive | Yes | 156,250 (6.3 years) | 31,250 (8.8 years) | Yes |
| 2 | c.del525T | c.del525T | Negative | Yes | 156,250 (3 years) | 31,250 (5.1 years) | Yes | |
| 3 | c.del525T | c.del525T | Negative | Yes | 781,250 (2.1 years) | 156,250 (3.6 years) | Yes | |
CRIM, cross-reactive immunologic material; IgG, immunoglobulin G; N/A, not available, ERT, enzyme replacement therapy; ITI, immune tolerance induction; GAA, gene encoding acid alpha-glucosidase.
The negative impact of IgG antibodies to ERT in patients with Pompe disease (Table 4)
Table 4. Impact of high and sustained IgG antibody titers to alglucosidase alfa.
| Article | Impact |
|---|---|
| Amalfitano et al. 2001 (2 CRIM-negative IPD patients) | Loss of motor skills |
| Decline in pulmonary function | |
| Decrease in ventilator-free survival | |
| Decline in muscle function | |
| Kishnani et al. 2009 (11 CRIM-negative and 4 CRIM-positive IPD patients) | Decrease in overall and ventilator-free survival |
| Less improvement in cardiac response | |
| Regression of motor milestones | |
| Increase in biomarker urinary Glc4 | |
| De Vries et al. 2010 (1 LOPD patient, age at ERT initiation of 50 years) | Inhibitory effect on the catalytic function or stability |
| Decline in distance walked at 6MWT | |
| Decline in FVC | |
| Increase in number infusion associated reactions | |
| Decline in muscle strength | |
| Banugaria et al. 2011 (11 CRIM-negative and 9 CRIM-positive IPD) | Shortened overall and ventilator-free survival |
| Worsening of left ventricular mass index | |
| Loss of motor gains | |
| Increase in biomarker urinary Glc4 | |
| Patel et al. 2012 (3 LOPD patients, age at ERT initiation of 37, 56, and 57 years respectively) | Decline in pulmonary function |
| Decline quality of life | |
| Decline in motor function | |
| Gelder et al. 2015 (2 CRIM-negative and 4 CRIM-positive) | Inhibition of enzyme uptake |
| Ability to walk | |
| Decreased catalytic activity | |
| Loss of motor gains | |
| Berrier et al. 2015 (17 CRIM-negative IPD) | Decline in ventilator-free and overall survival |
| Worsening of the cardiac parameters | |
| De Vries et al. 2016 (12 LOPD patients) | Increase in number of infusion associated reactions |
CRIM, cross-reactive immunologic material; IPD, infantile Pompe disease; LOPD, late-onset Pompe disease; 6MWT, six minute walk test; Glc4, glucose tetrasaccharide; ERT, enzyme replacement therapy.
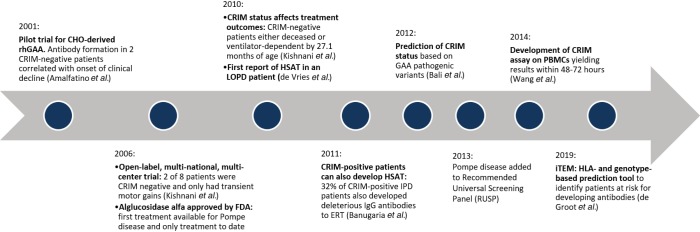
The deleterious effects of IgG antibodies to ERT in Pompe disease were first recognized in the pilot trial of alglucosidase alfa in three patients with IPD (18). Results from this study showed a decline in clinical outcome in two cross-reactive immunologic material (CRIM)-negative IPD patients which correlated with the development of HSAT. In comparison, the third IPD patient, a CRIM-positive patient, did not develop IgG antibodies and exhibited a good response to ERT. These findings were instrumental in advancing our understanding of the impact of HSAT on clinical outcome and revealing a correlation between CRIM status and ADA development (18). More specifically, the development of HSAT is strongly influenced by the presence (CRIM-positive) or absence (CRIM-negative) of endogenous GAA protein; although the GAA is nonfunctional, its presence is often sufficient to induce some degree of immune tolerance (19). In a seminal study assessing the impact of CRIM status on treatment outcomes in 32 IPD patients, CRIM-negative patients who received ERT were either deceased or ventilator-dependent by 27.1 months of age due to the loss of efficacy of ERT secondary to development of HSAT (19), whereas most CRIM-positive patients experienced continued and long-term benefits from ERT. In a follow-up study exploring the extent of the IgG antibody response in CRIM-positive IPD patients, 32% of CRIM-positive IPD patients also developed HSAT to ERT with poor clinical outcomes, similar to CRIM-negative IPD patients (20,21). These studies, among others, confirmed the negative impact of HSAT in response to ERT (Figure 1) (19,21) rather than the CRIM status per se. More specifically, the development HSAT can lead to the following effects on ERT and clinical outcomes in patients with Pompe disease: inhibition of enzyme uptake, decrease in catalytic activity, and mistargeting of ERT into FcR bearing cells, away from the intended muscle targets (Table 4) (18,19,21-25).
Figure 1.
Advances in our understanding of CRIM status and the impact of high and sustained IgG antibodies on clinical outcomes. CHO, Chinese hamster ovary cell; rhGAA, recombinant human acid alpha-glucosidase; CRIM, cross-reactive immunologic material; HSAT, high and sustained IgG antibody titers; LOPD, late-onset Pompe disease; IPD, infantile Pompe disease; PBMCs, peripheral blood mononuclear cells; iTEM, individualized T cell epitope measure; IgG, immunoglobulin G; ERT, enzyme replacement therapy; HLA, human leukocyte antigen.
While early evidence showed HSAT formation primarily in patients with IPD, it has been demonstrated that patients with late-onset Pompe disease (LOPD; symptom onset <12 months of age without cardiac involvement or ≥12 months of age) can similarly mount HSAT to ERT. In contrast to patients with IPD, the effect of IgG antibodies on the efficacy of ERT and the necessity for immunomodulation in patients with LOPD is still debated (22,23,25-27). Some studies have shown that development of HSAT can lead to a poor clinical outcome (23,25), while other studies have reported that IgG antibodies do not significantly impact clinical outcomes in patients with LOPD (22,26,27). Moreover, despite the hypothesis that the “leaky” splice site variant (c.-32-13T>G), commonly seen in patients with LOPD, reduces the risk of IgG antibody formation, there have been reported cases of LOPD patients with the “leaky” splice site variant who developed HSAT (22,25). Unlike in patients with IPD in whom clinical endpoints such as left ventricular mass index, overall and ventilator-free survival can be easily appreciated, there is a lack of sufficiently sensitive clinical endpoints to capture the more gradual changes seen in patients with LOPD. Further research is warranted to assess the development and impact of ADA to ERT in LOPD patients.
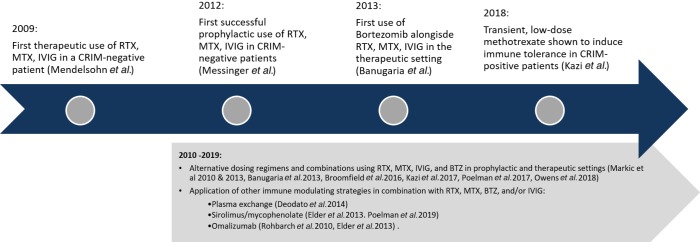
Prophylactic immunomodulation approaches (Table 1, Figure 2)
Figure 2.
