Abstract
Background:
West Nile fever, as an expanding zoonotic disease, has been reported from different creatures involved in the disease from Iran. In addition to biological mosquito-associated factors, various elements such as their activities, distribution, behavior and vectorial capacity could be affected by environmental factors. We determined the distribution of West Nile virus (WNV) vectors, the environmental factors affecting WNV transmission and the high-risk areas across West Azerbaijan Province (Northwestern Iran), regarding the potential of WNV transmission using Geographical Information System (GIS).
Methods:
Mosquitoes’ larvae and adults were collected from different habitats of the province in 2015 and identified using standard morphological keys. The data regarding the distribution of mosquitoes across the studied area were organized in ArcMap databases. Inverse Distance Weighted (IDW) interpolation analysis was conducted on the data of synoptic stations to find climatic variables in the collection sites of different mosquito species. Layers of transmission-related environmental factors were categorized and weighed based on their effects on disease transmission.
Results:
Overall, 2813 samples of different mosquito species from different regions of the province were collected and identified. According to the GIS analysis, areas in the northeastern province, which have lower altitudes and slopes with higher temperatures and more water bodies, were found to have better condition for the activity of mosquitoes (as high-risk areas: hot spots).
Conclusion:
The precision of our results was proven to be in line with previous study results that identified high-risk areas, where WNV-infected vectors were captured from these same areas.
Keywords: Mosquitoes, Aedes caspius, Culex pipiens, Iran
Introduction
Due to notable problems caused by mosquitoes and mosquito-borne diseases (MBDs), the studies of factors influencing the presence, activities, and distribution of mosquitoes and MBDs are important and form absolute parts of the epidemiology of MBDs, in which environmental conditions and their changes are components of this process (1). In addition to biological factors, mosquitoes’ activities, distribution, behavior, and even their vectorial capacity could be affected by environmental conditions (2–5).
Mosquitoes’ feeding rates vary expressively with temperature. Moreover, feeding behavior and host availability are affected by climate. Additionally, length of the gonotrophic cycle as an important factor in diseases transmission could be influenced by precipitation patterns (6). Finally, a positive correlation between temperature and West Nile and West Nile virus (WNV) incidence in mosquitoes have been reported (7, 8).
Among the different tools used to identify the effects of environmental factors on MBDs and their epidemiology, is Geographical Information System (GIS) (9). In addition, global positioning systems (GPS), remote sensing, and spatial statistics could also play important role in MBDs research, surveillance, and control programs (10).
Among the MBDs, West Nile fever is an emerging zoonosis rapidly spread and caused expansive health threat in different parts of the world (11, 12). The transmission cycle of WNV includes a wide range of migratory birds as reservoirs (13), equines (14) and humans (15) as dead-end hosts, and numerous mosquito species including different species e.g. Culex pipiens, Cx. restuans, Cx. salinarius, Cx. tarsalis, Aedes vexans, Ae. albopictus, and Coquillettidia perturbans as biological vectors (16–18). WNV has been isolated and identified in numerous mosquito species including different species of Culex, Aedes, and Anopheles (19–21). Due to the wide use of GIS in the study of vector-borne diseases (including MBDs) (22, 23), several studies have employed GIS across the world to predict, risk assessment (18), surveillance (25, 26) and the environmental factors influencing WNV transmission (27, 28).
Because of the presence of theoretically favorable environments for the establishment of WNV across Iran, the presence of WNV has been investigated and showed the sero-prevalence rate of (1.3%) in human (29–33), 23.7% in equines (34), overall (15%) infection in birds (35) and recently among the potential vector species, in which among them WNV has been isolated and reported from Ae. caspius (36) and Culex spp (37).
Iranian West Azerbaijan Province in the northwestern part of the country, could be considered as one of the most suspicious areas in Iran regarding the possibility of establishment and transmission of WNV, because of its abundant water resources and wetlands for migratory birds from different parts around the world, which serve as reservoirs of WNV (38, 39) and also, the presence of potential vector species of mosquitoes in this region e.g. Culex pipiens s.l., Ae. caspius, Anopheles maculipennis s.l., Culiseta longiareolata (40–42). As the first isolation of WNV from its potential vectors was reported from this region (36), and this region borders several countries such as Turkey, Iraq, Armenia, and the Republic of Azerbaijan, more attention is needed at this area.
Due to the special circumstances mentioned above about West Azerbaijan Province, we aimed to determine: 1) the distribution of probable WNV vectors, 2) the environmental factors affecting WNV transmission and 3) the high-risk areas across the province regarding the potential of WNV transmission using GIS.
Materials and Methods
Study area
West Azerbaijan Province is located in the northwest of Iran between latitudes 35° 58′–39° 46′ N and longitudes 44° 3′–47° 23′ E. This province formally includes 17 counties. It is bordered by Turkey, Iraq, Armenia, and the Republic of Azerbaijan. In addition, it is also bordered by Iranian provinces such as East Azerbaijan, Zanjan and Kurdistan (Fig. 1). According to information obtained from Forests, Range and Watershed management organization, West Azerbaijan Province have five types of Micro-climates, including Highly semi-arid (HSA), Moderate semi-arid (MSA), Slight semi-arid (SSA), semi-wet (SW) and wet (W).
