Abstract
The formation of o-quinones from direct 2-electron oxidation of catechols and/or two successive one electron oxidations could explain the cytotoxic/genotoxic and/or chemopreventive effects of several phenolic botanical extracts. For example, poison ivy contains urushiol, an oily mixture, which is oxidized to various o-quinones likely resulting in skin toxicity through oxidative stress and alkylation mechanisms resulting in immune responses. Green tea contains catechins which are directly oxidized to o-quinones by various oxidative enzymes. Alternatively, phenolic botanicals could be o-hydroxylated by P450 to form catechols in vivo which are oxidized to o-quinones. Examples include, resveratrol which is oxidized to piceatannol and further oxidized to the o-quinone. Finally, botanical o-quinones can be formed by O-dealkylation of O-alkoxy groups or methylenedioxy rings resulting in catechols which are further oxidized to o-quinones. Examples include safrole, eugenol, podophyllotoxin and etoposide, as well as methysticin. Once formed these o-quinones have a variety of biological targets in vivo resulting in various biological effects ranging from chemoprevention - > no effect - > toxicity. This U-shaped biological effect curve has been described for a number of reactive intermediates including o-quinones. The current review summarizes the latest data on the formation and biological targets of botanical o-quinones.
Keywords: Quinones, P450, Bioactivation, Botanicals, Chemoprevention, Carcinogen
1. Introduction
o-Quinones are reactive metabolites of a variety of catechol natural products that could be responsible for the cytotoxic/genotoxic and chemopreventive effects of the parent catechols (Bolton, 2002; Dietz et al., 2016). They are electrophilic species that are often not detected, since they rapidly react with a variety of nucleophiles via non-enzymatic Michael addition (Fig. 1). o-Quinone formation can be inferred by trapping them with reactive thiol nucleophiles, such as GSH.
Fig. 1.
Formation of o-quinones through two and one electron oxidation mechanisms. Reaction with GSH and generation of ROS.
There are three major pathways by which these intermediates are formed in vivo; direct two electron enzymatic oxidation or two successive one electron chemical oxidations of the parent catechol (Fig. 1), aromatic hydroxylation followed by catechol oxidation (Fig. 2), and O-dealkylation and oxidation of the resulting catechol (Fig. 3). The successive removal of two electrons (or alternatively, an electron and a hydrogen atom) from catechols is usually catalyzed by cytochromes P450 (P450) or other oxidative enzymes, such as peroxidases (Bolton and Dunlap, 2017). Chemical autoxidation can occur generating semiquinone radical and ultimately the quinone. Reactive oxygen species (ROS) are formed during this reaction. Alternatively, phenols can be first hydroxylated at the o-position forming catechols catalyzed by P450 followed by oxidation to o-quinones as described above (Fig. 2). Finally, o-methoxyphenols and methylenedioxy compounds can undergo O-dealkylation reactions catalyzed by P450 resulting in a catechol that is subsequently oxidized to the o-quinone (Fig. 3).
Fig. 2.

O-Hydroxylation of phenols and oxidation to o-quinone.
Fig. 3.

O-Dealkylation of o-alkoxyphenols and methylenedioxy rings followed by o-quinone formation.
Once formed o-quinones have a variety of biological targets (Fig. 4) (Aggarwal and Shishodia, 2006; Bolton and Dunlap, 2017; Pierce et al., 2016). Initial reaction with GSH the major non-protein thiol leads to GSH depletion either through direct alkylation and/or through oxidation generating GSSG (Fig. 4) (Ishii et al., 2009). Once GSH is depleted reaction with cysteine residues in proteins such as heat shock protein (HSP), protein disulfide isomerase (PDI), and binding immunoglobin protein (BiP) occurs (Liu and Sok, 2003). Alkylation/oxidation of cysteine residues on key proteins can lead to o-quinone signaling. For example, a major protein target of o-quinones is Keap1 leading to induction of detoxification enzymes including NAD(P)H-quinone oxidoreductase 1 (NQO1), glutathione-S-transferase (GST), and heme oxygenase-1 (HO-1) (Pierce et al., 2016). Alkylation/oxidation of IκB kinase (IKK) results in modulation of NF-κB and inhibition of inflammatory pathways (Bolton and Dunlap, 2017). Finally, some o-quinones form DNA adducts and oxidized DNA bases which could lead to genotoxic effects (Bolton and Dunlap, 2017).
Fig. 4.

Biological targets of o-quinones.
The various targets of o-quinones lead to biological activities, including detoxification, chemoprevention, and toxicity. o-Quinones can deplete GSH causing oxidative stress or directly alkylate proteins (PSH) to elicit chemopreventive activity (e.g. Keap1 and IKK) or stress response (e.g. HSP, PDI, BiP, GST-P1). The toxic effects from o-quinones arise from DNA adducts/oxidation which leads to mutagenesis and P450 oxidative enzyme inhibition or from alkylation of key proteins that can lead to toxicity, such as liver toxicity in the case of kava lactones.
The focus of this mini-review is on botanicals and their bioactive compounds which generate reactive o-quinones either directly or after enzyme catalyzed bioactivation mechanisms. Several examples of natural products whose biological effects could be attributed to o-quinone formation are given. The resulting biological targets of o-quinones are predicted (Fig. 4) (Aggarwal and Shishodia, 2006).
2. Two electron oxidation of catechols
Catechols are very readily oxidized to o-quinones catalyzed by virtually any oxidative enzyme, metal ion, or in some cases molecular oxygen. A semi-quinone radical is initially formed which is readily converted to the o-quinone. This process is characterized by random oxidative damage generating ROS and causing oxidative stress within cells. However, depending on the prooxidant/antioxidant balance within cells, the catechols can quench ROS protecting cells from oxidative stress.