Clinical applications of immune modulation strategies. RTX, rituximab; MTX, methotrexate; IVIG, intravenous immunoglobulin; CRIM, cross-reactive immunologic material; BTZ, bortezomib.
Rituximab, methotrexate and/or IVIG (5-week short course)
With the success of rituximab, methotrexate and IVIG in preventing or eliminating IgG antibodies to ERT and resultant immune tolerance to ERT (1,8,28), Kazi et al. evaluated the long-term safety of prophylactic immunomodulation with rituximab, methotrexate and IVIG in a cohort of 19 CRIM-negative IPD patients, with the longest follow-up assessment of IgG antibody titer at 285 weeks (6). Of the 19 CRIM-negative IPD patients, 15 either did not develop IgG antibodies (n=8) or maintained low IgG antibody titers (n=7) (LT; low antibody titers defined as titers of ≤6,400) (Table 2). Three patients developed peak IgG antibody titers in the sustained intermediate titer rage (SIT; defined as titers of ≥12,800 and <51,200) and one patient developed HSAT (defined as titers of ≥51,200) with a peak titer of 51,200 (6). Fourteen of the 19 patients received one cycle of immunomodulation and five patients required more than one cycle. Fifteen of 16 evaluable patients demonstrated B-cell recovery, one patient was still receiving rituximab at study’s end. Four patients reported infections in the peri-immunomodulation period, requiring antibiotic treatment; two developed central line infections and bacteremia, managed by line removal and antibiotic treatment; one experienced respiratory syncytial virus infection; and one suffered an episode of aspiration pneumonia and enterovirus/rhinovirus infection.
Broomfield et al. reported the use of combined rituximab and methotrexate for immunomodulation in nine IPD patients, eight CRIM-negative and one CRIM-positive (29). The immunomodulation regimen was similar to the approach by Banugaria et al. only without IVIG (28). Of the nine patients, eight patients did not seroconvert and one CRIM-negative patient developed IgG antibodies with peak titers of 12,800 (Table 2). All but one patient who received immunomodulation had B cell recovery at study termination. Additionally, two CRIM-negative patients who did not receive any immunomodulation at the time of ERT initiation developed HSAT with titers of 204,800. The authors reported significantly improved survival in CRIM-negative patients who received immunomodulation compared to the CRIM-negative patients who received ERT alone (29).
Poelman et al. utilized a combination of rituximab, methotrexate, and IVIG in three patients with IPD—one CRIM-negative and two CRIM-positive (30). While the immunomodulatory agents used in this protocol were identical to those described in Kazi et al., (6) the two differ in terms of dose and duration of methotrexate administration—while Kazi et al. utilized 3 cycles (3 doses/cycle, total 9 doses) of methotrexate (0.4 mg/kg/dose) (6), Poelman et al. used a less intensive dosing schedule including weekly methotrexate (1 mg/kg/dose) with each ERT infusion. All three patients seroconverted with highest observed IgG antibody titers in each patient of 800,000, 6,250, and 200,000 (Table 2). All three patients showed B cell recovery after rituximab discontinuation; however, B cell recovery coincided with rising IgG antibody titers. A bacterial infection was reported in one patient around the time of immunomodulation. All three patients were alive and ventilator-free at the study end. Thus, two of three IPD patients were not immune tolerant to ERT; this could be related to differences in the dosage and administration regimen from the successful immunomodulation approaches used by Kazi et al. and Broomfield et al. (Table 2) (6,29,30).
Rituximab, sirolimus or mycophenolate, and IVIG
Elder et al. used rituximab in combination with mycophenolate or sirolimus to induce B cell depletion and T cell modulation prior to exposure to ERT in five IPD patients (four CRIM-negative and one CRIM-positive) (31). After an initial pre-ERT course of immunomodulation, ERT was initiated alongside monthly IVIG and maintenance rituximab every 12 weeks. One CRIM-negative patient (who did not receive maintenance rituximab) developed HSAT (Table 2). In this patient, B cell recovery was associated with a rapid increase in IgG antibody titer with subsequent clinical decline and death. In four of the five remaining patients, the immunomodulation prevented antibody formation. These four patients were alive at the end of the study and none had developed bacterial infections attributable to immunomodulation, which was somewhat surprising in that long-term use of rituximab as in this regimen can lead to skewing to naïve B cell subpopulations, delayed B cell recovery, inability to mount a response to vaccines, and consequent increased risk of infection. A limitation of this approach was the delay in initiation of ERT by 3 weeks during the initial induction of rituximab, which can lead to irreversible muscle damage in such high-risk patients (32,33) and lack of response to ERT.
Transient low-dose methotrexate (TLD-MTX) as a single agent
Studies in GAA knock-out mice showed that TLD-MTX initiated with ERT reduced IgG antibodies to ERT through an IL-10 and B regulatory cell-dependent mechanism (5,34). Based on these results, an immunomodulation approach with TLD-MTX was administered to 14 patients with IPD (13 CRIM-positive and 1 CRIM-negative) (7). Of the 14 IPD patients, 12 maintained LT, one patient developed SIT, and one patient developed HSAT. The CRIM-negative IPD patient maintained LT with the highest observed titer of 6,400 (Table 2). Infections in the timeframe of methotrexate administration were seen in two patients; one patient suffered rhinovirus infection and the other experienced respiratory infections. Two IPD patients died due to cardiorespiratory failure associated with disease progression and unrelated to immunomodulation. In comparison, 32.4% of CRIM-positive IPD patients from a historical cohort treated with ERT monotherapy developed IgG antibody titers of ≥12,800 (20). Further studies in larger cohorts of IPD patients are warranted to evaluate the efficacy of this approach in achieving long-term immune tolerance to ERT.
Therapeutic immunomodulation approaches (Table 1, Figure 2)
Prophylactic immunomodulation approaches co-initiated alongside ERT have shown considerable success in minimizing IgG antibody responses and in many cases, achieving immune tolerance to ERT with a short course of immunomodulation. On the other hand, the elimination of pre-existing HSAT has required prolonged use of immunomodulating agents and ongoing immunosuppression, yielding varying degrees of success. The success of such approaches is primarily limited by failure to target antibody-secreting plasma cells after the immune response has been established and the lack of biomarkers for durable immune tolerance.
Rituximab, methotrexate, and IVIG for treatment of patients with non-HSAT ADA to ERT
In a case report, Mendelsohn et al. first described the use of rituximab, methotrexate, and IVIG for eliminating IgG antibodies to ERT in a CRIM-negative IPD patient with titers of 1,600 after 16 weeks on ERT (1). The IgG antibody titer declined after initiation of immunomodulation and became negative by 7.5 months following initiation of ERT and within 3.5 months after initiation of immunomodulation (1). The same authors described successful immune tolerance induction in two additional CRIM-negative IPD patients treated therapeutically with this combination regimen (8) whose highest antibody titers were 1,600 and 12,800 after 4 months and 1 month on ERT (Table 3). IgG antibodies were fully eliminated in both patients after 3 and 9 months of immunomodulation. At study’s end, both patients had discontinued all immune therapy and maintained negative IgG antibody titers with complete B cell recovery. The success of eliminating ADA in these cases may be attributable to the relatively low titer at the initiation of therapy and the prolonged course of immunomodulation, though given the lack of biomarkers of immune tolerance, it is not clear at what point in their respective treatment courses tolerance had been established. In contrast, such treatment was not successful in the face of HSAT (see section Bortezomib, rituximab, methotrexate and IVIG). Additionally, Markic et al. utilized a similar regimen in a CRIM-positive patient in whom severe IARs resolved after induction of immunomodulation (Table 4) (9,10).