Fig. 1.
Study area in northwestern Iran
Distribution of potential West Nile Vectors in West Azerbaijan Province
Mosquitoes were collected during May–Nov 2015 from 24 localities (wetlands) across the province (Table 1). Adults and larvae were collected from different habitats using standard methods (43). Collected samples were quickly identified using a stereo microscope and a standard morphological key at the collection sites (44).
Table 1.
Collection sites of mosquitoes in West Azerbaijan Province, northwestern Iran, 2015
| County | Collection site | Longitude | Latitude | Altitude | Ae. caspius | An. maculipennis | Cx. pipiens | Cx. theileri | Cs. longiareolata |
|---|---|---|---|---|---|---|---|---|---|
| Bazargan | Bazargan | 44.38944 | 39.38833 | 1400 | 0 | 25 | 1 | 13 | 0 |
| Yarim-Ghiye | 44.43656 | 39.44604 | 1409 | 175 | 65 | 0 | 420 | 0 | |
| Khoy | Hashiyeh rood | 45.06315 | 38.57117 | 1058 | 3 | 0 | 6 | 18 | 0 |
| Mahabad | Mahabad | 45.71667 | 36.75 | 1351 | 0 | 10 | 89 | 25 | 4 |
| Khoor-khooreh | 45.72341 | 36.98732 | 1279 | 205 | 2 | 120 | 0 | 30 | |
| Kani barazan-wetland | 45.77716 | 36.98689 | 1275 | 0 | 0 | 68 | 1 | 0 | |
| Hajib khosh | 45.79718 | 36.90788 | 1288 | 0 | 0 | 136 | 0 | 0 | |
| Gapis | 45.74795 | 36.93805 | 1286 | 0 | 0 | 4 | 7 | 0 | |
| Beytas | 45.69427 | 36.67645 | 1396 | 3 | 0 | 28 | 39 | 0 | |
| Makoo | Makoo | 44.43333 | 39.3333 | 1411 | 6 | 7 | 82 | 23 | 3 |
| Sangar | 44.43429 | 39.31578 | 1355 | 288 | 132 | 100 | 169 | 90 | |
| Milan | 44.4332 | 39.34351 | 1445 | 37 | 115 | 25 | 22 | 0 | |
| Keshmesh tappeh | 44.40093 | 39.33351 | 1385 | 0 | 0 | 150 | 94 | 0 | |
| Glik gadim | 44.66767 | 39.71264 | 807 | 31 | 0 | 26 | 0 | 0 | |
| Deim-Gheshlagh | 45.07119 | 39.34766 | 784 | 0 | 58 | 0 | 0 | 0 | |
| Deimgeshlag | 44.7987 | 39.62488 | 797 | 190 | 0 | 20 | 0 | 0 | |
| Miandoab | Miandoab | 46.06947 | 36.98592 | 1291 | 0 | 0 | 98 | 0 | 0 |
| Naghadeh | Naghadeh | 45.41667 | 36.95 | 1338 | 0 | 56 | 0 | 0 | 7 |
| Yadegarloo | 45.52839 | 37.03822 | 1284 | 0 | 10 | 0 | 23 | 19 | |
| Plodasht | Poldasht | 45.07111 | 39.34778 | 788 | 0 | 10 | 55 | 89 | 0 |
| Khol-kholeh | 44.74524 | 39.67784 | 784 | 0 | 3 | 0 | 40 | 0 | |
| Ghooch-Ali | 44.74391 | 39.66296 | 1401 | 0 | 25 | 1 | 5 | 0 | |
| Sardasht | Sardasht | 45.48333 | 36.15 | 1556 | 0 | 18 | 0 | 0 | 0 |
| Urmia | Urmia | 45.05 | 37.66667 | 1328 | 0 | 128 | 151 | 163 | 246 |
| Naz-loo | 44.98442 | 37.65172 | 1358 | 0 | 0 | 441 | 67 | 10 | |
| Ghahraman-loo | 45.17485 | 37.64896 | 1000 | 170 | 0 | 24 | 0 | 0 | |
| Koor-Abad | 44.64239 | 37.72749 | 1545 | 0 | 68 | 34 | 0 | 6 | |
| Silvana | 44.85142 | 37.42867 | 1577 | 0 | 120 | 0 | 0 | 0 | |