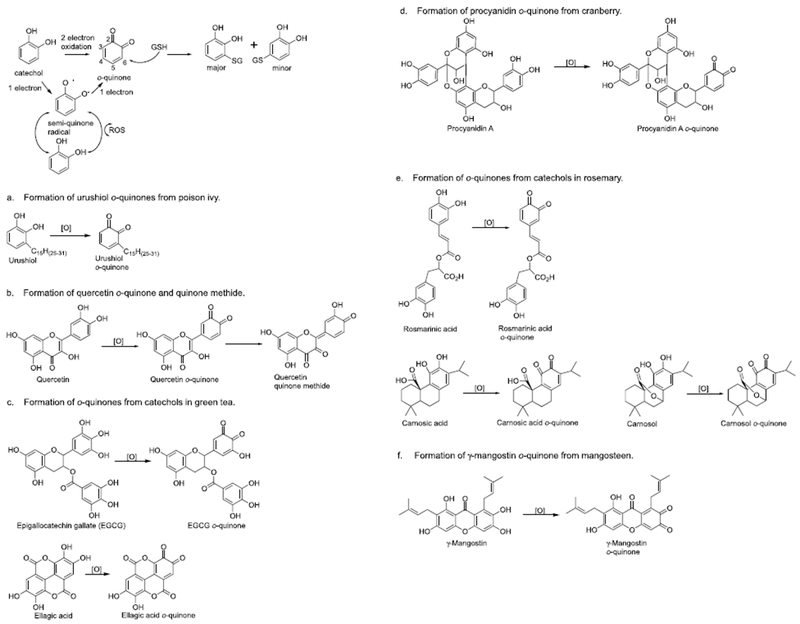
2.1. Urushiol (poison ivy, Toxicodendron radicans)
Urushiol, an oily mixture of catechols with an alkyl side chain, are present in poison ivy and poison oak (Fig. 1a). Brushing up against these plants results in skin toxicity leading to an itchy rash (Hershko et al., 2005). It is likely that oxidation of urushiols to o-quinones followed by depletion of cellular GSH in skin cells and reaction with cysteine residues on proteins leads to skin toxicity (Dunn et al., 1982; Liberato et al., 1981). The allergic contact dermatitis induced by urushiols is known to be mediated by T lymphocytes that recognize urushiol bound to proteins (Dunn et al., 1982). Urushiol o-quinones are likely also responsible for the DNA fragmentation observed in ovarian cancer cells treated with urushiol leading to induction of apoptosis (Choi et al., 2001).
2.2. Quercetin (ubiquitous)
Quercetin occurs in a variety of brightly colored plants including capers, onions, cranberries, and blueberries (Fig. 1b) (Rietjens et al., 2005). It has been demonstrated in several bacterial and mammalian mutagenicity experiments that quercetin has mutagenic properties that could be related to quinoid formation (Brown, 1980; MacGregor and Jurd, 1978). Quercetin is initially oxidized to an o-quinone that rapidly isomerizes to di-quinone methides which could also be called extended quinones (Boersma et al., 2000; Rietjens et al., 2005). These di-quinone methides can be trapped with GSH, although the GSH conjugates are unstable and equilibrate over time producing an isomeric mixture of both quinone and quinone methide GSH conjugates. Protein and DNA adducts have also been observed in Caco-2 and HepG2 cells exposed to 14C-labelled quercetin although these adducts were also unstable (Walle et al., 2003). The transient nature of the quercetin quinoid adducts may partially explain that extrapolating quercetin genotoxicity in vitro to carcinogenicity in vivo is problematic (van der Woude et al., 2005). It has also been shown that quercetin has anti-inflammatory, anti-proliferative, and anti-atherosclerotic effects in human in vivo and in vitro models (Kleemann et al., 2011). For example, quercetin and/or its quinoids inhibit the IKK/NF-κB signaling pathway leading to decreased inflammation and enhanced apoptosis in colon cancer cells (Peng et al., 2017; Zhang et al., 2015). Quercetin quinoids likely are responsible for the reduced levels of Keap1 protein, which increases Nrf2 and Nrf2 dependent antioxidant response element (ARE) activity leading to induction of several detoxification enzymes (Tanigawa et al., 2007). Finally, it has been shown that the prooxidant effect of flavonoids such as quercetin leads to ARE induction, which was inversely dependent on GSH concentration (Lee-Hilz et al., 2006).
2.3. Catechin (green tea, Camellia sinensis)
Extracts of green tea are reported to have anti-cancer, anti-atherosclerotic, anti-diabetic, anti-bacterial, anti-viral, and anti-obesity effects (Cabrera et al., 2006; Poddar et al., 2011; Singh et al., 2011; Suzuki et al., 2012). Most if not all of the beneficial health effects of green tea are probably due to catechins such as catechol (—)-epigallocatechin gallate (EGCG) (Singh et al., 2011) (Fig. 1c). They are likely responsible for the antioxidant, anti-inflammatory, and chemopreventive properties of green tea. Catechins can be oxidized by tyrosinase, peroxidase, and P450 to an o-quinone which forms a variety of conjugates with GSH (Moridani et al., 2001). It has been shown that EGCG covalently modifies proteins through initial autoxidation to the o-quinone and reaction with cysteine residues (Ishii et al., 2008). These thiol protein adducts especially on Keap1 could influence Keap1-Nrf2 signaling and stimulate detoxification pathways through induction of HO-1, NQO1, and GST (Aggarwal and Shishodia, 2006; Sriram et al., 2009; Wang et al., 2015). Due to its ability to scavenge ROS, antioxidative activities have also been described for ellagic acid (Fig. 1c) (Rios et al., 2018). During this process ellagic acid is oxidized to the o-quinone. Similar to EGCG, other cytoprotective activities including anti-inflammatory properties have been depicted for ellagic acid.
2.4. Procyanidin (cranberry, Vaccinium macrocarpon)
Procyanidins are bioactive compounds present in cranberry (Fig. 1d). They are likely responsible for the potent antioxidant, anti-inflammatory, and chemopreventive properties of cranberry (Aggarwal and Shishodia, 2006; Neto, 2007a,b). It has been shown that phenolic fractions of cranberry prevent oxidative stress, inflammation, and mitochondrial dysfunction in the intestine (Denis et al., 2015). The targets of procyanidins are likely NF-κB deactivation and Nrf2 up-regulation.