Plasma exchange, rituximab, and IVIG
Plasma exchange is a procedure in which the patient’s blood is passed through an apheresis machine where the filtered plasma, along with its content of antibodies, is removed and discarded with reinfusion of red blood cells along with replacement fluid. The hypothesis is that removal of pathogenic antibodies and immune complexes from the plasma can further reduce damage and potentially reverse the biological mechanisms generating pathology. Deodato et al. assessed plasma exchange together with rituximab in a CRIM-negative IPD patient with severe IARs (11). Prior to this combined treatment, the patient’s severe IARs led to discontinuation of ERT for two months. Though IgE antibodies were absent, the patient’s IgG antibody titers peaked at 25,600 at week 16. After three plasma exchange sessions followed by a single dose of rituximab (375 gm/m2) and IVIG (11), the patient’s IgG antibody titer declined and remained below 100 with complete B cell recovery (Table 3). Contrary to the success observed in this case, in another CRIM-negative patient, albeit one with HSAT, treatment with cyclophosphamide, IVIG, plasmapheresis, rituximab and an increased dose of ERT was not successful in reducing HSAT (35). This approach requires a great deal of caution in such fragile infants with severe cardiomyopathy as it is a high-risk procedure with additional complications (36).
Bortezomib, rituximab, methotrexate, and IVIG
Rituximab is a monoclonal antibody directed against the CD20 molecule expressed on B cells in early to late stages of normal development. However, CD20 is lost when B cells differentiate into antibody-producing plasma cells. Thus, once B cells differentiate into plasma cells, alternative and/or additional agents must be utilized to address HSAT (37). Based on the clear need to eliminate antibody-producing plasma cells, Banugaria et al. added a proteasome inhibitor, bortezomib, which targets plasma cells in an attempt to reduce lethal HSAT and potentially to achieve immune tolerance in Pompe patients. The protocol was initiated in three patients whose highest observed IgG antibody titers were 204,800, 819,200, and 204,800 (Table 3) and in severe clinical decline. After multiple cycles of immunomodulation (3–6 cycles), including bortezomib, all three patients exhibited a significant decline in their IgG antibody titers to 100, 6,400, and 3,200, respectively, accompanied by dramatic clinical improvement. Critically, no serious adverse events or infections related to this therapy were reported in these patients. In a follow-up study, Kazi et al. reported all three patients were maintaining low IgG antibody titers at 0, 1,600, and 800 with full B cell recovery (Table 3) (14). Two of three patients had discontinued immunomodulating agents and were up-to-date on routine vaccinations with adequate humoral responses to diphtheria and tetanus. One patient was still receiving maintenance rituximab and methotrexate, however, frequency of rituximab has been tapered significantly and patient had been off rituximab for 41 weeks at the end of study. The addition of bortezomib to the rituximab, methotrexate and IVIG regimen was the first protocol to successfully eliminate IgG antibodies in patients with HSAT and also to achieve immune tolerance.
Despite the success of Banugaria et al. and Kazi et al. in reversing HSAT with the addition of bortezomib, Owens et al. reported two patients with IPD in whom bortezomib failed to eliminate HSAT and prevent clinical decline (12). Both IPD patients (one CRIM-negative and one CRIM-positive) developed HSAT leading to clinical decline and were initiated on immunomodulation. Despite the reduction in IgG antibody titers with immunomodulation, both patients continued to decline and died. In contrast to Banugaria et al., the failure of bortezomib to rescue these patients can be attributed to administration of a limited number of bortezomib cycles and advanced disease progression at the time of bortezomib initiation. These cases highlight the importance of close and continuous monitoring of IgG antibody titers, the need for multiple cycles of immunomodulation in HSAT settings, and timely initiation of ERT and immunomodulatory therapy prior to rapid progression of irreversible damage.
Bortezomib, rituximab, rapamycin, and IVIG
Poelman et al. reported their experience with a combination of bortezomib, rituximab, rapamycin, and IVIG in three IPD patients, one CRIM-positive and two CRIM-negative (38). One CRIM-negative IPD patient had received immunomodulation with rituximab, methotrexate and IVIG in the ERT-naïve setting (30). At study onset, IgG antibody titers were 156,250, 156,250, and 781,250. Following immunomodulation with the combination therapies, IgG antibody titers declined to 31,250, 31,250, and 156,250 (Table 3). Interestingly, all patients had experienced IARs prior to the immunomodulatory therapy, but these did not persist following immunomodulation. No serious adverse event related to immunomodulation was reported. All three patients had B cell recovery and were alive at conclusion of the study (38). Although the regimen with bortezomib, rituximab, rapamycin, and IVIG was able to reduce IgG antibody titers, it was not successful in eliminating IgG antibodies and inducing immune tolerance to ERT.
High-dose IVIG
IVIG at doses of 0.5 g/kg has been widely used for IgG replacement therapy in various immune therapy regimens to prevent microbial infections in immunocompromised patients or, at higher doses (1–3 g/kg), as an immunomodulator in autoimmune diseases. Rairikar et al described an alternative, novel approach towards immunomodulation with high-dose IVIG alone in a CRIM-negative patient who broke tolerance after rituximab, methotrexate, and IVIG combination therapy (16). Following prophylactic immunomodulation, this patient developed IgG antibodies to ERT with a peak titer of 1,600. However, on initiation of a second round of the combination immunomodulatory regimen, the patient suffered severe diarrhea, Clostridium difficile colitis, respiratory failure, and sepsis, and the treatment was thus discontinued. After 265 weeks of ERT, the patient’s antibody levels increased rapidly, to a peak titer of 204,800. Due to previous adverse events associated with the standard immunomodulation regimen, high-dose IVIG (1 g/kg weekly) was initiated, which lead to a steady decline in antibody titers to 12,800 (16). At this time, this patient continued to receive IVIG every 2–3 months. Although high dose IVIG successfully reduced IgG antibodies, it did not induce immune tolerance to ERT in this patient. Thus, further studies are required to verify the efficacy of high dose IVIG alone or in combination with other immunomodulatory agents.
Omalizumab
Rohrbach et al. reported a CRIM-negative patient who developed anaphylaxis and was switched to a reduced dose at a decreased infusion rate alongside pre-treatment consisting of corticosteroids and anti-histamines. Because anaphylaxis was attributable to an IgE antibody response, omalizumab, a monoclonal antibody to IgE, was added to the pretreatment prophylaxis, due to the inability of conservative premedication measures to control the life-threatening hypersensitivity response. The addition of omalizumab allowed safe ERT administration in this patient who was then weaned off all other treatments except for omalizumab. Interestingly, the patient was not only able to then receive ERT safely without any serious IARs, but also maintained a low IgG antibody titer throughout the course of treatment, with the highest IgG antibody titer of 400. The authors concluded that omalizumab may have curtailed isotype switching, hence acting as an immune modulator in this CRIM-negative patient. However, the underlying mechanism by which omalizumab may affect the development of IgG antibodies is unclear.