| Gojar | 44.83373 | 39.48785 | 1736 | 0 | 7 | 15 | 19 | 7 | |
| Mavana | 44.79643 | 37.56658 | 1617 | 0 | 13 | 0 | 32 | 24 | |
| Shaharchay dam | 44.98628 | 37.4952 | 1433 | 0 | 11 | 0 | 10 | 3 | |
| Talebin | 44.83398 | 37.54025 | 1608 | 0 | 0 | 0 | 149 | 0 | |
Climatic and environmental data
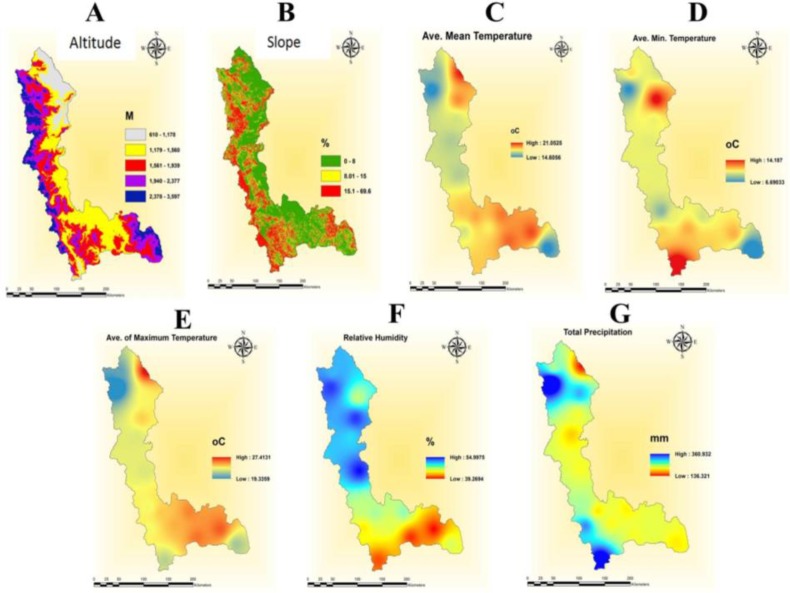
As distribution of mosquitoes and their transmitted diseases are notably dependent on environmental and climatic factors, the effective climatic data, including maximum monthly temperature, minimum monthly temperature, mean monthly temperature, mean relative humidity and rainfall data of decade (2004–2014) were obtained from 16 stations of West Azerbaijan Meteorological Organization (Table 2). Datasets at district level were created in Excel sheets for further analysis by ArcGIS 10.3. The pattern of recent decade of Maximum, Mean and Minimum temperature of different areas in West Azerbaijan Province was analyzed and mapped in Fig. 2 and summarized in Table 2.
Table 2.
Average of environmental variables in meteorological stations of West Azerbaijan Province of Iran, 1997–2016
| Localities | Geographical properties | Environmental variable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longitude | Latitude | Altitude | Total Rainfall | Mean Relative Humidity | Mean Temp | Min Temp | Max Temp | ||||||
| Bookan | 46.21667 | 36.53333 | 1386.1 | 360.5 | 46 | 14.5 | 6.3 | 19.9 | |||||
| Chaldoran | 44.38333 | 39.06667 | 1788 | 449.5 | 58 | 9.2 | 2.7 | 13.9 | |||||
| Ghareh Ziahadin | 45.01667 | 38.9 | 1108 | 357.3 | 52 | 13.6 | 8 | 18 | |||||
| Khoy | 44.96667 | 38.55 | 1103 | 289.2 | 59 | 12.1 | 5.5 | 18.6 | |||||
| Mahabad | 45.71667 | 36.75 | 1351.8 | 403.8 | 53 | 13.0 | 6.9 | 19.15 | |||||
| Makoo | 44.43333 | 39.3333 | 1411.3 | 302.8 | 57 | 10.6 | 5.5 | 15.6 | |||||
| Miandoab | 46.05 | 36.96667 | 1300 | 303.8 | 53 | 14.4 | 5.6 | 20.0 | |||||
| Naghadeh | 45.41667 | 36.95 | 1338 | 339.0 | 53 | 13.9 | 5.8 | 19.2 | |||||
| Oshnavie | 45.13333 | 37.05 | 1415.9 | 437.3 | 52 | 13.5 | 4.5 | 18.6 | |||||
| Piranshahr | 45.15 | 36.7 | 1443.5 | 672.7 | 52 | 12.0 | 6.2 | 17.9 | |||||
| Poldasht | 45.07119 | 39.34778 | 787 | 198.8 | 51 | 14.7 | 4.8 | 20.2 | |||||
| Salmas | 44.85 | 38.21667 | 1337 | 247.5 | 57 | 11.5 | 5.2 | 17.8 | |||||
| Sardasht | 45.48333 | 36.15 | 1556.8 | 841.2 | 49 | 13.1 | 9.2 | 16.8 | |||||
| Shahindej | 46.73333 | 36.6667 | 1395 | 334.8 | 47 | 14.9 | 6.7 | 20.4 | |||||
| Takab | 47.1 | 36.4 | 1817.2 | 338.6 | 55 | 9.4 | 2.5 | 16.4 | |||||
| Urmia | 45.05 | 37.66667 | 1328 | 338.9 | 61 | 11.6 | 5.4 | 17.7 | |||||
Fig. 2.