2.5. Rosmarinic acid and carnosol (rosemary, Rosmarinus officinalis)
Rosmarinic acid, carnosic acid, and carnosol are major bioactive compounds found in rosemary (Fig. 1e). They have a variety of biological effects including antioxidant, anti-inflammatory, and chemopreventive properties (Gao et al., 2005; Munoz-Munoz et al., 2013). Rosmarinic acid is oxidized by two electrons to give the rosmarinic acid o-quinone which reacts with a variety of cellular proteins mainly at cysteine residues (Tang et al., 2016). For example, tumor necrosis factor α (TNFα)-induced NF-κB activation was suppressed by rosmarinic acid through inhibition of IKK activity (Lee et al., 2006). Carnosol suppressed inducible nitric oxide synthase by down-regulating NF-κB in macrophages (Lo et al., 2002). The mechanism likely involves carnosol o-quinone mediated covalent modification of IKK leading to down regulation of IKK activity preventing NF-κB activation (Fig. 4). Carnosol was also reported to be a potent inducer of cytoprotective enzymes likely through carnosol o-quinone mediated modulation of the Keap1-Nrf2 pathway (Ostreicher et al., 2017; Wu et al., 2014). Similar cytoprotective properties have been reported for carnosic acid, which include inhibition of inflammatory responses and induction of detoxification pathways (Yanagitai et al., 2012). These activities were confirmed in an in vivo model (Kocak et al., 2016).
2.6. γ-Mangostin (mangosteen, Garcinia mangostana)
γ-Mangostin is a bioactive compound present in mangosteen that could contribute to the numerous reported beneficial biological effects of mangosteen including antioxidant, anti-inflammatory, anticancer, and chemopreventive properties (Fig. 1f) (Chang and Yang, 2012; Chin and Kinghorn, 2008; Obolskiy et al., 2009). As a catechol it readily undergoes two electron oxidation catalyzed by numerous oxidative enzymes as well as by ROS. γ-Mangostin has been shown to inhibit IKK activity and decrease cyclooxygenase-2 (COX-2) gene expression likely through γ-mangostin o-quinone modification of IKK (Nakatani et al., 2004). Several other catechols are present in mangosteen which likely have similar biological effects (Obolskiy et al., 2009). In addition, mangosteen contains a variety of o-methoxy phenols which could form o-quinones through O-dealkylation followed by two electron oxidation mechanism (Obolskiy et al., 2009).
3. O-Hydroxylation of phenols followed by two electron oxidation
This pathway tends to be more specific since catechol formation must occur catalyzed by P450s in the endoplasmic reticulum (Fig. 2). The resulting catechols will act as pro- and/or antioxidants depending on the redox state of the cell. Given they are in the endoplasmic reticulum they will likely be readily oxidized to o-quinones directly catalyzed by P450 without generating ROS. More stable quinones can leave the endoplasmic reticulum and react with a variety of biological targets (Fig. 4).
3.1. Genistein (red clover, Trifolium pratense; soy, Glycine max)
Genistein which is present in red clover and soy is a potent bioactive isoflavone which is known for its preferential estrogen receptor β estrogenic effects (Hajirahimkhan et al., 2013). Genistein can also be oxidized by cytochrome P450 1A2 producing the catechol orobol which is further oxidized to an o-quinone (Fig. 2a) (Bolton and Dunlap, 2017; Breinholt et al., 2003; Lee et al., 2016; Roberts-Kirchhoff et al., 1999; Zhang et al., 2009). Studies show that orobol induced oxidative damage to DNA through metal catalyzed Fenton chemistry, whereas genistein had no effect (Murata et al., 2004). These data suggest that genistein could be carcinogenic via oxidative stress and formation of ROS generated upon autoxiation of orobol to orobol o-quinone. However, such a mode of action would have a threshold and not present a cancer risk at low intake when antioxidant protection is not overwhelmed.
3.2. Resveratrol (grapes, red wine, Vitis vinifera)
Resveratrol is present in the skin of red grapes and in red wine (Bhat et al., 2001). It is a potent antioxidant, anti-inflammatory, and inducer of detoxification enzymes including NQO1 (Brisdelli et al., 2009; de la Lastra and Villegas, 2005; Singh et al., 2014). Resveratrol can be hydroxylated by P450 resulting in the catechol piceatannol which is further oxidized to an o-quinone (Fig. 2b) (Piotrowska et al., 2012; Piver et al., 2004; Potter et al., 2002). It has been shown that phorbol ester-induced NF-κB activation and COX-2 expression can be inhibited by piceatannol both in vitro and in vivo (Liu et al., 2014; Son et al., 2010). It appears that alkylation of cysteine 179 of IKKβ is responsible for the inhibitory activity. Similarly, piceatannol prevents lipopolysaccharide induced nitric oxide production and NF-κB activation by alkylation of IKK (Fig. 4) (Islam et al., 2004). Piceatannol also targets the Keap1-Nrf2 pathway (Fig. 4). For example, it has been shown that piceatannol induces HO-1 expression in human mammary epithelial cells through the activation of the Keap1-Nrf2-ARE-driven signaling pathway (Lee et al., 2010). Piceatannol also blocks c-Jun N-terminal kinase (JNK) activation and protects cells against hydrogen peroxide and peroxynitrite-induced apoptosis (Kim et al., 2008). Taken together these data suggest that a number of biological targets are available to piceatannol o-quinone which may explain some of the numerous biological effects of resveratrol.
4. O-Dealkylation followed by two electron oxidation
Because the catechol is masked as an alkyl ether, catechol formation is targeted to the endoplasmic reticulum where P450 catalyzes O-dealkylation. The catechol can act as a pro- and/or antioxidant depending on the redox state of the cell. As described in 3, it is likely that the quinone is formed by direct 2-electron oxidation catalyzed by P450. Once it escapes the endoplasmic reticulum a variety of biological targets are available (Fig. 4).
4.1. Safrole (sassafras, Sassafras albidum)
Safrole is a major constituent of the oil of sassafras. It contains a methylenedioxy ring which can be oxidized by P450 forming the catechol hydroxychavicol (Fig. 3a) (Benedetti et al., 1977). Hydroxychavicol is a major component of the Indian betel leaf which is consumed by millions of people every year. Chewing betel quid has been implicated as a major risk factor for the development of oral squamous-cell carcinoma (IARC Working Group, 1985). Hydroxychavicol is readily oxidized by a variety of oxidative enzymes including cytochrome P450 and peroxidases, forming a relatively stable o-quinone (t1/2 = 9 min, pH 7.4) that is readily trapped by thiol nucleophiles including GSH (Bolton et al., 1994). It has been shown that hydroxychavicol significantly inhibits growth and proliferation through ROS formation in human prostate cancer cells. ROS-induced DNA damage was also observed likely through redox cycling of the o-quinone (Gundala et al., 2014).