Nonclinical studies (Table 5)
Table 5. Overview of preclinical development of immunomodulation strategies.
| Publication | Drug/treatment approach | Method | Results | Implication/potential clinical application |
|---|---|---|---|---|
| Better understanding of FDA-approved agents already tested in IPD patients | ||||
| Shimada et al. 2011 | BTZ | BTZ + NB-DNJ (pharmacological chaperone) administered in patient fibroblasts carrying c.546 C>T pathogenic variant | Improved stability, maturation, cellular trafficking and enzymatic activity in mutant GAA protein (c.546C>T) | In patients carrying c.546C>T pathogenic variant, BTZ may have a therapeutic function beyond its role in immune modulation |
| Joly et al. 2014 | Transient, low-dose MTX | Prophylactic administration of transient, low-dose MTX in single- and three-cycle regimens in Pompe mice | Reduction in antibody formation (single-cycle 78%, three-cycle 71%) | MTX may improve regulatory B cell expression (a novel function), therefore may be an effective alternative to rituximab where it may be inaccessible, or if there is a concern for safety |
| Increased regulatory B cell expression | ||||
| Adoptive transfer of B cells from MTX-treated mice appeared to transfer tolerance to naive hosts | ||||
| Shimada et al. 2014 | BTZ | BTZ + PC administered in patient fibroblasts carrying PC-responsive and PC-unresponsive mutations | 1.3–5.9-fold increase in mutant GAA enzyme activity | BTZ may enhance the activity of mutant GAA proteins in patients carrying pathogenic variants with varying degrees of responsiveness to pharmacological chaperone |
| Antigen targeted approaches | ||||
| Ohashi et al. 2012 | Non-depleting anti-CD3 monoclonal antibodies | Anti-CD3 monoclonal antibodies administered in wild-type and Pompe mice prior to ERT | Greater reduction in antibodies to ERT compared to hamster immunoglobulin-treated group | Anti-CD3+ monoclonal antibodies may be an effective prophylactic immune modulator (compared to IVIG) in patients with IPD |
| Sun et al. 2014 | Non-depleting anti-CD4 monoclonal antibodies | Anti-CD4 monoclonal antibodies administered in Pompe mice that developed high levels of IgG antibodies to ERT | 94.3% decrease in IgG antibodies, compared to 84.5% decrease in MTX-treated group | Anti-CD4+monoclonal antibodies may be an effective immune modulator (compared to methotrexate) in patients with IPD with pre-existing HSAT |
| Doerfler et al. 2015 | BAFF Blockade | Anti-BAFF antibodies administered in Pompe mice prior to ERT | Delayed IgG antibody formation and enhanced GAA activity | BAFF inhibition may eliminate B cells in the transitional phase, preventing response to T cells. This would hypothetically leave pre-existing immunity intact, therefore improving safety of immune modulation in IPD patients |
| Resolved antibody formation during an immune response | ||||
| Plasmablasts prevented from maturing and migrating to bone marrow | ||||
| Lim et al. 2017 | SVP-Rapa | IV injection of (SVP-Rapa) during first three weeks of ERT in GAA-KO mice | Reduced and delayed of IgG antibody formation. This response was more durable than in methotrexate-treated controls | Co-administration of SVP-Rapa and ERT with rhGAA may produce a more durable immunosuppressive effect compared to methotrexate |
| Higher efficacy of glycogen clearance and better motor function | ||||
| Antigen-specific approaches | ||||
| Ohashi et al. 2011 | Oral administration of rhGAA | 5 doses of 16 mg rhGAA administered orally prior to rhGAA injection in Pompe mice | Significant reduction in IgG antibodies to ERT | Cellular and humoral immune responses to rhGAA may be suppressed by prior oral administration of the therapeutic protein |
| Cremel et al. 2015 | Antigen encapsulated RBCs | GAA-encapsulated aged RBCs administered prior to ERT in Pompe mice | Reduced IgG titers by up to 92.7% | Aged RBCs can be used to present rhGAA as a self-antigen to T cells in the liver and spleen, thereby inducing antigen-specific tolerance |
| No reduction in anti-cholera toxin antibodies (response was specific to rhGAA) | ||||
| Su et al. 2015 | CTB-GAA expressed in plant chloroplasts | Prophylactic oral administration of N-terminal 410-amino acids of GAA fused with transmucosal carrier CTB and expressed in tobacco transplastomic lines | Substantially suppressed GAA-specific IgG1 antibody formation (three-fold lower than control group treated with ERT only) | Oral delivery of plant cells may be effective in reducing antibody response to ERT in IPD patients |
| Schneider & Balu-Iyer 2016 | PS-rhGAA | PS-rhGAA administered in Pompe mice prior to ERT | Reduced antibodies to ERT | Phosphatidylserine, when co-displayed with rhGAA by antigen-presenting cells, may convert rhGAA from an immunogenic to a tolerogenic form |
| Titer levels remained unchanged in 4 of 8 PS-rhGAA treated mice upon re-challenge by free rhGAA protein | ||||
| Gene therapy as an immune modulator to ERT (rhGAA) | ||||
| Sun et al. 2007 | AAV-LSPhGAApA | AAV2/8 vector-mediated therapy (liver specific) delivered in subtherapeutic dose prior to ERT in Pompe mice | Prevented immune response, compared to high titer formation in control mice | Liver directed AAV vector-mediated gene therapy may be an effective primary therapy as well as an effective prophylactic immune modulator to rhGAA in Pompe patients treated with ERT |
| Douillard-Guilloux et al. 2009 |
HSC gene transfer | Lentiviral vector-mediated HSC gene transfer by bone marrow transplantation | Achieved immunological unresponsiveness to ERT | Lentiviral vector-mediated HSC gene transfer may provide partial metabolic correction and induce tolerance to ERT in Pompe patients |
| GAA enzyme activity partially restored | ||||
| Sun et al. 2010 | AAV-LSPhGAApA | AAV2/8 vector-mediated therapy (liver specific) delivered in low dose prior to, following, and simultaneously with ERT | Significantly lowered IgG and IgE antibodies to GAA | Liver directed AAV vector-mediated gene therapy may suppress immune response both prophylactically and therapeutically in Pompe patients. Tregs may play a significant role in immune tolerance by gene therapy |
| Reduced hypersensitivity reactions, regardless of administration timing | ||||
| Treg depleted mice had stronger allergic reactions | ||||
| Better mechanistic understanding of immune response to ERT | ||||
| Nayak et al. 2012 | T cell mapping | CD4+ T cell epitopes mapped in GAA-KO mice using ELISpot | Identified 3 CD4+ T cell epitopes contributing to immune response to ERT | Multiple T cell epitopes contribute to CD4+ T cell response to rhGAA during ERT |
| Peptide IFLGPEPKSVVQ predicted to contain dominant epitope deriving CD4+ T cell response | Th2 immunity drives formation of antibodies | |||
| Frequencies of IL-4 producing T cells were highest, suggesting predominantly Th2 cell mediated response | Immune tolerance protocols targeting immunodominant CD4+ T cell epitopes may be effective | |||
BTZ, bortezomib; NB-DNJ, N-butyl-deoxynojirimycin; ERT, enzyme replacement therapy; MTX, methotrexate; PC, pharmacological chaperone; IgG, immunoglobulin G; BAFF, B cell activating factor; RBCs, red blood cells; SVP-Rapa, synthetic vaccine particle carrying rapamycin; rhGAA, recombinant human acid alpha-glucosidase; GAA, acid alpha-glucosidase; CTB, transmucosal carrier cholera toxin B subunit; PS-rhGAA, phosphatidylserine-associated recombinant human acid alpha-glucosidase; AAV, adeno-associated virus; HSC, hematopoietic stem cell; IgE, immunoglobulin E; Treg, regulatory T cell; GAA-KO, acid alpha-glucosidase gene knock out (Pompe disease mouse model); Th2, T helper cell 2.