Environmental and meteorological variables affecting mosquito distribution in West Azerbaijan Province, northwestern Iran, (A): Altitude, (B): Slope, (C): Average of Mean Temperature, (D): Average of Minimum Temperature, (E): Average of Maximum Temperature, (F): Relative Humidity and (G): Total Precipitation.
Spatial Analysis
For proper analysis, the data regarding distribution of mosquitoes across West Azerbaijan Province were acquired from previous studies (30, 33) and added to the findings of this survey in database created in ArcMap. Inverse distance weighted (IDW) interpolation analysis was conducted on the data of synoptic stations to find the climatic variables in the collection sites of different mosquito species. This analysis interpolates a raster surface from points and estimates cell values by averaging the values of nearby sample data points. The closer a point is to the center of the cell which is being estimated, the more weight it is given. The equation for IDW analysis is:
where
v̂= value to be estimated
vi= known value
di..., dn= distances from the n data points to the point estimated n
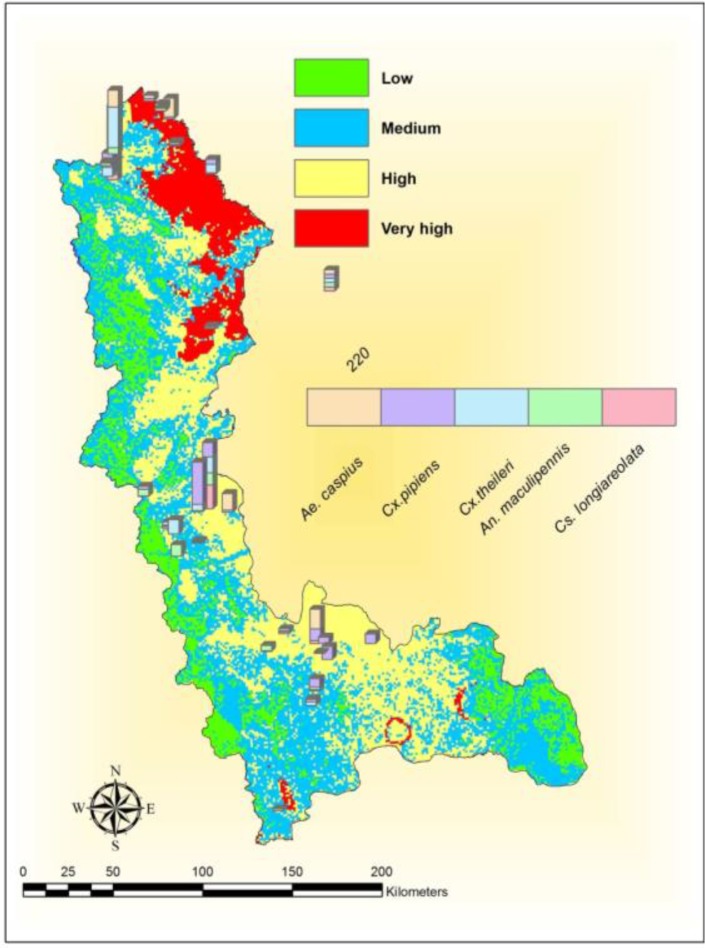
Layers of environmental factors that are important in transmitting the WNV were categorized and weighted based on their effects on disease transmission, and important impact on vector-borne diseases (3, 45–47) (Table 3). The categorized and weighted environmental factors were overlaid with the vectors distribution across the West Azerbaijan Province for determination of high-risk areas for the establishment of the transmission cycle of WNV based on the mentioned environmental factors. As the five species (Ae. caspius, An. maculipennis, Cs. longiareolata, Cx. pipiens and Cx. theileri) reported from study area, also are known proven and suspected vectors of WNV in different parts of the world, they have been included in final analysis (Fig. 3).
Table 3.
Categorizing the environmental factors based on their effects on disease transmission, and important impact on vector-borne diseases (3, 45–47)
| Environmental Factor | Ratio | Standard weight | Category | Group | Effect on diseases Transmission |
|---|---|---|---|---|---|
| Mean Temperature (°Celsius) | 55 | 0.55 | 26–30 | 4 | Very High |
| 20–26 | 3 | High | |||
| 18–20 | 2 | Medium | |||
| 14–18 | 1 | Low | |||
| Relative Humidity (%) | 35 | 0.35 | 60< | 4 | Very High |
| 50–60 | 3 | High | |||
| 40–50 | 2 | Medium | |||
| <40 | 1 | Low | |||
| Altitude (m) | 5 | 0.05 | 0–800 | 4 | Very High |
| 800–1200 | 3 | High | |||
| 1200–2000 | 2 | Medium | |||
| <2000 | 1 | Low | |||
| Slope (%) | 5 | 0.05 | <8 | 3 | Very High |
| 8–15 | 2 | Medium | |||
| 15< | 1 | Low | |||
Fig. 3.