4.2. Eugenol (cloves, Syzygium aromaticum)
Cloves are commonly used as a spice. Eugenol is the major bioactive phenol in cloves (Fig. 3a). Eugenol undergoes direct two electron oxidation to an electrophilic quinone methide which is likely responsible for GSH depletion and protein alkylation (Bolton, 2014). Eugenol also contains an o-methoxy substituent which can be oxidized by P450 producing hydroxychavicol which is further oxidized to an o-quinone (Bertrand et al., 1997). Like safrole, the hydroxychavicol o-quinone formed from eugenol is probably responsible for oxidative damage to cells (Atsumi et al., 2005; Bezerra et al., 2017). As far as signaling is concerned, it has been reported that eugenol inhibits cell proliferation by suppressing NF-κB through IKK modification (Manikandan et al., 2011). It is not clear if the quinone methide and/or the o-quinone are responsible for eugenol signaling.
4.3. Methysticin (kava, Piper methysticum)
Kava is consumed as a tea in Polynesia for anxiety and insomnia (Cote et al., 2004). In North America kava is marketed as a dietary supplement and there have been multiple case reports of kava toxicity including liver toxicity requiring liver transplantation (Sarris et al., 2011). It appears that the administration of a traditional kava extract (aqueous) versus kava dietary supplements that are often kava ethanol extracts may explain the different biological effects between the kava preparations (Cote et al., 2004). Kava contains lactones such as methysticin which contains a methylenedioxy ring that can be oxidized to a catechol and quinone similar to safrole (Fig. 3b) (Chen et al., 2011; Johnson et al., 2001, 2003). Formation of the kava quinones leads to GSH depletion and toxicity to hepatocytes (Whitton et al., 2003). Methysticin has also been shown to be a mechanism based inhibitor of numerous P450s likely through quinone formation (Mathews et al., 2002, 2005). Other potential biological targets of kava quinones include inhibition of NF-κB through IKK modification (Folmer et al., 2006). Interestingly, a comparison of a traditional kava preparation with commercial kava extracts using ethanol, acetone, or methanol as a solvent demonstrated a much higher concentration of kavalactones including methysticin in the commercial preparations (Cote et al., 2004). The commercial extracts also lead to higher inhibition of P450 enzymes indicating an enhanced potential of protein alkylation.
4.4. Curcumin (turmeric, Curcuma longa)
Curcumin is the major bioactive compound in the spice turmeric which has been used as an anti-inflammatory remedy in Ayurvedic medicine for centuries. O-Demethylation of the methoxy substituent gives a catechol which is readily oxidized to an o-quinone (Fig. 3c). These oxidized metabolites of curcumin covalently modify IKK leading to inhibition of NF-κB and anti-inflammatory activity (Edwards et al., 2017). Curcumin also modifies Keap1 which leads to activation of the Keap1-Nrf2-ARE pathway causing the increased synthesis of detoxification enzymes such as GST, NQO1, and HO-1 (Balogun et al., 2003; Satoh et al., 2013).
4.5. Capsaicin (chili peppers, Capsicum annuum)
Capsaicin is the spicy component of chili peppers. It undergoes a variety of oxidative reactions including direct two electron oxidation giving a quinone methide. O-Dealkylation of the O-methoxy substituent gives the catechol which is readily oxidized to an o-quinone (Fig. 3d) (Reilly et al., 2013). The catechol and the o-quinone could contribute to a variety of biological effects of chili peppers including antioxidant, chemopreventive, anti-inflammatory properties, as well as DNA damaging effects (Reyes-Escogido Mde et al., 2011). For example, it has been shown that capsaicin induces HO-1 in HepG2 cells through activation of the Keap1-Nrf2 pathway (Joung et al., 2007). Capsaicin has been shown to induce oxidative damage to DNA likely through redox cycling of the o-quinone (Oikawa et al., 2006). It is possible that capsaicin-mediated DNA damage contributes to potential carcinogenic properties. Epidemiological studies have shown a correlation between red chili pepper consumption and gastric, gallbladder, liver, and pancreatic cancers (Lee et al., 1995; Lopez-Carrillo et al., 2003; Pandey and Shukla, 2002; Serra et al., 2002).
4.6. Podophyllotoxin and etoposide (mayapple, Podophyllum peltatum)
Podophyllotoxin (4’-demethylepipodophyllotoxin) is a potent toxin isolated from the mayapple tree (Montecucco et al., 2015; Sinkule, 1984) (Fig. 3e). As podophyllotoxin is too toxic for internal clinical applications, etoposide, a semisynthetic derivative of podophyllotoxin has been developed. Etoposide has been successfully used as a chemotherapeutic agent against various malignancies (Sinkule, 1984). A major metabolite of etoposide involves O-demethylation catalyzed by P450 giving the catechol which is readily oxidized to an o-quinone (Fig. 3e) (Gibson et al., 2016; Jacob et al., 2013; Mans et al., 1992; Smith et al., 2014; van Maanen et al., 1988). The o-quinone of etoposide causes depletion of GSH and oxidative stress within cancer cells inducing apoptosis (Mans et al., 1992; Usami et al., 1998). Similar reactions occur in normal cells which contributes to side effects. The specific target of the etoposide o-quinone is topoisomerase and the etoposide o-quinone is considered a classical topoisomerase II poison (Jacob et al., 2013; Smith et al., 2014). Other biological targets of etoposide o-quinone include stress proteins such as BiP (Wang et al., 2016), GST P1-1 and the JNK signaling pathway (Bernardini et al., 2002), and IKK and the NF-κB signaling pathway (Choi et al., 2006) (Fig. 4).