Immunomodulatory agents implemented successfully in the clinical setting, though antigen targeted, are broadly immune suppressive, clearly indicating the need for antigen-specific approaches to improve the safety of immune tolerance induction (Table 5).
In antigen-specific approaches, the antigen is the bait and only the antigen-relevant lymphocyte populations are the catch. For example, presentation of ERT encapsulated in nanoparticles that enhance presentation of the ERT in a tolerogenic fashion should theoretically induce tolerance to rhGAA alone, even when the immune system is challenged by other antigens. Preclinical antigen specific tolerance studies in mouse models of Pompe disease have included oral tolerance induction strategies (39,40), encapsulation of GAA in aged red blood cells (41), phosphatidylserine-associated rhGAA (42), and gene therapy(43-47) (Table 5).
In antigen-targeted approaches, lymphocyte populations are broadly targeted, but the relevant lymphocyte populations pertaining to specific antigens are targeted by the close proximity of the tolerizing therapeutic in time and location with the target antigens and their antigen reactive lymphocytes. For example, co-administration of rapamycin and ERT nanoparticles may induce tolerance via the close proximity of the ERT nanoparticles, ERT-specific lymphocytes and rapamycin in the liver or lymph node. However, this strategy may incidentally target and thus tolerize cells mediating immune responses to other antigens for which tolerance would not be desirable (e.g., tumor neoantigens). Published literature has demonstrated promising results in such approaches, including the use of non-depleting anti-CD3 and anti-CD4 monoclonal antibodies (3,4), inhibition of B cell activating factors (BAFF) (2), and synthetic vaccine particles carrying rapamycin (SVP-Rapa) (Table 5) (48).
Other preclinical studies have further investigated the mechanisms of immunomodulatory agents such as methotrexate and bortezomib, which have been administered clinically in patients with Pompe disease (Table 5). Findings from these studies can potentially help refine current treatment algorithms and combination drug protocols. Furthermore, mechanistic in vitro and in silico studies of immune response in Pompe disease can reveal potential targets for future immunomodulatory therapies (Table 5). For example, it has been shown that regulatory T cell epitopes (Tregitopes) play an important role in immune tolerance induction using liver-specific vector-mediated gene therapy in Pompe mice (45), but the specific mechanisms of T cell response remain largely unstudied. Nayak et al. identified three CD4+ T cell epitopes contributing to Th2 immunity in Pompe mice, driving the formation of antibodies to ERT (49). Therapeutic approaches targeting these epitopes may further improve the specificity and efficacy of immune modulation in patients with Pompe disease.
A better understanding of agents currently used in immunomodulation
Transient, low-dose methotrexate
Joly et al. compared two low-dose methotrexate regimens (single cycle vs. three cycles) in Pompe mice treated with rhGAA, which yielded similar reduction in antibodies to ERT (78% and 71%, respectively) (5). These mice generated normal antibody responses upon subsequent challenge with an irrelevant antigen (antithymocyte globulin). The mechanism of tolerance appeared to be induction of regulatory B cells rather than cell depletion, confirmed by increased numbers of IL-10 and TGF-β secreting B cells, and adoptive transfer of tolerance from methotrexate-tolerized mice to naïve hosts via splenic B cells. Because of the heightened infection risk associated with rituximab (relative to that of low dose methotrexate) and its often limited availability, low dose methotrexate would hence be an attractive alternative to rituximab. However, further studies are still needed to verify the consistency and magnitude of these effects.
Bortezomib in addition to chaperone therapy
Shimada et al. 2011 reported that Bortezomib given alongside a pharmacological chaperone NB-DNJ [to rescue folding, trafficking, and activity of the mutated GAA protein (50)] improved the function of mutant GAA protein in patient fibroblasts carrying GAA pathogenic variant c.546C>T (51). The proposed mechanism of action is that, as a proteasome inhibitor, bortezomib effectively suppresses the degradation of misfolded proteins by ERAD through the ubiquitin-proteasome degradation system, thus enhancing interaction between GAA mutant protein and molecular chaperones. The same group showed a response to Bortezomib in GAA proteins from patient fibroblasts carrying several other pathogenic variants (52). These findings suggest that alongside its immunomodulatory function, bortezomib, together with therapeutics that address protein folding and stability, can potentially enhance enzyme activity in CRIM positive IPD patients with certain pathogenic variants.
Antigen targeted approaches in preclinical studies
Non-depleting anti-CD3+ and anti-CD4+ monoclonal antibodies
Ohashi et al. 2012 prophylactically administered non-depleting anti-CD3 monoclonal antibodies, resulting in significantly reduced IgG antibodies in wild-type and Pompe mice, compared to high antibody formation in control mice treated with hamster immunoglobulins and ERT (3). Importantly, this effect was also seen in mice that had already mounted antibodies to ERT (therapeutic setting). Additionally, Sun et al. 2014 demonstrated that in Pompe mice with high levels of ERT specific IgG antibody titers, treatment with 3 doses of non-depleting anti-CD4 monoclonal antibody yielded a significant decrease (94.3%) in antibody levels (4). This reduction was greater than that seen in GAA-KO mice treated with methotrexate (84.5%), suggesting improved efficacy compared to a clinically available immunomodulatory agent.
BAFF inhibition
Doerfler et al. showed that prophylactic administration of anti-B cell activating factor (BAFF) antibody delayed and reduced anti-rhGAA antibody formation, and enhanced GAA activity in Pompe mice (2). Additionally, a high proportion of splenic B-cells were blocked at the transitional stage of development, suggesting intervention at a crucial checkpoint to immune competence. While maintenance anti-BAFF treatment was required for prolonged beneficial effects, plasmablasts were prevented from maturing and migrating to the bone marrow, thereby avoiding the formation of longer-lasting, plasma cell driven immunity.
Synthetic vaccine particles carrying rapamycin (SVP-Rapa)
In a recent pilot study, Lim et al. showed that IV injection of synthetic vaccine particles carrying rapamycin (SVP-Rapa) during the first 3 weeks of ERT in GAA-KO mice resulted in the inhibition of antibody formation (48). Compared to methotrexate treatment, SVP-Rapa was more durable and resulted in improved glycogen clearance and motor function. However, antibody formation did occur in a subset of animals at 12 weeks on ERT (compared to antibody formation at 6 weeks on ERT methotrexate-treated mice). In this model, SVP delivers rapamycin to antigen-presenting cells, limiting systemic exposure and enhancing its uptake. Results of this study suggest that SVP-Rapa may have a more durable immunosuppressive effective than methotrexate, although additional preclinical studies are needed to further confirm and characterize these results.