Spatial distribution of mosquitoes and hot spots for vectors of West Nile virus in West Azerbaijan Province regarding environmental factors affecting the transmission of WNV, northwestern Iran, 2015
Ethics approval and consent to participate
Prior to the approval of all projects by the Urmia University of Medical Sciences (UMSU), they are reviewed and endorsed by the Ethics Committee of the UMSU. Sample collection was carried out from private human and animal dwellings. At least one day prior to any sample collection, the owners were informed by the Local Health System officers. The whole process was coordinated, managed and documented by the “Local Health System officer” in the study areas.
Results
Overall, 2813 mosquito specimens from different regions of the province were collected and identified (Table 1). Analysis of some climatic variables across the collection sites for different mosquito species is summarized in Table 4. The most frequent species in our study was Cx. pipiens (22 out of 24 collection sites), while Ae. caspius was found only in 10 localities.
Table 4.
Average of some environmental and climatic variables in the collection sites of mosquitoes, West Azerbaijan Province, northwestern Iran, 2015
| Species | No. of collection sites | Precipitation (mm) | Average of Altitude (M) | Average of Relative Humidity (%) | Average of Maximum Temperature (°C) | Average of Minimum Temperature (°C) | Average of Mean Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Ae. caspius | 10 | 326.3 | 1214.7 | 56 | 17.21 | 5.61 | 11.74 |
| An. maculipennis | 20 | 347.3 | 1273.9 | 54.91 | 17.75 | 5.66 | 12.4 |
| Cx. pipiens | 22 | 328.6 | 1227.5 | 55.33 | 17.89 | 5.6 | 12.3 |
| Cx. theileri | 21 | 330.65 | 1275.1 | 55.78 | 17.65 | 5.6 | 12.1 |
| Cs. longiareolata | 12 | 345.1 | 1364.25 | 56.14 | 17.95 | 5.66 | 12.3 |
The altitudinal activity of suspected vectors was determined across the areas with an average of 1214m for Ae. caspius to 1364m for Cs. longiareolata (Table 4). Considering the altitude of the study area which ranges from 605m to 3600m, the values of the altitude were classified into five categories, by overlying the presence of potential vectors and their distribution map on altitude values of the province, height less than 1615m (605–1500m), are more prepared and favored places for mosquitoes in the study areas. Finally using the scale of the most important environmental variables (Table 3), determination of high risk areas for WNV and distribution of potential vectors across the studied areas was analyzed and revealed in Fig. 3. The map of the mosquitoes including proven and suspected vectors of WNV, ie Cx. pipiens, Cx. theileri and Ae. caspius were collected mostly in hot spots, determined by the model (Fig. 3).
Discussion
The study area has a large number of wet-lands that host of migratory birds. Although WNV is an enzootic disease among birds (48), however, humans transmission is possible through bites of infected mosquitoes (49). WNV is responsible for disease outbreaks among human in the United States, Europe, and the Middle East (50). It has been detected in recent studies from birds, horse, human and mosquitoes in Iran (31–36).
In the present study, environmental factors affecting the transmission of WNV were studied and high-risk areas were determined (Fig. 3). Earlier study on the impact of climate and environmental variables on WNV in Iran, using data from seropositive horses, found four studied factors that correlated with WNV infection in equine, these factors were temperature, distance to wetlands, and local and regional Normalized Difference Vegetation Index (NDVI) (27). Results from previous studies also indicated the presence of WNV vectors (Cx. pipiens, Cx. theileri, Ae. caspius) in the region (36, 40) and their infection with WNV in Iran (36). In addition, the studied areas have the potential for establishment of WNV transmission. The results of current study regarding the determination of the high-risk areas are interesting because they are in accordance with results from previous study that isolated West Nile virus from vectors (36). The isolation of the virus in the mentioned study has been reported from the high-risk areas identified in current study and this point shows the acceptable accuracy of the results of the current study which analyzed environmental factors (temperature, relative humidity, Altitude and Slope). These areas should be considered in planning of WNV epidemic control programs. Among the probable reasons increasing the risk of establishing transmission cycle of WNV in these areas is the wide range of wetlands in the area and the presence of migratory birds as the reservoirs of disease and also the appropriateness of environmental conditions for the presence and abundance of various species of mosquitoes, as potential vectors of WNV. Also it seems northeastern areas of the province, which have lower altitudes and slopes with warmer temperatures and more water bodies, were found to have better condition for the activity of mosquitoes.
Diversity of environmental conditions in West Azerbaijan Province, which provides suitable environment for the establishment of various species of mosquitoes (Table 1) have been found in this current and related studies (36, 40, 42). Other results have shown the effect of temperature in transmission of WNV by Cx. pipiens, and the effect of temperature on the replication of the virus within the mosquito’s body and the incubation period, as well (51). WNV has the ability to replicate in mosquitoes in wider range of temperatures between 14 °C in mosquitoes (52) to 45 °C in birds (53). By increasing the temperature, WNV propagation rate could be increased.