5. Conclusions and future prospects
These are several examples of both structurally-simple and complex catechols for which data strongly implicate o-quinone intermediates as mediators of toxicity, carcinogenesis, and/or chemoprevention. These electrophiles/redox active compounds could be considerably more important to the metabolism and biological properties of the parent naturally occurring catechols or catechol metabolites than is currently recognized. o-Quinones are formed both enzymatically and non-enzymatically, but the details of these processes and relationships to the structures of phenolic compounds are just beginning to emerge. It is clear that binding to both proteins and DNA competes with detoxification, and that o-quinones are capable of inducing cytotoxic and possibly genotoxic responses (Fig. 4), although it is not clear if genotoxic data from in vitro studies translates to in vivo effects. Alternatively, quinone formation could represent a chemopreventive mechanism for example through induction of detoxification enzymes. The various biological activities of these reactive o-quinones can be seen as a function of their reactivity, concentration, and time of exposure (table of content graphic). Based on these functions different biological targets are anticipated. o-Quinones are reactive intermediates with modest reaction rates that can induce detoxification and chemopreventive responses, for example Nrf2 activation and NF-κB reduction, at low concentrations (Dietz and Bolton, 2011) (Fig. 4). However, higher concentrations can also lead to toxic responses, as in the case of kavalactones. In rare cases, when the quinones can reach the DNA, genotoxic or mutagenic effects might occur. Future studies will seek to clarify relationships between reactivities and biological actions of these electrophiles/redox active compounds and to gain insight into the mechanisms involved in cell damage. The data obtained will assist in clarifying the complex biological properties of phenolic compounds and provide new information on intracellular targets as a function of electrophile/redox reactivity which may be applicable to other types of reactive intermediates. Future in vivo studies will also clarify which of the observed in vitro data can translate to meaningful in vivo and clinical results.
Acknowledgements
Work cited from the authors’ laboratories was supported by NIH Grant P50 AT000155.
Abbreviations:
- ARE
antioxidant response element
- BiP
binding immunoglobin protein
- COX-2
cyclooxygenase-2
- JNK
c-Jun N-terminal kinase
- EGCG
(—)-epigallocatechin gallate
- GST
glutathione-S-transferase
- GSH
glutathione
- HSP
heat shock protein
- HO-1
heme oxygenase-1
- IKK
IκB kinase
- NQO1
NAD(P)H-quinone oxidoreductase 1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PDI
protein disulfide isomerase
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor α
Footnotes
Conflicts of interest
The authors report no conflict of interest.
References
- Aggarwal BB, Shishodia S, 2006. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol 71, 1397–1421. [DOI] [PubMed] [Google Scholar]
- Atsumi T, Fujisawa S, Tonosaki K, 2005. A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicol. Vitro 19, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R, 2003. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J 371, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti MS, Malnoe A, Broillet AL, 1977. Absorption, metabolism and excretion of safrole in the rat and man. Toxicology 7, 69–83. [DOI] [PubMed] [Google Scholar]
- Bernardini S, Bellincampi L, Ballerini S, Ranalli M, Pastore A, Cortese C, Federici G, 2002. Role of GST P1-1 in mediating the effect of etoposide on human neuroblastoma cell line Sh-Sy5y. J. Cell. Biochem 86, 340–347. [DOI] [PubMed] [Google Scholar]
- Bertrand F, Basketter DA, Roberts DW, Lepoittevin JP, 1997. Skin sensitization to eugenol and isoeugenol in mice: possible metabolic pathways involving ortho-quinone and quinone methide intermediates. Chem. Res. Toxicol 10, 335–343. [DOI] [PubMed] [Google Scholar]
- Bezerra DP, Militao GCG, de Morais MC, de Sousa DP, 2017. The dual anti-oxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 9, 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KPL, Kosmeder ,JW 2nd, Pezzuto JM., 2001. Biological effects of resveratrol. Antioxidants Redox Signal. 3, 1041–1064. [DOI] [PubMed] [Google Scholar]
- Boersma MG, Vervoort J, Szymusiak H, Lemanska K, Tyrakowska B, Cenas N, Segura-Aguilar J, Rietjens IM, 2000. Regioselectivity and reversibility of the glutathione conjugation of quercetin quinone methide. Chem. Res. Toxicol 13, 185–191. [DOI] [PubMed] [Google Scholar]
- Bolton JL, 2002. Quinoids, quinoid radicals, and phenoxyl radicals formed from estrogens and antiestrogens. Toxicology 177, 55–65. [DOI] [PubMed] [Google Scholar]
- Bolton JL, 2014. Quinone methide bioactivation pathway: contribution to toxicity and/or cytoprotection? Curr. Org. Chem 18, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Acay NM, Vukomanovic V, 1994. Evidence that 4-allyl-o-quinones spontaneously rearrange to their more electrophilic quinone methides: potential bioactivation mechanism for the hepatocarcinogen safrole. Chem. Res. Toxicol 7, 443–450. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Dunlap T, 2017. Formation and biological targets of quinones: cytotoxic versus cytoprotective effects. Chem. Res. Toxicol 30, 13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinholt VM, Rasmussen SE, Brosen K, Friedberg TH, 2003. In vitro metabolism of genistein and tangeretin by human and murine cytochrome P450s. Pharmacol. Toxicol 93, 14–22. [DOI] [PubMed] [Google Scholar]
- Brisdelli F, D’Andrea G, Bozzi A, 2009. Resveratrol: a natural polyphenol with multiple chemopreventive properties. Curr. Drug Metabol 10, 530–546. [DOI] [PubMed] [Google Scholar]
- Brown JP, 1980. A review of the genetic effects of naturally occurring flavonoids, anthraquinolines and related compounds. Mutat. Res 75, 243–277. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Artacho R, Gimenez R, 2006. Beneficial effects of green tea–a review. J. Am. Coll. Nutr 25, 79–99. [DOI] [PubMed] [Google Scholar]