Targeting antigen-presenting pathways to induce antigen-specific tolerance
Antigen-specific approaches aim to convert immunogenic antigen presenting cells to tolerogenic antigen presenting cells, reducing immune reaction while fully preserving immune response to irrelevant antigens.
Ohashi and colleagues administered rhGAA orally in several doses to Pompe mice prior to rhGAA injection (39). This resulted in a significant reduction in IgG antibodies, suggesting that oral tolerance induction may be able to suppress the cellular and humoral immune response to rhGAA when administered prophylactically. A related approach was taken up by Su and colleagues: oral administration of N-terminal 410-amino acids of GAA, fused with transmucosal carrier CTB and expressed in transplastomic lines of plant chloroplast, substantially suppressed GAA-specific IgG1 antibody formation in Pompe mice (40). The level of antibody formation in GAA-CTB + ERT treated mice was three-fold lower than a control group treated with ERT only. It is hypothesized that oral delivery of antigen-encapsulated plant cells can safely display GAA as an auto-antigen prior to ERT initiation, thereby inducing antigen-specific tolerance. Importantly, the researchers emphasize that the truncated GAA incorporated into plant chloroplasts should include relevant CD4+ T-cell epitopes shown to play a role in B-cell activation and the formation of antibodies to ERT in Pompe (49).
Cremel and colleagues investigated the use of GAA-encapsulated red blood cells (RBCs) when injected in Pompe mice (41). Aged RBCs are physiologically removed from the spleen and liver by antigen-presenting cells and are presented in a tolerogenic manner as self-antigens to T cells. Administration of these antigen encapsulated RBCs in three doses, followed by ERT, reduced anti-GAA IgG titers up to 92.7% (53). Upon subsequent challenge by cholera toxin (CT), no reduction in anti-CT antibodies was observed, indicating that the GAA-encapsulated RBCs were able to specifically target rhGAA.
In a different approach, Schneider and Balu-Iyer aimed to utilize phosphatidylserine (PS) associated with rhGAA to induce rhGAA-specific tolerance in Pompe mice (42). First, free rhGAA protein was shown to associate with PS liposomes (PS-rhGAA) upon incubation by intercalating within its phospholipid bilayer. Then, mice were treated with PS-rhGAA and subsequently re-challenged with free rhGAA protein to determine any change in antibody response. Compared to control groups treated with phosphatidylglycerol (PG)-rhGAA and free rhGAA only, PS-rhGAA mice produced fewer antibody titers to ERT. Importantly, in four of eight PS-rhGAA treated mice, titer levels remained unchanged upon re-challenge, suggesting that tolerance was successfully induced in these animals.
Taken together, results from these innovative antigen-specific methods should be further investigated in the pre-clinical setting in order to (I) verify whether the tolerance induced and/or immunosuppressive effect produced is truly specific to the rhGAA protein, (II) compare their safety and efficacy to currently, clinically used immunomodulatory agents, and (III) evaluate their potential clinical application.
Gene therapy to induce immune tolerance to ERT
AAV vector-mediated gene therapy is a promising new treatment strategy on the horizon for patients with Pompe disease. Beyond its importance as a novel treatment for the primary manifestations of the disease, experiments in GAA KO mice have demonstrated liver-targeted AAV2/8 vector-mediated gene therapy as a viable strategy for inducing immune tolerance to rhGAA (43-46). This method expresses GAA exclusively in the liver and activates regulatory T cells (Tregs), which have been shown to be critical for tolerance induction to rhGAA in preclinical experiments. Immune tolerance to ERT has also been demonstrated in experiments using self-inactivated-lentiviral vector-mediated hematopoietic stem cell gene transfer by bone marrow transplantation, although glycogen clearance was only partially reduced (47). The therapeutic aim of this method is to systemically increase GAA enzyme activity by its secretion from HSCs and distribution through the bloodstream.
Critical issues and future directions in basic and clinical research
Various immunomodulation strategies, both prophylactic and therapeutic, have been employed to induce tolerance to ERT in patients with Pompe disease. Such immunomodulatory approaches have not only prevented or reduced the deleterious effects of IgG antidrug antibodies but have also alleviated events such as hypersensitivity reactions associated with ongoing therapy. Prophylactic administration has been associated with a favorable safety profile due to the short course of immunomodulation. In particular, the regimen described by Kazi et al. has shown the most success in achieving long-term immune tolerance, without long-term toxicity pertaining to the period of immune suppression or any delay in ERT initiation (6). Patients so treated have exhibited immune tolerance as evidenced by absent/minimal IgG antibody response with complete B cell recovery, ability to mount a humoral response to other antigens such as vaccines, and without the need of ongoing immune therapy. Evidence shows that immune tolerance can also be achieved in the entrenched setting, yet this requires a longer duration and higher intensity of immunosuppressive therapy. It is clear that the prophylactic approach is preferable as it allows the patient with Pompe disease to safely receive ERT and leads to much better clinical outcomes. Further studies are warranted to assess the long-term safety of these approaches, especially in regimens recommending the long-term use of rituximab in patients with Pompe disease (30).
Based on lessons learned from published literature in the field, the general success of current immunomodulation approaches is limited by a number of factors yet to be resolved. Critically, there remains a need for (I) novel approaches to immune tolerance induction, especially those that are antigen-specific and/or more highly antigen targeted, (II) a better understanding of the specific mechanisms and dynamics of the immune response to ERT, (III) more personalized treatment approaches including immunogenicity prediction prior to ERT initiation, especially in CRIM-positive IPD and LOPD patients, (IV) standardized biomarkers and clinical endpoints to quantify and compare response to therapy in patients across the Pompe disease spectrum, especially in LOPD, and (V) next-generation therapeutics with improved muscle targeting, with considerations for immunogenicity in mind. Many of these challenges continue to be addressed in recent ongoing research.
The efficacy of currently available immunomodulation strategies is also limited by our incomplete understanding of how immune response to rhGAA is specifically generated in patients with Pompe disease. Better characterization of activation pathways and cell types that are involved in the immune response is needed, as well as the progression of such responses to generation of neutralizing antibodies that inhibit the uptake and/or catalytic activity of ERT. However, patients with high and sustained IgG antibody titers can have suboptimal response to ERT even in the absence of neutralizing antibodies (21,54) based on uptake into FcR expressing cells rather than uptake by muscle cells . Further studies in these areas, as well as the characterization of other targets such as T cell epitopes and regulatory B cells, will help refine and improve the efficacy and safety of future approaches.
CRIM-positive IPD patients exhibit widely varied immune responses to ERT, and there is currently no method to accurately predict such responses prior to ERT initiation. It has been recently hypothesized that the development of ADAs is driven by certain sequences in the infused rhGAA protein that are recognized by the immune system as foreign, and may be based on the HLA haplotype of the patient. A prediction tool, “individualized T cell epitope measure (iTEM)”, has been developed, which compares the patient’s native GAA protein, described by HLA haplotype and GAA pathogenic variants, to the specific T cell epitope content of the rhGAA sequence (55). The availability of prediction tools like iTEM may allow clinicians to identify CRIM-positive IPD and LOPD patients at high risk for HSAT/SIT development and initiate immunomodulation accordingly, while immunomodulation may be avoided in low-risk patients.