The suitable temperature for WNV replication is provided in the northeastern and southern parts of the provinces of Iran. Therefore, these areas need more investigation on blood feeding pattern of mosquitoes and their infection with WNV. Recent study on the feeding patterns of potential WNV vectors in South-West Spain showed that Cx. modestus, Cx. perexiguus and Cx. pipiens mainly feed on birds, while Cx. theileri and Ae. caspius mainly feed on mammals. Cx. perexiguus had the highest potential for enzootic virus transmission, followed by Cx. modestus and Cx. pipiens. According to results of the South-West Spanish study, potential transmission risk to humans was low for Cx. pipiens, Cx. theileri and Ae. caspius (54). The frequency of feeding on humans was only affected by season, while the low number of human blood meals was related to their study site. On the other hand, the researchers worked in natural areas with very low anthropic presence (54). Most of these mosquito species have been reported from our study area as well (36), therefore, they may have some roles in both avian to avian enzootic cycle and avian-to-mammal transmission.
Certainly, the current study did not cover all important environmental factors affecting the potential of WNV transmission regarding the environmental factors and the effect of other important factors such as wetland, Normalized Difference Vegetation Index (NDVI) and land use should be analyzed and determined in future studies.
Conclusion
The present study serves as a preliminary guide that shows the important effects of environmental conditions on one of the important members (mosquitoes) in the transmission cycle of WNV and should be continued with supplementary studies. Taking into account the vector bio-ecologic conditions, other environmental factors and the interaction of the virus and the vectors as well as other important rings in the transmission of disease, the data obtained from this current study will be very useful and effective in knowing the exact nature of the disease transmission pathways and help in designing its control strategies.
Acknowledgements
This article is part of the results of the first author’s dissertation for fulfillment of MSc degree in Medical Entomology and Vector Control from the Department of Medical Entomology and Vector Control, School of Public Health, Urmia University of Medical Sciences, Urmia, Iran.
This study was financially supported by Urmia University of Medical Sciences, Urmia, Iran (Project no. 1579). The authors would like to thank the health staff of the studied regions for their kind support and contributions.
The authors declare that there is no conflict of interests.
References
- 1.Khormi HM, Kumar L. (2011) Examples of using spatial information technologies for mapping and modelling mosquitoborne diseases based on environmental, climatic, socioeconomic factors and different spatial statistics, temporal risk indices and spatial analysis: a review. J Food Agr Environ. 9: 41–49. [Google Scholar]
- 2.Drakeley CJ, Carneiro I, Reyburn H, Malima R, Lusingu JP, Cox J, Theander TG, Nkya WM, Lemnge MM, Riley EM. (2005) Altitude-dependent and independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J Infect Dis. 191: 1589–1598. [DOI] [PubMed] [Google Scholar]
- 3.Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Charrahy Z, Haghdoost A, Zamani G, Abedi F, Sedaghat MM, Soltani M, Shahi M, Raeisi A. (2012) Spatial analysis and mapping of malaria risk in an endemic area, south of Iran: a GIS based decision making for planning of control. Acta Trop. 122(1): 132–137. [DOI] [PubMed] [Google Scholar]
- 4.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. (2008) Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerg Infect Dis. 14(10): 1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paaijmans KP, Blanford S, Chan BH, Thomas MB. (2011) Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett: 8(3): 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. (2014) The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol. 51: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, Haramis L, Goldberg TL, Kitron UD. (2010) Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 3(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn MB, Monaghan AJ, Hayden MH, Eisen RJ, Delorey MJ, Lindsey N, Nasci RS, Fischer M. (2015) Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 92(5): 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen L, Eisen RJ. (2011) Using geographic information systems and decision support systems for the prediction, prevention, and control of vector-borne diseases. Annu Rev Entomol. 56: 41–61. [DOI] [PubMed] [Google Scholar]
- 10.Kitron U. (1998) Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. J Med Entomol. 35: 435–445. [DOI] [PubMed] [Google Scholar]