- Chang HF, Yang LL, 2012. Gamma-mangostin, a micronutrient of mangosteen fruit, induces apoptosis in human colon cancer cells. Molecules 17, 8010–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Serag ES, Sneed KB, Zhou SF, 2011. Herbal bioactivation, molecular targets and the toxicity relevance. Chem. Biol. Interact 192, 161–176. [DOI] [PubMed] [Google Scholar]
- Chin YW, Kinghorn AD, 2008. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini-Rev. Org. Chem 5, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Park CS, Choi J, Rhim H, Chun HJ, 2001. Cytotoxic effect of urushiol on human ovarian cancer cells. J. Microbiol. Biotechnol 11, 399–405. [Google Scholar]
- Choi YS, Park H, Jeong S, 2006. Role of PI3-kinase/Akt pathway in the activation of etoposide-induced NF-kappa B transcription factor. J. Microbiol. Biotechnol 16, 391–398. [Google Scholar]
- Cote CS, Kor C, Cohen J, Auclair K, 2004. Composition and biological activity of traditional and commercial kava extracts. Biochem. Biophys. Res. Commun 322, 147–152. [DOI] [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I, 2005. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol. Nutr. Food Res 49, 405–430. [DOI] [PubMed] [Google Scholar]
- Denis MC, Desjardins Y, Furtos A, Marcil V, Dudonne S, Montoudis A, Garofalo C, Delvin E, Marette A, Levy E, 2015. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin. Sci. (Lond.) 128, 197–212. [DOI] [PubMed] [Google Scholar]
- Dietz BM, Bolton JL, 2011. Biological reactive intermediates (BRIs) formed from botanical dietary supplements. Chem. Biol. Interact 192, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz BM, Hajirahimkhan A, Dunlap TL, Bolton JL, 2016. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev 68, 1026–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn IS, Liberato DJ, Castagnoli N, Byers VS, 1982. Contact sensitivity to urushiol: role of covalent bond formation. Cell. Immunol 74, 220–233. [DOI] [PubMed] [Google Scholar]
- Edwards RL, Luis PB, Varuzza PV, Joseph AI, Presley SH, Chaturvedi R, Schneider C, 2017. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem 292, 21243–21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, Diederich M, 2006. Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem. Pharmacol 71, 1206–1218. [DOI] [PubMed] [Google Scholar]
- Gao LP, Wei HL, Zhao HS, Xiao SY, Zheng RL, 2005. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie 60, 62–65. [PubMed] [Google Scholar]
- Gibson EG, King MM, Mercer SL, Deweese JE, 2016. Two-mechanism model for the interaction of etoposide quinone with topoisomerase IIalpha. Chem. Res. Toxicol 29, 1541–1548. [DOI] [PubMed] [Google Scholar]
- Gundala SR, Yang C, Mukkavilli R, Paranjpe R, Brahmbhatt M, Pannu V, Cheng A, Reid MD, Aneja R, 2014. Hydroxychavicol, a betel leaf component, inhibits prostate cancer through ROS-driven DNA damage and apoptosis. Toxicol. Appl. Pharmacol 280, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirahimkhan A, Dietz BM, Bolton JL, 2013. Botanical modulation of menopausal symptoms: mechanisms of action? Planta Med 79, 538–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko K, Weinberg I, Ingber A, 2005. Exploring the mango-poison ivy connection: the riddle of discriminative plant dermatitis. Contact Dermatitis 52, 3–5. [DOI] [PubMed] [Google Scholar]
- IARC Working Group, 1985. International agency for research on cancer: betel-quid and areca nut chewing. IARC Monograph 37, 141–291. [Google Scholar]
- Ishii T, Ishikawa M, Miyoshi N, Yasunaga M, Akagawa M, Uchida K, Nakamura Y, 2009. Catechol type polyphenol is a potential modifier of protein sulfhydryls: development and application of a new probe for understanding the dietary polyphenol actions. Chem. Res. Toxicol 22, 1689–1698. [DOI] [PubMed] [Google Scholar]
- Ishii T, Mori T, Tanaka T, Mizuno D, Yamaji R, Kumazawa S, Nakayama T, Akagawa M, 2008. Covalent modification of proteins by green tea polyphenol (-)-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med 45, 1384–1394. [DOI] [PubMed] [Google Scholar]
- Islam S, Hassan F, Mu MM, Ito H, Koide N, Mori I, Yoshida T, Yokochi T, 2004. Piceatannol prevents lipopolysaccharide (LPS)-induced nitric oxide (NO) production and nuclear factor (NF)-kappaB activation by inhibiting IkappaB kinase (IKK). Microbiol. Immunol 48, 729–736. [DOI] [PubMed] [Google Scholar]
- Jacob DA, Gibson EG, Mercer SL, Deweese JE, 2013. Etoposide catechol is an oxidizable topoisomerase II poison. Chem. Res. Toxicol 26, 1156–1158. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Bolton JL, van Breemen RB, 2001. Screening botanical extracts for quinoid metabolites. Chem. Res. Toxicol 14, 1546–1551. [DOI] [PubMed] [Google Scholar]
- Johnson BM, Qiu SX, Zhang S, Zhang F, Burdette JE, Yu L, Bolton JL, van Breemen RB, 2003. Identification of novel electrophilic metabolites of piper methysticum Forst (Kava). Chem. Res. Toxicol 16, 733–740. [DOI] [PubMed] [Google Scholar]
- Joung EJ, Li MH, Lee HG, Somparn N, Jung YS, Na HK, Kim SH, Cha YN, Surh YJ, 2007. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxidants Redox Signal. 9, 2087–2098. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Leeb KW, Kim MS, Lee HJ, 2008. Piceatannol attenuates hydrogen-peroxide- and peroxynitrite-induced apoptosis of PCI2 cells by blocking down-regulation of Bcl-X-L and activation of JNK. J. Nutr. Biochem 19, 459–466. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T, 2011. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 218, 44–52. [DOI] [PubMed] [Google Scholar]
- Kocak C, Kocak FE, Akcilar R, Isiklar OO, Kocak H, Bayat Z, Simsek H, Taser F, Altuntas I, 2016. Molecular and biochemical evidence on the protective effects of embelin and carnosic acid in isoproterenol-induced acute myocardial injury in rats. Life Sci. 147, 15–23. [DOI] [PubMed] [Google Scholar]
- Lee HH, Park SA, Almazari I, Kim EH, Na HK, Surh YJ, 2010. Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling. Arch. Biochem. Biophys 501, 142–150. [DOI] [PubMed] [Google Scholar]