With the inclusion of Pompe disease in newborn screening (NBS), patients with LOPD are being identified and monitored, leading to the emergence of a new phenotypic spectrum. Drastically varying presenting features and clinical outcomes in patients with IPD and LOPD can make the clinical impact of antidrug antibodies difficult to quantify and compare across multiple patient cohorts. Additionally, currently published studies in immunomodulation utilize clinical endpoints that differ from one another, resulting in difficulty comparing the efficacy of various immunomodulation strategies. For these reasons, standardized clinical endpoints are needed to assess the deleterious effect of ADAs across the disease spectrum.
While patients with Pompe disease are living longer and can expect much better prognoses compared to just a decade ago, there are still many aspects of the disease yet to be explored. For example, in long-term survivors of IPD, it has become apparent that white matter lesions in the CNS develop over time. This was previously unseen due to early fatality during the pre-ERT era. Thus, penetration of the CNS by ERT therapeutics will likely become a prominent challenge for optimization of treatment. With the addition of Pompe disease in NBS, as well as the development of gene therapy and second-generation therapeutic proteins on the horizon, lessons learned from the various immunomodulation approaches to date will help further improve therapeutic efficacy and general treatment outcome in individuals living with Pompe disease.
Acknowledgments
None.
Footnotes
Conflicts of Interest: AK Desai has received grant support from Sanofi Genzyme and the Lysosomal Disease Network. C Li and AS Rosenberg have no financial or proprietary interest in the material presented herein. PS Kishnani has received grant support from Sanofi Genzyme, Valerion Therapeutics, Shire Pharmaceuticals, and Amicus Therapeutics. PS Kishnani has received consulting fees and honoraria from Sanofi Genzyme, Shire Pharmaceuticals, Amicus Therapeutics, Vertex Pharmaceuticals, and Asklepios BioPharmaceutical, Inc. (AskBio). PS Kishnani is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme. PS Kishnani has equity in AskBio, which is developing gene therapy for Pompe disease.
References
- 1.Mendelsohn NJ, Messinger YH, Rosenberg AS, et al. Elimination of antibodies to recombinant enzyme in Pompe's disease. N Engl J Med 2009;360:194-5. 10.1056/NEJMc0806809 [DOI] [PubMed] [Google Scholar]
- 2.Doerfler PA, Nayak S, Herzog RW, et al. BAFF blockade prevents anti-drug antibody formation in a mouse model of Pompe disease. Clin Immunol 2015;158:140-7. 10.1016/j.clim.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohashi T, Iizuka S, Shimada Y, et al. Administration of anti-CD3 antibodies modulates the immune response to an infusion of alpha-glucosidase in mice. Mol Ther 2012;20:1924-31. 10.1038/mt.2012.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun B, Banugaria SG, Prater SN, et al. Non-depleting anti-CD4 monoclonal antibody induces immune tolerance to ERT in a murine model of Pompe disease. Mol Genet Metab Rep 2014;1:446-50. 10.1016/j.ymgmr.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly MS, Martin RP, Mitra-Kaushik S, et al. Transient low-dose methotrexate generates B regulatory cells that mediate antigen-specific tolerance to alglucosidase alfa. J Immunol 2014;193:3947-58. 10.4049/jimmunol.1303326 [DOI] [PubMed] [Google Scholar]
- 6.Kazi ZB, Desai AK, Berrier KL, et al. Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease. JCI Insight 2017. doi: . 10.1172/jci.insight.94328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazi ZB, Desai AK, Troxler RB, et al. An immune tolerance approach using transient low-dose methotrexate in the ERT-naive setting of patients treated with a therapeutic protein: experience in infantile-onset Pompe disease. Genet Med 2019;21:887-95. 10.1038/s41436-018-0270-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messinger YH, Mendelsohn NJ, Rhead W, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med 2012;14:135-42. 10.1038/gim.2011.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markic J, Polic B, Kuzmanic-Samija R, et al. Immune Modulation Therapy in a CRIM-Positive and IgG Antibody-Positive Infant with Pompe Disease Treated with Alglucosidase Alfa: A Case Report. JIMD Rep 2012;2:11-5. 10.1007/8904_2011_34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markic J, Polic B, Stricevic L, et al. Effects of immune modulation therapy in the first Croatian infant diagnosed with Pompe disease: a 3-year follow-up study. Wien Klin Wochenschr 2014;126:133-7. 10.1007/s00508-013-0475-3 [DOI] [PubMed] [Google Scholar]
- 11.Deodato F, Ginocchio VM, Onofri A, et al. Immune tolerance induced using plasma exchange and rituximab in an infantile Pompe disease patient. J Child Neurol 2014;29:850-4. 10.1177/0883073813485819 [DOI] [PubMed] [Google Scholar]
- 12.Owens P, Wong M, Bhattacharya K, et al. Infantile-onset Pompe disease: A case series highlighting early clinical features, spectrum of disease severity and treatment response. J Paediatr Child Health 2018;54:1255-61. 10.1111/jpc.14070 [DOI] [PubMed] [Google Scholar]
- 13.Banugaria SG, Prater SN, McGann JK, et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med 2013;15:123-31. 10.1038/gim.2012.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazi ZB, Prater SN, Kobori JA, et al. Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses. JCI Insight 2016. doi: . 10.1172/jci.insight.86821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenger EO, Kazi Z, Lisi E, et al. Immune Tolerance Strategies in Siblings with Infantile Pompe Disease-Advantages for a Preemptive Approach to High-Sustained Antibody Titers. Mol Genet Metab Rep 2015;4:30-4. 10.1016/j.ymgmr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rairikar M, Kazi ZB, Desai A, et al. High dose IVIG successfully reduces high rhGAA IgG antibody titers in a CRIM-negative infantile Pompe disease patient. Mol Genet Metab 2017;122:76-9. 10.1016/j.ymgme.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishnani PS, Nicolino M, Voit T, et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2006;149:89-97. 10.1016/j.jpeds.2006.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med 2001;3:132-8. [DOI] [PubMed] [Google Scholar]
- 19.Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26-33. 10.1016/j.ymgme.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai AK, Kazi ZB, Kishnani PS. Cross-reactive immunologic material positive infantile pompe disease: Characterization of immune responses in patient treated with enzyme replacement therapy. Mol Genet Metab 2016;117:S41 10.1016/j.ymgme.2015.12.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banugaria SG, Prater SN, Ng YK, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med 2011;13:729-36. 10.1097/GIM.0b013e3182174703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries JM, Kuperus E, Hoogeveen-Westerveld M, et al. Pompe disease in adulthood: effects of antibody formation on enzyme replacement therapy. Genet Med 2017;19:90-7. 10.1038/gim.2016.70 [DOI] [PubMed] [Google Scholar]