- 11.Hayes EB, Gubler DJ. (2006) West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 57: 181–194. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie JS, Gubler DJ, Petersen LR. (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 10: S98–S109. [DOI] [PubMed] [Google Scholar]
- 13.Rappole JH, Derrickson SR, Hubálek Z. (2000) Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis. 6: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autorino GL, Battisti A, Deubel V, Ferrari G, Forletta R, Giovannini A, Lelli R, Murri S, Scicluna MT. (2002) West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 8(12): 1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsey NP, Staples JE, Lehman JA, Fischer M. (2010) Surveillance for human West Nile virus disease-United States, 1999–2008. MMWR Surveill Summ. 59(2): 1–17. [PubMed] [Google Scholar]
- 16.Hubalek Z, Halouzka J, Juricova Z, Sebesta O. (1998) First isolation of mosquito-borne West Nile virus in the Czech Republic. Acta Virol. 42: 119–120. [PubMed] [Google Scholar]
- 17.Hubalek Z, Rudolf I, Bakonyi T, Kazdova K, Halouzka J, Sebesta O, Sikutová S, Juricová Z, Nowotny N. (2010) Mosquito (Diptera: Culicidae) surveillance for arboviruses in an area endemic for West Nile (Lineage Rabensburg) and Tahyna viruses in Central Europe. J Med Entomol. 47(3): 466–472. [DOI] [PubMed] [Google Scholar]
- 18.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. (2000) Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 38(11): 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romi R, Pontuale G, Ciufolini M, Fiorentini G, Marchi A, Nicoletti L, Cocchi M, Tamburro A. (2004) Potential vectors of West Nile virus following an equine disease outbreak in Italy. Med Vet Entomol. 18(1): 14–19. [DOI] [PubMed] [Google Scholar]
- 20.Turell MJ, Sardelis MR, Dohm DJ, O’Guinn ML. (2001) Potential North American vectors of West Nile virus. Ann NY Acad Sci. 951: 317–324. [DOI] [PubMed] [Google Scholar]
- 21.Ergünay K, Litzba N, Brinkmann A, Günay F, Sarıkaya Y, Kar S, Örsten S, Öter K, Domingo C, Erisoz Kasap Ö, Özkul A, Mitchell L, Nitsche A, Alten B, Linton YM. (2017) Co-circulation of West Nile virus and distinct insect-specific flaviviruses in Turkey. Parasit Vectors. 10(1): 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcantonio M, Rizzoli A, Metz M, Rosà R, Marini G, Chadwick E, Neteler M. (2015) Identifying the environmental conditions favouring West Nile Virus outbreaks in Europe. PLoS One. 10(3): e0121158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valiakos G, Papaspyropoulos K, Giannakopoulos A, Birtsas P, Tsiodras S, Hutchings MR, Spyrou V, Pervanidou D, Athanasiou LV, Papadopoulos N, Tsokana C, Baka A, Manolakou K, Chatzopoulos D, Artois M, Yon L, Hannant D, Petrovska L, Hadjichristodoulou C, Billinis C. (2014) Use of wild bird surveillance, human case data and GIS spatial analysis for predicting spatial distributions of West Nile virus in Greece. PloS One. 9(5): e96935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Gómez A, Amela C, Fernández-Carrión E, Martínez-Avilés M, Sánchez-Vizcaíno JM, Sierra-Moros MJ. (2017) Risk mapping of West Nile virus circulation in Spain, 2015. Acta Trop. 169: 163–169. [DOI] [PubMed] [Google Scholar]
- 25.Eisen RJ, Eisen L. (2014) Use of geographic information systems in infectious disease surveillance. In: M’ikanatha NM, Iskander J. (Eds) Concepts and Methods in Infectious Disease Surveillance, Wiley-Blackwell, pp. 219–229. [Google Scholar]
- 26.Moore M. (2014) Geographic Analysis of West Nile Virus in the Upper Minnesota River Valley: A GIS and Multi-temporal Remote Sensing Approach. [MSc dissertation]. Minnesota State University, Mankato Mankato, Minnesota, USA. [Google Scholar]
- 27.Ahmadnejad F, Otarod V, Fathnia A, Ahmadabadi A, Fallah MH, Zavareh A, Miandehi N, Durand B, Sabatier P. (2016 ) Impact of climate and environmental factors on West Nile virus circulation in Iran. J Arthropod Borne Dis. 10(3): 315–27. [PMC free article] [PubMed] [Google Scholar]
- 28.Roiz D, Ruiz S, Soriguer R, Figuerola J. (2015) Landscape effects on the presence, abundance and diversity of mosquitoes in Mediterranean Wetlands. PloS One. 10(6): e0128112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naficy K, Saidi S. (1970) Serological survey on viral antibodies in Iran. Trop Geogr Med. 22: 183–188. [PubMed] [Google Scholar]
- 30.Saidi S, Tesh R, Javadian E, Nadim A. (1976) The prevalence of human infection with West Nile virus in Iran. Iran J Public Health. 5: 8–13. [Google Scholar]
- 31.Talebian A. (2010) A study of West Nile virus infection in Iranian blood donors. Arch Iran Med. 13(1): 1–4 [PubMed] [Google Scholar]