- Lee J, Jung E, Kim Y, Lee J, Park J, Hong S, Hyun CG, Park D, Kim YS, 2006. Rosmarinic acid as a downstream inhibitor of IKK-beta in TNF-alpha-induced upregulation of CCL11 and CCR3. Br. J. Pharmacol 148, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Park BJ, Yoo KY, Ahn YO, 1995. Dietary factors and stomach cancer: a case-control study in Korea. Int. J. Epidemiol 24, 33–41. [DOI] [PubMed] [Google Scholar]
- Lee SH, Baek K, Lee JE, Kim BG, 2016. Using tyrosinase as a monophenol mono-oxygenase: a combined strategy for effective inhibition of melanin formation. Biotechnol. Bioeng 113, 735–743. [DOI] [PubMed] [Google Scholar]
- Lee-Hilz YY, Boerboom AM, Westphal AH, Berkel WJ, Aarts JM, Rietjens IM, 2006. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol 19, 1499–1505. [DOI] [PubMed] [Google Scholar]
- Liberato DJ, Byers VS, Dennick RG, Castagnoli N, 1981. Regiospecific attack of nitrogen and sulfur nucleophiles on quinones derived from poison oak/ivy catechols (urushiols) and analogues as models for urushiol-protein conjugate formation. J. Med. Chem 24, 28–33. [DOI] [PubMed] [Google Scholar]
- Liu L, Li J, Kundu JK, Surh YJ, 2014. Piceatannol inhibits phorbol ester-induced expression of COX-2 and iNOS in HR-1 hairless mouse skin by blocking the activation of NF-kappaB and AP-1. Inflamm. Res 63, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Liu XW, Sok DE, 2003. Identification of alkylation-sensitive target chaperone proteins and their reactivity with natural products containing Michael acceptor. Arch Pharm. Res. (Seoul) 26, 1047–1054. [DOI] [PubMed] [Google Scholar]
- Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK, 2002. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis 23, 983–991. [DOI] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Lopez-Cervantes M, Robles-Diaz G, Ramirez-Espitia A, Mohar-Betancourt A, Meneses-Garcia A, Lopez-Vidal Y, Blair A, 2003. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int. J. Cane 106, 277–282. [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Jurd L, 1978. Mutagenicity of plant flavonoids: structural requirements for mutagenic activity in Salmonella typhimurium. Mutat. Res 54, 297–309. [DOI] [PubMed] [Google Scholar]
- Manikandan P, Vinothini G, Vidya Priyadarsini R, Prathiba D, Nagini S, 2011. Eugenol inhibits cell proliferation via NF-kappaB suppression in a rat model of gastric carcinogenesis induced by MNNG. Invest. N. Drugs 29, 110–117. [DOI] [PubMed] [Google Scholar]
- Mans DR, Lafleur MV, Westmijze EJ, Horn IR, Bets D, Schuurhuis GJ, Lankelma J, Retel J, 1992. Reactions of glutathione with the catechol, the ortho-quinone and the semi-quinone free radical of etoposide. Consequences for DNA inactivation. Biochem. Pharmacol 43, 1761–1768. [DOI] [PubMed] [Google Scholar]
- Mathews JM, Etheridge AS, Black SR, 2002. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab. Dispos 30, 1153–1157. [DOI] [PubMed] [Google Scholar]
- Mathews JM, Etheridge AS, Valentine JL, Black SR, Coleman DP, Patel P, So J, Burka LT, 2005. Pharmacokinetics and disposition of the kavalactone kawain: interaction with kava extract and kavalactones in vivo and in vitro. Drug Metab. Dispos 33, 1555–1563. [DOI] [PubMed] [Google Scholar]
- Montecucco A, Zanetta F, Biamonti G, 2015. Molecular mechanisms of etoposide. EXCLI. J 14, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moridani MY, Scobie H, Salehi P, O’Brien PJ, 2001. Catechin metabolism: glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome p450. Chem. Res. Toxicol 14, 841–848. [DOI] [PubMed] [Google Scholar]
- Munoz-Munoz JL, Garcia-Molina F, Ros E, Tudela J, Garcia-Canovas F, Rodriguez-Lopez JN, 2013. Prooxidant and antioxidant activities of rosmarinic acid. J. Food Biochem 37, 396–408. [Google Scholar]
- Murata M, Midorikawa K, Koh M, Umezawa K, Kawanishi S, 2004. Genistein and daidzein induce cell proliferation and their metabolites cause oxidative DNA damage in relation to isoflavone-induced cancer of estrogen-sensitive organs. Biochemistry 43, 2569–2577. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yamakuni T, Kondo N, Arakawa T, Oosawa K, Shimura S, Inoue H, Ohizumi Y, 2004. gamma-Mangostin inhibits inhibitor-kappaB kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol. Pharmacol 66, 667–674. [DOI] [PubMed] [Google Scholar]
- Neto CC, 2007a. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res 51, 652–664. [DOI] [PubMed] [Google Scholar]
- Neto CC, 2007b. Cranberry and its phytochemicals: a review of in vitro anti cancer studies. J. Nutr 137, 186S–193S. [DOI] [PubMed] [Google Scholar]
- Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M, 2009. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother Res. 23, 1047–1065. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Nagao E, Sakano K, Kawanishi S, 2006. Mechanism of oxidative DNA damage induced by capsaicin, a principal ingredient of hot chili pepper. Free Radic. Res 40, 966–973. [DOI] [PubMed] [Google Scholar]
- Ostreicher C, Bartenbacher S, Pischetsrieder M, 2017. Targeted proteome analysis with isotope-coded protein labels for monitoring the influence of dietary phyto-chemicals on the expression of cytoprotective proteins in primary human colon cells. J.Proteomics 166, 27–38. [DOI] [PubMed] [Google Scholar]
- Pandey M, Shukla VK, 2002. Diet and gallbladder cancer: a case-control study. Eur. J. Cane. Prev 11, 365–368. [DOI] [PubMed] [Google Scholar]
- Peng Z, Gong X, Yang Y, Huang L, Zhang Q, Zhang P, Wan R, Zhang B, 2017. Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-kappaB and MAPK signal pathways. Int. Immunopharm 52, 281–289. [DOI] [PubMed] [Google Scholar]
- Pierce EN, Piyankarage SC, Dunlap T, Litosh V, Siklos MI, Wang YT, Thatcher GR, 2016. Prodrugs bioactivated to quinones target NF-kappaB and multiple protein networks: identification of the quinonome. Chem. Res. Toxicol 29, 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska H, Kucinska M, Murias M, 2012. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat. Res 750, 60–82. [DOI] [PubMed] [Google Scholar]
- Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F, Lucas D, 2004. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem. Pharmacol 68, 773–782. [DOI] [PubMed] [Google Scholar]