- 23.de Vries JM, van der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab 2010;101:338-45. 10.1016/j.ymgme.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 24.van Gelder CM, Poelman E, Plug I, et al. Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe disease: an open-label single-center study. J Inherit Metab Dis 2016;39:383-90. 10.1007/s10545-015-9912-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel TT, Banugaria SG, Case LE, et al. The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol Genet Metab 2012;106:301-9. 10.1016/j.ymgme.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 26.Masat E, Laforet P, De Antonio M, et al. Long-term exposure to Myozyme results in a decrease of anti-drug antibodies in late-onset Pompe disease patients. Sci Rep 2016;6:36182. 10.1038/srep36182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filosto M, Cotti Piccinelli S, Ravaglia S, et al. Assessing the Role of Anti rh-GAA in Modulating Response to ERT in a Late-Onset Pompe Disease Cohort from the Italian GSDII Study Group. Adv Ther 2019;36:1177-89. 10.1007/s12325-019-00926-5 [DOI] [PubMed] [Google Scholar]
- 28.Banugaria SG, Prater SN, Patel TT, et al. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: a step towards improving the efficacy of ERT. PLoS One 2013;8:e67052. 10.1371/journal.pone.0067052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broomfield A, Fletcher J, Davison J, et al. Response of 33 UK patients with infantile-onset Pompe disease to enzyme replacement therapy. J Inherit Metab Dis 2016;39:261-71. 10.1007/s10545-015-9898-5 [DOI] [PubMed] [Google Scholar]
- 30.Poelman E, Hoogeveen-Westerveld M, Kroos-de Haan MA, et al. High Sustained Antibody Titers in Patients with Classic Infantile Pompe Disease Following Immunomodulation at Start of Enzyme Replacement Therapy. J Pediatr 2018;195:236-43.e3. 10.1016/j.jpeds.2017.11.046 [DOI] [PubMed] [Google Scholar]
- 31.Elder ME, Nayak S, Collins SW, et al. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2013;163:847-54.e1. 10.1016/j.jpeds.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CF, Yang CC, Liao HC, et al. Very Early Treatment for Infantile-Onset Pompe Disease Contributes to Better Outcomes. J Pediatr 2016;169:174-80.e1. 10.1016/j.jpeds.2015.10.078 [DOI] [PubMed] [Google Scholar]
- 33.Chien YH, Lee NC, Chen CA, et al. Long-term prognosis of patients with infantile-onset Pompe disease diagnosed by newborn screening and treated since birth. J Pediatr 2015;166:985-91.e1-2. [DOI] [PubMed]
- 34.Joseph A, Munroe K, Housman M, et al. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol 2008;152:138-46. 10.1111/j.1365-2249.2008.03602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banugaria SG, Patel TT, Mackey J, et al. Persistence of high sustained antibodies to enzyme replacement therapy despite extensive immunomodulatory therapy in an infant with Pompe disease: need for agents to target antibody-secreting plasma cells. Mol Genet Metab 2012;105:677-80. 10.1016/j.ymgme.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokrzycki MH, Balogun RA. Therapeutic apheresis: a review of complications and recommendations for prevention and management. J Clin Apher 2011;26:243-8. 10.1002/jca.20303 [DOI] [PubMed] [Google Scholar]
- 37.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol 2011;24:203-16. 10.1016/j.beha.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poelman E, Hoogeveen-Westerveld M, van den Hout JMP, et al. Effects of immunomodulation in classic infantile Pompe patients with high antibody titers. Orphanet J Rare Dis 2019;14:71. 10.1186/s13023-019-1039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohashi T, Iizuka S, Shimada Y, et al. Oral administration of recombinant human acid alpha-glucosidase reduces specific antibody formation against enzyme in mouse. Mol Genet Metab 2011;103:98-100. 10.1016/j.ymgme.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 40.Su J, Sherman A, Doerfler PA, et al. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol J 2015;13:1023-32. 10.1111/pbi.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cremel M, Guerin N, Campello G, et al. Innovative approach in Pompe disease therapy: Induction of immune tolerance by antigen-encapsulated red blood cells. Int J Pharm 2015;491:69-77. 10.1016/j.ijpharm.2015.05.062 [DOI] [PubMed] [Google Scholar]
- 42.Schneider JL, Balu-Iyer SV. Phosphatidylserine Converts Immunogenic Recombinant Human Acid Alpha-Glucosidase to a Tolerogenic Form in a Mouse Model of Pompe Disease. J Pharm Sci 2016;105:3097-104. 10.1016/j.xphs.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun B, Bird A, Young SP, et al. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet 2007;81:1042-9. 10.1086/522236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koeberl DD, Kishnani PS. Immunomodulatory gene therapy in lysosomal storage disorders. Curr Gene Ther 2009;9:503-10. 10.2174/156652309790031094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun B, Kulis MD, Young SP, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther 2010;18:353-60. 10.1038/mt.2009.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bond JE, Kishnani PS, Koeberl DD. Immunomodulatory, liver depot gene therapy for Pompe disease. Cell Immunol 2017. [Epub ahead of print]. 10.1016/j.cellimm.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douillard-Guilloux G, Richard E, Batista L, et al. Partial phenotypic correction and immune tolerance induction to enzyme replacement therapy after hematopoietic stem cell gene transfer of alpha-glucosidase in Pompe disease. J Gene Med 2009;11:279-87. 10.1002/jgm.1305 [DOI] [PubMed] [Google Scholar]
- 48.Lim HH, Yi H, Kishimoto TK, et al. A pilot study on using rapamycin-carrying synthetic vaccine particles (SVP) in conjunction with enzyme replacement therapy to induce immune tolerance in Pompe disease. Mol Genet Metab Rep 2017;13:18-22. 10.1016/j.ymgmr.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nayak S, Sivakumar R, Cao O, et al. Mapping the T helper cell response to acid alpha-glucosidase in Pompe mice. Mol Genet Metab 2012;106:189-95. 10.1016/j.ymgme.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okumiya T, Kroos MA, Vliet LV, et al. Chemical chaperones improve transport and enhance stability of mutant alpha-glucosidases in glycogen storage disease type II. Mol Genet Metab 2007;90:49-57. 10.1016/j.ymgme.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 51.Shimada Y, Nishida H, Nishiyama Y, et al. Proteasome inhibitors improve the function of mutant lysosomal alpha-glucosidase in fibroblasts from Pompe disease patient carrying c.546G>T mutation. Biochem Biophys Res Commun 2011;415:274-8. 10.1016/j.bbrc.2011.10.038 [DOI] [PubMed] [Google Scholar]
- 52.Shimada Y, Nishimura E, Hoshina H, et al. Proteasome Inhibitor Bortezomib Enhances the Activity of Multiple Mutant Forms of Lysosomal alpha-Glucosidase in Pompe Disease. JIMD Rep 2015;18:33-9. 10.1007/8904_2014_345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ing R, Segura M, Thawani N, et al. Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J Immunol 2006;176:441-50. 10.4049/jimmunol.176.1.441 [DOI] [PubMed] [Google Scholar]
- 54.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007;68:99-109. 10.1212/01.wnl.0000251268.41188.04 [DOI] [PubMed] [Google Scholar]
- 55.De Groot AS, Kazi ZB, Martin RF, et al. HLA- and genotype-based risk assessment model to identify infantile onset pompe disease patients at high-risk of developing significant anti-drug antibodies (ADA). Clin Immunol 2019;200:66-70. 10.1016/j.clim.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]