- 32.Chinikar S, Javadi A, Ataei B, Shakeri H, Moradi M, Mostafavi E, Ghiasi SM. (2012) Detection of West Nile virus genome and specific antibodies in Iranian encephalitis patients. Epidemiol Infect. 140(8): 1525–1529. [DOI] [PubMed] [Google Scholar]
- 33.Meshkat Z, Chinikar S, Shakeri M, Manavifar L, Moradi M, Mirshahabi H, Jalali T, Khakifirouz S, Shahhosseini N. (2015) Prevalence of West Nile virus in Mashhad, Iran: A population–based study. Asian Pac J Trop Med. 8(3): 203–205. [DOI] [PubMed] [Google Scholar]
- 34.Ahmadnejad F, Otarod V, Fallah M, Lowenski S, Sedighi-Moghaddam R, Zavareh A, Durand B, Lecollinet S, Sabatier P. (2011) Spread of West Nile virus in Iran: A cross-sectional serosurvey in equines, 2008–2009. Epidemiol Infect. 139(10): 1587–1593. [DOI] [PubMed] [Google Scholar]
- 35.Fereidouni SR, Ziegler U, Linke S, Niedrig M, Modirrousta H, Hoffmann B, Groschup MH. (2011) West Nile virus monitoring in migrating and resident water birds in Iran: are common coots the main reservoirs of the virus in wetlands? Vector Borne Zoonotic Dis. 11 (10): 1377–1381. [DOI] [PubMed] [Google Scholar]
- 36.Bagheri M, Terenius O, Oshaghi MA, Motazakker M, Asgari S, Dabiri F, Vatandoost H, Mohammadi Bavani M, Chavshin AR. (2015) West Nile virus in mosquitoes of Iranian wetlands. Vector Borne Zoonotic Dis. 15(12): 750–754. [DOI] [PubMed] [Google Scholar]
- 37.Shahhosseini N, Chinikar S, Moosa-Kazemi SH, Sedaghat MM, Kayedi MH, Lühken R, Schmidt-Chanasit J. (2017) West Nile Virus lineage-2 in Culex specimens from Iran. Trop Med Int Health. 22(10): 1343–1349. [DOI] [PubMed] [Google Scholar]
- 38.Salmanzadeh R, Majidi A, Jabbari H, Abbasnejad H, Saket A. (2011) The Study of Mahabad’ s Kanibarazan Wetland Biodiversity Indexes, Iran. Int Proc Chem Biol Environ. 24: 73–77. [Google Scholar]
- 39.John R. (2005) “Birds of Azerbaijan” by Michael Patrikeev (book review). Can field-nat. 119: 299. [Google Scholar]
- 40.Khoshdel-Nezamiha F, Vatandoost H, Azari-Hamidian S, Bavani MM, Dabiri F, Entezar-Mahdi M, Chavshin AR. (2014) Fauna and larval habitats of mosquitoes (Diptera: culicidae) of west Azerbaijan Province, northwestern Iran. J Arthropod Borne Dis. 8(2): 163–173. [PMC free article] [PubMed] [Google Scholar]
- 41.Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE. (2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 23(2): 111–21. [DOI] [PubMed] [Google Scholar]
- 42.Khoshdel-Nezamiha F, Vatandoost H, Oshaghi MA, Azari-Hamidian S, Mianroodi RA, Dabiri F, Bagheri M, Terenius O, Chavshin AR. (2016) Molecular characterization of mosquitoes (Diptera: Culicidae) in Northwestern Iran by using rDNA-ITS2. Jpn J Infect Dis. 69(4): 319–322. [DOI] [PubMed] [Google Scholar]
- 43.Silver JB. (2008) Mosquito ecology: field sampling methods: Springer. [Google Scholar]
- 44.Azari-Hamidian S, Harbach RE. (2009) Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa. 2078: 1–33 [Google Scholar]
- 45.Dohm DJ, O’Guinn ML, Turell MJ. (2002) Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 39: 221–225. [DOI] [PubMed] [Google Scholar]
- 46.Dohm DJ, Turell MJ. (2001) Effect of incubation at overwintering temperatures on the replication of West Nile virus in New York Culex pipiens (Diptera: Culicidae). J Med Entomol. 38: 462–4. [DOI] [PubMed] [Google Scholar]
- 47.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. (2008) Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 4: e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malkinson M, Banet C. (2002) The role of birds in the ecology of West Nile virus in Europe and Africa. In: Mackenzie JS, Barrett ADT, Deubel V. (Eds) Japanese Encephalitis and West Nile Viruses, Springer; pp. 309–322. [DOI] [PubMed] [Google Scholar]
- 49.Murgue B, Murri S, Zientara S, Durand B, Durand JP, Zeller H. (2001) West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg Infect Dis. 7: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer LD, Styer LM, Ebel GD. (2008) A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 53: 61–81. [DOI] [PubMed] [Google Scholar]
- 51.Hess A, Cherubin C, LaMotte L. (1963) Relation of temperature to activity of western and St. Louis encephalitis viruses. Am J Trop Med Hyg. 12: 657–667. [Google Scholar]
- 52.Cornel AJ, Jupp PG, Blackburn NK. (1993) Environmental temperature on the vector competence of Culex univittatus (Diptera: Culicidae) for West Nile virus. J Med Entomol. 30: 449–456. [DOI] [PubMed] [Google Scholar]
- 53.Kinney RM, Huang CY-H, Whiteman MC, Bowen RA, Langevin SA, Miller BR, Brault AC. (2006) Avian virulence and thermostable replication of the North American strain of West Nile virus. J Gen Virol. 87(Pt 12): 3611–3622. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz J, Ruiz S, Soriguer R, Alcaide M, Viana DS, Roiz D, Vázquez A, Figuerola J. (2012) Feeding patterns of potential West Nile virus vectors in south-west Spain. PloS One. 7: e39549. [DOI] [PMC free article] [PubMed] [Google Scholar]