- Poddar K, Kolge S, Bezman L, Mullin GE, Cheskin LJ, 2011. Nutraceutical supplements for weight loss: a systematic review. Nutr. Clin. Pract 26, 539–552. [DOI] [PubMed] [Google Scholar]
- Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T, Ruparelia KC, Lamb JH, Farmer PB, Stanley LA, Burke MD, 2002. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br. J. Cane 86, 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly CA, Henion F, Bugni TS, Ethirajan M, Stockmann C, Pramanik KC, Srivastava SK, Yost GS, 2013. Reactive intermediates produced from the metabolism of the vanilloid ring of capsaicinoids by P450 enzymes. Chem. Res. Toxicol 26, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E, 2011. Chemical and pharmacological aspects of capsaicin. Molecules 16, 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietjens IM, Boersma MG, van der Woude H, Jeurissen SM, Schutte ME, Alink GM, 2005. Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat. Res 574, 124–138. [DOI] [PubMed] [Google Scholar]
- Rios JL, Giner RM, Marin M, Recio MC, 2018. A pharmacological update of ellagic acid. Planta Med. 10.1055/a-0633-9492. [DOI] [PubMed] [Google Scholar]
- Roberts-Kirchhoff ES, Crowley JR, Hollenberg PF, Kim H, 1999. Metabolism of genistein by rat and human cytochrome P450s. Chem. Res. Toxicol 12, 610–616. [DOI] [PubMed] [Google Scholar]
- Sarris J, LaPorte E, Schweitzer I, 2011. Kava: a comprehensive review of efficacy, safety, and psychopharmacology. Aust. N. Z. J. Psychiatr 45, 27–35. [DOI] [PubMed] [Google Scholar]
- Satoh T, McKercher SR, Lipton SA, 2013. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med 65, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K, Watanabe H, Tajima K, 2002. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int. J. Cane 102, 407–411. [DOI] [PubMed] [Google Scholar]
- Singh B, Shoulson R, Chatterjee A, Ronghe A, Bhat NK, Dim DC, Bhat HK, 2014. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 35, 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BN, Shankar S, Srivastava RK, 2011. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol 82, 1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkule JA, 1984. Etoposide: a semisynthetic epipodophyllotoxin. Chemistry, pharmacology, pharmacokinetics, adverse effects and use as an antineoplastic agent. Pharmacotherapy 4, 61–73. [DOI] [PubMed] [Google Scholar]
- Smith NA, Byl JA, Mercer SL, Deweese JE, Osheroff N, 2014. Etoposide quinone is a covalent poison of human topoisomerase II beta. Biochemistry 53, 3229–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son PS, Park SA, Na HK, Jue DM, Kim S, Surh YJ, 2010. Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-{kappa}B activation and cyclooxygenase-2 expression in human breast epithelial cells: cysteine 179 of IKK {beta} as a potential target. Carcinogenesis 31, 1442–1449. [DOI] [PubMed] [Google Scholar]
- Sriram N, Kalayarasan S, Sudhandiran G, 2009. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem. Biol. Interact 180, 271–280. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Miyoshi N, Isemura M, 2012. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 88, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CB, Zhang WG, Wang YS, Xing LJ, Xu XL, Zhou GH, 2016. Identification of rosmarinic acid-adducted sites in meat proteins in a gel model under oxidative stress by triple TOF MS/MS. J. Agric. Food Chem 64, 6466–6476. [DOI] [PubMed] [Google Scholar]
- Tanigawa S, Fujii M, Hou DX, 2007. Action of Nrf2 and Keap1 in are-mediated NQO1 expression by quercetin. Free Radic. Biol. Med 42, 1690–1703. [DOI] [PubMed] [Google Scholar]
- Usami I, Kubota M, Bessho R, Kataoka A, Koishi S, Watanabe K, Sawada M, Lin YW, Akiyama Y, Furusho K, 1998. Role of protein tyrosine phosphorylation in etoposide-induced apoptosis and NF-kappa B activation. Biochem. Pharmacol 55, 185–191. [DOI] [PubMed] [Google Scholar]
- van der Woude H, Alink GM, van Rossum BE, Walle K, van Steeg H, Walle T, Rietjens IM, 2005. Formation of transient covalent protein and DNA adducts by quercetin in cells with and without oxidative enzyme activity. Chem. Res. Toxicol 18, 1907–1916. [DOI] [PubMed] [Google Scholar]
- van Maanen JM, Verkerk UH, Broersen J, Lafleur MV, De Vries J, Retel J, Pinedo HM, 1988. Semi-quinone formation from the catechol and ortho-quinone metabolites of the antitumor agent VP-16–213. Free Radic. Res. Commun 4, 371–384. [DOI] [PubMed] [Google Scholar]
- Walle T, Vincent TS, Walle UK, 2003. Evidence of covalent binding of the dietary flavonoid quercetin to DNA and protein in human intestinal and hepatic cells. Biochem. Pharmacol 65, 1603–1610. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang F, Cao Y, Zhang M, Wang A, Xu M, Su M, Zhang M, Zhuge Y, 2016. Etoposide induces apoptosis in activated human hepatic stellate cells via ER stress. Sci. Rep 6, 34330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang Y, Wan X, Yang CS, Zhang J, 2015. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol 283, 65–74. [DOI] [PubMed] [Google Scholar]
- Whitton PA, Lau A, Salisbury A, Whitehouse J, Evans CS, 2003. Kava lactones and the kava-kava controversy. Phytochemistry 64, 673–679. [DOI] [PubMed] [Google Scholar]
- Wu KC, McDonald PR, Liu J, Klaassen CD, 2014. Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med. 80, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagitai M, Itoh S, Kitagawa T, Takenouchi T, Kitani H, Satoh T, 2012. Carnosic acid, a pro-electrophilic compound, inhibits LPS-induced activation of microglia. Biochem. Biophys. Res. Commun 418, 22–26. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tu T, d’Avignon DA, Gross ML, 2009. Balance of beneficial and deleterious health effects of quinones: a case study of the chemical properties of genistein and estrone quinones. J. Am. Chem. Soc 131, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Zhang S, Yin Q, Zhang J, 2015. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B pathway. Phcog. Mag 11, 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]


